Abstract
Purpose
This study compares late effects of treatment on physical well-being and utilization of health care resources between ovarian germ cell tumor (OGCT) survivors and age/race/education-matched controls.
Patients and Methods
Eligible patients had OGCT treated with surgery and chemotherapy and were disease-free for at least 2 years at time of enrollment. The matched control group was selected from acquaintances recommended by survivors. Symptoms and function were measured using previously validated scales. Health care utilization was assessed by questions regarding health insurance coverage and health services utilization.
Results
One hundred thirty-two survivors and 137 controls completed the study. Survivors were significantly more likely to report a diagnosis of hypertension (17% v 8%, P = .02), and marginally hypercholesterolemia (9.8% v 4.4%, P = .09), and hearing loss (5.3% v 1.5%, P = .09) compared with controls. There were no significant differences in the rates of self-reported arthritis, heart, pulmonary or kidney disease, diabetes, non-OGCT malignancies, anxiety, hearing loss, or eating disorders between groups. Among chronic functional problems, numbness, tinnitus, nausea elicited by reminders of chemotherapy (v general nausea triggers for controls), and Raynaud's symptoms were reported more frequently by survivors. Patients who received vincristine, dactinomycin, and cyclophosphamide in addition to cisplatin therapy had increased functional complaints, particularly numbness and nausea. Health care utilization was similar, but 15.9% of survivors reported being denied health insurance versus 4.4% of controls (P < .001).
Conclusion
Although a few sequelae of treatment persist, in general, OGCT survivors enjoy a healthy life comparable to that of controls.
INTRODUCTION
Ovarian germ cell tumors (OGCTs) are rare tumors diagnosed at a median age of 16 to 20 years. Over the past three decades, significant progress has been achieved in their treatment. The major advances relate to improved chemotherapy, the current standard being cisplatin, etoposide, and bleomycin.1–3 With this treatment, most patients are cured.3–5 Given the excellent prognosis and long-term survivorship, it is essential to understand the long-term effects of treatment. The purpose of this study was to compare OGCT survivors with age/race/education-matched controls on physical well-being and utilization of health care resources.
Because of the rarity of these tumors, limited information on late effects of treatment and quality of life of survivors is available.6,7 One pediatric study described long-term effects of treatment on 73 children with germ cell tumors treated between 1962 and 1988.6 Late effects were frequent, with more than two thirds of patients having at least one complication, including hearing loss, neuropathy, cardiovascular, endocrine, musculoskeletal, gastrointestinal, and urinary abnormalities. Assumptions regarding long-term outcomes of adult OGCT survivors have been extrapolated from the literature describing testicular cancer survivors.8–13 In male patients who received cisplatin-based regimens, late toxicities included high-tone hearing loss, neurotoxicity, Raynaud's phenomenon, ischemic heart disease, hypertension, renal dysfunction, and pulmonary toxicity.8–14 Fortunately, despite these, most testis cancer survivors have excellent overall health and functional status.12 Although treatment for OGCT and testis cancer is similar, OGCT survivors differ by having had abdominal surgery for tumor debulking, as OGCTs tend to disseminate intraperitoneally, unlike testis cancer. There are other sex-specific psychosocial and reproductive differences, justifying the need for investigating late treatment effects in OGCT survivors.
The first study to address this issue is the recent protocol of the Gynecologic Oncology Group (GOG), which investigated social, psychological, sexual, physical, and reproductive sequelae of treatment. Patients had been treated on prospective clinical trials of the GOG or similar protocols at The University of Texas M. D. Anderson Cancer Center.5 The objectives were to compare self-reported health, symptoms, and function of long-term survivors of OGCT with controls matched on age, race, and education. Several reports have resulted from this analysis.14–16 Compared with controls, survivors were well-adjusted, free of depression, and able to develop strong relationships.15,16 The impact on fertility was modest in patients undergoing fertility-sparing surgery.14 The current article, using the same data set, describes characteristics related to physical well-being and health care utilization.
PATIENTS AND METHODS
Study Population
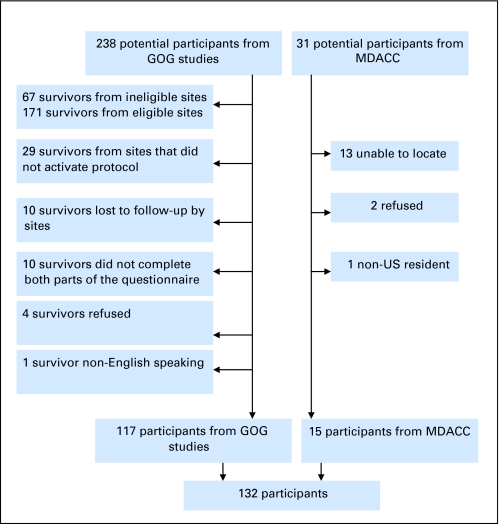
Institutional review boards at Indiana University, M. D. Anderson Cancer Center, and each participating site approved this protocol. Eligible candidates had a history of OGCT and were disease-free for at least 2 years. All histologic types and stages were eligible. Treatment consisted of surgery followed by platinum-based chemotherapy on GOG protocols 45, 78, 90, or 116, or similar protocols at M. D. Anderson, as summarized in Table 1. Protocols 45 and 90 included patients with incompletely resected tumor. Protocols 78 and 116 were adjuvant therapy trials. Patients who received other chemotherapy or radiotherapy were excluded. Patients were required to complete a written questionnaire and telephone interview in English. Every effort was made to contact and enroll all potentially eligible patients. Those that were not enrolled either could not be located, had been treated at institutions no longer in the GOG, or, in a few instances, did not consent, as indicated in Appendix Figure A1 (online only). Their characteristics compared with participants are noted in this figure. The control group consisted of acquaintances recommended by survivors and was frequency matched for age, race, and education.
Table 1.
Chemotherapy Regimens
| Protocol | Chemotherapy | Survivors |
|
|---|---|---|---|
| No. | % | ||
| 45 | Cisplatin, vinblastine, bleomycin | 15 | 11.4 |
| 78 | BEP | 51 | 38.6 |
| 90 | BEP, then VAC | 49 | 37.1 |
| 116 | Carboplatin and etoposide | 16 | 12.1 |
| Other | Bleomycin, then VAC | 1 | 0.8 |
| Total | 132 | 100 | |
Abbreviations: BEP, etoposide, bleomycin, cisplatin; VAC, vincristine, dactinomycin, cyclophosphamide.
Study Design
Survivors and controls were contacted by trained interviewers from Indiana University Melvin and Bren Simon Cancer Center (IUSCC) and mailed an informed consent and background questionnaire. After receipt of the background questionnaire, a 60-minute standardized computer-assisted telephone interview was conducted by graduate level nurses or social workers. Interview questions were sent to participants before interview and interviewers entered responses directly into a computer. Cancer-related questions were deleted from questionnaires directed to controls. Interviews, database management, and analyses were conducted at IUSCC.
Information Collected
Cancer diagnosis and treatment variables were obtained from existing GOG and M. D. Anderson databases. Other sociodemographic characteristics were extracted from questionnaires.
Physical well-being: Assessment of chronic physical effects of treatment included 12 questions regarding systemic chronic illness. Symptoms associated with peripheral neuropathy were assessed using the Functional Assessment of Cancer Therapy (FACT)/GOG-Neurotoxicity (NTX) scale, which is part of the Functional Assessment of Chronic Illness Therapy Measurement System.17 For this study, three items were added to reflect late effects commonly associated with chemotherapy (difficulty concentrating; nausea when thoughts, sights, or smells remind survivors of chemotherapy; and Raynaud's symptoms). Controls were asked about nausea from certain thoughts, sights, and smells. Each item from the FACT/GOG-NTX scale was examined separately. Health Care Utilization was measured by questions regarding insurance coverage and frequency with which survivors used physical and mental health care services, including outpatient, emergency room, and in-patient services. Questions regarding insurance coverage and work discrimination were based on prior studies.18
Statistical Analysis
α of 0.05 and two-sided tests were used for all statistical tests. Differences between survivors and controls on categoric variables were tested using Fisher's exact test. Differences on continuous variables were tested with the Wilcoxon Rank Sum test. Exact-probability multiple logistic regression was used to statistically compare survivors and controls on health care utilization variables after adjusting for the two demographic variables that survivors and controls differed on, specifically, income (< $65,000 v ≥ $65,000) and marital status (married/relationship v others). SAS was used for all statistical analyses (SAS Institute, Cary, NC). When comparing groups on separate items of the FACT/GOG-NTX scale, the false discovery rate method was used to adjust for multiple comparisons, and levels of significance were reported for unadjusted and adjusted results.19 For items identified as different between groups, multivariable exact-probability logistic regression was performed to determine whether group (survivors v controls) remained a significant predictor after adjusting for confounding covariates: smoking, age, race, married status, insurance status (yes/no), and income (≥ $65,000 v others). Each covariate was adjusted for in separate models.
RESULTS
Study Population
The survivors were selected from a pool of 238 eligible women treated at GOG sites and of 31 women treated at M. D. Anderson, with 132 survivors ultimately enrolled (117 from GOG and 15 from M. D. Anderson). Table 2 indicates that subjects and controls were well matched on demographic characteristics, yielding similar distributions for age, race, and educational status. Survivors were approximately 10 years out from diagnosis. The majority (87%) had received cisplatin and bleomycin. There were no differences in regard to smoking. Fewer survivors were married or involved in a relationship (P = .042) or earning in excess of $65,000 annually (P = .022).
Table 2.
Demographic Characteristics
| Characteristic | Survivors (n = 132) |
Controls (n = 137) |
Difference | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Age, years | 0.2 | −2.0 to 2.4 | |||||
| Mean | 35.9 | 35.7 | |||||
| SD | 9.1 | 9.2 | |||||
| Range | 19-64 | 19-58 | .90 | ||||
| Age at diagnosis, years | |||||||
| Mean | 25.8 | ||||||
| SD | 8.7 | ||||||
| Range | 11-53 | ||||||
| Years since diagnosis | |||||||
| Mean | 10.2 | ||||||
| SD | 4.2 | ||||||
| Range | 3-21 | ||||||
| Race | .88 | ||||||
| White | 106 | 80.3 | 108 | 78.8 | 1.5 | −8.2 to 11.1 | |
| African American | 15 | 11.4 | 14 | 10.2 | 1.1 | −6.3 to 8.6 | |
| Asian/Pacific Islander | 3 | 2.3 | 1 | 0.7 | 1.5 | −1.4 to 4.5 | |
| Other | 8 | 6.1 | 14 | 10.2 | −4.2 | −10.7 to 2.4 | |
| Education | .22 | ||||||
| Some college or less | 70 | 53.0 | 62 | 45.3 | 7.7 | −4.1 to 19.7 | |
| College graduate or more | 62 | 47.0 | 75 | 54.7 | −7.7 | −19.7 to 4.1 | |
| Income, $ | .02 | ||||||
| < 25,000 | 30 | 22.7 | 22 | 16.1 | 6.7 | −2.8 to 16.1 | |
| 25,000-44,999 | 39 | 29.5 | 28 | 20.4 | 9.1 | −1.2 to 19.4 | |
| 45,000-64,999 | 20 | 15.2 | 24 | 17.5 | −2.4 | −11.2 to 6.5 | |
| ≥ 65,000 | 39 | 29.5 | 59 | 43.1 | −13.5 | −24.9 to −2.2 | |
| Missing | 4 | 3.0 | 4 | 2.9 | 0.1 | −4.0 to 4.1 | |
| Marital status | .04 | ||||||
| Single | 38 | 28.8 | 26 | 19.0 | 9.8 | −0.3 to 20.0 | |
| Married/relationship | 75 | 56.8 | 95 | 69.3 | −12.5 | −24.0 to −1.1 | |
| Separated/divorced/widowed | 19 | 14.4 | 16 | 11.7 | 1.2 | −6.7 to 9.1 | |
| Currently smoke | .36 | ||||||
| No | 109 | 82.6 | 106 | 77.4 | 5.2 | −4.3 to 14.7 | |
| Yes | 23 | 17.4 | 31 | 22.6 | −5.2 | −14.7 to 4.3 | |
| Chemotherapy regimen | |||||||
| VAC | 35 | 26.5 | |||||
| No VAC | 97 | 73.5 | |||||
| Chemotherapy regimen | |||||||
| Bleomycin (BEP or PVB) | 116 | 87.9 | |||||
| Not (116-carboplatin) | 16 | 12.1 | |||||
NOTE. P value is for two-sided tests comparing survivors versus controls: age, Wilcoxon Rank Sum Test (t approximation); smoking and education, Fisher's exact test; race, Fisher's exact test for white versus others; income, Fisher's exact test for < $65,000 versus ≥ $65,000; marital status, Fisher's exact test for married/relationship versus others. Difference = observed difference (survivors–controls) in means or proportions (95% CI).
Abbreviations: SD, standard deviation; VAC, vincristine, dactinomycin, cyclophosphamide; BEP, etoposide, bleomycin, cisplatin; PVB, cisplatin, vinblastine, bleomycin.
Chronic Health Issues
There were no significant differences in the rates of chronic arthritis, heart disease, pulmonary problems, kidney disease, diabetes, anxiety, or eating disorders between the groups (Table 3). Hypertension was reported at a significantly higher rate for survivors (17%) than controls (8%; P = .027).
Table 3.
Chronic Disease
| Chronic Disease | Survivors(n = 132) |
Controls(n = 137) |
Difference | 95% CI | P* | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Arthritis | 10 | 7.6 | 5 | 3.6 | 3.9 | −1.6 to 9.4 | .19 |
| Heart disease or problem | 1 | 0.8 | 3 | 2.2 | −1.4 | −4.3 to 1.4 | .62 |
| High blood pressure or stroke | 23 | 17.4 | 11 | 8.0 | 9.4 | 1.5 to 17.3 | .03 |
| Serious respiratory distress or problem | 8 | 6.1 | 4 | 2.9 | 3.1 | −1.8 to 8.1 | .25 |
| Kidney disease or problem | 3 | 2.3 | 2 | 1.5 | 0.8 | −2.4 to 4.1 | .68 |
| High cholesterol | 13 | 9.8 | 6 | 4.4 | 5.5 | −0.7 to 11.6 | .10 |
| Diabetes | 5 | 3.8 | 5 | 3.6 | 0.1 | −4.4 to 4.7 | 1.00 |
| Hearing loss | 7 | 5.3 | 2 | 1.5 | 3.8 | −0.5 to 8.2 | .10 |
| Leukemia/cancer (not ovarian germ cell) | 3 | 2.3 | 2 | 1.5 | 0.8 | −2.4 to 4.1 | .68 |
| Anxiety/panic disorders | 15 | 11.4 | 11 | 8.0 | 3.3 | −3.7 to 10.4 | .41 |
| Eating disorders | 8 | 6.1 | 6 | 4.4 | 1.7 | −3.6 to 7.0 | .59 |
| None of the above | 76 | 57.6 | 97 | 70.8 | −13.2 | -24.6 to −1.9 | .03 |
P value is for comparing survivors versus controls using two-sided Fisher's exact test.
Hypercholesterolemia and hearing loss were reported marginally more frequently by survivors compared with controls (P < .10). Multivariable logistic regression showed that group remained a significant predictor of high blood pressure (P < .05), and a marginally (.05 < P < .10) significant predictor of high cholesterol and hearing loss. None of the covariates adjusted for were significant predictors of the outcomes.
The occurrence of non-OGCT cancers was not different between groups, with three reports among survivors during a mean follow-up period of 10.2 years compared with two cases of cancer among controls (P = .68). Those second malignancies reported by OGCT survivors included two breast cancers and one vulvar cancer in situ.
Chronic Functional Problems
Survivors were more likely to experience numbness or tingling in hands (P = .035) and marginally more likely to experience numbness or tingling in feet (P = .095) compared with controls (Table 4). Comparison of intensity measured on a scale from 0 to 4 is shown in Appendix Table A1 (online only). Intensity of numbness and tingling was also higher in cancer survivors. Ringing in the ears was more common (29% v 17%, P = .037) and intense (0.59 v 0.25, P = .014) in survivors versus controls, but there were no significant differences in self-reported hearing loss. Raynaud's symptoms were recorded by 36% of survivors compared with 18% of controls (P = .002), with higher intensity in survivors (P = .0005; Appendix Table A1). Nausea elicited by thoughts, sights, or smells of chemotherapy was more common (52% v 18%, P < .0001) and more intense (P < .0001) in survivors than any type of nausea experienced by controls. Survivors also had significantly greater problems buttoning buttons than controls (11% v 2%). Interestingly, a significantly higher percentage of controls reported joint or muscle pains compared with OGCT survivors, and the level of discomfort was higher in controls than survivors, possibly reflecting a higher tolerance level for mundane discomfort by survivors. These differences in function persisted after adjusting for multiple comparisons, with four differences remaining significant for frequency and intensity, one item (ringing in ears) remaining significant only on intensity, and one item (numbness in hands) losing significance on both presence and intensity. Survivors did not differ from controls in regard to fatigue, perception of discomfort in hands or feet, difficulty hearing or concentrating, or altered ability to walk or feel small objects. In our previous report of summed-item NTX intensity score, survivors had significantly greater intensity than controls when the three additional items were included.17
Table 4.
Presence of Chronic Functional Problems
| Item on the FACT/GOG–NTX Scale | Survivors(n = 132) |
Controls(n = 137) |
Difference | 95% CI | P * | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| 1. Numbness or tingling in hands | 44 | 33.3 | 26 | 21.0 | 12.4 | 1.6 to 23.1 | .04 |
| 2. Numbness or tingling in feet | 34 | 25.8 | 21 | 16.9 | 8.8 | −1.1 to 18.8 | .10 |
| 3. Discomfort in hands | 27 | 20.5 | 30 | 24.6 | −4.1 | −14.4 to 6.2 | .45 |
| 4. Discomfort in feet | 26 | 19.7 | 34 | 27.4 | −7.7 | −18.1 to 2.7 | .18 |
| 5. Joint pain or muscle cramps† | 41 | 31.1 | 63 | 50.8 | −19.8 | −31.6 to −7.9 | .0015 |
| 6. Weak all over | 32 | 24.2 | 23 | 18.7 | 5.5 | −4.5 to 15.6 | .29 |
| 7. Trouble hearing | 28 | 21.4 | 28 | 22.6 | −1.2 | −11.4 to 9.0 | .88 |
| 8. Ringing or buzzing in ears | 38 | 28.8 | 21 | 17.1 | 11.7 | 1.5 to 21.9 | .04 |
| 9. Trouble buttoning buttons† | 14 | 10.8 | 3 | 2.4 | 8.4 | 2.4 to 14.3 | .01 |
| 10. Trouble feeling shape of small objects in hand | 8 | 6.1 | 3 | 2.4 | 3.7 | −1.2 to 8.6 | .22 |
| 11. Trouble walking | 17 | 12.9 | 16 | 12.9 | −0.02 | −8.2 to 8.2 | 1.00 |
| 12. Difficulty concentrating | 48 | 36.4 | 52 | 41.9 | −5.6 | −17.5 to 6.4 | .37 |
| 13. Nausea when certain thoughts, sights, or smells remind me of chemotherapy† | 69 | 52.3 | 22 | 17.9 | 34.4 | 23.5 to 45.3 | .00 |
| 14. Fingers turn blue or white when it is cold† | 47 | 35.6 | 22 | 17.7 | 17.9 | 7.3 to 28.4 | .0018 |
Abbreviation: FACT/GOG–NTX, Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Neurotoxicity scale.
P value is for comparing survivors versus controls on the percentage of participants endorsing any level of the symptom using the two-sided Fisher's exact test. For controls, item 13 read “nausea from certain thoughts, sights or smells.”
Items that remained significant after adjusting for 14 multiple comparisons with the false discovery rate method.
In Table 5, factors analyzed for possible associations with development of late toxicity are based on prior reports and include age at diagnosis, type of treatment (eg, VAC v no VAC, bleomycin v no bleomycin), and time since completing chemotherapy.6 Additionally, we analyzed the association between smoking and late toxicity of treatment, as smoking is a known risk factor for vascular and pulmonary disorders.
Table 5.
Correlations Among Survivors Between Presence of Chronic Functional Problems and Demographic/Treatment-Related Variables
| Item on the FACT/GOG-NTX Scale | Chemotherapy Regimen |
Age in Years | Age at Diagnosis | Years Since Diagnosis | Currently Smoke | |
|---|---|---|---|---|---|---|
| VAC/No | Bleo/No | |||||
| 1. Numbness or tingling in hands | 0.23*† | 0.21‡ | 0.02 | 0.04 | −0.05 | 0.27*† |
| 2. Numbness or tingling in feet | 0.31§† | 0.22‡ | 0.01 | 0.08 | −0.13 | 0.14 |
| 3. Discomfort in hands | 0.29§† | 0.19‡ | 0.03 | 0.06 | −0.06 | 0.16 |
| 4. Discomfort in feet | 0.31§† | 0.18‡ | 0.17‡ | 0.25*† | −0.14 | 0.07 |
| 5. Joint pain or muscle cramps | 0.23*† | 0.15 | 0.06 | 0.12 | −0.10 | −0.05 |
| 6. Weak all over | 0.14 | 0.10 | −0.05 | −0.03 | −0.04 | 0.07 |
| 7. Trouble hearing | 0.19‡† | 0.19‡ | 0.08 | 0.04 | 0.09 | 0.21‡ |
| 8. Ringing or buzzing in ears | 0.22*† | 0.13 | −0.05 | −0.10 | 0.09 | 0.10 |
| 9. Trouble buttoning buttons | 0.18‡ | 0.05 | 0.16 | 0.20‡ | −0.07 | 0.16 |
| 10. Trouble feeling shape of small objects in hand | 0.06 | 0.00 | 0.09 | 0.10 | −0.01 | 0.13 |
| 11. Trouble walking | 0.28*† | 0.14 | 0.24* | 0.25*† | 0.02 | −0.06 |
| 12. Difficulty concentrating | 0.19‡† | 0.14 | −0.04 | 0.01 | −0.11 | 0.03 |
| 13. Nausea when certain thoughts, sights, or smells remind me of chemotherapy | 0.16 | 0.06 | −0.21‡ | −0.19‡ | −0.06 | 0.04 |
| 14. Fingers turn blue or white when it is cold | 0.16 | 0.13 | 0.01 | −0.03 | 0.08 | 0.12 |
NOTE. Values in the table are ϕ correlation coefficients for binary variables (VAC/no, Bleo/no, currently smoking) and point-biserial correlations for continuous variables (age, age at diagnosis, and years since diagnosis).
Abbreviations: FACT/GOG–NTX, Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Neurotoxicity scale; VAC, vincristine, dactinomycin, cyclophosphamide; Bleo, bleomycin.
P < .01.
Denote items that remained significant after adjusting for 14 multiple comparisons with the false discovery rate method.
P < .05.
P < .001.
Treatment with VAC in addition to platinum (ie, some patients treated on protocol GOG 90) was significantly associated with 10 of 14 chronic functional problems; nine of the 10 differences remaining significant after adjusting for multiple comparisons. The strongest magnitude of correlation with VAC was with numbness or tingling in feet (r = 0.31, P < .001), reflecting added toxicity from vincristine in this group of patients. Likewise, intensity of nausea triggered by reminders of chemotherapy was associated with exposure to VAC, although not after adjusting for multiple comparisons.19 Survivors that had received VAC reported increased difficulty concentrating (significant after adjustment for multiple comparisons). Absence of bleomycin and cisplatin in the regimen (ie, patients from protocol 116) was associated with decreased frequency of numbness or tingling, discomfort in hands and feet, and trouble hearing, reflecting a more favorable toxicity profile of this regimen. However, possibly because of low power from a small sample size of only 16 patients in this subgroup, those differences became nonsignificant after adjusting for multiple comparisons.
Older age was associated with development of discomfort in feet, trouble walking, and lower likelihood to report nausea on reminders of chemotherapy; discomfort in feet and trouble walking remained significant after adjusting for multiple comparisons. Smoking was associated with numbness in hands and trouble hearing; numbness remained significant after multiple comparisons adjustment. Interestingly, neither use of bleomycin nor smoking were correlated with Raynaud's symptoms.
Health Care Utilization and Insurance Issues
No significant group differences were found in hospital-based health or mental health care use for non–cancer-related purposes (Table 6). Survivors had seen a health care provider for non–cancer-related problems less than controls (P = .046). However, 80% of survivors had seen a health care provider for cancer-related reasons at least annually, and it is possible that survivors may have taken care of non–cancer-related health care needs at that time. Survivors were more likely than controls to identify that professional counseling or a support group would be helpful (P = .041). However, after adjustment for income and marital status, this difference was no longer significant (P = .156).
Table 6.
Health Care Utilization
| Category | Survivors (n = 132) |
Controls (n = 137) |
Difference | 95% CI | P* | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Times to emergency room for problem not related to having had cancer | −0.0 | −0.2 to 0.20 | .10 | ||||
| Mean | 0.4 | 0.4 | |||||
| SD | 1.0 | 0.9 | |||||
| Times admitted to hospital for problem not related to having had cancer | −0.1 | −0.3 to 0.1 | .13 | ||||
| Mean | 0.3 | 0.3 | |||||
| SD | 0.9 | 0.8 | |||||
| Times seen physician or other health provider for problem not related to having had cancer | −0.9 | −2.1 to 0.2 | .05 | ||||
| Mean | 2.4 | 3.4 | |||||
| SD | 3.4 | 5.8 | |||||
| Times seen mental health professional for reasons not related to having had cancer | 0.7 | −0.5 to 1.8 | .14 | ||||
| Mean | 1.6 | 0.9 | |||||
| SD | 5.9 | 3.6 | |||||
| Counseling or support group helpful | .04 | ||||||
| No | 88 | 66.7 | 106 | 77.9 | −11.3 | −21.9 to −0.6 | |
| Yes | 44 | 33.3 | 30 | 22.1 | 11.3 | 0.6 to 21.9 | |
| Health insurance | .84 | ||||||
| No | 14 | 10.6 | 13 | 9.6 | 1.1 | −6.2 to 8.3 | |
| Yes | 118 | 89.4 | 123 | 90.4 | −1.1 | −8.3 to 6.2 | |
| Main reason for no insurance | 14 | 13 | 27 | .43 | |||
| Cannot afford the premiums | 7 | 53.8 | 4 | 33.3 | 20.5 | −17.5 to 58.5 | |
| Other | 6 | 46.2 | 8 | 66.7 | −20.5 | −58.5, 17.5 | |
| Satisfied with health insurance | .46 | ||||||
| No | 27 | 22.3 | 33 | 26.4 | −4.1 | −14.8, 6.6 | |
| Yes | 94 | 77.7 | 92 | 73.6 | 4.1 | −6.6 to 14.8 | |
| Ever been denied health insurance | .0002 | ||||||
| No | 106 | 80.3 | 131 | 95.6 | −15.3 | −22.9 to −7.7 | |
| Yes | 21 | 15.9 | 6 | 4.4 | 11.5 | 5.5 to 18.7 | |
| Unsure | 5 | 3.8 | 0 | 0.0 | 3.8 | 0.5 to 7.0 | |
| Main reason for being denied health | 21 | 6 | 27 | .27 | |||
| Cancer or other medical/health | 16 | 84.2 | 3 | 60.0 | 24.2 | −21.8 to 70.2 | |
| Other | 3 | 15.8 | 2 | 40.0 | −24.2 | −70.2 to 21.8 | |
| Health Insurance policy being canceled | .71 | ||||||
| No | 123 | 95.3 | 127 | 92.7 | 2.7 | −3.0 to 8.3 | |
| Yes | 4 | 3.1 | 7 | 5.1 | −2.0 | −6.8 to 2.7 | |
| Unsure | 2 | 1.6 | 3 | 2.2 | −0.6 | −3.9 to 2.6 | |
| Afraid to change job for losing insurance | .02 | ||||||
| No | 103 | 78.0 | 121 | 89.0 | −10.9 | −19.8 to −2.1 | |
| Yes | 29 | 22.0 | 15 | 11.0 | 10.9 | 2.1 to 19.8 | |
| Staying in job for providing insurance | .12 | ||||||
| No | 108 | 81.8 | 122 | 89.1 | −7.2 | −15.6 to 1.2 | |
| Yes | 24 | 18.2 | 15 | 10.9 | 7.2 | −1.2 to 15.6 | |
Abbreviation: SD, standard deviation.
P value is for two-sided tests comparing survivors versus controls: continuous variables, Wilcoxon rank sum test (t approximation); categorical variables, Fisher's exact test.
Rates of lack of health insurance coverage were nearly identical for survivors and controls (10.6% v 9.6%), despite the controls' higher mean income and greater likelihood of being married. There was no difference between groups related to satisfaction with health insurance or insurance cancellation. However, 15.9% of survivors reported being denied health insurance, compared with 4.4% of controls (P < .001). In addition, 22% of survivors reported being afraid to change jobs for fear of losing health insurance, compared with 11% of controls (P = .02). After adjusting for income and marital status, survivors remained significantly more likely than controls to have been denied health insurance (P = .0004) or to be afraid to change jobs (P = .019), and had fewer non–cancer-related visits to health care providers (P = .032). Most study participants were enrolled in a health maintenance organization, preferred provider organization, or other private insurance entity, rather than Medicaid or Medicare. Occupation at the time of study was similar between groups. Fewer survivors were students at study time than at diagnosis time, as most had completed their education. No differences in unemployment rates were identified (3% v 2.2%), consistent with prior research indicating cancer survivors perform their jobs as well as other employees.20,21 Fourteen percent of survivors reported they changed occupations because their illness or treatment affected their ability to perform their work, consistent with prior reports.20 Survivors were marginally more likely to report participating in support groups (27.3% v 17.5%, P = .057). Of 36 survivors who reported attending support groups, 25 (69.4%) attended cancer-related groups.
DISCUSSION
The general health of OGCT survivors was comparable to that of age-matched controls. There were a few notable differences, as detailed in Tables 3 and 4, particularly, a higher incidence of hypertension, hypercholesterolemia, and neuropathy. Likewise, symptoms suggestive of Raynaud's disease, tinnitus, and nausea elicited by thoughts of chemotherapy were more commonly reported by survivors. However, chronic cardiovascular, endocrine, and musculoskeletal disorders did not differ from controls. Reassuringly, pulmonary disorders were not more common in OGCT survivors, despite prior reports suggesting that female patients are more sensitive to bleomycin compared with testis cancer patients.22 Even when controlling for type of chemotherapy received (bleomycin in survivor group v control group) and smoking status, self-reported pulmonary disorders were rare and equally frequent in the control group. The incidence of second non-OGCT cancers was low, not different than the incidence of malignancy in the control population, and likely unrelated to the treatment received. Not captured here, as these women were not included in the survey, are one case of lung cancer among patients treated on protocol GOG 116, one etoposide-induced leukemia, and one non-Hodgkin's lymphoma among patients treated on protocol GOG 78.2,3
Although we did not address in detail whether survivors developed cognitive dysfunction, we included one question regarding difficulty concentrating and found no difference between survivors and controls on self-reported ability to concentrate. However, among survivors, the inclusion of VAC was associated with difficulty concentrating (P < .05). This observation raises the possibility that cognitive dysfunction may be related to use of specific cytotoxics (eg, alkylating agents), as suggested by studies in breast cancer.23,24
These observations are consistent with reports in the testis cancer literature citing neuropathy, hypercholesterolemia, and Raynaud's symptoms as the most common late effects of treatment.25–27 As expected, we found that higher intensity treatment (eg, addition of VAC to platinum-based therapy in protocol 90) was associated with increased functional problems, reflecting higher toxicity. However, caution should be used in interpreting these results, because the magnitude of correlations was generally low. Notably, this regimen (cisplatin, etoposide, and bleomycin plus VAC) has not been accepted as standard of care for OGCT.
Our findings contrast with some reports in the pediatric literature, particularly among patients with OGCT, where late effects of treatment are more frequent.6 Specifically, neurotoxicity, growth abnormalities, and pulmonary and gastrointestinal toxicity have been reported in children receiving treatment for OGCT, reflecting a negative impact of chemotherapy on growth and development. Cisplatin-related ototoxicity, pulmonary fibrosis related to chemotherapy, and excess mortality from secondary malignancies have been observed more frequently in pediatric cancer survivors.28–30 Interestingly, we found very little correlation between age and incidence of chronic functional problems, reflecting that women included here had completed their growth. The difference may also be explained by lesser intensity and shorter duration chemotherapy and no prior radiation as compared with the pediatric study.6 However, included in our analysis were 28 patients diagnosed and treated before age 18 years, following adult-type protocols. Reassuringly, our analysis shows no age-related differences in long-term outcomes.
The similarity in insurance coverage between groups may relate to the high employment, education, and income levels in both groups. Although it has been suggested that young adults sometimes face problems because of preexisting conditions, this did not happen in this study. Although cancer survival sometimes affects long-term employment, the percentage of unemployed survivors was small and similar to controls (3% v 2.2%). In a previously reported study, 21.3% of breast cancer survivors reported being denied insurance, the primary reason for denial being their cancer.29,18 Although our study did not inquire about reason for denial, a similar number (22%) reported being denied insurance. The significant difference in being afraid to change jobs for fear of losing health insurance (“job lock”) is consistent with other research, such as the breast cancer study, where 31.6% of respondents indicated they stayed in a job to keep insurance.18
One limitation of our analysis is its reliance on self-reporting; responder bias is possible because chronic disease diagnoses were not confirmed by medical record review. However, the large sample size of a rare patient population and completeness of data make this study unique. Our findings are significant to the pre- and post-treatment counseling of women with OGCT. Results should provide reassurance that late toxicity from treatment is infrequent and by and large does not impact the future general health of survivors. Health care utilization does not differ from controls, although more survivors report being denied insurance. In our view, and based on the current findings, interventions to limit toxicity are not warranted and curative treatment with standard doses should be pursued.
Appendix
The following Gynecologic Oncology Group member institutions participated in this study: University of Alabama at Birmingham; Duke University Medical Center; Abington Memorial Hospital; University of Minnesota Medical School; University of Mississippi Medical Center; Colorado Gynecologic Oncology Group P.C.; Milton S. Hershey Medical Center; University of Cincinnati; University of North Carolina School of Medicine; University of Iowa Hospitals and Clinics; Indiana University Medical Center; Wake Forest University School of Medicine; University of California Medical Center at Irvine; Tufts-New England Medical Center; Rush-Presbyterian–St Luke's Medical Center; State University of New York Downstate Medical Center; University of Kentucky; Community Clinical Oncology Program; The Cleveland Clinic Foundation; Johns Hopkins Oncology Center; State University of New York at Stony Brook; Eastern Pennsylvania GYN/ONC Center, P.D.; Washington University School of Medicine; Memorial Sloan-Kettering Cancer Center; Cooper Hospital/University Medical Center; Columbus Cancer Council; Fox Chase Cancer Center; University of Oklahoma; Tacoma General Hospital; Christiana Health Care; Carolinas Medical Care Center; Grant No./Riverside Cancer Services; Hinsdale Hospital; Massachusetts General Hospital; Long Beach Memorial Medical Center; Miami Valley Hospital; University of New Mexico; University of Wisconsin; Women & Infants Hospital of Rhode Island; Women's Hospital, Baton Rouge.
Fig A1.
Derivation of the final set of participants. Participants and nonparticipants did not differ based on age and type of chemotherapy received. Mean age of participants was 25.7 years (range, 10.8 to 53 years) compared with 23.9 years (range, 9 to 50 years) for nonrespondents (P = .22). There was no difference in the type of chemotherapy regimens received by participants versus nonparticipants (two-sided Fisher exact test, P = .44). Nonrespondents were further from diagnosis compared with respondents. The mean period since diagnosis for nonparticipants was 13.75 years (range, 4.8 to 24 years) compared with 10.2 years (range, 2.8 to 21) for the respondents (P < .0001). GOG, Gynecologic Oncology Group; MDACC, The University of Texas M. D. Anderson Cancer Center.
Table A1.
Intensity of Chronic Functional Problems
| Item on the FACT/GOG-NTX Scale | Survivors (n = 132) |
Controls (n = 137) |
Difference | 95% CI | P* | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| 1. Numbness or tingling in hands | 0.63 | 1.09 | 0.31 | 0.68 | 0.31 | 0.09 to 0.54 | .0182 |
| 2. Numbness or tingling in feet | 0.49 | 1.01 | 0.33 | 0.84 | 0.16 | −0.07 to 0.39 | .0982 |
| 3. Discomfort in hands | 0.36 | 0.84 | 0.36 | 0.73 | 0.003 | −0.19 to 0.20 | .5432 |
| 4. Discomfort in feet | 0.41 | 0.96 | 0.58 | 1.11 | −0.17 | −0.43 to 0.08 | .1440 |
| 5. Joint pain nor muscle cramps† | 0.65 | 1.13 | 0.85 | 1.05 | −0.20 | −0.46 to 0.07 | .0146 |
| 6. Weak all over | 0.44 | 0.90 | 0.26 | 0.63 | 0.18 | −0.01 to 0.37 | .1971 |
| 7. Trouble hearing | 0.42 | 0.95 | 0.31 | 0.66 | 0.11 | −0.09 to 0.32 | .9504 |
| 8. Ringing or buzzing in ears† | 0.59 | 1.10 | 0.25 | 0.66 | 0.34 | 0.11 to 0.57 | .0140 |
| 9. Trouble buttoning buttons† | 0.15 | 0.48 | 0.03 | 0.22 | 0.11 | 0.02 to 0.21 | .0086 |
| 10. Trouble feeling shape of small objects in hand | 0.08 | 0.37 | 0.02 | 0.15 | 0.06 | −0.01 to 0.13 | .1462 |
| 11. Trouble walking | 0.16 | 0.46 | 0.21 | 0.64 | −0.05 | −0.19 to 0.09 | .9295 |
| 12. Difficulty concentrating | 0.71 | 1.14 | 0.58 | 0.79 | 0.13 | −0.11 to 0.37 | .8490 |
| 13. Nausea when certain thoughts, sights, or smells remind me of chemotherapy† | 1.28 | 1.48 | 0.24 | 0.57 | 1.04 | 0.76 to 1.33 | .0000 |
| 14. Fingers turn blue or white when it is cold† | 0.80 | 1.28 | 0.27 | 0.70 | 0.52 | 0.27 to 0.78 | .0005 |
Abbreviations: FACT/GOG–NTX, Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Neurotoxicity scale; SD, standard deviation.
P value is for comparing survivors versus controls using the two-sided Wilcoxon rank sum test (t approximation).
These items remained significant after adjusting for 14 multiple comparisons with the false discovery rate method.
Footnotes
Supported by National Cancer Institute R01 Grant No. CA 77470 and the National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (Grant No. CA 27469) and the Gynecologic Oncology Group Statistical Office (Grant No. CA 37517).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: David Gershenson, Gynecologic Oncology Group (U) Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: David Gershenson, David Cella, Victoria L. Champion, Stephen D. Williams
Provision of study materials or patients: David Gershenson
Collection and assembly of data: Daniela Matei, Anna M. Miller, Victoria L. Champion, Stephen D. Williams
Data analysis and interpretation: Daniela Matei, Patrick Monahan, David Gershenson, Qianqian Zhao, David Cella, Victoria L. Champion, Stephen D. Williams
Manuscript writing: Daniela Matei, Anna M. Miller, Patrick Monahan, David Gershenson, Qianqian Zhao, David Cella, Victoria L. Champion, Stephen D. Williams
Final approval of manuscript: Daniela Matei, Anna M. Miller, David Gershenson, David Cella, Victoria L. Champion, Stephen D. Williams
REFERENCES
- 1.Williams SD, Blessing JA, Moore DH, et al. Cisplatin, vinblastine, and bleomycin in advanced and recurrent ovarian germ-cell tumors: A trial of the Gynecologic Oncology Group. Ann Intern Med. 1989;111:22–27. doi: 10.7326/0003-4819-111-1-22. [DOI] [PubMed] [Google Scholar]
- 2.Williams SD, Kauderer J, Burnett AF, et al. Adjuvant therapy of completely resected dysgerminoma with carboplatin and etoposide: A trial of the Gynecologic Oncology Group. Gynecol Oncol. 2004;95:496–499. doi: 10.1016/j.ygyno.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 3.Williams S, Blessing JA, Liao SY, et al. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: A trial of the Gynecologic Oncology Group. J Clin Oncol. 1994;12:701–706. doi: 10.1200/JCO.1994.12.4.701. [DOI] [PubMed] [Google Scholar]
- 4.Matei D RA, Horowitz C, Gershenson DM, et al. Ovarian Germ Cell Tumors. In: Hoskins WJ, Young RC, Perez CA, et al., editors. Principles and Practice of Gynecologic Oncology. ed 4. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 989–1011. [Google Scholar]
- 5.Gershenson DM, Morris M, Cangir A, et al. Treatment of malignant germ cell tumors of the ovary with bleomycin, etoposide, and cisplatin. J Clin Oncol. 1990;8:715–720. doi: 10.1200/JCO.1990.8.4.715. [DOI] [PubMed] [Google Scholar]
- 6.Hale GA, Marina NM, Jones-Wallace D, et al. Late effects of treatment for germ cell tumors during childhood and adolescence. J Pediatr Hematol Oncol. 1999;21:115–122. doi: 10.1097/00043426-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Swenson MM, MacLeod JS, Williams SD, et al. Quality of life after among ovarian germ cell cancer survivors: A narrative analysis. Oncol Nurs Forum. 2003;30:380. doi: 10.1188/03.ONF.E48-E54. [DOI] [PubMed] [Google Scholar]
- 8.Hansen SW, Helweg-Larsen S, Trojaborg W. Long-term neurotoxicity in patients treated with cisplatin, vinblastine, and bleomycin for metastatic germ cell cancer. J Clin Oncol. 1989;7:1457–1461. doi: 10.1200/JCO.1989.7.10.1457. [DOI] [PubMed] [Google Scholar]
- 9.Hansen SW, Olsen N. Raynaud's phenomenon in patients treated with cisplatin, vinblastine, and bleomycin for germ cell cancer: Measurement of vasoconstrictor response to cold. J Clin Oncol. 1989;7:940–942. doi: 10.1200/JCO.1989.7.7.940. [DOI] [PubMed] [Google Scholar]
- 10.Hansen SW, Groth S, Sorensen PG, et al. Enhanced pulmonary toxicity in smokers with germ-cell cancer treated with cis-platinum, vinblastine and bleomycin: A long-term follow-up. Eur J Cancer Clin Oncol. 1989;25:733–736. doi: 10.1016/0277-5379(89)90211-3. [DOI] [PubMed] [Google Scholar]
- 11.Roth BJ, Greist A, Kubilis PS, et al. Cisplatin-based combination chemotherapy for disseminated germ cell tumors: Long-term follow-up. J Clin Oncol. 1988;6:1239–1247. doi: 10.1200/JCO.1988.6.8.1239. [DOI] [PubMed] [Google Scholar]
- 12.Boyer M, Raghavan D, Harris PJ, et al. Lack of late toxicity in patients treated with cisplatin-containing combination chemotherapy for metastatic testicular cancer. J Clin Oncol. 1990;8:21–26. doi: 10.1200/JCO.1990.8.1.21. [DOI] [PubMed] [Google Scholar]
- 13.Stoter G, Koopman A, Vendrik CP, et al. Ten-year survival and late sequelae in testicular cancer patients treated with cisplatin, vinblastine, and bleomycin. J Clin Oncol. 1989;7:1099–1104. doi: 10.1200/JCO.1989.7.8.1099. [DOI] [PubMed] [Google Scholar]
- 14.Gershenson DM, Miller AM, Champion VL, et al. Reproductive and sexual function after platinum-based chemotherapy in long-term ovarian germ cell tumor survivors: A Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:2792–2797. doi: 10.1200/JCO.2006.08.4590. [DOI] [PubMed] [Google Scholar]
- 15.Champion V, Williams SD, Miller A, et al. Quality of life in long-term survivors of ovarian germ cell tumors: A Gynecologic Oncology Group study. Gynecol Oncol. 2007;105:687–694. doi: 10.1016/j.ygyno.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monahan PO, Champion VL, Zhao Q, et al. Case-control comparison of quality of life in long term ovarian germ call tumors survivors: A Gynecologic Oncology Group Study. J Psychosoc Oncol. 2008;26:19–42. doi: 10.1080/07347330802115715. [DOI] [PubMed] [Google Scholar]
- 17.Cella D. Factors influencing quality of life in cancer patients: Anemia and fatigue. Semin Oncol. 1998;25:43–46. [PubMed] [Google Scholar]
- 18.Kinney ED, Freund DA, Camp ME, et al. Serious illness and private health coverage: A unique problem calling for unique solutions. J Law Med Ethics. 1997;25:180–191. doi: 10.1111/j.1748-720x.1997.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 19.Curran-Everett D. Multiple comparisons: Philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- 20.Cella DF. Cancer survival: Psychosocial and public issues. Cancer Invest. 1987;5:59–67. doi: 10.3109/07357908709020308. [DOI] [PubMed] [Google Scholar]
- 21.McKenna RJ, Black B, Hughes R, et al. American Cancer Society Workshop on Adolescents and Young Adults with Cancer: Workgroup #2. Insurance and employability. Cancer. 1993;71:2414–2418. doi: 10.1002/1097-0142(19930401)71:7<2414::aid-cncr2820710738>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Chambers SK, Flynn SD, Del Prete SA, et al. Bleomycin, vincristine, mitomycin C, and cis-platinum in gynecologic squamous cell carcinomas: A high incidence of pulmonary toxicity. Gynecol Oncol. 1989;32:303–309. doi: 10.1016/0090-8258(89)90629-x. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda T, Takayama T, Tashiro M, et al. Mild cognitive impairment after adjuvant chemotherapy in breast cancer patients: Evaluation of appropriate research design and methodology to measure symptoms. Breast Cancer. 2005;12:279–287. doi: 10.2325/jbcs.12.279. [DOI] [PubMed] [Google Scholar]
- 24.Taillibert S, Voillery D, Bernard-Marty C. Chemobrain: Is systemic chemotherapy neurotoxic? Curr Opin Oncol. 2007;19:623–627. doi: 10.1097/CCO.0b013e3282f0e224. [DOI] [PubMed] [Google Scholar]
- 25.Kollmannsberger C, Kuzcyk M, Mayer F, et al. Late toxicity following curative treatment of testicular cancer. Semin Surg Oncol. 1999;17:275–281. doi: 10.1002/(sici)1098-2388(199912)17:4<275::aid-ssu9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 26.Berger CC, Bokemeyer C, Schuppert F, et al. Endocrinological late effects after chemotherapy for testicular cancer. Br J Cancer. 1996;73:1108–1114. doi: 10.1038/bjc.1996.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen SW. Late-effects after treatment for germ-cell cancer with cisplatin, vinblastine, and bleomycin. Dan Med Bull. 1992;39:391–399. [PubMed] [Google Scholar]
- 28.Bertolini P, Lassalle M, Mercier G, et al. Platinum compound-related ototoxicity in children: Long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26:649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 29.Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2002;95:2431–2441. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]
- 30.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]