Abstract
Traditional evaluation of pain in animals has primarily used reflexive withdrawal or nocifensive response from singly presented stimulation. However, daily experience of thermal sensation involves situations in which rapid temperature changes from cold to hot can occur. Therefore, in order to better understand integration of competing stimuli and their role in the motivational character of pain perception, behavioral tasks have been adapted to evaluate treatment-driven changes in hindpaws when exposed to two or more stimuli. However, such assessments of craniofacial sensitivity are lacking. In this study, we sought to characterize thermal preference for facial stimulation when rats are given the option of experiencing a hot or cold stimulus to obtain a milk reward, or abstaining from stimulation. We found that when both cold and hot stimuli were either non-noxious or where both stimuli were noxious the hot stimulus was preferred. When the hot stimulus was noxious, non-noxious cold was preferred. Unstimulated time was dependent on the combined aversiveness of the two stimuli, such that unstimulated time was the greatest with a highly aversive stimulus pair (−4 and 48°C). We also found that pairing stimuli modulated successful task completion for each stimulus, but for nociceptive heat, this was not solely a consequence of thermal preference. Finally, we found that previous preference could both induce and abolish subsequent thermode preference independent of stimulus cues. The findings in this study will allow us to evaluate experimental pain states and analgesic treatments in a manner more relatable to the experience of the patient.
Keywords: thermal preference, operant, behavior, cold, hot, orofacial, pain, avoidance
1. Introduction
Traditional behavioral testing methods for evaluating thermal processing in animals have primarily used reflexive withdrawal from a single hot or cold stimulus. However, in our daily experience we encounter a mix of thermal stimuli that produce complex sensations. Even in a non-pathological state, the summation of disparate stimuli can produce unpleasant or painful sensations that are greater than might be produced by an individual stimulus, as occurs in the thermal grill illusion [9]. Recent molecular and electrophysiological studies indicate that this phenomenon may be due in part to the co-expression of molecular mediators of hot and cold thermal transduction within a single nociceptor, such as overlapping expression among transient receptor potential channels vanilloid 1 (TRPV1), melastatin 8 (TRPM8), and ankyrin 1 (TRPA1) [15, 36]. Populations of heat- and/or cold-sensing nociceptors can also provide convergent input to second order neurons that transmit noxious signals to higher centers [10, 37, 38]. The central integration of these stimuli, as well as the context in which they are presented, influences pain perception and motivation.
Thus it is important to accompany molecular and electrophysiological studies with behavioral assays that evaluate pain-related decision making in the presence of differing stimuli. Some groups have used the thermal preference task to evaluate sensory integration of stimuli detected in the hindpaws of rodents either with two stimulating options [18, 31, 32, 34] or a gradient of stimuli [18]. While this is sufficient to evaluate pain states targeting the hindpaw, no such method exists to evaluate pain within the head and face. It is important to evaluate facial pain specifically because there are forms of chronic pain unique to the trigeminal system, such as headaches and trigeminal neuralgia. Some differences have also been noted between the trigeminal and sciatic nervous systems, including differences in basal expression of the TRPM8 important for cold perception [15], expression of the inflammatory mediator interleukin-6 following chronic constriction injury [17], and the efficacy of serotonin antagonists [13]. These differences could have implications for how pain is processed in the craniofacial region.
We previously characterized an operant assay that assesses the ability of rats to obtain a milk reward while self-stimulating the face with a single stimulus [23, 24]. We have also presented thermal preference for a hot stimulus with a combination of −4 and 48°C [24], which are both noxious, i.e. capable of causing tissue damage with sufficient duration of contact. Our goal in this study was to expand on these findings and characterize thermal preference with various hot and cold stimuli, including those that range from unpleasant, but not painful (e.g. 24°C) to potentially painful, but non-noxious (e.g. 10, 45°C).
In humans, cold pain thresholds are reported at about 18°C [35], but unpleasantness thresholds have been reported within +6°C from pain thresholds [11]. Reflex and nocifensive tests of rats often identify statistically significant responses lower, near ∼5°C [2], but this may reflect the threshold for pain intolerance occurring just prior to noxious stimulation. Or findings and an operant escape assay stimulating the hindpaw indicate that innocuous cold stimuli can modestly reduce operant outcome measures, suggesting aversion to these stimuli relative to equivalent warmth [20, 24]. Heat pain thresholds in humans are reported at about 43°C [35] and unpleasantness is more tightly coupled with pain, occurring within ∼1°C of the pain threshold [11]. Our work in rats similarly indicates a threshold of response around 45°C, with sharp decline in reward attainment as temperature increases [23]. Based on these data, we paired cold stimuli (24, 18, and 10°C) with hot stimuli ranging from 42 to 52°C.
We also examined the effect of previous experience on modulating subsequent preference and these findings suggest previous experience can serve to condition an aversion or preference, which could have implications for evaluating affect of pain. This assay could therefore provide a useful tool for evaluating perceptual and motivational aspects of pain states and analgesic treatments in a manner more relatable to the experience of the patient.
2. Materials and Methods
2.1 Animals
Male hairless Sprague-Dawley rats (seven weeks old, Charles River, Raleigh, NC) were housed in groups of five in enriched housing (see ref for description) and were maintained in a standard 12-hour light/dark cycle and were allowed access to food (Harlan Teklad LM-485 Mouse/Rat Sterilizable Diet, Harlan Labs, Tampa, FL) and water ad libitum when not being tested. Rats’ weights were recorded every week to monitor general health. Animal testing procedures and general handling complied with the ethical guidelines and standards established by the Institutional Animal Care & Use Committee at the University of Florida, and all procedures complied with the Guide for Care and Use of Laboratory Animals (1996).
2.2 Thermal Preference Testing of the Face
Facial testing was completed using a reward-conflict operant testing paradigm as described previously [23, 24]. Briefly, the rats were trained to drink sweetened condensed milk while making facial contact with a thermode. During the training period (approximately 2 weeks) their baseline intake was recorded, and the rats were considered ready for experimental testing once their average baseline intake was 10g or greater at 37°C. Training was performed in the single stimulus condition for all rats. Once trained, the facial testing region for each animal was depilitated under light isoflurane anesthesia (inhalation, 2.5 %) once a week to maximize thermal stimulus contact. The rats were fasted over night (13−15hrs) prior to each recorded session.
The thermal preference of the rats was recorded as previously described in Rossi et al. 2006 [24]. Rats were trained in the single task condition and initially placed in the thermal preference apparatus with both thermodes set at 37°C to allow them to become accustomed to this new task. A second such session was recorded to ensure rats did not demonstrate a side preference. Rats were able to move freely from one side of the compartment to the other and explore both thermodes at will. Subsequently, rats (n = 5−10) were tested on separate days at the following temperature combinations: 10/42°C, 10/10°C, 10/45°C, 18/45°C, 18/52°C, 18/48°C, 10/48°C, 24/45°C, and 24/48°C. Additional animals were also tested at 10/45°C (n =5), and 10/48°C (n =15), and found to exhibit the same pattern of preferences as the first group, thus their data were pooled. Cold and hot thermodes were alternated across testing sessions to prevent a learned side preference. Two days or more separated the introduction of a new stimulus pair, except for the conditioned aversion/preference experiments described in section 3.3 of the Results, where 10/42°C, was followed one day later by 10/10°C, and 52/18°C was followed by 18/48°C. We also present data previously published (thermal preference for −4 and 48°C, n = 7 rats) for comparison with the other stimulus combinations and to illustrate the percentage of unstimulated time not reported in the previous publication [24].
The number of licking contacts with each thermode, were calculated for and totaled to determine the percentage of licks obtained on each side and at each temperature. The duration of time spent on each thermode was used to determine the percentage of time spent stimulating with the hot or cold stimulus, as well as the time spent not performing the reward task (i.e. unstimulated). An assessment of successful task completion for each stimulus was made by dividing the number of licks (rewarded attempts) by the number of stimulus contacts (the number of total contacts or attempts) for each stimulus. In order to evaluate the effect of temperature combination on the success ratios, the single stimulus ratios were compared with the ratios calculated for the same stimulus when paired with another. Single stimulus data were collected in a separate group of rats.
2.3 Statistical Analysis
All statistical analyses were performed using SPSS (v. 16.0, SPSS, Inc.). Rats were excluded from consideration if they failed to switch sides. For within stimulus pair comparisons, paired t-tests were used to determine the difference between the percentage of licks on the cold and hot thermodes (or right and left for neutral conditions) and repeated measures analysis of variance (ANOVA) was used to determine significant differences in the percentage of time spent on either thermode or unstimulated. For cold stimuli paired with 45°C stimulation, One-way ANOVAs were used to compare licks and time across the different stimulus pairs on the cold stimuli, on the 45°C stimulus, or off the stimuli. This was also true for cold stimuli paired with 48°C stimulation. For the hot stimuli, 45 and 48°C, one-way ANOVAs with post-hoc Tukey's test were used to compare success ratios for the single stimulus condition with success ratios for that stimulus when paired with various cold stimuli (i.e. success at 45°C alone versus success at 45°C when paired with 10°C, with 18°C, etc.). For the cold stimuli, t-tests were used to compare success ratios for the stimulus presented alone versus success ratios at that cold stimulus when paired with a hot stimulus (e.g. success at 10°C alone versus success at 10°C when paired with 45°C, etc.). Repeated measures ANOVA was used to assess the effect of previous experience on duration spent on the thermode (left or right) targeted by the previous stimulating conditions, as well as unstimulated time. When significant effects were found with one-way ANOVA, post-hoc comparisons were made using Tukey's test and for the repeated measures ANOVA, with least squared difference test (LSD). Statistical significant was set to p<0.05.
3. Results
3.1. Thermal Preference and Unstimulated Time
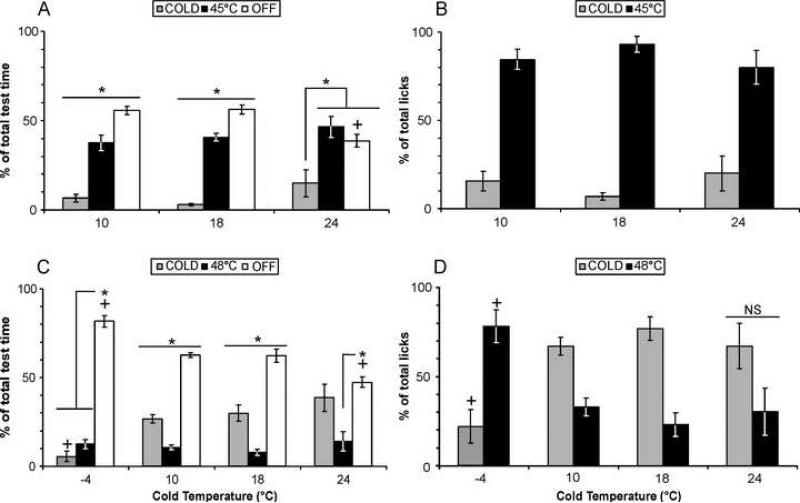
Although individuals may exhibit a side preference when exposed to a pair of neutral temperatures, this preference was not consistent across testing sessions. When all rats percentage of licks and time spent on each thermode were averaged no side bias was observed, as we have previously reported [17]. With few exceptions, individual rats exhibit a temperature preference each time they are exposed to hot and cold pair of stimuli, and most individuals exhibit the same preference, predictive of the group mean (Table 1). We tested a range of cold stimuli paired either with 45 or 48°C. For all pairs including 45°C as the hot stimulus, 45°C is strongly preferred, with approximately 40% of testing time and >80% of total licks spent in contact with this stimulus (Fig. 1A, B, see Table 2 for within-pair statistics). There was no significant difference in the percentage of time or licks spent on the 45°C stimulus or the cold stimulus when compared across stimulus pairs (Fig. 1A, B). However, the unstimulated time is significantly lower when 24°C is the cold option than for the other two stimulus pairs (Fig. 1A).
Table 1.
Number of rats exhibiting a cold, hot, or no preference at the stimulus combinations tested, as determined by the percentage of licks; individuals in agreement with the group preference are denoted in bold.
| Temperature Pair (°C) | Cold Preference | Hot Preference | No Preference* | Total n |
|---|---|---|---|---|
| −4 & 48 | 2 | 11 | 1 | 14 |
| 10 & 48 | 37 | 15 | 2 | 54 |
| 18 & 48 | 8 | 1 | 0 | 9 |
| 24 & 48 | 7 | 2 | 0 | 9 |
| 10 & 45 | 2 | 12 | 0 | 14 |
| 18 & 45 | 0 | 9 | 0 | 9 |
| 24 & 45 | 2 | 10 | 0 | 12 |
| 37 & 37** | 11 | 14 | 2 | 27 |
“No preference” is defined as 50 ± 5% of licks spent in contact with either stimulus
For 37&37°C, the count in the “cold preference” column reflects number of rats with a left thermode preference
Fig. 1. Distribution of time spent on the cold, hot, or off the thermode and percentage of licks spent at either thermode when cold stimuli were paired with 45 or 48°C.
(A) More time is spent on the 45°C stimulus when paired with a 10, 18, or 24°C cold stimulus (see Table 2 for F statistics). Significantly less time is spent unstimulated (“Off”) when the cold stimulus is 24°C relative to the unstimulated time for the other two pairs (F 2, 30 = 7.016). (B) The majority of licks also occur in contact with the 45°C stimulus (see Table 2), but there is no significant difference among licks in contact with cold thermode across stimulus pairs (F 2, 30 = 1.192). (C) More time is spent on the non-noxious cold stimulus (10, 18, 24°C) when paired with 48°C. In contrast, more time is spent at 48°C when the cold stimulus is noxious (−4°C). The lowest percentage of unstimulated time occurs when the cold stimulus is 24°C and the greatest when it is −4°C (F 3, 76 = 20.481, p<0.001). The percentage of time spent on the cold stimulus when it is −4°C is significantly less than the percentage of time on all other cold stimuli when paired with 48°C (F 3, 76 = 20.481, p<0.001). (D) More licks occur at 10 or 18°C than at 48°C (although with 24°C as the cold stimulus this does not reach statistical significance), and the opposite is true when −4 and 48°C are paired (see Table 2). * indicates significant difference (i.e. preference) in time spent on either stimulus or unstimulated (repeated-measures ANOVAs with post-hoc LSD test) or between licks spent on the two stimuli (paired t-tests). + indicates a significant difference in percentage of licks or testing time across stimulus pairs for cold stimuli, hot stimuli, or unstimulated duration (one-way ANOVA with post-hoc Tukey's test). All data are mean ± SEM, significance is p < 0.05.
Table 2.
Statistical determination of group preferences for each stimulus pair, as indicated by percentage of total licks (paired t-tests) and by percentage of total testing duration (repeated measures ANOVA, with post-hoc least squared differences, or LSD, test).
| Temperature Pair (°C) | Licks Hot - Cold t statistics | Test Time Hot, Cold, Off F statistics | LSD Test | df |
|---|---|---|---|---|
| −4 & 48 | −2.975* | 142.073** | H=C<Off | 13 |
| 10 & 48 | −3.453* | 147.75** | H<C <Off | 53 |
| 18 & 48 | −4.096* | 37.86** | H<C <Off | 8 |
| 24 & 48 | −1.425 | 6.034* | H=C=Off (H<Off) | 8 |
| 10 & 45 | 6.17** | 52.335** | Off>H>C | 13 |
| 18 & 45 | 21.352** | 100.667** | Off>H>C | 8 |
| 24 & 45 | 3.049* | 5.075* | Off=H>C | 11 |
Abbreviations: H = hot stimulus, C = cold stimulus, df = degrees of freedom.
p<0.05
p<0.001
As indicated by individual preferences, non-noxious cold stimuli (10, 18, 24°C) are preferred when paired with a noxious hot stimulus, 48°C (Fig. 1C, D, and see Table 2 for within-pair statistics). However, these cold preferences are moderate, with <50% of time and ∼ 70% of licks spent in contact with the cold thermode. In contrast, when 48°C is paired with a noxious cold stimulus (−4°C), 48°C is preferred (Fig. 1C, D).
When comparing across stimulus pairs, there was no significant difference in the percentage of time spent on the 48°C stimulus (10−15%, Fig. 1C), even when the licking preference switches from cold to hot (at 48 with −4°C). In contrast, both time and licks are significantly lower for the −4°C cold stimulus than the non-noxious cold stimuli (Fig. 1D). As was the case for 45°C, the percentage of unstimulated time is modulated by the intensity of the cold stimulus; unstimulated time at 24/48°C was significantly lower, and at −4/48°C was significantly greater than with all other pairs (Fig. 1C).
3.2. Effect of Pairing Stimuli on Successful Task Completion
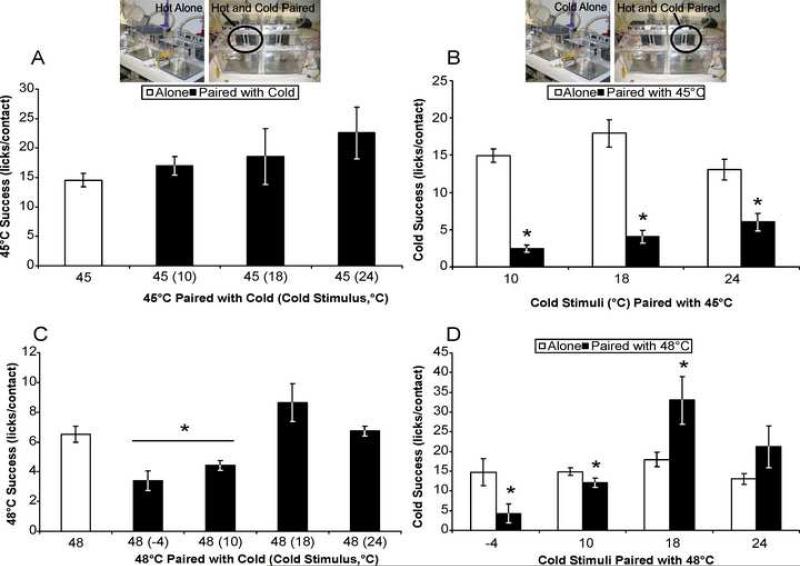
We also evaluated the effect of pairing stimuli on successful task completion relative to success when each stimulus is presented individually. Successful task completion is measured by calculating the ratio of licks to stimulus contacts for each stimulus. The success ratios at 45°C when paired with any cold stimulus were not significantly different than success ratios at 45°C alone (Fig. 2A). In contrast, success ratios at all of the cold stimuli paired with 45°C were decreased relative to when the stimuli were presented alone (Fig. 2B). Interestingly, the success ratios for 10, 18, and 24°C are significantly different from each other in the presence of 45°C (one-way ANOVA with post-hoc Tukey's test, F 2,27 = 4.72), but not when these cold stimuli are presented individually, which we have demonstrated previously regarding individually presented cold stimuli [17].
Fig. 2. Successful task completion when stimuli are paired or presented alone.
(A) Success at 45°C is not significantly increased by pairing with non-noxious cold stimuli as compared to when it is presented alone (n = 29; F 3, 62 = 2.457, p = 0.103). (B) Success ratios at the cold stimuli (10, 18, 24°C) are significantly decreased when paired with 45°C (10°C: t51 = 12.264, for single stimulus, n = 41; 18°C: t17 = 6.633, n = 12; 24°C: t22 = 3.866, n = 14). (C) Success ratio at 48°C is significantly reduced when paired with −4 and 10°C, but not significantly different when paired with 18 or 24°C as compared to when it is presented alone (n = 28). (D) Success ratio is significantly decreased at −4 and 10°C when paired with 48°C (−4°C t13 = 2.708; 10°C: t105 = 2.706), significantly increased at 18°C (t11 = −2.486), and not significantly increased at 24°C (t9 = −1.46). * indicates p<0.05 for success alone versus paired, (for success ratios with hot stimuli significance was determined by ANOVA and Tukey's test post-hoc, and by t tests for success ratios with cold stimuli). All data are mean ± SEM.
The success ratio at 48°C was modulated by cold in an intensity dependent fashion, with a significant decrease in success at 48°C noted when paired with −4 or 10°C as compared to success 48°C alone (Fig. 2C). In contrast, no differences in success ratios were found when 48°C was paired with 18 or 24°C relative to when presented alone (Fig. 2C). Conversely, success ratios at −4 and 10°C are lower with 48°C than the success ratios when these stimuli are presented alone (Fig. 2D). Success ratios at 18 and 24°C are greater when paired with 48°C than the success ratios when these stimuli are presented alone, but only significantly so for 18°C (Fig. 2D). Taken together, these data indicate that pairing stimuli can modulate successful task completion at either stimulus as compared to when that stimulus is presented alone.
3.3. Effect of Previous Experience on Thermal Preference
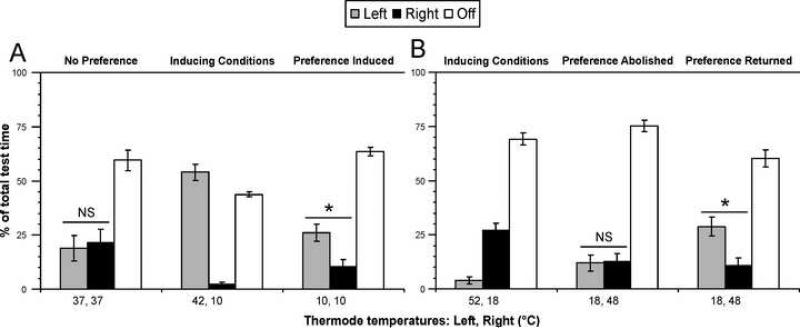
In addition to characterizing thermal preference, time distribution, and the effect of stimulus interactions on successful task completion, we also evaluated if prior experience in the thermal testing apparatus could affect thermode preference the following day. Rats (n =10) were tested first at 37/37°C, exhibiting no side preference (Fig. 3A). They were then tested at 42/10°C and preferred the 42°C, left thermode. Twenty four hours later, the rats were tested at 10/10°C, spent significantly more time on the left thermode than the right (Fig. 3A), and exhibited a bias for starting on the left thermode (Table 2A). Taken together, these findings suggest that the left-thermode preference (right thermode avoidance) of the previous day was sufficient to produce a conditioned preference in the presence of two equal stimuli.
Fig. 3. Preference can be conditioned or abolished by stimulus exposure 24 hours prior.
(A) Two equivalent stimuli normally do not produce a preference, as indicated by 37 and 37°C (“NS” indicates no significant difference between thermodes). However, when preceded by a preference inducing combination (42 and 10°C; F2, 9 = 50.39), the next day more time is spent on the left 10°C thermode (previously preferred) than the right 10°C thermode (previously avoided, F2, 9 = 48.09). When comparing the right thermode across the stimulus pairs, the percentage of time spent on the right 10°C thermode was also significantly different from the right thermode set at 37°C (F2, 9 = 6.96). (B) Previous experience can also hinder the presentation of a stimulus driven preference. In this case, an avoidance of the left thermode (52°C) and preference for the right thermode (18°C) was established (F2, 8 = 91.28). The following day, the cold and hot sides were switched such that the left thermode was 18°C and the right, 48°C. Rats spend an equal percentage of time on the left and right thermodes (p = 0.909 for post-hoc LSD comparison of stimulus duration). In contrast, when tested two days later at with the same stimulus configuration, they exhibited a preference for the left 18°C stimulus (F2, 8 = 37.86). When comparing duration on the 18°C stimulus across these stimulus pairs, the time spent on 18°C (black bar) when paired with 52°C was significantly greater than the time spent on 18°C (grey bar) for the first 18/48°C pair (no preference), but not for the second 18/48°C pair (preference returned; F2, 8 = 10.73). * indicates within pair significant difference, p < 0.05, with repeated measures ANOVA and post-hoc LSD test. All data are mean ± SEM.
We also determined that previous experience can obscure subsequent preference. Rats were first tested at 52/18°C, which induced a preference for 18°C, on the right (Fig. 3B). We generally observe that simply switching the hot and cold stimuli the following day does not have a significant effect on thermal preference. However, changing one of the stimuli by a few degrees in addition to alternating hot and cold sides could have a significant impact on behavior. Therefore, the next day 18/48°C was used. Rats spent an equal percentage of testing time on either stimulus, indicating no stimulus preference (Fig. 3B). After being exposed to 52°C on the left thermode, fewer rats started on the left side when it was switched to 18°C (Table 2B). Rats also spent significantly less time on the left thermode at 18°C when preference was abolished, relative to the time spent at 18°C in either the inducing conditions or when preference returned two days later (Fig. 3B). Unstimulated time was also significantly greater when preference for 18°C was abolished than when it was present. Taken together, these data indicate that previous experience can condition avoidance of one thermode that leads to avoidance of that side and thus an apparent lack of preference.
4. Discussion
Thermal preference tasks that stimulate the paws have been used to reveal shifts in profiles produced by genetic manipulation [6, 16, 21, 26], models of neuropathic injury [31, 34], and sex differences [32] but no such evaluations exist for competing stimuli applied to the face. In this study, we sought to fully evaluate the role of competing thermal stimuli on operant pain behavior using a thermal preference paradigm adapted from our single-stimulus facial pain assay [23, 24]. We paired several cold stimuli (24, 18, 10, and −4°C) with either 45 or 48°C. These cold stimuli range from innocuous (24°C), near painful (18°C), painful, but non-noxious (10°C), and painful and noxious (−4°C). Of the two hot stimuli, 45°C is at the pain threshold, but non-noxious, while 48°C is both painful and noxious.
The painful 45°C stimulus was always preferred when paired with any cold stimulus, including the innocuous 24°C. This was somewhat unexpected given that similar hindpaw preference studies demonstrate either a slight cold preference in male rats, or no overt preference, as given by equal time spent on either a 45 or 10°C stimulus [31, 32]. Also, based on the fact that we typically observe no difference in success ratios for cold stimuli and 45°C when these stimuli are presented individually (compare white bars in Fig. 2 A&B), we had expected to observe a less pronounced preference for 45°C stimulation of the face with these stimulus pairs.
These findings indicate that for hungry male rats, painful sensations induced by a 45°C stimulus are insufficient to overcome the thermoregulatory drive to avoid cold, even when that stimulation may be unpleasant but non-painful (24°C). In contrast and not unexpected, non-noxious cold stimuli are preferred over 48C, although, the more moderate nature of this preference also indicates the aversive capacity of these cold stimuli. Taken together, thermal preferences revealed by this assay support a role for thermal pain perception as a part of homeostatic, interoceptive processing system [8] and can be used to evaluate changes in motivational influence of thermal stimuli in pathological, genetically, or pharmacologically modified states.
Additionally, the design of this assay allows the animal to “escape” from both stimuli (avoid stimulation), which is reflected in the percentage of unstimulated time. It has been suggested that escape from stimulation can be used to indicate affective evaluation of painful stimuli by animals [33], and demonstrated that stressful applications including social defeat [19], restraint [14], and previous pain testing [33] can increase escape from nociceptive stimuli. In this study, 40−50% of the testing time was spent unstimulated for 24/45°C, 24/48°C, and 10/42°C pairs (one is painful and/or aversive one is not), 50−60% for 10 or 18/45°C (both near or just within pain thresholds), and 60−80% for all painful cold with 48°C or 18/52°C (one stimulus is noxious and the other painful and aversive). Thus the percentage of unstimulated time (escape from stimulation) increases as the aversive and painful qualities of both stimuli increase in the absence of treatment or conditioning experience. This association of unstimulated time or escape with the aversivness of the stimulus pairs may support the idea of this measure as tool for affect evaluation.
In future work this assay could be used to examine the capacity for different classifications of compounds, pain experience, or lesions of specific neuroanotomical regions to change the percentage of unstimulated time independent of or in conjunction with a change in thermal preference. For example, an anxyolitic compound might have no effect on the perceptual and motivational factors underlying thermal preference, but it might reduce stress that may be caused by the presence of painful stimuli, enhancing operant responding, and decreasing unstimulated time. Alternatively, pain states which preferentially effect cold sensitivity, but not heat may alter both thermal preference and unstimulated time. There are certainly many potential applications of this assay relatable to the study of addiction, experience, and motivation.
In addition to the central perceptual, motivational and affective factors that influence behavior in this preference task, there is also potential for interaction among nociceptors populations that might cross sensitize or desensitize the animal to the stimui. In order to resolve this issue, we compared the success ratio (licks/stimulus contact) for the cold stimulus when paired with a hot stimulus to the success ratio obtained when the cold stimulus was presented alone. This was also done for the hot stimulus. Pairing 45°C with cold stimuli insignificantly increased success ratios relative to 45°C alone. Success ratios for cold stimuli were significantly decreased when paired with 45°C, and this decrease was proportional to decreasing temperature. The latter effect may be the result of a temporally induced paradoxical heat effect, in which the burning character of cold is unmasked by an imbalance in the activity of polymodal nociceptors versus cool-sensitive cells in lamina I of the dorsal horn [8, 9].
The capacity for both 48°C and painful cold to cross suppress successful task completion also suggests that painful cold activates substrates that can facilitate heat pain, and vice verse. This cold-facilitation of heat pain could be due to the activation of peripheral nociceptors that respond to both cold and heat, or the convergent, facilatory input of distinct cold and heat-responsive nociceptors onto second order neurons in the dorsal horn of the trigeminal nucleus caudalis. Two molecular mediators of cold and heat, TRPM8 and TRPV1 respectively, are co-expressed in naïve trigeminal ganglia [15, 36]. Although their co-expression is reported to be very low in naïve trigeminal ganglia [15], this does not necessarily mean that such a population could not have a significant influence on thermal processing in the context of alternating or mixed hot and cold stimulation. It is also possible that the cold and hot nociceptors have convergent inputs within the trigeminal spinal nucleus. Lingual application of the TRPM8 agonist menthol has been shown to cross-sensitize subjects to the irritation produced capsaicin, a TRPV1 agonist [5]. TRPA1 has also been proposed to respond to nociceptive cold stimulation [3, 16, 25, 27], but this is debated [4, 12, 22, 39]. TRPA1 and TRPV1 are highly co-expressed [15] and agonists of this channel have been shown to weakly enhance cold pain [1]. Additionally, local field potentials in the trigeminal nucleus caudalis respond to mixed thermal stimuli and agonists of TRPM8, TRPA1, and TRPV1, which is suggestive of convergent inputs onto the same second order neuron [1, 37, 38]. Assessing changes in success in our thermal preference task could provide a means of studying the interaction between cold and hot nociceptors in vivo.
Thus far, we have established that this thermal preference assay can assess the motivational capacity of competing stimuli by behavioral outcomes that indicate degree of preference, as well as avoidance. We have also determined that interactions between cold and hot nociceptors may be inferred by examining changes success ratios. We also demonstrated that previous experience within the preference task could influence behavior in the task one day later, such that preference could be artificially induced or abolished by previous experience. This effect is similar to conditioned place aversion, where an inflammatory pain state becomes associated with one compartment in a two or three chambered box and avoided by the animal in future trials [28, 29]. This paradigm is often used to evaluate the capacity of treatments to modulate the affective component of pain independent of the intensity component, necessitating separate sensory testing to evaluate intensity [7, 30]. In contrast, this thermal preference assay has the potential to assess both intensity and affect aspects of pain simultaneously. Future experimentation could examine the capacity of a treatment to enhance or reduce the conditioned preference effect demonstrated here. Alternatively, place preference or aversion could be pharmacologically induced and the capacity of different stimulus pairs to change the conditioned preference could be examined.
5. Conclusions
In the current study, we characterize a thermal preference assay that can assess both intensity and affective aspects of facial pain simultaneously. This method of assessment provides a clinically relevant examination of pain mechanisms because it can assess both the direct response to the stimuli and the avoidance of the stimuli. Additionally, there are potential applications of this assay in other areas related to pain, such as the study of addiction, Understanding the mechanisms underling these relationships will allow for future studies to improve treatment for chronic pain.
Table 3.
Influence of previous experience on frequency of start side and thermal preference 24 hours later.
| A | Preference Induced | |
|---|---|---|
| First Day | Left 42°C | Right 10°C |
| n rats started on this side | 4 | 6 |
| n rats preferred this side | 10 | 0 |
| Second Day | Left 10°C | Right 10°C* |
| n rats started on this side | 8 | 2 |
| n rats preferred this side | 8 | 2 |
| Total n | 10 | |
| B | Preference Abolished | |
|---|---|---|
| First Day | Left 52°C | Right 18°C |
| n rats started on this side | 5 | 4 |
| n rats preferred this side | 1 | 8 |
| Second Day | Left 18°C | Right 48°C |
| n rats started on this side | 3 | 6 |
| n rats preferred this side | 4 | 5 |
| Total n | 9 | |
Please note that the two rats who started on the right exhibited a left-side preference and the two rats that preferred the right started on the left.
Acknowledgments
This research was supported by grant 5R21DE016704−02, from the National Institute of Dental and Craniofacial Research. We thank Jean Kauffman for her technical assistance and care of the animals, as well as Charles Vierck for his insights into evaluating thermal preference.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albin KC, Carstens MI, Carstens E. Modulation of oral heat and cold pain by irritant chemicals. Chemical Senses. 2008;33:3–15. doi: 10.1093/chemse/bjm056. [DOI] [PubMed] [Google Scholar]
- 2.Allchorne AJ, Broom DC, J. WC Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol Pain. 2005 Dec 14;1 doi: 10.1186/1744-8069-1-36. 2005 <doi: 2010.1186/1744-8069-2001-2036>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel trpa1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 4.Bautista DM, Jordt S-E, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. Trpa1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Cliff MA, Green BG. Sensitization and desensitization to capsaicin and menthol in the oral cavity: Interactions and individual differences. Physiology & Behavior. 1996;59:487–494. doi: 10.1016/0031-9384(95)02089-6. [DOI] [PubMed] [Google Scholar]
- 6.Colburn RW, Lubin ML, Stone JDJ, Wang Y, Lawrence D, D'Andrea Michael R, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in trpm8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Colpaert FC, Deseure K, Stinus L, Adriaensen H. High-efficacy 5-hydroxytryptamine 1a receptor activation counteracts opioid hyperallodynia and affective conditioning. J Pharmacol Exp Ther. 2006;316:892–899. doi: 10.1124/jpet.105.095109. [DOI] [PubMed] [Google Scholar]
- 8.Craig AD. A new view of pain as a homeostatic emotion. Trends in Neurosciences. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 9.Craig AD, Bushnell MC. The thermal grill illusion: Unmasking the burn of cold pain. Science. 1994;265:252–255. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- 10.Green BG, Akirav C. Individual differences in temperature perception: Evidence of common processing of sensation intensity of warmth and cold. Somatosens Mot Res. 2007;24:71–84. doi: 10.1080/08990220701388117. [DOI] [PubMed] [Google Scholar]
- 11.Greenspan J, Roy E, Caldwell P, Farooq N. Thermosensory intensity and affect throughout the perceptible range. Somatosens Mot Res. 2003;20:19–26. doi: 10.1080/0899022031000083807. [DOI] [PubMed] [Google Scholar]
- 12.Jordt S-E, Bautista DM, Chuang H-h, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the trp channel anktm1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 13.Kayser V, Aubel B, Hamon M, Bourgoin S. The antimigraine 5ht 1b/1d receptor agonists, sumatriptan, zolmitriptan and dihydroergotamine, attenuate pain-related behaviour in a rat model of trigeminal pain. Br J Pharmacol. 2002;137:1287–1297. doi: 10.1038/sj.bjp.0704979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King CD, Devine DP, Vierck CJ, Rodgers J, Yezierski RP. Differential effects of stress on escape and reflex responses to nociceptive thermal stimuli in the rat. Brain Res. 2003;987:214–222. doi: 10.1016/s0006-8993(03)03339-0. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of trpm8, trpa1, and trpv1 mrnas in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 16.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, Corey DP. Trpa1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Latremoliere A, Mauborgne A, Masson J, Bourgoin S, Kayser V, Hamon M, Pohl M. Differential implication of proinflammatory cytokine interleukin-6 in the development of cephalic versus extracephalic neuropathic pain in rats. J Neurosci. 2008;28:8489–8501. doi: 10.1523/JNEUROSCI.2552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcinkiewcz CA, Green MK, Devine DP, Duarte P, Vierck CJ, Yezierski RP. Social defeat stress potentiates thermal sensitivity in operant models of pain processing. Brain Research. 2009;1251:112–120. doi: 10.1016/j.brainres.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauderli AP, Acosta-Rua A, Vierck CJ. An operant assay of thermal pain in conscious, unrestrained rats. Journal of Neuroscience Methods. 2000;97:19–29. doi: 10.1016/s0165-0270(00)00160-6. [DOI] [PubMed] [Google Scholar]
- 21.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KSR, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking trpv3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 22.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of trpa1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck JCJ, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Rossi HL, Vierck CJ, Caudle RM, Neubert JK. Characterization of cold sensitivity and thermal preference using an operant orofacial assay. Mol Pain. 2006;2 doi: 10.1186/1744-8069-2-37. doi: 10.1186/1744-8069-1182-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant trpa1 channels. Brain Research. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu I, Iida T, Guan Y, Zhao C, Raja SN, Jarvis MF, Cockayne DA, Caterina MJ. Enhanced thermal avoidance in mice lacking the atp receptor p2x3. Pain. 2005;116:96–108. doi: 10.1016/j.pain.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. Anktm1, a trp-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 28.Sufka K. Conditioned place preference paradigm: A novel approach for analgesic drug assessment against chronic pain. Pain. 1994;58:355–366. doi: 10.1016/0304-3959(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 29.Vaccarino AL, Plamondon H, Melzack R. Analgesic and aversive effects of naloxone in balb/c mice. Experimental Neurology. 1992;117:216–218. doi: 10.1016/0014-4886(92)90130-i. [DOI] [PubMed] [Google Scholar]
- 30.van der Kam EL, Vry JD, Schiene K, Tzschentke TM. Differential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the rat. Pain. 2008;136:373–379. doi: 10.1016/j.pain.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 31.Vierck CJ, Acosta-Rua AJ, Johnson RD. Bilateral chronic constriction of the sciatic nerve: A model of long-term cold hyperalgesia. The Journal of Pain. 2005;6:507–517. doi: 10.1016/j.jpain.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Vierck CJ, Acosta-Rua AJ, Rossi HL, Neubert JK. Sex differences in thermal pain sensitivity and sympathetic reactivity for two strains of rat. The Journal of Pain. 2008;9:739–749. doi: 10.1016/j.jpain.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vierck CJ, Green M, Yezierski RP. Pain as a stressor: Effects of prior nociceptive stimulation on escape responding of rats to thermal stimulation. European Journal of Pain. 2009 doi: 10.1016/j.ejpain.2009.01.009. doi:10.1016/j.ejpain.2009.01.009. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walczak J-S, Beaulieu P. Comparison of three models of neuropathic pain in mice using a new method to assess cold allodynia: The double plate technique. Neuroscience Letters. 2006;399:240–244. doi: 10.1016/j.neulet.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 35.Wasner GL, Brock JA. Determinants of thermal pain thresholds in normal subjects. Clinical Neurophysiology. 2008;119:2389–2395. doi: 10.1016/j.clinph.2008.07.223. [DOI] [PubMed] [Google Scholar]
- 36.Xing H, Ling J, Chen M, Gu JG. Chemical and cold sensitivity of two distinct populations of trpm8-expressing somatosensory neurons. J Neurophysiol. 2006;95:1221–1230. doi: 10.1152/jn.01035.2005. [DOI] [PubMed] [Google Scholar]
- 37.Zanotto KL, Iodi Carstens M, Carstens E. Cross-desensitization of responses of rat trigeminal subnucleus caudalis neurons to cinnamaldehyde and menthol. Neuroscience Letters. 2008;430:29–33. doi: 10.1016/j.neulet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanotto KL, Merrill AW, Carstens MI, Carstens E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol, and other irritant stimuli. J Neurophysiol. 2007;97:966–978. doi: 10.1152/jn.00996.2006. [DOI] [PubMed] [Google Scholar]
- 39.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel trpa1 by ca2+. Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]