Abstract
Depression ranks as the primary emotional problem for which help is sought. Depressed people often have severe, complex problems, and rumination is a common feature. Depressed people often believe that their ruminations give them insight into their problems, but clinicians often view depressive rumination as pathological because it is difficult to disrupt and interferes with the ability to concentrate on other things. Abundant evidence indicates that depressive rumination involves the analysis of episode-related problems. Because analysis is time consuming and requires sustained processing, disruption would interfere with problem-solving. The analytical rumination (AR) hypothesis proposes that depression is an adaptation that evolved as a response to complex problems and whose function is to minimize disruption of rumination and sustain analysis of complex problems. It accomplishes this by giving episode-related problems priority access to limited processing resources, by reducing the desire to engage in distracting activities (anhedonia), and by producing psychomotor changes that reduce exposure to distracting stimuli. Because processing resources are limited, the inability to concentrate on other things is a tradeoff that must be made to sustain analysis of the triggering problem. The AR hypothesis is supported by evidence from many levels, including genes, neurotransmitters and their receptors, neurophysiology, neuroanatomy, neuroenergetics, pharmacology, cognition and behavior, and the efficacy of treatments. In addition, we address and provide explanations for puzzling findings in the cognitive and behavioral genetics literatures on depression. In the process, we challenge the belief that serotonin transmission is low in depression. Finally, we discuss implications of the hypothesis for understanding and treating depression.
Keywords: adaptation, analysis, anxiety, attentional control, counterfactual thinking, depression, disorder, evolution, rumination, serotonin, social dilemmas, ventrolateral prefrontal cortex, vigilance, working memory
Introduction
Depression is an affective state characterized by sad mood, anhedonia (the inability to derive pleasure from activities such as eating or sex), and changes in psychomotor, sleeping, and eating patterns (American Psychiatric Association [APA], 2000). Depression is commonly thought to be caused by severe problems or stressors, often of a social nature (Brown & Harris, 1978; Hammen, 1992; Kendler, Karkowski, & Prescott, 1999). Depressed people are often concerned and ruminate about these problems, which they perceive to be complex, severe, and difficult to solve (Edwards & Weary, 1993; Lyubomirsky, Tucker, Caldwell, & Berg, 1999; Rudolph & Conley, 2005; Treynor, Gonzalez, & Nolen-Hoeksema, 2003). Depressive rumination is intrusive, persistent, resistant to distraction, and difficult to suppress (Lam, Smith, Checkley, Rijsdijk, & Sham, 2003; Nolen-Hoeksema, 1990, 1991; Wenzlaff & Luxton, 2003).
The predominant medical view is that depression is a mental disorder (APA, 2000). But a recent critique argues that many episodes that meet DSM-IV-TR criteria for major depression are erroneously classified as disorder (Horwitz & Wakefield, 2007), and there have been calls for greater research into the possibility that depression is an evolved adaptation (Kennair, 2003; Nesse, 2000). Conceptions of mental disorder are usually defined in terms of biological dysfunction (APA, 2000; Kraepelin, 1907; Wakefield, 1992). All body systems are susceptible to malfunctioning, and on this level the idea that depression exists as a disorder has a good foundation. However, what is considered evidence of biological dysfunction has not been well specified. With the DSM-IV, the presence of “clinically significant impairment or distress” was added as a general criterion for the purpose of ensuring that a psychological condition was a disorder (APA, 2000).
The clinical significance criterion has been criticized on a number of grounds, and it probably fails in its primary purpose—to prevent erroneous diagnoses of disorder (Spitzer & Wakefield, 1999). According to one epidemiological survey, 46.4% of people in the United States have met DSM-IV-TR criteria for at least one mental disorder at some point in their lives, and 16.6% have met criteria for major depressive disorder (Kessler, Berglund, Demler, Jin, & Walters, 2005). Like this survey, most epidemiological estimates of lifetime risk for mental disorders are based on a single wave of data collection. However, people have poor recall of prior symptoms at a single point in time, so lifetime estimates increase when longitudinal information on psychiatric traits are collected from an epidemiological sample and aggregated across multiple waves (Wells & Horwood, 2004). One longitudinal study of adolescents living in Christchurch, New Zealand found that 37% met criteria for a lifetime DSM-III-R or DSM-IV episode of major depression by age 21 (Wells & Horwood, 2004). Another indication that even lifetime estimates may be conservative comes from a recent epidemiological survey in which over 45% of young adults in the United States met DSM-IV-TR criteria for at least one mental disorder within the last year, with 7% meeting criteria for major depression (Blanco et al., 2008).
In addition to high prevalence estimates in industrialized societies, the characteristic features of depression have been found in every society in which the issue has been examined closely (Horwitz & Wakefield, 2007; Patel, 2001). Information from small-scale societies is sorely lacking, but what exists indicates that depression is present there as well (Hadley & Patil, 2008; Kohrt et al., 2005; Patil & Hadley, 2008; Pike & Patil, 2006).
Such evidence suggests that much of what is currently classified as depressive disorder represents normal psychological functioning (Horwitz & Wakefield, 2007). One likely factor contributing to overdiagnosis is that clinically significant impairment is not conclusive evidence of disorder (Spitzer & Wakefield, 1999). Impairment can be caused by biological dysfunction, but it can also be caused by properly functioning stress response mechanisms. Organisms have limited energy, attention, and other resources that can be mobilized and allocated to different body systems to deal with adaptive challenges. Some stressors are important and severe enough to tax limited resources, and it is not possible to simultaneously devote resources to all problems. Organisms evolved stress response mechanisms that are triggered by particular stressors, prioritize fitness-related goals, make coordinated tradeoffs in the functioning of body systems, and allocate limited resources accordingly. Negative emotions are stress response mechanisms they are involuntary responses to environmental challenges with important fitness consequences, and they evolved to coordinate changes in physiology, immune function, attention and cognition, physical activity and other body systems to meet those challenges (Cosmides & Tooby, 2000; Ekman, 1999; Frijda, 1986; LeDoux, 1996; Levenson, 1999; Tooby & Cosmides, 1990).
Stress response mechanisms can produce impairments when making tradeoffs between different body systems to respond to a stressor. For instance, fever is metabolically expensive and causes significant impairment in multiple domains (work, sexual functioning, social relations, etc.), but these impairments are not usually the product of biological dysfunction. Rather, fever is an adaptation that evolved to coordinate aspects of the immune system in response to infection (Blatteis, 2003; Hasday, Fairchild, & Shanholtz, 2000; Kluger, Kozak, Conn, Leon, & Soszynski, 1996), and the impairments are the adaptive outcome of tradeoffs in body systems needed to produce an effective response (Nesse & Williams, 1994). If the clinical significance criterion were applied to fever, it would be erroneously classified as a disorder.
Like fever, depression causes distress and impairment in many domains of life, including sexual functioning, work, and social relations. One mechanism by which depression is thought to cause impairment is through the production of maladaptive cognitions that interfere with the ability to solve problems (Beck, 1967; Coyne, 1976a, 1976b; Kramer, 2005; Nettle, 2004; Nolen-Hoeksema, 1991; Seligman, 1975). This view is not universally held, however. Depressed people believe their ruminations give them insight into their problems (Lyubomirsky & Nolen-Hoeksema, 1993; Watkins & Baracaia, 2001; Watkins & Moulds, 2005). Clinicians also do not present a unified front on the question of whether depression has any beneficial cognitive effects. The issue is commonly debated in the therapeutic trenches, but it also reaches the clinical literature. Neil Jacobson argued that depression helps people detach from unrewarding social environments, but it also promotes avoidance of aspects of the social environment with antidepressant or problem-solving qualities (N. S. Jacobson, Martell, & Dimidjian, 2001). Emmy Gut argued that depression is a functional response to problems in the environment (Gut, 1989). It facilitates problem solving by drawing attention to and promoting the analysis of problems, but it can turn unproductive if people develop avoidant strategies. Peter Kramer acknowledges the lack of unity about depression, but eschews any benefits, cognitive or otherwise (Kramer, 2005).
Empirical research also differs with respect to whether depression improves or impairs problem-solving. One body of research shows that depression is associated with reduced accuracy on tasks that tap memory, intelligence, and executive functioning (Austin, Mitchell, & Goodwin, 2001; Hartlage, Alloy, Vazquez, & Dykman, 1993; Veiel, 1997). However, another large literature shows that depressed affect promotes an analytical processing style that enhances accuracy on complex tasks (Alloy & Abramson, 1979; Ambady & Gray, 2002; Au, Chan, Wang, & Vertinsky, 2003; Braverman, 2005; Forgas, 1998, 2007; Gasper & Clore, 2002; G. Hertel, Neuhof, Theuer, & Kerr, 2000; Sinclair, 1988; Sinclair & Mark, 1995; Storbeck & Clore, 2005).
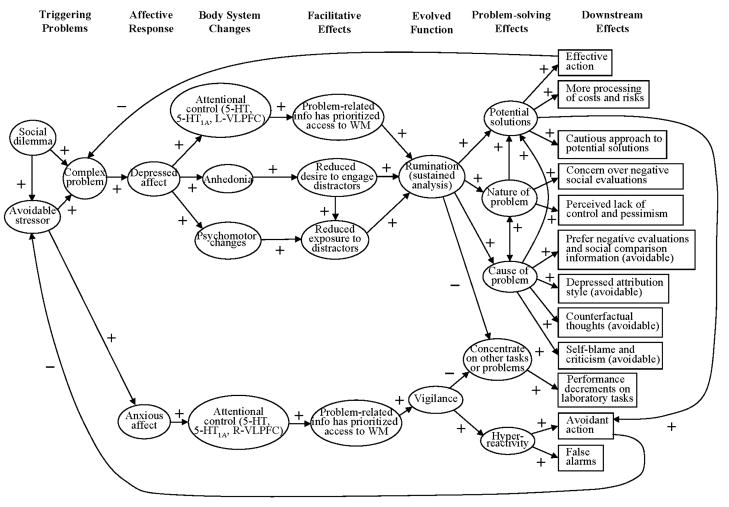
In this paper, we explain the impairments associated with depression, cognitive and otherwise, by hypothesizing that depression is an evolved stress response mechanism. The hypothesis consists of a series of claims, depicted diagrammatically in Figure 1, of which we now give an overview, and which are discussed in greater detail below.
Figure 1.
A diagram of the proposed causal relationships between the variables and constructs that are prominent in the AR hypothesis. The diagram uses the terminology of structural equation modeling where the circles represent latent constructs, the rectangles represent manifest variables, and the arrows denote the hypothesized direction of causation.
Different environmental stressors trigger different emotions, which coordinate body systems in different ways because those challenges require different responses (Cosmides & Tooby, 2000; Tooby & Cosmides, 1990). It is therefore important to specify the features of problems that trigger depressed affect. The first claim is that complex problems that influence important fitness-related goals trigger depressed affect. For the purposes of this paper, a “complex problem” is an analytically difficult problem. The American Heritage Dictionary (2006) defines analysis as “[t]he separation of an intellectual or material whole into its constituent parts for individual study” and “[t]he study of such constituent parts and their interrelationships in making up a whole”. An analytical problem is therefore one that can be solved by breaking it into smaller, more manageable components and studying each component in turn. The analytical difficulty of a task increases with the number of components that must be studied. In Figure 1, we list two analytically difficult problems that are thought to trigger depression social dilemmas and exposure to stressors that were otherwise avoidable.
When selection causes a trait to evolve, it does so because the trait has a gene-propagating effect, which is called the trait’s function (Andrews, Gangestad, & Matthews, 2002a; Thornhill, 1990; G. C. Williams, 1966). The cumulative effects of selection for genes that promote a specific effect often leaves the tell-tale signs of its workings on a trait, and such evidence can be used to make inferences about past selection pressures (Andrews et al., 2002a; Thornhill, 1990; G. C. Williams, 1966). When a trait has features that proficiently promote a specific effect, that will go a long way to demonstrating that the effect is an evolved function of the trait because it is highly unlikely that chance processes could be completely responsible for the trait’s features (Andrews et al., 2002a; Thornhill, 1990; G. C. Williams, 1966).
One effect of sad or depressed mood is to promote an analytical reasoning style in which greater attention is paid to detail and information is processed more slowly, methodically, thoroughly, and in smaller chunks (Ambady & Gray, 2002; Edwards & Weary, 1993; Forgas, 1998; Gasper, 2004; Gasper & Clore, 2002; Schwarz, 1990; Schwarz & Bless, 1991; Yost & Weary, 1996). Conversely, positive mood states promote heuristic or creative processing (Ambady & Gray, 2002; Isen, Daubman, & Nowicki, 1987). The second claim is that depression coordinates a suite of changes in body systems that promote rumination, the evolved function of which is to analyze the triggering problem. While analysis is used in science and many areas of modern life, this claim proposes that it is part of the evolved human cognitive repertoire.
Analysis is time consuming and requires sustained processing, so it is susceptible to disruption, which interferes with problem-solving. Depression induces changes in body systems, producing effects that facilitate analytical rumination by reducing disruption (listed as body system changes and facilitative effects in Figure 1). Specifically, depressed affect: (1) activates neurological mechanisms that promote attentional control, which gives problem-related information prioritized access to limited processing resources and makes depressive rumination intrusive, persistent, resistant to distraction, and difficult to suppress; (2) induces anhedonia, which reduces the desire to think about and engage in hedonic activities that could disrupt problem-related processing; and (3) promotes psychomotor changes that reduce exposure to stimuli that could disrupt processing (e.g., desire for social isolation, loss of appetite).
The third claim is that, over evolutionary time, depressive rumination often helped people solve the problems that triggered their episodes (listed as problem-solving effects in Figure 1). This claim is at odds with the commonly held view that depressive cognition is maladaptive and impairs problem-solving (Beck, 1967; Coyne, 1976a, 1976b; Kramer, 2005; Nettle, 2004; Nolen-Hoeksema, 1991; Seligman, 1975). However, the strength with which this view is held surpasses the evidence that can currently be mustered in support of it. Most importantly, the view is held without the support of a single study showing that depressed affect impairs solving the problems that actually trigger the depressed episode. Nearly all the evidence that depression impairs cognitive problem-solving comes from laboratory tasks. In contrast, this claim predicts that depression promotes resolution of the triggering problem.
Stress response mechanisms often have to make tradeoffs between fitness-related goals to produce an effective response to the triggering problem. Like fever, then, the impairments associated with depression are usually the outcome of adaptive tradeoffs rather than disorder. For instance, because processing resources are limited, a decreased ability to concentrate on other things is a necessary tradeoff that has to be made in order to sustain analysis of a complex, depressogenic problem (listed as a problem-solving effect in Figure 1). The fourth claim is that depression reduces accuracy on laboratory tasks because depressive rumination takes up limited processing resources (a downstream effect in Figure 1).
In summary, we hypothesize that depression is a stress response mechanism: (1) that is triggered by analytically difficult problems that influence important fitness-related goals; (2) that coordinates changes in body systems to promote sustained analysis of the triggering problem, otherwise known as depressive rumination; (3) that helps people generate and evaluate potential solutions to the triggering problem; and (4) that makes tradeoffs with other goals in order to promote analysis of the triggering problem, including reduced accuracy on laboratory tasks. Collectively, we refer to this suite of claims as the analytical rumination (AR) hypothesis.
In Figure 1, we list as downstream effects many other features of depression that have been reported in the literature. We argue that these features are interpretable in terms of the AR hypothesis as well. In the course of discussing the AR hypothesis, we also discuss anxiety because it is often comorbid with depression (Belzer & Schneier, 2004), and it shares genetic covariance and common triggers with depression (Kendler, Hettema, Butera, Gardner, & Prescott, 2003; Kendler & Prescott, 2006). In Figure 1, we flesh out some of the triggers and effects of anxiety to the extent it helps us discuss the AR hypothesis.
The AR hypothesis does not preclude other evolutionary accounts for depression, and several important hypotheses have been proposed (Allen & Badcock, 2003; P. Gilbert, 2006; Hagen, 1999, 2002; Nesse, 2000; Price, Sloman, Gardner, Gilbert, & Rohde, 1994; Watson & Andrews, 2002). In principle, it is possible for different selection pressures to shape a trait for multiple functions (Andrews et al., 2002a; Andrews, Gangestad, & Matthews, 2002b). Emotions may be good examples of such traits because they are thought to have evolved to coordinate the activity of multiple body systems to meet challenges in the environment (Frijda, 1986; Levenson, 1999; Tooby & Cosmides, 1990). Since new body systems are constructed from pre-existing designs, their coordination may have evolved in a stepwise fashion over evolutionary time as new systems were constructed. If so, emotions could be viewed as adaptations that gradually accumulated multiple coordinating functions over evolutionary time. Elucidating that a trait has multiple functions minimally requires demonstrating that the trait has some features unique to one function, and other features unique to another function (Andrews et al., 2002a).
We argue that depression has some unique features to it that are best explained by the AR hypothesis, and are difficult for other hypotheses to explain. In doing so, we integrate research on genes, neurotransmitters, receptors, neuroanatomy, neurophysiology, functional neuroimaging, pharmacology, behavior, cognition, comparative research, and the efficacy of therapies. We describe some of the likely neurological mechanisms involved in making depressive rumination analytical and resistant to distraction. This supports the point that depression evolved by natural selection because there is a neurological orderliness that appears to specifically and proficiently promote analysis in depressive rumination and is not likely to have evolved by chance. In the course of this review, we are led to examine the evidence for the widely held view that depression is a state of low brain serotonin. The evidence for this view is indirect, and we conclude that there are perhaps more compelling reasons to suspect the opposite i.e., that depression is a state of high serotonergic transmission. We also consider a puzzling finding in the behavioral genetics literature on depression and provide a possible resolution to it. Finally, we consider a number of paradoxical findings in the scientific literature on the cognitive effects of depression (Table 1). To our knowledge, this list has not been compiled before or targeted as worthy of scientific study. The AR hypothesis provides plausible or compelling explanations for each of them.
Table 1.
| Paradoxical Findings |
|---|
|
Crucial Concepts and Caveats
It is not uncommon to see arguments that depression might be adaptive at low levels, but is maladaptive at levels that reach DSM criteria (Dobson & Pusch, 1995; P. Gilbert & Allan, 1998; L. Lee, Harkness, Sabbagh, & Jacobson, 2005; Markman & Miller, 2006; Nettle, 2004; Price et al., 1994; Wolpert, 2008). These arguments implicitly assume that clinical and subclinical episodes are qualitatively different. Because we think that the clinical significance criterion leads to the overdiagnosis of depressive disorder, we intend our arguments to apply to a range of depressive symptoms, from transient sadness to much of what would currently satisfy DSM-IV-TR criteria for major depression. A good deal of evidence supporting the AR hypothesis comes from samples that satisfy DSM criteria, and there is little evidence that clinical depression is qualitatively different from subclinical depression, a point to which we now turn.
Is Major Depression Qualitatively Different from Subclinical Depression?
Our comments here are restricted to unipolar depression. For instance, we are not challenging the view that bipolar depression is qualitatively different from unipolar depression.
Epidemiological and quantitative studies explicitly testing the issue find that unipolar depressive symptoms are better characterized on a single continuum of severity, duration, and liability (Aggen, Neale, & Kendler, 2005; Korszun et al., 2004; Krueger & Markon, 2006). Depressive symptoms are continuously distributed through large populations (Hankin, Fraley, Lahey, & Waldman, 2005), and depressive rumination increases continuously with symptom severity (Just & Alloy, 1997; Lam et al., 2003; Nolen-Hoeksema & Morrow, 1991).
While it is sometimes argued that impairment distinguishes clinical and subclinical depression, psychosocial impairment increases monotonically with the number of depressive symptoms (Kessler, Zhao, Blazer, & Swartz, 1997; Sakashita, Slade, & Andrews, 2007) as does the future liability for major depression (Aggen et al., 2005). Even the performance decrements on cognitive tests increase with the severity of episodes (Elderkin-Thompson et al., 2003). Finally, while chronic stress-related reductions in hippocampal volume have been found in people with major depression, the relationship between stress and reduced hippocampal volume is continuous and is not limited to those who satisfy clinical criteria (Gianaros et al., 2007).
The symptoms of depression show some variability, and this variability has been used to argue for depressive subtypes. For instance, the signs of melancholia include anhedonia, psychomotor disturbance, weight loss, feelings of guilt, and early morning wakening (Akiskal & Akiskal, 2007). However, the melancholic subtype is not heritable, except as part of a general liability to depression (Maier, Lichtermann, Heun, & Hallmayer, 1992). Moreover, individuals with melancholia in one episode often show different patterns in other episodes (Akiskal & Akiskal, 2007). Altogether, such variability is consistent with evidence of modulation of symptoms in response to different triggering stressors (M. C. Keller, Neale, & Kendler, 2007; M. C. Keller & Nesse, 2006). It is not strong evidence that the symptoms are discontinuous.
It could be argued that depression is not continuous based on evidence that variability in specific genes, such as the serotonin transporter gene, are risk factors for depression (Murphy, Lerner, Rudnick, & Lesch, 2004). However, the problem lies in understanding how genes with discrete effects can code for traits with continuous phenotypic variability. The resolution is that the effects of such genes are usually small and do not account for all the genetic variability (Conner & Hartl, 2004). When the phenotypic effects are summed across all loci, their overall influence looks continuous (Conner & Hartl, 2004). Thus, evidence that individual genes have small discrete effects on the risk of depression is not inconsistent with the evidence of continuity.
In this paper, we also rely on studies of people who, instead of coming into the lab with pre-existing depression, are exposed to a sad experimental mood induction. The issue of whether experimentally induced sadness is qualitatively different from pre-existing depression is more complicated. There are many methods that are used to induce sad mood, but they all seem to increase depressed affect as assessed by validated instruments (Westermann, Spies, Stahl, & Hesse, 1996). They also tend to increase other forms of negative affect, such as anxiety and anger (Westermann et al., 1996). But because pre-existing depression is often naturally comorbid with anxiety and anger (Belzer & Schneier, 2004; P. Gilbert, Gilbert, & Irons, 2004), it is not clear that this makes experimentally induced sadness qualitatively different. Indeed, from a practical standpoint, it is impossible to study pre-existing depression or experimentally induced sadness without some degree of comorbidity with other negative emotions.
Functional neuroimaging studies tend to show a great deal of similarity in the brain activation patterns of people with experimentally induced sadness and major unipolar depression, but there are some differences (Drevets & Raichle, 1998; Liotti, Mayberg, McGinnis, Brannan, & Jerabek, 2002). Some differences may stem from the fact that experimentally induced sadness usually does not involve actual exposure to a stressor (Drevets & Raichle, 1998). Other differences may result from the fact that the neuroimaging signal becomes a less reliable indicator of neuronal activity as the intensity of depressive episodes increases (Andrews & Neale, n.d.; Conca et al., 2000; Dunn et al., 2005).
Finally, as we discuss in more detail below, the cognitive effects of experimentally induced sadness vary with the mood induction procedure. Some methods produce a state similar to pre-existing depression by virtue of the fact that they increase depressed affect as measured by validated instruments, elicit intrusive thoughts (ruminations), and affect performance on laboratory tasks in similar ways (Seibert & Ellis, 1991b). Other methods increase depressed affect, but probably do not elicit ruminative thoughts, and affect performance on laboratory tasks in different ways from pre-existing depression. We argue that the differences in the methods and their effects provide strong experimental evidence relevant to the AR hypothesis.
More generally, even if most episodes of DSM-IV-TR major depression were qualitatively different from subclinical forms in some ways, we show in this paper that the evidence that depression promotes the sustained analysis of problem-related information comes from research on clinical, subclinical, and experimentally induced samples (see Second, Third and Fourth Claims).
Terminology
Symptom
To some, “symptom” may imply an underlying disorder or disease. We do not mean to imply this and instead use the term merely to refer to a characteristic or feature of a trait.
Depressed affect or depression
Because depressive episodes that satisfy DSM-IV-TR criteria are often thought to be qualitatively different from subclinical episodes, terms like “dysphoria” are sometimes used to describe subclinical depression, while “depression” is reserved for episodes that reach clinical criteria. Because this categorical approach is unsupported, we avoid “dysphoria”. Instead, we use depressed affect and depression interchangeably to describe an affective continuum that ranges from transient sadness (including experimentally induced sadness) to severe, chronic depression. When referring to studies, we still use “major depression”, “clinical depression”, “subclinical depression”, and “sad” or “induced” mood to let the reader know whether the depressed state was pre-existing or induced and to provide the reader with information about the intensity of the affective state. For instance, “major depression” and “clinical depression” are used to describe pre-existing depressive states that satisfy DSM criteria for episodes of major depression. But we view the thresholds that separate clinical and subclinical depression as arbitrary. As such, to us, episodes of major depression fall on the severe end of the continuum. Future research may prove the assumption of continuity wrong or in need of modification. However, it currently enjoys substantial empirical support, and we find it useful for organizing this paper.
Cognitive resources
In a general sense, the AR hypothesis proposes that depression influences the allocation of cognitive resources to problems or tasks. We define “cognitive resources” as the neurological machinery that is involved in monitoring, processing or storing information (Reisberg, 2006). Abundant evidence indicates that cognitive resources are limited (Baddeley, 2007; Cowan, 2005; Kahneman, 1973; Marois & Ivanoff, 2005; Reisberg, 2006). This is commonly demonstrated by interference patterns when subjects are given dual tasks that utilize the same pool of cognitive resources (Kane & Engle, 2002; Marois & Ivanoff, 2005; Reisberg, 2006).
Attention
We occasionally use some terms to help us describe the allocation of limited cognitive resources. In general, we use “attention” to refer to cognitive resources that could be devoted to some task or problem. When used in association with attention, “focus” and its derivatives refer to an increase in the allocation of limited cognitive resources toward a task at the expense of other potential tasks to which the resources could be devoted; whereas “distract” and its derivatives refer to a diversion of cognitive resources away from one task by another task. Given two tasks, A and B, that utilize the same pool of cognitive resources, it is in principle equivalent to say that attention focuses on task A at the expense of task B, or that task A distracts attention from task B. From the viewpoint of the AR hypothesis, however, the most interesting task is the problem that triggered the depressive episode. Thus, we typically use focus and distract in reference to the allocation of resources towards, or away from, the triggering problem.
First Claim: Complex Problems Trigger Depressed Affect
The AR hypothesis proposes that depressed affect is triggered by problems: (1) that are complex (analytically difficult); and (2) that affected fitness in evolutionary environments. While we spend more effort on the first component as it requires greater explanation, this is not to minimize the importance of the second component. The first component implies the existence of mechanisms that register whether a problem is ‘complex’. The features of such mechanisms are largely outside of the scope of this paper, but one way a problem could be registered as ‘complex’ is if it resists simple attempts to solve it.
While this claim has not been directly tested, we know of no contradictory evidence, and it provides new interpretations of many findings in the depression literature.
Research on Treatments for Depression
The first line of research that we interpret with our hypothesis involves the efficacy of depression treatments. A fundamental principle in medicine is that it is more effective to treat the cause of an illness than to treat its symptoms (Nesse & Williams, 1994). For instance, treating fever with antipyretics does not treat the infection that caused the fever, and antipyretics actually impair recovery from infection (Hasday et al., 2000; Kluger et al., 1996; Plaisance, Kudaravalli, Wasserman, Levine, & Mackowiak, 2000). An antibiotic will be more effective for a bacterial infection. The principle may be reversed: the factors that make treatments effective can give insight into the cause of a condition. For instance, the cause of asexual reproduction in a species of wasp was attributed to a bacterial infection when it reverted to sexual reproduction upon administration of an antibiotic (Stouthamer, Luck, & Hamilton, 1990). We use the same kind of logic to get insight into the causes of depression.
Depression is commonly considered to be a neurochemical disorder, and it is often treated with antidepressant medications. Relative to placebo, the response to antidepressants often fails to reach clinical significance except for the most severely depressed patients (Kirsch et al., 2008). Moreover, controlled experiments have yielded no evidence that they prevent relapse once treatment stops (Hollon, Thase, & Markowitz, 2002), which is precisely what one would expect if medications were only treating symptom.
Unlike medications, some psychotherapies try to help people solve problems in their lives, and controlled experiments have shown that they work just as well as medications in the acute phase and have lasting post-treatment effects (Hollon et al., 2002). Cognitive behavioral therapy (CBT) is based on the cognitive triad hypothesis, which proposes that depression is caused by negative cognitions about the self, the future, and the world (Beck, Rush, Shaw, & Emery, 1979). These cognitions are thought to arise in response to negative events and lead to social withdrawal, which reinforces the cognitions. In CBT, intervention is possible at a number of points, including: (1) helping depressed people solve the problems that cause their cognitions; (2) helping depressed people stay engaged in their social environment so they can test the veracity of their cognitions (the behavioral activation [BA] component); and (3) directly helping them change the way they think about their situation (the automatic thoughts [AT] component) (Greenberger & Padesky, 1995). CBT has positive effects in the acute and post-treatment phases (Hollon et al., 2002).
Because CBT is heterogeneous, some components may not be therapeutic. Neil Jacobson and his colleagues randomly assigned depressed subjects to three conditions: (a) full CBT; (b) AT plus BA; and (c) BA only. They found no differences in outcome in the either the acute or post-treatment phases (E. T. Gortner, Gollan, Dobson, & Jacobson, 1998; N. S. Jacobson et al., 1996). They reasoned that BA was the primary therapeutic component because it was the only thing common to all conditions. Based on these results, Jacobson and his colleagues developed and tested an enhanced behavioral activation therapy (EBA) (N. S. Jacobson et al., 2001). The goal of EBA is to identify the punishing or non-rewarding aspects of the environment that the depressed person attempts to avoid and help the person find ways to make them more rewarding. In the acute phase, EBA worked better than CBT and just as well as antidepressants (Dimidjian et al., 2006), and just as well as CBT in the post-treatment phase (Dobson et al., 2008). Moreover, this study found that patients with severe, chronic depression did not respond well to CBT, whereas they responded better to EBA (Coffman, Martell, Gallop, Dimidjian, & Hollon, 2007).
Such evidence suggests that the attempt to change depressed people’s cognitions may not be the most fruitful therapeutic approach to treating depression. Indeed, a study found that the degree to which a CBT therapist focused on changing cognitions during treatment was associated with worse long-term outcomes, possibly because patients can perceive CBT as dismissive of their real troubles (Castonguay, Goldfried, Wiser, Raue, & Hayes, 1996).
Another effective psychotherapy is interpersonal therapy (IPT), and one of its primary goals is to assess the interpersonal problems that depressed people face and help them develop strategies and skills for solving them (Hollon et al., 2002). Like EBA, there is some evidence that IPT may work better than CBT, and IPT appears to work just as well as medications in the acute phase (Cuijpers, van Straten, Andersson, & van Oppen, 2008; Hollon et al., 2002).
In short, CBT, EBA and IPT are effective psychotherapies for depression, and a common feature is that they attempt to identify and help solve problems that depressed patients face. Unlike medications, they have enduring effects even after treatment has ended. Applying the principle that treating the cause of a psychological condition works better than treating a sign or symptom of it, this research suggests that depressive episodes are not usually caused by negative cognitions, but rather by problems that people have difficulty solving on their own.
What Depressed People Think About Their Situation
The second line of research that we interpret in light of the first claim involves the beliefs that depressed people have of their situation. Depressed people often report that they face severe, complex problems that are difficult to solve, they report less confidence in finding solutions to their problems, and they focus more on them (Lyubomirsky et al., 1999). Depressed people also tend to report having lost control over their lives (Edwards & Weary, 1998; J. A. Jacobson, Weary, & Edwards, 1999; Lyubomirsky et al., 1999). Perceived lack of control over negative events is often called hopelessness (Abramson, Alloy, & Metalsky, 1989), which is misleading because the term suggests a binary variable in which one either has some hope or no hope at all. In practice, it is treated as a semi-continuous variable that reflects a negative outlook for the self and the degree of perceived lack of control over it (Abramson et al., 1989; Beck, Weissman, Lester, & Trexler, 1974). The perception of having complex problems, and of having lost control over outcomes, also suggests that depression may be caused by complex problems.
Social Dilemmas
Complex social problems may be the primary evolutionarily relevant trigger of depression in human beings (Watson & Andrews, 2002). The fitness benefits of living in groups include, among other things, food sharing, assistance in raising children, protection from enemies, and close proximity to mates. But the accrual of such benefits requires a certain amount of cooperation among group members. At the same time, selection favors those who effectively pursue their self-interest, and group members also compete for limited resources, social status, and mates. Over evolutionary time, humans have faced social dilemmas in which fitness depended upon effectively pursuing self-interest, but without breaking the cooperative bonds that make group living possible (Andrews, 2001; Humphrey, 1976).
Social dilemmas have an analytical structure, in part, because there are multiple goals that must be satisfied (e.g., maintaining cooperative bonds, pursuing self-interest). In essence, the problem must be broken down and studied with respect to each goal. However, the analytical structure of social dilemmas is not defined simply by goal number. The goals merely provide the context that creates the dilemma. For every social dilemma, there may be many possible tactics that one may take, and each must be analyzed for their effects on goals. The number of tactics that must be evaluated increases the analytical complexity of the dilemma.
Another facet of a social dilemma that increases its analytical difficulty is that the goals tend to work against each other. For instance, the pursuit of self-interest can often be accomplished with the use of coercion or deception, yet such tactics tend to have the effect of eroding cooperative bonds (Humphrey, 1976). When goals work against each other, it becomes more difficult to find a solution that satisfies all the goals, and tradeoffs may have to be made. This forces the level of analysis to go a level deeper in which the specific costs and benefits of each possible option must be considered and weighed against each other to ascertain the best likely choice. Finding the best solution to a social dilemma becomes more analytically difficult when there are more tradeoffs because the number of elements that must be studied increases.
Human social groups are not simply composed of dyadic relations they are webs in which people are also indirectly connected to each other through others (Andrews, 2001; Watson & Andrews, 2002). Dilemmas that reach wider into a social web are more analytically difficult to solve because more players must be considered (Humphrey, 1976).
Finally, in human social groups, individuals interact repeatedly over their lifetimes (Humphrey, 1976; Trivers, 1971). A possible solution to a dilemma must not only be evaluated with respect to how it influences present outcomes, but it must also be evaluated for how one’s partner-opponents are likely to respond in future interactions (Humphrey, 1976). As in chess, the successful social player is often one who can see further into the future of possible moves and responses (Humphrey, 1976; Watson & Andrews, 2002). But this should also be an analytical task because it requires decomposing the problem into a decision tree, studying the possible moves and responses at each decision node, and considering their value not only to oneself, but also to one’s partner-opponents (Andrews, 2001; Watson & Andrews, 2002).
Social dilemmas have probably been recurrent features of the human evolutionary environment. Here are some examples. First, sexual infidelity may pose dilemmas for men and women (Buss, 2000). Consider a woman with dependent children who discovers her husband is having an affair with a younger woman. Is the wife’s best strategy to ignore it and tolerate the time and investment that the husband devotes to this other woman or confront him, force him to choose between her and the other woman, and risk abandonment? Conversely, consider a man who discovers that his wife is having an affair with a stronger, more dominant man. Is the best strategy to ignore the affair, and if his wife gets pregnant, help her raise offspring that may not be his? Or is it to confront the two and risk a potentially dangerous conflict with a more powerful man? In both scenarios, the answer is not obvious because there are benefits and risks associated with each option, and the best solution may depend on the specifics of the situation.
Second, for men, gaining dominance over other men yields benefits with respect to access to mates and the distribution of resources (Geary, Byrd-Craven, Hoard, Vigil, & Numtee, 2003). In humans, dominance is partly achieved by physical ability. But men also form cooperative coalitions, which are often successful in neutralizing the physical advantage of stronger males (Geary et al., 2003). Achieving within-group dominance in a cooperative coalition often requires great political skill, because indiscriminate violence and aggression can erode the support of group members. Conflict over dominance-striving is inevitable (Geary et al., 2003), but the dilemma is to find ways of negotiating it that do not erode coalitional bonds.
Finally, women also have several fitness-related goals that tend to work against each other. First, bearing and raising offspring is one of the most energetically expensive activities that a female mammal can undertake, primarily due to lactation (Millar, 1977). In hunter-gatherer groups, which are thought to exist in conditions similar to those of the human evolutionary past, reproductively aged women expend more energy than they produce through their own resource acquisition activities (Kaplan, Hill, Lancaster, & Hurtado, 2000). Women’s energy budgets are therefore subsidized, primarily by men (Kaplan et al., 2000; Marlowe, 2003). Second, unlike other female primates, women need assistance during childbirth (Rosenberg & Trevathan, 2002). Third, they need protection because they tend to be physically weaker, which makes them vulnerable to rape, capture by warring bands, and homicide by jealous lovers and husbands (Bleske-Rechek & Buss, 2001; Wilson & Mesnick, 1997). Finally, they need to minimize social stress because it appears to cause infertility and negative pregnancy outcomes (Berga & Loucks, 2006; Berga et al., 2003; Hobel & Culhane, 2003; Wasser & Place, 2001). In the human evolutionary past, the difficulty of achieving these goals was probably exacerbated by the fact that, unlike men, women tended to migrate away from their natal group when they reached reproductive age (Geary et al., 2003). Migration put young women in the dilemma of trying to elicit resources, assistance, and protection without causing social stress from people they were genetically unrelated to, and with whom they had few prior interactions, both of which increase competition and reduce cooperation (Hamilton, 1964a, 1964b; Trivers, 1971).
Is there any evidence that social dilemmas are depressogenic? Many of the problems that depressed people face are social in nature (Brown & Harris, 1978; Hammen, 1992; Kendler et al., 2003). Interpersonal conflict is commonly associated with depression (Hammen, 1992), but it is associated with higher levels of depression if it occurs with close social partners. For instance, people have higher levels of depressive symptoms if the conflict occurs in their most intimate circle of partners as opposed to less intimate circles (Antonucci, Akiyama, & Lansford, 1998). In married couples, the risk for major depression is about 40 times greater if the couple is unhappily married (Weissman, 1987). Moreover, conflict with close social partners is associated with more depression if the relationship is otherwise characterized by helpfulness and cooperation (Major, Zubek, Cooper, Cozzarelli, & Richards, 1997; Pagel, Erdly, & Becker, 1987). The fact that conflict within an otherwise cooperative relationship is associated with higher levels of depression strongly suggests that social dilemmas are depressogenic.
Defeat in a struggle for dominance is thought to be depressogenic to many organisms, including humans, primates, and rodents (P. Gilbert, 2006). The social defeat hypothesis proposes that depression is a response to being socially uncompetitive, and its function is to down-regulate behavior that could be perceived as a challenge to dominants and signal that the depressed individual is not a threat (P. Gilbert, 2006). We agree that social defeat is depressogenic and an ecologically and evolutionarily relevant stressor. But we are unconvinced that depression serves the proposed appeasement functions. While depression elicits negative responses from people whose relationship to the depressed person is distant (Segrin & Dillard, 1992), it appears to suppress aggression and elicit supportive and sympathetic responses from partners when the partner and the depressed person are in conflict and in a close relationship (e.g., mates, parent-child) (Sheeber, Hops, & Davis, 2001). Reduced aggression is consistent with the social defeat hypothesis, but the elicitation of support and sympathy is more consistent with the idea that depression is useful in negotiating a personally advantageous outcome when in conflict with close social partners (Hagen, 1999, 2002; Hagen & Thomson, 2004; Watson & Andrews, 2002). We suggest that social defeat is depressogenic because the lives of subordinate organisms are complicated and they have to rely more on their wits to pursue self-interested goals (R. Byrne & Whiten, 1988; de Waal, 2007). They have greater difficulty getting mates and resources, and they are more vulnerable to stress-related disease and aggression by dominants (Alcock, 2001; Sapolsky, 2004).
Preventing Recurrences of Avoidable Stressors
Social problems are often thought to be avoidable with foresight or planning (Hammen, 1992; Kendler et al., 1999). Many organisms have been under selection to prevent the recurrence of avoidable stressors. For instance, operant conditioning mechanisms allow organisms to learn, through repeated exposure to negative outcomes, to associate those outcomes with concurrent environmental stimuli, and use that information to behave in ways that help them avoid recurrences (Kandel, Schwartz, & Jessell, 1991). However, operant conditioning often requires multiple exposures to a negative outcome, and repeated exposure to a negative outcome can sometimes be inefficient, costly, or deadly. In such situations, selection may favor the evolution of more complex mechanisms that foster quicker learning or that help anticipate stressors so that preventative action may be taken before they occur.
With respect to human beings, one reason why avoidable stressors occur is because the person lacked an accurate causal understanding of the situation and how they could influence it (Roese & Olson, 1997; Weary, Marsh, Gleicher, & Edwards, 1993). This, in turn, suggests that the person could experience similar stressors again. Consequently, people who have been exposed to avoidable stressors may devote greater cognitive effort to understanding why the event occurred and how it could have been avoided (Roese & Olson, 1997; Weary et al., 1993). Such thoughts are called upward counterfactual thoughts (Roese & Olson, 1997). They are counterfactual thoughts because they focus on how the present situation could have turned out differently if different action had been taken. And they are upward counterfactual thoughts because they are focused on how the situation could have turned out better than it actually did.
Counterfactual thoughts are sometimes considered unproductive because it is impossible to alter the past (Roese & Olson, 1997). However, upward counterfactual thoughts appear to help people understand why the problem was not avoided and how similar problems could be avoided in the future (Roese & Olson, 1997). Consistent with this, several studies have found that counterfactual thinking may help prevent recurrences of avoidable stressors (Nasco & Marsh, 1999; Page & Colby, 2003; Roese, 1994).
Experiments show that avoidable stressors trigger negative affect, which in turn triggers upward counterfactuals about the avoidable stressor (Roese & Olson, 1997). This is also supported by correlational studies on depression. Exposure to an avoidable stressor appears to trigger depression (Hammen, 1992; Kendler et al., 1999), and depressed people tend to have more upward counterfactuals about recent avoidable stressors (Markman & Weary, 1996).
The generation of counterfactual thoughts involves analysis. When an avoidable stressor occurs, understanding why it occurred requires the individual to reverse the causal order of events to understand at what points he or she could have made different decisions that would have avoided the problem (Roese & Olson, 1997). This is an analytical task because it requires breaking up the causal chain of events into different decision nodes and studying the various decisions that could have been made and evaluating their likely consequences. Consistent with this, the longer the causal chain, the more difficult this becomes, and people have greater difficulty generating counterfactual thoughts (R. M. J. Byrne, 1998; German & Nichols, 2003).
To summarize, exposure to an avoidable stressor poses the problem of understanding why the stressor occurred so that similar events can be avoided in the future. Acquiring this understanding is a complex problem that requires counterfactual analysis. Consistent with our claim that depression is a response to complex problems, epidemiological evidence suggests that exposure to an avoidable stressor is more depressogenic than exposure to an unavoidable stressor (Hammen, 1992; Kendler et al., 1999), and experimental evidence indicates that it triggers higher levels of negative affect (Roese & Olson, 1997).
Future Directions
A direct test of the first claim would experimentally manipulate the analytical difficulty of a task and measure depressed affect during the task. The depression-triggering effects of a task are predicted to be higher during the task (as opposed to after its completion) because depression is hypothesized to be a process-oriented emotion. We recently conducted a preliminary test (Andrews et al., 2007). Subjects who came into the testing situation with low depression reported an increase in depressed affect after they had been given difficult practice questions from an analytically difficult test. Conversely, control subjects who did not take the practice questions did not report an increase in depressed affect. Moreover, subjects who reported higher levels of depressed affect after taking the practice questions then proceeded to perform better on the test. These results suggest that the practice questions elicited depressed affect because they were analytically difficult, and not because they made subjects fatigued or frustrated, or because they found the questions too difficult and gave up. (Depression was measured after the practice questions but before administration of the test to facilitate detecting process-oriented effects.)
The claim should be tested with social dilemmas as they are posited to be an evolutionarily relevant trigger of depressed affect. As noted above, real-life social dilemmas are positively associated with depression. Several experimental paradigms have been developed in which cooperation must be balanced with the pursuit of self-interest (Axelrod, 1984; Doebeli & Hauert, 2005; G. Hertel et al., 2000). The AR hypothesis predicts that experimentally manipulating the analytical difficulty of the dilemma by changing the degree to which tradeoffs must be made between cooperating and pursuing self-interest, the number of people to keep track of, and so on, will induce depressed affect.
Second Claim: Depression Coordinates Changes in Body Systems that Promote Sustained Analysis of the Triggering Problem
Intrusive thoughts are not unique to depression they also occur in other negative emotions (anxiety, fear, anger), post-traumatic stress, bipolar disorder, obsessive-compulsive disorder, etc. Negative emotions are thought to be responses to problems or threats in the environment that can be solved or alleviated with attention to the problem (Alexander, 1986; Thornhill & Thornhill, 1989), so they are all predicted to orient cognitive resources on the triggering problem. This distinguishes negative emotions from some psychological states such as bipolar disorder in which thoughts race in the mind without focusing on a single issue.
Adaptationist hypotheses for emotions propose that there is a concordance between the triggering problem and the effects promoted by the emotional response (Cosmides & Tooby, 2000; Tooby & Cosmides, 1990). Negative emotions are predicted to be distinguishable from each other in that they affect cognition in different ways because they have different triggers that require different responses. For instance, a severe drought is likely to require a different cognitive response from the theft of a valuable object or the dissolution of a cherished romantic relationship. The AR hypothesis predicts that depression coordinates changes in body systems to promote an analytical problem-solving approach that is concordant with the analytical difficult problems that trigger it. Consistent with this, studies of pre-existing and experimentally induced mood have consistently found that depressed affect promotes an analytical processing style in which information is processed more carefully, thoroughly, methodically, and in smaller chunks (Ambady & Gray, 2002; Edwards & Weary, 1993; Gasper, 2004; Gasper & Clore, 2002; Schwarz, 1990; Schwarz & Bless, 1991; Yost & Weary, 1996). Depressive rumination is therefore predicted to be uniquely different from other forms of intrusive thinking in that it involves analysis. To the extent analytical rumination is associated with other psychological states, such as post-traumatic stress (Martin & Tesser, 1996; Tedeschi & Calhoun, 2004), such states should be comorbid with depression and the event that triggered the comorbid state should pose complex problems.
Two rumination factors have been identified in depression (Treynor et al., 2003), both of which involve analysis. The first factor is focused on analyzing the problems that depressed people currently face (Treynor et al., 2003). This rumination style is often called pondering or reflection (Treynor et al., 2003), but we refer to it as problem analysis to emphasize its analytical nature. Depressed people often perceive the problems that they face as severe and complex (Lyubomirsky et al., 1999), and they attempt to analyze them (Lyubomirsky et al., 1999; Treynor et al., 2003).
The second factor is focused on regretful thoughts about the episode, especially understanding why the episode happened and what could have been done to prevent it (Markman & Weary, 1996; Treynor et al., 2003; Watkins & Mason, 2002). As discussed above, such counterfactual thoughts are generated through analysis. We therefore refer to this rumination style as counterfactual analysis, though it is often called brooding (Treynor et al., 2003).
The AR hypothesis predicts that the key factor for promoting sustained analysis is to minimize the disruption of rumination (see Figure 1). In this section, we first explain why analysis is vulnerable to disruption, and then we turn to discussing how depression coordinates body system changes to minimize the disruption of analytical rumination.
Analysis and Working Memory
Working memory (WM) is a memory system that maintains problem-relevant information in an active, accessible state because it is used in ongoing mental work (Baddeley, 2007). Analysis requires the use of WM because: (1) complex problems are broken down into smaller components; (2) components are studied sequentially; and (3) the results must be kept in an active state to study other components or to solve the larger problem.
Maintaining information in an active, accessible state requires cognitive resources (Kane, 2005; Kane & Engle, 2002). For this reason, WM tasks are vulnerable to interruption, which can interfere with effective problem-solving. Organisms are constantly bombarded with information from the environment. Salient, but task-irrelevant, stimuli can displace information from WM and draw cognitive resources away from the task. Because the interruption of processing may have negative consequences to the organism, WM is thought to be functionally linked to mechanisms that allocate cognitive resources to maintaining information in WM to sustain task-relevant processing under conditions where processing could be interrupted (Baddeley, 2007; Gray, Chabris, & Braver, 2003; Kane, 2005; Kane & Engle, 2002). We refer to such mechanisms as attentional control mechanisms, where “attentional control” refers to the maintenance of information in WM under disruptive conditions by giving it prioritized access to WM.
Working memory load refers to the amount of information that must be held in working memory. As WM load increases, more cognitive resources must be devoted to keeping information in WM, there is less of a margin for cognitive resources to be diverted from the task before performance suffers, and distracting stimuli are more likely to interfere with task performance (Carpenter, Just, & Shell, 1990; Gray et al., 2003; Kane & Engle, 2002). Thus, WM tasks become more vulnerable to interruption, and require greater attentional control, when WM load is high (Gray et al., 2003; Kane & Engle, 2002).
An example of an analytical task in which WM load varies is Raven’s Advanced Progressive Matrices (RAPM) (Raven, Court, & Raven, 1994). The RAPM is considered one of the best measures of nonverbal analytical reasoning ability and fluid intelligence (Carroll, 1993). Each item is a nonverbal pattern completion task in which one of eight choices correctly completes a two-dimensional visual array, and test items become progressively more difficult. One reason that difficulty increases is that the number of elements in the array increases and the rules for how they vary across the two-dimensional array can be different for each element (Carpenter et al., 1990). The rule for each element must be solved independently, so once subjects figure out the rule for how one element varies across the array, they must keep the solution in their WM while they figure out the rules for the remaining elements. Simply put, the problems are solved by analysis. The number of elements that must be analyzed and held in WM varies from 1 to 5, and the proportion of people getting a test item correct is negatively related to the number of elements that must be analyzed (Carpenter et al., 1990).
A recent study used a combination of cognitive and neurobiological evidence to provide evidence that analysis of RAPM problems is related to attentional control (Gray et al., 2003). The researchers gave participants a modified 3-back-matching task, in which individuals are sequentially presented with stimuli such as words or faces. Participants must indicate as quickly as possible whether each stimulus matched the stimulus three items back. In the sequence A-B-C-A-D, when presented with the fourth item, the correct response is a match because it matches the first item (A). But when presented with the fifth item (D), the correct response is a non-match because it does not match the second item (B). This task requires greater attentional control when false lures are presented. For instance, in the sequence A-B-C-D-E-D, the sixth item (D) requires a non-match because the third item back is a C. But the D is a false lure because it was also presented two items back. In this version of the 3-back-matching task, the presence of lures makes it more difficult to remember and update in WM the position of prior items relative to the current stimulus, and people tend to make more errors (Gray et al., 2003).
The researchers first gave participants the RAPM and then measured their brain activity using fMRI while they completed two versions of the 3-back test one with lures and one without lures. Performance on the RAPM was more related to the percentage of correct answers that they made on the 3-back-with-lures than the 3-back-without-lures. This suggests that people who perform better on the RAPM (i.e., those who can hold more information in WM) also are better at resisting distracting stimuli. Indeed, many studies have shown that people with larger WM spans have greater attentional control (Kane, 2005; Kane & Engle, 2002). Moreover, the covariance between performance on the RAPM and the three-back-with lures test was largely explained by differential activity in a few brain regions implicated in attentional control. For the purposes of this paper, the most important region of activation was the left ventrolateral prefrontal cortex (VLPFC), also called the inferior frontal cortex, which encompasses Brodmann’s areas 44, 45, and 47. We now discuss the VLPFC and its function.
The Role of the Left VLPFC in Attentional Control
Functional neuroimaging studies show that activation of the left VLPFC increases with WM load (Braver et al., 1997; Glahn et al., 2002; Love, Haist, Nicol, & Swinney, 2006; Wolf, Vasic, & Walter, 2006), which strongly suggests that it is involved in attentional control (D'Esposito, Postle, & Rypma, 2000; Jonides & Nee, 2006). VLPFC neurons appear to promote attentional control by continuing to fire through periods of distraction and delay (D'Esposito et al., 2000; Dolcos, Miller, Kragel, Jha, & McCarthy, 2007; Jonides & Nee, 2006). In delayed memory tasks, the subject is to recall a stimulus after a delay. In such tasks, delay-sensitive cells in the VLPFC begin firing when the target stimulus is to be encoded and continue firing throughout the delay period (Funahashi, Inoue, & Kubota, 1997; Rao, Rainer, & Miller, 1997). Unlike delay-sensitive cells in other cortical areas, cells in the lateral PFC, especially the VLPFC, are resistant to distraction (Dolcos et al., 2007; Miller, Erickson, & Desimone, 1996; Miller, Li, & Desimone, 1993; Postle, 2006; Yoon, Curtis, & D'Esposito, 2006).
With respect to the study by Gray et al. (2003), the fact that differential activation of the left VLPFC largely mediated the correlation between performance on the 3-back-with-lures and the RAPM suggests that the region promotes the analysis of complex tasks by increasing attentional control and decreasing the risk of disruption.
The Role of Serotonin in Attentional Control
Serotonin (5-hydroxytryptamine or 5-HT) appears to influence attentional control, which should make it an important regulator of analytical reasoning. The dorsal raphe nucleus (DRN), located in the midbrain, is the source of serotonergic neurons that project to cortical and limbic areas (Amat et al., 2005; Barnes & Sharp, 1999). While there are 15 identified serotonin receptors (Albert & Lemonde, 2004; Barnes & Sharp, 1999), of primary importance to this paper are the 5-HT1A receptors. 5-HT1A receptors are found in two locations: (1) autoreceptors are found presynaptically on the soma of serotonergic neurons in the DRN that project to cortical areas; and (2) heteroreceptors are found postsynaptically on non-serotonergic neurons (Barnes & Sharp, 1999; Kia et al., 1996; Verge et al., 1986). 5-HT1A autoreceptors are inhibitory, so their activation inhibits the firing of DRN neurons (Sharp, Boothman, Raley, & Queree, 2007). The 5-HT1A heteroreceptor is present in the human VLPFC (Varnas, Halldin, & Hall, 2004).
Evidence that 5-HT influences attentional control comes from a recent study involving the 5-HT1A receptor (Carter et al., 2005). Oral administration of psilocybin, a 5-HT1A activator (agonist), significantly impaired performance on human subjects’ ability to track an object in the presence of distractors, probably by activating the 5-HT1A autoreceptor, thereby inhibiting the DRN (Carter et al., 2005). Moreover, the effect was load dependent. The impairments increased with the number of objects that had to be tracked and kept in WM, which parallels the findings implicating the left VLPFC as WM load increases.
Such evidence suggests that attentional control is enhanced by activation of the DRN and increased transmission of 5-HT to the left VLPFC. This interpretation is supported by experimental research on how rats’ performance on demanding WM tasks is affected by lesion of the DRN (Harrison, Everitt, & Robbins, 1997), manipulation of 5-HT1A autoreceptors (Carli & Samanin, 2000), and manipulation of post-synaptic 5-HT1A heteroreceptors in the ventral region of the medial prefrontal cortex (Winstanley et al., 2003). The ventral region of the medial prefrontal cortex (mPFCv) is a likely rodent homologue to the human VLPFC (Kesner, 2000).
Summary
Analytically complex tasks are vulnerable to disruption, at least in part, because they have high WM loads. Maintaining such information in WM requires greater attentional control, which is regulated by the left VLPFC, serotonin, and the 5-HT1A receptor.
How Depression Promotes Rumination (Sustained Analysis)
We have argued that an important requirement for sustaining analysis is to minimize disruption of information in WM. We now argue that depression produces three types of changes in body systems that promote sustained analysis by reducing the risk of disruption (Figure 1).
Depression Enhances Attentional Control
First, depression activates the left VLPFC (Figure 1), which enhances attentional control and keeps information in WM when performing analytically difficult tasks. Neuroimaging studies of humans have consistently found that, relative to controls, people with experimentally induced depression or outpatient samples with episodes of major depression usually show a high neuroimaging signal in the VLPFC, with a tendency towards left lateralized activation (Drevets, 1999, 2000; George et al., 1995; Pardo, Pardo, & Raichle, 1993). As discussed above, the left VLPFC is activated during processing that requires high WM loads, such as analysis. Left VLPFC activity should therefore be crucial in maintaining the attentional control necessary to sustain analytical rumination in depression.
Sustained Neuronal Firing and Neuronal Apoptosis
As discussed above, attentional control is achieved by sustained neuronal activity in the VLPFC during periods of distraction or delay (D'Esposito et al., 2000; Dolcos et al., 2007; Jonides & Nee, 2006). However, the sustained activation of VLPFC neurons in depression is costly. Roughly 80% of cortical neurons release glutamate, an important neurotransmitter (Somogyi, Tamas, Lujan, & Buhl, 1998). High levels of glutamate in the synapse are toxic and can induce apoptosis (programmed cell death) (Hara & Snyder, 2007). Thus, while sustained neuronal activity in the left VLPFC is crucial to prevent disruption of analytical rumination, it carries a risk of apoptosis. Here, we argue that depression also coordinates processes that reduce apoptosis.
To reduce this risk, glutamate must be quickly cleared out of the synapse (A. L. Lee, Ogle, & Sapolsky, 2002). This function is accomplished by nearby astrocytes an abundant type of glial cell with processes that appose capillaries, neurons, and their synapses (Magistretti & Ransom, 2002). Astrocytes are the only brain cells where glycogen, the stored form of glucose, can be found (Magistretti & Ransom, 2002). Astrocytes take up glutamate from the synapse and convert it to glutamine in an energy dependent process (Magistretti & Ransom, 2002).
Under resting activity, neurons derive most of their energy from the metabolism of blood-borne glucose (Nehlig & Coles, 2007). However, as neuronal activity becomes more intense and sustained, energy must be quickly mobilized to sustain firing and to clear synaptic glutamate and convert it to glutamine (Pellerin et al., 2007; Shulman, Hyder, & Rothman, 2001a, 2001b). Under these conditions, there is an increasing reliance on the metabolism of astrocytic glycogen (Pellerin et al., 2007; Shulman et al., 2001b), the predominant product of which is lactate, the preferred energy substrate for neurons (Pellerin et al., 2007).
Thus, sustained activation of glutamatergic neurons in the VLPFC in depression is likely to be supported by the production of astrocytic lactate. We know of no pertinent studies in humans that directly show astrocytic lactate production in depression. However, support comes from studies of behavioral depression in rats, which is often induced by repeated exposure to uncontrollable stress (Vollmayr & Henn, 2003). Uncontrollable stress triggers sustained release of 5-HT to the mPFCv and sustained firing of mPFCv neurons (Amat et al., 2005). As the mPFCv is a likely homologue to the human VLPFC (Kesner, 2000), this may be analogous to sustained VLPFC activity in human depression. Moreover, repeated exposure to uncontrollable stress also causes astrocytic lactate production to increase in the mPFCv via the 5-HT1A autoreceptor (Uehara, Sumiyoshi, Matsuoka, Itoh, & Kurachi, 2006).
The Role of 5-HT in Depression
Sustained 5-HT transmission in depression may reduce apoptosis caused by sustained VLPFC activity by increasing lactate production. But it conflicts with conventional wisdom that human depression is characterized by low brain 5-HT (Maes & Meltzer, 1999). Depression is widely thought to involve altered interactions between 5-HT and its receptors, with the 5-HT1A receptor playing an important role (Sharp et al., 2007). Mutant mice lacking the 5-HT1A receptor show an ‘antidepressant’ (non-depressed) behavioral profile (Heisler et al., 1998; Mayorga et al., 2001; Ramboz et al., 1998), which strongly suggests it plays a role in depression. Moreover, 5-HT1A agonists have antidepressant properties in animal models (Albert & Lemonde, 2004). The 5-HT1A receptor is also implicated by evidence that it shows enhanced binding properties in the VLPFC of clinically depressed people (Parsey et al., 2006).
However, the evidence for the low brain serotonin hypothesis is largely based on two circumstantial pieces of evidence (Albert & Lemonde, 2004). First, selective serotonin reuptake inhibitors (SSRIs) tend to be effective in alleviating depressive symptoms (Maes & Meltzer, 1999). When 5-HT is released into the synapse, it is taken back into the presynaptic neuron by the serotonin transporter molecule (Albert & Lemonde, 2004). Thus, the serotonin transporter tends to decrease extracellular levels of serotonin. SSRIs bind to the serotonin transporter, which inhibits the reuptake of serotonin, thereby increasing extracellular levels and increasing the binding to 5-HT receptors (Albert & Lemonde, 2004). Thus, depression is thought to be characterized by low levels of 5-HT because SSRIs have antidepressant properties and tend to increase synaptic levels of serotonin. Second, giving subjects diets that deplete levels of tryptophan, an amino acid needed to synthesize serotonin, tends to cause an increase in depressive symptoms, at least in subjects with recently remitted depression (Moore et al., 2000).
However, several lines of evidence suggest that 5-HT is high in depression. First, as noted above, experimental research on rats has found that behavioral depression (induced by repeated exposure to uncontrollable stress) causes sustained DRN activity and 5-HT transmission (Amat et al., 2005). Despite some debate about their validity, rodent models of behavioral depression have been extremely useful in screening for antidepressant medications, identifying the phenotypic effects of genes, and testing neurobiological hypotheses about depression and other psychiatric conditions (Kalueff, Wheaton, & Murphy, 2007). It would be really surprising if the role of 5-HT in depression was functionally opposite in rodents and humans.
Second, SSRIs have multiple effects and it has been difficult to establish the precise mechanism by which they achieve their antidepressant effects (Hjorth et al., 2000). For instance, SSRIs also tend to activate 5-HT1A autoreceptors, which tends to inhibit DRN activity (Hjorth et al., 2000). Thus, the serotonin transporter binding and 5-HT1A autoreceptor activation properties of SSRIs tend to have opposing effects on synaptic levels of serotonin, which may partly explain why they usually take several weeks before they reduce symptoms.
Third, there is variation in the serotonin transporter gene, with most people having either short (s) or long (l) alleles (Murphy et al., 2004). The variation has transcriptional and functional consequences, with the s-allele resulting in lower densities of transporter mRNA and protein, and slower clearance of 5-HT from the synaptic cleft (Murphy et al., 2004). By slowing the clearance of 5-HT, the s-allele mimics the serotonin transporter-binding effects of SSRIs. However, individuals with the s-allele are at greater risk of depression and anxiety, not less (Canli & Lesch, 2007), suggesting that depression is associated with high levels of synaptic 5-HT.
Fourth, a recent study of jugular blood flow in subjects with major depression found higher overflow of 5-hydroxyindoleacetic acid (5-HIAA) relative to non-depressed controls (Barton et al., 2008). 5-HIAA is the principal neuronal metabolite of 5-HT. Higher 5-HIAA overflow could reflect a clearing of 5-HT reserves from the brain, consistent with the low serotonin hypothesis. But this would not be a stable equilibrium (Barton et al., 2008). Clearance without a concomitant increase in synthesis and transmission would quickly deplete 5-HT and lead to an equilibrium in which 5-HIAA was lower in depressed subjects than in non-depressed controls. The prolonged nature of major depression argues against this interpretation. Rather, a more natural interpretation is that neuronal transmission of 5-HT is higher in depression (Barton et al., 2008). Moreover, the researchers found that 5-HIAA overflow was higher in subjects with the s-allele of the serotonin transporter gene. Since the s-allele tends to increase synaptic levels of serotonin, and is associated with a greater risk of depression, this further suggests that depression is associated with high levels of serotonin. Finally, 5-HIAA overflow was lower after treatment with an SSRI, consistent with studies reporting that a reduction in symptoms following SSRI treatment was associated with a reduction in 5-HIAA in the cerebral spinal fluid (Nikisch et al., 2004; Sheline, Bardgett, & Csernansky, 1997). This suggests that 5-HT production decreases with SSRI treatment (Barton et al., 2008).
Fifth, post-mortem studies have found that DRN cell number is higher, and 5-HT1A autoreceptor density is lower, in depressed subjects relative to non-depressed controls (Rajkowska, 2000). This suggests a greater capacity to transmit 5-HT in depression and less inhibition of serotonergic transmission.
Finally, a PET neuroimaging study of brain activity following tryptophan depletion found that DRN activity was increased, not decreased (Morris, Smith, Cowen, Friston, & Dolan, 1999). This surprising finding directly contradicts the hypothesis that tryptophan depletion induces depressive symptoms by decreasing serotonin. We predict that lowering blood levels of 5-HT through tryptophan depletion deactivates the 5-HT1A autoreceptor, reduces the inhibition of the DRN, and increases serotonergic transmission to cortical areas.
Summary
The distraction-resistant analysis of problem-related information that occurs in depressive rumination requires sustained left VLPFC activity. Based in part on evidence that behavioral depression causes sustained 5-HT release to the rodent homologue of the VLPFC and maintains neuronal activity there, we predict that brain 5-HT is high in human depression, not low. The sustained release of 5-HT to the VLPFC should promote the production of astrocytic lactate, sustain neuronal firing, and reduce apoptosis by supporting the clearance of synaptic glutamate.
Anhedonia
Depression also reduces disruption of rumination by inducing anhedonia (APA, 2000). The problems that depressed people face are often important, complex and difficult to solve, and sustained processing may not offer immediate reward. However, attending to activities that do offer more of an immediate reward (e.g., eating, sex, companionship) would tend to interfere with attempts to problem-solve. Anhedonia should promote uninterrupted rumination by reducing the motivation to engage in hedonic activities (Figure 1). It should also have the effect of reducing exposure to such activities because they will not be sought out (Figure 1).
If anhedonia reduces the salience of hedonic activities, then depressed people with higher levels of anhedonia should experience more focused and uninterrupted rumination. We know of no research testing this prediction. However, indirect support comes from research showing that those who score high in anhedonia tend to show a reduction in the amplitude of the P300 event-related potential (ERP) signals, which is often interpreted as evidence of a highly focused attentional state (Dubal, Pierson, & Jouvent, 2000; Yee & Miller, 1994). Such people may be ruminating about important problems in their lives that require sustained processing.
Psychomotor Changes
Depression may also decrease disruption of analysis through psychomotor changes, including a preference for solitude, fatigue, changes in appetite, and changes in sleep or activity patterns. Some of these symptoms can be easily seen to reduce exposure to distracting stimuli. For instance, social situations often demand attention, and this would tend to interfere with the depressive’s processing of episode-related problems. A preference for solitude would help the depressive avoid social interactions that could interrupt such processing.
Other activities may similarly interfere with sustained processing. Sleeping is not conducive to analytical processing, although it may allow divergent thinking or unconscious processing. Consistent with this, depressed people who ruminate more tend to sleep less (Guastella & Moulds, 2007). Similarly, neurobiological evidence indicates that oral or buccal activity interferes with the processing of stimuli (Jacobs & Fornal, 1999). The reduced appetite often seen in depression may sustain processing by reducing oral and buccal activity.
Finally, motor activity often requires cognitive resources to be devoted to how the body interacts with the environment. Psychomotor retardation might facilitate rumination by reducing the need to devote attention to the physical navigation of the environment and by keeping the depressed person in environments that are conducive to uninterrupted rumination. This predicts that symptoms of psychomotor retardation will be positively associated with more intense rumination. Indirect support for this comes from research showing that psychomotor retardation is positively associated with difficulty in attending to cognitive tasks in the laboratory (Lemelin & Baruch, 1998). As we discuss below, depressed people often have difficulty on laboratory tasks because their persistent ruminations about their personal life problems prevent them from devoting cognitive resources to laboratory tasks.
Future Directions
The AR hypothesis proposes that sustained left VLPFC activity is responsible for making depressive rumination intrusive, persistent, resistant to distraction, and difficult to suppress. The intensity of left VLPFC activity is therefore predicted to correlate positively with the intensity of depressive rumination. The DRN is predicted to be activated in depression and glycogenolysis is predicted to occur in the left VLPFC, especially during rumination, as this is hypothesized to reduce the risk of apoptosis that comes from sustained cortical activity.
Surprisingly, we know of no research testing whether anhedonia and rumination are linked. The AR hypothesis predicts that they will be positively correlated in natural samples. Anhedonia is also predicted to be triggered by analytically difficult problems in a dose-dependent fashion, especially problems that are evolutionarily relevant.
Another issue arises from the fact that effective therapies often appear to promote activity that is opposite from the psychomotor symptoms that depressed people present. For instance, depressed people are often lethargic (DSM-IV-TR), but behavioral activation is therapeutic (Dimidjian et al., 2006; N. S. Jacobson et al., 1996). Similarly, depressed people often socially isolate (DSM-IV-TR), but talking therapies are helpful (Hollon et al., 2002). It could be argued that the fact that such interventions work suggests that it is counter-productive to socially isolate and be physically inactive when depressed.
We suggest that this is a puzzle only when one describes therapies and symptoms in imprecise terms. On the therapy side, the AR hypothesis predicts that is not physical or social activity per se that is therapeutic (although they may be palliatives that temporarily alleviate symptoms). Rather, physical and social activity are predicted to be therapeutic to the extent they help the depressed person solve the complex problem that caused the episode. For instance, behavioral activation is not simply about getting people up and moving about. It is based on behaviorism modification of behavior through punishment and reward, which makes it useful in problem-solving (N. S. Jacobson et al., 2001). Similarly, the efficacious interactive talking therapies we have reviewed CBT, EBA, and IPT have problem-solving components to them.
On the symptom side, the psychomotor symptoms that depressed people present are variable and may be more context-dependent and related to problem-solving than is typically appreciated. Depressed people can show lethargic or agitated activity patterns; they may sleep either more or less than normal; and they may have increased or decreased appetite (APA, 2000). The AR hypothesis proposes that some of the variability is a response to circumstances of the depressed person. Depressed people are predicted to exhibit psychomotor symptoms that make them stay put (e.g., lethargy) when in environments conducive to high quality, uninterrupted rumination. When in environments that are not conducive to rumination they are predicted to show symptoms that motivate them to search out better environments (e.g., agitation).
The preference for social isolation is also variable. In general population and depressed samples, most people prefer psychotherapies to antidepressant medications as treatments for depression, because they believe they will yield insight and help them solve their problems (Prins, Verhaak, Bensing, & van der Meer, 2008; van Schaik et al., 2004). Thus, depressed people don’t prefer social isolation in all contexts. In general, the AR hypothesis predicts that depressed people seek interaction with those that they perceive as trustworthy and willing and competent to give good advice. Because social dilemmas are likely to be causes of many depressive episodes, depressed people may be more likely to avoid those to whom they are closest, because these people are likely to be part of the problem. People who are depressed about social dilemmas with close social partners are predicted to be more likely to seek help from a therapist.
Third Claim: Depressive Rumination Often Helps People Solve the Triggering Problem
This claim has not been thoroughly tested. However, there is one experiment directly on point, and other lines of evidence are interpretable in light of the claim.
Experimental Evidence
A study that attempts to tackle this issue experimentally must have a particular design. The laboratory task on which performance is to be evaluated must also be the problem that triggers depressed affect. We know of only one pertinent mood induction experiment. It explored the effects of mood in a simulated market where participants could buy and sell Deutsche Marks and Swiss Francs (Au et al., 2003). All participants were finance or economics students with knowledge of, or experience with, simulated or real financial trading. To help make decisions, participants were provided on each round with historically accurate charts about the daily closing prices in the last 3 years, and news items from the Dow Jones News Archive, which described influential market factors, how the market was moving, and comments from leading practitioners and economists from prominent investment banks. Careful analysis of this information would allow participants to make good predictions about the relative movement of Deutsche Marks and Swiss Francs. Performance was assessed by whether participants decided to buy or sell the correct currency on that round (accuracy), and how much money they gained or lost (profit), which in turn depended on accuracy and the amount invested.
Mood was manipulated by providing subjects with false feedback on the first round. In the positive mood induction, subjects received a high profit for their decision, regardless of what they actually did. In the sad mood induction, subjects took a substantial loss. In the neutral mood induction, subjects broke even. For all subsequent rounds, subjects’ payoffs were determined by their actual decisions and mood was maintained with positive, sad, or no music. People in positive moods made worse decisions by both standards: they were less accurate, and they lost more because they invested more. Sad participants made the most accurate decisions, but they tended to invest conservatively. Neutral participants were not as accurate as sad participants, but they received a higher profit because they invested more.
This experiment is very much in line with a model of depression’s causes and cognitive effects suggested by Weary et al. (1993). Subjects experienced an increase in sad affect when they received feedback that their causal understanding of their situation (the trading situation) was erroneous or in need of modification. Sad affect appeared to have focused their attention on the problem and helped them analyze it so that they could gain control over the situation. This experiment also explains why depressed people are sometimes cautious in implementing potential solutions (Lyubomirsky et al., 1999) (listed as a downstream effect in Figure 1). Caution is adaptive when uncertain about the cause-effect consequences of different options and making a mistake can make things worse. Because participants in the sad condition were unsure that their causal understanding of the situation was correct, they were conservative in their approach to the problem. But their processing of the problem led them to make more accurate decisions, because the problem was complex and required analysis.
Social Dilemmas
As discussed above, conflicts with cooperative partners appear to be particularly depressogenic. Is there any evidence that depression helps people resolve social dilemmas? Experimental research shows that people in depressed or sad mood states perform better in social dilemmas, because they show more context-dependent behavior and greater processing of information on costs and risks (G. Hertel et al., 2000; Hokanson, Sacco, Blumberg, & Landrum, 1980; Kirchsteiger, Rigotti, & Rustichini, 2006; Pietromonaco & Rook, 1987).
To make these points more salient, we describe one of these studies in detail. It involved a modified Prisoner’s Dilemma (PD) game in which subjects played against each other in dyads (Hokanson et al., 1980). The PD offers players a choice between cooperating and defecting. The payoff structure provides an incentive for defecting, and, since the payoffs are symmetrical for both players, they both have an incentive to defect. However, the payoff structure is designed so that both players do better if they both cooperate than if they both defect (Axelrod, 1984).
In the usual PD, both players make their choices simultaneously. However, in this study, one player made his choice first (the low power position) and the other made his choice second (the high power position). The player choosing second is in the high power position because he knows what his opponent has chosen and can make the choice that optimizes his payoff on that round. Each dyad played 60 rounds. There was no monetary payoff subjects played for points.
There were three subject groups: (1) a non-depressed group (N); (2) a subclinically depressed group who scored high on a depression inventory (D); and (3) a group who scored high for fear or other problems (O). Subjects were then grouped into dyads. There were 10 N-D pairings, 10 D-N pairings, 10 N-N pairings, 10 N-O pairings, and 10 O-N pairings (the first letter refers to the low power position and the second letter refers to the high power position). Since O subjects did not play against depressed subjects, we do not discuss them any further.
The average scores for players in each of the pairing types are presented in Table 2. Several things about the results are worth noting. First, the highest scores were accumulated by non-depressed subjects in the high power position who were paired with depressed subjects (160.9 pts). However, their depressed partners in the low power position did almost as well, accumulating the second highest score (139.7 pts). Second, the worst scores were accumulated by non-depressed subjects in the low power position (−38.6 pts), but their depressed partners in the high power position did not do nearly as bad (55.0 pts). When averaged across these two pairing types (i.e., N-D and D-N), depressed subjects outperformed normal subjects (97.4 pts vs. 61.2 pts). N-N pairings did even better, but there were no D-D pairings to which they could be compared. Still, if the N-N pairings are included, the average score for the non-depressed only rises to 88.5 (i.e., depressed subjects still outperformed the non-depressed).
Table 2.
A summary of the results of the modified Prisoner’s Dilemma game reported by Hokanson et al. (1980). Players were either subclinically depressed (D) or normal (N). In the Dyadic Pairing column, the first letter refers to which player is in the low power position, and the second letter refers to which player is in the high power position. Numbers in the Low Power Position and High Power Position columns refer to the average number of points accumulated by players in that position.
| Dyadic Pairing | Low Power Position | High Power Position |
|---|---|---|
| N-N | 112.1 | 119.7 |
| D-N | 139.7 | 160.9 |
| N-D | −38.6 | 55.0 |
| Mean | 71.1 | 111.9 |
The differences in performance occurred because non-depressed subjects tended to cooperate regardless of whether they were in the high power position. In contrast, the behavior of the depressed subjects was more sensitive to position. In the high power position, depressed subjects tended to defect more; when in the low power position, they tended to cooperate more.
Indeed, the studies exploring the effects on depressed affect in social dilemmas have all found that non-depressed subjects tend to cooperate regardless of their situation, whereas depressed subjects modulate their behavior with their situation in more rational ways (G. Hertel et al., 2000; Hokanson et al., 1980; Kirchsteiger et al., 2006). Specifically, depressed people are more sensitive to costs of cooperating than the non-depressed and are more likely to defect when it is costly to cooperate (G. Hertel et al., 2000; Hokanson et al., 1980; Kirchsteiger et al., 2006). This suggests that the depressed and the non-depressed may process cost-benefit information in different ways. Research explicitly exploring this issue has found that depressed and non-depressed people appear to give roughly the same weight to the potential benefits of an action, but the depressed appear to give greater weight to the costs (Pietromonaco & Rook, 1987).
That depression may help people solve social dilemmas is also supported by research on real-life dilemmas. When in conflict with close, cooperative social partners, people tend to show more sympathy, more support, and reduced aggression when their partner has depressive symptoms (Sheeber et al., 2001). The supportive response that the depressed get from their close social partners has led some researchers to argue that it reinforces depressive tendencies (Sheeber et al., 2001), which suggests that it may be useful in solving social dilemmas (Hagen, 1999, 2002, 2003; Hagen & Thomson, 2004; Watson & Andrews, 2002).
Problem Analysis Rumination in Therapy
Another line of research that we interpret in light of the third claim involves a therapy developed by Adele Hayes and her colleagues that facilitates processing of episode-related thoughts and feelings (Hayes, Beevers, Feldman, Laurenceau, & Perlman, 2005; Hayes, Feldman et al., 2007). Rather than trying to prevent depressed people from ruminating, the facilitated processing aspect of the therapy encourages it by having patients write about their strongest thoughts and feelings about their depressive episode in a journal. Journal entries were later coded by third-party raters for avoidance and processing. Avoidance was defined as having difficulty facing disturbing emotions or thoughts, whereas processing was defined as exploring and questioning issues related to the episode, with some change in perspective or insight. Long-term improvements in depression were associated with a peak in the frequency and intensity of processing and greater insight, while peak levels of avoidance were associated with poorer long-term outcomes (Hayes et al., 2005; Hayes, Feldman et al., 2007). Moreover, the peak in processing was also associated with a spike in depressive symptomatology. Thus, the authors viewed the temporary spike in depression as a positive sign of growth and insight during treatment (Hayes et al., 2005; Hayes, Feldman et al., 2007; Hayes, Laurenceau, Feldman, Strauss, & Cardaciotto, 2007). This suggests that depression may enhance processing that promotes growth and insight into problems and may facilitate the resolution of the episode.
While this research is correlational, controlled longitudinal experiments with subclinical and outpatient samples show that expressive writing about emotionally difficult topics facilitates the resolution of depressive symptoms over time (E. M. Gortner, Rude, & Pennebaker, 2006; Graf, Gaudiano, & Geller, 2008). Moreover, therapists rate their clients as having greater insight into the problems that they are working on in therapy (Graf et al., 2008).
Future Directions
Because it is crucial to the AR hypothesis, future research on how depression affects problem-solving should explicitly test how depressive cognition influences the triggering problem. As people with pre-existing depression will often have quite different problems, this issue may be best addressed experimentally. People who have depressed or sad mood triggered experimentally by an analytically difficult problem are predicted to perform better on that problem. The paper by Au et al. (2003) provides a model for conducting such experiments.
With respect to research on pre-existing depression, social dilemmas in which there is conflict with a close cooperative partner over a self-interested goal are particularly relevant. Effects on problem-solving can be assessed using longitudinal designs in which depressed people are randomly assigned to interventions that affect rumination: (1) antidepressant medications versus placebo; or (2) journal-based therapies that encourage depressive rumination versus therapies with elements that work against it, such as CBT. The AR hypothesis predicts that outcomes will be poorer for people assigned to antidepressant medications and therapies that interfere with depressive rumination. The crucial metric for assessing the outcome of a social dilemma is meeting the self-interested goal without breaking cooperative bonds.
Fourth Claim: Depression Reduces Performance on Laboratory Tasks Because Depressive Rumination Takes Up Limited Processing Resources
People in depressed mood states often show performance decrements on many laboratory tasks of cognition (listed as a downstream effect in Figure 1), and this is often interpreted as evidence that depression impairs problem-solving (Ackermann & DeRubeis, 1991; Austin et al., 2001; Gotlib & Asarnow, 1979; Oaksford, Morris, Grainger, & Williams, 1996; Veiel, 1997). However, our fourth claim is well supported. For instance, the performance decrements are unstable they can be alleviated by interventions that help depressed people disengage from their ruminations and focus on the task. In one study, the memory deficits exhibited by subclinically depressed people in a delay recall task were eliminated by giving them an extra instruction that forced them to focus on the task (P. T. Hertel & Rude, 1991). In another study comparing clinically depressed subjects to non-depressed controls, the performance decrements of clinically depressed subjects in a test of executive functioning (a random number generation task) were eliminated by first giving them a task that was designed to distract them from their ruminations (thinking for 5 minutes about a large black umbrella) (Watkins & Brown, 2002).
Relative to non-depressed controls, depressed people tend to recall fewer autobiographic memories in response to cue words and report more overgeneral memories (i.e., memories without details) (J. M. G. Williams & Scott, 1988). However, these decrements disappeared when clinically or subclinically depressed subjects were first given distracting tasks, such as thinking of a black umbrella (Watkins & Teasdale, 2001; Watkins, Teasdale, & Williams, 2000). Similarly, in a series of three studies, Lyubomirsky and her colleagues found that the decrements that subclinically depressed subjects showed relative to non-depressed controls on reading comprehension tasks were eliminated when they were first given tasks to distract them from their ruminations, and exacerbated when they were first instructed to ruminate about the symptoms and causes of their episode (Lyubomirsky, Kasri, & Zehm, 2003).
These studies indicate that the performance decrements on laboratory tasks in both clinical and subclinical samples occur because limited cognitive resources are allocated to something other than the laboratory task, probably the problem that triggered the depressive episode, and this interferes with performance on the laboratory task. This interpretation is strongly supported by experiments in which depressed mood is induced by self-referent statements that subjects read and apply to themselves (Ellis, Moore, Varner, Ottaway, & Becker, 1997; Ellis, Ottaway, Varner, Becker, & Moore, 1997; Ellis, Thomas, & Rodriguez, 1984; Oaksford et al., 1996; Seibert & Ellis, 1991b), which forms the basis of the Velten procedure and its variants (Seibert & Ellis, 1991a; Velten, 1968). Examples of such statements include: “I feel a little down today”, “My classes are harder than expected”, and “I wish I could be myself but nobody likes me when I am” (Seibert & Ellis, 1991a). These methods appear to induce depressed affect by leading subjects to imagine that they currently have difficult problems, and they commonly cause performance decrements on laboratory tasks (Ellis, Ottaway et al., 1997; Ellis et al., 1984; Oaksford et al., 1996; Seibert & Ellis, 1991b). Crucially, they cause off-task ruminations that appear to interfere with task-related processing (Ellis, Moore et al., 1997; Gunther, Ferraro, & Kirchner, 1996; Seibert & Ellis, 1991b). For instance, the degree to which the induction causes off-task ruminations is correlated with the performance decrement on the laboratory task (Ellis, Moore et al., 1997; Seibert & Ellis, 1991b).
In summary, studies of clinical, subclinical, and experimentally induced depression all show that when given a laboratory task, depressed people ruminate about other things, which takes up limited cognitive resources and interferes with their ability to perform well on the task. It is therefore illegitimate to conclude that depression generally impairs problem-solving from studies showing reduced performance on laboratory tasks. They have nothing to say about how successful depressed people are in solving the problems that they are ruminating about.
Other Features of Depression
According to the AR hypothesis, analysis in depression promotes greater understanding of the causes and the nature of the complex problem, and it helps generate and evaluate potential solutions (problem-solving effects in Figure 1). In this section, we argue that many other cognitions and behaviors associated with depression (downstream effects in Figure 1) are the product of this problem-solving process.
Understanding the Cause of the Problem
Since exposure to otherwise avoidable stressors is caused by one’s own decisions (Hammen, 1992; Kendler et al., 1999), self-blame and self-criticism in depression may indicate recognition of this fact. Similarly, upward counterfactual thoughts may help depressed people understand why avoidable problems occurred and how they could have been avoided.
Depressed people are also more likely than the non-depressed to attribute their own successes to external factors, such as luck, and their failures to internal factors, such as lack of ability (Sweeney, Anderson, & Bailey, 1986). The reverse pattern is seen when evaluating others’ successes and failures. The depressed are more likely than the non-depressed to attribute the others’ successes to internal factors (ability) and others’ failures to external factors (chance). This pattern is called the depressive attributional style, and it is often considered evidence that depressed people are unduly negative about their abilities and their life situation (Sweeney et al., 1986). However, concluding that the depressive attributional style is erroneous is problematic because it is difficult to obtain an objective measure of accuracy for attributions (Andrews, 2001; Harvey, Town, & Yarkin, 1981). Some evidence indicates that the non-depressed attributional style is driven by a self-serving bias (Sedikides, 1993), which may suggest that the depressive attributional style is unbiased. Indeed, when evaluating themselves and others on ability and the future, the depressed are more even-handed, and the non-depressed have positive illusions about themselves (Ahrens, Zeiss, & Kanfer, 1988; Alloy & Ahrens, 1987; Taylor & Brown, 1988).
Depressed people may also seek information that helps them understand why avoidable problems occurred. For instance, relative to non-depressed people, depressed people prefer to interact with people who give them negative evaluations of their personalities (Swann, Wenzlaff, Krull, & Pelham, 1992; Swann, Wenzlaff, & Tafarodi, 1992). They also prefer friends and romantic partners to give them negative evaluations of their attractiveness, intelligence, sociability, etc., and they prefer information about their weaknesses rather than their strengths (Swann, Wenzlaff, Krull et al., 1992; Swann, Wenzlaff, & Tafarodi, 1992). Depressed people’s preference for negative evaluations may be an important mechanism for gaining information that helps them understand why they are facing a problem and identify what difficult behavioral changes they may need to make to solve it. Indeed, the depressed are more interested in negative evaluations because they are believed to be more accurate (Giesler, Josephs, & Swann, 1996). Depressed people are also more interested in social comparison information indicating that they may be performing worse than others, but the effect appears to be specific to when they have performed poorly themselves (Swallow & Kuiper, 1992, 1993), which suggests they are interested in such information to the extent it helps them understand their reasons for failure.
The AR hypothesis predicts that self-blame, self-criticism, upward counterfactual thoughts, the depressive attribution style, the preference for negative evaluations, and the interest in social comparison information are more likely to occur when exposed to avoidable stressors, because preventing their recurrence requires an understanding of one’s own role in why they occurred.
Understanding the Nature of the Problem
Other cognitive features may help people understand what needs fixing and how difficult it may be to fix it. For instance, while depressed people are more likely to use defecting tactics in social dilemmas, they also are more concerned about negative evaluations from others (Kuiper, Olinger, & Swallow, 1987; Rudolph & Conley, 2005). This may indicate an awareness of the social consequences of using such tactics. Depressed people’s perception that they face problems that are difficult to solve and hard to control (Edwards & Weary, 1998; Lyubomirsky et al., 1999) may also be a realistic assessment of their situation.
Evaluating and Implementing Potential Solutions
Analysis of the cause and nature of problems may naturally suggest potential solutions, which is why they are linked in Figure 1. However, depressed people often appear to be reluctant to implement solutions to problems (Lyubomirsky et al., 1999). Above (see Third Claim), we hypothesized that a cautious approach to implementing solutions may be adaptive when uncertain that one adequately understands the cause-effect nature of the situation.
Depressed people often behave differently in social situations (Segrin, 2000). They show more irritability, are less likely to look someone in the eye, and are more likely to speak in monotone (Coyne, 1976a, 1976b; Segrin, 2000; Tse & Bond, 2004). Depressed people often elicit negative emotional reactions from others (Segrin & Dillard, 1992), and there is some evidence that they may be less cooperative than non-depressed people (G. Hertel et al., 2000; Hokanson et al., 1980; Kirchsteiger et al., 2006; Schaller & Cialdini, 1990). To some, these patterns do not appear to be promote social problem-solving (Segrin, 2000; Tse & Bond, 2004).
However, this interpretation is problematic for two reasons. First, this research does not directly measure problem-solving on the social problems that triggered the episode. As should be clear by now, we view performance on the triggering problem as a crucial metric for evaluating depressive cognition. Second, the conclusion that depression impairs social skills depends on accepting the notion that some behaviors, such as friendliness and cooperation, are always better for social problem-solving, regardless of the situation or context. A more direct definition of social competence is simply the ability to achieve social goals, especially in situations of social conflict (Green & Rechis, 2006). This instrumental definition argues that socially competent individuals choose from a suite of options to meet situational demands (Green & Rechis, 2006). Some social situations might warrant cooperation, but others might require the use of deceptive, coercive, or even aggressive tactics. The depressed might be more competent in this instrumental sense by analyzing possible tactics and strategies for dealing with their social dilemmas. As discussed (see Third Claim), depressed people use a wider range of tactics in social dilemmas, and they appear to choose the tactic that is best matched for social goals, which is a key feature of this instrumental definition of social competence. The AR hypothesis predicts that the judicious use of cooperative and non-cooperative tactics, displays of irritability and other behaviors that the depressed express in social situations are useful to them in trying to solve the social dilemmas that triggered their episodes (examples of effective action in Figure 1). This might be tested experimentally in laboratory social dilemmas by assessing how people in different mood states produce different facial displays in response to non-cooperative behavior by their partners and how those facial displays influence their partners’ subsequent behavior.
Implications
We now discuss several implications of the AR hypothesis.
Comorbidity of Anxiety and Depression
Depression is often comorbid with anxiety (Belzer & Schneier, 2004). Anxiety is an emotional reaction to a real or perceived threat that involves a worry or apprehension of the threat (Barlow, 2002). It coordinates changes in body systems to increase vigilance for potential threats (evolved function in Figure 1) and hyper-reactivity in which the threshold for taking preventative action is lowered (problem-solving effect in Figure 1) (Barlow, 2002). Hyper-reactivity increases false alarms, but it should also result in avoidant action that prevents threats from occurring (downstream effects in Figure 1). Perhaps for this reason, people with anxious personalities are less likely to die from accidents, (W. E. Lee, Wadsworth, & Hotopf, 2006).
We hypothesize that depression and anxiety often co-occur because some problems require both analysis (promoted by depressed affect) and vigilance (promoted by anxiety). For example, preventing the recurrence of an avoidable stressor poses several subproblems, some with analytical and vigilance components to them. First, it requires recognizing the avoidable nature of the problem. Above, we discussed evidence that people in depressed mood states recognize when their problems could have been avoided. Second, preventing a recurrence of an avoidable stressor requires gaining a better understanding of why the event occurred and how it could have been avoided, which requires counterfactual analysis (Roese & Olson, 1997). Consistent with this, depressed people report more upward counterfactual thinking about recent avoidable stressors than unavoidable stressors (Markman & Weary, 1996). Third, preventing a recurrence of an avoidable stressor requires one to be vigilant for circumstances that indicate the problem may be recurring. Fourth, to prevent a recurrence of an avoidable stressor, it is important to take preventative action before it happens, which is promoted by hyper-reactivity.
Based on the foregoing, we predict that exposure to avoidable stressors will trigger comorbid anxiety and depression. Consistent with this, we found evidence from a longitudinal study of an epidemiological sample of adult twins that avoidable stressors were more associated with comorbid episodes than unavoidable stressors were (Andrews & Hettema, n.d.).
A curious fact about depression and anxiety is that their underlying genetics appear to be virtually identical (Kendler & Prescott, 2006). While this fact, coupled with the high comorbidity, might be taken as evidence that depression and anxiety are not unique, they have different effects on body systems, including arousal and cognition (Barlow, 2002). And while they often have the same environmental triggers, they also appear to have unique triggers (Barlow, 2002; Kendler et al., 2003).
We attempt to explain the common genetic foundation of depression and anxiety through their neurobiology. Earlier, we argued that left VLPFC is recruited in depression to increase attentional control so that the analysis of complex problems with high WM loads is not interrupted. A parallel case can be made for vigilance and anxiety. Vigilance tasks require the use of some sensory modality to monitor the environment for stimuli that satisfy search criteria (Caggiano & Parasuraman, 2004; Davies & Parasuraman, 1982). They require WM because search criteria must be kept in an active, accessible state (Caggiano & Parasuraman, 2004; Davies & Parasuraman, 1982). Vigilance tasks require greater attentional control when search criteria must be kept in WM for a longer period of time (Caggiano & Parasuraman, 2004; Davies & Parasuraman, 1982), when search criteria are changed across trials (Aron, Robbins, & Poldrack, 2004), or when search stimuli are more difficult to distinguish from background (Marklund et al., 2007). Difficult vigilance tasks also recruit the VLPFC, but, unlike high WM load tasks, the recruitment is often right lateralized (Aron et al., 2004; Garavan, Ross, & Stein, 1999; Marklund et al., 2007; Pardo, Fox, & Raichle, 1991). Anxiety appears to regulate right VLPFC activity during exposure to stimuli that could be threatening (McClure et al., 2007; Monk et al., 2006), and this may increase attentional control so that search criteria are maintained in WM. Such findings suggest that anxiety and depression regulate the same neurological circuitry, but some structures are differentially lateralized to the right and left hemispheres, respectively. We hypothesize that depression and anxiety have a nearly identical genetic foundation is because the body plan is bilaterally symmetrical and many genes do not influence developmental processes in a lateralized way (Moller & Swaddle, 1998; J. E. Schmitt et al., 2008). Genetic variants that influence the susceptibility to depression through the structure of left lateralized circuitry should concomitantly influence the susceptibility to anxiety through right lateralized circuitry.
The Sex Difference in Depression
Females are more at risk for depression and anxiety than males, and this sex difference develops in adolescence (Rudolph & Conley, 2005). Depressive rumination mediates much of the sex difference, but counterfactual analysis rumination is the primary mediator (Treynor et al., 2003), which suggests that women are expending more analytical effort to prevent problems.
As discussed above (see First Claim), social strain can reduce young women’s ability to acquire resources, support, and protection. It may therefore be adaptive to down-regulate reproductive functioning under social stress (Wasser & Place, 2001), which may explain why it has an adverse effect on women’s fertility and the outcome of pregnancy (Berga & Loucks, 2006; Berga et al., 2003; Hobel & Culhane, 2003). For this reason, we hypothesize that women were under greater selection than men to avoid exposure to social stressors. This predicts that women spend more effort analyzing the future consequences of different decisions (promoted by depressed affect), and being vigilant for signs of potential stress (promoted by anxious affect).
Young women exhibit several traits that indicate enhanced analysis of, and vigilance for, threats to social relationships (Cross & Madson, 1997; Jenkins, Goodness, & Buhrmester, 2002; Maccoby, 1990; Rose & Rudolph, 2006; Rudolph & Conley, 2005). First, relative to boys, adolescent girls are more concerned about being negatively evaluated by others and they have a greater desire for social approval (Calvete & Cardenoso, 2005; La Greca, Dandes, Wick, Shaw, & Stone, 1988; La Greca & Lopez, 1998; Maccoby, 1990; Rudolph & Conley, 2005). The enhanced sensitivity to cues of social failure and success should draw girls’ attention to social problems before they become serious. Second, adolescent girls are generally more emotionally reactive than boys to interpersonal conflict (Rose & Rudolph, 2006), which should also draw their attention to signs of conflict earlier. Finally, adolescent girls are more empathic than boys (Geary, 1998; Rose & Rudolph, 2006), which probably allows them to anticipate how social partners are likely to feel and respond earlier to potential conflicts of interest.
There is also some evidence that vigilance-related traits may mediate the sex difference in depression, at least in adolescents. In one study of 11 year-olds, girls’ greater concern about how others may evaluate them completely mediated the sex difference in depression (Rudolph & Conley, 2005). In another study of 14–17 year-olds, girls’ greater need for social approval and success partially mediated their greater risk of depression (Calvete & Cardenoso, 2005).
Resolving the Cognitive Paradoxes
We now discuss how the AR hypothesis explains the paradoxical findings in Table 1.
Paradox 1: Rumination vs. difficulty concentrating
While depressed people often report difficulty concentrating (APA, 2000), they have persistent ruminations that reflect a state of highly focused attention. As discussed above (Fourth Claim), this paradox is explained by the fact that processing priority is given to problems related to the episode, which leaves fewer resources for other things.
Paradox 2: Analytical reasoning style vs. cognitive deficits
There is a large literature showing that depressed affect is associated with, and causes, performance decrements in a variety of cognitive domains, including memory, intelligence, and executive functioning (Ackermann & DeRubeis, 1991; Austin et al., 2001; Ellis, Moore et al., 1997; Ellis, Ottaway et al., 1997; Ellis et al., 1984; Hartlage et al., 1993; Oaksford et al., 1996; Seibert & Ellis, 1991b; Veiel, 1997). There is also a large literature showing that depressed affect promotes an analytical processing style that enhances accuracy on complex tasks (Ackermann & DeRubeis, 1991; Alloy & Abramson, 1979; Alloy, Abramson, & Viscusi, 1981; Ambady & Gray, 2002; Au et al., 2003; Bless, Mackie, & Schwarz, 1992; Braverman, 2005; Edwards & Weary, 1993; Forgas, 1998; Gasper, 2004; Gasper & Clore, 2002; Harkness, Sabbagh, Jacobson, Chowdrey, & Chen, 2005; G. Hertel et al., 2000; P. A. Keller, Lipkus, & Rimer, 2002; Lane & DePaulo, 1999; McCaul, 1983; Schwarz, 1990; Schwarz & Bless, 1991; Semmler & Brewer, 2002; Sinclair, 1988; Sinclair & Mark, 1995; Storbeck & Clore, 2005; Yost & Weary, 1996). Both literatures are well-established. How can the literature that depression causes performance decrements be reconciled with the literature that it promotes an analytical processing style?
The puzzle cannot be resolved by simply assuming that pre-existing depression causes cognitive deficits and experimentally induced sadness promotes analytical reasoning, because the puzzle cuts across each literature. For instance, while pre-existing depression is associated with performance decrements on certain tasks (Austin et al., 2001; Veiel, 1997), it is also associated with an analytical processing style and enhanced performance on other tasks (Ackermann & DeRubeis, 1991; Alloy & Abramson, 1979; Ambady & Gray, 2002; Harkness et al., 2005; Lane & DePaulo, 1999; McCaul, 1983; Yost & Weary, 1996). Similarly, while many studies show that experimentally induced sadness promotes an analytical processing style and enhances accuracy on many cognitive tasks (Ambady & Gray, 2002; Au et al., 2003; Bless et al., 1992; Braverman, 2005; Forgas, 1998, 2007; Gasper, 2004; Gasper & Clore, 2002; G. Hertel et al., 2000; Semmler & Brewer, 2002; Sinclair, 1988; Sinclair & Mark, 1995; Storbeck & Clore, 2005), other studies have shown that it causes cognitive deficits (Ellis, Ottaway et al., 1997; Ellis, Seibert, & Herbert, 1990; Ellis et al., 1984; Oaksford et al., 1996; Seibert & Ellis, 1991b).
How then is the puzzle to be solved? We have already explained that the performance decrements on laboratory tasks occur because depressive rumination takes up limited cognitive resources. The more difficult problem is explaining why depressed people ever show enhanced performance on some laboratory tasks. Given the evidence that depression promotes an analytical processing style, it is of interest that the tasks that depressed people perform better have an analytical structure to them. We discuss several of these tasks in detail.
Judgment of Control
A well-known example is the judgment of control task (Alloy & Abramson, 1979). In this task the subject is to assess the degree of control he or she has over the lighting of a bulb by pushing a button and seeing if the bulb lights up or not. To arrive at the correct answer the subject must estimate the probability that the bulb will light when the button is pushed, p(L|B), and then subtract the probability that the bulb lights when the button is not pushed, p(L|~B) (Alloy & Abramson, 1979).
There are too many studies that explore the effects of depression on the judgment of control task to review them all here. However, an early review of the literature found that most studies reported that people with depression outperformed the non-depressed on this task (Ackermann & DeRubeis, 1991). The current consensus appears to be that depressed people reliably perform better than the non-depressed in zero contingency situations (Msetfi, Murphy, Simpson, & Kornbrot, 2005). A zero contingency situation is one where the subject’s action does not change the probability of the outcome (i.e., the subject has no control over the outcome). For instance, people have no control over the bulb if it lights up 25% of the time when the button is pushed and 25% of the time when it is not pushed (a 25-25 zero contingency).
In zero contingency situations, non-depressed people report having more control over the outcome as the frequency of the outcome increases. For instance, they estimate a higher degree of control over the bulb in a 75-75 zero contingency situation than in a 25-25 zero contingency situation. In contrast, depressed people are relatively immune to outcome density effects in zero contingency situations. The difference between depressed and non-depressed participants in zero contingency situations has been demonstrated in experimentally induced (Alloy et al., 1981) and subclinical populations (Alloy & Abramson, 1979; Msetfi et al., 2005).
In summary, depressed people tend to outperform the non-depressed in situations where the outcome density is high and the degree of control is small or non-existent. They perform equally well when the outcome density is low or when the degree of control is large.
We propose that the situations in which depressed and the non-depressed perform equally well are computationally easier than the ones in which the depressed are more accurate. One way of estimating the relevant probabilities is to thoroughly sample two contingencies: (1) the frequency that the bulb lights up when the button is pushed; and (2) the frequency that the bulb lights up when the button is not pushed. If the sampling rate is high, p(L|B) and p(L|~B) may be estimated from these frequencies. This approach has a clear analytical component to it because it requires frequency information to be continually updated in WM while the sampling continues.
However, when the outcome density is low, simpler rules can be used to estimate control. If the bulb rarely lights up, even when the button is pushed, the degree of control cannot be great i.e., p(L|B)-p(L|~B) must be relatively small. But this does not make heavy demands on WM because every observation does not need to be retained and updated in WM. Low control can be inferred by seeing that the bulb rarely lights up when the button is pushed.
Similarly, simpler rules can also be used if the actual degree of control is high because this requires the bulb to light up frequently when the button is pushed, and rarely when it is not pushed i.e., p(L|B)-p(L|~B) must be relatively large. This also does not require every observation to be retained and updated in WM it only requires that the subject get a general sense of the relative difference in the probabilities.
Conversely, the problem becomes more difficult when the outcome density is high but the actual degree of control is low. The fact that the outcome density is high suggests that the subject may have a high degree of control over the outcome. However, this can only be verified by ascertaining the relative probability that the bulb lights up when the button is not pushed. But since the bulb also lights up frequently when the button is not pushed, it may not be clear whether there is any control over the outcome. To do this accurately, there is no clear shortcut to sampling both contingencies and updating them in WM. In other words, the task becomes more analytically challenging when outcome density is high and control is low.
Support for the idea that the judgment of control is analytically challenging in situations of high outcome density and low control comes from a recent study showing that accuracy decreased as subjects had to wait longer periods of time between trials (Msetfi et al., 2005). In WM tasks, longer intertrial intervals increase task difficulty because it increases the demands on attention (Dalley, Cardinal, & Robbins, 2004; Robbins, 2002). However, longer intertrial intervals only impaired the accuracy of the non-depressed in situations of high outcome density and low control, which suggests that the depressed were better at staying focused on the problem under analytically demanding conditions.
Mind-reading
Because people cannot directly observe the beliefs and intentions of others, they must often make inferences about an actor’s internal state based on observable features, such as the actor’s behavior and the situational context in which the behavior occurs. Mind-reading becomes an analytical task when people must cross-reference different pieces of information about an actor with each other, because each piece must be evaluated, and then kept in WM while others are evaluated, so that their implications can be compared.
Several different lines of research suggest that depressed people are better at mind-reading, at least when it requires analysis. First, depressed people are less likely to make the Fundamental Attribution Error (FAE) (Forgas, 1998; McCaul, 1983; Yost & Weary, 1996). The FAE is the tendency to infer that an actor’s internal state corresponds to expressed behavior more than appears to be logically warranted by the situation (Andrews, 2001; Ross, 1977). For instance, people make the FAE when they attribute a pro-Castro stance to the writer of a pro-Castro essay even when they know that the writer wrote the essay as part of a class assignment and was assigned the pro-Castro stance by the course instructor (Jones & Harris, 1967). The FAE is thought to be a pervasive part of judgment across a variety of social contexts (D. T. Gilbert & Malone, 1995; Jones, 1979). Avoiding the FAE requires multiple processing steps in which an initial attribution is made based on the actor’s behavior (the pro-Castro stance) and then a correction is made based on the situational context (the assignment of the stance by the course instructor). This approach is cognitively effortful. People are less likely to use situational information, and are more likely to make the FAE, under conditions of cognitive load (D. T. Gilbert, Pelham, & Krull, 1988; Trope & Alfieri, 1997). Moreover, those who avoid the FAE take longer on the task (Yost & Weary, 1996). Since information from the first step must be held in WM while the processing on the second step takes place, avoiding the FAE has an analytical component to it. Research on pre-existing, subclinical depression (McCaul, 1983; Yost & Weary, 1996) and experimentally induced sadness (Forgas, 1998) has found that people in depressed states were less likely to make the FAE.
Second, there is a large literature on how depressed people perceive facial expressions of emotion. It suggests that depressed people may be less accurate in recognizing facial expressions (Leppanen, 2006; Venn, Watson, Gallagher, & Young, 2006). However, anxiety, which is often comorbid with depression, also influences the processing of facial information, and research indicates that depression is unrelated to accuracy after controlling for anxiety (Bouhuys, Geerts, & Mersch, 1997). Moreover, many experiments involving the recognition of facial expressions do not allow analysis because they require subjects to recognize facial expressions based on very brief exposures and require quick judgments based on little processing (Frewen & Dozois, 2005). However, a recent study found that subclinically depressed people were better at interpreting facial clues of emotion when the clues are subtle and require analysis and attention to detail (Harkness et al., 2005). Importantly, this result held after controlling for anxiety.
Third, depressed people may also be better at detecting deception, at least under certain conditions (Lane & DePaulo, 1999). Detecting deception requires cross-referencing a statement or signal with other cues in order to evaluate the veracity of the signal. This is analytical because multiple pieces of information must be evaluated and compared, which requires holding them in WM. Accurately detecting deception is difficult, and most people perform only slightly better than chance (Bond & DePaulo, 2006). Experimental research suggests that people may be more primed to evaluate the possibility of deception, however, when the actor has a potential motive to deceive (Fein, 1996; Fein, Hilton, & Miller, 1990; Hilton, Fein, & Miller, 1993), and this may make it a more ecologically and evolutionarily relevant situation (Andrews, 2001). One study found that subclinically depressed people weren’t any better at detecting deception than the non-depressed when the actor had no apparent motive to deceive, but they outperformed the non-depressed when the actor did have an apparent motive to deceive (Lane & DePaulo, 1999). These conditions may prime depressed people to apply their analytical processing style to the task of scrutinizing the potential deceiver for clues of deception.
Decision-making
Analytical reasoning is an important component of decision-making in which multiple options are evaluated according to some metric of utility, such as monetary interest. This is analytical because making the most rational (i.e., utility-maximizing) decision requires the systematic evaluation of options. People in depressed mood states often behave more rationally in decision-making situations, including social dilemmas (G. Hertel et al., 2000; Hokanson et al., 1980; Kirchsteiger et al., 2006), complex economic experiments (Au et al., 2003), and the assessment of health risk (P. A. Keller et al., 2002).
Summary
We have not fully explained this paradox. We have merely noted that the laboratory tasks on which people in depressed mood states perform better than the non-depressed have an analytical structure to them. To understand why they perform better on such tasks, we turn to the next two paradoxes.
Paradox 3: Different procedures for inducing depressed affect enhance accuracy on some tasks and decrease accuracy on others
The literature on experimentally induced mood does not paint a consistent picture about the effects of depressed affect on laboratory task performance. Some experimental mood induction studies have found that depressed affect enhances performance on analytical laboratory tasks (Bless et al., 1992; Braverman, 2005; Forgas, 1998; G. Hertel et al., 2000; Storbeck & Clore, 2005), while others have found that it reduces performance (Ellis, Ottaway et al., 1997; Ellis et al., 1984; Oaksford et al., 1996; Seibert & Ellis, 1991b). We hypothesize that these apparently anomalous findings can be resolved by considering the different methods for inducing depressed affect and how they influence the allocation of cognitive resources.
There are a number of reliable methods for experimentally inducing depressed affect, including having subjects: (a) listen to sad music; (b) watch sad movies; (c) recall sad memories; (d) read self-referent statements to interpret his or her current life situation in negative, depressing ways; and (e) receive negative evaluations to elicit thoughts and feelings of failure (Westermann et al., 1996). Above, we reviewed evidence that self-referent methods cause performance decrements on laboratory tasks (Ellis, Moore et al., 1997; Ellis, Ottaway et al., 1997; Ellis et al., 1984; Oaksford et al., 1996; Seibert & Ellis, 1991b). These methods also induce people to have off-task ruminations (Ellis, Moore et al., 1997; Gunther et al., 1996; Seibert & Ellis, 1991b). The AR hypothesis predicts that they induce depressed mood by leading people to imagine that they have important, difficult problems in their lives. These ruminations interfere with task performance because they take up limited cognitive resources that could otherwise be devoted to the task (Ellis & Ashbrook, 1988; Ellis, Moore et al., 1997; Seibert & Ellis, 1991b).
Most experiments showing that depressed affect enhances performance on analytically challenging tasks used sad music or film clips to induce mood (Bless, Bohner, Schwarz, & Strack, 1990; Bless et al., 1992; Bless, Schwarz, Clore, Golisano, & Rabe, 1996; Braverman, 2005; Forgas, 1998; G. Hertel et al., 2000; Semmler & Brewer, 2002; Sinclair, 1988; Sinclair & Mark, 1995; Storbeck & Clore, 2005), although some of them have used self-referent statements (Alloy et al., 1981; Au et al., 2003). The AR hypothesis predicts that sad music or film clips inductions lead to enhanced performance on analytically challenging laboratory tasks because they don’t lead people to imagine that they have problems that they then ruminate about.
Paradox 4: Pre-existing depression is associated with increased accuracy on some tasks and reduced accuracy on others
The literature on the cognitive effects of pre-existing depression is also not uniform. Often, pre-existing depression is associated with reduced accuracy on laboratory tasks (Austin et al., 2001; Hartlage et al., 1993; Veiel, 1997). However, pre-existing depression is associated with greater accuracy on some tasks (Alloy & Abramson, 1979; Harkness et al., 2005; Lane & DePaulo, 1999; McCaul, 1983; Yost & Weary, 1996). Moreover, inverted u-shaped effects are found in some of these tasks (Marsh & Weary, 1994), with performance peaking with subclinical levels of pre-existing depression (Alloy & Abramson, 1979; Harkness et al., 2005) and declining at clinical levels (Dobson & Pusch, 1995; L. Lee et al., 2005). The inverted u-shaped effects are sometimes taken as evidence that clinical depression is qualitatively different from subclinical depression (L. Lee et al., 2005; Marsh & Weary, 1994).
According to the AR hypothesis, depressed people ruminate about important problems in their lives, and depression coordinates a suite of effects that prevent attention from engaging in processing that is unrelated to those problems. This predicts that people with pre-existing depression might have an easier time attending to tasks that are similar to the problems that they face. At the same time, even similar laboratory tasks will differ in important ways from the real problems that depressed people face. As depressive symptoms intensify, limited cognitive resources should become locked tighter on analyzing episode-related information, and it may become progressively more difficult for depressed people to attend to anything but the specifics of their problems. The AR hypothesis predicts that the inverted u-shaped effects will disappear with attentional interventions of the sort discussed above (see Fourth Claim), and that this will be primarily attributable to increased performance by those with clinical depression.
We review two domains in which people with pre-existing depression outperform non-depressed people social dilemmas and the judgment of control and discuss their similarity to the problems that depressed people face.
Social Dilemmas
Above, we reviewed evidence that social dilemmas are evolutionarily relevant, depressogenic stressors. Part of the evidence that depressed people behave more rationally, pay more attention to information about costs and risks, and perform better came from social dilemma experiments in which participants had subclinical levels of pre-existing depression (Hokanson et al., 1980; Pietromonaco & Rook, 1987).
People with moderate levels of pre-existing depression often perform better in other cognitive domains that are likely to be useful in social dilemmas. Close social relationships are maintained, in part, through interdependency and cooperation, and conflict threatens those bonds. Communication about the conflict may be indirect or avoided altogether, and may involve obfuscation, euphemistic communication, deception, self-deception, partial disclosure, or other avoidant behaviors (Pinker, 2007). The ability to detect deception and accurately infer the actual mental states of others may be particularly useful in such contexts. Consistent with this, people with moderate levels of pre-existing depression are better at detecting deception, at least when the actor has an apparent motive to deceive (Lane & DePaulo, 1999). They are also better on attribution tasks in which behavioral and situational information must be cross-referenced to make inferences about the mental states of others (McCaul, 1983; Yost & Weary, 1996), and they are better at reading emotional states from facial expressions (Harkness et al., 2005). Reading emotional states from facial expressions appears to show inverted u-shaped effects, with clinically depressed people performing worse than the non-depressed (L. Lee et al., 2005).
Judgment of Control
Depressed people often perceive that they have lost control of their lives, and they attempt to regain control, in part, through ruminative analysis (Edwards & Weary, 1998; J. A. Jacobson et al., 1999; Lyubomirsky et al., 1999). This suggests that depressed people may be particularly concerned about accurately assessing the degree of control they have over situations. Consistent with this, people with depressed affect perform better on the judgment of control task (Paradox 2), and most research has used samples with moderate levels of pre-existing depression (Ackermann & DeRubeis, 1991). The judgment of control task also appears to show inverted u-shaped effects with those with clinical depression performing no better than those without depression (Dobson & Pusch, 1995).
Paradox 5: Antidepressants enhance cognitive performance in people with depression, but they cause performance decrements in the non-depressed
Research on the cognitive effects of antidepressant medications is often not well designed (Stein & Strickland, 1998). One study gave the Wechsler Adult Intelligence Scale-Revised (WAIS-R) to two groups of clinically depressed subjects responders and non-responders to the SSRI fluvoxamine (Mandelli et al., 2006). Responders scored higher on the WAIS-R than non-responders and this may be because the SSRI improved cognition. As responders and non-responders may differ in other ways, the ability to draw inferences from this study is limited. In a better designed study, two groups of clinically depressed subjects were given a vigilance test before and after antidepressant treatment (Koetsier et al., 2002). One group received imipramine (a tricyclic) and another received fluvoxamine. The mood of both antidepressant groups alleviated (though not to subclinical levels) and their vigilance performance improved. Since this study lacked a control group, it is unclear that the improved performance was attributable to the antidepressant medications.
However, controlled experiments have shown that antidepressants have cognitive effects in non-depressed subjects. They improve performance in certain domains, such as reaction time (Nathan, Sitaram, Stough, Silberstein, & Sali, 2000), but they also reliably impair accuracy on tasks that require attentional control, such as vigilance tasks (O'Hanlon, Robbe, Vermeeren, Van Leeuwen, & Danjou, 1998; Ramaekers, Muntjewerff, & O'Hanlon, 1995; J. A. J. Schmitt et al., 2002; Schwenzer, Heitkamp, & Mathiak, 2006) and delayed recall tasks with high WM load (Riedel, Eikmans, Heldens, & Schmitt, 2005; J. A. J. Schmitt, Kruizinga, & Riedel, 2001).
Why do antidepressant medications appear to have different effects in depressed and non-depressed people? Under the AR hypothesis, depression and anxiety regulate attentional control mechanisms, which are activated in high WM load or sustained vigilance tasks. A completely effective antidepressant would disable attentional control mechanisms. Rumination on episode-related problems would cease, but patients would also be unable to perform well on laboratory tasks that required attentional control. A completely effective antidepressant medication should therefore not help depressed patients perform better on laboratory tasks that require attentional control. In practice, antidepressant medications are never completely effective at eliminating depressed affect (Hollon et al., 2002; Kirsch et al., 2008). The AR hypothesis proposes that antidepressants improve depressed subjects’ accuracy on difficult tasks by alleviating depression enough so that attentional control is partly diminished and cognitive resources can be diverted from personal problems, but attentional control is not diminished so much that they cannot refocus them on the laboratory task. Consistent with this, functional neuroimaging studies find that activation of the left VLPFC is reduced in depressed subjects following antidepressant treatment (Drevets, 1999). Conversely, the AR hypothesis proposes that antidepressant medications cause performance decrements in non-depressed subjects precisely because they reduce attentional control. Non-depressed subjects who have been administered antidepressants are predicted to have diminished activation of the left VLPFC on tasks with high WM loads.
Paradox 6: Disruption of depressive rumination causes a temporary alleviation of depressive symptoms, but systematic disruption is associated with longer depressive episodes
Consistent evidence shows that depressed affect alleviates, at least temporarily, when depressed people are given laboratory tasks that distract them from their ruminative thoughts (Andrews et al., 2007; Morrow & Nolen-Hoeksema, 1990; Nolen-Hoeksema & Morrow, 1993; Park, Goodyer, & Teasdale, 2004; Vickers & Vogeltanz-Holm, 2003).
Such evidence led Susan Nolen-Hoeksema and her colleagues to predict that systematically disrupting depressive rumination would shorten depressive episodes (Nolen-Hoeksema, Morrow, & Fredrickson, 1993). This prediction has not been supported. In one test, people with subclinical depression who reported a greater propensity to use distracting strategies to deal with their ruminations did not have shorter episodes (Nolen-Hoeksema et al., 1993). Another study with a clinical sample found that distraction at admission to a psychiatric hospital did not predict symptoms four months after discharge (Kuehner & Weber, 1999). Other research has found contradictory results. A study of people with minor depression found that distraction was associated with longer episodes (Schmaling, Dimidjian, Katon, & Sullivan, 2002), and other longitudinal studies of clinical and subclinical samples have found that depressed people who try to avoid or suppress depressive rumination tend to have longer episodes (Hayes et al., 2005; Wenzlaff & Luxton, 2003). In other words, if anything, longitudinal research indicates that the systematic disruption of rumination, through avoidance, distraction or suppression, is associated with longer, not shorter, depressive episodes.
We explain this paradox with the principle that treating cause is more effective than treating symptom. The fact that distraction temporarily alleviates depressive symptoms in experimental studies, but is not associated with shorter episodes, suggests that distraction does not treat cause. Rather, it appears to be a temporary palliative for depressive symptoms, in much the same way that aspirin will temporarily reduces a fever but does not treat the infection.
Paradox 7: Different rumination styles are associated with different longitudinal outcomes
Recent studies suggest that rumination style is associated with the different longitudinal outcomes. After controlling for initial depression at time 1 (assessed by a short version of the Beck Depression Inventory), higher problem analysis rumination at time 1 is associated with lower depression at time 2, while higher counterfactual analysis rumination at time 1 is associated with higher depression at time 2 (Treynor et al., 2003). These findings must be interpreted in light of the fact that depressive symptoms often resolve over time without therapeutic intervention (Beck, 1967). Thus, the symptoms of those with higher problem analysis rumination decline with time at a steeper rate than average, while the symptoms of those with higher counterfactual analysis rumination decline at a slower rate than average.
The evidence that counterfactual analysis is associated with longer episodes has been replicated (Burwell & Shirk, 2007; Nolen-Hoeksema & Davis, 2004). However, the evidence that problem analysis is associated with shorter episodes is less consistent. Two longitudinal studies have found that subjects whose depressive symptoms improved with time showed a corresponding increase in cognition and behavior targeted towards analyzing the cause of the problem or solving it (Matheson & Anisman, 2003; Yamada, Nagayama, Tsutiyama, Kitamura, & Furukawa, 2003). One of these studies used a clinical sample (Yamada et al., 2003). At the same time, two recent longitudinal studies involving adolescent and bereaved samples have not found the negative relationship between problem analysis and subsequent depressive symptomatology (Burwell & Shirk, 2007; Nolen-Hoeksema & Davis, 2004).
We suggest that problem analysis and counterfactual analysis serve different functions, and these functions help shed light on the apparently discrepant relationship between rumination style and longitudinal outcomes. First, since depressed people often have current problems that require analysis, problem analysis rumination may be associated with shorter depressive episodes because it helps them understand or solve their problems quicker. This is supported by research that interventions encouraging depressive rumination through expressive writing tend to shorten depressive episodes (see Third Claim).
Second, we have argued that counterfactual thinking and vigilance co-occur as part of a mixed depressed-anxious state that prevents the recurrence of avoidable stressors. Because vigilance requires attentional control, the duration of the episode will depend on the duration of vigilance. Thus, one possible reason counterfactual analysis is associated with longer episodes is because it is associated with vigilance, and vigilance is only effective in preventing avoidable stressors for as long as one is vigilant. If so, the relationship between episode length and counterfactual analysis should be mediated by anxiety-related vigilance.
Another possibility is that measures of counterfactual rumination are often confounded with avoidant thoughts thoughts in which the person attempts to suppress, avoid, or distract him/herself from normal, adaptive rumination because it is painful. For instance, counterfactual thoughts (“if only I had done X, I wouldn’t be in the position I’m in now”) are probably the product of the desire to avoid painful feelings (“if only I had done X, I wouldn’t feel this pain now”). So the desire to avoid pain and counterfactual rumination are probably closely related. Since the use of avoidant strategies, such as distraction and suppression, tends to be associated with longer episodes (Hayes et al., 2005; Schmaling et al., 2002; Wenzlaff & Luxton, 2003), the relationship between counterfactual rumination and episode length may be driven by closely related avoidant thought strategies.
Treating Depression
We briefly discuss several treatment implications of the AR hypothesis. Great emphasis is placed on antidepressants in current clinical practice. Our review suggests that medications treat symptoms, whereas psychotherapies are more likely to be treating cause. The AR hypothesis suggests that psychotherapies are productive when they help depressed people identify and solve important problems in their lives. It also suggests that depressive rumination is useful and that antidepressants may interfere with the ability to ruminate. For these reasons, the AR hypothesis would place greater emphasis on psychotherapy and less on medications.
CBT is one of the most widely used psychotherapies (Beck et al., 1979). One of the components of CBT attempts to help depressed people change cognitions that are assumed to be unproductive. In contrast, the AR hypothesis suggests that depressive rumination is useful. Above, we discussed evidence that the cognitive change component of CBT may not be the therapeutic component (Coffman et al., 2007; Dimidjian et al., 2006; E. T. Gortner et al., 1998; N. S. Jacobson et al., 1996), and may be counterproductive (Castonguay et al., 1996). Similarly, depressed people who systematically attempt to disrupt their ruminations tend to have longer episodes (Hayes et al., 2005; Schmaling et al., 2002; Wenzlaff & Luxton, 2003). We do not mean to imply that treating depression by trying to change cognitions will never be helpful. We agree that some depressed people may have erroneous or unproductive cognitions, but the AR hypothesis suggests that such cognitions should only play a causal role in triggering depression to the extent they cause complex social problems. Such depressions may be treated by helping patients change those cognitions, but the AR hypothesis suggests that depressive rumination will help the person analyze and re-evaluate beliefs and cognitions that may be causing social problems.
In the current DSM-IV-TR atmosphere, depression is frequently diagnosed and treated by DSM-IV-TR checklists, often by primary care physicians who are treating depression with increasing regularity (Gilbody, Whitty, Grimshaw, & Thomas, 2003; Hepner et al., 2007). According to some estimates, nearly 75% of antidepressant medications are prescribed by primary care physicians (Mojtabai & Olfson, 2008). If a patient cannot concentrate, that is listed as a symptom, and no further inquiry is made. Instead of the clinician pursuing what is impairing concentration and specifically asking about ruminations and their contents, some form of treatment is initiated therapy and/or medication.
In contrast, the AR hypothesis suggests that the primary therapeutic goal should be to help depressed people identify the social problems that triggered their episodes and help them solve those problems. Primary care physicians are ill-suited for this task, having neither the time nor the training to delve into patients’ ruminations and help them solve problems. The AR therapist would encourage interventions that promote depressive rumination, such as writing about one’s strongest depressive thoughts and feelings, with the idea that it might help one gain insight into the problem and promote the resolution of the episode (E. M. Gortner et al., 2006; Graf et al., 2008; Hayes et al., 2005). Depressive cognition is explored with an eye for understanding the complexities of the depressed person’s social situation, particularly the factors that create social dilemmas and must be considered when generating potential solutions.
Many people may be reluctant to disclose the reasons for their depression because the problem is embarrassing, reputationally damaging or otherwise sensitive, which is often why depressive episodes may appear to be endogenous (Leff, Roatch, & Bunney, 1970). In cases where the patient is unwilling to discuss the content of their ruminations and does not report an environmental trigger, the AR therapist would not assume that there is none, but would provide a safe environment that facilitates the disclosure of sensitive information. Often, time building trust with the patient over multiple sessions will facilitate disclosure (Leff et al., 1970). Comorbid feelings of anger, guilt, or shame will also imply a social cause.
Recapitulation and Integration
Depression coordinates body systems in ways that solve several problems associated with the sustained analysis of the triggering problem: (1) it reduces the chance that analytical processing will be interrupted by inducing anhedonia, psychomotor changes, and recruiting the left VLPFC to enhance attentional control; (2) by increasing serotonin, it stimulates lactate production in nearby astrocytes, providing the energy needed to sustain VLPFC activity; and (3) serotonin also drives glutamate-glutamine cycling so that glutamate is cleared out of the synapse and the loss of neuronal tissue that can occur under sustained glutamatergic transmission is reduced. Such coordination makes it very unlikely that depressive rumination is a byproduct of biological processes or is attributable to chance. Just as the highly structured and complex design of the vertebrate eye must have been constructed by selection and not by chance, it is difficult to see how chance biological processes could have generated such coordination. It suggests that depression evolved by natural selection, probably because it helped people analyze and solve the problems they were ruminating about.
The evolutionary benefits of depressive rumination must have been great enough to compensate for the substantial costs, many of which we have discussed. Some of these costs provide further evidence that is relevant to the AR hypothesis. For instance, a growing body of research indicates that stress-induced depression causes a loss of prefrontal gray matter (Gianaros et al., 2007; Konarski et al., 2008) via glutamatergic apoptosis (A. L. Lee et al., 2002). Given that the prefrontal areas are widely considered to be involved in higher cognition, the loss of prefrontal tissue can be considered further, albeit indirect, evidence that sustained higher order cognitive processing takes place in depression. The loss of neuronal tissue probably occurs because sustained glutamatergic activity eventually depletes astrocytic glycogen reserves, which are used to clear glutamate from the synapse. These reserves must be replenished (Shulman et al., 2001a), which may partly explain why depressed people prefer ‘comfort foods’ that are high in simple sugars (Christensen, 2001). The timing of replenishment is predicted to be organized around sleep when rumination cannot take place and demands for astrocytic lactate are low. Specifically, since oral activity interferes with sustained processing (Jacobs & Fornal, 1999), appetite and food intake should increase later in the day, which will minimize disruption by keeping it close to the time of sleep. This, in turn, predicts that sustained rumination will generally be most intense after waking, when glycogen reserves are high, which may explain why melancholia is associated with early morning waking (Akiskal & Akiskal, 2007).
A design analysis does not require depressive rumination to be currently adaptive because modern and evolutionary environments may differ in important ways (Thornhill, 1990, 1997). All that is required is that, on average, depressive rumination helped people analyze and solve the problems they were ruminating about in ancestral environments. Still, strong, replicable evidence that depressive rumination currently helps people analyze and solve the problems they ruminate about would support the evolutionary argument, and more research is needed here.
Also relevant to a design analysis is whether the trait exhibits features that match the environmental problem (Andrews et al., 2002a). With depression, this part of a design analysis asks whether the problems and situations that trigger depression are cognitively complex and require analysis and attentional control. We have argued that at least some depressogenic problems have these features social dilemmas and exposure to avoidable stressors.
Others have suggested that depressed affect may promote problem-solving (Carver & Scheier, 1990; Gut, 1989; Hagen, 2003; Pyszczynski & Greenberg, 1987; Schwarz & Bless, 1991; Thornhill & Thornhill, 1989; Watson & Andrews, 2002; Weary et al., 1993). There are multiple ways that depression could help people solve problems. It could motivate the depressed individual to engage in problem-solving behavior (Carver & Scheier, 1990; Pyszczynski & Greenberg, 1987). Because depressed people are often unable to attend to social relationships and obligations, depression imposes costs on close social partners who are dependent on the depressed individual. Depression could motivate close social partners to provide help or make concessions to the depressed individual in order to stop the imposition of costs (Hagen, 2003; Watson & Andrews, 2002). Depression could also reduce social aggression by signaling that the depressed individual is not a threat to more dominant individuals (P. Gilbert, 2006).
The AR hypothesis is a natural extension of hypotheses suggesting that depression promotes problem-solving by influencing cognition (Gut, 1989; Thornhill & Thornhill, 1989; Watson & Andrews, 2002; Weary et al., 1993), particularly by promoting an analytical processing style (Schwarz & Bless, 1991). It also is related to the resource allocation hypothesis, which argues that people with pre-existing depression often show performance decrements on laboratory tasks because they are ruminating about other things, which takes up limited cognitive resources and interferes with their ability to concentrate on laboratory tasks (Ellis & Ashbrook, 1988; Seibert & Ellis, 1991b). The AR hypothesis links these two literatures by arguing that analysis is vulnerable to interruption, and depression recruits attentional control mechanisms. Cognitive resources therefore stay focused on analyzing episode-related problems and processing is less likely to be interrupted by less important things, such as laboratory tasks.
Many influential hypotheses propose that depressive cognition is maladaptive. The cognitive triad hypothesis proposes that depression is caused by negative cognitions about the self, the future, and the world, and depression can be alleviated by changing those cognitions (Beck et al., 1979). Similarly, the depressive rumination hypothesis assumes that depression enhances negative thoughts (Nolen-Hoeksema, 1990), which exacerbates episodes by promoting pessimism, increasing recall for unhappy memories, enhancing sensitivity to negative information about situations, increasing the salience of negative interpretations of situations, and interfering with instrumental problem-solving behavior. It proposes that the key to alleviating depression is to distract or shift attention from self-focused thoughts to other domains. The learned helplessness hypothesis proposes that repeated exposure to uncontrollable stressors induces a depressed state in which it perceives that it is helpless to control its environment, even over aspects of the environment that it can in fact control (Seligman, 1975).
We have reviewed several bodies of evidence that appear to be inconsistent with these hypotheses. First, depression promotes analysis, which is not usually thought to be unproductive. Second, depression may help people solve the problems that triggered their depressive episode. This has been supported directly by experiment (Au et al., 2003), indirectly by research on experimental and real-life social dilemmas (G. Hertel et al., 2000; Hokanson et al., 1980; Sheeber et al., 2001), and indirectly by experimental and clinical research showing that interventions that promote depressive rumination by expressive writing are associated with greater insight and quicker resolution of symptoms (E. M. Gortner et al., 2006; Graf et al., 2008; Hayes et al., 2005). Finally, if depressive cognition were generally unproductive, then the disruption of depressive rumination should be associated with better outcomes. But, if anything, the reverse appears to be true (Hayes et al., 2005; Schmaling et al., 2002; Wenzlaff & Luxton, 2003). Should future research confirm these findings, these hypotheses should be put to rest.
The AR hypothesis must also be subjected to greater scrutiny. Throughout the paper, we have highlighted issues that we think warrant greater attention. We close with a final issue why people sometimes attempt to avoid depressive thoughts and feelings. For instance, obsessive-compulsive thoughts may reduce painful thoughts by overloading working memory (Boyer & Lienard, 2008). Some suicidal behaviors may also be motivated by the desire to avoid painful thoughts and feelings (Baumeister, 1990). Alcohol is sometimes used to avoid depressive thoughts and feelings (Kuo, Gardner, Kendler, & Prescott, 2006; Nolen-Hoeksema, Stice, Wade, & Bohon, 2007; Young-Wolff, Kendler, Sintov, & Prescott, 2009), possibly because it impairs working memory function (Schweizer & Vogel-Sprott, 2008). Like people who use distraction or suppression to cope with depressive thoughts and feelings (Hayes et al., 2005; Schmaling et al., 2002; Wenzlaff & Luxton, 2003), depressed people who abuse alcohol or are dependent on it tend to have longer episodes and are more likely to have relapses or recurrences (Howland et al., 2009). In any event, if depression is an adaptation for promoting analysis of a problem, then why do some people try to avoid it?
Painful feelings draw attention to problems and motivate problem-solving behavior (Carver & Scheier, 1990; Eccleston & Crombez, 1999; Thornhill & Thornhill, 1989; Wall, 2000). For many problems, it is not adaptive to endure physical or emotional pain for long periods of time. Action must be taken quickly to prevent damage (hand is in the fire, fist is approaching face, predator is preparing to pounce). For this reason, organisms are highly motivated to take action that reduces painful feelings quickly.
Perhaps more so than other painful emotions, people in the evolutionary past must have had to learn how to endure extended periods of depression. A complex problem, for instance, resists simple solution, and depressive pain persists despite attempts to quickly solve it. We suggest that when facing complex problems, organisms must learn to stop trying to quickly resolve their pain with simple solutions, accept a slower, analytical approach to problem-solving, and learn how to endure the pain until the problem is solved. The extended nature of depressive pain is useful. Without it, people would not be motivated to engage in the extended effort required to solve complex problems, and the pain should cease once the problem is solved. But another reason why it is important to learn to endure depressive pain is that people facing social dilemmas may anticipate further long-term pain if the best solutions require making tradeoffs (e.g., people contemplating divorce may lose children, money and home by leaving, and face continued marital problems by staying). Thus, effective decision-making will require accepting and enduring the pain that persists during analysis and the subsequent anticipated pain that arises from different courses of action.
In ancestral environments, there were probably few ways to bypass this learning process. The persistence of depressive pain despite attempts to quickly resolve it would eventually force the organism to adopt a slow problem-solving approach and learn how to accept and endure the pain. Learning may also be facilitated through interaction with close social partners, who demonstrate or encourage the acceptance of depressive pain. Yet in modern environments, there are many ways to temporarily reduce depressive pain without solving the complex triggering problem (e.g., drugs, alcohol, distracting activities like television, etc.). Blanket statements by professionals, pharmaceutical companies, and the media that depression is a disorder may also interfere with the learning process and promote avoidant behaviors.
The AR hypothesis proposes that avoidant behaviors: (a) bypass the process by which people learn to endure painful feelings that persist when taking a slow, analytical problem-solving approach and that arise as a consequence of considering and making tradeoffs; (b) are a maladaptive byproduct of the evolved propensity to take action that quickly reduces pain; and (c) occur in environments where the means to engage in avoidant behavior are available. It predicts that depressed people are more likely to use avoidant behaviors: (1) when they face difficult social dilemmas (because they are more likely to face painful tradeoffs that they will want to avoid); (2) in their adolescent years (because they have had less opportunity to learn how to deal with depressive feelings); (3) when they have close social partners who also engage in avoidant behaviors (because learning to be avoidant or non-avoidant is socially transmitted, in part); (4) if they feel depression and anxiety more intensely (because such people will feel greater urgency to reduce the pain quickly and be less tolerant of the learning process); and (5) if they are less intelligent (because their learning process will be slower and less efficient).
Acknowledgments
PWA was partly supported by a National Research Service Award from the NIH, MH-20030 (PI: Michael C. Neale) and partly by NIH grant DA-018673 (PI: Michael C. Neale). We thank Nalini Ambady, Ed Hagen, Lisa Halberstadt, Steve Hollon, W. Jake Jacobs, Matt Keller, Steve Maier, Mike Neale, Mark Reimers, Eric Schmitt, Jerome Wakefield, Paul Watson, and an anonymous reviewer for inspiration, comments, support or suggestions.
Literature Cited
- Abramson LY, Alloy LB, Metalsky GI. Hopelessness depression: A theory-based subtype of depression. Psychological Review. 1989;96(2):358–372. [Google Scholar]
- Ackermann R, DeRubeis RJ. Is depressive realism real? Clinical Psychology Review. 1991;11(5):565–584. [Google Scholar]
- Aggen SH, Neale MC, Kendler KS. DSM criteria for major depression: Evaluating symptom patterns using latent-trait item response models. Psychological Medicine. 2005;35(4):475–487. doi: 10.1017/s0033291704003563. [DOI] [PubMed] [Google Scholar]
- Ahrens AH, Zeiss AM, Kanfer R. Dysphoric deficits in interpersonal standards, self-efficacy, and social-comparison. Cognitive Therapy and Research. 1988;12(1):53–67. [Google Scholar]
- Akiskal HS, Akiskal KK. A mixed state core for melancholia: An exploration in history, art and clinical science. Acta Psychiatrica Scandinavica. 2007;115:44–49. doi: 10.1111/j.1600-0447.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: Guilt by association. Neuroscientist. 2004;10(6):575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- Alcock J. Animal Behavior. 7. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- Alexander RD. Ostracism and indirect reciprocity: The reproductive significance of humor. Ethology and Sociobiology. 1986;7(3–4):253–270. [Google Scholar]
- Allen NB, Badcock PBT. The social risk hypothesis of depressed mood: Evolutionary, psychosocial, and neurobiological perspectives. Psychological Bulletin. 2003;129(6):887–913. doi: 10.1037/0033-2909.129.6.887. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. Judgment of contingency in depressed and nondepressed students: Sadder but wiser? Journal of Experimental Psychology-General. 1979;108(4):441–485. doi: 10.1037//0096-3445.108.4.441. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Viscusi D. Induced mood and the illusion of control. Journal of Personality and Social Psychology. 1981;41(6):1129–1140. [Google Scholar]
- Alloy LB, Ahrens AH. Depression and pessimism for the future: Biased use of statistically relevant information in predictions for self versus others. Journal of Personality and Social Psychology. 1987;52(2):366–378. doi: 10.1037//0022-3514.52.2.366. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neuroscience. 2005;8(3):365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Ambady N, Gray HM. On being sad and mistaken: Mood effects on the accuracy of thin-slice judgments. Journal of Personality and Social Psychology. 2002;83(4):947–961. [PubMed] [Google Scholar]
- American Psychiatric Association. (DSM-IV-TR) Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Press, Inc; 2000. text revision ed. [Google Scholar]
- Andrews PW. The psychology of social chess and the evolution of attribution mechanisms: explaining the fundamental attribution error. Evolution and Human Behavior. 2001;22(1):11–29. doi: 10.1016/s1090-5138(00)00059-3. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Aggen SH, Miller GF, Radi C, Dencoff JE, Neale MC. The functional design of depression's influence on attention: A preliminary test of alternative control-process mechanisms. Evolutionary Psychology. 2007;5(3):584–604. [Google Scholar]
- Andrews PW, Gangestad SW, Matthews D. Adaptationism - How to carry out an exaptationist program. Behavioral and Brain Sciences. 2002a;25(4):489–504. doi: 10.1017/s0140525x02000092. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Gangestad SW, Matthews D. Adaptationism, exaptationism, and evolutionary behavioral science. Behavioral and Brain Sciences. 2002b;25(4):534–553. doi: 10.1017/s0140525x02000092. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Hettema J. Dependent stressors predict comorbid episodes of depression and anxiety (n.d.) [Google Scholar]
- Andrews PW, Neale MC. Solving Drevets' paradox: A cautionary note on the hunt for neuroimaging biomarkers of psychiatric traits (n.d.) [Google Scholar]
- Antonucci TC, Akiyama H, Lansford JE. Negative effects of close social relations. Family Relations. 1998;47(4):379–384. [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Au K, Chan F, Wang D, Vertinsky I. Mood in foreign exchange trading: Cognitive processes and performance. Organizational Behavior and Human Decision Processes. 2003;91(2):322–338. [Google Scholar]
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: Possible implications for functional neuropathology. British Journal of Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Axelrod R. The evolution of cooperation. New York, NY: Basic Books; 1984. [Google Scholar]
- Baddeley A. Working memory, thought, and action. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Barlow DH. Anxiety and its disorders. 2. New York: Guilford; 2002. [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Barton DA, Esler MD, Dawood T, Lambert EA, Haikerwal D, Brenchley C, et al. Elevated brain serotonin turnover in patients with depression. Archives of General Psychiatry. 2008;65(1):38–46. doi: 10.1001/archgenpsychiatry.2007.11. [DOI] [PubMed] [Google Scholar]
- Baumeister RF. Suicide as escape from self. Psychological Review. 1990;97(1):90–113. doi: 10.1037/0033-295x.97.1.90. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Clinical, experimental and theoretical aspects. New York, NY: Hoeber; 1967. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York, NY: The Guilford Press; 1979. [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L. Measurement of pessimism-hopelessness scale. Journal of Consulting and Clinical Psychology. 1974;42(6):861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- Belzer K, Schneier FR. Comorbidity of anxiety and depressive disorders: Issues in conceptualization, assessment, and treatment. Journal of Psychiatric Practice. 2004;10(5):296–306. doi: 10.1097/00131746-200409000-00003. [DOI] [PubMed] [Google Scholar]
- Berga SL, Loucks TL. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Women's Health and Disease: Gynecologic, Endocrine, and Reproductive Issues. 2006;1092:114–129. doi: 10.1196/annals.1365.010. [DOI] [PubMed] [Google Scholar]
- Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertility and Sterility. 2003;80(4):976–981. doi: 10.1016/s0015-0282(03)01124-5. [DOI] [PubMed] [Google Scholar]
- Blanco C, Okuda M, Wright C, Hasin DS, Grant BF, Liu SM, et al. Mental health of college students and their non-college-attending peers: Results from the National Epidemiologic Study on Alcohol and Related Conditions. Archives of General Psychiatry. 2008;65(12):1429–1437. doi: 10.1001/archpsyc.65.12.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatteis CM. Fever: Pathological or physiological, injurious or beneficial? Journal of Thermal Biology. 2003;28(1):1–13. [Google Scholar]
- Bleske-Rechek AL, Buss DM. Opposite-sex friendship: Sex differences and similarities in initiation, selection, and dissolution. Personality and Social Psychology Bulletin. 2001;27(10):1310–1323. [Google Scholar]
- Bless H, Bohner G, Schwarz N, Strack F. Mood and persuasion: A cognitive response analysis. Personality and Social Psychology Bulletin. 1990;16(2):331–345. [Google Scholar]
- Bless H, Mackie DM, Schwarz N. Mood effects on attitude judgments: Independent effects of mood before and after message elaboration. Journal of Personality and Social Psychology. 1992;63(4):585–595. doi: 10.1037//0022-3514.63.4.585. [DOI] [PubMed] [Google Scholar]
- Bless H, Schwarz N, Clore GL, Golisano V, Rabe C. Mood and the use of scripts: Does a happy mood really lead to mindlessness? Journal of Personality and Social Psychology. 1996;71(4):665–679. doi: 10.1037//0022-3514.71.4.665. [DOI] [PubMed] [Google Scholar]
- Bond CF, DePaulo BM. Accuracy of deception judgments. Personality and Social Psychology Review. 2006;10(3):214–234. doi: 10.1207/s15327957pspr1003_2. [DOI] [PubMed] [Google Scholar]
- Bouhuys AL, Geerts E, Mersch PPA. Relationship between perception of facial emotions and anxiety in clinical depression: Does anxiety-related perception predict persistence of depression? Journal of Affective Disorders. 1997;43:213–223. doi: 10.1016/s0165-0327(97)01432-8. [DOI] [PubMed] [Google Scholar]
- Boyer P, Lienard P. Ritual behavior in obsessive and normal individuals-Moderating anxiety and reorganizing the flow of action. Current Directions in Psychological Science. 2008;17(4):291–294. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Braverman J. The effect of mood on detection of covariation. Personality and Social Psychology Bulletin. 2005;31(11):1487–1497. doi: 10.1177/0146167205276152. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris T. Social origins of depression: A study of psychiatric disorder in women. New York, NY: The Free Press; 1978. [Google Scholar]
- Burwell RA, Shirk SR. Subtypes of rumination in adolescence: Associations between brooding, reflection, depressive symptoms, and coping. Journal of Child Clinical and Adolescent Psychology. 2007;36(1):56–65. doi: 10.1080/15374410709336568. [DOI] [PubMed] [Google Scholar]
- Buss DM. The dangerous passion: Why jealousy is as necessary as love and sex. New York, NY: The Free Press; 2000. [Google Scholar]
- Byrne R, Whiten A, editors. Machiavellian intelligence: Social expertise and the evolution of intellect in monkeys, apes, and humans. New York, NY: Oxford University Press; 1988. [Google Scholar]
- Byrne RMJ. Spatial mental models in counterfactual thinking of what might have been. Kognitionswissenschaft. 1998;7:19–26. [Google Scholar]
- Caggiano DM, Parasuraman R. The role of memory representation in the vigilance decrement. Psychonomic Bulletin & Review. 2004;11(5):932–937. doi: 10.3758/bf03196724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete E, Cardenoso O. Gender differences in cognitive vulnerability to depression and behavior problems in adolescents. Journal of Abnormal Child Psychology. 2005;33(2):179–192. doi: 10.1007/s10802-005-1826-y. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10(9):1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Carli M, Samanin R. The 5-HT1A receptor agonist 8-OH-DPAT reduces rats’ accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: Role of presynaptic 5-HT1A receptors. Psychopharmacology. 2000;149:259–268. doi: 10.1007/s002139900368. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Shell P. What one intelligence-test measures: A theoretical account of the processing in the Raven Progressive Matrices test. Psychological Review. 1990;97(3):404–431. [PubMed] [Google Scholar]
- Carroll JB. Human cognitive abilities: A survey of factor-analytic studies. New York, NY: Cambridge University Press; 1993. [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. Journal of Cognitive Neuroscience. 2005;17(10):1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. Origins and functions of positive and negative affect: A control-process view. Psychological Review. 1990;97(1):19–35. [Google Scholar]
- Castonguay LG, Goldfried MR, Wiser S, Raue PJ, Hayes AM. Predicting the effect of cognitive therapy for depression: A study of unique and common factors. Journal of Consulting and Clinical Psychology. 1996;64(3):497–504. [PubMed] [Google Scholar]
- Christensen L. The effect of food intake on mood. Clinical Nutrition. 2001;20:161–166. [Google Scholar]
- Coffman SJ, Martell CR, Gallop R, Dimidjian S, Hollon SD. Extreme nonresponse in cognitive therapy: Can behavioral activation succeed where cognitive therapy fails? Journal of Consulting and Clinical Psychology. 2007;75(4):531–541. doi: 10.1037/0022-006X.75.4.531. [DOI] [PubMed] [Google Scholar]
- Conca A, Fritzsche H, Peschina W, Konig P, Swoboda E, Wiederin H, et al. Preliminary findings of simultaneous F-18-FDG and Tc-99m-HMPAO SPECT in patients with depressive disorders at rest: differential correlates with ratings of anxiety. Psychiatry Research-Neuroimaging. 2000;98(1):43. doi: 10.1016/s0925-4927(99)00051-7. + [DOI] [PubMed] [Google Scholar]
- Conner JK, Hartl DL. A primer of ecological genetics. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Cosmides L, Tooby J. Evolutionary psychology and the emotions. In: Lewis M, Haviland-Jones JM, editors. Handbook of the Emotions. 2. New York, NY: The Guilford Press; 2000. pp. 91–115. [Google Scholar]
- Cowan N. Working memory capacity. New York, NY: Psychology Press; 2005. [Google Scholar]
- Coyne JC. Depression and response of others. Journal of Abnormal Psychology. 1976a;85(2):186–193. doi: 10.1037//0021-843x.85.2.186. [DOI] [PubMed] [Google Scholar]
- Coyne JC. Toward an interactional description of depression. Psychiatry-Interpersonal and Biological Processes. 1976b;39(1):28–40. doi: 10.1080/00332747.1976.11023874. [DOI] [PubMed] [Google Scholar]
- Cross SE, Madson L. Models of the self: Self-construals and gender. Psychological Bulletin. 1997;122(1):5–37. doi: 10.1037/0033-2909.122.1.5. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Andersson G, van Oppen P. Psychotherapy for depression in adults: A meta-analysis of comparative outcome studies. Journal of Consulting and Clinical Psychology. 2008;76(6):909–922. doi: 10.1037/a0013075. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Experimental Brain Research. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Davies DR, Parasuraman R. The psychology of vigilance. New York, NY: Academic Press; 1982. [Google Scholar]
- de Waal FBM. Chimpanzee politics: Power and sex among apes. Baltimore, MD: Johns Hopkins University Press; 2007. [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology. 2006;74(4):658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Dobson KS, Dimidjian S, Kohlenberg RJ, Rizvi SL, Dunner DL, Jacobson NS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. Journal of Consulting and Clinical Psychology. 2008;76(3):468–477. doi: 10.1037/0022-006X.76.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson KS, Pusch D. A test of the depressive realism hypothesis in clinically depressed subjects. Cognitive Therapy and Research. 1995;19(2):179–194. [Google Scholar]
- Doebeli M, Hauert C. Models of cooperation based on the Prisoner's Dilemma and the Snowdrift game. Ecology Letters. 2005;8(7):748–766. [Google Scholar]
- Dolcos F, Miller B, Kragel P, Jha A, McCarthy G. Regional brain differences in the effect of distraction during the delay interval of a working memory task. Brain Research. 2007;1152:171–181. doi: 10.1016/j.brainres.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Advancing from the Ventral Striatum to the Extended Amygdala. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biological Psychiatry. 2000;48(8):813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition & Emotion. 1998;12(3):353–385. [Google Scholar]
- Dubal S, Pierson A, Jouvent R. Focused attention in anhedonia: A P3 study. Psychophysiology. 2000;37(5):711–714. [PubMed] [Google Scholar]
- Dunn RT, Willis MW, Benson BE, Repella JD, Kimbrell TA, Ketter TA, et al. Preliminary findings of uncoupling of flow and metabolism in unipolar compared with bipolar affective illness and normal controls. Psychiatry Research-Neuroimaging. 2005;140(2):181–198. doi: 10.1016/j.pscychresns.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Crombez G. Pain demands attention: A cognitive-affective model of the interruptive function of pain. Psychological Bulletin. 1999;125(3):356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- Edwards JA, Weary G. Depression and the impression-formation continuum: Piecemeal processing despite the availability of category information. Journal of Personality and Social Psychology. 1993;64(4):636–645. doi: 10.1037//0022-3514.64.4.636. [DOI] [PubMed] [Google Scholar]
- Edwards JA, Weary G. Antecedents of causal uncertainty and perceived control: a prospective study. European Journal of Personality. 1998;12(2):135–148. [Google Scholar]
- Ekman P. Basic emotions. In: Dalgleish T, Power M, editors. Handbook of cognition and emotion. New York, NY: Wiley; 1999. pp. 3–19. [Google Scholar]
- Elderkin-Thompson V, Kumar A, Bilker WB, Dunkin JJ, Mintz J, Moberg PJ, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Archives of Clinical Neuropsychology. 2003;18(5):529–549. doi: 10.1016/s0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Ellis HC, Ashbrook PW. Resource allocation model of the effects of depressed mood states on memory. In: Fiedler K, Forgas JP, editors. Affect, cognition and social behavior. Gottingen, Germany: Hogrefe; 1988. pp. 25–43. [Google Scholar]
- Ellis HC, Moore BA, Varner LJ, Ottaway SA, Becker AS. Depressed mood, task organization, cognitive interference, and memory: Irrelevant thoughts predict recall performance. Journal of Social Behavior and Personality. 1997;12(2):453–470. [Google Scholar]
- Ellis HC, Ottaway SA, Varner LJ, Becker AS, Moore BA. Emotion, motivation, and text comprehension: The detection of contradictions in passages. Journal of Experimental Psychology-General. 1997;126(2):131–146. doi: 10.1037//0096-3445.126.2.131. [DOI] [PubMed] [Google Scholar]
- Ellis HC, Seibert PS, Herbert BJ. Mood state effects on thought listing. Bulletin of the Psychonomic Society. 1990;28(2):147–150. [Google Scholar]
- Ellis HC, Thomas RL, Rodriguez IA. Emotional mood states and memory: Elaborative encoding, semantic processing, and cognitive effort. Journal of Experimental Psychology-Learning Memory and Cognition. 1984;10(3):470–482. doi: 10.1037//0278-7393.10.3.470. [DOI] [PubMed] [Google Scholar]
- Fein S. Effects of suspicion on attributional thinking and the correspondence bias. Journal of Personality and Social Psychology. 1996;70(6):1164–1184. [Google Scholar]
- Fein S, Hilton JL, Miller DT. Suspicion of ulterior motivation and the correspondence bias. Journal of Personality and Social Psychology. 1990;58(5):753–764. doi: 10.1037//0022-3514.58.5.753. [DOI] [PubMed] [Google Scholar]
- Forgas JP. On being happy and mistaken: Mood effects on the fundamental attribution error. Journal of Personality and Social Psychology. 1998;75(2):318–331. doi: 10.1037//0022-3514.75.2.318. [DOI] [PubMed] [Google Scholar]
- Forgas JP. When sad is better than happy: Negative affect can improve the quality and effectiveness of persuasive messages and social influence strategies. Journal of Experimental Social Psychology. 2007;43(4):513–528. [Google Scholar]
- Frewen PA, Dozois DJA. Recognition and interpretation of facial expressions in dysphoric women. Journal of Psychopathology and Behavioral Assessment. 2005;27(4):5–15. [Google Scholar]
- Frijda NH. The emotions. New York, NY: Cambridge University Press; 1986. [Google Scholar]
- Funahashi S, Inoue M, Kubota K. Delay-period activity in the primate prefrontal cortex encoding multiple spatial positions and their order of presentation. Behavioural Brain Research. 1997;84(1–2):203–223. doi: 10.1016/s0166-4328(96)00151-9. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(14):8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasper K. Do you see what I see? Affect and visual information processing. Cognition & Emotion. 2004;18(3):405–421. [Google Scholar]
- Gasper K, Clore GL. Attending to the big picture: Mood and global versus local processing of visual information. Psychological Science. 2002;13(1):34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]
- Geary DC. Male, female: The evolution of human sex differences. Washington, DC: American Psychological Association; 1998. [Google Scholar]
- Geary DC, Byrd-Craven J, Hoard MK, Vigil J, Numtee C. Evolution and development of boys' social behavior. Developmental Review. 2003;23(4):444–470. [Google Scholar]
- George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. American Journal of Psychiatry. 1995;152(3):341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- German TP, Nichols S. Children's counterfactual inferences about long and short causal chains. Developmental Science. 2003;6(5):514–523. [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35(2):795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesler RB, Josephs RA, Swann WB. Self-verification in clinical depression: The desire for negative evaluation. Journal of Abnormal Psychology. 1996;105(3):358–368. doi: 10.1037//0021-843x.105.3.358. [DOI] [PubMed] [Google Scholar]
- Gilbert DT, Malone PS. The correspondence bias. Psychological Bulletin. 1995;117(1):21–38. doi: 10.1037/0033-2909.117.1.21. [DOI] [PubMed] [Google Scholar]
- Gilbert DT, Pelham BW, Krull DS. On cognitive busyness: When person perceivers meet persons perceived. Journal of Personality and Social Psychology. 1988;54(5):733–740. [Google Scholar]
- Gilbert P. Evolution and depression: Issues and implications. Psychological Medicine. 2006;36(3):287–297. doi: 10.1017/S0033291705006112. [DOI] [PubMed] [Google Scholar]
- Gilbert P, Allan S. The role of defeat and entrapment (arrested flight) in depression: an exploration of an evolutionary view. Psychological Medicine. 1998;28(3):585–598. doi: 10.1017/s0033291798006710. [DOI] [PubMed] [Google Scholar]
- Gilbert P, Gilbert J, Irons C. Life events, entrapments and arrested anger in depression. Journal of Affective Disorders. 2004;79(1–3):149–160. doi: 10.1016/S0165-0327(02)00405-6. [DOI] [PubMed] [Google Scholar]
- Gilbody S, Whitty P, Grimshaw J, Thomas R. Educational and organizational interventions to improve the management of depression in primary care - A systematic review. Jama-Journal of the American Medical Association. 2003;289(23):3145–3151. doi: 10.1001/jama.289.23.3145. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Kim J, Cohen MS, Poutanen VP, Therman S, Bava S, et al. Maintenance and manipulation in spatial working memory: Dissociations in the prefrontal cortex. Neuroimage. 2002;17(1):201–213. doi: 10.1006/nimg.2002.1161. [DOI] [PubMed] [Google Scholar]
- Gortner EM, Rude SS, Pennebaker JW. Benefits of expressive writing in lowering rumination and depressive symptoms. Behavior Therapy. 2006;37(3):292–303. doi: 10.1016/j.beth.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Gortner ET, Gollan JK, Dobson KS, Jacobson NS. Cognitive-behavioral treatment for depression: Relapse prevention. Journal of Consulting and Clinical Psychology. 1998;66(2):377–384. doi: 10.1037//0022-006x.66.2.377. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Asarnow RF. Interpersonal and impersonal problem-solving skills in mildly and clinically depressed university students. Journal of Consulting and Clinical Psychology. 1979;47(1):86–95. doi: 10.1037//0022-006x.47.1.86. [DOI] [PubMed] [Google Scholar]
- Graf MC, Gaudiano BA, Geller PA. Written emotional disclosure: A controlled study of the benefits of expressive writing homework in outpatient psychotherapy. Psychotherapy Research. 2008;18(4):389–399. doi: 10.1080/10503300701691664. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6(3):316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Green VA, Rechis R. Children's cooperative and competitive interactions in limited resource situations: A literature review. Journal of Applied Developmental Psychology. 2006;27(1):42–59. [Google Scholar]
- Greenberger D, Padesky C. Mind over mood: Change how you feel by changing the way you think. New York, NY: The Guilford Press; 1995. [Google Scholar]
- Guastella AJ, Moulds ML. The impact of rumination on sleep quality following a stressful life event. Personality and Individual Differences. 2007;42(6):1151–1162. [Google Scholar]
- Gunther DC, Ferraro FR, Kirchner T. Influence of emotional state on irrelevant thoughts. Psychonomic Bulletin & Review. 1996;3(4):491–494. doi: 10.3758/BF03214552. [DOI] [PubMed] [Google Scholar]
- Gut E. Productive and unproductive depression: Success or failure of a vital process. New York, NY: Basic Books; 1989. [Google Scholar]
- Hadley C, Patil CL. Seasonal changes in household food insecurity and symptoms of anxiety and depression. American Journal of Physical Anthropology. 2008;135(2):225–232. doi: 10.1002/ajpa.20724. [DOI] [PubMed] [Google Scholar]
- Hagen EH. The functions of postpartum depression. Evolution and Human Behavior. 1999;20(5):325–359. [Google Scholar]
- Hagen EH. Depression as bargaining - The case postpartum. Evolution and Human Behavior. 2002;23(5):323–336. [Google Scholar]
- Hagen EH. The bargaining model of depression. In: Hammerstein P, editor. Genetic and Cultural Evolution of Cooperation. Cambridge, MA: MIT Press; 2003. pp. 95–123. [Google Scholar]
- Hagen EH, Thomson JA. Social navigation hypothesis of depression revisited. Journal of Affective Disorders. 2004;83(2–3):285–286. doi: 10.1016/j.jad.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. I. Journal of Theoretical Biology. 1964a;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. II. Journal of Theoretical Biology. 1964b;7:17–32. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- Hammen C. Life events and depression: The plot thickens. American Journal of Community Psychology. 1992;20(2):179–193. doi: 10.1007/BF00940835. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Fraley RC, Lahey BB, Waldman ID. Is depression best viewed as a continuum or discrete category? A taxometric analysis of childhood and adolescent depression in a population-based sample. Journal of Abnormal Psychology. 2005;114(1):96–110. doi: 10.1037/0021-843X.114.1.96. [DOI] [PubMed] [Google Scholar]
- Hara MR, Snyder SH. Cell signaling and neuronal death. Annual Review of Pharmacology and Toxicology. 2007;47:117–141. doi: 10.1146/annurev.pharmtox.47.120505.105311. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Sabbagh MA, Jacobson JA, Chowdrey NK, Chen T. Enhanced accuracy of mental state decoding in dysphoric college students. Cognition & Emotion. 2005;19(7):999–1025. [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Doubly dissociable effects of median- and dorsal-raphe lesions on the performance of the five-choice serial reaction time test of attention in rats. Behavioural Brain Research. 1997;89(1–2):135–149. doi: 10.1016/s0166-4328(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Hartlage S, Alloy LB, Vazquez C, Dykman B. Automatic and effortful processing in depression. Psychological Bulletin. 1993;113(2):247–278. doi: 10.1037/0033-2909.113.2.247. [DOI] [PubMed] [Google Scholar]
- Harvey JH, Town JP, Yarkin KL. How fundamental Is the fundamental attribution error? Journal of Personality and Social Psychology. 1981;40(2):346–349. [Google Scholar]
- Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes and Infection. 2000;2(15):1891–1904. doi: 10.1016/s1286-4579(00)01337-x. [DOI] [PubMed] [Google Scholar]
- Hayes AM, Beevers CG, Feldman GC, Laurenceau JP, Perlman C. Avoidance and processing as predictors of symptom change and positive growth in an integrative therapy for depression. International Journal of Behavioral Medicine. 2005;12(2):111–122. doi: 10.1207/s15327558ijbm1202_9. [DOI] [PubMed] [Google Scholar]
- Hayes AM, Feldman GC, Beevers CG, Laurenceau JP, Cardaciotto L, Lewis-Smith J. Discontinuities and cognitive changes in an exposure-based cognitive therapy for depression. Journal of Consulting and Clinical Psychology. 2007;75(3):409–421. doi: 10.1037/0022-006X.75.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AM, Laurenceau JP, Feldman G, Strauss JL, Cardaciotto L. Change is not always linear: The study of nonlinear and discontinuous patterns of change in psychotherapy. Clinical Psychology Review. 2007;27(6):715–723. doi: 10.1016/j.cpr.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(25):15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepner KA, Rowe M, Rost K, Hickey SC, Sherbourne CD, Ford DE, et al. The effect of adherence to practice guidelines on depression outcomes. Annals of Internal Medicine. 2007;147(5):320–329. doi: 10.7326/0003-4819-147-5-200709040-00007. [DOI] [PubMed] [Google Scholar]
- Hertel G, Neuhof J, Theuer T, Kerr NL. Mood effects on cooperation in small groups: Does positive mood simply lead to more cooperation? Cognition & Emotion. 2000;14(4):441–472. [Google Scholar]
- Hertel PT, Rude SS. Depressive deficits in memory: Focusing attention improves subsequent recall. Journal of Experimental Psychology-General. 1991;120(3):301–309. doi: 10.1037/0096-3445.120.3.301. [DOI] [PubMed] [Google Scholar]
- Hilton JL, Fein S, Miller DT. Suspicion and dispositional inference. Personality and Social Psychology Bulletin. 1993;19(5):501–512. [Google Scholar]
- Hjorth S, Bengtsson HJ, Kullberg A, Carlzon D, Peilot H, Auerbach SB. Serotonin autoreceptor function and antidepressant drug action. Journal of Psychopharmacology. 2000;14(2):177–185. doi: 10.1177/026988110001400208. [DOI] [PubMed] [Google Scholar]
- Hobel C, Culhane J. Role of psychosocial and nutritional stress on poor pregnancy outcome. Journal of Nutrition. 2003;133(5):1709S–1717S. doi: 10.1093/jn/133.5.1709S. [DOI] [PubMed] [Google Scholar]
- Hokanson JE, Sacco WP, Blumberg SR, Landrum GC. Interpersonal-behavior of depressive individuals in a mixed-motive game. Journal of Abnormal Psychology. 1980;89(3):320–332. doi: 10.1037//0021-843x.89.3.320. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Thase ME, Markowitz JC. Treatment and prevention of depression. Psychological Science in the Public Interest. 2002;3(2):39–77. doi: 10.1111/1529-1006.00008. [DOI] [PubMed] [Google Scholar]
- Horwitz AV, Wakefield JC. The loss of sadness: How psychiatry transformed normal sorrow into depressive disorder. New York, NY: Oxford University Press; 2007. [DOI] [PubMed] [Google Scholar]
- Howland RH, Rush AJ, Wisniewski SR, Trivedi MH, Warden D, Fava M, et al. Concurrent anxiety and substance use disorders among outpatients with major depression: Clinical features and effect on treatment outcome. Drug and Alcohol Dependence. 2009;99(1–3):248–260. doi: 10.1016/j.drugalcdep.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Humphrey NK. The social function of intellect. In: Bateson PPG, Hinde RA, editors. Growing points in ethology. Cambridge, UK: Cambridge University Press; 1976. pp. 303–317. [Google Scholar]
- Isen AM, Daubman KA, Nowicki GP. Positive affect facilitates creative problem-solving. Journal of Personality and Social Psychology. 1987;52(6):1122–1131. doi: 10.1037//0022-3514.52.6.1122. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21(2):S9–S15. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Jacobson JA, Weary G, Edwards JA. Certainty-related beliefs and depressive symptomatology: Concurrent and longitudinal relationships. Social Cognition. 1999;17(1):19–45. [Google Scholar]
- Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollan JK, et al. A component analysis of cognitive-behavioral treatment for depression. Journal of Consulting and Clinical Psychology. 1996;64(2):295–304. doi: 10.1037//0022-006x.64.2.295. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Martell CR, Dimidjian S. Behavioral activation treatment for depression: Returning to contextual roots. Clinical Psychology-Science and Practice. 2001;8(3):255–270. [Google Scholar]
- Jenkins SR, Goodness K, Buhrmester D. Gender differences in early adolescents' relationship qualities, self-efficacy, and depression symptoms. Journal of Early Adolescence. 2002;22(3):277–309. [Google Scholar]
- Jones EE. Rocky road from acts to dispositions. American Psychologist. 1979;34(2):107–117. doi: 10.1037//0003-066x.34.2.107. [DOI] [PubMed] [Google Scholar]
- Jones EE, Harris VA. Attribution of attitudes. Journal of Experimental Social Psychology. 1967;3(1):1–24. [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139(1):181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Just N, Alloy LB. The response styles theory of depression: Tests and an extension of the theory. Journal of Abnormal Psychology. 1997;106(2):221–229. doi: 10.1037//0021-843x.106.2.221. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Attention and effort. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- Kalueff AV, Wheaton M, Murphy DL. What's wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behavioural Brain Research. 2007;179(1):1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. 3. Norwalk, CT: Appleton & Lange; 1991. [Google Scholar]
- Kane MJ. Full frontal fluidity? Looking in on the neuroimaging of reasoning and intelligence. In: Wilhelm O, Engle RW, editors. Handbook of Understanding and Measuring Intelligence. Thousand Oaks, CA: Sage; 2005. pp. 141–163. [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology. 2000;9(4):156–185. [Google Scholar]
- Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. American Journal of Psychiatry. 2007;164(10):1521–1529. doi: 10.1176/appi.ajp.2007.06091564. [DOI] [PubMed] [Google Scholar]
- Keller MC, Nesse RM. The evolutionary significance of depressive symptoms: Different adverse situations lead to different depressive symptom patterns. Journal of Personality and Social Psychology. 2006;91(2):316–330. doi: 10.1037/0022-3514.91.2.316. [DOI] [PubMed] [Google Scholar]
- Keller PA, Lipkus IA, Rimer BK. Depressive realism and health risk accuracy: The negative consequences of positive mood. Journal of Consumer Research. 2002;29:57–69. [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Archives of General Psychiatry. 2003;60(8):789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156(6):837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, environment, and psychopathology: Understanding the causes of psychiatric and substance use disorders. New York, NY: The Guilford Press; 2006. [Google Scholar]
- Kennair LEO. Evolutionary psychology and psychopathology. Current Opinion in Psychiatry. 2003;16(6):691–699. [Google Scholar]
- Kesner RP. Subregional analysis of mnemonic functions of the prefrontal cortex in the rat. Psychobiology. 2000;28(2):219–228. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Zhao SY, Blazer DG, Swartz M. Prevalence, correlates, and course of minor depression and major depression in the national comorbidity survey. Journal of Affective Disorders. 1997;45(1–2):19–30. doi: 10.1016/s0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, ElMestikawy S, et al. Immunocytochemical localization of serotonin(1A) receptors in the rat central nervous system. Journal of Comparative Neurology. 1996;365(2):289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kirchsteiger G, Rigotti L, Rustichini A. Your morals might be your moods. Journal of Economic Behavior & Organization. 2006;59(2):155–172. [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: A meta-analysis of data submitted to the food and drug administration. Plos Medicine. 2008;5(2):260–268. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. The adaptive value of fever. Infectious Disease Clinics of North America. 1996;10(1):1–&. doi: 10.1016/s0891-5520(05)70282-8. [DOI] [PubMed] [Google Scholar]
- Koetsier GC, Volkers AC, Tulen JHM, Passchier J, van den Broek WW, Bruijn JA. CPT performance in major depressive disorder before and after treatment with imipramine or fluvoxamine. Journal of Psychiatric Research. 2002;36(6):391–397. doi: 10.1016/s0022-3956(02)00026-2. [DOI] [PubMed] [Google Scholar]
- Kohrt BA, Kunz RD, Baldwin JL, Koirala NR, Sharma VD, Nepal MK. “Somatization” and “comorbidity”: A study of jhum-jhum and depression in rural Nepal. Ethos. 2005;33(1):125–147. [Google Scholar]
- Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disorders. 2008;10(1):1–37. doi: 10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- Korszun A, Moskvina V, Brewster S, Craddock N, Ferrero F, Gill M, et al. Familiality of symptom dimensions in depression. Archives of General Psychiatry. 2004;61(5):468–474. doi: 10.1001/archpsyc.61.5.468. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. In: Clinical psychiatry. 2. Diefendorf AR, translator. Norwood, MA: Norwood Press; 1907. [Google Scholar]
- Kramer PD. Against depression. New York, NY: Viking; 2005. [Google Scholar]
- Krueger RF, Markon KE. Understanding psychopathology: Melding behavior genetics, personality, and quantitative psychology to develop an empirically based model. Current Directions in Psychological Science. 2006;15(3):113–117. doi: 10.1111/j.0963-7214.2006.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C, Weber I. Responses to depression in unipolar depressed patients: an investigation of Nolen-Hoeksema's response styles theory. Psychological Medicine. 1999;29(6):1323–1333. doi: 10.1017/s0033291799001282. [DOI] [PubMed] [Google Scholar]
- Kuiper NA, Olinger LJ, Swallow SR. Dysfunctional attitudes, mild depression, views of self, self-consciousness, and social perceptions. Motivation and Emotion. 1987;11(4):379–401. [Google Scholar]
- Kuo PH, Gardner CO, Kendler KS, Prescott CA. The temporal relationship of the onsets of alcohol dependence and major depression: Using a genetically informative study design. Psychological Medicine. 2006;36(8):1153–1162. doi: 10.1017/S0033291706007860. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Dandes SK, Wick P, Shaw K, Stone WL. Development of the Social Anxiety Scale for Children: Reliability and concurrent validity. Journal of Clinical Child Psychology. 1988;17(1):84–91. [Google Scholar]
- La Greca AM, Lopez N. Social anxiety among adolescents: Linkages with peer relations and friendships. Journal of Abnormal Child Psychology. 1998;26(2):83–94. doi: 10.1023/a:1022684520514. [DOI] [PubMed] [Google Scholar]
- Lam D, Smith N, Checkley S, Rijsdijk F, Sham P. Effect of neuroticism, response style and information processing on depression severity in a clinically depressed sample. Psychological Medicine. 2003;33(3):469–479. doi: 10.1017/s0033291702007304. [DOI] [PubMed] [Google Scholar]
- Lane JD, DePaulo BM. Completing Coyne's cycle: Dysphorics' ability to detect deception. Journal of Research in Personality. 1999;33(3):311–329. [Google Scholar]
- LeDoux J. The emotional brain: The mysterious underpinnings of emotional life. New York, NY: Simon & Schuster; 1996. [Google Scholar]
- Lee AL, Ogle WO, Sapolsky RM. Stress and depression: Possible links to neuron death in the hippocampus. Bipolar Disorders. 2002;4(2):117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- Lee L, Harkness KL, Sabbagh MA, Jacobson JA. Mental state decoding abilities in clinical depression. Journal of Affective Disorders. 2005;86(2–3):247–258. doi: 10.1016/j.jad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Lee WE, Wadsworth MEJ, Hotopf A. The protective role of trait anxiety: A longitudinal cohort study. Psychological Medicine. 2006;36(3):345–351. doi: 10.1017/S0033291705006847. [DOI] [PubMed] [Google Scholar]
- Leff MJ, Roatch JF, Bunney WE. Environmental factors preceding onset of severe depressions. Psychiatry. 1970;33(3):293–&. doi: 10.1080/00332747.1970.11023630. [DOI] [PubMed] [Google Scholar]
- Lemelin S, Baruch P. Clinical psychomotor retardation and attention in depression. Journal of Psychiatric Research. 1998;32(2):81–88. doi: 10.1016/S0022-3956(98)00002-8. [DOI] [PubMed] [Google Scholar]
- Leppanen JM. Emotional information processing in mood disorders: A review of behavioral and neuroimaging findings. Current Opinion in Psychiatry. 2006;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Levenson RW. The intrapersonal functions of emotion. Cognition & Emotion. 1999;13(5):481–504. [Google Scholar]
- Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: Mood challenge in patients with remitted unipolar depression. American Journal of Psychiatry. 2002;159(11):1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Love T, Haist F, Nicol J, Swinney D. A functional neuroimaging investigation of the roles of structural complexity and task-demand during auditory sentence processing. Cortex. 2006;42(4):577–590. doi: 10.1016/s0010-9452(08)70396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S, Kasri F, Zehm K. Dysphoric rumination impairs concentration on academic tasks. Cognitive Therapy and Research. 2003;27(3):309–330. [Google Scholar]
- Lyubomirsky S, Nolen-Hoeksema S. Self-perpetuating properties of dysphoric rumination. Journal of Personality and Social Psychology. 1993;65(2):339–349. doi: 10.1037//0022-3514.65.2.339. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Tucker KL, Caldwell ND, Berg K. Why ruminators are poor problem solvers: Clues from the phenomenology of dysphoric rumination. Journal of Personality and Social Psychology. 1999;77(5):1041–1060. doi: 10.1037//0022-3514.77.5.1041. [DOI] [PubMed] [Google Scholar]
- Maccoby EE. Gender and relationships: A developmental account. American Psychologist. 1990;45(4):513–520. doi: 10.1037//0003-066x.45.4.513. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY. The serotonin hypothesis of major depression. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The fourth generation of progress. Raven Press; 1999. CD-ROM edition ed. [Google Scholar]
- Magistretti PJ, Ransom BR. Astrocytes. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The fifth generation of progress. Philadelphia, PA: Lipincott Williams & Wilkins; 2002. pp. 133–145. [Google Scholar]
- Maier W, Lichtermann D, Heun R, Hallmayer J. The risk of minor depression in families of probands with major depression: Sex differences and familiality. European Archives of Pschiatry and Clinical Neuroscience. 1992;242:89–92. doi: 10.1007/BF02191553. [DOI] [PubMed] [Google Scholar]
- Major B, Zubek JM, Cooper ML, Cozzarelli C, Richards C. Mixed messages: Implications or conflict and social support within close relationships for adjustment to a stressful life. Journal of Personality and Social Psychology. 1997;72(6):1349–1363. doi: 10.1037//0022-3514.72.6.1349. [DOI] [PubMed] [Google Scholar]
- Mandelli L, Serretti A, Colombo C, Florita M, Santoro A, Rossini D, et al. Improvement of cognitive functioning in mood disorder patients with depressive symptomatic recovery during treatment: An exploratory analysis. Psychiatry and Clinical Neurosciences. 2006;60(5):598–604. doi: 10.1111/j.1440-1819.2006.01564.x. [DOI] [PubMed] [Google Scholar]
- Marklund P, Fransson P, Cabeza R, Petersson KM, Ingvar M, Nyberg L. Sustained and transient neural modulations in prefrontal cortex related to declarative long-term memory, working memory, and attention. Cortex. 2007;43(1):22–37. doi: 10.1016/s0010-9452(08)70443-x. [DOI] [PubMed] [Google Scholar]
- Markman KD, Miller AK. Depression, control, and counterfactual thinking: Functional for whom? Journal of Social and Clinical Psychology. 2006;25(2):210–227. [Google Scholar]
- Markman KD, Weary G. The influence of chronic control concerns on counterfactual thought. Social Cognition. 1996;14(4):292–316. [Google Scholar]
- Marlowe FW. The mating system of foragers in the standard cross-cultural sample. Cross-Cultural Research. 2003;37(3):282–306. [Google Scholar]
- Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends in Cognitive Sciences. 2005;9(6):296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Marsh KL, Weary G. Severity of depression and responsiveness to attributional information. Journal of Social and Clinical Psychology. 1994;13(1):15–32. [Google Scholar]
- Martin LL, Tesser A. Some ruminative thoughts. In: Wyer RS Jr, editor. Advances in social cognition. Vol. 9. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. pp. 1–47. [Google Scholar]
- Matheson K, Anisman H. Systems of coping associated with dysphoria, anxiety and depressive illness: A multivariate profile perspective. Stress-the International Journal on the Biology of Stress. 2003;6(3):223–234. doi: 10.1080/10253890310001594487. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant. Journal of Pharmacology and Experimental Therapeutics. 2001;298(3):1101–1107. [PubMed] [Google Scholar]
- McCaul KD. Observer attributions of depressed students. Personality and Social Psychology Bulletin. 1983;9(1):74–82. [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Millar JS. Adaptive features of mammalian reproduction. Evolution. 1977;31(2):370–386. doi: 10.1111/j.1558-5646.1977.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. Journal of Neuroscience. 1996;16(16):5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term-memory task. Journal of Neuroscience. 1993;13(4):1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M. National patterns in antidepressant treatment by psychiatrists and general medical providers: Results from the National Comorbidity Survey Replication. Journal of Clinical Psychiatry. 2008;69(7):1064–1074. doi: 10.4088/jcp.v69n0704. [DOI] [PubMed] [Google Scholar]
- Moller AP, Swaddle JP. Asymmetry, developmental instability and evolution. Oxford, UK: Oxford University Press; 1998. [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Moore P, Landolt HP, Seifritz E, Clark C, Bhatti T, Kelsoe J, et al. Clinical and physiological consequences of rapid tryptophan depletion. Neuropsychopharmacology. 2000;23(6):601–622. doi: 10.1016/S0893-133X(00)00161-5. [DOI] [PubMed] [Google Scholar]
- Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10(2):163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- Morrow J, Nolen-Hoeksema S. Effects of responses to depression on the remediation of depressive affect. Journal of Personality and Social Psychology. 1990;58(3):519–527. doi: 10.1037//0022-3514.58.3.519. [DOI] [PubMed] [Google Scholar]
- Msetfi RM, Murphy RA, Simpson J, Kornbrot DE. Depressive realism and outcome density bias in contingency judgments: The effect of the context and intertrial interval. Journal of Experimental Psychology-General. 2005;134(1):10–22. doi: 10.1037/0096-3445.134.1.10. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: Gene, genetic disorders, and pharmacogenetics. Molecular Interventions. 2004;4(2):109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Nasco SA, Marsh KL. Gaining control through counterfactual thinking. Personality and Social Psychology Bulletin. 1999;25(5):556–568. [Google Scholar]
- Nathan PJ, Sitaram C, Stough C, Silberstein RB, Sali A. Serotonin, noradrenaline and cognitive function: A preliminary investigation of the acute pharmacodynamic effects of a serotonin versus a serotonin and noradrenaline reuptake inhibitor. Behavioural Pharmacology. 2000;11(7–8):639–642. doi: 10.1097/00008877-200011000-00011. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Coles JA. Cellular pathways of energy metabolism in the brain: Is glucose used by neurons or astrocytes? Glia. 2007;55(12):1238–1250. doi: 10.1002/glia.20376. [DOI] [PubMed] [Google Scholar]
- Nesse RM. Is depression an adaptation? Archives of General Psychiatry. 2000;57(1):14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Williams GC. Why we get sick: The new science of Darwinian medicine. New York, NY: Times Books; 1994. [Google Scholar]
- Nettle D. Evolutionary origins of depression: A review and reformulation. Journal of Affective Disorders. 2004;81(2):91–102. doi: 10.1016/j.jad.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Nikisch G, Mathe AA, Czernik A, Eap CB, Jimenez-Vasquez P, Brawand-Amey M, et al. Stereoselective metabolism of citalopram in plasma and cerebrospinal fluid of depressive patients - Relationship with S-HIAA in CSF and clinical response. Journal of Clinical Psychopharmacology. 2004;24(3):283–290. doi: 10.1097/01.jcp.0000125680.89843.a6. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Sex differences in depression. Stanford, CA: Stanford University Press; 1990. [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Davis CG. Theoretical and methodological issues in the assessment and interpretation of posttraumatic growth. Psychological Inquiry. 2004;15:60–64. [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective-study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma-Prieta earthquake. Journal of Personality and Social Psychology. 1991;61(1):115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. Effects of rumination and distraction on naturally-occurring depressed mood. Cognition & Emotion. 1993;7(6):561–570. [Google Scholar]
- Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. Journal of Abnormal Psychology. 1993;102(1):20–28. doi: 10.1037//0021-843x.102.1.20. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Stice E, Wade E, Bohon C. Reciprocal relations between rumination and bulimic, substance abuse, and depressive symptoms in female adolescents. Journal of Abnormal Psychology. 2007;116(1):198–207. doi: 10.1037/0021-843X.116.1.198. [DOI] [PubMed] [Google Scholar]
- O'Hanlon JF, Robbe HWJ, Vermeeren A, Van Leeuwen C, Danjou PE. Venlafaxine's effects on healthy volunteers' driving, psychomotor, and vigilance performance during 15-day fixed and incremental dosing regimens. Journal of Clinical Psychopharmacology. 1998;18(3):212–221. doi: 10.1097/00004714-199806000-00006. [DOI] [PubMed] [Google Scholar]
- Oaksford M, Morris F, Grainger B, Williams JMG. Mood, reasoning, and central executive processes. Journal of Experimental Psychology-Learning Memory and Cognition. 1996;22(2):476–492. [Google Scholar]
- Page CM, Colby PM. If only I hadn't smoked: The impact of counterfactual thinking on smoking-related behavior. Psychology & Marketing. 2003;20(11):955–976. [Google Scholar]
- Pagel MD, Erdly WW, Becker J. Social networks: We get by with (and in spite of) a little help from our friends. Journal of Personality and Social Psychology. 1987;53(4):793–804. doi: 10.1037//0022-3514.53.4.793. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349(6304):61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Raichle ME. Neural correlates of self-induced dysphoria. American Journal of Psychiatry. 1993;150(5):713–719. doi: 10.1176/ajp.150.5.713. [DOI] [PubMed] [Google Scholar]
- Park RJ, Goodyer IM, Teasdale JD. Effects of induced rumination and distraction on mood and overgeneral autobiographical memory in adolescent Major Depressive Disorder and controls. Journal of Child Psychology and Psychiatry. 2004;45(5):996–1006. doi: 10.1111/j.1469-7610.2004.t01-1-00291.x. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HTIA receptor binding potential during a major depressive episode predicts poor treatment response: Preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31(8):1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- Patel V. Cultural factors and international epidemiology. British Medical Bulletin. 2001;57:33–45. doi: 10.1093/bmb/57.1.33. [DOI] [PubMed] [Google Scholar]
- Patil C, Hadley C. Symptoms of anxiety and depression and mother's marital status: An exploratory analysis of polygyny and psychosocial stress. American Journal of Human Biology. 2008;20(4):475–477. doi: 10.1002/ajhb.20736. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, et al. Activity-dependent regulation of energy metabolism by astrocytes: An update. Glia. 2007;55(12):1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pietromonaco PR, Rook KS. Decision style in depression: The contribution of perceived risks versus benefits. Journal of Personality and Social Psychology. 1987;52(2):399–408. [Google Scholar]
- Pike IL, Patil CL. Understanding women's burdens: Preliminary findings on psychosocial health among Datoga and Iraqw women of northern Tanzania. Culture Medicine and Psychiatry. 2006;30(3):299–330. doi: 10.1007/s11013-006-9022-2. [DOI] [PubMed] [Google Scholar]
- Pinker S. The stuff of thought: Language as a window into human nature. New York, NY: Viking; 2007. [Google Scholar]
- Plaisance KI, Kudaravalli S, Wasserman SS, Levine MM, Mackowiak PA. Effect of antipyretic therapy on the duration of illness in experimental influenza A, Shigella sonnei, and Rickettsia rickettsii infections. Pharmacotherapy. 2000;20(12):1417–1422. doi: 10.1592/phco.20.19.1417.34865. [DOI] [PubMed] [Google Scholar]
- Postle BR. Distraction-spanning sustained activity during delayed recognition of locations. Neuroimage. 2006;30(3):950–962. doi: 10.1016/j.neuroimage.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Price J, Sloman L, Gardner R, Gilbert P, Rohde P. The social competition hypothesis of depression. British Journal of Psychiatry. 1994;164:309–315. doi: 10.1192/bjp.164.3.309. [DOI] [PubMed] [Google Scholar]
- Prins MA, Verhaak PFM, Bensing JM, van der Meer K. Health beliefs and perceived need for mental health care of anxiety and depression: The patients' perspective explored. Clinical Psychology Review. 2008;28(6):1038–1058. doi: 10.1016/j.cpr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Pyszczynski T, Greenberg J. Self-regulatory perseveration and the depressive self-focusing style: A self-awareness theory of reactive depression. Psychological Bulletin. 1987;102(1):122–138. [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biological Psychiatry. 2000;48(8):766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Muntjewerff ND, O'Hanlon JF. A comparative-study of acute and subchronic effects of dothiepin, fluoxetine and placebo on psychomotor and actual driving performance. British Journal of Clinical Pharmacology. 1995;39(4):397–404. doi: 10.1111/j.1365-2125.1995.tb04468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Serotonin receptor 1A knockout: An animal model of anxiety-related disorder. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(24):14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276(5313):821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Advanced Progressive Matrices: Sets I & II. Manual for Raven’s progressive matrices and vocabulary scales. Oxford, England: Oxford Psychologists Press Ltd; 1994. [Google Scholar]
- Reisberg D. Cognition: Exploring the science of the mind. New York, NY: W. W. Norton & Company; 2006. [Google Scholar]
- Riedel WJ, Eikmans K, Heldens A, Schmitt JA. Specific serotonergic reuptake inhibition impairs vigilance performance acutely and after subchronic treatment. Journal of Psychopharmacology. 2005;19(1):12–20. doi: 10.1177/0269881105048887. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163(3–4):362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Roese NJ. The functional basis of counterfactual thinking. Journal of Personality and Social Psychology. 1994;66(5):805–818. [Google Scholar]
- Roese NJ, Olson JM. Counterfactual thinking: The intersection of affect and function. Advances in Experimental Social Psychology. 1997;29:1–59. [Google Scholar]
- Rose AJ, Rudolph KD. A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin. 2006;132(1):98–131. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg K, Trevathan W. Birth, obstetrics and human evolution. Bjog-an International Journal of Obstetrics and Gynaecology. 2002;109(11):1199–1206. doi: 10.1046/j.1471-0528.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- Ross L. The intuitive psychologist and his shortcomings: Distortions in the attribution process. In: Berkowitz L, editor. Advances in experimental psychology. Vol. 10. New York, NY: Academic Press; 1977. pp. 173–220. [Google Scholar]
- Rudolph KD, Conley CS. The socioemotional costs and benefits of social-evaluative concerns: Do girls care too much? Journal of Personality. 2005;73(1):115–137. doi: 10.1111/j.1467-6494.2004.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakashita C, Slade T, Andrews G. Empirical investigation of two assumptions in the diagnosis of DSM-IV major depressive episode. Australian and New Zealand Journal of Psychiatry. 2007;41(1):17–23. doi: 10.1080/00048670601050440. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why zebras don't get ulcers. 3. New York, NY: Henry Holt and Company; 2004. [Google Scholar]
- Schaller M, Cialdini RB. Happiness, sadness, and helping: A motivational integration. In: Higgins ET, Sorrentino RM, editors. Handbook of motivation & cognition: Foundations of social behavior. Vol. 2. New York, NY: Guilford Press; 1990. pp. 265–296. [Google Scholar]
- Schmaling KB, Dimidjian S, Katon W, Sullivan M. Response styles among patients with minor depression and dysthymia in primary care. Journal of Abnormal Psychology. 2002;111(2):350–356. doi: 10.1037//0021-843x.111.2.350. [DOI] [PubMed] [Google Scholar]
- Schmitt JAJ, Kruizinga MJ, Riedel WJ. Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors. Journal of Psychopharmacology. 2001;15(3):173–179. doi: 10.1177/026988110101500304. [DOI] [PubMed] [Google Scholar]
- Schmitt JAJ, Ramaekers JG, Kruizinga MJ, van Boxtel MPJ, Vuurman E, Riedel WJ. Additional dopamine reuptake inhibition attenuates vigilance impairment induced by serotonin reuptake inhibition in man. Journal of Psychopharmacology. 2002;16(3):207–214. doi: 10.1177/026988110201600303. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Lenroot RK, Wallace GL, Ordaz S, Taylor KN, Kabani N, et al. Identification of genetically mediated cortical networks: A multivariate study of pediatric twins and siblings. Cerebral Cortex. 2008;18(8):1737–1747. doi: 10.1093/cercor/bhm211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N. Feelings as information: Informational and motivational functions of affective states. In: Higgins ET, Sorrentino RM, editors. Handbook of motivation & cognition: Foundations of social behavior. Vol. 2. New York, NY: Guilford Press; 1990. pp. 527–561. [Google Scholar]
- Schwarz N, Bless H. Happy and mindless, but sad and smart? The impact of affective states on analytic reasoning. In: Forgas JP, editor. Emotion and social judgments. Elmsford, NY: Pergamon Press; 1991. pp. 55–71. [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: A review of acute tolerance and recovery of cognitive performance. Experimental and Clinical Psychopharmacology. 2008;16(3):240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Schwenzer M, Heitkamp HC, Mathiak K. Differential effects of indoleamines on auditory choice reaction, selective attention, and pitch discrimination. Neuroscience Letters. 2006;398(3):310–313. doi: 10.1016/j.neulet.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Sedikides C. Assessment, enhancement, and verification determinants of the self-evaluation process. Journal of Personality and Social Psychology. 1993;65(2):317–338. [Google Scholar]
- Segrin C. Social skills deficits associated with depression. Clinical Psychology Review. 2000;20(3):379–403. doi: 10.1016/s0272-7358(98)00104-4. [DOI] [PubMed] [Google Scholar]
- Segrin C, Dillard JP. The interactional theory of depression: A meta-analysis of the research literature. Journal of Social and Clinical Psychology. 1992;11(1):43–70. [Google Scholar]
- Seibert PS, Ellis HC. A convenient self-referencing mood induction procedure. Bulletin of the Psychonomic Society. 1991a;29(2):121–124. [Google Scholar]
- Seibert PS, Ellis HC. Irrelevant thoughts, emotional mood states, and cognitive task-performance. Memory & Cognition. 1991b;19(5):507–513. doi: 10.3758/bf03199574. [DOI] [PubMed] [Google Scholar]
- Seligman MEP. Helplessness: On depression, development and death. New York, NY: W. H. Freeman; 1975. [Google Scholar]
- Semmler C, Brewer N. Research report: Effects of mood and emotion on juror processing and judgments. Behavioral Sciences & the Law. 2002;20(4):423–436. doi: 10.1002/bsl.502. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Queree P. Important messages in the 'post': Recent discoveries in 5-HT neurone feedback control. Trends in Pharmacological Sciences. 2007;28(12):629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Sheeber L, Hops H, Davis B. Family processes in adolescent depression. Clinical Child and Family Psychology Review. 2001;4(1):19–35. doi: 10.1023/a:1009524626436. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Bardgett ME, Csernansky JG. Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. Journal of Clinical Psychopharmacology. 1997;17(1):11–14. doi: 10.1097/00004714-199702000-00003. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: Neurochemical basis of functional imaging. Proceedings of the National Academy of Sciences of the United States of America. 2001a;98(11):6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. Nmr in Biomedicine. 2001b;14(7–8):389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- Sinclair RC. Mood, categorization breadth, and performance-appraisal: The effects of order of information acquisition and affective state on halo, accuracy, information-retrieval, and evaluations. Organizational Behavior and Human Decision Processes. 1988;42(1):22–46. [Google Scholar]
- Sinclair RC, Mark MM. The effects of mood state on judgmental accuracy: Processing strategy as a mechanism. Cognition & Emotion. 1995;9(5):417–438. [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Research Reviews. 1998;26(2–3):113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Wakefield JC. DSM-IV diagnostic criterion for clinical significance: Does it help solve the false positives problem? American Journal of Psychiatry. 1999;156(12):1856–1864. doi: 10.1176/ajp.156.12.1856. [DOI] [PubMed] [Google Scholar]
- Stein RA, Strickland TL. A review of the neuropsychological effects of commonly used prescription medications. Archives of Clinical Neuropsychology. 1998;13(3):259–284. [PubMed] [Google Scholar]
- Storbeck J, Clore GL. With sadness comes accuracy; With happiness, false memory - Mood and the false memory effect. Psychological Science. 2005;16(10):785–791. doi: 10.1111/j.1467-9280.2005.01615.x. [DOI] [PubMed] [Google Scholar]
- Stouthamer R, Luck RF, Hamilton WD. Antibiotics cause parthenogenetic Trichogramma (Hymenoptera, Trichogrammatidae) to revert to sex. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(7):2424–2427. doi: 10.1073/pnas.87.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow SR, Kuiper NA. Mild depression and frequency of social- comparison behavior. Journal of Social and Clinical Psychology. 1992;11(2):167–180. [Google Scholar]
- Swallow SR, Kuiper NA. Social-comparison in dysphoria and nondysphoria: Differences in target similarity and specificity. Cognitive Therapy and Research. 1993;17(2):103–122. [Google Scholar]
- Swann WB, Wenzlaff RM, Krull DS, Pelham BW. Allure of negative feedback: Self-verification strivings among depressed persons. Journal of Abnormal Psychology. 1992;101(2):293–306. doi: 10.1037/0021-843x.101.2.293. [DOI] [PubMed] [Google Scholar]
- Swann WB, Wenzlaff RM, Tafarodi RW. Depression and the search for negative evaluations: More evidence of the role of self-verification strivings. Journal of Abnormal Psychology. 1992;101(2):314–317. doi: 10.1037//0021-843x.101.2.314. [DOI] [PubMed] [Google Scholar]
- Sweeney PD, Anderson K, Bailey S. Attributional style in depression: A meta-analytic review. Journal of Personality and Social Psychology. 1986;50(5):974–991. doi: 10.1037//0022-3514.50.5.974. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Brown JD. Illusion and well-being: A social psychological perspective on mental-health. Psychological Bulletin. 1988;103(2):193–210. [PubMed] [Google Scholar]
- Tedeschi RG, Calhoun LG. Posttraumatic growth: Conceptual foundations and empirical evidence. Psychological Inquiry. 2004;15(1):1–18. [Google Scholar]
- Thornhill R. The study of adaptation. In: Bekoff M, Jamieson D, editors. Interpretation and explanation in the study of animal behavior: Explanation, evolution and adaptation. Boulder, CO: Westview Press; 1990. pp. 31–62. [Google Scholar]
- Thornhill R. The concept of an evolved adaptation. In: Bock G, Cardew G, editors. Characterizing human psychological adaptations. London, UK: CIBA Foundation; 1997. pp. 4–13. [DOI] [PubMed] [Google Scholar]
- Thornhill R, Thornhill NW. The evolution of psychological pain. In: Bell R, Bell N, editors. Sociobiology and the social sciences. Lubbock, TX: Texas Tech University; 1989. pp. 73–103. [Google Scholar]
- Tooby J, Cosmides L. The past explains the present: Emotional adaptations and the structure of ancestral environments. Ethology and Sociobiology. 1990;11(4–5):375–424. [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27(3):247–259. [Google Scholar]
- Trivers RL. Evolution of reciprocal altruism. Quarterly Review of Biology. 1971;46(1):35–&. [Google Scholar]
- Trope Y, Alfieri T. Effortfulness and flexibility of dispositional judgment processes. Journal of Personality and Social Psychology. 1997;73(4):662–674. [Google Scholar]
- Tse WS, Bond AJ. The impact of depression on social skills: A review. Journal of Nervous and Mental Disease. 2004;192(4):260–268. doi: 10.1097/01.nmd.0000120884.60002.2b. [DOI] [PubMed] [Google Scholar]
- Uehara T, Sumiyoshi T, Matsuoka T, Itoh H, Kurachi M. Role of 5-HT1A receptors in the modulation of stress-induced lactate metabolism in the medial prefrontal cortex and basolateral amygdala. Psychopharmacology. 2006;186(2):218–225. doi: 10.1007/s00213-006-0370-y. [DOI] [PubMed] [Google Scholar]
- van Schaik DJF, Klijn AFJ, van Hout HPJ, van Marwijk HWJ, Beekman ATF, de Haan M, et al. Patients' preferences in the treatment of depressive disorder in primary care. General Hospital Psychiatry. 2004;26(3):184–189. doi: 10.1016/j.genhosppsych.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Human Brain Mapping. 2004;22(3):246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiel HOF. A preliminary profile of neuropsychological deficits associated with major depression. Journal of Clinical and Experimental Neuropsychology. 1997;19(4):587–603. doi: 10.1080/01688639708403745. [DOI] [PubMed] [Google Scholar]
- Velten E. A laboratory task for induction of mood states. Behaviour Research and Therapy. 1968;6(4):473–&. doi: 10.1016/0005-7967(68)90028-4. [DOI] [PubMed] [Google Scholar]
- Venn HR, Watson S, Gallagher P, Young AH. Facial expression perception: an objective outcome measure for treatment studies in mood disorders? International Journal of Neuropsychopharmacology. 2006;9(2):229–245. doi: 10.1017/S1461145705006012. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Marcinkiewicz M, Patey A, Elmestikawy S, Gozlan H, et al. Quantitative autoradiography of multiple 5-Ht1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. Journal of Neuroscience. 1986;6(12):3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers KS, Vogeltanz-Holm ND. The effects of rumination and distraction tasks on psychophysiological responses and mood in dysphoric and nondysphoric individuals. Cognitive Therapy and Research. 2003;27(3):331–348. [Google Scholar]
- Vollmayr B, Henn FA. Stress models of depression. Clinical Neuroscience Research. 2003;3(4–5):245–251. [Google Scholar]
- Wakefield JC. The concept of mental disorder: On the boundary between biological facts and social values. American Psychologist. 1992;47:373–388. doi: 10.1037//0003-066x.47.3.373. [DOI] [PubMed] [Google Scholar]
- Wall P. Pain: The science of suffering. New York, NY: Columbia University Press; 2000. [Google Scholar]
- Wasser SK, Place NJ. Reproductive filtering and the social environment. In: Ellison PT, editor. Reproductive ecology and human evolution. Hawthorne, NY: Aldine de Gruyter; 2001. [Google Scholar]
- Watkins E, Baracaia S. Why do people ruminate in dysphoric moods? Personality and Individual Differences. 2001;30(5):723–734. [Google Scholar]
- Watkins E, Brown RG. Rumination and executive function in depression: An experimental study. Journal of Neurology Neurosurgery and Psychiatry. 2002;72(3):400–402. doi: 10.1136/jnnp.72.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E, Mason A. Mood as input and rumination. Personality and Individual Differences. 2002;32(4):577–587. [Google Scholar]
- Watkins E, Moulds M. Positive beliefs about rumination in depression: A replication and extension. Personality and Individual Differences. 2005;39(1):73–82. [Google Scholar]
- Watkins E, Teasdale JD. Rumination and overgeneral memory in depression: Effects of self-focus and analytic thinking. Journal of Abnormal Psychology. 2001;110(2):353–357. doi: 10.1037/0021-843x.110.2.333. [DOI] [PubMed] [Google Scholar]
- Watkins E, Teasdale JD, Williams RM. Decentring and distraction reduce overgeneral autobiographical memory in depression. Psychological Medicine. 2000;30(4):911–920. doi: 10.1017/s0033291799002263. [DOI] [PubMed] [Google Scholar]
- Watson PJ, Andrews PW. Toward a revised evolutionary adaptationist analysis of depression: The social navigation hypothesis. Journal of Affective Disorders. 2002;72(1):1–14. doi: 10.1016/s0165-0327(01)00459-1. [DOI] [PubMed] [Google Scholar]
- Weary G, Marsh KL, Gleicher F, Edwards JA. Social-cognitive consequences of depression. In: Weary G, Marsh KL, editors. Control motivation and social cognition. New York, NY: Springer-Verlag; 1993. pp. 255–287. [Google Scholar]
- Weissman MM. Advances in psychiatric epidemiology: Rates and risks for major depression. American Journal of Public Health. 1987;77(4):445–451. doi: 10.2105/ajph.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JE, Horwood LJ. How accurate is recall of key symptoms of depression? A comparison of recall and longitudinal reports. Psychological Medicine. 2004;34(6):1001–1011. doi: 10.1017/s0033291703001843. [DOI] [PubMed] [Google Scholar]
- Wenzlaff RM, Luxton DD. The role of thought suppression in depressive rumination. Cognitive Therapy and Research. 2003;27(3):293–308. [Google Scholar]
- Westermann R, Spies K, Stahl G, Hesse FW. Relative effectiveness and validity of mood induction procedures: A meta-analysis. European Journal of Social Psychology. 1996;26(4):557–580. [Google Scholar]
- Williams GC. Adaptation and natural selection: A critique of some current evolutionary thought. Princeton, NJ: Princeton University Press; 1966. [Google Scholar]
- Williams JMG, Scott J. Autobiographical memory in depression. Psychological Medicine. 1988;18(3):689–695. doi: 10.1017/s0033291700008370. [DOI] [PubMed] [Google Scholar]
- Wilson M, Mesnick S. An empirical test of the bodyguard hypothesis. In: Gowaty PA, editor. Feminism and evolutionary biology: Boundaries, intersections, and frontiers. New York, NY: Chapman Hall; 1997. pp. 505–511. [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DEH, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology. 2003;167(3):304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Vasic N, Walter H. Differential activation of ventrolateral prefrontal cortex during working memory retrieval. Neuropsychologia. 2006;44(12):2558–2563. doi: 10.1016/j.neuropsychologia.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Depression in an evolutionary context. Philosophy, Ethics, and Humanities in Medicine. 2008;3:8–10. doi: 10.1186/1747-5341-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Nagayama H, Tsutiyama K, Kitamura T, Furukawa T. Coping behavior in depressed patients: A longitudinal study. Psychiatry Research. 2003;121(2):169–177. doi: 10.1016/s0165-1781(03)00249-x. [DOI] [PubMed] [Google Scholar]
- Yee CM, Miller GA. A dual-task analysis of resource-allocation in dysthymia and anhedonia. Journal of Abnormal Psychology. 1994;103(4):625–636. doi: 10.1037//0021-843x.103.4.625. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Curtis CE, D'Esposito M. Differential effects of distraction during working memory on delay-period activity in the prefrontal cortex and the visual association cortex. Neuroimage. 2006;29(4):1117–1126. doi: 10.1016/j.neuroimage.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Yost JH, Weary G. Depression and the correspondent inference bias: Evidence for more effortful cognitive processing. Personality and Social Psychology Bulletin. 1996;22(2):192–200. [Google Scholar]
- Young-Wolff KC, Kendler KS, Sintov SD, Prescott CA. Mood-related drinking motives mediate the familial association between major depression and alcohol dependence. Alcoholism: Clinical and Experimental Research. 2009 doi: 10.1111/j.1530-0277.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]