Abstract
Purpose
To evaluate the safety and efficacy of high-dose [131I]metaiodobenzylguanidine ([131I]MIBG) in the treatment of malignant pheochromocytoma (PHEO) and paraganglioma (PGL).
Methods
Fifty patients with metastatic PHEO or PGL, age 10 to 64 years, were treated with [131I]MIBG doses ranging from 492 to 1,160 mCi (median, 12 mCi/kg). Cumulative [131I]MIBG administered ranged from 492 to 3,191 mCi. Autologous hematopoietic stem cells were collected and cryopreserved before treatment with [131I]MIBG greater than 12 mCi/kg or with a total dose greater than 500 mCi. Sixty-nine [131I]MIBG infusions were given, which included infusions to 35 patients treated once and infusions to 15 patients who received two or three treatments. Response was evaluated by [123I]MIBG scans, computed tomography/magnetic resonance imaging, urinary catecholamines/metanephrines, and chromogranin A.
Results
The overall complete response (CR) plus partial response (PR) rate in 49 evaluable patients was 22%. Additionally, 35% of patients achieved a CR or PR in at least one measure of response without progressive disease, and 8% of patients maintained stable disease for greater than 12 months. Thirty-five percent of patients experienced progressive disease within 1 year after therapy. The estimated 5-year overall survival rate was 64%. Toxicities included grades 3 to 4 neutropenia (87%) and thrombocytopenia (83%). Grades 3 to 4 nonhematologic toxicity included acute respiratory distress syndrome (n = 2), bronchiolitis obliterans organizing pneumonia (n = 2), pulmonary embolism (n = 1), fever with neutropenia (n = 7), acute hypertension (n = 10), infection (n = 2), myelodysplastic syndrome (n = 2), and hypogonadism (n = 4).
Conclusion
Although serious toxicity may occur, the survival and response rates achieved with high-dose [131I]MIBG suggest its utility in the management of selected patients with metastatic PHEO and PGL.
INTRODUCTION
Pheochromocytomas (PHEOs) are tumors derived from the adrenal medulla. Non–head/neck paragangliomas (PGLs) arise from sympathetic ganglia. Approximately 5% to 10% of PHEOs metastasize, whereas 30% to 40% of non–head/neck PGLs metastasize.1 The 5-year survival rate for metastatic PHEO/PGL has been less than 50%.2
Metaiodobenzlguanidine (MIBG) is a norepinephrine analog that concentrates in the adrenal medulla and in certain neoplasms of neuroectodermal origin. Radioiodinated MIBG can be used both diagnostically and therapeutically for patients with metastatic PHEO/PGL.3–6 [131I]MIBG therapy with a mean dose of 388 mCi provided symptomatic relief, but the 5-year survival rate after treatment was less than 45%.3 Patients may be treated with higher doses of [131I]MIBG if peripheral-blood stem cells are harvested before treatment for reinfusion for prolonged myelosuppression.4 Higher doses of [131I]MIBG have produced a greater response rate with tolerable toxicity in children with neuroblastoma.2,7
We are reporting the results of 50 patients on a phase II prospective study of high-dose [131I]MIBG treatment for malignant PHEO/PGL.
METHODS
Patient Population
Patients with PHEO/PGL were required to have the following: metastatic tumor with [123I]MIBG avidity at least twice that of soft tissue background; a measurable lesion ≥ 10 mm in diameter, or elevated levels of a tumor marker accompanying lesions less than 10 mm or exclusive bone lesions; and no major surgery, chemotherapy, radiation therapy, or investigational agents within 30 days before treatment with [131I]MIBG. Eligible patients were ≥ 4 years of age with a life expectancy ≥ 12 weeks and a Karnofsky performance status ≥ 60%. Exclusion criteria included pregnancy or nursing, uncontrolled intercurrent illness, and active second malignancies. Patients were required to have the following: absolute neutrophil count (ANC)≥ 1,000/μL; platelet count ≥ 80,000/μL, AST ≤ 2.5 times the upper limit of normal (ULN); total bilirubin ≤ 2.5 × ULN; and creatinine ≤ 2.5 × ULN. Between December 3, 1992 and November 1, 2007, the study enrolled 50 patients at the University of California, San Francisco (UCSF). The cutoff date for analysis was July 1, 2008. Data on the first 12 patients were previously reported in detail, and preliminary response data on the first 30 patients were summarized in a review.1,4 The study was approved by the UCSF Committee on Human Research. Informed consent was obtained from all patients or guardians.
Study Design
The primary objective was to determine the efficacy of [131I]MIBG on the basis of the percentage of patients with a complete, partial, or minor response and without progressive disease (PD) 1 year from the initial treatment with [131I]MIBG. Secondary aims included determination of the following: significant factors for response, including tumor markers, age, sex, diagnosis, prior chemotherapy, prior radiation therapy, extent of disease, and succinate dehydrogenase subunit B (SDHB) mutation; overall survival (OS) and event-free survival (EFS) rates; and grades 3 and 4 toxicity.
Pretreatment assessment included the following: [123I]MIBG or [131I]MIBG scan; computed tomography (CT) or magnetic resonance imagine (MRI); serum chromogranin A; and 24-hour urine collection for fractionated catecholamines, metanephrines, and creatinine. Dosing was dictated by the presence of stem cells and by radiation safety guidelines. Successful peripheral-blood stem cell harvest was required for patients to receive greater than 12 mCi/kg of body weight and greater than 500 mCi [131I]MIBG. Patients with cryopreserved stem cells received 18 mCi/kg up to a total dose dictated by institutional radiation safety guidelines; guidelines limited [131I]MIBG preparation to 800 mCi (during 1992-1996), 1,000 mCi (during 1997-2004), and 1,200 mCi (during 2004-present) at different times during the study. Subsequent therapies were prescribed to replicate the first dose patients had received. [131I]MIBG was prepared on-site at UCSF through November 2006, in accordance with institutional licensing that allowed a maximum preparation of 800 to 1,200 mCi with specific activity of 10 to 12 Ci/mmol and with free iodide content at time of administration of less than 5%.5 The quality control for radiochemical purity was determined by the Sep-Pak method (Waters Sep-Pak cartridges, Milford, MA). After November 2006, doses were obtained from Nuclear Diagnostic Products (NDP; Rockaway, NJ).
[131I]MIBG was infused intravenously in a lead-shielded room over 2 hours with intravenous hydration, a Foley catheter for bladder protection, and potassium iodide and potassium perchlorate for thyroid protection.6 Granisetron and lorazepam were given for radiation-induced nausea. Blood pressure was monitored throughout the [131I]MIBG infusion, and nifedipine was given for systolic blood pressure ≥ 170 mmHg. Patients were discharged when their radiation levels met institutional guidelines.
Filgrastim (granulocyte colony-stimulating factor; GCSF) was administered for ANC less than 500/μL; platelet transfusions were administered for platelet counts less than 10,000/μL or for significant bleeding with counts less than 40,000/μL; blood transfusions were administered for significant anemia; and erythropoietin was administered at the discretion of the primary oncologist. Patients received an autologous hematopoietic stem-cell transfusion (HSCT) for prolonged myelosuppression (defined as ANC < 200/μL, hematocrit < 25%, and/or platelets < 20,000/μL) for greater than 2 weeks. Response evaluation was required at 3 to 6 months after treatment and was repeated at 6-month intervals, and an overall best response was determined after [131I]MIBG therapy and before the onset of PD.
Response criteria were defined prospectively in our Comprehensive Cancer Center protocol. An overall complete response (CR) was defined as the disappearance of all lesions on both CT/MRI and [123I]MIBG scans as well as normalization of all tumor markers.
CT/MRI responses were rated as a partial response (PR) if the sum of the longest diameter of index lesions decreased ≥ 30%, PD if the sum of the longest diameter of target lesions increased ≥ 20%, and stable disease (SD) if the sum was between these parameters.7 For lesions in which exact measurements were not available, the radiologist's interpretation was used.
[123I]MIBG scans were evaluated on the basis of the general impression of the interpreting radiologist, as follows: CR was described as the disappearance of all lesions; PR, a decrease in the number and/or intensity of lesions; SD, no discernible change; and PD, the appearance of new lesions.4 Radiologists were not formally blinded to clinical data in their assessments of MIBG scans. Single photon emission computed tomography was available for the majority of MIBG scans.
Tumor markers were considered PR for decreases ≥ 50% and PD for increases greater than 20%. Overall tumor marker response was a PR if all markers achieved at least a PR, was a PD if any marker indicated PD, was an SD if all modalities maintained SD, and was a minor response (MR) if any marker achieved PR or CR while others remained SD.
Toxicity was graded with Common Terminology Criteria for Adverse Events version 3.0; grades 3 to 4 toxicities were reported.
Statistical Analysis
EFS and OS were determined by using the Kaplan-Meier method. Cox proportional hazards models evaluated the effect of prognostic variables on OS and EFS in univariate and multivariable analysis. The χ2 test was employed to evaluate differences in response.
RESULTS
Patient and Treatment Characteristics
The on-study characteristics of 49 patients are listed in Table 1. Of 50 patients enrolled and treated, one patient was lost to follow-up. Thirty-four patients (69%) enrolled on the study were diagnosed with PGL, whereas 15 patients (31%) were diagnosed with PHEO. Of the 24 patients who underwent genetic testing, 12 (50%) had an SDHB mutation. Additionally, one patient had neurofibromatosis-1.
Table 1.
On-Study Patient and Treatment Characteristics
| Characteristic | Patients |
|
|---|---|---|
| No. | % | |
| Diagnosis | ||
| Pheochromocytoma | 15 | 31 |
| Paraganglioma | 34 | 69 |
| Sex | ||
| Male | 29 | 59 |
| Female | 20 | 41 |
| Age at entry, years | ||
| Median | 42.6 | |
| Range | 10.3-64.4 | |
| Time from diagnosis to entry, years | ||
| Median | 3.4 | |
| Range | 0.2-26.6 | |
| Prior treatment | ||
| Chemotherapy | 15 | 31 |
| Radiation | 16 | 33 |
| Surgery | 44 | 90 |
| No. of infusions of [131I]MIBG | ||
| 1 | 34 | |
| 2 | 11 | |
| 3 | 4 | |
| [131I]MIBG activity infused, mCi | ||
| Median, first treatment | 818 | |
| Range, first treatment | 492-1,160 | |
| Range, all treatments | 492-3,191 | |
| [131I]MIBG activity infused, mCi/kg | ||
| Median, first treatment | 12 | |
| Range, first treatment | 6-19 | |
| Secretory tumor | 37 | 76 |
| SDHB mutation | 12* | 50 |
Abbreviations: MIBG, metaiodobenzylguanidine; SDHB, succinate dehydrogenase subunit B.
Twelve patients were positive of 24 patients who underwent genetic testing.
The median activities of [131I]MIBG infused were 12 mCi/kg and 818 mCi. Doses ranged from 6 to 19 mCi/kg and 492 to 1,160 mCi total for the first therapy. Three patients did not have a hematopoietic stem-cell leukapheresis because of marrow contamination by tumor; one did not have leukapheresis because of sickle cell disease; and two did not because of insufficient harvests; these six patients were required to receive [131I]MIBG less than 500 mCi (± 10%).
Toxicity
The primary toxicity was hematologic. After the first treatment, grades 3 to 4 hematologic toxicity included WBC count less than 2.0 × 109/L in 85%, ANC less than 1.0 × 109/L in 87%, platelet count less than 50.0 × 109/L in 83%, and hemoglobin less than 8.0 g/dL in 8% of patients. Four patients experienced prolonged myelosuppression that required autologous stem-cell rescue after treatments of 733 mCi, 820 mCi, 976 mCi, and 1,030 mCi. Four patients developed fever and neutropenia after a single [131I]MIBG infusion.
Grades 3 to 4 nonhematologic toxicities are reported in Table 2. The most serious and unusual toxicities were pulmonary. Grade 4 acute respiratory distress syndrome (ARDS) developed in two patients who had large, secretory, hepatic metastases. A 22-year-old woman developed ARDS 4 days after her third treatment with [131I]MIBG (total dose, 3,190 mCi). She required mechanical ventilation for 4 days and fully recovered from ARDS, but she later died as a result of PD. A 42-year-old woman had pre-existent nephrotic syndrome and ascites; she developed ARDS 11 days after [131I]MIBG (1,030 mCi) and died as a result of multisystem organ failure after 14 days of mechanical ventilation.
Table 2.
Nonhematologic Toxicity With 68 Treatments in 49 Evaluable Patients
| Characteristic | No. of Grades 3 to 4 Toxicities in 68 MIBG Infusions |
|
|---|---|---|
| First Treatment (n = 49) | All Treatments (n = 68) | |
| BOOP/ARDS | 3* | 4 |
| DVT/PE | 1 | 1 |
| Fever and neutropenia | 4 | 7 |
| Hypertension | 7 | 10 |
| Infection | 1 | 1 |
| MDS | 0 | 2† |
| Ovarian failure/hypogonadism | 2 | 4 |
| Pneumonia, infectious | 1 | 1 |
Abbreviations: MIBG, metaiodobenzylguanidine; BOOP, bronchiolitis obliterans organizing pneumonia; ARDS, acute respiratory distress syndrome; DVT, deep vein thrombosis; PE, pulmonary embolism; MDS, myelodysplastic syndrome.
One patient died as a result of ARDS after first treatment.
Two patients died as a result of MDS after two and three treatments, respectively.
Two patients experienced bronchiolitis obliterans organizing pneumonia (BOOP). One man had pre-existent hepatic metastases, nephrotic syndrome, and a history of pulmonary edema; he developed BOOP 6 months after [131I]MIBG (845 mCi). He was discharged on prednisone, and his symptoms resolved. One woman developed BOOP 2.5 months after treatment with [131I]MIBG (1,062 mCi) and was treated with prednisone and in-home oxygen.
Myelodysplastic syndrome (MDS) occurred in two patients after multiple infusions of [131I]MIBG. Both patients developed concurrent acute myeloid leukemia. Neither patient had received prior chemotherapy or radiation. The first patient developed MDS 5 years after her third treatment with [131I]MIBG (cumulative dose, 1,712 mCi) and died suddenly after allogeneic bone marrow transplantation. The second patient developed MDS 2 years after his second treatment with [131I]MIBG (cumulative dose, 1,832 mCi) and died as a result of busulfan-induced lung disease 3 months after an autologous stem-cell transplantation. The bone marrow cytogenetics for these patients were monosomy 7 and 45,XY,-5,del(7)(q22q36)/46,XY,t(2,5)(p21p13), respectively.
Seven patients experienced grade 3 acute hypertension during [131I]MIBG infusion; four of these patients had a secretory tumor with previously diagnosed hypertension. Hypertension reoccurred in three of these patients during a subsequent infusion. When hypertension occurred, its onset generally was noted approximately 30 minutes into the 2-hour infusion, and it was reversible with nifedipine.
Four patients developed hypogonadism after [131I]MIBG therapy. Hypogonadism in women was suspected by lack of menses and was documented by elevated gonadotropins and low serum estradiol. One woman developed ovarian failure 12 months after one treatment (1,010 mCi). One adolescent had primary amenorrhea after three treatments (cumulative dose, 1,712 mCi). Hypogonadism in men was suspected by symptoms of diminished libido or erectile dysfunction with elevated gonadotropins and low serum testosterone. One man developed hypogonadism 6 months after one treatment (930 mCi). Another man had hypogonadism 6 years after two treatments (cumulative dose, 1,656 mCi).
No patients became hypothyroid. However, three patients became hyperthyroid after treatment with [131I]MIBG, presumably because of the large doses of potassium iodide given to competitively inhibit thyroidal uptake of free [131I]MIBG.1
Response
Responses after the first and second treatments are listed in Table 3 and 4. The overall response rate, including complete, partial, and minor responses, for all evaluable patients was 57% (CR, 8%; PR, 14%; and MR, 35%). Another 8% achieved SD, whereas 35% had PD within 1 year after [131I]MIBG therapy. Chromogranin A CR/PR was 74%, catecholamine/metanephrine CR/PR/MR was 66%, and CR/PR by [123I]MIBG was 52%. Only 27% of patients achieved CR/PR by CT/MRI. The overall response rate (ie, CR, PR, and MR) after the second treatment was 73%, and the best overall response rate including all treatments (ie, CR, PR, and MR) was 63%.
Table 3.
Response by Individual Response Modality After First Treatment and Best Overall Response After All Treatments
| Response | First Treatment |
Overall |
Best Overall |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scan |
Catecholamine/Metanephrine |
CgA |
||||||||||
| MIBG |
CT/MRI |
|||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| CR | 7 | 15 | 4 | 9 | 6 | 19 | 8 | 24 | 4 | 8 | 5 | 10 |
| PR | 17 | 37 | 8 | 18 | 5 | 16 | 17 | 50 | 7 | 14 | 10 | 20 |
| MR | — | — | — | — | 10 | 31 | — | — | 17 | 35 | 16 | 33 |
| SD | 20 | 43 | 24 | 53 | 5 | 16 | 9 | 26 | 4 | 8 | 3 | 6 |
| PD | 2 | 4 | 9 | 20 | 6 | 19 | 0 | 0 | 17 | 35 | 15 | 31 |
NOTE. Minor response by catecholamines/metanephrines indicates complete/partial response in some, but not all, hormone measurements without progressive disease in any.
Abbreviations: MIBG, metaiodobenzylguanidine; CT, computed tomography; MRI, magnetic resonance imaging; CgA, chromogranin A; CR, complete response; PR, partial response; MR, minor response; SD, stable disease; PD, progressive disease.
Table 4.
Response by Individual Response Modality After Second Treatment
| Response | Second Treatment |
Overall |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scan |
Catecholamine/Metanephrine |
CgA |
||||||||
| MIBG |
CT/MRI |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| CR | 2 | 14 | 2 | 17 | 4 | 57 | 1 | 20 | 1 | 7 |
| PR | 8 | 57 | 4 | 33 | 1 | 14 | 1 | 20 | 5 | 33 |
| MR | — | — | — | — | 1 | 14 | — | — | 5 | 33 |
| SD | 3 | 21 | 3 | 25 | 1 | 14 | 2 | 40 | 1 | 7 |
| PD | 1 | 7 | 3 | 25 | 0 | 0 | 1 | 20 | 3 | 20 |
NOTE. Response after second treatment was relative to disease status after the first treatment.
Abbreviations: MIBG, metaiodobenzylguanidine; CT, computed tomography; MRI, magnetic resonance imaging; CgA, chromogranin A; CR, complete response; PR, partial response; MR, minor response; SD, stable disease; PD, progressive disease.
Pretreatment predictors of response are presented in Table 5. Chromogranin A, catecholamine and/or metanephrine secretion, sites of metastases, age at first treatment (< 40 versus ≥ 40 years), sex, prior chemotherapy, prior radiation, and diagnosis were not significant predictors of CR/PR. Univariate analysis showed that patients with the SDHB mutation were significantly more likely to achieve CR/PR (χ2 P = .012). Multivariate analyses to investigate this association were not possible, because all patients who achieved CR/PR and had genetic testing had an SDHB mutation.
Table 5.
Univariate Analysis of Prognostic Indicators of Survival and Response
| Indicator | Analyses |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survival |
Response |
|||||||||
| Survival Type Evaluated | Hazard Ratio | 95% CI | P | Indicator Category | CR/PR |
MR/SD/PD |
P | |||
| No. | % | No. | % | |||||||
| Chromogranin A | EFS | 1.9 | 0.4 to 8.4 | .383 | Normal | 2 | 40.0 | 3 | 60.0 | .2 |
| OS | 2.1 | 0.3 to 17 | .471 | Elevated | 6 | 16.2 | 31 | 83.8 | ||
| SDHB mutation | EFS | 0.5 | 0.2 to 1.9 | .336 | Normal | 0 | 0.0 | 12 | 100.0 | .01* |
| OS | 2.0 | 0.2 to 20.7 | .558 | Mutation | 5 | 41.7 | 7 | 58.3 | ||
| Secretory | EFS | 2.4 | 0.8 to 7 | .123 | Nonsecretory | 3 | 27.3 | 8 | 72.7 | .66 |
| OS | 2.0 | 0.4 to 9.4 | .362 | Secretory | 8 | 21.1 | 30 | 78.9 | ||
| Bone and soft tissue | EFS | 1.4 | 0.6 to 3.3 | .454 | One site involved | 5 | 25.0 | 15 | 75.0 | .72 |
| OS | 0.4 | 0.1 to 1.4 | .179 | Both involved | 6 | 20.7 | 23 | 79.3 | ||
| Age, years | EFS | 1.9 | 0.9 to 4.4 | .115 | < 40 | 5 | 23.8 | 16 | 76.2 | .84 |
| OS | 2.7 | 0.8 to 9.2 | .106 | ≥ 40 | 6 | 21.4 | 22 | 78.6 | ||
| Sex | EFS | 0.9 | 0.4 to 1.9 | .696 | F | 4 | 20.0 | 16 | 80.0 | .73 |
| OS | 0.4 | 0.1 to 1.2 | .112† | M | 7 | 24.1 | 22 | 75.9 | ||
| Prior chemotherapy | EFS | 1.9 | 0.9 to 4.3 | .113 | No | 9 | 26.5 | 25 | 73.5 | .31 |
| OS | 3.4 | 1.1 to 10.3 | .031* | Yes | 2 | 13.3 | 13 | 86.7 | ||
| Prior radiation | EFS | 2.9 | 1.2 to 7.1 | .017* | No | 10 | 30.3 | 23 | 69.7 | .06 |
| OS | 2.2 | 0.7 to 6.9 | .166 | Yes | 1 | 6.3 | 15 | 93.8 | ||
| Diagnosis | EFS | 1.9 | 0.8 to 4.8 | .172 | PHEO | 4 | 26.7 | 11 | 73.3 | .64 |
| OS | 2.8 | 0.6 to 12.6 | .188 | PGL | 7 | 20.6 | 27 | 79.4 | ||
NOTE. Increased hazard ratio represents worse survival in patients who are positive for the trait. For the purposes of this analysis, patients with the following conditions were considered to be positive for the trait: elevated chromogranin A, mutated SDHB, secretory tumors, disease present in bone and soft tissue, age ≥ 40 years; male sex, prior chemotherapy or radiation therapy; diagnosis of paraganglioma.
Abbreviations: CR, complete response; PR, partial response; MR, minor response; SD, stable disease; PD, progressive disease; EFS, event-free survival; OS, overall survival; SDHB, succinate dehydrogenase subunit B; PHEO, pheochromocytoma; PGL, paraganglioma.
Significant in univariate analysis.
Significant in multivariate analysis that included sex, prior chemotherapy, prior radiation, and bone or soft tissue involvement.
OS and EFS
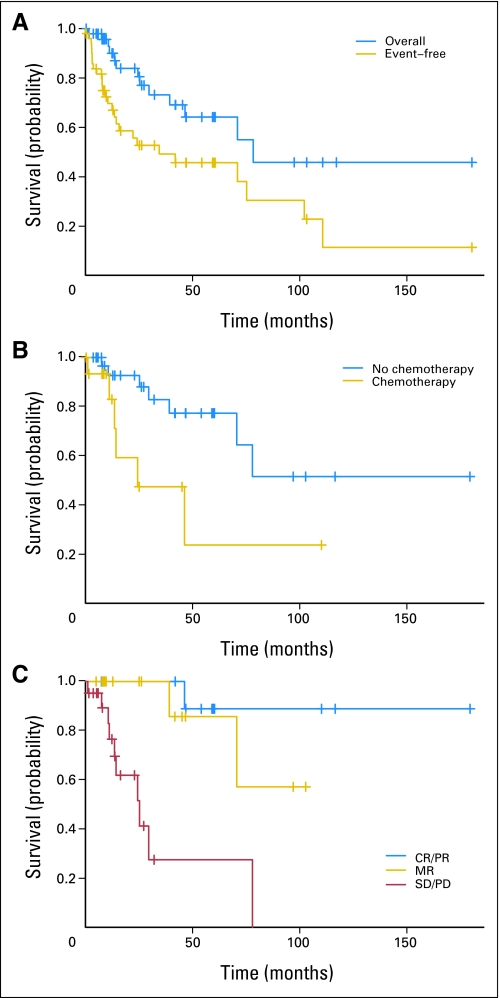
The estimated 5-year OS for all patients was 64% (SE, ± 9%), and 5-year EFS was 47% (SE, ± 8%; Fig 1A). Median follow-up from time of MIBG treatment for all patients was 2 years, and the range was 0.1 to 15 years.
Fig 1.
Kaplan-Meier survival curves. (A) Overall and event-free survival for all patients enrolled on the study. (B) Overall survival according to prior chemotherapy. (C) Overall survival according to overall response. CR, complete response; PR, partial response; MR, minor response, SD, stable disease; PD, progressive disease.
Univariate analysis of pretreatment predictors of survival are listed in Table 5. Chromogranin A, SDHB status, catecholamines and metanephrines, sites of metastases, age, sex, and diagnosis were not significant predictors of OS or EFS in univariate analysis. Prior chemotherapy (P = .031) was a significant predictor of poor OS (Fig 1B) and prior radiation therapy (P = .017) was a significant predictor of poor EFS. In multivariate analysis (that included prior chemotherapy, prior radiation therapy, sex, and sites of metastases), poor OS was predicted by both prior chemotherapy (P = .025) and female sex (P = .016).
We evaluated the usefulness of different measures of response as predictors of survival (Table 6). Significant predictors of survival included overall response (P = .039; Fig 1C), response by MIBG scan (P = .014), and response by CT/MRI (P = .038). Catecholamine/metanephrine response and chromogranin A response were not significant predictors of survival.
Table 6.
Univariate Analysis of Response by Individual Modalities as Predictors of Survival
| Modality | Response Compared With CR/PR | Hazard Ratio | 95% CI | P |
|---|---|---|---|---|
| MIBG scan | SD/PD | 4.9 | 1.4 to 17.3 | .014* |
| CT/MRI | SD/PD | 9.0 | 1.1 to 70.9 | .038* |
| Catecholamine/metanephrine | SD/PD | 3.4 | 0.3 to 39.7 | .324 |
| MR | 4.8 | 0.5 to 46.4 | .176 | |
| MR/SD/PD | 4.2 | 0.5 to 36.5 | .196 | |
| Chromogranin A | SD/PD | 1.9 | 0.4 to 9 | .405 |
| Overall | SD/PD | 22.9 | 2.7 to 192.8 | .004* |
| MR | 2.6 | 0.2 to 29.3 | .431 | |
| MR/SD/PD | 8.8 | 1.1 to 69.1 | .039* |
Abbreviations: CR, complete response; PR, partial response; MIBG, metaiodobenzylguanidine; SD, stable disease; PD, progressive disease; CT, computed tomography; MRI, magnetic resonance imaging; MR, minor response.
Significant in univariate analysis.
DISCUSSION
This prospective phase II study shows that high-dose [131I]MIBG produced objective responses in patients with metastatic PHEO/PGL; 22% of patients attained CR/PR, 35% of patients attained an objective MR, and 8% of patients maintained SD for greater than 12 months. The 5-year OS was 64% (SE, ± 9%), which compares favorably to historical data.
Several studies have reported lower-dose [131I]MIBG treatment for patients with malignant PHEO/PGL. Loh et al8 reported a retrospective meta-analysis of 116 patients with metastatic PHEO/PGL who were treated with [131I]MIBG (mean dose, 158 mCi; mean cumulative dose, 490 mCi). The rate of progressive disease was 45% after a median interval of 19 months. The tumor response rate was 30% and the hormonal response rate was 45%.8 However, the criteria for responses were not presented. The results presented here show a comparable objective tumor response rate by strict criteria. In addition, 66% of patients in this study achieved a hormone response (CR, PR, or MR), which is markedly improved relative to the meta-analysis.
In this study, we assessed biochemical responses by using six different tumor markers: chromogranin A, catecholamines (ie, epinephrine, norepinephrine, dopamine) and metanephrines (ie, metanephrine, normetanephrine). Several patients achieved CR/PR by several tumor markers, but not by all six; as such, the MR category was necessary to distinguish the many patients who responded by several tumor markers from those who failed to respond at all.
Safford et al3 reported that hormone response was a significant predictor of survival in a retrospective review of 33 patients with malignant PHEO/PGL treated with a mean dose of 388 mCi of [131I]MIBG. In their study, tumor volume declined more than 50% in 38% of patients, and hormone levels declined more than 50% in 60% of patients. The 5-year survival rate for patients was 45% after treatment. In their report, a hormone response predicted improved survival, whereas a reduction in tumor volume did not.3 In contrast, the data presented here show that hormone response was not predictive of OS but that a CR/PR by MIBG scans or CT/MRI imaging was predictive of OS. Safford et al3 also identified a survival benefit in patients who received greater than 500 mCi as their initial [131I]MIBG doses, which supports the use of higher doses.3 The 5-year OS in our study, in which 98% of patients were treated with greater than 500 mCi, was 64%.
Chemotherapy is an option for patients with metastatic PHEO/PGL, but large clinical trials are lacking. One study reported a combination chemotherapy regimen that included cyclophosphamide, vincristine, and dacarbazine (CVD).2,9 In 14 patients with metastatic pheochromocytoma, this therapy produced CR/PR in 57%.9 More recently, results from a 22-year follow-up of 18 patients treated with CVD chemotherapy showed a CR rate of 11% and a PR rate of 44%, but 5-year survival of patients was less than 50%.10 Although CVD chemotherapy response rates may compare favorably with those for [131I]MIBG therapy, estimated 5-year OS is substantially less than the 64% achieved in this study of high-dose [131I]MIBG. In addition, antiangiogenic therapy with sunitinib may prove useful in the treatment of malignant PHEO/PGL.11 Although Safford et al3 showed an increased survival in patients who received additional chemotherapy after MIBG therapy, the data presented here show that prior chemotherapy portends poor survival. Tumors that are resistant to chemotherapy and radiation may be more aggressive and resistant to multiple therapeutic approaches, including [131I]MIBG, as seen in this study.
SDHB mutations are autosomal dominant and are characterized by the development of paragangliomas with significant metastatic potential.12 In this study, patients with known SDHB mutations were significantly more likely to achieve CR/PR. SDHB status was not, however, predictive of OS or EFS, though only 50% of patients had known SDHB status. Patients with an SDHB mutation may have an improved response to [131I]MIBG but may have a more aggressive natural history of their disease, which may explain the discrepancies between response analysis and hazards ratios for OS and EFS.
Two patients developed BOOP after [131I]MIBG. A previous study reported that BOOP occurred 4 months after high-dose [131I]MIBG in a child with neuroblastoma.13 BOOP may have occurred as a delayed pulmonary reaction to radiation exposure from [131I]MIBG. BOOP has also been reported in patients after thoracic radiation therapy.14 Two patients developed ARDS after [131I]MIBG. Although ARDS has not been reported previously after previously MIBG therapy, it has occurred spontaneously in patients with PHEO without prior [131I]MIBG therapy.15–17 Because two of the four patients with pulmonary toxicity had nephrotic syndrome, it is possible that delayed clearance of MIBG may have contributed to this toxicity; however, neither patient had an elevated creatinine at the time of treatment. Nevertheless, we now restrict patients with nephrotic syndrome from receiving high-dose [131I]MIBG. In addition, because three of four patients with pulmonary toxicity in this study received greater than 1,000 mCi per dose, we suggest that doses of [131I]MIBG should not exceed 800 mCi.
MDS/leukemia was a fatal complication experienced by two patients at 2 and 6 years, respectively, after high-dose [131I]MIBG. Radiation is a known risk factor for secondary malignancy, and the large cumulative doses received by these two patients likely placed them at increased risk. The observed cytogenetic abnormalities (ie, loss of chromosomes 5 and 7) have been reported in secondary leukemia after treatment with alkylating agents and radiation. MDS was previously reported in three of 95 children treated at UCSF with [131I]MIBG for neuroblastoma, and a literature review revealed an additional three patients.18
In summary, high-dose [131I]MIBG produced objective responses in patients with metastatic PHEO/PGL. Myelosuppression was common but well tolerated. Four patients developed pulmonary complications, and two developed MDS/leukemia after high-dose [131I]MIBG. Nevertheless, the estimated 5-year OS was 64%, an improvement over the historical 5-year survival of 44% without such therapy.19 [131I]MIBG at doses of approximately 12 mCi/kg, with total doses that range from 500 to 800 mCi, may provide the maximum therapeutic benefit while minimizing risk of pulmonary toxicity. We conclude that high-dose [131I]MIBG is an effective therapy for selected patients with metastatic PHEO/PGL.
Acknowledgment
We thank Alekist Quach for collection of data in this study.
Footnotes
Supported in part by the National Institutes of Health Grant No. 2MO1 RR0127; by donations from the Campini Foundation, the Conner Research Fund, the Krantz Memorial Fund, and the Katie Dougherty Foundation; and by National Institutes of Health/National Center for Research Resources UCSF-Clinical and Translational Sciences Institute Grant No. UL1 RR024131.
These contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Robert Goldsby, Katherine K. Matthay, Randall Hawkins, John Huberty, Paul Fitzgerald
Administrative support: Aimee Sznewajs, Paul Fitzgerald
Provision of study materials or patients: Robert Goldsby, Katherine K. Matthay, David Price, John Huberty, Lloyd Damon, Charles Linker, Paul Fitzgerald
Collection and assembly of data: Sara Gonias, Robert Goldsby, Katherine K. Matthay, Randall Hawkins, Aimee Sznewajs, Paul Fitzgerald
Data analysis and interpretation: Sara Gonias, Robert Goldsby, Katherine K. Matthay, Steve Shiboski, Paul Fitzgerald
Manuscript writing: Sara Gonias, Robert Goldsby, Katherine K. Matthay, Lloyd Damon, Paul Fitzgerald
Final approval of manuscript: Sara Gonias, Robert Goldsby, Katherine K. Matthay, Randall Hawkins, David Price, John Huberty, Lloyd Damon, Charles Linker, Aimee Sznewajs, Steve Shiboski, Paul Fitzgerald
REFERENCES
- 1.Fitzgerald PA, Goldsby RE, Huberty JP, et al. Malignant pheochromocytomas and paragangliomas: A phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG) Ann N Y Acad Sci. 2006;1073:465–490. doi: 10.1196/annals.1353.050. [DOI] [PubMed] [Google Scholar]
- 2.Ahlman H. Malignant pheochromocytoma: State of the field with future projections. Ann N Y Acad Sci. 2006;1073:449–464. doi: 10.1196/annals.1353.049. [DOI] [PubMed] [Google Scholar]
- 3.Safford SD, Coleman RE, Gockerman JP, et al. Iodine -131 metaiodobenzylguanidine is an effective treatment for malignant pheochromocytoma and paraganglioma. Surgery. 2003;134:956–962. doi: 10.1016/s0039-6060(03)00426-4. discussion 962–963. [DOI] [PubMed] [Google Scholar]
- 4.Rose B, Matthay KK, Price D, et al. High-dose 131I-metaiodobenzylguanidine therapy for 12 patients with malignant pheochromocytoma. Cancer. 2003;98:239–248. doi: 10.1002/cncr.11518. [DOI] [PubMed] [Google Scholar]
- 5.Matthay KK, Panina C, Huberty J, et al. Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I-MIBG. J Nucl Med. 2001;42:1713–1721. [PubMed] [Google Scholar]
- 6.Matthay KK, Tan JC, Villablanca JG, et al. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: A new approach to Neuroblastoma Therapy Consortium Study. J Clin Oncol. 2006;24:500–506. doi: 10.1200/JCO.2005.03.6400. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Loh KC, Fitzgerald PA, Matthay KK, et al. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): A comprehensive review of 116 reported patients. J Endocrinol Invest. 1997;20:648–658. doi: 10.1007/BF03348026. [DOI] [PubMed] [Google Scholar]
- 9.Averbuch SD, Steakley CS, Young RC, et al. Malignant pheochromocytoma: Effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine. Ann Intern Med. 1988;109:267–273. doi: 10.7326/0003-4819-109-4-267. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Abraham J, Hung E, et al. Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: Recommendation from a 22-year follow-up of 18 patients. Cancer. 2008;113:2020–2028. doi: 10.1002/cncr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshua AM, Ezzat S, Asa SL, et al. Rationale and evidence for sunitinib in the treatment of malignant paraganglioma/pheochromocytoma. J Clin Endocrinol Metab. 2009;94:5–9. doi: 10.1210/jc.2008-1836. [DOI] [PubMed] [Google Scholar]
- 12.Karagiannis A, Mikhailidis DP, Athyros VG, et al. Pheochromocytoma: An update on genetics and management. Endocr Relat Cancer. 2007;14:935–956. doi: 10.1677/ERC-07-0142. [DOI] [PubMed] [Google Scholar]
- 13.Howard JP, Maris JM, Kersun LS, et al. Tumor response and toxicity with multiple infusions of high dose 131I-MIBG for refractory neuroblastoma. Pediatr Blood Cancer. 2005;44:232–239. doi: 10.1002/pbc.20240. [DOI] [PubMed] [Google Scholar]
- 14.Cornelissen R, Senan S, Antonisse IE, et al. Bronchiolitis obliterans organizing pneumonia (BOOP) after thoracic radiotherapy for breast carcinoma. Radiat Oncol. 2007;2:2. doi: 10.1186/1748-717X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winter C, Schmidt-Mutter C, Cuny R, et al. Fatal form of phaeochromocytoma presenting as acute pyelonephritis. Eur J Anaesthesiol. 2001;18:548–553. doi: 10.1046/j.1365-2346.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- 16.Feldman JM. Adult respiratory distress syndrome in a pregnant patient with a pheochromocytoma. J Surg Oncol. 1985;29:5–7. doi: 10.1002/jso.2930290103. [DOI] [PubMed] [Google Scholar]
- 17.Spencer E, Pycock C, Lytle J. Phaeochromocytoma presenting as acute circulatory collapse and abdominal pain. Intensive Care Med. 1993;19:356–357. doi: 10.1007/BF01694713. [DOI] [PubMed] [Google Scholar]
- 18.Weiss B, Vora A, Huberty J, et al. Secondary myelodysplastic syndrome and leukemia following 131I-metaiodobenzylguanidine therapy for relapsed neuroblastoma. J Pediatr Hematol Oncol. 2003;25:543–547. doi: 10.1097/00043426-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Samaan NA, Hickey RC, Shutts PE. Diagnosis, localization, and management of pheochromocytoma: Pitfalls and follow-up in 41 patients. Cancer. 1988;62:2451–2460. doi: 10.1002/1097-0142(19881201)62:11<2451::aid-cncr2820621134>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]