Abstract
Purpose
The primary objective of this study was to assess the 1-year survival of patients with locally advanced, unresectable pancreatic cancer treated with the combination of bevacizumab, capecitabine, and radiation. Secondary end points were toxicity, progression-free survival (PFS), and response rate (RR).
Patients and Methods
Patients with locally advanced pancreatic cancer without duodenal invasion were treated with 50.4 Gy per 28 fractions to the gross tumor with concurrent capecitabine 825 mg/m2 orally twice daily on days of radiation and bevacizumab 5 mg/kg on days 1, 15, and 29 followed by maintenance gemcitabine 1 g/m2 weekly for 3 weeks and bevacizumab 5 mg/kg every 2 weeks, both in 4-week cycles until progression. Treatment plans were reviewed for quality assurance (QA).
Results
Between January 2005 and February 2006, 82 eligible patients were treated. The median and 1-year survival rates were 11.9 months (95% CI, 9.9 to 14.0 months) and 47% (95% CI, 36% to 57%). Median PFS was 8.6 months (95% CI, 6.9 to 10.5), and RR was 26%. Overall, 35.4% of patients had grade 3 or greater treatment-related gastrointestinal toxicity (22.0% during chemoradiotherapy, 13.4% during maintenance chemotherapy). Unacceptable radiotherapy protocol deviations (ie, inappropriately generous volume contoured) correlated with grade 3 or greater gastrointestinal toxicity during chemoradiotherapy (45% v 18%; adjusted odds ratio, 3.7; 95% CI, 0.98 to 14.1; P = .05).
Conclusion
The addition of bevacizumab to chemoradiotherapy followed by bevacizumab and gemcitabine resulted in a similar median survival to previous Radiation Therapy Oncology Group studies in patients with locally advanced pancreatic cancer. Prospective QA may help limit toxicity in future trials.
INTRODUCTION
Locally advanced pancreatic cancer is a challenging malignancy to treat. Approaches that use chemotherapy, chemoradiotherapy, or both have significant limitations. Anti–vascular endothelial growth factor (VEGF) –based regimens have been successful in combination with chemotherapy in the metastatic setting in colorectal,1 lung,2 renal,3,4 and breast carcinomas.5 When this trial was developed, bevacizumab in combination with gemcitabine was considered a promising regimen for patients with metastatic pancreatic cancer on the basis of a phase II trial that showed a median survival of 9.2 months.6
Ionizing radiation induces VEGF expression,7 which may protect endothelial cells exposed to radiation. Inhibition of VEGF with bevacizumab, therefore, may enhance the cytotoxicity of radiation because of the potentiation of endothelial cell death. In vivo studies have shown that a radioresistant phenotype can be overcome by using agents that neutralize VEGF activity or prevent its signaling.7–9 Alternatively, enhancement of radiotherapy by bevacizumab could occur through the prevention of VEGF binding to VEGF receptors present on pancreatic tumor cells.10,11
In a phase I trial at the M. D. Anderson Cancer Center, 47 patients were treated with escalating doses of bevacizumab in combination with capecitabine and radiation. During that trial, three of the first 30 patients were found to have duodenal bleeding at the tumor site. All three were suspected of having tumor that invaded the duodenum at presentation. Subsequent protocol modification to exclude such patients led to the successful accrual of 17 more patients without subsequent bleeding events. At the dose level of bevacizumab 5 mg/kg, six of 12 patients had partial response, and there were no grade 3 gastrointestinal toxicities. This study, Radiation Therapy Oncology Group (RTOG) 0411, was designed to assess the 1-year overall survival rate and safety of bevacizumab with radiation in a cooperative group setting.
PATIENTS AND METHODS
Patient Eligibility Criteria
Cytologic or histologic proof of localized unresectable adenocarcinoma of the pancreas and a Zubrod performance score of 0 or 1 were required. Unresectability was based on institutional criteria that used either computed tomography (CT) or magnetic resonance imaging (MRI) and chest x-ray within 4 weeks of protocol entry. Patients who had received chemotherapy more than 2 years before enrollment for diseases other than pancreatic cancer were eligible provided they had no evidence of disease. There was no upper age restriction. Patients were required to have an absolute granulocyte count of ≥ 1,500 cells/μL, a platelet count ≥ 100,000 cells/μL, a calculated creatinine clearance greater than 50/mL/min, an AST level less than three times the upper limit of normal, an ALT level less than three times upper limit of normal, a serum bilirubin level of less than 2.0 mg/dL, and an international normalized ratio ≤ 1.5 before registration. All patients had to sign a study-specific consent form, and the study was approved by the Human Investigations Committee of participating institutions. Exclusion criteria are listed in Table 1.
Table 1.
Exclusion Criteria
| Evidence of duodenal invasion on imaging or upper endoscopy |
| Patients with unequivocal non-nodal, extrapancreatic, metastatic disease seen on CT, MRI, or chest x-ray |
| History of a gastrointestinal fistula or perforation |
| Prior radiation to the upper abdomen |
| History of aneurysm or arteriovenous malformations |
| Clinically significant cardiovascular or peripheral vascular disease (eg, uncontrolled hypertension, myocardial infarction, cerebrovascular event, transient ischemic attack, unstable angina within 6 months, congestive heart failure, or serious cardiac arrhythmia requiring medication) |
| Current pregnancy |
| Age younger than 18 years |
| Organ transplantation |
| Major surgical procedure, open biopsy, or significant traumatic injury within 28 days of study entry; or serious nonhealing wound, ulcer, or bone fracture |
| Patients discovered to have ≥ 1+ proteinuria at baseline were required to have a 24-hour urine protein < 1,000 mg |
| Fine-needle aspiration or core biopsy within 7 days of study entry |
| Requirement for or use of low-molecular-weight heparin at prophylactic and therapeutic dosages for patients requiring anticoagulation |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
Study Design and Treatment Plan
Patients received 50.4 Gy in 28 fractions on Monday through Friday over 5.5 weeks with concurrent capecitabine 825 mg/m2 orally twice daily on days of radiation. Bevacizumab 5 mg/kg was administered on days 1, 15, and 29 of chemoradiotherapy. Patients with stable or responding disease at the first post-treatment evaluation (4 to 7 weeks after the completion of chemoradiotherapy) were offered gemcitabine 1 g/m2 weekly for 3 weeks and bevacizumab 5 mg/kg every 2 weeks in 4-week cycles until disease progression occurred.
Protocol-Specified Conformal Radiation Technique
The gross primary tumor and any regional lymph nodes greater than 1 cm identified on CT or MRI scans were treated. Regional lymph nodes were not electively treated. A radial block margin of 2 cm and a cranial and caudal block margin of 3 cm were used. The dose was prescribed to the 95% isodose line by using greater than 6-MV photons. The volume of the liver to receive 30 Gy was required to be less than 40%, and the volume to receive 20 Gy was required to be less than 67%. At least 75% of an entire functioning kidney was required to receive less than 18 Gy, and the maximum dose to the spinal cord was 45 Gy. A four-field technique with equal beam weighting was suggested, but customization of beam angles and weighting was allowed.
Dose Modifications for Toxicity
Toxicities were scored by using the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. During the concurrent chemoradiotherapy phase, dose adjustments were required for capecitabine-related toxicity. Once a dose was reduced, it was not increased at a later time or made up. Capecitabine was withheld for grade 3 or greater neutropenia and grade 2 hand-foot syndrome, mucositis, or gastrointestinal toxicities and was restarted after recovery to grade 1. The dose adjustment was made according to the number of occurrences of gastrointestinal toxicity: for first occurrence, dose was reduced to 75% of starting dose; for second and third occurrences, dose was reduced to 50% of starting dose. Interruption of radiotherapy was required for grade 3 gastrointestinal toxicity until the toxicity resolved to grade 1.
There was no dose reduction for bevacizumab-related toxicity. However, bevacizumab was discontinued permanently for any patient who experienced bowel perforation or fistula, arterial thrombosis, hemorrhage, grade 4 nephrotic syndrome, or hypertension related to bevacizumab.
During the maintenance phase, a permanent 25% dose reduction of gemcitabine was made for any treatment-related grade 3 or 4 hematologic or nonhematologic toxicity. Treatment resumed after recovery to grade 2 or lower. Subsequent occurrences of the same toxicity required a second, permanent, 25% reduction; gemcitabine and bevacizumab were permanently discontinued if toxicity recurred a third time. Dose reduction was not made for fatigue or cholangitis during either the concurrent or maintenance phases.
Patient Monitoring
During chemoradiotherapy, patients were assessed weekly on protocol for toxicity by physical exam and blood counts. Full chemistries, chest x-ray, CA 19-9, and CT or MRI of the abdomen were required 4 to 7 weeks after the completion of chemoradiotherapy and every 2 months thereafter until disease progression occurred.
Criteria for Discontinuing Therapy
Treatment (including postchemoradiation gemcitabine and bevacizumab) was discontinued after local, regional, or distant disease progression was diagnosed on the basis of radiographic imaging; if patients decided to discontinue protocol therapy for any reason; or if patients experienced any drug-related toxicity, as described above.
Quality Assurance
Radiographic images (ie, CT or MRI), digitally reconstructed radiographs or simulation films, dose-volume histograms, and dosimetry that showed the central axis isodose distributions were submitted for post-treatment central review. This quality assurance review included evaluation of criteria of dose homogeneity and the accuracy of gross tumor volume contouring.
End Points and Statistics
The primary objective was 1-year overall survival rate. In the previous RTOG protocol for unresectable pancreatic cancer (RTOG 9812), the 1-year survival was 43%.12 When this trial was designed, there were 109 assessable patients on RTOG 9812, and 61 were still at risk of death by 1 year. By using the method of Dixon and Simon,13 a sample size of 74 assessable patients who were observed during 12 months ensured at least 90% probability of detecting a minimum of 15% improvement in the 1-year survival rate compared with RTOG 9812 at the .10 significance level (with a one-sided log-rank test14). After the sample size was adjusted by 10% to allow for patient ineligibility or loss, the total sample size was 82 patients. Overall survival was measured from the date of study entry to the date of death or last follow-up and was estimated by using the Kaplan-Meier method.15
Secondary end points included adverse events, scored by the CTCAE version 3.0, and progression-free survival (PFS). PFS was defined as local, regional, or distant progression or death as a result of any cause. PFS was measured from the date of study entry to the date of first progression, the date of death (without progression), or the date of last follow-up, and it was estimated by using the Kaplan-Meier method.15 Local and regional failure were estimated by using the cumulative incidence method. Response and progression were based on RECIST (Response Evaluation Criteria in Solid Tumors) criteria. Imaging was not reviewed centrally.
Adverse events were monitored throughout the trial, and rules were set for evaluation of the rates of unacceptable serious adverse events (USAEs) and unacceptable adverse events (UAEs). USAEs were defined per the CTCAE version 3.0 as grade 3 or greater bowel perforation, grade 3 or greater bleeding, grade 4 or greater thrombotic event, or grade 3 or greater arterial event (including vessel injury, visceral arterial ischemia, cardiac ischemia/infarction) occurring at any time; UAEs also were defined per the CTCAE version 3.0 and included grade 3 or greater gastrointestinal bleeding occurring at any time or grade 4 or greater nonhematologic adverse events occurring within 90 days of the start of treatment. A rate of ≥ 15% USAEs was considered unacceptable on the basis of the method by Fleming16; if four or more of 25, five or more of 50, or 6 or more of 74 assessable patients experienced a USAE, then the USAE rate was at least 15%.
Similarly, a rate of ≥ 35% UAEs was considered unacceptable on the basis of the method by Fleming16; if 10 or more of 25, 15 or more of 50, or 19 or more of 74 assessable patients experienced a UAE, then the UAE rate was at least 35%.
RESULTS
Patient Characteristics
Between January 18, 2005 and February 7, 2006, 94 patients were enrolled from 36 institutions. Eleven patients were ineligible on the basis of inability to meet eligibility criteria (most were inadequate lab studies), and one patient withdrew consent. Therefore, 82 patients were eligible for inclusion in the analysis. Pretreatment characteristics for eligible patients are listed in Table 2.
Table 2.
Pretreatment Characteristics
| Characteristic | Patients (N = 82) |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 63 | |
| Range | 32-83 | |
| Sex | ||
| Male | 32 | 39 |
| Female | 50 | 61 |
| Ethnicity | ||
| Hispanic or Latino | 2 | 2 |
| Not Hispanic or Latino | 77 | 94 |
| Unknown* | 3 | 4 |
| American Indian or Alaskan Native | 2 | 2 |
| African American | 4 | 5 |
| White | 75 | 91 |
| More than one ethnicity | 1 | 1 |
| Zubrod performance score | ||
| 0 | 38 | 46 |
| 1 | 44 | 54 |
| Radiographic T stage† | ||
| 1 | 3 | 4 |
| 2 | 5 | 6 |
| 3 | 17 | 21 |
| 4 | 57 | 70 |
| X | 0 | 0 |
| Radiographic N stage† | ||
| 0 | 47 | 57 |
| 1 | 21 | 26 |
| X | 14 | 17 |
| Tumor stage† | ||
| IA | 3 | 4 |
| IB | 2 | 2 |
| IIA | 9 | 11 |
| IIB | 8 | 10 |
| III | 57 | 70 |
| Unknown | 3 | 4 |
No reported ethnicity.
Staging according to American Joint Committee on Cancer Staging Manual (ed 6).17
Efficacy End Points
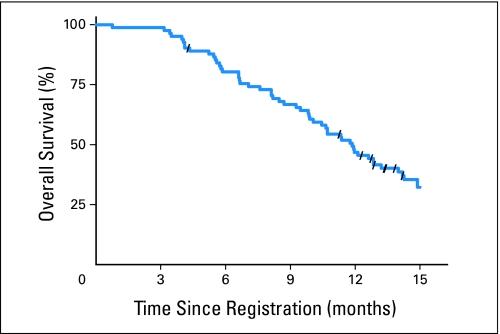
The estimated 1-year survival rate was 47% (95% CI, 36% to 57%; Fig 1) and was not statistically higher than in RTOG 9812 (one-sided log-rank test P = .49 for this study v RTOG 9812). The median survival time for patients in this study was 11.9 months (95% CI, 9.9 to 14.0 months). At the time of analysis, two patients were alive with less than 1 year of follow-up. The estimated 1-year PFS rate was 30% (95% CI, 20% to 40%). The median PFS time was 8.6 months (95% CI, 6.9 to 10.5 months). The best local responses was stable disease (50 of 82; 61%), followed by partial response (21 of 82; 26%).
Fig 1.
The estimated 1-year survival rate was 47% (95% CI, 36% to 57%).
The 12-month, actuarial, radiographic, local and regional progression rates were 29.7% (95% CI, 19.6% to 40.0%) and 8.6% (95% CI, 2.5% to 14.8%), respectively.
Events Possibly Related to Bevacizumab
Bleeding occurred in five patients (6.1%) at 83, 127, 179, 180, and 316 days after the start of chemoradiotherapy. None of these events occurred during chemoradiotherapy; two occurred during maintenance chemotherapy. None of these events were related to the acute effects of chemoradiotherapy at the tumor site. Gastrointestinal perforation occurred in three patients (3.7%) at 195 (grade 5), 231 (grade 4), and 286 (grade 3) days after the start of chemoradiotherapy. The grade 4 event resulted in discontinuation of protocol therapy, and the other two occurred after protocol therapy had been discontinued. No arterial thrombotic events were reported, but deep venous thrombosis was reported in three patients; one was grade 3, and two were grade 4. Two instances of grade 3 hypertension were reported. One patient died 23 days after the first dose of bevacizumab from an abdominal bleed that was confirmed at autopsy to be related to an endoscopic retrograde cholangiopancreatography injury.
Overall Toxicity
Sixty-six (80%) of 82 patients experienced grade 3 or greater toxicity reported as definitely, probably, or possibly related to treatment. The worst nonhematologic toxicity was grade 3 or greater in 54 (65.9%) of 82 patients. The most common events were gastrointestinal toxicity in 29 (35.4%) of 82 patients and constitutional symptoms in 22 (26.8%) of 82 patients (Table 3). During chemoradiotherapy, 18 (22.0%) of 82 patients experienced grade 3 or greater gastrointestinal toxicity (Table 4) compared with 14 (17.3%) of 81 during maintenance chemotherapy (Table 5). There were three deaths reported as definitely, probably, or possibly related to treatment. One patient experienced sudden death 175 days after the initiation of treatment; one patient experienced infection of the peritoneal cavity and died 110 days after beginning treatment; and one patient experienced colonic perforation and died 195 days after the initiation of treatment. There were an additional two deaths reported as unlikely to be related to treatment or as unrelated to treatment 23 and 155 days after treatment initiation.
Table 3.
Select Adverse Events Reported As Definitely, Probably, or Possibly Related to Treatment and Occurring at Any Time (N = 82)
| Adverse Event Category | Event Grade |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2 |
3 |
4 |
5 |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Blood/bone marrow | 15 | 18 | 29 | 35 | 8 | 10 | 0 | 0 |
| Hemoglobin | 14 | 17 | 6 | 7 | 0 | 0 | 0 | 0 |
| Leukopenia NOS | 18 | 22 | 24 | 29 | 1 | 1 | 0 | 0 |
| Lymphopenia | 2 | 2 | 5 | 6 | 3 | 4 | 0 | 0 |
| Neutrophil count | 17 | 21 | 15 | 18 | 5 | 6 | 0 | 0 |
| Platelet count | 16 | 20 | 10 | 12 | 0 | 0 | 0 | 0 |
| Cardiac, general | 4 | 5 | 3 | 4 | 0 | 0 | 0 | 0 |
| Constitutional symptom | 29 | 35 | 22 | 27 | 0 | 0 | 0 | 0 |
| Death not associated with CTCAE 3.0 toxicity | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Dermatologic/skin | 2 | — | 2 | — | 0 | 0 | 0 | 0 |
| Gastrointestinal | 33 | 40 | 26 | 32 | 2 | 2 | 1 | 1 |
| Hemorrhage/bleeding | 3 | 4 | 4 | 5 | 1 | 1 | 0 | 0 |
| Infection | 3 | 4 | 2 | 2 | 1 | 1 | 1 | 1 |
| Musculoskeletal/soft tissue | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 |
| Neurologic | 4 | 5 | 2 | 2 | 0 | 0 | 0 | 0 |
| Ocular/visual | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Pain | 16 | 20 | 5 | 6 | 2 | 2 | 0 | 0 |
| Pulmonary/upper respiratory | 5 | 6 | 4 | 5 | 0 | 0 | 0 | 0 |
| Syndrome | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Vascular | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 |
Abbreviations: NOS, not otherwise specified; CTCAE 3.0, Common Terminology Criteria of Adverse Events, version 3.0.
Table 4.
Select Adverse Events Reported As Definitely, Probably, or Possibly Related to Treatment and Occurring During Chemoradiation (N = 82)
| Adverse Event Category | Event Grade |
|||||
|---|---|---|---|---|---|---|
| 2 |
3 |
4 |
||||
| No. | % | No. | % | No. | % | |
| Blood/bone marrow | 12 | 15 | 6 | 7 | 3 | 4 |
| Hemoglobin | 4 | 5 | 0 | 0 | 0 | 0 |
| Leukopenia NOS | 7 | 9 | 3 | 4 | 0 | 0 |
| Lymphopenia | 2 | 2 | 3 | 4 | 3 | 4 |
| Neutrophil count | 3 | 4 | 0 | 0 | 0 | 0 |
| Platelet count | 1 | 1 | 0 | 0 | 0 | 0 |
| Cardiac, general | 4 | 5 | 1 | 1 | 0 | 0 |
| Constitutional symptom | 32 | 39 | 8 | 10 | 0 | 0 |
| Dermatologic/skin | 2 | 2 | 1 | — | 0 | 0 |
| Gastrointestinal | 28 | 34 | 18 | 22 | 0 | 0 |
| Hemorrhage/bleeding | 1 | 1 | 0 | 0 | 0 | 0 |
| Infection | 4 | 5 | 1 | 1 | 0 | 0 |
| Musculoskeletal/soft tissue | 1 | 1 | 0 | 0 | 0 | 0 |
| Neurologic | 4 | 5 | 0 | 0 | 0 | 0 |
| Ocular/visual | 1 | 1 | 1 | 1 | 0 | 0 |
| Pain | 9 | 11 | 2 | 2 | 0 | 0 |
| Pulmonary/upper respiratory | 0 | 0 | 1 | 1 | 0 | 0 |
| Syndrome | 0 | 0 | 1 | 1 | 0 | 0 |
Abbreviation: NOS, not otherwise specified.
Table 5.
Select Adverse Events Reported As Definitely, Probably, or Possibly Related to Therapy Occurring During Maintenance Chemotherapy (N = 81)
| Adverse Event Category | Event Grade |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2 |
3 |
4 |
5 |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Blood/bone | 12 | 15 | 30 | 37 | 5 | 6 | 0 | 0 |
| Hemoglobin | 13 | 16 | 6 | 7 | 0 | 0 | 0 | 0 |
| Leukopenia NOS | 14 | 17 | 24 | 29 | 1 | 1 | 0 | 0 |
| Lymphopenia | 2 | 2 | 5 | 6 | 0 | 0 | 0 | 0 |
| Neutrophil count | 17 | 21 | 15 | 18 | 5 | 6 | 0 | 0 |
| Platelet count | 16 | 20 | 10 | 12 | 0 | 0 | 0 | 0 |
| Cardiac, general | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 |
| Constitutional symptom | 18 | 22 | 17 | 21 | 0 | 0 | 0 | 0 |
| Death not associated with a CTCAE 3.0 toxicity | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Dermatologic/skin | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 23 | 28 | 11 | 13 | 2 | 2 | 1 | 1 |
| Hemorrhage/bleeding | 2 | 2 | 4 | 5 | 1 | 1 | 0 | 0 |
| Infection | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Musculoskeletal/soft tissue | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 |
| Neurologic | 3 | 4 | 1 | 1 | 0 | 0 | 0 | 0 |
| Pain | 12 | 15 | 3 | 4 | 1 | 1 | 0 | 0 |
| Pulmonary/upper respiratory | 5 | 6 | 3 | 4 | 0 | 0 | 0 | 0 |
| Vascular | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 |
Abbreviations: NOS, not otherwise specified; CTCAE 3.0, Common Terminology Criteria of Adverse Events, version 3.0.
When the first 25 and the first 50 assessable patients were evaluated for toxicity, neither the early stopping criterion for USAEs nor that for UAEs was met. Additionally, only four of the first 74 assessable patients experienced UAEs; however, at the final analysis, 9 patients of the first 74 assessable patients had USAEs. Therefore, on the basis of these data, the rate of USAEs as defined in this protocol is greater than 15% but not greater than 35%.
Chemotherapy Dose Adjustments for Toxicity
During chemoradiotherapy, capecitabine was adjusted for toxicity in 29% of patients, and bevacizumab was held or discontinued in 16% of patients. A median of three cycles of maintenance chemotherapy was given (range, 0 to 10.3 cycles). The gemcitabine dose was modified, held, or discontinued in 89% of patients, and the bevacizumab dose was held or discontinued in 50% of patients during the maintenance phase.
Radiotherapy Quality Assurance
All the radiation treatment plans were reviewed after the completion of protocol therapy, and deviations in either tumor contouring or treatment field design were noted. Major (unacceptable) deviation was defined as contoured gross tumor volume 5 cm greater than the actual tumor size on the basis of diagnostic imaging in any dimension, inability to contour gross tumor, or the use of block margin greater than 5 cm. There were 11 (13.4%) unacceptable deviations. All were related to excessive inclusion of normal tissue in the contoured volume. There was a significant correlation between major deviation and the incidence of grade 3 or greater gastrointestinal toxicity both during chemoradiotherapy (45% v 18%; adjusted odds ratio, 3.7; 95% CI, 0.98 to 14.1; P = .05) and during maintenance chemotherapy (45% v 13%; adjusted odds ratio, 5.7; 95% CI, 1.45 to 22.8; P = .01).
Surgical Resection
Ten patients underwent surgical exploration and attempted resection at a median of 7.1 weeks after the last dose of bevacizumab (range, 4.1 to 14.7 weeks). Seven of these underwent pancreaticoduodenectomy, and one underwent distal pancreatectomy. Five of the eight patients who underwent resection had margin-negative (R0) resection, and three had resection with indeterminate margins. Two were determined to be unresectable. There was one perioperative complications reported: a grade 3 perioperative infection.
DISCUSSION
Treatments for locally advanced pancreatic adenocarcinoma have significant efficacy limitations. Concurrent chemoradiotherapy is a standard treatment option that appears to modestly prolong median survival through enhanced tumor control.18 However, objective treatment response is uncommon, and tumor control using conventional doses of radiotherapy is anecdotal. Agents that target aberrant overexpression of growth regulatory molecules, such as growth factor receptors and their ligands, are being evaluated now in combination with chemoradiotherapy as a strategy to enhance local tumor control. To our knowledge, this study is the first study to demonstrate the safety of the combination of anti-VEGF therapy given concurrently with chemoradiotherapy in a multi-institutional setting.
In an effort to reduce the rate of acute gastrointestinal toxicity, this trial was the first from the RTOG trials to use treatment fields targeted to the gross tumor alone. Although the grade 3, treatment-related, acute gastrointestinal toxicity rate that occurred during chemoradiotherapy was acceptable (22.0%), the rate was even lower in the patients treated without protocol deviation related to the treatment field (18.0%). Unacceptable protocol deviations (13.4%) were identified in a retrospective quality assurance review, and all were related to the contouring of excessive amounts of gastrointestinal tissue rather than to the inability to treat the primary tumor. The overtreatment of the gastrointestinal mucosa in these patients likely caused excessive gastrointestinal toxicity. Although it is speculation, the impression of the investigators was that the overcontouring was related, at least in part, to poor tumor visualization on the diagnostic images that were used for treatment planning. Increased use of higher-quality imaging techniques, such as pancreatic cancer–specific CT imaging as well as oral and intravenous contrast,19 likely would contribute to more accurate tumor visualization and targeting. Pancreatic protocol diagnostic imaging and prospective quality assurance will be incorporated into future RTOG pancreatic cancer trials.
There were a small number of bleeding events (6.1%) that were at least possibly related to bevacizumab. However, none of these events occurred at the tumor site, and all but one occurred at least 3 months after chemoradiotherapy was completed. Therefore, the exclusion of patients whose tumors appeared to be clinically invading the duodenal mucosa effectively limited the occurrence of bleeding at the site of the primary tumor in this trial, as it did in the last 18 patients of the phase I study on which it was based.20 Therefore, the exclusion of patients whose tumors appeared to be clinically invading the duodenal mucosa effectively limited the occurrence of bleeding at the site of the primary tumor.
The 1-year overall survival rate in this study is comparable to that of prior RTOG phase II studies. Therefore, the primary end point was negative, and additional study of this regimen in locally advanced pancreatic cancer is not warranted. The maintenance chemotherapy component of this trial was designed to address the risk of the development of metastatic disease from presumed micrometastatic disease. The role of the addition of bevacizumab to gemcitabine in metastatic pancreatic cancer was addressed in a recently reported, phase III trial (CALGB 80303).21 That trial failed to demonstrate an improvement in median survival with the addition of bevacizumab. From these two trials, it appears that the addition of bevacizumab does not have a role in the treatment of either locally advanced or metastatic pancreatic cancer. However, anti-VEGF therapy could still have a role in combination with radiotherapy. Ongoing phase II studies are evaluating the ability of bevacizumab to enhance the local treatment effect in other solid tumors in which local control is a larger component of the overall failure risk than it is in pancreatic cancer, such as esophageal, head and neck, cervical, and lung cancers.
In summary, the addition of bevacizumab to a regimen of capecitabine-based chemoradiotherapy followed by gemcitabine did not result in an improvement in overall survival in this phase II study. However, bevacizumab-related adverse events were uncommon and were similar to those expected on the basis of previous trials. In addition, our findings highlight the importance of prospective incorporation of radiotherapy quality assurance review in future pancreatic chemoradiotherapy trials to minimize treatment-related toxicity.
Footnotes
Supported by Grants No. RTOG U10 CA21661, CCOP U10 CA37422, and Stat U10 CA32115 from the National Cancer Institute and by Genentech through the National Cancer Institute (provided bevacizumab).
Presented at 49th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, October 28, 2007 to November 1, 2007, Los Angeles, CA.
This contents of this manuscript are the sole responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
Clinical trial information can be found for the following: NCT00114179.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Walter Curran, Genentech (C) Stock Ownership: None Honoraria: Christopher H. Crane, Roche Research Funding: Christopher H. Crane, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Christopher H. Crane, William F. Regine, Howard Safran, Christopher G. Willett
Financial support: Walter Curran, Christopher G. Willett
Administrative support: Kathryn Winter, Walter Curran, Christopher G. Willett
Provision of study materials or patients: Christopher H. Crane, William F. Regine, Howard Safran, Tyvin A. Rich, Robert A. Wolff
Collection and assembly of data: Christopher H. Crane, Kathryn Winter
Data analysis and interpretation: Christopher H. Crane, Kathryn Winter, Howard Safran, Christopher G. Willett
Manuscript writing: Christopher H. Crane, Kathryn Winter
Final approval of manuscript: Christopher H. Crane, Kathryn Winter, William F. Regine, Howard Safran, Tyvin A. Rich, Walter Curran, Robert A. Wolff, Christopher G. Willett
REFERENCES
- 1.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 5.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 6.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 7.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 8.Geng L, Donnelly E, McMahon G, et al. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res. 2001;61:2413–2419. [PubMed] [Google Scholar]
- 9.Kozin SV, Boucher Y, Hicklin DJ, et al. Vascular endothelial growth factor receptor-2–blocking antibody potentiates radiation-induced long-term control of human tumor xenografts. Cancer Res. 2001;61:39–44. [PubMed] [Google Scholar]
- 10.Wey J, Fan F, Gray M, et al. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer. 2005;104:427–438. doi: 10.1002/cncr.21145. [DOI] [PubMed] [Google Scholar]
- 11.Yang AD, Camp ER, Fan F, et al. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Res. 2006;66:46–51. doi: 10.1158/0008-5472.CAN-05-3086. [DOI] [PubMed] [Google Scholar]
- 12.Rich T, Harris J, Abrams R, et al. Phase II study of external irradiation and weekly paclitaxel for nonmetastatic, unresectable pancreatic cancer: RTOG-9812. Am J Clin Oncol. 2004;27:51–56. doi: 10.1097/01.coc.0000046300.88847.bf. [DOI] [PubMed] [Google Scholar]
- 13.Dixon D, Simon R. Sample size considerations for studies comparing curves using historical controls. J Clin Epidemiol. 1988;41:1209–1213. doi: 10.1016/0895-4356(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 14.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chem Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 15.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Fleming T. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 17.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. ed 6. New York, NY: Springer; 2002. Exocrine pancreas; pp. 157–164. [Google Scholar]
- 18.Loehrer P, Powell M, Cardenes H, et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201. J Clin Oncol. 2008;26(suppl):214s. doi: 10.1097/COC.0b013e3181e9c103. abstr 4506. [DOI] [PubMed] [Google Scholar]
- 19.Tamm E, Loyer E, Faria S, et al. Staging of pancreatic cancer with multidetector CT in the setting of preoperative chemoradiation therapy. Abdom Imaging. 2006;31:568–574. doi: 10.1007/s00261-005-0194-y. [DOI] [PubMed] [Google Scholar]
- 20.Crane CH, Ellis LM, Abbruzzese JL, et al. Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol. 2006;24:1145–1151. doi: 10.1200/JCO.2005.03.6780. [DOI] [PubMed] [Google Scholar]
- 21.Kindler HL, Niedzwiecki D, Hollis DR, et al. A double-blind, placebo-controlled, randomized Phase III trial of gemcitabine plus bevacizumab versus gemcitabine plus placebo in patients with advanced pancreatic cancer: A preliminary analysis of Cancer and Leukemia Group B (CALGB) J Clin Oncol. 2007;25(suppl):199s. abstr 4508. [Google Scholar]