Abstract
Chronic alcohol abuse is associated with changes in stress and reward pathways that could alter vulnerability to emotional stress and alcohol craving. This study examines whether chronic alcohol abuse is associated with altered stress and alcohol craving responses. Treatment-engaged, 28-day abstinent alcohol-dependent individuals (ADs; 6F/22M), and social drinkers (SDs; 10F/18M) were exposed to a brief guided imagery of a personalized stressful, alcohol-related and neutral-relaxing situation, one imagery condition per session, presented in random order across 3 days. Alcohol craving, anxiety and emotion ratings, behavioral distress responses, heart rate, blood pressure, and salivary cortisol measures were assessed. Alcohol patients showed significantly elevated basal heart rate and salivary cortisol levels. Stress and alcohol cue exposure each produced a significantly enhanced and persistent craving state in alcohol patients that was marked by increased anxiety, negative emotion, systolic blood pressure responses, and, in the case of alcohol cue, behavioral distress responses, as compared to SDs. Blunted stress-induced cortisol responses were observed in the AD compared to the SD group. These data are the first to document that stress and cue exposure induce a persistent negative emotion-related alcohol craving state in abstinent alcoholics accompanied by dysregulated HPA and physiological arousal responses. As laboratory models of stress and negative mood-induced alcohol craving are predictive of relapse outcomes, one implication of the current data is that treatments targeting decreases in stress and alcohol cue-induced craving and regulation of stress responses could be of benefit in improving alcohol relapse outcomes.
Keywords: alcohol dependence, social drinking, stress, craving, alcohol cues, cortisol
INTRODUCTION
Chronic alcohol abuse is associated with alterations in stress systems, namely the corticotrophin releasing factor (CRF), hypothalamic-pituitary adrenal (HPA) axis and autonomic arousal pathways, and also the mesolimbic dopamine pathways involved in the rewarding properties of drugs, including alcohol (Koob et al, 2004; Sinha, 2005). Alterations in HPA axis responses have been observed during active drinking, acute withdrawal, and 4 weeks post-withdrawal from alcohol (Wand and Dobs, 1991; Adinoff et al, 1991, 2005; Koob et al, 2004). Furthermore, autonomic dysregulation has been noted during acute alcohol withdrawal, and altered cardiovascular and noradrenergic responsivity has been seen during the protracted alcohol abstinence period (Krystal et al, 1996; Bernardy et al, 2003; Rasmussen et al, 2006).
These alcohol-related adaptations in the stress, reward, and autonomic arousal systems raise the question of whether such alterations are accompanied by an enhanced sensitivity to emotional stress and alcohol craving, which could promote persistent alcohol craving associated with compulsive alcohol seeking. Preclinical studies indicate that specific stress-induced anxiety-like behaviors as well as alcohol cues are associated with increased alcohol self-administration, and CRF antagonists and noradrenergic agents that decrease brain CRF and reduce norepinephrine, respectively, also decrease stress-induced alcohol reinstatement (Le et al, 1998; Le et al, 1999; Matsuzawa et al, 1998; Le et al, 2000; Liu and Weiss, 2002). In humans, stress and negative affect are known to increase alcohol and drug craving and relapse susceptibility (Brown et al, 1990; Cooney et al, 1997; Breese et al, 2005; Sinha et al, 2006, Brady et al, 2006; Sinha, 2007). Koob and colleagues proposed that chronic alcohol and withdrawal-related neuroadaptation produces a negative affect state representing a shift from positive to negative reinforcement mechanisms in addiction (Koob, 2003; Koob et al, 2004). We have further hypothesized that such neuroadaptations contribute to an increased sensitivity to stress and drug craving, which in turn enhances the risk of relapse (Sinha, 2001a; Breese et al, 2005), but these hypotheses have not been fully tested in alcohol samples, thus far.
It is well-known that alcohol cue exposure increases alcohol craving (Cooney et al, 1987; Laberg and Ellertsen, 1987; Monti et al, 1987; Payne et al, 1992; Rohsenow et al, 1992) with a greater craving response in alcoholics compared to social drinkers (SDs) (Pomerleau et al, 1983; Grusser et al, 2006). However, the hedonic state associated with such increases in alcohol cue-induced craving has not been previously assessed. If, indeed, there is a shift from positive to negative reinforcement in the motivational aspects of alcohol seeking, as suggested by preclinical studies (Koob et al, 2004), one would hypothesize that in alcoholics, alcohol cue exposure would increase negative emotions and such increases would be associated with alcohol craving. Conversely, in SDs one may expect minimal changes in negative emotion with cue exposure and no negative emotion-related increases in alcohol craving. As stress and negative affect also increase alcohol craving and relapse susceptibility in alcoholics (Cooney et al, 1997; Fox et al, 2007; Breese et al, 2005; Brady et al, 2006), it may be hypothesized that the distress state associated with stress-induced alcohol craving is similar to that associated with alcohol cue-induced craving in alcoholics and not in SDs. Finally, as dysregulation of stress and reward pathways are associated with chronic alcohol abuse, we expected that the stress and cue-induced changes in hedonic state and craving in alcoholics would be accompanied by dysregulation of behavioral distress and physiological responses not observed in SDs. Although we have previously shown that both stress and drug cue exposure similarly increase craving and produces a negative emotional state in addicted individuals (Sinha et al, 2000; Sinha et al, 2003; Fox et al, 2007), whether these changes are indicative of a dysregulation in stress system function compared with SDs has not previously been examined. Thus, the current study tested the above hypotheses in a treatment-engaged group of 28-day abstinent alcoholics who were compared to a demographically matched group of light to moderate SDs.
METHODS
Subjects
Twenty-eight treatment-seeking individuals (22M/6W) who met DSM-IV criteria for current alcohol dependence were admitted to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) for 4−6 weeks of inpatient treatment and research participation. Twenty-eight demographically matched SDs (18M/10W) between the ages of 21−50 years were also recruited from the community through local advertisements with documented multiple negative urine toxicology screens on admission into the study. The SD group reported drinking up to 25 drinks or less per month and was classified into light to moderate SD categories using the quantity frequency variability index (Cahalan et al, 1969). They were excluded, if they met current or lifetime abuse or dependence criteria for alcohol or any other illicit drug. Alcoholic participants did not meet current DSM-IV criteria for dependence on other psychoactive substances, other than nicotine and were also excluded, if they met current criteria for any other DSM-IV axis I psychiatric disorder. Women were excluded from the study, if they were using any form of birth control or were either peri/postmenopausal. In addition, individuals on medications for medical or psychiatric problems were excluded from the study. All subjects underwent a complete medical evaluation to ensure good physical health and the study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Procedures
Alcohol patients were admitted to the CNRU, a locked inpatient treatment research facility with no access to alcohol or drugs and limited access to visitors. Urine and breathalyzer testing was also conducted regularly to ensure continued abstinence. The CNRU is a smoke-free unit and patients are allowed four smoke breaks of 15 min each and are escorted out to a smoking area where they smoke about 1−2 cigarettes. All patients participated in specialized substance abuse treatment for 4 weeks before the laboratory sessions. During the second week of admission, all patients completed demographic, diagnostic, and alcohol-related assessments. Healthy SDs completed demographic, diagnostic, and alcohol-related assessments in two to three assessment appointments and were then admitted for a 3-day hospital stay to the Yale General Clinical Research Center (GCRC) at Yale-New Haven Hospital for participation in the laboratory study. During this period, they were required to stay on the unit, within a similar controlled environment as that of the alcohol patients.
The laboratory study for alcohol patients was conducted 28 days after admission to the inpatient unit to allow for normalization of neurobiological changes associated with alcohol withdrawal and adaptation to reduced access to nicotine smoking. The SD group had been abstinent from alcohol use on an average of 55 days (SE = 28) before the laboratory sessions. The laboratory sessions involved all participants being exposed to three imagery conditions (stress, alcohol cue, and neutral/relaxing). The conditions were presented across three separate consecutive testing days with only one stimulus presentation per day. Condition order was assigned randomly and counterbalanced across participants by group. This procedure accounts for any nonspecific effects of order on the response measures. Research staff conducting the experiments and handling data were blind to imagery condition and the content of the scripts assigned to each laboratory session. Subjects also remained blind until imagery presentation.
Imagery script development procedures
Before the laboratory sessions, guided imagery scripts for stress, alcohol cue and neutral relaxing states were developed. The stress imagery script was based on subjects’ description of a recent personal stressful event that was experienced as ‘most stressful’. Most stressful was determined by having the subjects rate their individual level of distress on a 10-point Likert scale where ‘1 = not at all stressful’ and ‘10 = the most stress they felt recently in their life’. Only situations rated as eight or above were accepted as appropriate for script development. Examples include a breakup with a significant other or unemployment-related stress. Trauma-related situations and those with explicit alcohol cues were not allowed. The alcohol cue's script was based on individual situations that included alcohol-related stimuli and resulted in subsequent alcohol use (eg, buying alcohol and being at a bar, watching others drink alcohol). Alcohol-related situations that occurred in the context of negative affect or psychological distress were not allowed. A neutral script was developed from the participants’ individual experiences of commonly experienced neutral-relaxing situations, such as a summer day relaxing at the beach or a fall day reading at the park.
Details of each elicited situation was described using the scene construction questionnaire, based on methods developed by Lang et al (1980, 1983) and further adapted in our previous studies (Sinha and O'Malley, 1999a; Sinha et al, 1999b, 2000; Sinha, 2001a; Sinha et al, 2003). Scripts were developed using a standardized format, based on specific stimulus and response details of each situation and then audiotaped for presentation in the laboratory sessions.
Habituation and imagery training session
On a day before the laboratory sessions subjects were brought into the testing room where they were acclimatized to specific aspects of the study procedures, such as the subjective rating forms and then trained in relaxation and imagery procedures (Sinha et al, 2003; Sinha, 2001b).
Laboratory sessions
On each day, subjects abstained from breakfast and were brought into the testing room at 0800 hours by the research nurse. All subjects were allowed an initial smoke break at 0730 hours to address potential nicotine craving. A blood pressure cuff was placed on the subject's preferred arm to monitor blood pressure and a pulse sensor was placed on the subject's forefinger to obtain a measure of pulse. This was followed by a 1-h adaptation period. At 0900 hours, subjects were provided headphones and the audiotape presented the instructions for the imagery procedure and the script for guided imagery. The length of each script was approximately 5 min. After imagery, subjects remained in the testing room for an additional 75 min to examine recovery from the imagery exposure. If participants’ anxiety remained above baseline levels following the final time point, they were taken through another series of relaxation procedures until their ratings returned to baseline levels. After the last assessment at 1030 hours, the subject was disconnected from the apparatus and was served breakfast.
Laboratory assessments
Subjective Measures: Alcohol craving and Anxiety were assessed using a 10-point visual analog scale (VAS) in which 1 was anchored at ‘not at all’ and 10 at ‘extremely high’. Subjects rated their ‘desire for an alcoholic drink’ and how ‘anxious, tense and/or jittery’ they felt in that moment.
Differential emotion scale (DES -Izard, 1972)
Participants were required to rate on a 5-point scale the extent to which 30 emotional words described the way s/he felt at the current time. The ratings were summed and collapsed into two subscales: negative (anger, fear, and sadness) and positive emotion (joy and relaxation).
All subjective rating scales were administered at baseline (−5), immediately following imagery (0 time point) and at + 5, + 10, + 15, + 30, + 45, and + 60 min after imagery.
The Revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar; Sullivan et al, 1989). This is a widely-used measure of alcohol withdrawal symptoms and has shown excellent inter-rater reliability and good validity, correlating with physicians’ ratings of withdrawal behaviors (Shaw et al, 1981). The scale was used to assess alcohol withdrawal in alcohol dependent (AD) patients on day 1 of their admission to inpatient treatment.
Behavioral Distress Responses
Behavioral observation scale (BOS): Two independent raters, blind to imagery condition, rated participants’ responses on muscle twitching, muscle tremor, restlessness, muscle tension, muscle ache, headache, quickened breathing, yawning, talking/facial movements, crying, sweating, and stomach/abdominal changes by direct observation and questioning of participants. Both raters stood behind a partition to discreetly observe the aspects of participants’ behavior. Presence of these behaviors was then checked off on an itemized sheet of behavioral responses. The rater then asked participants a series of structured questions regarding bodily arousal responses. Inter-rater reliabilities for the presence of these behaviors were excellent (for stress condition, K = 0.88; for neutral/relaxing, K = 0.83; for alcohol cue, K = 0.88). The total rating score was used as the measure of behavioral and bodily response. BOS ratings were taken at baseline (−5), immediately following imagery exposure (0) and at + 10 min after imagery.
Cardiovascular Measures
A Critikon Dinamap monitor was used to assess multiple measures of heart rate (HR) and systolic and diastolic blood pressure (SBP and DBP) averaged to obtain mean responses for the baseline (four readings during the 5-min preimagery baseline period) during the imagery period (four readings averaged for the 0 time point) and single readings at + 5, + 10, + 15, + 30, + 45, and + 60 min time points postimagery.
Salivary Cortisol
At the baseline (−5), imagery (0) and for five additional recovery time points (every 15 min: + 15, + 30, + 45, + 60,and + 75), participants placed a cotton roll between their tongue and cheek for approximately 2−3 min until the swab was completely saturated (Salivette, Sarstedt Inc.). Saliva samples were stored in a −20 freezer and assayed in duplicate following standard radioimmunoassay kits with no modifications (Diagnostic Products Corporation, CA) at the Yale GCRC Core Laboratories. The intra-assay coefficients of variation ranged from 3.0 to 5.1%.
Statistical analysis
Linear mixed effect (LME; Laird and Ware, 1982) models were implemented to analyze the data, using the SAS software package (Version 9, 2006; SAS Institute, Cary, NC). Between-subjects factor of group 2 (SD vs AD) and Within-subjects factors of Condition 3 (stress, alcohol cue, and neutral) and Time-point (varying levels) were the fixed effects, and subjects was the random effect. In the case of baseline differences for any particular measure, the LME models included the baseline values as a covariate and baseline adjusted mean scores were presented in figures. Spearman's Rho correlation coefficients were used to assess the associations between stress and cue-induced alcohol craving, the composite negative and positive emotion subscales of the DES, the BOS, and physiological responses in both AD and SD groups. An averaged response across time points was computed for each measure to assess correlations.
RESULTS
Participants
The AD and the SD groups were matched for gender, race, age, and years of education. There were no differences between groups on lifetime prevalence of major depression, but alcohol patients had a higher lifetime prevalence of anxiety disorders and they were more likely to be smokers as compared to the SD group (Table 1).
Table 1.
Demographics and sample characteristics of alcohol dependent (AD) and social drinkers (SDs)
| Alcoholic patients (AD) (n = 28) | Social drinkers (SD) (n = 28) | |
|---|---|---|
| Demographics | ||
| Gender: male; number (%) | 22 (78.6%) | 18 (64.3%) |
| Race: Caucasian; number (%) | 20 (71.4%) | 13 (46.4%) |
| African American | 6 (21.4%) | 8 (28.6%) |
| Hispanic | 1 (3.6%) | 6 (21.4%) |
| Other | 1 (3.6%) | 1 (3.6%) |
| Age: mean (SD) | 36.96 (7.7) | 34.57 (10.6) |
| Years in education: mean (SD) | 13.25 (1.6) | 13.85 (1.6) |
| Before alcohol use: mean (SD) | ||
| Number of days used in the last 30 days** | 21.78 (10.2) | 4.83 (6.6) |
| Number of drinks per day** | 15.90 (6.9) | 1.67 (0.8) |
| Number of drinks per month** | 358.44 (247.6) | 8.30 (7.4) |
| Years of alcohol use** | 16.93 (7.7) | 9.72 (10.0) |
| Number of smokers** | 25 (82%) | 7 (25%) |
| Prevalence of psychiatric disorders: No. (%) | ||
| Lifetime depression | 5 (17.9%) | 2 (7.1%) |
| Lifetime PTSD | 3 (10.7%) | 2 (7.1) |
| Lifetime anxiety disorders (without PTSD)* | 5 (17.9%) | 0 (0%) |
Note:
p<0.05
p<0.001.
Baseline Differences
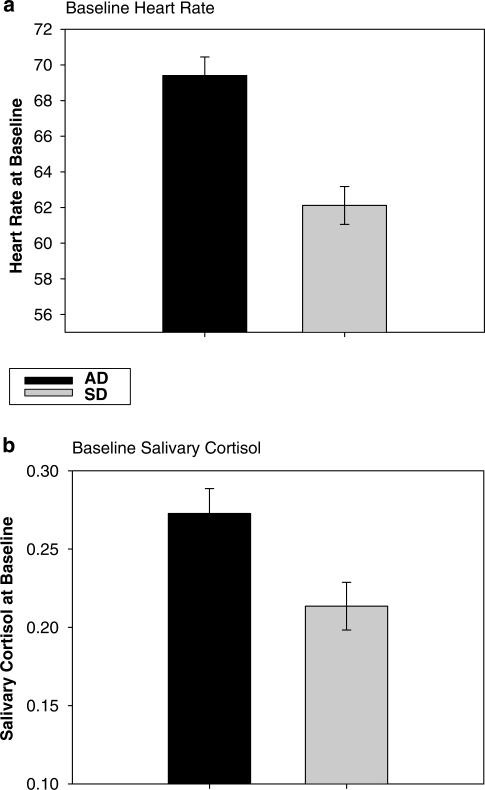
Alcohol patients showed significantly higher basal HR (1, 51 = 8.3; p<0.006) and salivary cortisol (1, 54 = 4.8; p = 0.03) compared with SDs (see Figure 1a and b). No other dependent measures were different at baseline between groups. Post hoc analyses indicated that the basal HR was significantly correlated with higher withdrawal ratings observed on day 1 of admission (r = 0.47; p = 0.02) as measured by the Revised Clinical Institute Withdrawal Assessment for Alcohol (R-CIWA, 40). Basal salivary cortisol levels were not significantly associated with the R-CIWA.
Figure 1.
Differences between alcohol dependent (AD) patients and social drinkers (SDs) in (a) baseline heart rate (b.p.m.; p<0.006) and (b) baseline salivary cortisol levels (μg 100 ml; p<.03). Note that the levels represent average baseline and SE across the three laboratory sessions.
Response Findings
Condition main effect
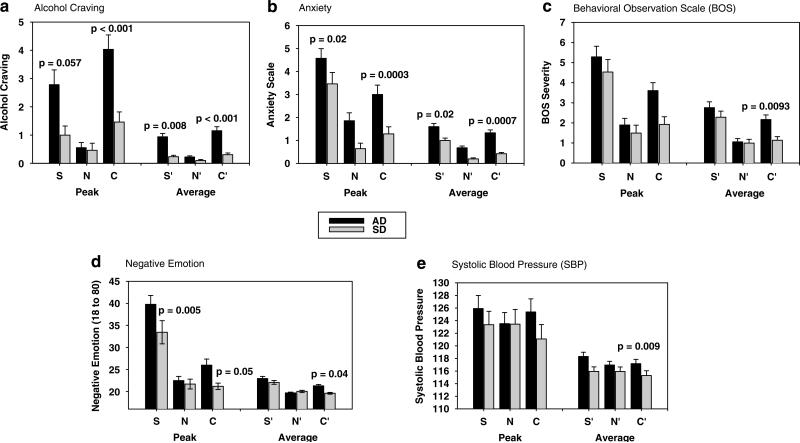
A significant effect of condition was seen for alcohol craving (2, 108 = 47.07 p<0.0001), anxiety (2, 108 = 86.65 p < 0.0001), negative emotion (2, 108 = 100.67; p<0.0001), positive emotion (2, 108 = 94.26; p<0.0001), SBP (2, 108 = 3.5; p = 0.03), salivary cortisol (2, 97 = 6.05; p<0.003), and behavioral distress responses (2, 108 = 43.7; p<0.0001).
Significantly greater alcohol craving, anxiety, and negative emotion ratings were observed in the stress and the alcohol cue compared with the neutral condition (stress: p<0.0001, in all cases; alcohol cue: craving: p<0.0001; anxiety: p<0.0001; negative emotion: p<0.003). Significantly greater anxiety (p<0.0001) and negative emotion was reported in the stress compared with the cue condition (p<0.0001), but greater alcohol craving was reported in the alcohol cue compared with the stress condition (p<0.02). Participants reported significantly greater positive emotion in the neutral compared with the stress condition, the neutral compared with the alcohol cue condition, and in the alcohol cue compared with the stress condition (p<0.0001 in all cases).
Significantly higher behavioral distress responses were recorded in the stress (p<0.0001) and alcohol cue (p = 0.002) compared with the neutral condition and in the stress condition compared with the alcohol cue (p<0.0001, see Figure 2c). Significantly higher behavioral distress responses and SBP were also observed in the stress compared with the alcohol cue condition (BOS: p<0.0001; SBP: p = 0.01). Salivary cortisol response was significantly higher in the cue condition compared with both the neutral (p = 0.001) and the stress (p<0.01) conditions.
Figure 2.
Mean and SE for peak and average (across 75 min and six time points postimagery exposure) alcohol craving, anxiety, behavioral distress, and emotional responses following exposure to stress (S, S’), alcohol cues (C, C’), and neutral (N, N’) conditions in alcohol-dependent (AD) vs social drinking (SD) participants. For the average responses, condition contrasts within groups are as follows: (a) alcohol craving: in the AD group, S’ >N’ (p<0.0001) and C’ >N’ (p<0.0001); in the SD, only C’ >N’, p<0.02; (b) anxiety: in the AD, S’ >N’ (p<0.0001), C’ >N’ (p<0.0001), and S’ >C’ (p<0.005) and in SD, S’ >N’ (p<0.0001), C’ >N’ (p<0.02), and S’ >C’ (p<0.0001). (c) Behavioral distress responses (BOS): in the AD group, S’ >N’ (p<0.0001), C’ >N’ (p<0.001), and S’ >C’ (p<0.01) and in the SD, S’ >N’ (p<0.0001) and S’ >C’ (p<0.0001). (d) Negative emotion: in the AD group, S’ >N’ (p<0.0001), C’ >N’ (p<0.0001),and S’ >C’ (p<0.0001) and in SD, S’ >N’ (p<0.0001) and S’ >C’ (p<0.0001). (e) Systolic blood pressure (SBP in mmHg): in the AD group, S’ >N’ (p<0.009) and S’ >C’ (p<0.02), and no differences between conditions in the SD group. The significance between group contrasts in conditions is shown in the graphs.
Group main effect
A main effect of group was observed in alcohol craving (1, 54 = 8.4; p = 0.005) and anxiety (1, 54 = 7.02; p = 0.01) responses, with the AD group showing higher responses compared to the SD group (p<.01) (see Figure 2a and b).
Interaction effects (group X condition)
Significant group X condition interactions were observed in alcohol craving (2, 108 = 20.1; p<0.0001), anxiety (2, 108 = 5.86; p<0.004), negative emotion (2, 108 = 12.21; p<0.0001), behavioral distress responses (2, 108 = 4.5; p = 0.01), HR (2,108 = 5.63, p<0.005), and salivary cortisol (2,97 = 11.7, p<0.0001).
Significantly increased alcohol craving and anxiety were observed in the AD vs the SD following both stress (alcohol craving: p = 0.008; anxiety: p = 0.02), and alcohol cue (alcohol craving: p<0.0001; anxiety: p = 0.0007) exposure. Although the AD group had higher anxiety ratings in the neutral condition, this difference was not statistically significant (p<0.08). The AD patients also reported significantly higher negative emotion compared with the SDs following exposure to alcohol cue (p = 0.04) and demonstrated an alcohol cue-related increase=in negative emotion (C>N, p<0.0001), not observed in the SDs. While both groups reported increased alcohol cue-induced craving (alcohol patients, p<0.0001; controls, p<0.02), only AD patients reported significantly increased alcohol craving in the stress compared with the neutral condition (p<0.0001; see Figure 2a–d).
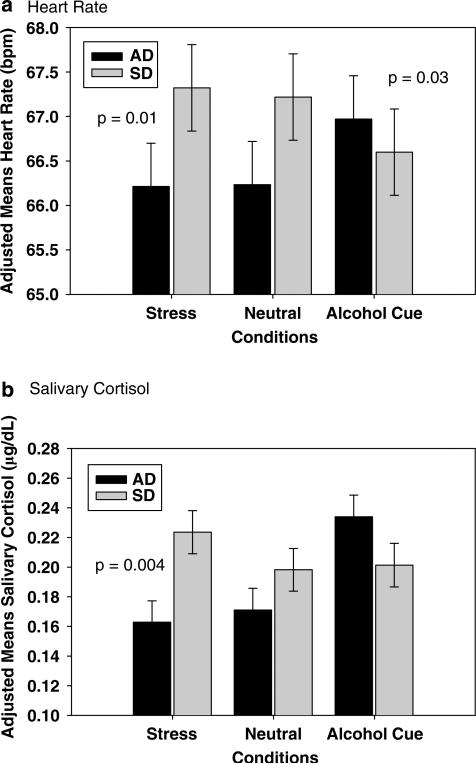
Both the AD and the SD group showed significantly higher behavioral distress responses in the stress vs the neutral condition (p <0.0001; no differences between groups) and in the stress vs the cue condition (AD: p<0.01; SD: p<0.0001), but significantly higher behavioral distress responses to cues were only seen in the AD group (C>N, p<0.0001; see Figure 2c). After accounting for baseline differences, HR responses were significantly increased in the AD group for the cue condition as compared to the neutral (p<0.04) and the stress (p<0.03) condition, but in the SD group average HR was higher in the stress compared to the cue condition (p<0.03). For salivary cortisol, SDs were higher than ADs in the stress condition (p<0.004) and within each group, AD subjects showed higher responses in the cue compared to stress (p<0.0001) and neutral (p<0.0001) conditions, while the SD groups showed higher responses in stress vs neutral (p<0.06) condition only (Figure 3a and b).
Figure 3.
Mean and SE (adjusted for group differences in baseline) in response to stress (S), alcohol cue (C), and neutral (N) imagery exposure in alcohol-dependent individuals (AD) and social drinkers (SDs) in: (a) heart rate (b.p.m.); in the AD group, C’ >N’ (p<0.04) and C’ >S’ (p = 0.03) and in the SD, S’ >C’ (p<0.04). (b) Salivary cortisol (μg per 100 ml); in the AD group, C’ >N’ (p<0.0001) and C’ >S’ (p<0.0001) and in the SD, S’ >N’ (p = 0.06). The Significance between group contrasts in conditions is shown on the graphs.
Persistent Elevation of Craving, Anxiety and Blood Pressure Following Stress and Cue Exposure
A significant group X condition X time-point interaction was observed in alcohol craving (14, 754 = 2.6; p = 0.001), resulting from higher levels of alcohol craving at the imagery (stress: p<0.0001; cue: p<0.0001), the initial recovery (stress: p = 0.0003; cue: p = 0.0009), the second recovery (stress: p<0.005; cue: p = 0.01), and the third recovery (stress: p<0.05; cue: p = 0.03) time points in the AD vs the SD groups in the stress and the alcohol cue, but not the neutral, conditions.
Significant group X time point interactions were found for anxiety (7, 378 = 2.1; p = 0.04), negative emotion (7, 387 = 3.15; p = 0.003), SBP (8, 432 = 2.0; p<0.05), and behavioral distress scores (2, 108 = 3.8; p<0.03). Increased anxiety in the AD vs SD groups was evident at the imagery (+0; p<0.0001) and all recovery time points ( + 5; p = 0.01; + 10; p<0.05; + 15; p<0.05; + 30; p = 0.02; + 45; p = 0.03; + 60; p = 0.01) for the stress and cue conditions only. Similarly, in AD patients, ratings of negative emotion remained increased compared with baseline at the initial (p<0.0001), second (p = 0.0009), and third (p = 0.01) recovery time points. In SD, increased rating were only observed at the first imagery time point (p<0.02). A significant group X Time point interaction for SBP indicated that the AD patients’ blood pressure remained significantly elevated compared with their baseline until the final recovery time point in both the stress ( + 10: p<0.003; + 15: p = 0.02; + 30: p = 0.005; + 60: p<0.009) and cue ( + 15: p = 0.05; + 30: p = 0.0002; + 45: p<0.0001; + 60: p = 0.002) imagery conditions. In the SDs, no significant elevations relative to baseline were observed in the cue condition and increases were only seen at the first ( + 5: p = 0.03) and second ( + 10: p = 0.006) recovery time point in the stress condition (see Figure 2e). Higher behavioral distress scores were observed at the imagery time point only for the AD vs SD groups.
Correlational Analyses
In AD patients
Significant correlations were obtained between stress-induced alcohol craving and anxiety (r = 0.49; p<0.009) and negative emotion (r = 0.38; p<0.05), and for cue-induced alcohol craving with anxiety (r 0.53; p<0.0004) and negative emotion (r = 0.52; p<0.005).
In SD group
Significant correlations were obtained between cue-induced alcohol craving and anxiety (r = 0.48; p = 0.01), and HR (r = 0.43, p = 0.02).
Secondary Analyses
As the AD group had higher basal cortisol levels, we examined whether higher basal levels were associated with the blunted cortisol response observed in the stress condition. Significant negative association between basal cortisol levels and peak cortisol response (differences from baseline) following stress exposure was observed indicating that increased basal levels of cortisol were associated with a decrease in peak cortisol following exposure to the stress imagery condition (r = 0.61; p<0.001). To account for the potential influence of higher lifetime prevalence of anxiety disorders in the AD vs SD groups, we reanalyzed the data for all dependent measures excluding the five individuals with a history of anxiety disorders, and findings remained unchanged. To assess whether the above findings in the AD patients were due to smoking status of the subjects, we also reanalyzed the data using smoking status as a covariate in the baseline data and in the LME models, and the significance levels of the analyses presented in the previous section remained unchanged.
DISCUSSION
The current findings demonstrate an increased sensitivity to emotional distress and alcohol craving with exposure to stress and to alcohol cues in treatment engaged AD individuals as compared to SDs. Stress exposure produced significant increases in alcohol craving in alcohol patients but not in controls, and stress-induced alcohol craving was significantly associated with increased anxiety and negative emotion in the alcohol patients. Alcohol cue exposure also resulted in increased negative emotion that was associated with cue-induced alcohol craving in alcoholics. Although, as expected, the SD group showed an increased stress response in the stress condition, they had minimal increases in stress-induced alcohol craving. Furthermore, in alcoholics only, stress-induced and cue-induced alcohol craving, anxiety, negative emotion, and blood pressure responses remained significantly elevated over multiple assessments during the recovery period, indicating a persistence of distress and alcohol craving in the context of stress and cue exposure. Altogether, the findings support our hypothesis that stress and cue exposure increases negative emotions and anxiety more so in alcoholics than controls. Moreover, these increases in negative emotion and anxiety were positively associated with sustained increases in alcohol craving only in alcoholics.
Greater severity of alcohol abuse is associated with higher levels of distress and craving (Fox et al, 2005; Grusser et al, 2006, 2007; Yoon et al, 2006; Rosenberg and Mazzola, 2007). Preclinical studies and human positron emission tomography (PET) studies indicate chronic alcohol-related changes in mesolimbic dopamine transmission. Furthermore, these changes in dopaminergic transmission are associated with alcohol craving in alcoholics and compulsive alcohol seeking in animal models of alcohol dependence (Koob et al, 2004; Koob and Kreek, 2007; Heinz et al, 2004, 2005; Martinez et al, 2005). This previous research is consistent with the current findings indicating that only alcoholics and not SDs show enhanced and persistent context-dependent craving. Although there is less evidence that alterations in mesolimbic dopamine transmission may enhance sensitivity to emotional distress, stress and glucocorticoid modulation of mesolimbic dopamine has been demonstrated (Oswald et al, 2005; Wand et al, 2007), and chronic alcohol abuse-associated CRF changes in limbic and dopaminergic regions such as the amygdala, nucleus accumbens, and the ventral tegmental area has been reported (Koob et al, 2004; Koob and Kreek, 2007).
While the above-mentioned literature supports the notion that chronic alcohol abuse-related changes may underlie the enhanced sensitivity to stress, negative emotions, and associated alcohol craving found in this study, it could also be argued that greater stress and cue-induced negative emotion and alcohol craving is due to factors that predate alcoholism. While the AD group was compared to a demographic and intellectually matched SD group, and other factors such as lifetime history of anxiety and smoking status did not appear to contribute to the findings, there is evidence of genetic and individual difference variables contributing to greater stress sensitivity and to the risk of alcohol dependence (Hariri et al, 2002; Goldman et al, 2005; Measelle et al, 2006). On the other hand, there is little evidence in the literature that in the absence of regular and chronic alcohol use, higher distress is associated with greater alcohol craving, especially in both stress and cue-related contexts. Thus, future studies would benefit from examining interactions of vulnerability factors with severity of alcohol abuse to assess their combined effects on alcohol craving and relapse risk.
Alcohol patients also demonstrated significantly higher basal HR compared with SDs. Increased basal HR was associated with higher acute alcohol withdrawal symptoms on day 1 after admission. Furthermore, unlike in the SD group, where higher HRs were associated with cue-induced alcohol craving as previously reported in the literature (Carter and Tiffany, 1999), the AD group showed increased HR in the cue condition but no association between increased HR and craving levels. Elevated cardiac output, increased HR, and decreased HR variability have been reported during acute alcohol withdrawal and during alcohol abstinence up to 4 weeks (Rechlin et al, 1996; Ingjaldsson et al, 2003; Bar et al, 2006; Thayer et al, 2006; Kahkonen and Bondarenko, 2000). Consistent with these human data, recent preclinical findings in nonhuman primates also indicate that chronic use of moderate levels of alcohol significantly increased HR, decreased HR variability, and altered HR responses to stress of a novel environment (Shively et al, 2007). This previous work along with the current findings indicate that chronic alcohol abuse and alcohol withdrawal are associated with over-activity of the sympathetic nervous system and decreased parasympathetic cardiac modulation, which could contribute to altered stress and cue-induced arousal during challenge conditions.
Higher basal levels of cortisol and a blunted response to stress were observed in the AD compared to the SD group. Blunted stress-related cortisol response in abstinent alcoholics has been previously documented (Errico et al, 1993; Bernardy et al, 1996; Lovallo et al, 2000; Junghanns et al, 2003; Adinoff et al, 2005), with lower responses in those who relapsed vs those who did not (Adinoff et al, 2005; Junghanns et al, 2005). Current findings are consistent with these data and extend them by documenting a significant negative correlation between high basal levels and lower stress-related cortisol responses. Heavy alcoholic drinking (Adinoff et al, 2003; Beresford et al, 2006), acute withdrawal (Kutscher et al, 2002), and recent withdrawal (Costa et al, 1996; Adinoff et al, 1991; Keedwell et al, 2001) have been associated with elevated basal cortisol levels, possibly related to increased CRH release (Holsboer et al, 1987). Increased basal cortisol levels are consistent with an overactive CRH–HPA system associated with chronic alcohol abuse and acute and protracted withdrawal (Becker, 1999; Koob et al, 2004; Breese et al, 2005; Menzaghi et al, 1994; Koob et al, 1998; Funk et al, 2006). In this study, elevated salivary cortisol levels even after 28 days of abstinence suggests continued dysregulation of CRF–HPA function, as has been reported with other chronic distress states, and with habitual smoking (Bhagwagar et al, 2003; Steptoe and Ussher, 2006; Li et al, 2007). An inability to mount an adequate stress response may also reflect altered HPA axis function, which could, in turn, contribute to the enhanced emotional distress and craving state seen in this study, and support the notion of a weakened ability to effectively cope with stress during protracted abstinence (Adinoff et al, 2005).
On the other hand, we found the enhanced salivary cortisol response to alcohol cues relative to neutral imagery in the AD group, indicating a differential HPA response in an alcohol-related context. Alcohol-related cues are more salient and arousing to alcoholics than controls, and our data also indicate that such cues increase negative emotions and anxiety in alcoholics. Somewhat different neurobiological pathways have been associated with stress and cue-induced drug seeking in animal models of reinstatement (Shaham et al, 2003; Weiss, 2005), and drugs such as alcohol and psychostimulants are known to activate the HPA axis (King et al, 2006). Drug-induced cortisol increases are associated with mesolimbic dopaminergic transmission and drug-related euphoria ratings in humans (Oswald et al, 2005), suggesting a role for cortisol in appetitive drug-related states. Although it is unclear whether cue-related cortisol increases in this study are related to drug-like effects or the associated anxiety and distress-related state, or both, the findings suggest the need to further investigate the role of cue-related increases in cortisol in drinking and alcohol seeking in future studies.
Significant correlations between alcohol craving and negative emotions were found in alcoholics, and HR and craving in the SD group, but significant associations across behavioral, subjective, and physiological response domains were not observed. Lack of significant association between these domains is not uncommon (Baum et al, 1982; Sinha et al, 2003; Back et al, 2005) and may be related to the fact that stress and cue-related subjective, physiological, and behavioral responses represent semi-independent response domains specific to different aspects of stress adaptation (Sinha et al, 2003). While subjective measures may be related to the cognitive component of adaptation, cardiovascular or autonomic and HPA markers may be more related to the mobilization of energy sources and physiological and behavioral adaptation associated with stress.
Finally, several study limitations are noteworthy. While stress scenarios were individually calibrated on experience of distress between alcoholic patients and social controls, the alcohol cue exposure scenarios were elicited primarily on the basis of enhanced desire for alcohol and subsequent alcohol use. Thus, alcohol cues may encompass largely positive situations in SDs, while representing more negative situations in alcohol patients. Nonetheless, alcohol patients showed comparable levels of enhanced stress and craving sensitivity in response to both stress and to alcohol cue exposure. It is also important to note that the findings are based on a predominantly male sample, due to the limited number of alcoholic females. As prior studies in alcohol (Brady et al, 2006), cocaine, and healthy samples (Horrocks et al, 1990; Fox et al, 2008) have shown gender differences in stress and cue-related responses, future research that examines gender differences in these responses is warranted. Similarly, while secondary analyses were conducted to account for group differences in smoking status and lifetime prevalence of anxiety disorders, future studies may also benefit from testing the effects of these factors a priori as part of the experimental design. Without prospective premorbid data, it is also difficult to ascertain for certain whether the changes in basal stress arousal markers and stress responses are solely due to neuroadaptations associated with chronic alcohol abuse.
Despite these caveats, findings from this study provide the first evidence of an enhanced and persistent subjective and behavioral distress state associated with alcohol craving in the context of stress and alcohol cue exposure in chronic alcoholics when compared to a well-matched sample of SDs. These findings could have significant treatment implications. Careful assessment of the increased and persistent stress and alcohol craving sensitivity in the clinical context would be useful in identifying individuals susceptible to relapse. Furthermore, as supported by previous preclinical research (Le et al, 1999; Matsuzawa et al, 1998; Le et al, 2000; Liu and Weiss, 2002), the current findings suggest the need to further develop pharmacological therapies such as CRF antagonists and noradrenergic agents to specifically target the regulation of distress and distress-related alcohol craving during protracted abstinence to improve alcohol relapse outcomes.
ACKNOWLEDGEMENTS
This study was supported in part by Grants R0I-AA13892 (Sinha), UL1-RR24925 (Sinha), K02-DA17232 (Sinha) and the NIH/NCRR/CTSA Program Grant no.1 UL1 RR024139 (Yale GCRC). We also thank the staff at the Substance Abuse Treatment Unit, Clinical Neuroscience Research Unit, the General Clinical Research Center at Yale University School of Medicine, and those at the Yale General Clinical Research Center Laboratory for their assistance in completing this study.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that they have no competing financial interests or conflict of interest relating to the data included in this manuscript. Dr Rajita Sinha declares that she serves on the scientific advisory board of Embera NeuroTherapeutics, Inc., and Dr Zubin Bhagwagar declares that he serves on the speakers panel of AstraZeneca, BMS, and Janssen. The author(s) declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity relevant to the data in this manuscript over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest for this manuscript.
REFERENCES
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;30:585. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GH, Nutt DJ, et al. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res. 2003;27:1420–1427. doi: 10.1097/01.ALC.0000087581.13912.64. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Boettger MK, Neubauer R, Groteluschen M, Jochum T, Baier V, et al. Heart rate variability and sympathetic skin response in male patients suffering from acute alcohol withdrawal syndrome. Alcohol Clin Exp Res. 2006;30:1592–1598. doi: 10.1111/j.1530-0277.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- Baum A, Grunberg NE, Singer JE. The use of psychological and neuroendocrinological measurements in the study of stress. Health Psychol. 1982;1:217–236. [Google Scholar]
- Becker HC. Alcohol withdrawal: neuroadaptation and sensitization. CNS Spectrums. 1999;4:38–65. [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Beresford HF, et al. Hypercortisolism in alcohol dependence and its relation to hippocampal volume loss. J Stud Alcohol. 2006;67:861–867. doi: 10.15288/jsa.2006.67.861. [DOI] [PubMed] [Google Scholar]
- Bernardy NC, King AC, Lovallo WR. Cardiovascular responses to physical and psychological stress in female alcoholics with transitory hypertension after early abstinence. Alcohol Clin Exp Res. 2003;27:1489–1498. doi: 10.1097/01.ALC.0000085587.00498.38. [DOI] [PubMed] [Google Scholar]
- Bernardy NC, King AC, Parsons OA, Lovallo WR. Altered cortisol response in sober alcoholics: an examination of contributing factors. Alcohol. 1996;13:493–498. doi: 10.1016/0741-8329(96)00043-2. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Increase in concentration of waking salivary cortisol in recovered patients with depression. Am J Psychiatry. 2003;160:1890–1891. doi: 10.1176/appi.ajp.160.10.1890. [DOI] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, et al. Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol Clin Exp Res. 2006;30:938–946. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, McQuaid JR, Patterson TL, Irwin MR, Grant I. Severity of psychosocial stress and outcome of alcoholism treatment. J Abnorm Psychol. 1990;99:344–348. doi: 10.1037//0021-843x.99.4.344. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. Monographs of the Rutgers Center of Alcohol Studies. Vol. 6. New Brunswick, NJ: 1969. American drinking practices. A national study of drinking behaviors and attitudes. pp. 185–260. [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Cooney NL, Gillespie RA, Baker LH, Kaplan RF. Cognitive changes after alcohol cue exposure. J Consult Clin Psychol. 1987;55:150–155. doi: 10.1037//0022-006x.55.2.150. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G. An assessment of hypothalamo-pituitary-adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology. 1996;21:263–275. doi: 10.1016/0306-4530(96)00001-7. [DOI] [PubMed] [Google Scholar]
- Errico AL, Parsons OA, King AC, Lovallo WR. Attenuated cortisol response to biobehavioral stressors in sober alcoholics. J Stud Alcohol. 1993;54:393–398. doi: 10.15288/jsa.1993.54.393. [DOI] [PubMed] [Google Scholar]
- Fox HC, Berquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong K-I, Siedlarz KM, Sinha R. Enhanced Sensitivity to Stress and Drug/Alcohol Craving in Abstinent Cocaine Dependent Individuals Compared to Social Drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated response to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Morsen CP, Flor H. Alcohol craving in problem and occasional alcohol drinkers. Alcohol Alcohol. 2006;41:421–425. doi: 10.1093/alcalc/agl035. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Morsen CP, Wolfling K, Flor H. The relationship of stress, coping, effect expectancies and craving. Eur Addict Res. 2007;13:31–38. doi: 10.1159/000095813. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, et al. Correlation of alcohol craving with striatal dopamine synthesis capacity and d2/3 receptor availability: A combined [18f]dopa and [18f]dmfp pet study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser S, et al. Correlation between dopamine d(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Holsboer F, von Bardeleben U, Buller R, Heuser I, Steiger A. Stimulation response to corticotropin-releasing hormone (CRH) in patients with depression, alcoholism and panic disorder. Horm Metab Res Suppl. 1987;16:80–88. [PubMed] [Google Scholar]
- Horrocks PM, Jones AF, Ratcliffe WA, Holder G, White A, Holder R, et al. Patterns of ACTH and cortisol pulsatility over twenty-four hours in normal males and females. Clin Endocrinol (Oxf) 1990;32:127–134. doi: 10.1111/j.1365-2265.1990.tb03758.x. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biol Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Izard C. Patterns of emotions: A new analysis of anxiety and depression. Academic Press; New York: 1972. [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, et al. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, Kuether M, Jurth R, Ehrenthal D, et al. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–85. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Kahkonen S, Bondarenko BB. Cardiovascular changes in alcoholic patients during withdrawal phase. German J Psychiatry. 2000;3:1–6. [Google Scholar]
- Keedwell PA, Poon L, Papadopoulos AS, Marshall EJ, Checkley SA. Salivary cortisol measurements during a medically assisted alcohol withdrawal. Addict Biol. 2001;6:247–256. doi: 10.1080/13556210120056580. [DOI] [PubMed] [Google Scholar]
- King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006;59:203–209. doi: 10.1016/j.ijpsycho.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, et al. Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry. 1996;153:83–92. doi: 10.1176/ajp.153.1.83. [DOI] [PubMed] [Google Scholar]
- Kutscher S, Heise DJ, Banger M, Saller B, Michel MC, Gastpar M, et al. Concomitant endocrine and immune alterations during alcohol intoxication and acute withdrawal in alcohol-dependent subjects. Neuropsychobiology. 2002;45:144–149. doi: 10.1159/000054955. [DOI] [PubMed] [Google Scholar]
- Laberg JC, Ellertsen B. Psychophysiological indicators of craving in alcoholics: effects of cue exposure. Br J Addict. 1987;82:1341–1348. doi: 10.1111/j.1360-0443.1987.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for long-itudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: The problem of affective response integration. J Abnorm Psychol. 1983;92:276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr Emotional imagery: Conceptual structure and pattern of somatovisceral response. Psychophysiology. 1980;17:179–192. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Lifetime socioeconomic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32:824–833. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M. Conditioned fear stress induces ethanol-associated place preference in rats. Eur J Pharmacol. 1998;341:127–130. doi: 10.1016/s0014-2999(97)01456-8. [DOI] [PubMed] [Google Scholar]
- Measelle JR, Stice E, Springer DW. A prospective test of the negative affect model of substance abuse: moderating effects of social support. Psychol Addict Behav. 2006;20:225–233. doi: 10.1037/0893-164X.20.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzaghi F, Rassnick S, Heinrichs S, Baldwin H, Pich EM, Weiss F, et al. The role of corticotropin-releasing factor in the anxiogenic effects of ethanol withdrawal. Ann NY Acad Sci. 1994;739:176–184. doi: 10.1111/j.1749-6632.1994.tb19819.x. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, et al. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Rychtarik RG, Rappaport NB, Smith PO, Etscheidt M, Brown TA, et al. Reactivity to alcohol-relevant beverage and imaginal cues in alcoholics. Addict Behav. 1992;17:209–217. doi: 10.1016/0306-4603(92)90026-r. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Fertig J, Baker L, Cooney N. Reactivity to alcohol cues in alcoholics and non-alcoholics: implications for a stimulus control analysis of drinking. Addict Behav. 1983;8:1–10. doi: 10.1016/0306-4603(83)90048-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA. Chronic daily ethanol and withdrawal: 6. Effects on rat sympathoadrenal activity during ‘abstinence’. Alcohol. 2006;38:173–177. doi: 10.1016/j.alcohol.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechlin T, Orbes I, Weis M, Kaschka WP. Autonomic cardiac abnormalities in alcohol-dependent patients admitted to a psychiatric department. Clin Auton Res. 1996;6:119–122. doi: 10.1007/BF02291234. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Abrams DJ, Rubonis AV, Niaura RS, Sirota AD, et al. Cue-elicited urge to drink and salivation in alcoholics: Relationship to individual differences. Adv Beh Res Th. 1992;14:195–210. [Google Scholar]
- Rosenberg H, Mazzola J. Relationships among self-report assessments of craving in binge-drinking university students. Addict Behav. 2007;32:2811–2818. doi: 10.1016/j.addbeh.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shaw JM, Kolesar GS, Sellers EM, Kaplan HL, Sandor P. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1:328–388. doi: 10.1097/00004714-198111000-00006. [DOI] [PubMed] [Google Scholar]
- Shively CA, Mictus JE, Grant KA, Goldberger AL, Bennett AJ, Willard SL. Effects of chronic moderate alcohol consumption and novel environment on heart rate variability in primates. Psychopharmacology (Berl) 2007;192:183–191. doi: 10.1007/s00213-007-0709-z. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001a;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Imagery Development Procedures Manual. Yale University; 2001b. unpublished manual. [Google Scholar]
- Sinha R. Stress and drug abuse. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook of Stress and the Brain. Part 2 Stress: Integrative and Clinical Aspects. Elsevier; Amsterdam: 2005. pp. 333–356. [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999b;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympathoadreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, O'Malley SS. Craving for Alcohol: Findings from the clinic and the laboratory. Alcohol Alcohol. 1999a;34:223–230. doi: 10.1093/alcalc/34.2.223. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Ussher M. Smoking, cortisol and nicotine. Int J Psychophysiol. 2006;59:228–235. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hall M, Sollers JJ, III, Fischer JE. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int J Psychophysiol. 2006;59:244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pituitary-adrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, et al. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32:2310–2320. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Yoon G, Kim SW, Thuras P, Grant JE, Westermeyer J. Alcohol craving in outpatients with alcohol dependence: Rate and clinical correlates. J Stud Alcohol. 2006;67:770–777. doi: 10.15288/jsa.2006.67.770. [DOI] [PubMed] [Google Scholar]