Abstract
Production of mRNA from the cocaine- and amphetamine-regulated transcript (CART) gene is regulated by cocaine and other drugs of abuse in the nucleus accumbens (NAc), a brain reward region. Current hypotheses postulate that CART peptides there oppose the rewarding actions of cocaine by opposing the effects of dopaminergic transmission. Since over expression of CREB was shown to decrease cocaine-mediated reward, we hypothesized that CART could be a target gene for CREB in the NAc and that over expression of CREB would increase CART peptide levels. Transcription factor (TF) binding to DNA is influenced by sequences adjacent to consensus TF binding sites and other factors. We thus examined CREB binding to a 27mer oligonucleotide containing the CRE sequence from the CART gene proximal promoter. Using electrophoretic mobility shift assays and TF-antibody super shift assays, CREB was found to bind to the CRE sequence from the CART promoter. To test if over expression of CREB in the NAc affected CART peptide levels, Herpes simplex virus-1 vectors over expressing CREB (HSV-CREB), or a vector that expressed LacZ (HSV-LacZ) as a control, were injected into the NAc of rats. Western blotting and in situ hybridization showed that HSV-CREB injections increased CART mRNA and peptide levels. Injections of a dominant negative CREB mutant (HSV-mCREB) did not alter either CART mRNA or peptide levels. The finding that CREB can regulate the levels of CART mRNA and peptides in vivo in the NAc supports a role for CART peptides in psychostimulant-induced reward and reinforcement.
Keywords: CREB, CART peptide, Psychostimulant, Nucleus accumbens
1. Introduction
Psychostimulant-induced reward, reinforcement and dependence are thought to be mediated by specific molecular changes in the nucleus accumbens (NAc) and other brain regions (Nestler, 2004). The transcription factor (TF) CREB is one of the molecules regulated by rewarding drugs such as cocaine (Hope et al., 1992; Carlezon et al., 2005). Over expression of CREB in the rat NAc reduced cocaine-induced reward and reinforcement, while over expression of a serine-133 to alanine dominant negative mutant of CREB (mCREB) resulted in an increase in cocaine's rewarding effects as shown by conditioned place preference assays (Carlezon et al., 1998). This form of mutant CREB is able to dimerize with other CREB family members and bind to CRE cis-regulatory sites on gene promoters (Lonze and Ginty, 2002). It cannot, however, recruit the transcriptional co-activator CREB-Binding Protein (CBP) and its paralogue P300 to initiate transcription because mCREB lacks an essential serine-133 that must be phosphorylated for phospho-CREB/CBP interactions at the promoter (Mayr and Montminy, 2001; Lonze and Ginty, 2002).
While CREB can influence the expression of many genes (Mayr and Montminy, 2001; McClung and Nestler, 2003), it is not yet clear which genes targeted by CREB in the NAc influence reward and reinforcement. In this study we examined CREB's effects on the expression of CART mRNA and peptides in the NAc, which are proposed to play a key role in regulating cocaine's action in that brain region (Jaworski et al., 2003, 2007, 2008; Kim et al., 2003, 2007).
CART peptides are neurotransmitters that influence many physiologic processes, including psychostimulant-induced reward and reinforcement (Douglass et al., 1995; Rogge et al., 2008; Jaworski and Jones, 2006; Jaworski et al., 2008). Injections of active CART 55−102 peptides into the NAc resulted in reduced cocaine-, dopamine- and amphetamine-induced locomotor activity (Jaworski et al., 2003; Kim et al., 2003), cocaine-induced behavioral sensitization (Kim et al., 2007), and cocaine self-administration (Jaworski et al., 2008). An interesting connection between CART and CREB is that the CART gene contains a consensus cyclic AMP response element (CRE) site in its 5′ proximal promoter region (Dominguez et al., 2002; Dominguez, 2006; Barrett et al., 2002), and CART gene expression is regulated by CREB in cultured cells (Barrett et al., 2002; Dominguez and Kuhar, 2004; de Lartigue et al., 2007). This suggests that over expression of CREB in the rat NAc might increase CART peptide levels in vivo. In this study, we examined if CREB isolated from the rat NAc would bind to an oligonucleotide identical in sequence to the CART gene consensus CRE cis-element and adjacent flanking sequences, and if over expression of CREB in the rat NAc influenced CART mRNA and peptide levels. Since CART peptide injections seem to oppose cocaine's actions, and since CREB over expression reduces cocaine reward, we hypothesized that the CART gene is a physiologic target of CREB, such that CREB over expression will increase CART peptide levels in vivo in the NAc.
2. Results
An oligonucleotide of 27 base pairs in length, containing the CRE cis-regulatory element and adjacent sequences identical to those found in the rat CART gene promoter (Barrett et al., 2002), were examined in EMSA and antibody super shift assays, because CRE cis-element core sequences and viability vary from species to species and gene to gene (Holmberg et al., 1995; Nestler et al., 2001). In addition, DNA sequences that immediately flank consensus cis-regulatory sequences can influence transcription factor (TF) binding (Yanagisawa and Schmidt, 1999; Mayr and Montminy, 2001; Faiger et al., 2006, 2007). We thus used a synthetic oligonucleotide to test if the CRE cis-regulatory element present within the rat CART gene promoter, flanked by sequences from the CART gene promoter, permitted specific binding of CREB and phospho-CREB ser-133 isolated from the rat NAc.
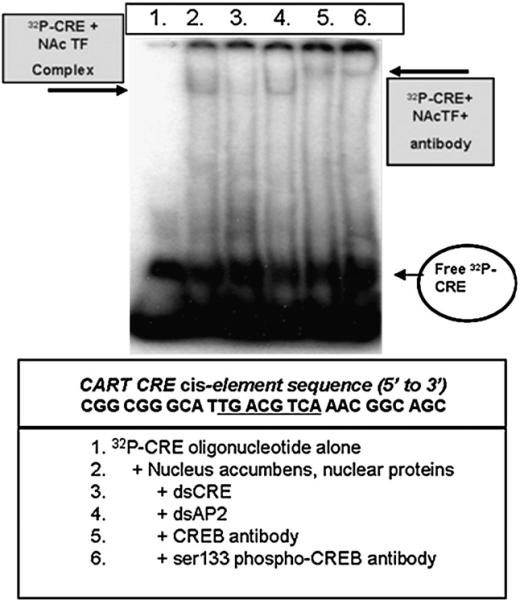
Nuclear extract proteins from the rat NAc added to radiolabeled oligonucleotides containing the consensus CRE core binding site with flanking sequences (32P-CRE) resulted in a shift in the mobilities of the 32P-CRE oligonucleotides in polyacrylamide gel electrophoresis separations. The binding of 32P-CRE oligonucleotides to nuclear extract proteins was blocked by excess double-stranded CRE oligonucleotides but not by unrelated, double-stranded AP2 oligonucleotides, which indicated specificity of the binding. Addition of anti-CREB or -phospho-CREB antibodies to the 32P-CRE + TF complex resulted in super shifted bands (Fig. 1). In contrast, the 32P-CRE-binding bands were not super shifted by antibodies to Pit-1, JunD or c-fos antibodies (data not shown).
Fig. 1.
Oligonucleotides containing the CART promoter CRE cis-regulatory element bind to CREB and phospho-CREB from the rat NAc. EMSA and super shift analyses were performed using nuclear proteins from the NAc as described in the Experimental procedures (the gel shown is representative of the data from at least five other animals assayed separately in independent experiments). The sequence of the regions of the CART promoter containing the CRE cis-element (the 27mer is referred to as the “CRE”) is given below the picture of the gel in the box, and the core binding sequence is underlined. 32P-CRE+NAc transcription factor (TF) complexes were visualized by incubating proteins extracted from cell nuclei with a 32P-radiolabeled oligonucleotide identical in sequence to the CART gene CRE cis-regulatory element along with its flanking sequence, and separating free 32P-CRE (see figure's bottom right) from the 32P-CRE+NAc TF complex on a polyacrylamide gel (complex is denoted by boxed arrow at the figure's top left). The identity of the protein in the 32P-CRE+NAc TF complex was determined by super shift analysis using either a CREB- or ser133 phospho-CREB-specific antibody (see box at top right denoting the 32P-CRE+NAc TF+antibody complex). See text for additional details.
These findings demonstrate that phospho-CREB ser133, the active form of the CREB protein, is at least part of the protein complex that binds to the CART promoter CRE sequence under these conditions. In addition, the quantity of radiolabeled oligonucleotide shifted in the gels was increased or decreased when the quantity of nuclear extract was increased or decreased, respectively (not shown). Thus, TF levels in nuclear extracts from the NAc that bind to CART gene promoter cis-elements can be detected in a semi-quantitative fashion in these procedures.
Taken together, these data suggest that CREB and phospho-CREB in the NAc can bind to the CRE cis-element of the CART gene promoter. The data also support the idea that changing CREB levels in the NAc in vivo could influence transcriptional regulation of the CART gene by binding to the gene promoter CRE cis-regulatory element.
In the following experiments, we tested that hypothesis directly by determining if over expression of CREB in the rat NAc resulted in increased CART peptide levels as predicted using Western blot analyses. Western blots were performed as opposed to other techniques such as the radioimmunoassay (RIA) because CART peptides are processed into various different peptide fragments from a pro-peptide precursor molecule in neurons. RIA does not discriminate between the differently processed CART peptide fragments, some of which show physiological activity when exogenously administered to animals (Dylag et al., 2006). Western blots allowed us to both semi-quantify the amount of active CART peptides (i.e. CART 55−102 and 62−102) present in animal tissues and also to identify the specific CART peptide fragments present by their molecular weights, which are shown clearly in Westerns but not in RIAs.
In order to manipulate CREB levels, animals were injected intra-NAc with viral vectors (Fig. 2) as described in the Experimental procedures. To minimize inter-experiment variability, all experimental sessions utilized pairs of animals that were treated on the same day, within hours of one another; the treated (HSV-CREB or HSV-mCREB) animals’ values were divided by values from the control animals (HSV-LacZ injected), and the resulting ratios were analyzed. Ratios greater than 1.0 indicated an increase over control in that pair, and ratios less than 1.0 indicated a decrease (see Experimental procedures).
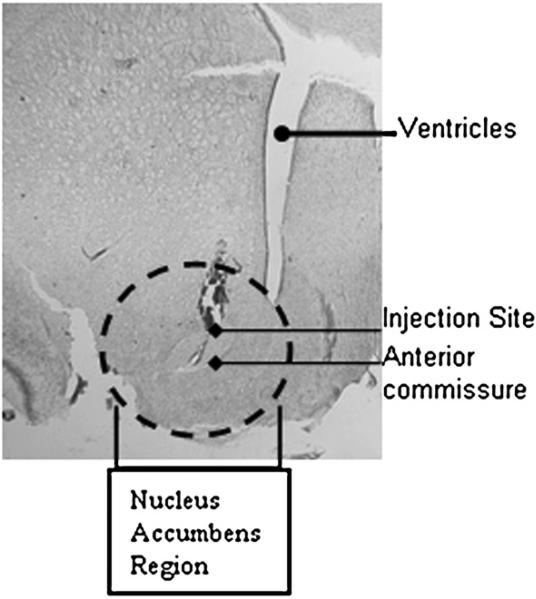
Fig. 2.
Nissl-stained hemisection in the coronal plane shows an example of the site of injection. HSV vectors were injected into the NAc as described in the Experimental procedures. Stereotaxic coordinates were determined from the Rat Atlas (Paxinos and Watson, 2002). The section is at A/V +1.5, M/L±1.6 and D/V−7.6 and 1.6×1000 magnification. The anterior commissure, ventricles and injection site are delineated and the accumbens region has been circled to orient the reader.
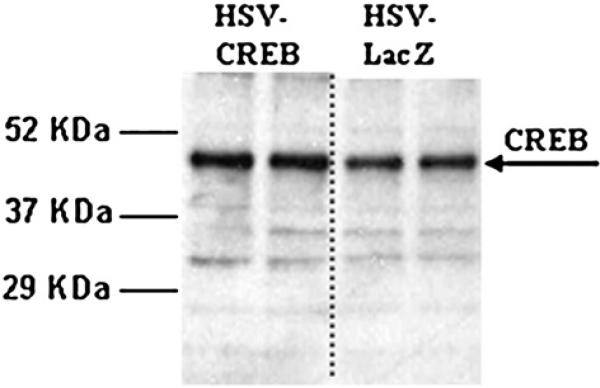
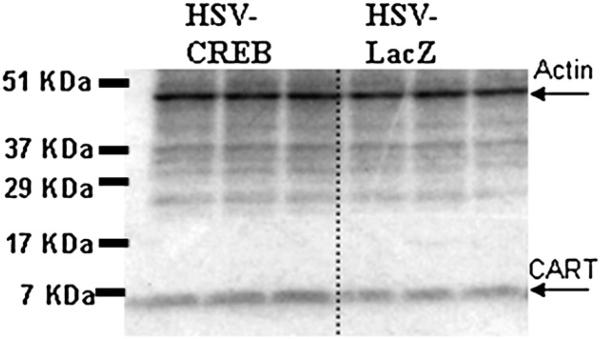
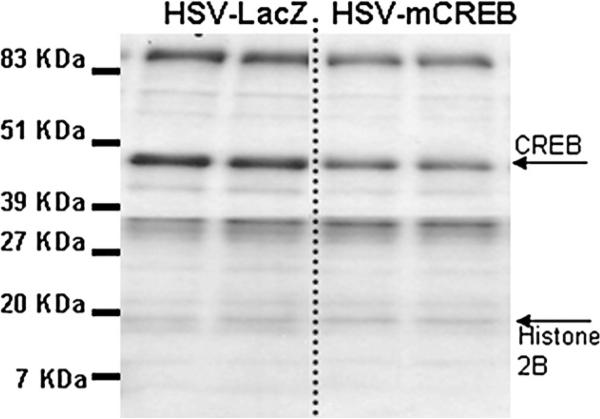
In the NAc of animals treated with HSV-CREB viral vectors, there was an over expression of CREB protein as measured by Western blot analysis (example in Fig. 3, Table 1). The ratios of CREB levels in treated animals to control animals were consistently greater than one (ratio of 1.42±052), and significant (P=0.0013, n=5 pairs). Phospho-CREB levels were not significantly changed (ratio of 0.81±0.171, P=0.355, n=4). Importantly, though, CART peptide levels were significantly increased in these same animals as determined by Western blot analysis (ratio of 1.32±0.073, P=0.0074, n=6 pairs, example in Fig. 4, Table 1). Under these conditions for Western blotting, the two active CART peptides (CART 55−102 and CART 62−102) are not clearly separated in the blots, and they run together as a wide band with a molecular weight less than 7 kilodaltons (kDa) (the predicted molecular weight of CART 62−102 is 6.5 kDa and CART 55−102 is 5.72 kDa). The specificity of the CART antibody was determined previously using antigen pre-absorption by Koylu et al. (1997).
Fig. 3.
HSV-CREB injections into the rat NAc increase CREB protein levels. The levels of CREB protein (approximately 45 kDa) in NAc from an HSV-CREB treated animal was determined by Western blot and compared to CREB levels in a control animal treated with HSV-LacZ (ratio of 1.42±052, p<0.01). The two lanes on the left show duplicate repeats using tissue from one of a pair of animals, and the two lanes on the right are duplicate repeats from the other, control animal of the pair. All lanes were loaded with the same amount of tissue protein as measured by Bradford assay previous to loading the gel. Quantification and statistical analysis of immunoreactive bands were performed using the Scion Image software (NIH, Bethesda, MD) as described in the Experimental procedures. This result is representative for a total of five pairs of animals prepared and assayed separately in independent experiments. See text for details.
Table 1.
Summary of CREB, Phospho-CREB and CART protein level changes in the rat NAc injected with either HSV-CREB or HSV-mCREB Vs. HSV-LacZ, paired controls
| Protein ID | Treatment |
|
|---|---|---|
| HSV-CREB | HSV-mCREB | |
| CREB | Increase p=0.0013*, n=5 | Trend to a decrease p=0.143, n=4 |
| P-CREB | No change p=0.355, n=4 | Decrease p=0.0440*, n=7 |
| CART | Increase p=0.0074*, n=6 | No change p=0.842, n=7 |
To test for changes in rat NAc protein levels subsequent to treatment with either the HSV-CREB over expressing virus or its dominant-negative mutant, HSV-mCREB, animals were paired during treatment procedures (one received either HSV-CREB or HSV-mCREB and the other received HSV-LacZ) as described in the Experimental procedures (see text for additional details). Asterisks indicate p values significantly less than 0.05.
Fig. 4.
HSV-CREB injections into the rat NAc increase CART peptide levels. The levels of CART peptide (approximately 6.5 kDa) in NAc from an HSV-CREB treated animal were determined by Western blot and compared to CART peptide levels in a control animal treated with HSV-LacZ (ratio of 1.32±0.073, p<0.01). The three lanes on the left show triplicate repeats using tissue from one of a pair of animals, and the three lanes on the right are triplicate repeats from the other, control animal of the pair. Each lane is normalized to whole tissue actin (approximately 50 kDa) as measured by Western blot. After normalization, the ratio of the pair of values were calculated and analyzed as described in the Experimental procedures. This result is representative of a total of six pairs of animals prepared and assayed separately in independent experiments. See text for details.
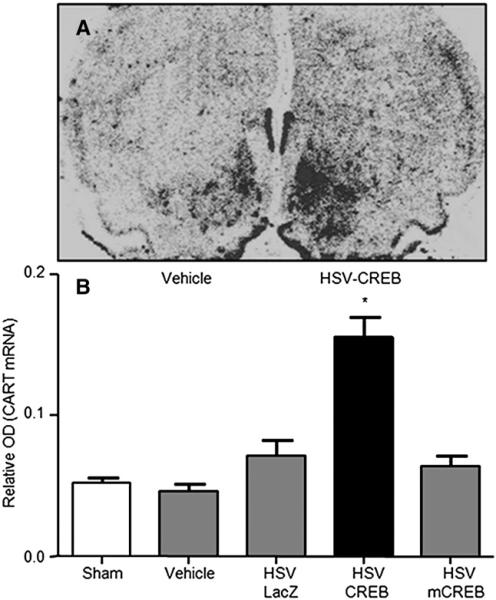
In order to explore the mechanism of HSV-CREB induced increases in CART peptide abundance, in situ hybridization using a molecular probe complementary to the CART gene mRNA transcript (see Experimental procedures for details) was performed with a separate group of animals. These data demonstrated that HSV-CREB intra-NAc injections increased CART mRNA levels in the rat NAc (Fig. 5). Concurrent increases in NAc mRNA and peptide levels subsequent to HSV-CREB intra-NAc injections likely indicate that CART gene expression is positively regulated by CREB in the NAc by transcriptional mechanisms.
Fig. 5.
Over-expression of CREB increases CART mRNA in the rat NAc. CART mRNA levels were measured by in situ hybridization 36 h following viral infection with either HSV-CREB or mCREB. Animals received HSV-CREB or HSV-mCREB in one hemisphere and a control (sham injection or HSV-LacZ vehicle) in the contralateral hemisphere thus allowing each animal to serve as its own control. (A) Representative autoradiogram showing increased radioactive CART mRNA signal in the HSV-CREB treated hemisphere. (B) Quantitative analysis was conducted by measuring the relative OD of the radioactive signal in the NAc. Data is expressed as the mean± SEM and significance was tested with a one-way ANOVA and Newman–Keuls post hoc test. HSV-infected animals had significantly higher CART mRNA levels compared to all controls and HSV-mCREB-infected animals (*p<0.01). CART mRNA levels in the NAc of HSV-mCREB treated rats did not differ from those in rats treated with sham injections or HSV-LacZ alone.
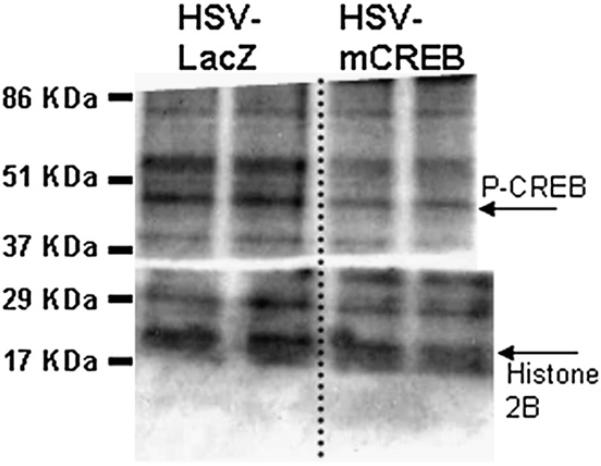
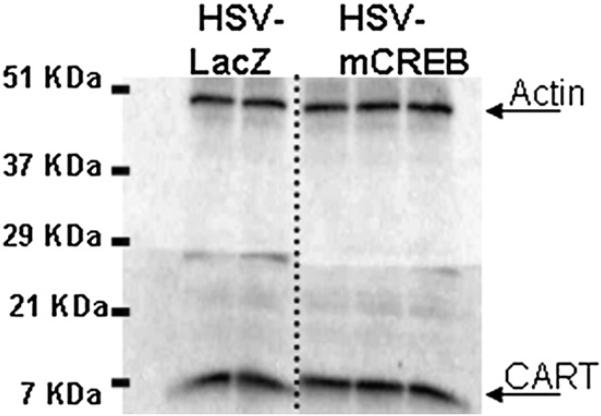
To further test the hypothesis that CREB levels affect CART mRNA and peptide levels, a viral vector over expressing a dominant-negative mutant of CREB, HSV-mCREB (serine-133 to alanine mutated) was injected into the NAc of rats. As above, the animals were treated in pairs, one with HSV-mCREB and the other with a control HSV-LacZ expressing vector. HSV-mCREB injections significantly reduced the amount of phospho-CREB in treated animals compared to their paired, HSV-LacZ treated controls (ratio of 0.64±0.145, P=0.0440, n=7), but the amount of CREB was not significantly reduced although there was a trend for reduction (ratio of 0.73±0.148, p=0.143, n=4) (Figs. 6, 7, Table 1). However, CART levels were not changed significantly after injections of HSV-mCREB compared to HSV-LacZ injections (ratio of 1.02±0.071, P=0.842, n=7 pairs, example in Fig. 8, Table 1). Consistent with the lack of change in CART peptide levels, CART mRNA levels in the rat NAc remained unchanged after HSV-mCREB administration when examined in a separate group of animals by in situ hybridization (Fig. 5B).
Fig. 6.
HSV-mCREB injections into the rat NAc trend towards decreasing CREB protein levels. The levels of CREB protein (approximately 45 kDa) in NAc from an HSV-mCREB treated animal was determined by Western blot and compared to CREB levels in an HSV-LacZ treated control animal (ratio of 0.73±0.148, p>0.05). The two lanes on the left show duplicate repeats using tissue from one of a pair of animals, and the two lanes on the right are duplicate repeats from the other, control animal of the pair. All lanes were normalized to its histone 2B content using an antibody specific to H2B. After normalization, the ratio of the pair of values were calculated and analyzed as described in the Experimental procedures. Quantification and statistical analysis of immunoreactive bands were performed using the Scion Image software (NIH, Bethesda, MD) as described in the Experimental procedures. This result is representative of a total of four pairs of animals prepared and assayed in independent experiments. See text for additional details.
Fig. 7.
HSV-mCREB injections into the rat NAc decrease phospho-CREB levels. The levels of ser133 phospho-CREB (approximately 45 kDa) in NAc from an HSV-mCREB treated animal was determined by Western blot and compared to phospho-CREB levels in an HSV-LacZ treated control animal (ratio of 0.64±0.145, p<0.05). In this representative blot showing phospho-CREB levels in a pair of animals, all lanes were normalized to the histone 2B content. After normalization, the ratio of the pair of values were calculated and analyzed as described in Methods. The two lanes on the left show duplicate repeats using tissue from one of a pair of animals, and the two lanes on the right are duplicate repeats from the other, control animal of the pair. Quantification and statistical analysis of immunoreactive bands were performed using the Scion Image software (NIH, Bethesda, MD) as described in the Experimental procedures. This result is representative for a total of seven pairs of animals prepared and assayed separately in independent experiments. See text for additional details.
Fig. 8.
HSV-mCREB injections into the rat NAc do not change CART peptide levels. The levels of CART peptide (approximately 6.5 kDa) in tissue from an HSV-mCREB treated animal were determined by Western blot and compared to CART peptide levels in an HSV-LacZ treated control animal (ratio of 1.02±0.071, P=0.842). In this representative blot showing CART levels in a pair of animals, each lane is normalized to whole tissue actin (approximately 50 kDa). After normalization, the ratio of the pair of values were calculated and analyzed as described in the Experimental procedures. This result is representative of a total of seven pairs of animals prepared and assayed separately in independent experiments. See text for additional details.
In conclusion, we determined that over expression of CREB in the rat NAc via injection of the HSV-CREB viral construct significantly increased the levels of CART mRNA and peptide in that tissue. This increase was concomitant with increases in rat NAc CREB protein levels. Although injections of HSV-mCREB, a dominant-negative mutant of CREB, did significantly decrease phospho-CREB protein levels in the rat NAc and exhibited a trend towards reducing CREB protein levels, HSV-mCREB had no significant effect on rat NAc CART mRNA or peptide levels relative to HSV-LacZ controls (see Table 1 for summary).
3. Discussion
Injections of active CART 55−102 peptides into the NAc have been shown to blunt or reduce the locomotor, sensitizing, and rewarding effects of cocaine, amphetamine, or dopamine in rodents (Jaworski et al., 2003, 2007, 2008; Kim et al., 2003, 2007). CART peptides are therefore hypothesized to play a homeostatic role in that brain nucleus (Jaworski et al., 2008), and the mechanisms of CART gene regulation are of interest. There have been several studies of the CART gene proximal promoter in cultured cells (Dominguez et al., 2002; Dominguez and Kuhar 2004; Lakatos et al., 2002; Barrett et al., 2002; de Lartigue et al., 2007) and one paper showed that CART mRNA production in vivo, in the rat NAc, is regulated by the cyclic adenosine 5′ monophosphate/protein kinase A (cAMP/PKA) second messenger system (Jones and Kuhar, 2006). The current study, however, focused specifically on the role of CREB (a downstream target of PKA) in regulating CART mRNA and peptide levels in vivo in the rat NAc.
In terms of understanding the molecular mechanisms of drug reward and reinforcement, a related observation is that over expression of CREB in the NAc reduced the rewarding effects, or perhaps increased the aversive effects, of cocaine (Carlezon et al., 1998). This behavior was correlated with increases in mRNA and protein from the CREB target gene preprodynorphin in the rat NAc (Carlezon et al., 1998). The behavioral similarities between animals which over expressed CREB in the NAc, and animals injected intra-NAc with active CART peptides (i.e. reduced cocaine reward), led us to hypothesize that because the CART gene promoter has a consensus CRE cis-element, and because CREB can regulate expression of CART mRNA in cultured cells (Barrett et al., 2002; Dominguez and Kuhar, 2004; de Lartigue et al., 2007), then CART may be a physiologic target gene for CREB-mediated transcription in the NAc, such that over expressing CREB in this region could increase CART peptides in vivo. That is, we hypothesized that CREB in vivo could regulate CART peptide levels in vivo which in turn could influence cocaine's rewarding effects.
To address the hypothesis, we first wanted to show that the consensus CRE cis-element in the CART gene promoter was able to bind CREB and phospho-CREB isolated from the NAc of rats. This is because DNA cis-regulatory elements not only vary in their core binding sequences from gene to gene, but also are surrounded by flanking stretches of DNA which also differ in sequence from gene to gene (Yanagisawa and Schmidt, 1999; Faiger et al., 2006). CRE sequences are not different (Mayr and Montminy, 2001; Nestler et al., 2001). Together, both the core binding sequences and flanking sequences of cis-elements can determine the strength of TF-DNA cis-element interactions, either enhancing or diminishing them (Hai and Curran, 1991; Liu et al., 1994; Boonyaratanakornkit et al., 1998; Yanagisawa and Schmidt, 1999; Faiger et al., 2006, 2007). We showed that the CART gene promoter consensus CRE cis-element was able to bind CREB and phospho-CREB TFs from the NAc of rats, which suggests that over expression of CREB in the NAc could result in regulation of CART expression at the CART gene consensus CRE cis-regulatory site in vivo.
We also determined that over expression of CREB in the NAc via injection of the HSV-CREB viral construct significantly increased the levels of NAc CART peptide as we hypothesized. In addition, the idea that CREB may bind to the CRE cis-element to the CART gene promoter in the NAc was further bolstered with in situ observations of CART mRNA increases in the NAc after HSV-injections. The above facets of evidence indicate a possible role for CREB in the transcriptional regulation of CART mRNA and peptide production in the rat NAc.
Of interest was the finding that HSV-mCREB injections significantly decreased phospho-CREB levels but not those of CART mRNA or peptide. Findings from other investigators, however, support our observations and make it seem unlikely that methodological errors account for HSV-mCREB's null effects on CART mRNA and peptide levels. First, not all genes up regulated by CREB over expression are down regulated by mCREB over expression in the NAc (McClung and Nestler, 2003). In a microarray study, 23% of the mRNAs that were up regulated by HSV-CREB injections into the NAc were not down regulated by HSV-mCREB intra-NAc injections (McClung and Nestler, 2003), which indicates that there are many genes in the NAc whose expression can only be up regulated upon manipulation of CREB activity.
Second, in vitro studies indicate that basal CART promoter-driven gene expression can occur in the presence of dominant negative mutants of CREB, such as mCREB (Dominguez and Kuhar, 2004; de Lartigue et al., 2007). In experiments with luciferase plasmids driven by the mouse CART gene proximal promoter in locus coeruleus-derived, cultured CATH.a cells, A-CREB (ser-133 mutated), a dominant negative mutant of CREB similar to mCREB, did not reduce basal levels of gene transcription (Dominguez and Kuhar, 2004). A-CREB did, however, block increases in CART gene promoter-driven luciferase expression after stimulation with the adenylyl cyclase activator forskolin. Stimulus-induced elevations of CART promoter-driven luciferase were also ablated by A-CREB in cholecystokinin-stimulated, cultured rat vagal afferent neurons (de Lartigue et al., 2007). It would seem that pharmacologically-stimulated expression of CART requires CREB, but basal expression does not (Dominguez et al., 2002; Dominguez and Kuhar, 2004; de Lartigue et al., 2007).
A third series of studies found that non-sense mutations in the mouse CRE DNA cis-element itself abolished basal as well as forskolin-stimulated CART promoter-driven luciferase expression in CATH.a cells, although A-CREB had no effect on basal activity in those same cells (Dominguez and Kuhar, 2004). Similar experiments yielded identical results in GH3, AtT20 and SH-SY5Y cultured cells using CART promoter-driven luciferase plasmids with non-sense mutations in the rat CART gene promoter CRE cis-element (Barrett et al., 2002). The mutated rat CART gene promoter, like the mutated mouse promoter, was unable to initiate either basal or forskolin-stimulated luciferase gene expression in all three cell lines.
Since A-CREB, a dominant-negative mutant of CREB, did not reduce basal expression of CART promoter-driven lucifer-ase plasmids in cultured cells, yet point mutations in either the mouse or rat CART proximal promoter CRE elements did reduce that basal expression, it is possible that TFs closely related to CREB may bind to the CRE site. For example, the mammalian family of CRE activators comprises a substantial number of closely related proteins arising from genes encoding CREB, CRE modulators (CREM) and activating transcription factors (ATF-1, 2 and 3). The family also includes numerous alternative splice variants of CREB, CREM and the ATFs which can all bind gene promoter CRE cis-elements and/or homo- and heterodimerize with CREB itself (Mayr and Montminy, 2001; Nestler et al., 2001). Interestingly, several of these CREB family TFs are regulated in the NAc by exposure to psychostimulants and influence behavioral responses to the drugs when over expressed in this brain region (Green et al., 2006, 2008). It is possible that under conditions where mCREB is present, CREB homodimers can no longer initiate transcription at gene promoter CREs and the brain compensates for decreased CREB TF activity by increasing the activity of other CRE cis-element binding proteins such as one of the various CREB family members or splice variants (Nestler et al., 2001). One piece of evidence indicating that other CREB family members may be responsible for un-stimulated CART gene expression is the finding that in vivo, in the rat NAc, basal as well as forskolin-stimulated CART mRNA expression was blocked with a non-hydrolyzable cAMP analogue as well as the protein kinase A inhibitor H89 (Jones and Kuhar, 2006). mCREB was not used in those studies.
Another phenomenon that may occur in conjunction with binding of non-CREB protein CRE activators (such as CREM) to the CART gene promoter CRE sequence may involve cooperativity amongst one or more other cis-regulatory elements present in the CART gene promoter. Cis-elements most likely function in concert with one another (Nestler et al., 2001) and numerous other cis-elements, such as AP-1, Pit-1, STAT3 and E Box co-exist within the CART gene proximal promoter and probably influence one another consequent to the effects of multiple signaling pathways convergent on the nucleus. Complex TF-TF and TF-co-factor interactions which require the CART gene promoter CRE cis-element, but not necessarily CREB TFs themselves, may be necessary for basal transcriptional activity of the CART gene in some tissues.
In this study, the fact that mCREB did not drive down basal expression of rat NAc CART mRNA or peptide seems to indicate that CREB TFs do not regulate basal levels of CART expression. It may regulate only the stimulated expression of the gene. Such an observation aligns with the idea that CREB is widely accepted as a stimulus-regulated transcription factor responsible for the expression of stimulus-inducible genes such as neurotransmitters (Mayr and Montminy, 2001; Nestler et al., 2001; Lonze and Ginty, 2002).
The results presented in this paper, which show that over expression of CREB increases the quantity of CART mRNA and peptide in the rat NAc, support our overall hypothesis that CART peptides in the NAc appear to be homeostatic regulators with functional effects opposing the locomotor, sensitizing and rewarding effects of psychostimulants and elevated dopaminergic neurotransmission (Jaworski et al., 2008). One mechanism by which CART may attenuate the behavioral effects of cocaine, amphetamine and dopaminergic signaling is via modulation of dopamine neurotransmission. Although it is not clear if CART peptides over expressed in the rat nucleus accumbens (NAc) directly affect dopamine release from the ventral tegmental area (VTA), neuroanatomical data show that a majority of the CART neurons which project from the NAc to the VTA form synapses with putative GABAergic interneurons (Philpot and Smith, 2006). CART may disinhibit dopaminergic mesofugal neurons, causing an efflux of dopamine from the VTA, by inhibiting these GABAergic interneurons.
In support of the hypothesis that CART released from NAc projection neurons in the VTA can influence DA output in the ventral mid-brain, in vivo microdialysis was performed on rats after intra-VTA injections with active CART peptides (Kuhar et al., 2005). These CART peptide injections into the rat VTA did slightly increase DA efflux from the VTA to the NAc as shown by in vivo microdialysis. In a separate set of experiments performed by Kimmel et al. (2000), rats exhibited increased locomotion and conditioned-place preference behaviors after high-dose intra-VTA injections with active CART peptides. Those behaviors were pharmacologically blocked in a dose-dependent manner by the D2 receptor antagonist haloperidol (Kimmel et al., 2000).
The remaining CART immunoreactive nerve terminals projecting from the NAc to the VTA, approximately 30% of the total, directly appose dopaminergic neurons there (Philpot and Smith, 2006). Nothing is known about the functions of CART released from these NAc projections to the VTA. As a result, the precise mechanism by which CART functionally antagonized the behavioral effects of dopamine and psychostimulants in previous studies thus remains obscure due to a limited scope of knowledge regarding CART receptor identity (s), function(s), associated signaling cascades in discreet CNS tissues and the complex neurocircuitry of CART neurons interconnected throughout the various regions of the rat brain associated with reward and reinforcement.
It is to be noted that we cannot be sure that increased CREB in the NAc acts solely and directly on the consensus CRE cis-element in the CART gene promoter to increase CART mRNA and peptide levels; an indirect effect is possible as well. However, the body of scientific knowledge regarding CART gene regulation in cultured cells (Barrett et al., 2002; Dominguez et al., 2002; Lakatos et al., 2002; Dominguez and Kuhar, 2004; de Lartigue et al., 2007) and in vivo in the rat NAc (Jones and Kuhar 2006), strongly suggests that the CART gene consensus CRE cis-regulatory element can bind phospho-CREB in the NAc and that that binding has an effect on the regulation of CART mRNA and peptide levels in vivo in that tissue.
In conclusion, a number of groups have used luciferase constructs transcriptionally driven by the CART gene 5′ flanking region to determine that in various different cultured cell lines the 5′ CRE cis-elements identified in both the mouse and rat CART gene promoters do regulate stimulus-induced transcription (Barrett et al., 2002; Lakatos et al., 2002; Dominguez and Kuhar, 2004; de Lartigue et al., 2007). Barrett et al. (2002) and Dominguez and Kuhar (2004) also showed that mutations of the mouse and rat CART gene CRE cis-elements abolished basal transcriptional activity in a number of cell lines. Dominguez and Kuhar (2004) and de Lartigue et al. (2007) also over expressed a dominant negative mutant of CREB, A-CREB, which inhibited stimulus-induced transcriptional activity.
Both Lakatos et al. (2002) and Dominguez and Kuhar (2004) also determined that phospho-CREB isolated from cultured cells could bind to an oligonucleotide identical in sequence to the mouse CART gene promoter CRE cis-regulatory element in EMSA/super shift analyses. Data from the current study extend that finding to include phospho-CREB from the rat NAc as a binding partner for the rat CART gene promoter CRE oligonucleotide. In addition, previous in vivo work by Jones and Kuhar (2006) demonstrated regulation of rat CART gene mRNA by the cAMP/PKA cell signaling pathway in the rat NAc. Our current work concludes that CREB, a cAMP-responsive TF, positively regulates rat CART gene mRNA and peptide production in the rat NAc. CREB regulation of the CART gene in the rat NAc is an important finding because, although psychostimulants regulate CREB activity in the brain reward pathway and these TFs play an essential role in drug reward and reinforcement, the complete gamut of genes regulated by CREB in the NAc by psychostimulants which may mediate their behavioral actions have not yet been fully elucidated. Identifying candidate CREB-regulated genes in the NAc, such as CART, will further our understanding of the molecular mechanisms of the reinforcing effects of psychostimulants such as cocaine and amphetamine.
4. Experimental procedures
4.1. Animals
Male, Sprague–Dawley rats weighing 250−325 g, aged 6−8 weeks were housed on a 7:00 to 19:00 light–dark cycle and fed and watered ad libitum. All animal care and experimentation were performed in accordance with the National Institutes of Health guide for the care and use of laboratory animals.
4.2. Intra-accumbal infusions
Rats were anesthetized with a mixture of medetomidine (0.5 mg/kg, i.p.) and ketamine (75 mg/kg, i.p.). The target stereotaxic coordinates for the bilateral infusions (relative to Bregma) were; A/P +1.6, M/L±1.5 and D/V −7.6 (Paxinos and Watson, 2002). Infusions were performed using 10 μl Hamilton microsyringes (Hamilton Co., Reno, NV) secured onto an UltraMicroPump II microsyringe injector and Micro4 Controller (World Precision Instruments, Inc., Sarasota, FL) mounted directly onto the stereotaxic frame. The bilateral NAc's were infused separately with 2 μl over 10 min of HSV-CREB, HSV-LacZ, or HSV-mCREB. Unilateral injections were performed on animals used in the in situ hybridization studies. This dosing regimen was optimized to reduce neurotoxicity in Carlezon et al. (2000). Dose–response analyses were not performed using graded doses of the viral vectors because our study aimed to look at the neurochemical changes associated with CREB over expression under the same conditions as those used by Drs Carlezon and Nestler in their conditioned-place preference studies showing blunted effects of cocaine after intra-accumbal HSV-CREB injections (Carlezon et al., 1998).
Non-replicative, HSV viral vectors were generated according to published protocols. For comprehensive reviews and further details of how the vectors were constructed, how CREB and mCREB were incorporated and how treatment times/doses were optimized, please see references 1) Neve et al. (2005) and 2) Carlezon et al. (2000). The dominant-negative mutant, mCREB, contains a serine-133 to alanine point mutation which prevents an activating phosphorylation at that ser-133 site. In effect, mCREB is not able to bind to CREB-Binding Protein (CBP), a transcription factor co-regulator essential for the initiation of transcription at gene promoter CRE cis-regulatory elements. As a dominant-negative mutant, though, the serine-133 to alanine mutation in the mCREB gene does not affect hetero- and homo-dimerization with other, wild-type CREB TF family members or DNA binding to gene promoter CRE elements. The point mutation in mCREB does, however, prevent the initiation of transcription when it binds to gene promoter CRE cis-regulatory sites as a dimer because it cannot interact with CBP (Lonze and Ginty, 2002).
Experiments, except for in situ hybridization, were done with pairs of animals, each treated within hours of one another; for example, one member of the pair was treated with HSV-CREB and the other member of the pair with HSV-LacZ on the same day, within 1−2 h as a control. The order of injections was reversed in alternate pairs. The pairs were handled together in all assays and housed individually after the surgeries until approximately 36 h post-injection when they were euthanized by rapid decapitation (see below also in the Quantification of data and statistical analyses section).
4.3. NAc dissections
Rats were anesthetized with isofluorane (Abbot, Chicago, Il), decapitated by guillotine, and their brains removed from the skull. The NAc shell and core were microdissected according to coordinates delineated in a rat atlas (Paxinos and Watson, 2002). Dissected NAc were immediately frozen at −80 °C.
4.4. In situ hybridization and autoradiogram image analysis
In situ hybridization was preformed as previously described (Jones and Kuhar, 2006). Rats were sacrificed 36 h following viral infection and 14 μm brain slices around the injection site were cut and slide mounted. Sections were fixed in 4% paraformaldehyde followed by consecutive washes in 2×SSC, triethanolamine/0.5% acetic anhydride (0.1 M), H20, 70%, 95%, and 100% ethanol, 5% chloroform, 95% and 70% ethanol, and then air dried. Slides were incubated at 37 °C in pre-hybridization buffer (50% deionized formamide, 4×SSC, 1×Denhardt's solution, 0.02 M NaPO4 [pH 7.0], 1% N-lauroylsarcosine, 10% dextran sulfate) in a humidifying chamber for 2 h. An oligodeoxynucleotide probe complementary to rat CART mRNA (nucleotides 223−270 (Douglass et al., 1995); synthesized by Emory Microchemical Facility; Emory University, Atlanta, GA) was labeled on the 3′ end with 35S-dATP (NEN, Boston, MA) to a specific activity of 5×109 cpm/μl using terminal deoxynucleotide transferase (Amersham Biotech, Piscataway, NJ) and then purified with a QIAquick Nucleotide removal kit (Qiagen Inc, Valencia, CA). Hybridization solution (pre-hybridization buffer plus 500 mg/l denatured salmon testis DNA and 200 mM dithiothreitol) containing the CART probe (∼5×105 cpms/slide) was applied to each section. Slides were hybridized overnight in a humidifying chamber at 42°. Slides were then washed in 2×SSC, 50% ethanol/0.3 M ammonium acetate, 85% ethanol/0.3 M ammonium acetate, 100% ethanol, and H20. Sections were air dried and exposed to Kodak BioMax MR autoradiography film for 10 days. CART mRNA levels were quantified by capturing the autoradiograms with a Photometrics CoolSNAP camera (Photometrics, Roper Scientific Inc, Tucson, AZ) and analyzing with MCID Basic imaging software (Imaging Research Inc, Ontario, Canada). Relative OD was measured using an outline with a consistent area (60×80 pixels) centered over the NAc shell/core junction. Magnification and illumination were held constant throughout the analysis so that OD's were within the linear portion of a standard curve. Measurements were taken through the injection site and without knowledge of treatment in brain slices at 2.2, 1.7, 1.6, and 1.2 mM from bregma, which represents a major portion of the NAc.
4.5. Electrophoretic mobility shift assay (EMSA)
DNA–protein interactions were studied by EMSA. Nuclear protein extracts were separated from cytoplasmic proteins in preparations detailed by Xu and Cooper (1995) Total nuclear protein (15 μg) was determined by Bradford assays, and incubated for 45 min at room temperature with 2 ng of 32P-5′ end-labeled oligonucleotide which was identical in sequence to the CART promoter CRE cis-regulatory element (5′-CGG CGG GCA TTG ACG TCA AAC GGC AGC-3′), in binding buffer composed of 10 mM Tris–HCl (pH 7.5), 50 mM NaCl, 1 mM EDTA, 5% glycerol, and 0.05 μg/μl Poly[d(I-C)] (Roche, Indianapolis, IN). Oligonucleotides were synthesized and HPLC purified by the Emory Core facility (Emory University, Atlanta, GA). In some cases, a 50-fold molar excess of a specific competitor (non-radiolabeled CART oligonucleotide of the same sequence as the 32P-labeled oligonucleotide), or 50-fold molar excess of a non-specific competitor, was added to the mixture prior to the addition of the 32P-labeled CART CRE cis-element oligonucleotide. After a 45-minute incubation with unlabeled oligonucleotide at room temperature, the 32P-labeled CART CRE oligonucleotide was added and incubation was continued for another 45 min.
In the case of antibody super shift assays, 2 μg of transcription factor-specific antibody were incubated with the nuclear extract for 45 min at room temperature followed by further incubation with the 32P-labeled CART CRE oligonucleotide for 45 min at room temperature. The antibodies used were raised against CREB (Cell signaling technology, Boston, MA), and phospho-CREB ser-133 (Santa Cruz Biotechnology, CA; this company's antibody has been shown to be better for EMSA that for western blots).
The 32P-labeled CART CRE oligonucleotide + protein complex was separated by electrophoresis on a 6% non-denaturing (80:1) polyacrylamide gel (1× TBE, 2.5% glycerin). Gels were run at 200 V in the presence of 0.5× TBE buffer for 45-min at 4 °C. Dried gels were exposed to Kodak BioMax MR Film (Eastman Kodak Company, Rochester, NY) and image quantification was performed using Scion Image software (NIH, Bethesda, MD).
4.6. Western blots
The nuclear proteins from dissected NAc were separated from their cytoplasmic counterparts and each fraction was separately frozen at −80 °C as detailed by Xu and Cooper (1995). 25 μg of total protein (either nuclear or cytoplasmic), determined by Bradford assay, were boiled for 5 min in 1:3 diluted 3×SDS sample buffer (187.5 mM Tris [pH 6.8 at 25 °C], 6% [w/v] SDS, 30% glycerol and 0.03% [w/v] bromophenol blue supplemented with 125 mM DTT [Cell signaling technology, Boston, MA]). The mixture was quickly centrifuged and run at 120-V for two-hours on a 4−20% Biorad, pre-cast SDS–Tris–Glycine gel (Biorad, Hercules, CA). After overnight transfer at 30-V and 4 °C, the membranes were blocked with 5% non-fat milk in 1× TBS-T (Tris-Buffered Saline, 0.1% Tween-20 [pH 7.6]) for 2 h, and then incubated with the primary antibody overnight. The primary antibodies used were: CREB anti-rabbit mAb (Cell signaling technology, Boston, MA); phospho-CREB ser133 anti-mouse mAb (Cell signaling technology, Boston, MA; very good for western blots); actin anti-mouse mAb (Sigma-Aldritch, St. Louis, MO); histone 2B anti-rabbit mAb (Cell signaling technology, Boston, MA); and CART anti-rabbit polyclonal Ab (produced by the Kuhar lab, Emory University, Atlanta, GA). The signal was detected by using horseradish peroxidase (HRP)-conjugated anti-rabbit antibodies (Cell signaling technology, Boston, MA), or HRP-conjugated anti-mouse antibodies (Cell signaling technology, Boston, MA) and an enhanced chemiluminescence kit (Amersham, Arlington Heights, IL). CART peptide immunoreactive bands were normalized to the signals from actin immunoreactive bands because actin is a cytoskeletal protein isolated along with CART peptides in the cytoplasmic fraction during sample preparation from NAc tissues. In contrast, actin was not found in the nuclear fractions of tissue preparations (data not shown) and CREB and phospho-CREB ser133 immunoreactive bands were thus normalized to signals from the nuclear localized histone 2B proteins identified in nuclear fractions of samples prepared from NAc tissues but not in the cytoplasmic fractions of those same samples (data not shown).
4.7. Quantification of data and statistical analyses
Western and EMSA raw data were digitally scanned into computer files. Scion Image software (NIH, Bethesda, MD) was subsequently used to quantify the optical densities of individual Western bands. Briefly, the optical densities were obtained by first outlining the Western band of interest and measuring its optical density. Three geometrically identical areas above or below the band of interest, but separate from other bands in the same lane as well as the band of interest, were measured as background. The average of those background optical densities was subtracted from the band of interest's optical density to calculate the Western band of interest optical density. As noted in the text, experiments were carried out with pairs of animals. A “pair” was defined as two sequentially treated animals, one with the LacZ vector (HSV-LacZ) as a control, and the other with a CREB expressing vector (HSV-CREB) or with a dominant negative mutant vector of CREB (HSV-mCREB) within hours. The experimental values from HSV-CREB or HSV-mCREB animals in each pair were divided by the value from the HSV-LacZ vector controls to produce a ratio. These ratios of results from paired animals (4−7 pairs and ratios/group depending on the protein examined) were subjected to a one-sample student's t-test to determine if the ratio significantly differed from 1.00 (Motulsky, 2003). Ratios were considered significantly different from 1.00 if p<0.05. A ratio greater than 1.0 indicated a significant increase over control, and a ratio less than 1.0 indicated the opposite. Working with individual pairs and utilizing the ratio of each pair minimized inter-experiment variability such as that due to diurnal variations in CART levels with time of day (Vicentic et al., 2005), small variations in the time of sacrifice, possible decay of viral vectors over time, and the use of different batches of viral vectors. The statistical tests were performed using Graphpad Prizm software (Graphpad Software, Inc., La Jolla, CA).
Acknowledgments
This work was supported by NIH/NIDA grants RR00165, DA00418, DA15162, DA015040, DA08227, and 5F31DA0219. We would like to sincerely thank Li Ling Chen for her outstanding technical expertise, Rachel L. Neve who generously supplied the HSV viral constructs, Dr. Mark Moffett, and Dr. Geraldina Dominguez for her helpful thoughts, ideas and training.
REFERENCES
- Barrett P, Davidson J, Morgan P. CART gene promoter transcription is regulated by a cyclic adenosine monophosphate response element. Obes. Res. 2002;10:1291–1298. doi: 10.1038/oby.2002.175. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Melvin V, Prendergast P, Altmann M, Ronfani L, Bianchi ME, Taraseviciene L, Nordeen SK, Allegretto EA, Edwards DP. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol. Cell. Biol. 1998;18:4471–4487. doi: 10.1128/mcb.18.8.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Nestler EJ, Neve RL. Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research. Crit. Rev. Neurobiol. 2000;4(1):47–67. doi: 10.1080/08913810008443546. Review. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. Review. [DOI] [PubMed] [Google Scholar]
- de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J. Neurosci. 2007;27:2876–2882. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez G. The CART gene: structure and regulation. Peptides. 2006;27:1913–1918. doi: 10.1016/j.peptides.2006.01.025. Review. [DOI] [PubMed] [Google Scholar]
- Dominguez G, Kuhar MJ. Transcriptional regulation of the CART promoter in CATH.a cells. Brain Res. Mol. Brain Res. 2004;126:22–29. doi: 10.1016/j.molbrainres.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Dominguez G, Lakatos A, Kuhar MJ. Characterization of the cocaine- and amphetamine-regulated transcript (CART) peptide gene promoter and its activation by a cyclic AMP-dependent signaling pathway in GH3 cells. J. Neurochem. 2002;80:885–893. doi: 10.1046/j.0022-3042.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J. Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylag T, Kotlinska J, Rafalski P, Pachuta A, Silberring J. The activity of CART peptide fragments. Peptides. 2006;27(8):1926–1933. doi: 10.1016/j.peptides.2005.10.025. Review. [DOI] [PubMed] [Google Scholar]
- Faiger H, Ivanchenko M, Cohen I, Haran TE. TBP flanking sequences: asymmetry of binding, long-range effects and consensus sequences. Nucleic Acids Res. 2006;34:104–119. doi: 10.1093/nar/gkj414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiger H, Ivanchenko M, Haran TE. Nearest-neighbor non-additivity versus long-range non-additivity in TATA-box structure and its implications for TBP-binding mechanism. Nucleic Acids Res. 2007;35:4409–4419. doi: 10.1093/nar/gkm451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Hommel JD, DiLeone RJ, Kumar A, Theobald DE, Neve RL, Nestler EJ. Induction of ICER expression in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. J. Neurosci. 2006;26:8235–8242. doi: 10.1523/JNEUROSCI.0880-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Unterberg S, Neve RL, Ghose S, Tamminga CA, Nestler EJ. Induction of activating transcription factors ATF2, ATF3, and ATF4 in the nucleus accumbens and their regulation of emotional behavior. J. Neurosci. 2008;28:2025–2032. doi: 10.1523/JNEUROSCI.5273-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg M, Leonardsson G, Ny T. The species-specific differences in the cAMP regulation of the tissue-type plasminogen activator gene between rat, mouse and human is caused by a one-nucleotide substitution in the cAMP-responsive element of the promoters. Eur. J. Biochem. 1995;231(2):466–474. doi: 10.1111/j.1432-1033.1995.tb20720.x. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc. Natl. Acad. Sci. U. S. A. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Jones DC. The role of CART in the reward/reinforcing properties of psychostimulants. Peptides. 2006;27:1993–2004. doi: 10.1016/j.peptides.2006.03.034. Review. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J. Pharmacol. Exp. Ther. 2003;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Kimmel HL, Mitrano DA, Tallarida RJ, Kuhar MJ. Intra-VTA CART 55−102 reduces the locomotor effect of systemic cocaine in rats: an isobolographic analysis. Neuropeptides. 2007;41:65–72. doi: 10.1016/j.npep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Hansen ST, Kuhar MJ, Mark GP. Injection of CART (cocaine- and amphetamine-regulated transcript) peptide into the nucleus accumbens reduces cocaine self-administration in rats. Behav. Brain Res. 2008;191:266–271. doi: 10.1016/j.bbr.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DC, Kuhar MJ. Cocaine-amphetamine-regulated transcript expression in the rat nucleus accumbens is regulated by adenylyl cyclase and the cyclic adenosine 5′-monophosphate/protein kinase a second messenger system. J. Pharmacol. Exp. Ther. 2006;317:454–461. doi: 10.1124/jpet.105.096123. [DOI] [PubMed] [Google Scholar]
- Kim JH, Creekmore E, Vezina P. Microinjection of CART peptide 55−102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides. 2003;37:369–373. doi: 10.1016/j.npep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kim S, Yoon HS, Kim JH. CART peptide 55−102 microinjected into the nucleus accumbens inhibits the expression of behavioral sensitization by amphetamine. Regul. Pept. 2007;144:6–9. doi: 10.1016/j.regpep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55−102 induces locomotor activity and promotes conditioned place preference. J. Pharmacol. Exp. Ther. 2000;294(2):784–792. [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J. Neuroendocrinol. 1997;9(11):823–833. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Jaworski JN, Hubert GW, Philpot KB, Dominguez G. Cocaine- and amphetamine-regulated transcript peptides play a role in drug abuse and are potential therapeutic targets. AAPS J. 2005;7(1):E259–E265. doi: 10.1208/aapsj070125. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos A, Dominguez G, Kuhar MJ. CART promoter CRE site binds phosphorylated CREB. Brain Res. Mol. Brain Res. 2002;104:81–85. doi: 10.1016/s0169-328x(02)00321-2. [DOI] [PubMed] [Google Scholar]
- Liu JL, Papachristou DN, Patel YC. Glucocorticoids activate somatostatin gene transcription through co-operative interaction with the cyclic AMP signalling pathway. Biochem. J. 1994;301(Pt 3):863–869. doi: 10.1042/bj3010863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev., Mol. Cell. Biol. 2001;2(8):599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ. Prism 4 Statistics Guide–Statistical Analyses For Laboratory and Clinical Researchers. GraphPad Software Inc.; San Diego, CA: 2003. [Google Scholar]
- Nestler EJ. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol. Sci. 2004;25:210–218. doi: 10.1016/j.tips.2004.02.005. Review. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE, Malenka RC. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience. The McGraw-Hill Companies, Inc.; 2001. pp. 129–136. Chapter 6. [Google Scholar]
- Neve RL, Neve KA, Nestler EJ, Carlezon WA., Jr. Use of herpes virus amplicon vectors to study brain disorders. Biotechniques. 2005;39(3):381–391. doi: 10.2144/05393PS01. Review. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 2002. [Google Scholar]
- Philpot K, Smith Y. CART peptide and the mesolimbic dopamine system. Peptides. 2006;27(8):1987–1992. doi: 10.1016/j.peptides.2005.11.028. Review. [DOI] [PubMed] [Google Scholar]
- Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat. Rev., Neurosci. 2008;9(10):747–758. doi: 10.1038/nrn2493. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentic A, Lakatos A, Hunter R, Philpot K, Dominguez G, Kuhar MJ. CART peptide diurnal rhythm in brain and effect of fasting. Brain Res. 2005;1032:111–115. doi: 10.1016/j.brainres.2004.10.053. [DOI] [PubMed] [Google Scholar]
- Xu W, Cooper GM. Identification of a candidate c-mos repressor that restricts transcription of germ cell-specific genes. Mol. Cell. Biol. 1995;15:5369–5375. doi: 10.1128/mcb.15.10.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S, Schmidt RJ. Diversity and similarity among recognition sequences of Dof transcription factors. Plant J. 1999;17:209–214. doi: 10.1046/j.1365-313x.1999.00363.x. [DOI] [PubMed] [Google Scholar]