Abstract
Purpose
Recent changes have occurred in the presurgical planning for breast cancer, including the introduction of preoperative breast magnetic resonance imaging (MRI). We sought to analyze the trends in mastectomy rates and the relationship to preoperative MRI and surgical year at Mayo Clinic, Rochester, MN.
Patients and Methods
We identified 5,405 patients who underwent surgery between 1997 and 2006. Patients undergoing MRI were identified from a prospective database. Trends in mastectomy rate and the association of MRI with surgery type were analyzed. Multiple logistic regression was used to assess the effect of surgery year and MRI on surgery type, while adjusting for potential confounding variables.
Results
Mastectomy rates differed significantly across time (P < .0001), and decreased from 45% in 1997% to 31% in 2003, followed by increasing rates for 2004 to 2006. The use of MRI increased from 10% in 2003% to 23% in 2006 (P < .0001). Patients with MRI were more likely to undergo mastectomy than those without MRI (54% v 36%; P < .0001). However, mastectomy rates increased from 2004 to 2006 predominantly among patients without MRI (29% in 2003% to 41% in 2006; P < .0001). In a multivariable model, both MRI (odds ratio [OR], 1.7; P < .0001) and surgical year (compared to 2003 OR: 1.4 for 2004, 1.8 for 2005, and 1.7 for 2006; P < .0001) were independent predictors of mastectomy.
Conclusion
After a steady decline, mastectomy rates have increased in recent years with both surgery year and MRI as significant predictors for type of surgery. Further studies are needed to evaluate the role of MRI and other factors influencing surgical planning.
INTRODUCTION
Prospective randomized trials have demonstrated no significant difference in the survival of patients who undergo mastectomy compared to lumpectomy followed by radiation (breast-conserving therapy [BCT]). Results from six randomized trials1–6 led to a consensus statement by the National Institutes of Health Consensus Development Panel in 19907 that breast conservation surgery (BCS) is the preferred method of primary surgical therapy for women with early-stage breast cancer.
After this consensus statement, the percentage of women undergoing BCT increased from 35% to 60% for stage 1 and 19% to 29% for stage 2 breast cancer from 1989 to 1995.8,9 There are few data regarding the rates of mastectomy during the past 10 years.
Recent changes have occurred in the presurgical evaluation and treatment of patients with breast cancer. Some of these changes have included the introduction of genetic testing to detect disease causing mutations in BRCA1 and BRCA2, increased education regarding surgical treatment options, and the introduction of new imaging modalities, such as ultrasound and magnetic resonance imaging (MRI).
Breast MRI provides an additional tool to visualize mammographically occult breast lesions in the ipsilateral and contralateral breast. In a randomized trial, at least one additional suspicious focus in the ipsilateral breast was found in 19.6% of the women undergoing MRI compared to 8.6% undergoing mammography.10 In addition, MRI identifies up to 3% of mammographically occult contralateral breast cancers in women who present with an ipsilateral invasive breast cancer.11
Because of its high sensitivity in identifying multifocal disease, MRI is being used more commonly for preoperative surgical planning.12–17 Breast MRI is reported to have a sensitivity of 88% to 95%, but specificity is variable, ranging from 30% to 80%.18–21 While guidelines for the use of MRI to screen high-risk women have been developed,22 there are no standardized recommendations for the use of MRI in the preoperative setting. Concern has been raised that preoperative MRI may influence surgical treatment of patients without adequate long-term data on local recurrence or survival.23,24
In light of the multiple changes occurring in the preoperative evaluation and treatment of patients with early-stage breast cancer, we sought to analyze the trends in mastectomy rates at Mayo Clinic Rochester for women with early-stage breast cancer over a 10-year period from 1997 to 2006 and to compare mastectomy rates in women who did or did not undergo preoperative MRI.
PATIENTS AND METHODS
This study was approved by the institutional review board of Mayo Clinic Rochester, MN. A cohort of 5,583 breast cancers, stages 0 to 2, in 5,405 patients who had surgery during the period of 1997 to 2006 at Mayo Clinic, Rochester, MN, were identified retrospectively using the Mayo Clinic Rochester Cancer Registry. The study population included women who had definitive surgical treatment for breast cancer at Mayo Clinic, Rochester, and, using the American Joint Cancer Committee Cancer Staging Manual, sixth edition,25 met the criteria of stage 0, I, or II disease (TisN0M0, T1N0M0, T2N0M0, or T2N1M0). Surgery type (mastectomy v lumpectomy) was defined using the Cancer Registry. This was cross-referenced with two other available databases: the Mayo Clinic Surgical Index and a separate breast cancer database maintained within the Department of Surgery. Discrepancies among databases with respect to the type of definitive surgery were manually reviewed.
The surgical cohort was then matched with a breast MRI database. This database was initiated in 2003 to prospectively collect data on all patients diagnosed with breast cancer undergoing breast MRI. Initially, practice guidelines for preoperative MRI were developed but not restricted to the following patient scenarios: biopsy-proven invasive lobular carcinoma; biopsy-proven invasive breast cancer that was palpable but not visible by mammogram; axillary metastasis from presumed breast primary with negative mammogram and clinical breast exam; and problem-solving situations in the setting of biopsy-proven breast cancer.
MRIs were classified as preoperative if the date of breast MRI was within 30 days before the date of surgery. When the date of breast MRI was more than 30 days prior but within 1 year of the date of breast cancer surgery, the medical record was reviewed to determine whether the breast MRI was related to the preoperative evaluation for the breast cancer surgery in question (eg, in the case of neoadjuvant chemotherapy).
The following variables were also obtained from institutional databases: patient age, TNM stage, histology, laterality (left v right), bilateral breast cancer (yes/no), history of contralateral breast cancer, breast density and family history, defined as a first-degree relative (parent, sibling, offspring). Breast density was obtained by matching the surgical cohort with the mammography database maintained within the Department of Radiology. The grading system was based on the American College of Radiology Breast Imaging Reporting and Data System which contains a 4-point grading of breast density (1 = fatty breasts, 2 = scattered fibroglandular tissue, 3 = heterogeneously dense, 4 = extremely dense) recorded by the radiologist at the time the exam was read. Breast density readings from mammograms performed within 1 year before the date of definitive surgery was available in 53% of the patients in the cohort.
Statistical Analysis
Descriptive statistics are reported using mean (95% CI) or frequency (percentage), as appropriate. Trends over time in the mastectomy rate and in the use of preoperative breast MRI are reported descriptively and graphically with 95% CI. χ2 tests were used to compare proportions, whereas Cochrane-Armitage trend tests were used to test trends over time in proportions. Logistic regression was used to assess the association of breast MRI and surgical year on the type of surgery (mastectomy v lumpectomy). Multiple logistic regression was used to assess these associations while adjusting for age, TNM stage, histology, breast density, laterality, the presence of concurrent or prior contralateral breast cancer, and family history of breast cancer. Results are reported with odds ratios (OR) and 95% CI. P values lower than .05 were considered statistically significant. All analysis was performed using SAS version 9.1.3 (SAS Institute Inc, Cary, NC).
Because some patients had both a right and left breast cancer during the time period under study and thus had two observations in the data, a generalized estimating equations approach was also applied using PROC GENMOD (SAS Institute Inc) with binomial distribution and logit link to take within-subject correlation into account in model fitting. These results were consistent with those found using standard logistic regression, so the logistic regression results were presented for simplicity.
RESULTS
Patient and tumor characteristics are outlined in Table 1. There were a total of 5,583 breast cancers identified between years 1997 to 2006 in 5,405 patients; 17% were stage 0, 49% were stage I, and 34% were stage II. The mean age at diagnosis was 61 years (range, 23 to 95).
Table 1.
Demographic and Baseline Characteristics of 5,583 Cancers in 5,405 Patients
| Characteristic | No. (N = 5,583) | % |
|---|---|---|
| Age, years | ||
| Mean | 60.6 | |
| 95% CI | 60.2 to 60.9 | |
| < 50 | 1,279 | 23 |
| ≥ 50 | 4,304 | 77 |
| TNM stage | ||
| 0 | 954 | 17 |
| 1 | 2,716 | 49 |
| 2 | 1,913 | 34 |
| Primary histology among TNM stage 1-2 patients | ||
| Invasive ductal | 3,758 | 81 |
| Invasive lobular | 523 | 11 |
| Other invasive | 348 | 8 |
| Breast density | ||
| 1, fatty breasts | 282 | 5 |
| 2, scattered fibroglandular | 1,222 | 22 |
| 3, heterogeneous dense | 1,102 | 20 |
| 4, extremely dense | 347 | 6 |
| Unknown | 2,630 | 47 |
| Concurrent or prior contralateral breast cancer | ||
| Yes | 580 | 10 |
| No | 5,003 | 90 |
| Laterality | ||
| Right | 2,709 | 49 |
| Left | 2,874 | 51 |
| Preoperative breast MRI | ||
| Yes | 346 | 6 |
| No | 5,237 | 94 |
| First-degree family history of breast cancer | ||
| Yes | 1,217 | 78 |
| No | 4,366 | 22 |
Abbreviation: MRI, magnetic resonance imaging.
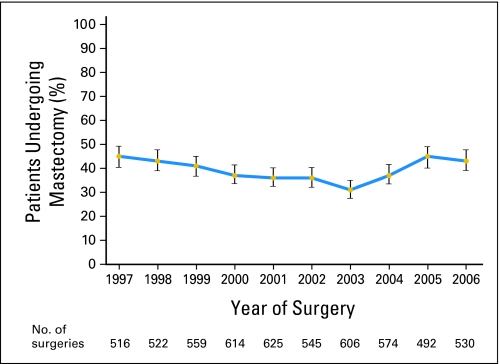
Mastectomy rates varied significantly according to surgical year. Beginning in 1997, mastectomy rates gradually decreased from 45% in 1997% to 31% in 2003 (P < .0001), followed by increase from 37% to 43% for the period of 2004 to 2006 years (Table 2; Fig 1). Although each stage showed some increase in mastectomy rates for 2004 to 2006 compared to 2003 (each P ≤ .01when testing for trend within a stage), the change was most marked for stage 2 cancers.
Table 2.
Breast Cancer Mastectomy and Preoperative MRI Rates and by Stage Over Time From 1997 to 2006
| Parameter | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of cancers | 516 | 522 | 559 | 614 | 625 | 545 | 606 | 574 | 492 | 530 |
| % with mastectomy | 45 | 43 | 41 | 37 | 36 | 36 | 31 | 37 | 45 | 43 |
| % with MRI | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 12 | 19 | 23 |
| % with mastectomy by TNM stage | ||||||||||
| TNM stage, % | ||||||||||
| 0 | 37 | 41 | 33 | 36 | 33 | 32 | 26 | 30 | 35 | 42 |
| 1 | 35 | 37 | 35 | 30 | 31 | 29 | 27 | 28 | 42 | 33 |
| 2 | 61 | 55 | 53 | 48 | 45 | 48 | 41 | 56 | 54 | 58 |
| With mastectomy by MRI, % | ||||||||||
| MRI | 53 | 54 | 57 | 52 | ||||||
| No MRI | 29 | 35 | 42 | 41 |
Abbreviation: MRI, magnetic resonance imaging.
Fig 1.
Proportion of patients undergoing mastectomy from 1997 to 2006; vertical bars represent 95% CIs for the estimated proportion.
From 2003 through 2006, preoperative breast MRI was performed for a total of 346 cancers in 337 patients from this cohort. In these 337 patients, MRI was performed before first biopsy (n = 22), before earliest definitive surgery (either lumpectomy or mastectomy; n = 295), and between lumpectomy and mastectomy (n = 20). In the nine patients with bilateral cancer, the right and left definitive surgeries were performed on the same day but MRI occurred after the biopsy date (n = 1) and before the biopsy date (n = 8).
The percentage of patients undergoing MRI from 2003 to 2006 by stage were: ductal carcinoma in situ, 7%; stage 1, 15%; and stage 2, 22% (P < .0001). The use of MRI also varied significantly by histology (P < .0001), with 32% of invasive lobular cancers undergoing MRI compared with only 16% for invasive ductal and 20% for other invasive histologies during this time period. The percentage of patients with MRI increased from 10% in 2003 to 23% in 2006 (P < .0001) (Fig 2). Overall during this time period, those who underwent preoperative MRI were more likely to undergo mastectomy than those without preoperative MRI (54% v 36%; P < .0001).
Fig 2.
Proportion of patients undergoing preoperative breast magnetic resonance imaging from 2003 to 2006; vertical bars represent 95% CIs for the estimated proportion.
Mastectomy rates increased over 2003 to 2006 both in patients with and without MRI (Fig 3). This increase was not statistically significant in the MRI group (P = .92); however, a significant increase was observed among those without MRI (29% in 2003 to 41% in 2006; P < .0001).
Fig 3.
Proportion of patients undergoing mastectomy from 2003 to 2006 according to utilization of preoperative magnetic resonance imaging; vertical bars represent 95% CIs for the estimated proportion.
Table 3 lists the univariate and multivariate analyses for factors associated with mastectomy. In the multivariate model, the following made a significant contribution to the mastectomy rate: age (< 50 v ≥ 50), TNM stage, lobular histology, breast density, concurrent or prior contralateral breast cancer, laterality, family history, MRI, and year of surgery. In this model, women undergoing preoperative MRI were significantly more likely to undergo mastectomy than women who did not have preoperative MRI (OR, 1.7; 95% CI, 1.3 to 2.2; P < .0001). In addition, surgical year remained a significant predictor of surgery type, with each year from 2004 to 2006 having significantly increased odds of mastectomy compared with 2003, the year with the lowest mastectomy rate.
Table 3.
Results of Univariate and Multivariable Logistic Regression Models for the Log Odds of Undergoing Mastectomy As Opposed to BCS
| Variable | Univariate Results |
Multivariable Results |
||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P | Odds Ratio | 95% CI | P | |
| Preoperative breast MRI, yes v no | 1.9 | 1.5 to 2.4 | < .0001 | 1.7 | 1.3 to 2.2 | < .0001 |
| Calendar year | ||||||
| 1997 v 2003 | 1.8 | 1.4 to 2.3 | < .0001 | 2.0 | 1.5 to 2.6 | < .0001 |
| 1998 v 2003 | 1.7 | 1.3 to 2.2 | < .0001 | 2.1 | 1.6 to 2.7 | < .0001 |
| 1999 v 2003 | 1.5 | 1.2 to 1.9 | .0007 | 1.7 | 1.3 to 2.2 | < .0001 |
| 2000 v 2003 | 1.3 | 1.0 to 1.7 | .02 | 1.4 | 1.1 to 1.8 | .01 |
| 2001 v 2003 | 1.3 | 1.0 to 1.6 | .06 | 1.4 | 1.1 to 1.8 | .01 |
| 2002 v 2003 | 1.2 | 1.0 to 1.6 | .08 | 1.4 | 1.1 to 1.8 | .02 |
| 2004 v 2003 | 1.3 | 1.0 to 1.7 | .02 | 1.4 | 1.1 to 1.8 | .01 |
| 2005 v 2003 | 1.8 | 1.4 to 2.3 | < .0001 | 1.8 | 1.4 to 2.4 | < .0001 |
| 2006 v 2003 | 1.7 | 1.3 to 2.2 | < .0001 | 1.7 | 1.3 to 2.2 | .0001 |
| Age, years | ||||||
| ≥ 50 v < 50 | 0.74 | 0.65 to 0.84 | < .0001 | 0.80 | 0.70 to 0.92 | .001 |
| TNM stage | ||||||
| TNM 1 v 0 | 0.9 | 0.8 to 1.1 | .34 | 0.9 | 0.7 to 1.0 | .09 |
| TNM 2 v 0 | 2.1 | 1.8 to 2.4 | < .0001 | 2.0 | 1.7 to 2.4 | < .0001 |
| Primary histology | ||||||
| Lobular v ductal | 1.6 | 1.4 to 2.0 | < .0001 | 1.6 | 1.3 to 1.9 | < .0001 |
| Other invasive v ductal | 1.1 | 0.9 to 1.4 | .40 | 1.2 | 1.0 to 1.6 | .09 |
| Breast density | ||||||
| 4 v 1, 2, 3 | 1.6 | 1.2 to 2.0 | .0001 | 1.5 | 1.1 to 1.9 | .002 |
| Unknown v 1, 2, 3 | 1.1 | 1.0 to 1.3 | .04 | 1.0 | 0.9 to 1.1 | .86 |
| Concurrent or prior contralateral breast cancer | ||||||
| Yes v no | 3.2 | 2.6 to 3.8 | < .0001 | 3.8 | 3.1 to 4.5 | < .0001 |
| Laterality | ||||||
| Left v right | 1.2 | 1.0 to 1.3 | .007 | 1.2 | 1.0 to 1.3 | .005 |
| First-degree family history of breast cancer | ||||||
| Yes v no | 1.1 | 1.0 to 1.3 | .05 | 1.2 | 1.0 to 1.3 | .04 |
Abbreviation: MRI, magnetic resonance imaging.
Interactions between surgery year and MRI, surgery year and stage, MRI and stage, and surgery year and family history were also assessed in this multivariable model, but none were statistically significant and thus were not included in the final model. In addition, the effect of individual surgeons was analyzed. There were no significant differences in mastectomy rates among individual surgeons for any year from 2003 to 2006 (P values ranged from 0.21 to 0.79). Further, adding surgeon to the multivariable model did not change the conclusions with respect to either MRI or the time trend from 2003 to 2006.
DISCUSSION
BCS followed by radiation has been widely accepted as a surgical treatment option for women with early-stage breast cancer since the National Institutes of Health Consensus statement in 19907 and led to a steady rise in BCT in the years after 1990.8,9 Consistent with these data, mastectomy rates at Mayo Clinic Rochester declined from the years 1997 to 2003. However, beginning in 2004, we observed a reversal in this trend. From 2003 to 2006, mastectomy rates in women who did not undergo preoperative MRI increased from 29% to 41% (P < .0001).
In recent years, changes have occurred in the preoperative management and counseling of breast cancer patients that may have contributed to our observed increase. First, there is increased knowledge about the importance of family history and the role of genetic testing for hereditary breast cancer syndromes. Approximately 5% to 6% of breast cancers are associated with germline mutations in BRCA1/226,27 and women with deleterious BRCA mutations carry an increased lifetime risk of breast and ovarian cancer.28,29 Commercial testing for BRCA1 and BRCA2 mutations has become available since 1999, and one option available for women at high risk of developing breast cancer includes prophylactic bilateral mastectomy, which has decreases the risk of breast cancer by 90% in high-risk women.30 These data may be influencing the surgical choices of patients with a diagnosis of early stage breast cancer in favor of mastectomy. Although patients in our cohort with a family history of breast cancer were more likely to undergo mastectomy compared to those without a family history (P = .04), we did not observe a significant interaction between surgical year and family history in the multivariate analysis, suggesting that mastectomy rates were not rising preferentially in those women with a family history of breast cancer.
Another possible explanation for the rise in mastectomy rates may be increased education regarding reconstruction options. Recent data suggests that women are four times more likely to choose mastectomy when presented with options for breast reconstruction.31 This finding was more commonly seen in younger and highly educated patients.32 Our current practice includes a referral to plastic and reconstructive surgeons for women with breast cancer who are considering all surgical options. However, given the retrospective nature of this study, the potential impact of such preoperative counseling on the patient's choice of surgical treatment could not be assessed.
While our mastectomy rates during the period 1998 to 2003 were similar to that observed from a Surveillance, Epidemiology and End Results (SEER) registry,26 there are no comparable published data for the years 2004 to 2006. The higher mastectomy rates observed in the later years could reflect referral bias, since these data were derived from a single academic institution which is likely to differ from the SEER population. In a study that did use the SEER database, Tuttle et al reported an increase in contralateral mastectomy rate from 1.8% in 1998 to 4.5% in 2003.33 Taken together, these findings suggest a possible global swing in the pendulum of breast surgical treatment preferences back toward mastectomy, and may reflect growing patient choice for a preventive surgical approach against local recurrence and new primary breast cancers despite any expected survival benefit with more aggressive surgery. Given the fact that patients who are most well informed and involved in decision making are more likely to choose mastectomy,34 further studies are necessary to determine whether surgical choice improves patient satisfaction and/or quality of life.
Despite the possibility of a recent increase in patient preference for mastectomy, our data suggest that preoperative breast MRI may be associated with higher mastectomy rates. Since 2003, the percentage of patients undergoing preoperative breast MRI at our institution increased up to 23% in 2006. Overall, the rate of mastectomy was 18% higher in women who underwent MRI compared to those who did not have MRI (P < .0001). The higher mastectomy rate seen in women who underwent MRI may be due to the detection of additional lesions which in turn led to more extensive surgical resection, although we were not able to confirm this with our retrospective study. In comparison to mammography, breast MRI is more sensitive in identifying additional lesions in both ipsilateral and contralateral breasts.11,12,19,35 Recent studies15,36–39 have demonstrated that the use of MRI resulted in a change in surgical management in up to 30% of patients, with 15.5% to 25% being converted from lumpectomy to mastectomy. While our findings are in keeping with these other studies, notably, the differences in mastectomy rate between those with and without MRI appears to lessen over time; with CIs that overlap for 2005 and 2006. This observation could reflect a learning curve effect, with increased experience with MRI resulting in fewer false positives and less impact on surgical treatment.
Since MRI has been shown to alter the surgical decision making in favor of mastectomy, serious concern has been raised regarding the routine use of MRI in the preoperative treatment of patients without adequate long-term outcomes data to document improvement in local recurrence or survival.23,24,40 A recent prospective study (COMICE) demonstrated no reduction in re-operation rates for women randomly assigned to receive preoperative MRI versus not.41 However, in agreement with our findings, the women randomly assigned to MRI in this study had significantly higher mastectomy rates (7.1%) versus those without MRI (1.2%). Other groups have reported no differences in local recurrence, distant recurrence, or overall survival between patients who did or did not undergo a breast MRI.23,24,40,42Another disadvantage of breast MRI is cost, ranging from $2,000 to $4,000 per diagnostic evaluation. Despite these limitations, breast MRI has gained widespread use and is increasingly expected by patients. A patient satisfaction survey conducted on 227 patients who underwent breast MRI at our institution demonstrated a very high level of satisfaction with breast MRI, with 91% reporting that it was reassuring to them and had a positive impact on their overall care (unpublished data). Therefore, the routine use of MRI in breast cancer patients remains a complex mire of medical, economic, and political issues that result when new technology is utilized more rapidly than its evidence-based science can justify.
One important limitation to this study is that we cannot determine causality regarding the association of MRI and mastectomy. Given that our data were retrospectively obtained, we are unable to determine whether the MRI findings influenced the decision-making process of the patient, physician, or both. Decision making by a woman diagnosed with early-stage breast cancer is complex. In addition to the anxiety involved with diagnosis of cancer, many factors may affect the decision making process, including age, family history, personal experiences, risk of local recurrence, physical appearance, social factors, concern about radiation exposure, and educational status.32,34,43 Furthermore, the anxiety associated with additional testing for false positive lesions detected by MRI may lead some women to consider mastectomy to avoid future follow-up studies. Another limitation is that the practice guidelines developed for obtaining MRI at our institution were not routinely implemented by all clinicians. This was evident based on the increasing number of MRIs obtained over the 4-year time period. Finally, there is the possibility that the increased rate of mastectomy among patients undergoing preoperative breast MRI is due to confounding by indication, with women selected to undergo MRI having characteristics associated with higher rates of mastectomy (eg, stage and histology). For example, MRI use varied significantly by histology and stage, and each was an independent factor associated with mastectomy in our data set. However, in the multivariate model which adjusted for histology as well as stage (Table 3), MRI remained significantly associated with mastectomy (OR, 1.7; P < .0001).
In summary, this study demonstrates a change in the surgical treatment of breast cancer at a single institution, with significantly higher rates of mastectomy in the three most recent years compared to 2003. While MRI was associated with higher rates of mastectomy, the rise in mastectomy rates was most apparent in women who did not undergo MRI. New studies will be necessary to evaluate whether these changes in surgical management lead to improvements in quality of life and/or patient satisfaction.
Footnotes
Supported by Grant No. CA 90628 to the Paul Calabresi Program in Clinical-Translational Research at Mayo Clinic (M.P.G.).
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Rajini Katipamula, Amy C. Degnim, Tanya Hoskin, Judy C. Boughey, Charles Loprinzi, Christopher G. Chute, Matthew P. Goetz
Financial support: Matthew P. Goetz
Administrative support: Tanya Hoskin, Clive S. Grant, Kathleen R. Brandt, Sandhya Pruthi, Christopher G. Chute, Matthew P. Goetz
Provision of study materials or patients: Tanya Hoskin, Charles Loprinzi, Clive S. Grant, Kathleen R. Brandt, Christopher G. Chute, Janet E. Olson, Fergus J. Couch, James N. Ingle, Matthew P. Goetz
Collection and assembly of data: Rajini Katipamula, Amy C. Degnim, Tanya Hoskin, Christopher G. Chute, Janet E. Olson, Fergus J. Couch, Matthew P. Goetz
Data analysis and interpretation: Rajini Katipamula, Amy C. Degnim, Tanya Hoskin, Judy C. Boughey, Charles Loprinzi, Kathleen R. Brandt, Sandhya Pruthi, James N. Ingle, Matthew P. Goetz
Manuscript writing: Rajini Katipamula, Amy C. Degnim, Tanya Hoskin, Judy C. Boughey, Charles Loprinzi, Kathleen R. Brandt, Sandhya Pruthi, James N. Ingle, Matthew P. Goetz
Final approval of manuscript: Rajini Katipamula, Amy C. Degnim, Tanya Hoskin, Judy C. Boughey, Charles Loprinzi, Clive S. Grant, Kathleen R. Brandt, Sandhya Pruthi, Christopher G. Chute, Janet E. Olson, Fergus J. Couch, James N. Ingle, Matthew P. Goetz
REFERENCES
- 1.Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: Six years of life-table analysis: Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr. 1992:19–25. [PubMed] [Google Scholar]
- 2.Lichter AS, Lippman ME, Danforth DN, Jr, et al. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: A randomized trial at the National Cancer Institute. J Clin Oncol. 1992;10:976–983. doi: 10.1200/JCO.1992.10.6.976. [DOI] [PubMed] [Google Scholar]
- 3.Sarrazin D, Le MG, Arriagada R, et al. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol. 1989;14:177–184. doi: 10.1016/0167-8140(89)90165-5. [DOI] [PubMed] [Google Scholar]
- 4.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organisation for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 6.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 7.JAMA; NIH Consensus Conference: Treatment of early-stage breast cancer; 1991. pp. 391–395. [PubMed] [Google Scholar]
- 8.Du X, Freeman DH, Jr, Syblik DA. What drove changes in the use of breast conserving surgery since the early 1980s? The role of the clinical trial, celebrity action and an NIH consensus statement. Breast Cancer Res Treat. 2000;62:71–79. doi: 10.1023/a:1006414122201. [DOI] [PubMed] [Google Scholar]
- 9.Lazovich D, Solomon CC, Thomas DB, et al. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early stage invasive breast carcinoma. Cancer. 1999;86:628–637. [PubMed] [Google Scholar]
- 10.Schnall MD, Blume J, Bluemke DA, et al. MRI detection of distinct incidental cancer in women with primary breast cancer studied in IBMC 6883. J Surg Oncol. 2005;92:32–38. doi: 10.1002/jso.20381. [DOI] [PubMed] [Google Scholar]
- 11.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 12.Boetes C, Mus RD, Holland R, et al. Breast tumors: Comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology. 1995;197:743–747. doi: 10.1148/radiology.197.3.7480749. [DOI] [PubMed] [Google Scholar]
- 13.Drew PJ, Turnbull LW, Chatterjee S, et al. Prospective comparison of standard triple assessment and dynamic magnetic resonance imaging of the breast for the evaluation of symptomatic breast lesions. Ann Surg. 1999;230:680–685. doi: 10.1097/00000658-199911000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esserman L, Hylton N, Yassa L, et al. Utility of magnetic resonance imaging in the management of breast cancer: Evidence for improved preoperative staging. J Clin Oncol. 1999;17:110–119. doi: 10.1200/JCO.1999.17.1.110. [DOI] [PubMed] [Google Scholar]
- 15.Fischer U, Kopka L, Grabbe E. Breast carcinoma: Effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999;213:881–888. doi: 10.1148/radiology.213.3.r99dc01881. [DOI] [PubMed] [Google Scholar]
- 16.Liberman L, Morris EA, Dershaw DD, et al. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. Am J Roentgenol. 2003;180:901–910. doi: 10.2214/ajr.180.4.1800901. [DOI] [PubMed] [Google Scholar]
- 17.Mumtaz H, Hall-Craggs MA, Davidson T, et al. Staging of symptomatic primary breast cancer with MR imaging. Am J Roentgenol. 1997;169:417–424. doi: 10.2214/ajr.169.2.9242745. [DOI] [PubMed] [Google Scholar]
- 18.Bone B, Pentek Z, Perbeck L, et al. Diagnostic accuracy of mammography and contrast-enhanced MR imaging in 238 histologically verified breast lesions. Acta Radiol. 1997;38:489–496. doi: 10.1080/02841859709174374. [DOI] [PubMed] [Google Scholar]
- 19.Bone B, Wiberg MK, Szabo BK, et al. Comparison of 99mTc-sestamibi scintimammography and dynamic MR imaging as adjuncts to mammography in the diagnosis of breast cancer. Acta Radiol. 2003;44:28–34. [PubMed] [Google Scholar]
- 20.Kacl GM, Liu P, Debatin JF, et al. Detection of breast cancer with conventional mammography and contrast-enhanced MR imaging. Eur Radiol. 1998;8:194–200. doi: 10.1007/s003300050362. [DOI] [PubMed] [Google Scholar]
- 21.Malur S, Wurdinger S, Moritz A, et al. Comparison of written reports of mammography, sonography and magnetic resonance mammography for preoperative evaluation of breast lesions, with special emphasis on magnetic resonance mammography. Breast Cancer Res. 2001;3:55–60. doi: 10.1186/bcr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 23.Morrow M. Limiting breast surgery to the proper minimum. Breast. 2005;14:523–526. doi: 10.1016/j.breast.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Morrow M, Freedman G. A clinical oncology perspective on the use of breast MR. Magn Reson Imaging Clin N Am. 2006;14:363–378. vi. doi: 10.1016/j.mric.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 25.American Joint Committee on Cancer. AJCC Cancer Staging Manual. ed 6. New York, NY: Springer; 2002. [Google Scholar]
- 26.John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298:2869–2876. doi: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 27.Malone KE, Daling JR, Doody DR, et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006;66:8297–8308. doi: 10.1158/0008-5472.CAN-06-0503. [DOI] [PubMed] [Google Scholar]
- 28.Eerola H, Vahteristo P, Sarantaus L, et al. Survival of breast cancer patients in BRCA1, BRCA2, and non-BRCA1/2 breast cancer families: A relative survival analysis from Finland. Int J Cancer. 2001;93:368–372. doi: 10.1002/ijc.1341. [DOI] [PubMed] [Google Scholar]
- 29.Stoppa-Lyonnet D, Ansquer Y, Dreyfus H, et al. Familial invasive breast cancers: Worse outcome related to BRCA1 mutations. J Clin Oncol. 2000;18:4053–4059. doi: 10.1200/JCO.2000.18.24.4053. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77–84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- 31.Alderman AK, Hawley ST, Waljee J, et al. Understanding the impact of breast reconstruction on the surgical decision-making process for breast cancer. Cancer. 2008;112:489–494. doi: 10.1002/cncr.23214. [DOI] [PubMed] [Google Scholar]
- 32.Janz NK, Mujahid M, Lantz PM, et al. Population-based study of the relationship of treatment and sociodemographics on quality of life for early stage breast cancer. Qual Life Res. 2005;14:1467–1479. doi: 10.1007/s11136-005-0288-6. [DOI] [PubMed] [Google Scholar]
- 33.Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: A trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25:5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 34.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005;23:5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- 35.Hata T, Takahashi H, Watanabe K, et al. Magnetic resonance imaging for preoperative evaluation of breast cancer: A comparative study with mammography and ultrasonography. J Am Coll Surg. 2004;198:190–197. doi: 10.1016/j.jamcollsurg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Bedrosian I, Mick R, Orel SG, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003;98:468–473. doi: 10.1002/cncr.11490. [DOI] [PubMed] [Google Scholar]
- 37.Lee JM, Orel SG, Czerniecki BJ, et al. MRI before reexcision surgery in patients with breast cancer. Am J Roentgenol. 2004;182:473–480. doi: 10.2214/ajr.182.2.1820473. [DOI] [PubMed] [Google Scholar]
- 38.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–849. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 39.Deurloo EE, Peterse JL, Rutgers EJ, et al. Additional breast lesions in patients eligible for breast-conserving therapy by MRI: Impact on preoperative management and potential benefit of computerised analysis. Eur J Cancer. 2005;41:1393–1401. doi: 10.1016/j.ejca.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Morrow M, Schmidt R, Hassett C. Patient selection for breast conservation therapy with magnification mammography. Surgery. 1995;118:621–626. doi: 10.1016/s0039-6060(05)80027-3. [DOI] [PubMed] [Google Scholar]
- 41.Turnbull L, Drew P, Walker L, et al. The UK NIHR multicentre randomised COMICE trial of MRI planning for breast conserving treatment for breast cancer. Cancer Res. 2009;69:51. [Google Scholar]
- 42.Solin LJ, Orel SG, Hwang WT, et al. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol. 2008;26:386–391. doi: 10.1200/JCO.2006.09.5448. [DOI] [PubMed] [Google Scholar]
- 43.Lantz PM, Janz NK, Fagerlin A, et al. Satisfaction with surgery outcomes and the decision process in a population-based sample of women with breast cancer. Health Serv Res. 2005;40:745–767. doi: 10.1111/j.1475-6773.2005.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]