Abstract
The perifornical (PF) region of the posterior hypothalamus plays an important role in the regulation of sleep-wake states and motor activity. Disinhibition of PF neurons by the GABAA receptor antagonist, bicuculline, has been used to study the mechanisms of wake- and motor activity-promoting effects that emanate from the PF region. Bicuculline activates PF neurons, including the orexin-containing cells that have major excitatory projections to brainstem noradrenergic and serotonergic neurons. Since premotor aminergic neurons are an important source of motoneuronal activation, we hypothesized that they mediate the excitation of motoneurons that results from disinhibition of PF neurons with bicuculline. In urethane-anesthetized, paralyzed and artificially ventilated rats, we found that PF bicuculline injections (1 mM, 20 nl) made after combined microinjections into the hypoglossal (XII) nucleus of α1-adrenergic and serotonergic receptor antagonists (prazosin and methysergide) increased XII nerve activity by 80% ±16(SE) of the control activity level. Thus, activation of XII motoneurons originating in the hypothalamic PF region was not abolished despite effective elimination by the aminergic antagonists of the endogenous noradrenergic and serotonergic excitatory drives to XII motoneurons and abolition of XII motoneuronal activation by exogenous serotonin or phenylephrine. These results show that a major component of XII motoneuronal activation originating in the posterior hypothalamus is mediated by pathways other than the noradrenergic and serotonergic projections to motoneurons.
Keywords: GABA, noradrenergic neurons, orexin, serotonin, sleep
Introduction
The posterior, lateral hypothalamus plays an important role in the maintenance of vigilance [7]. Pharmacological inhibition of neurons located in the hypothalamic perifornical (PF) region enhances sleep, whereas their activation increases wakefulness [1, 29, 30]. GABA-containing and sleep-active neurons located in the anterior hypothalamus send axonal projections to the posterior hypothalamic PF region [31]. The PF region is the only site in the brain containing neurons that synthesize the excitatory peptides orexins (ORX, also known as hypocretins) [17]. ORX cells are active during wakefulness [8, 19, 23], and ORX loss causes narcolepsy/cataplexy, a disorder characterized by excessive sleepiness and sudden spells of motor atonia [5, 14]. ORX cells have widespread efferent projections that primarily target other wake-related neuronal groups, including brainstem noradrenergic and serotonergic neurons [26].
In urethane-anesthetized rats, microinjections of the GABAA antagonist, bicuculline, into the hypothalamic PF region activates ORX and other local cells and elicits a systemic response that comprises desynchronized cortical electroencephalogram (EEG), hippocampal theta rhythm, increased hypoglossal (XII) nerve activity and accelerated respiratory rate [21]. A concomitant increase in expression of the immediate early gene, Fos, in pontine noradrenergic neurons suggests that these calls also are activated [21].
Brainstem noradrenergic and serotonergic neurons send axons to all brainstem and spinal motor nuclei, including the medullary XII nucleus [2, 15, 27]. Data show that the endogenous aminergic drive to XII motoneurons is a major determinant of their wake-related activation [4, 10]. This drive is mainly mediated by α1-adrenergic and type 2 serotonergic receptors [18]. Since ORX activates aminergic neurons [3, 16, 17, 22], we hypothesized that the excitatory motor effects of PF bicuculline are mainly mediated by noradrenergic and serotonergic projections to motoneurons. To test this hypothesis, we assessed the effect of PF bicuculline on XII nerve activity following antagonism of α1-adrenergic and serotonergic receptors in the XII nucleus. Preliminary results have been published [12].
Materials and methods
Experiments were performed on 12 adult (320–430 g), male Sprague-Dawley rats obtained from Charles River Laboratories (Wilmington, MA). All animal procedures followed the guidelines established by the National Institutes of Health (NIH Publication 80–23 with subsequent revisions) and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Animal preparation, recording and microinjections
Rats were pre-anesthetized with isoflurane followed by urethane (1.0 g/kg, i.p.), tracheotomized and had a femoral artery and vein catheterized for arterial blood pressure monitoring and fluid injections, respectively. The right XII nerve was dissected and placed in a cuff electrode for recording [9]. Both cervical vagi were cut to enhance XII nerve activity and make it independent of lung volume feedback. The animal’s head was placed in a stereotaxic holder and openings were made in the parietal bones for inserting bicuculline-containing pipette into the posterior hypothalamus on the left side and a recording electrode into the hippocampus on the right side. The caudal medulla was exposed by a posterior fossa craniotomy to insert a glass pipette filled with aminergic antagonists into the right XII nucleus.
The cortical, hippocampal and XII nerve activities were recorded together with end-expiratory CO2, blood pressure and rectal temperature (35.5–36.5°C), as described previously [21]. The animals were paralyzed with pancuronium bromide (2 mg/kg i.v., Sigma) and artificially ventilated with an air-oxygen mixture (30–60% O2). The end-expiratory CO2 was kept constant and sufficient to maintain steady respiratory modulation of XII nerve activity (5–6%). Adequate level of anesthesia was maintained based on stable amplitude and constant rate of inspiratory bursts recorded from the XII nerve, steady blood pressure and slow-wave cortical EEG. If needed, supplemental injections of urethane and pancuronium bromide were administered in 40 mg and 1 mg/kg increments, respectively.
All drugs (Sigma) were diluted from frozen aliquots in 0.9% NaCl before each experiment. We used (−)-bicuculline methiodide, a GABAA receptor antagonist (l mM); 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl) piperazine hydrochloride (prazosin), an α1-adrenoceptor antagonist (0.2 mM); methysergide maleate, a broad-spectrum serotonergic receptor antagonist (1.0 mM); phenylephrine, an α1-adrenoceptor agonist (2 mM); and serotonin creatinine sulfate (5 mM). Prazosin and methysergide were combined in one solution. To mark the PF injection sites, the bicuculline solution contained 2% of Pontamine sky blue dye (ICN Biomedicals, Aurora, OH). Injections sites in the XII nucleus were marked at the end of the experiment by injections of 2% Pontamine.
Microinjections were made from beveled glass pipettes (A-M Systems, Carlsborg, WA; tip diameters of 25–30 μm). The bicuculline pipette was inserted into the hypothalamic PF region aiming at: 3.1 mm caudal to bregma, 1.3 mm lateral to the midline, and a depth of 8.2 mm from the brain surface [25]. Drugs were injected by applying pressure to the fluid in the pipette while monitoring the movement of the meniscus with a calibrated microscope (1 nl resolution). The antagonist mix was injected into the XII nucleus at three sites separated by 0.65 mm in antero-posterior (A-P) direction to cover the entire XII nucleus [10].
Experimental protocol and measurements
After recording of baseline activity, each animal received three injections of the antagonist mix into the XII nucleus (30–40 nl each) made over 5.7 min ±0.4. This was followed 28.9 min ±2.9 later by one bicuculline injection into the PF region (20 nl). The ~30 min delay was chosen because the antagonists exert their maximal effect on XII nerve activity around this time [10].
The magnitude of XII nerve activity was measured from the moving average of the signal (time constant: 100 ms) as the difference between the peak of inspiratory burst and the level of activity during central expiration when activity was minimal or absent. XII nerve activity measured during subsequent stages of the experiment was normalized by its level measured before injections of the antagonist mix into the XII nucleus. The central respiratory rate was determined from XII nerve activity recording. All measurements were derived from 2 min periods at three time points during the experiment: before the antagonist injections into the XII nucleus, after the antagonist injections into the XII nucleus but before bicuculline injection into the PF region, and during the peak of the response to PF bicuculline. The latency of the response to PF bicuculline was measured between the onset of the injection and the time when XII nerve activity started to increase. The power of hippocampal activity in the theta-like frequency range (3–5 Hz) [32] was determined in successive 15 s intervals (Spike-2; CED, Cambridge, UK and Somnologica, MedCare, Buffalo, NY; acquisition rate: 100 Hz).
Histology and statistics
At the conclusion of the experiment, the animal received an additional dose of urethane (1 g/kg) and was perfused with cold phosphate-buffered 0.9% NaCl (pH 7.4) followed by 10% formalin. The brain was extracted, postfixed and 50 μm sections were cut in coronal plane through the hypothalamus and in parasagittal plane through the medulla. Sections containing the blue dye were serially mounted and stained with Neutral red.
Following verification that the variables were normally distributed, a paired, two-tailed Student’s t-test was used for statistical analysis (SigmaPlot, Jandel, San Rafael, CA). Differences were considered significant when P was less than 0.05. The variability of the means is characterized by the standard error (SE) throughout the report.
Results
Injections of prazosin and methysergide into the XII nucleus
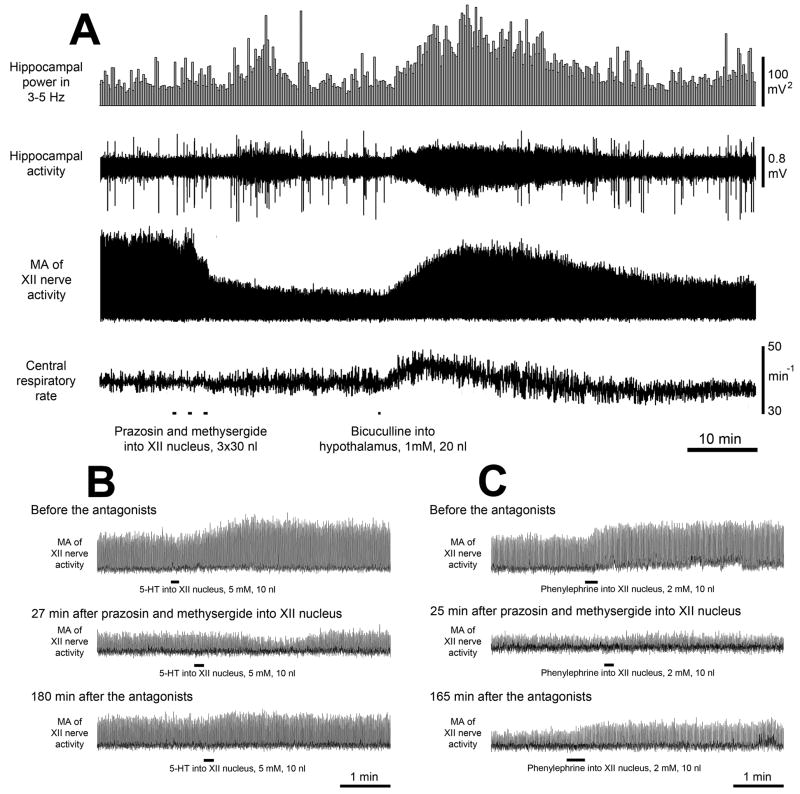
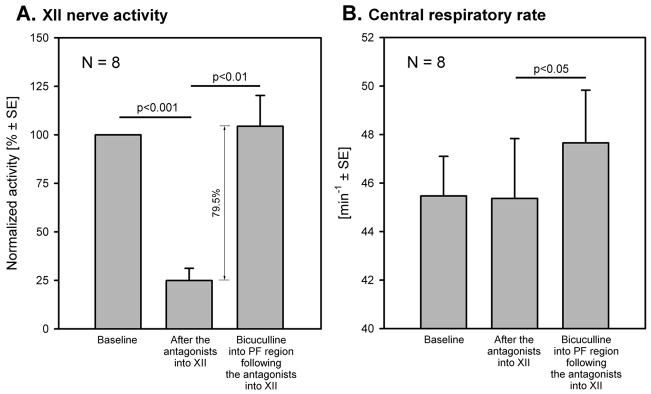
The three injections of the antagonist mix into the XII nucleus reduced XII nerve activity to 24.9% ±6.3 of the pre-antagonist level (n=8, P<0.001); in two experiments, XII nerve activity was abolished at the peak of the response. The depressant effect of the antagonists on XII nerve activity was not associated with any changes in cortical or hippocampal activity or central respiratory rate (45.5 min−1 ±1.6 before, and 45.4 min−1 ±2.5 after, the antagonists). The initial part of the record in Fig. 1A shows XII nerve activity decline following antagonist injections into the XII nucleus.
Figure 1. Example of the effect of combined prazosin and methysergide injections into the XII nucleus followed by bicuculline injection into the perifornical (PF) hypothalamus on XII nerve activity (A), and control experiments conducted to verify the effectiveness of the aminergic antagonists (B and C).
A: Three injections of the antagonist mix into the XII nucleus depress XII nerve activity, whereas bicuculline injected into the hypothalamic PF region 29 min later increases it, elicits hippocampal theta rhythm and accelerates the central respiratory rate. B: excitatory effect of serotonin (5-HT) injected into the XII nucleus (top trace) is abolished by the antagonist mix (middle trace) and then partially recovers ~3 h later (bottom trace). C: excitatory effect of phenylephrine injected into the XII nucleus (top trace) is also abolished by the antagonist mix (middle trace) and then partially recovers ~3 h later (bottom trace). MA – moving average.
Bicuculline injections into the hypothalamic PF region after prazosin and methysergide injections into the XII nucleus
Hypothalamic bicuculline injections made 28.9 min ± 2.9 after the antagonist mix injections into the XII nucleus elicited hippocampal theta rhythm, accelerated the respiratory rate, increased XII nerve activity (Fig. 1A) and activated cortical EEG (not shown; see ref. [21]). The mean latency and duration of the responses were 2.3 min ± 0.6 and 38.5 min ±1.3, respectively. XII nerve activity was significantly increased, from 24.9% ±6.3 to 104% ±16 of its baseline level at the beginning of the experiment (n=8, P<0.01) (Fig. 2A). The average increase from the level of XII nerve activity attained following microinjections of the antagonist mix into the XII nucleus (24.9%) to the peak of the response to PF bicuculline was 80% ±16 (Fig.2A). Following bicuculline, the central respiratory rate increased from 45.4 min−1 to 47.7 min−1 ±2.2 (P<0.05) (Fig. 2B).
Figure 2. Mean effects of the combined injections of prazosin and methysergide into the XII nucleus and then bicuculline into the perifornical hypothalamus on XII nerve activity and central respiratory rate.
A: the antagonist mix injected into the XII nucleus reduced XII nerve activity to 24.9% of its pre-antagonists baseline level. The subsequent hypothalamic injections of bicuculline increased XII nerve activity by 80% of the same baseline level despite antagonism of adrenergic and serotonergic receptors in the XII nucleus. B: the central respiratory rate was not affected by the antagonist injections into the XII nucleus but was increased by the subsequent hypothalamic bicuculline injection.
Control experiments
In four rats, the ability of the antagonist mix to block the corresponding receptors was tested by observing the excitatory effects of either serotonin (5 mM, 10 nl; 2 animals) or phenylephrine (2 mM, 10 nl; 2 animals) injected into XII nucleus before, and at different times after, injections of the antagonist mix. The excitatory effects of both agonists were abolished when tested 20–27 min after the antagonists and partially recovered 2.5–3.0 h later (Figs. 1B and C). The long duration of the antagonist mix action was consistent with its long-lasting effect on endogenous aminergic activation of XII motoneurons described previously [10].
Injection sites
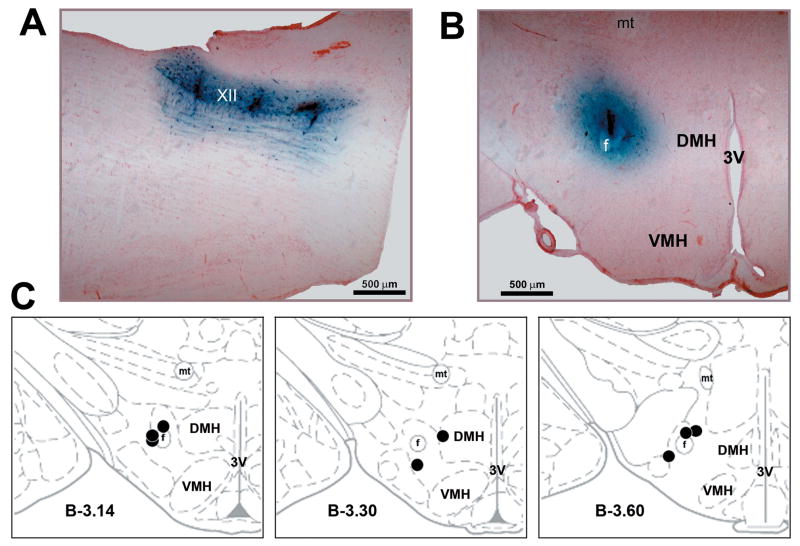
Examples of the aminergic antagonist mix and bicuculline injection sites are shown in Figs. 3A and B, respectively. Figure 3C shows the distribution of all PF bicuculline injection sites. They were located at A-P levels −2.80 to −3.80 mm from bregma [25].
Figure 3. Medullary and hypothalamic injection sites.
A: three antagonist mix injection sites in the XII nucleus shown in a parasagittal plane. B: hypothalamic bicuculline injection site shown in coronal plane. The sites shown in A and B are from the experiment illustrated in Fig. 1A. C: the distribution of all bicuculline injection sites superimposed onto the closest standard cross-sections from a rat brain atlas [25]. Abbreviations: 3V – third ventricle; DMH – dorsomedial hypothalamic nucleus; f – fornix; mt – mammillothalamic tract; VMH – ventromedial hypothalamic nucleus; XII – hypoglossal nucleus.
Discussion
Contrary to our hypothesis, we found that antagonism of α1-adrenergic and serotonergic receptors within and around the XII nucleus did not significantly diminish the excitatory effect of hypothalamic bicuculline injections on motoneuronal activity. This shows that the effect of PF bicuculline on XII motoneurons is predominantly mediated by pathways other than those that converge on premotor noradrenergic and serotonergic brainstem neurons with axonal projections to the XII nucleus. Thus, despite compelling neuroanatomical and pharmacological data suggesting that ORX-mediated activation of brainstem aminergic premotor neurons is a major source of wake-related motoneuronal activation [3, 16, 17, 22], our direct assessment of the role of this pathway reveals that it is not essential for mediation of the XII motoneuronal activation from the hypothalamic PF region. Whether this finding also applies to other motoneuronal groups remains to be determined in future studies.
Injections of bicuculline into the hypothalamic PF region of urethane-anesthetized rats elicit cortical and hippocampal activation, increased motoneuronal discharge, and accelerated central respiratory rhythm [21]. At the bicuculline injection site, many cells are activated, as indicated by increased Fos expression in ORX-containing and other neurons [21]. Since brainstem noradrenergic and serotonergic cells are major efferent targets of hypothalamic ORX neurons [22, 26] and themselves send dense projections to motor nuclei [2, 15, 27], one would expect the motoneuronal activation in response to PF bicuculline to be mediated to a major extent by noradrenergic and serotonergic afferents to motoneurons. To assess this construct, we quantified the activation of XII motoneurons that occurs in response to PF bicuculline following pharmacological blockade of adrenergic and serotonergic receptors in the XII nucleus. We chose to use this model system because the location and shape of the XII nucleus and the presence of consistent spontaneous activity in XII motoneurons under anesthesia make it particularly suitable for an antagonist microinjection study. We previously developed and characterized the methodology of antagonist microinjections into the XII nucleus [10, 11], and our present control experiments (Figs. 1B and C) demonstrate that this approach results in a complete antagonism of relevant aminergic receptors. We also previously quantified XII nerve activation by PF bicuculline in experiments without antagonist injections into the XII nucleus using the same animal model [21]. In that study, XII nerve activity was increased by PF bicuculline by 100% ±24 of its pre-bicuculline level.
In the present study, bicuculline was injected into PF region after injections of α1-adrenergic and serotonergic antagonists into the XII nucleus. These injections reduced XII nerve activity to 24.9% of the control level, which was identical to the decrease obtained in the same animal model in our previous study (24.9% ±3.6) [10]. The aminergic antagonist injections created a new, lower baseline level of XII nerve activity against which we then assessed the effect of PF bicuculline (Fig. 2A). When measured relative to the new baseline, the magnitude of XII nerve activation following PF bicuculline would be as high as 260% ±98 for the 6 rats in which some activity remained after the antagonists. This is considerably more than the 100% increase that we previously observed without the aminergic antagonists [21], but one should note that the baseline used in this method of quantification was reduced which causes an overestimation of the magnitude of the excitatory effect of PF bicuculline. A more conservative approach that is also compatible with our previous study is to express the XII nerve activity increase elicited by PF bicuculline relative to XII nerve activity baseline prior to any pharmacological interventions. Using this approach, we determined that PF bicuculline increased XII nerve activity in the presence of the aminergic receptor antagonists in the XII nucleus by 80% ±16. This is only slightly less than the 100% ±24 activation produced by PF bicuculline in our previous study without the aminergic antagonists [21], and not significantly different from that activation (P = 0.46). Thus, the antagonists had lesser effect on the bicuculline response than would be expected based on the hypothesis that brainstem noradrenergic and serotonergic cells with projections to motor nuclei mediate a major portion of motoneuronal activation that emanates from the PF region of the posterior hypothalamus.
If brainstem premotor noradrenergic and serotonergic neurons mediate only a small fraction of motoneuronal activation from the PF region of the posterior hypothalamus, one needs to consider other pathways. Those that may be proposed based on the existing neuroanatomical and pharmacological data include direct excitatory projections of ORX neurons to motoneurons [13, 22, 33], ORX-mediated activation of hypothalamic histaminergic neurons that may, in turn, activate motoneurons [24, 34], brainstem cholinergic neurons [20, 28], or other non-adrenergic/non-serotonergic premotor neurons that are excited by pathways that descend from the posterior, lateral hypothalamus [6]. Since PF bicuculline also excites many non-ORX cells in the PF region [21], such neurons may mediate motoneuronal activation from the posterior hypothalamus through pathways that use transmitters other than those classically considered important for wake-related motor activation.
Acknowledgments
The study was supported by NIH grants HL-071097 and HL-047600.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurons during sleep in rats. J Physiol. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldes LD, Chapman ME, Chronister RB, Haycock JW. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res Bull. 1992;29:931–942. doi: 10.1016/0361-9230(92)90168-w. [DOI] [PubMed] [Google Scholar]
- 3.Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 4.Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep–wake states. Am J Respir Crit Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 5.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 6.Dutschmann M, Kron M, Morschel M, Gestreau C. Activation of Orexin B receptors in the pontine Kölliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir Physiol Neurobiol. 2007;159:232–235. doi: 10.1016/j.resp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 7.von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–259. [Google Scholar]
- 8.Estabrooke IV, McCarthy MT, Ko E, Chou T, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenik V, Fenik P, Kubin L. A simple cuff electrode for nerve recording and stimulation in acute experiments on small animals. J Neurosci Meth. 2001;116:147–150. doi: 10.1016/s0165-0270(01)00340-5. [DOI] [PubMed] [Google Scholar]
- 10.Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenik VB, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep-like depression of hypoglossal motoneuronal activity. J Sleep Res. 2005;14:419–429. doi: 10.1111/j.1365-2869.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- 12.Fenik VB, Rukhadze I, Kubin L. Antagonism of α1-adrenergic and serotonergic receptors in the hypoglossal (XII) nucleus does not abolish activation of XII motoneurons elicited from the posterior lateral hypothalamus. Sleep. 2008;31(Suppl):A17. doi: 10.1016/j.neulet.2009.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung SJ, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: a double-labeling immunohistochemical study. Brain Res. 2001;903:257–262. doi: 10.1016/s0006-8993(01)02318-6. [DOI] [PubMed] [Google Scholar]
- 14.Gerashchenko D, Kohls MD, Greco MA, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry JN, Manaker S. Colocalization of substance P or enkephalin in serotonergic neuronal afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1998;391:491–505. [PubMed] [Google Scholar]
- 16.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 17.Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. TINS. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- 18.Kubin L, Volgin DV. Developmental profiles of neurotransmitter receptors in respiratory motor nuclei. Respir Physiol Neurobiol. 2008;164:64–71. doi: 10.1016/j.resp.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Sood S, Liu H, Horner RL. Opposing muscarinic and nicotinic modulation of hypoglossal motor output to genioglossus muscle in rats in vivo. J Physiol. 2005;565:965–980. doi: 10.1113/jphysiol.2005.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu JW, Fenik VB, Branconi JL, Mann GL, Rukhadze I, Kubin L. Disinhibition of perifornical hypothalamic neurones activates noradrenergic neurones and blocks pontine carbachol-induced REM sleep-like episodes in rats. J Physiol. 2007;582:553–567. doi: 10.1113/jphysiol.2007.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 23.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuzeret PC, Sakai K, Gormand F, Petitjean T, Buda C, Sastre JP, Parrot S, Guidon G, Lin JS. Application of histamine or serotonin to the hypoglossal nucleus increases genioglossus muscle activity across the wake-sleep cycle. J Sleep Res. 2009;18:113–121. doi: 10.1111/j.1365-2869.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego: 1991. [Google Scholar]
- 26.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rukhadze I, Kubin L. Differential pontomedullary catecholaminergic projections to hypoglossal motor nucleus and viscerosensory nucleus of the solitary tract. J Chem Neuroanat. 2007;33:23–33. doi: 10.1016/j.jchemneu.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Rukhadze I, Kubin L. Mesopontine cholinergic projections to the hypoglossal motor nucleus. Neurosci Lett. 2007;413:121–125. doi: 10.1016/j.neulet.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sallanon M, Denoyer M, Kitahama K, Aubert C, Gay N, Jouvet M. Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neurosci. 1989;32:669–683. doi: 10.1016/0306-4522(89)90289-3. [DOI] [PubMed] [Google Scholar]
- 30.Sallanon M, Sakai K, Buda C, Puymartin M, Jouvet M. Posterior hypothalamic lesion inducing transient hypersomnia. Arch Ital Biol. 1988;126:87–97. [PubMed] [Google Scholar]
- 31.Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience. 2007;150:104–120. doi: 10.1016/j.neuroscience.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vertes RP, Colom LV, Fortin WJ, Bland BH. Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat. Exp Brain Res. 1993;96:419–429. doi: 10.1007/BF00234110. [DOI] [PubMed] [Google Scholar]
- 33.Volgin DV, Saghir M, Kubin L. Developmental changes in the orexin 2 receptor mRNA in hypoglossal motoneurons. NeuroReport. 2002;13:433–436. doi: 10.1097/00001756-200203250-00014. [DOI] [PubMed] [Google Scholar]
- 34.Yamanaka A, Tsujino N, Funahashi H, Honda K, Guan JL, Wang QP, Tominaga M, Goto K, Shioda S, Sakurai T. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Comm. 2002;290:1237–1245. doi: 10.1006/bbrc.2001.6318. [DOI] [PubMed] [Google Scholar]