Abstract
Bacterial mRNAs begin with a triphosphate on the first transcribed nucleotide, but the endonuclease long thought to initiate mRNA decay in E. coli (RNase E) only works well on RNA with a 5′ monophosphate. The first committed step in the degradation of mRNA in that organism now appears to be the conversion of the 5′ triphosphate to a monophosphate, a process that is functionally similar to mRNA decapping in eukaryotes.
The 5′ end of newly synthesized RNAs bears a triphosphate derived from the first transcribed nucleotide. In eukaryotes the distal phosphate is replaced by an inverted, methylated GMP to form the m7GpppX- cap; however, no such modification occurs in prokaryotes. The hydrolysis of the cap to a 5′ monophosphate is the first committed step in eukaryotic mRNA decay, and a paper by Celesnik et al.1 that appeared in the July 6, 2007 issue of Molecular Cell shows that a remarkably similar process occurs in E. coli.
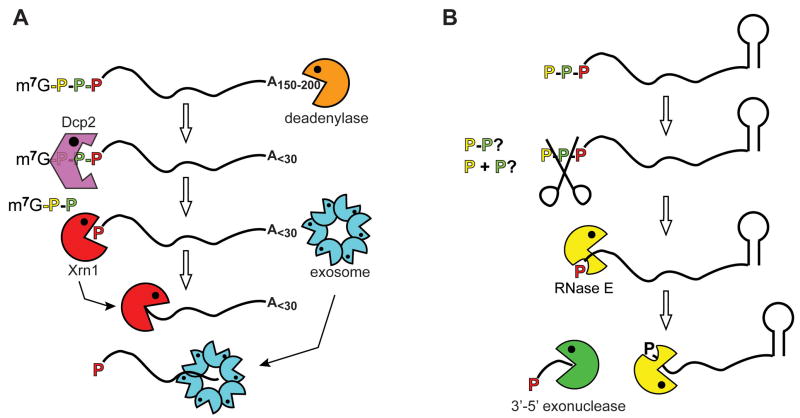
In eukaryotes the process of mRNA decay generally involves loss of the 3′ poly(A) tail and removal of the cap by a decapping enzyme (Dcp2). The body of the mRNA is then degraded from the 5′ end by the 5′-3′ exonuclease Xrn1 and from the 3′ end by the exosome2. Prokaryotes such as E. coli use a distinctly different process in which mRNA is cleaved by the endonuclease RNase E to generate an upstream product with a 3′ hydroxyl and a downstream product with a 5′ monophosphate. The upstream product is rapidly cleared by 3′–5′ exonucleases and the 5′ monophosphate end of each downstream cleavage product activates subsequent rounds of endonuclease cleavage and exonuclease clearance (see bottom of Fig. 1). Thus, on the surface bacterial mRNA decay appears to be distinctly different from the major decay process in eukaryotes.
Fig. 1. E. coli mRNA decay is activated by the conversion of the mRNA 5′ end from triphosphate to monophosphate.
Most bacterial mRNAs begin with a 5′ triphosphate and end with a stem-loop structure, and decay starts with endonuclease cleavage by RNase E. The preferred substrate for RNase E is RNA with a 5′ monophosphate, a property that is determined by the presence of a monophosphate-binding pocket within the catalytic domain of the enzyme, and Celesnik et al.1 describe a previously unknown step in mRNA decay in which pyrophosphate is removed from the 5′ end to generate a 5′ monophosphate substrate for cleavage by RNase E. This is the rate limiting step in decay, and subsequent cleavage by RNase E generates an upstream product with a 3′ hydroxyl that is degraded by 3′–5′ exonucleases and a downstream product with a 5′ monophosphate. This cycle is then repeated to complete degradation of the mRNA.
The 5′ end of substrate mRNA dramatically affects its ability to be cleaved by RNase E, with the rate constant for 5′ monophosphate RNA more than an order of magnitude greater than that for RNA with a 5′ triphosphate or 5′ hydroxyl3,4. The basis for this 5′ end selectivity became clear from the crystal structure of the homotetrameric catalytic domain5, which showed the 5′ monophosphate end is hydrogen bonded into a pocket at the base of an RNA-binding channel. This interaction is thought to cause an allosteric change in the protein that juxtaposes a magnesium-coordinated hydroxyl with the scissile phosphate at a downstream site in the RNA substrate.
Given that the 5′ end of bacterial mRNA is a 5′ triphosphate Celesnik et al.1 asked whether in E. coli there might be a previously unidentified step that triggers RNase E cleavage by converting the 5′ terminus to a monophosphate. To address this they developed a splinted ligation assay in which an antisense DNA oligonucleotide that extends past the RNA 5′ end is used to position another oligonucleotide (oligo X) adjacent to 5′ most nucleotide. DNA ligase will covalently join oligo X to the 5′ end of the RNA if it has a 5′ monophosphate, but not a 5′ hydroxl or 5′ triphosphate. By carefully optimizing reaction conditions and comparing ligated with unligated RNA this assay yields quantitative data of the amount of RNA with a 5′ monophosphate end. Using this approach the authors showed that a significant portion of the full-length mRNA is 5′ monophosphorylated. If mRNA with a 5′ monophosphate is indeed the proximal substrate for RNase E the upstream product generated by the first cleavage event should be entirely monophosphorylated, and several approaches both confirmed this and showed that pyrophosphate removal is the rate-determining step in mRNA decay (Fig. 1). Based on this, decay should be impaired if the mRNA 5′ end cannot be processed to a 5′ monophosphate. To test this they joined the mRNA to a hammerhead ribozyme, which after self-cleavage generated mRNA with a 5′ hydroxyl end. The half-life of this mRNA was 6-times greater than the same mRNA with a 5′ triphosphate terminus, thus proving that a 5′ monophosphate is required to activate the decay process.
mRNA decay is an important and tightly regulated process, and the results in this study provide a new and unexpected link with eukaryotic mRNA decay, where decapping to generate mRNA with a 5′ monophosphate end is considered the first irreversibly committed step in the decay process. Decapping is tightly regulated and coordinated by changes in nutritional state, various signaling processes and translation. The functional similarity of pyrophosphate removal in bacteria raises the possibility that this too is a tightly regulated process.
The results of this study raise many questions. What enzyme(s) catalyzes pyrophosphate removal? Does a single enzyme release pyrophosphate from the mRNA, or do multiple enzymes act to remove the two phosphates sequentially? Since RNase E forms a complex (the RNA degradosome) with the ATPase RhlB and other proteins6, might this ATPase moonlight as an RNA pyrophosphatase? Does the RNA pyrophosphatase have to be part of the degradosome at all? How universal is this decay mechanism, and what controls its rate? Do the small untranslated RNAs that regulate gene expression in prokaryotes impact pyrophosphate removal? Much as the discovery of decapping led to new discoveries in eukaryotic mRNA decay, the results of this study are likely to generate new insights into prokaryotic decay for some time to come.
References
- 1.Celesnik H, Deana A, Belasco JG. Initiation of RNA decay in Escherichia coli by 5′ pyrophosphate removal. Mol Cell. 2007;27:79–90. doi: 10.1016/j.molcel.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 3.Mackie GA. Ribonucleaes E is a 5′-end-dependent endonuclease. Nature. 1998;395:720–723. doi: 10.1038/27246. [DOI] [PubMed] [Google Scholar]
- 4.Tock MR, Walsh AP, Carroll G, McDowall KJ. The CafA protein required for the 5′-maturation of 16 S rRNA is a 5′-end-dependent ribonuclease that has context-dependent broad sequence specificity. J Biol Chem. 2000;275:8726–8732. doi: 10.1074/jbc.275.12.8726. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan AJ, et al. Structure of Escherichia coli RNase E catalytic domain and implications for RNA turnover. Nature. 2005;437:1187–1191. doi: 10.1038/nature04084. [DOI] [PubMed] [Google Scholar]
- 6.Marcaida MJ, DePristo MA, Chandran V, Carpousis AJ, Luisi BF. The RNA degradosome: life in the fast lane of adaptive molecular evolution. Trends Biochem Sci. 2006;31:359–365. doi: 10.1016/j.tibs.2006.05.005. [DOI] [PubMed] [Google Scholar]