Abstract
We used the patch-clamp technique to study the effect of arachidonic acid (AA) on basolateral 18-pS K channels in the principal cell of the cortical collecting duct (CCD) of the rat kidney. Application of AA inhibited the 18-pS K channels in a dose-dependent manner and 10 μM AA caused a maximal inhibition. The effect of AA on the 18-pS K channel was specific because application of 11,14,17-eicosatrienoic acid had no effect on channel activity. Also, the inhibitory effect of AA on the 18-pS K channels was abolished by blocking cytochrome P-450 (CYP) epoxygenase with N-methylsulfonyl-6-(propargyloxyphenyl)hexanamide (MS-PPOH) but was not affected by inhibiting CYP ω-hydroxylase or cyclooxygenase. The notion that the inhibitory effect of AA was mediated by CYP epoxygenase-dependent metabolites was further supported by the observation that application of 100 nM 11,12-epoxyeicosatrienoic acid (EET) mimicked the effect of AA and inhibited the basolateral 18-pS K channels. In contrast, addition of either 5,6-, 8,9-, or 14,15-EET failed to inhibit the 18-pS K channels. Moreover, application of 11,12-EET was still able to inhibit the 18-pS K channels in the presence of MS-PPOH. This suggests that 11,12-EET is a mediator for the AA-induced inhibition of the 18-pS K channels. We conclude that AA inhibits basolateral 18-pS K channels by a CYP epoxygenase-dependent pathway and that 11,12-EET is a mediator for the effect of AA on basolateral K channels in the CCD.
Keywords: eicosatrienoic acid; 11,12-epoxyeicosatrienoic acid; basolateral potassium conductance; sodium-potassium-adenosinetriphosphate; sodium transport
BASOLATERAL K CHANNELS serve several important cell functions in the cortical collecting duct (CCD) (28). First, they participate in generating the cell membrane potential. Because both Na reabsorption and K secretion are electrogenic processes (13, 24), alterations in cell membrane potentials are expected to affect both Na reabsorption and K secretion in the CCD. Indeed, it has been reported that inhibition of basolateral K conductance reduces the transepithelial Na transport rate in the isolated rabbit CCD (23). Second, the basolateral K channels play a key role in K recycling across the basolateral membrane, and basolateral K recycling has been shown to be coupled to the Na-K-ATPase, which is essential for Na extrusion across the basolateral membrane (18). Third, the basolateral K channels could provide the second route for K entering in the cell across the basolateral membrane in the CCD when the cell membrane potential exceeds the K equilibrium potential (22).
The CCD is responsible for hormone-regulated Na absorption. Because basolateral K channel activity is closely related to Na-K-ATPase turnover, which is stimulated by luminal Na entry (18), basolateral K channel activity may be indirectly correlated with apical Na transport. Therefore, the factor regulating apical Na transport is also expected to affect basolateral K channels. We have previously demonstrated that arachidonic acid (AA) inhibits epithelial Na channels (ENaC) through a cytochrome P-450 (CYP)- and epoxygenase-dependent metabolic pathway and that 11,12-epoxyeicosatrienoic acid (EET) mediates the effect of AA on ENaC (31, 32). Thus increased EET is expected to suppress Na absorption in the CCD. We speculate that EET blocks Na transport by both inhibition of ENaC and also by suppressing basolateral K channels, leading to reduction of the driving force for Na entry. The aim of the present study is to test the hypothesis that CYP epoxygenase-dependent AA metabolism plays a role in the regulating basolateral K channels.
METHODS
Preparation of CCDs
Pathogen-free Sprague-Dawley rats of both sex (age 5 wk) were used in experiments. The animals were purchased from Taconic Farms (Germantown, NY) and maintained on a high-K diet (10% wt/wt) for 7 days. The reason for maintaining animals on a high-K diet is that the basolateral membrane of principal cells is easier to patch than that from animals on a normal chow due to increased area of the lateral membrane. The weight of animals used for experiments was <90 g. Rats were killed by cervical dislocation, and kidneys were removed immediately. Several thin slices of the kidney (<1 mm) were cut and placed on the ice-cold Ringer solution until dissection. The dissection was carried out at room temperature, and two watchmaker forceps were used to isolate single CCDs. To immobilize the tubules, they were placed onto a 5 × 5-mm cover glass coated with “Cell-Tak” (Becton-Dickinson, Bedford, MA). The cover glass was transferred to a chamber (1,000 μl) mounted on an inverted Nikon microscope. The CCDs were superfused with HEPES-buffered NaCl solution, and the temperature of the chamber was maintained at 37 ± 1°C by circulating warm water surrounding the chamber. We followed the methods described previously to prepare the basolateral membrane for patch-clamp experiments (30).
Patch-clamp technique
An Axon200A patch-clamp amplifier was used to record channel current. The current was low-pass filtered at 1 KHz using an eight-pole Bessel filter (902LPF; Frequency Devices, Haverhill, MA) and digitized using an interface, digidata1200 (Axon). The data were then collected with an IBM-compatible Pentium computer (Gateway 2000) at a rate of 4 kHz and analyzed using the pClamp software system 7.0 (Axon Instruments, Burlingame, CA). Channel activity was defined as NPo, a product of channel number (N) and open probability (Po), that was calculated from data samples of 60-s duration in the steady state as follows
| (1) |
where ti is the fractional open time spent at each of the observed current levels. The channel conductance was determined by measuring the current amplitude over several holding potentials.
Experimental solution and statistics
The pipette solution contained (in mM) 140 KCl, 1.8 MgCl2, and 10 HEPES (pH = 7.4). The bath solution for cell-attached patches was composed of (in mM) 140 NaCl, 5 KCl, 1.8 CaCl2, 1.8 MgCl2, and 10 HEPES (pH = 7.4). AA and 11,14,17-eicosatrienoic acid (EA) were obtained from Nu-Check (Elysian, MN) while EETs and N-methylsulfonyl-12,12-dibromododec-11-enamide (DDMS) were purchased from Biomol. N-methylsulfonyl-6-(propargyloxyphenyl)hexanamide (MS-PPOH), an inhibitor of CYP epoxygenase (26), was synthesized in Falck's laboratory (University of Texas Southwestern Medical Center at Dallas). Indomethacin was obtained from Sigma (St. Louis, MO). Data are shown as means ± SE, and the paired Student's t-test was used to calculate the significance between the control and experimental groups. Statistical significance was taken as P < 0.05.
RESULTS
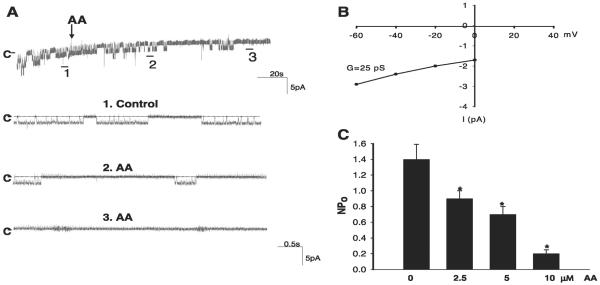
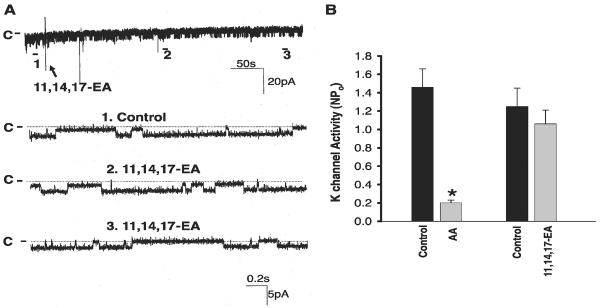
Figure 1A is a recording showing the activity of the basolateral small-conductance K channel in a cell-attached patch, and Fig. 1B is a current and voltage curve for this particular patch, showing that the channel conductance is 25 pS between -60 to -40 mV. Also, we confirmed the previous finding that the small-conductance K channel is the most abundant K channel in the basolateral membrane of the CCD (27). Because the channel slope conductance was 18 pS when measured in excised patches with symmetrical KCl solutions (27), we name the small-conductance K channel as the 18-pS K channel in the present study. We then examined the effect of AA on the 18-pS K channels in cell-attached patches. Figure 1A is a channel recording demonstrating that application of 10 μM AA inhibited the basolateral 18-pS K channels. Data summarized in Fig. 1C show that 10 μM AA decreased channel activity defined by NPo from 1.4 ± 0.2 to 0.2 ± 0.05 (N = 6). Figure 1B also demonstrates that application of 2.5 and 5 μM AA decreased channel activity to 0.9 ± 0.1 and 0.7 ± 0.1 (N = 4), respectively. To test whether the effect of AA on the basolateral K channels was specific, we examined the effect of 11,14,17-EA on the 18-pS K channels in the basolateral membrane. Figure 2A is a channel recording demonstrating that application of 10 μM 11,14,17-EA did not inhibit the basolateral K channels. Data summarized in Fig. 2B show that channel activity before and after 11,14,17-EA was not significantly altered. Thus AA-induced inhibition of basolateral K channels was specific and not due to unspecific lipid effect on K channels.
Fig. 1.
A: a channel recording demonstrates the effect of arachidonic acid (AA, 10 μM) on the basolateral 18-pS K channels in a cell-attached patch at 0 mV holding potential. The trace on top shows the time course of the experiment, and three parts of the recording indicated by nos. are extended at a fast time resolution. The addition of AA is indicated by an arrow, and channel closed level is indicated by C or a dotted line. B: the current (I) and voltage (V) relationship curve of the K channel in a cell-attached patch. The voltage was applied to the pipette, and the cell membrane potential was not taken into consideration. C: dose-dependent AA effect on the basolateral K channels. *Significant difference between the control (0 μM AA) and experimental groups.
Fig. 2.
A: channel recording demonstrating the effect of 11,14,17-11,14,17-eicosatrienoic acid (EA, 10 μM) on the basolateral 18-pS K channels in a cell-attached patch at 0 mV holding potential. The trace on top shows the time course of the experiment, and three parts of the recording indicated by nos. are extended at a fast time resolution. The addition of 11,14,17-EA is indicated by an arrow, and channel closed level is indicated by C or a dotted line. B: effect of AA and 11,14,17-EA on the basolateral K channels. *Significant difference.
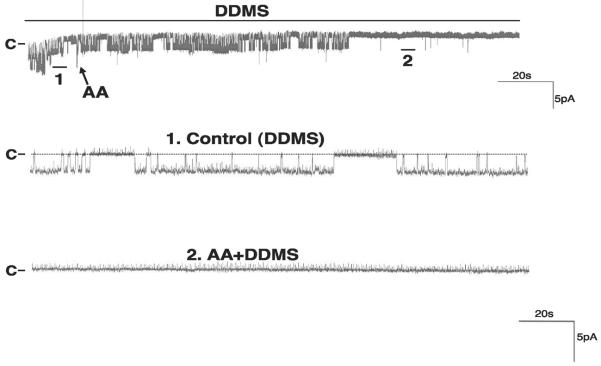
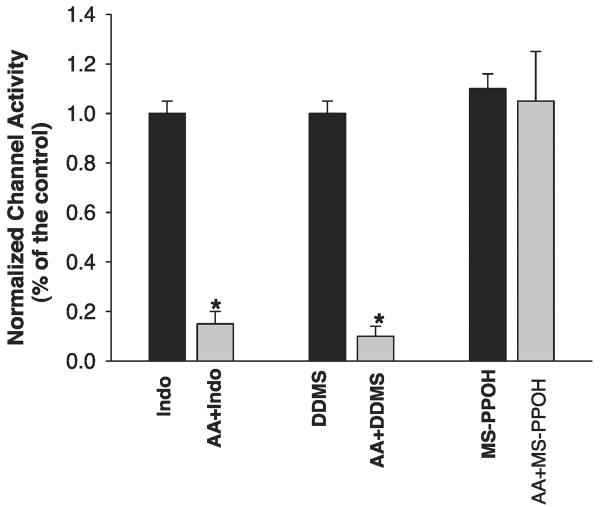
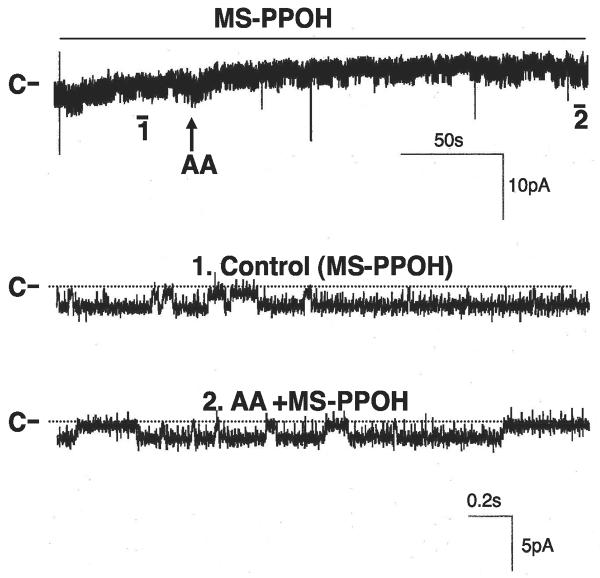
We then examined whether AA per se or AA metabolites are responsible for inhibiting the basolateral K channels. It is well established that the major metabolic enzymes of AA in the CCD include cyclooxygenase (COX), CYP ω-hydroxylase, and CYP epoxygenase (11, 19). Thus we examined the effect of AA in the presence of 5 μM DDMS, an inhibitor of CYP ω-hydroxylase (26). Figure 3 is a channel recording showing that DDMS did not abolish the effect of AA on the basolateral K channels since 10 μM AA still decreased channel activity by 89 ± 10% from 1.8 ± 0.5 to 0.2 ± 0.05 (N = 4). We also examined the effect of AA on the basolateral K channels in the presence of 5 μM indomethacin, an agent that inhibits COX-dependent AA metabolism. Similar to DDMS, inhibition of COX did not have a significant effect on channel activity (Fig. 4). Moreover, treatment of the CCD with indomethacin failed to abolish the inhibitory effect of AA on the basolateral 18-pS K channels. Data summarized in Fig. 4 demonstrate that application of 10 μM AA inhibited the basolateral K channels and decreased NPo by 85 ± 8% (N = 4). We then examined the effect of AA on basolateral K channels in the presence of MS-PPOH (26), an agent that blocks the CYP epoxygenase. Figure 5 is a channel recording demonstrating that AA failed to inhibit the 18-pS K channels in the CCD treated with 5 μM MS-PPOH. Data summarized in Fig. 4 demonstrate that inhibition of CYP epoxygenase completely blocks the effect of AA on the 18-pS K channels. Thus our data strongly indicate that the inhibitory effect of AA on basolateral K channels was mediated by CYP epoxygenase-dependent AA metabolites.
Fig. 3.
Channel recording demonstrating the effect of AA (10 μM) on the basolateral 18-pS K channels in the presence of DDMS (5 μM). The experiment was conducted in a cell-attached patch with 0 mV holding potential. The trace on top shows the time course of the experiment, and two parts of the recording indicated by nos. are extended at a fast time resolution. The addition of AA is indicated by an arrow, and channel closed level is indicated by C or a dotted line.
Fig. 4.
Effect of AA (10 μM) on the basolateral 18-pS K channels in the presence of DDMS (5 μM), indomethacin (indo) (5 μM), or N-methylsulfonyl-6-(propargyloxyphenyl)hexanamide (MS-PPOH, 5 μM). *Significant difference.
Fig. 5.
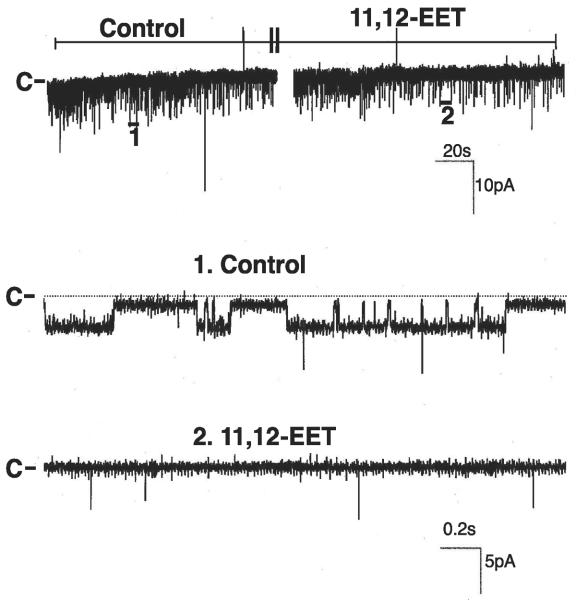
Channel recording demonstrating the effect of 100 nM 11,12-epoxyeicosatrienoic acid (EET) on the basolateral 18-pS K channels in a cell-attached patch with 0 mV holding potential. The trace on top shows the time course of the experiment, and two parts of the recording indicated by nos. are extended at a fast time resolution. The gap between traces was 60 s, and channel closed level is indicated by C or a dotted line.
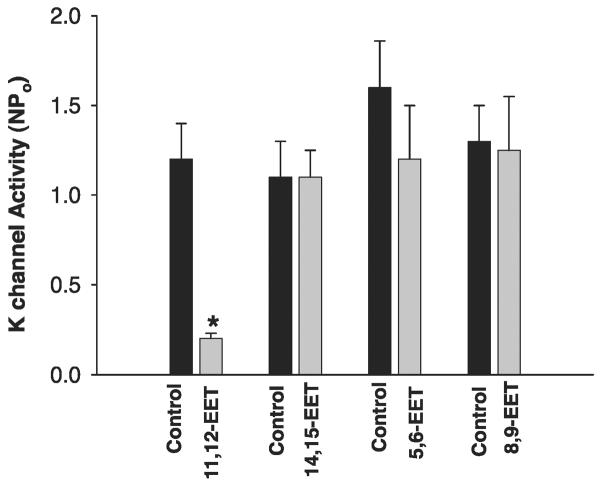
The major AA metabolites of the CYP epoxygenase-dependent pathway are 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET. It has been shown that 11,12-EET is the major product of CYP epoxygenase-dependent AA metabolites (12). We have also previously demonstrated that 11,12-EET inhibited the ENaC in the CCD (31). Thus we examined whether 11,12-EET could mimic the effect of AA and inhibit the basolateral K channels in the CCD. Figure 6 is a recording showing the effect of 11,12-EET on the basolateral K channels in a cell-attached patch. Application of 100 nM 11,12-EET mimicked the effect of AA and inhibited the 18-pS K channels. Data summarized in Fig. 7 demonstrate that 100 nM 11,12-EET decreased channel activity defined by NPo from 1.1 ± 0.2 to 0.2 ± 0.05 (N = 4). To further determine whether EET regioisomers other than 11,12-EET could also mimic the effect of AA and inhibit the basolateral K channels, we examined the effects of 100-200 nM 5,6-EET, 8,9-EET, and 14,15-EET. Figure 7 summarized the effects of 5,6-EET, 8,9-EET, and 14,15-EET on the basolateral 18-pS K channels. In contrast to 11,12-EET, addition of 100-200 nM 14,15-EET had no significant effect of the 18-pS K channels, and NPo was 1.1 ± 0.2 (control) and 1.1 ± 0.2 (14,15-EET). Similar to 14,15-EET, application of 100-200 nM 5,6-EET did not have a significant effect on the basolateral K channels, and NPo was 1.6 ± 0.2 (control) and 1.3 ± 0.2 (5,6-EET). Also, application of 8,9-EET had no effect on the basolateral K channels, and NPo was 1.3 ± 0.2 (control) and 1.25 ± 0.2 (8,9-EET). Moreover, 11,12-EET can inhibit the basolateral 18-pS K channels in the presence of MS-PPOH (data not shown). Thus 11,12-EET is responsible for mediating the effect of AA on the basolateral K channels in the CCD.
Fig. 6.
Channel recording demonstrating the effect of AA (10 μM) on the basolateral 18-pS K channels in the presence of MS-PPOH (5 μM). The experiment was conducted in a cell-attached patch with 0 mV holding potential. The trace on top shows the time course of the experiment, and two parts of the recording indicated by nos. are extended at a fast time resolution. The addition of AA is indicated by an arrow, and channel closed level is indicated by C or a dotted line.
Fig. 7.
Effect of 100 nM 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET on the basolateral 18-pS K channels. Experiments were performed in cell-attached patches. *Significant difference.
DISCUSSION
The main finding of the present study is that AA inhibits basolateral 18-pS K channels in the CCD. Three types of basolateral K channels, large conductance (>100 pS), intermediate conductance (45-85 pS), and small conductance (18-28 pS), have been found in cell-attached patches in the basolateral membrane of the CCD (10, 30). When the channel conductance was measured in inside-out patches with symmetrical 140 mM KCl in the bath as well as in the pipette, conductance of the three K+ channels was 85, 28, and 18 pS, respectively. We and others have shown that nitric oxide and cGMP stimulate the basolateral K channels (9, 15). Although three types of K channels are expressed in the basolateral membrane of the CCD, the 18-pS K channel has a high channel open probability and is the most frequently observed K channel in our experiments. Thus it is possible that the 18-pS K channel is a major type of K channel in the basolateral membrane of principal cell in the CCD from the rat kidney. The notion that 18-pS K channels may be major contributors to basolateral K conductance has also been shown with whole cell patch experiments in which noise analysis reveals that 18-pS K channels may account for 65% of whole cell K current (6). Thus inhibition of 18-pS K channels by AA is expected to have an effect on the basolateral membrane potential. However, we have observed either no change or a small decrease in the single channel current amplitude during the experiments. One possible interpretation is that AA stimulates Ca2+-activated big-conductance K (BK) channel in the apical membrane (Wang, unpublished observation). Because our experimental setting was not exactly mimicking the in vivo condition in which apical and basolateral membrane potential are different, stimulation of apical BK channel may also cause a hyperpolarization and offset the effect of AA on the basolateral membrane potential.
The inhibitory effect of AA on the basolateral K channels was not the result of nonspecific lipid effect because addition of 11,14,17-EA did not mimic the effect of AA. Moreover, inhibition of CYP epoxygenase activity abolished the effect of AA on basolateral K channels, suggesting that metabolites of AA mediated the effect of AA on basolateral 18-pS K channels. The major enzymes responsible for AA metabolism in the kidney include COX, lipooxygenase, CYP ω-hydroxylase and CYP epoxygenase (20). However, a large body of evidence indicates that both COX-dependent and CYP enzyme-dependent metabolites of AA play an important role in the regulation of membrane transport in the kidney (4, 16, 20, 29). The main metabolites of COX1- or -2-dependent pathways of AA are prostaglandins such as PGE2, which has been shown to inhibit transepithelial Cl transport (3), bicarbonate transport (5), apical 70-pS K channels in the thick ascending limb (TAL) (14), and Na transport in the rabbit collecting tubule (25). The metabolites of the CYP-dependent ω oxidation pathway are 19- and 20-hydroxyeicosatetraenoic acid (20), which have been shown to inhibit the apical K channels and Na-Cl-K cotransporter in the TAL (4, 7). However, it is unlikely that COX or CYP ω-hydroxylation-dependent metabolites were responsible for the AA-induced inhibition of basolateral K channels because neither indomethacin nor DDMS was able to block the effect of AA on the basolateral K channels.
Two lines of evidence suggest that the inhibitory effect of AA on the 18-pS K channel was mediated by a CYP epoxygenase-dependent pathway: 1) inhibition of CYP epoxygenase with MS-PPOH abolished the effect of AA on the basolateral K channels; and 2) addition of 11,12-EET, a metabolite of the CYP epoxygenase-dependent pathway, mimicked the effect of AA and inhibited the 18-pS K channels. Thus CYP epoxygenase-dependent AA metabolites are responsible for the AA-mediated inhibition of the basolateral K channels. The role of EET in the regulation of ion channels and membrane transport is well established. EET has been shown to inhibit cardiac L-type Ca2+ channels (1) and activate the Ca2+-dependent large-conductance K channel in smooth muscle cells of renal vessels (34). EET has been shown to inhibit Na/H exchanger viaaCa2+-dependent pathway in the rabbit CCD (21) and diminish the effect of vasopressin on water permeability in the CCD (11).
CYP epoxygenase is able to convert AA to four types of EETs: 5,6-, 8,9-, 11,12-, and 14,15-EET. Each type of EET has been shown to play a role in the regulation of renal function: 14,15-EET has been demonstrated to stimulate proliferation of renal epithelial cells by a c-Src-dependent mechanism (2), whereas 5,6-EET has been reported to inhibit the Na transport in the isolated CCD (21). However, the effect of 5,6-EET was most likely mediated by COX-dependent metabolite because indomethacin abolished the effect of 5,6-EET on the Na transport. This view is also supported by the observation that PGE2 inhibits the Na transport and suppresses the vasopressin-induced increase in water channels (8). Our present study shows that 11,12-EET-induced inhibition of the 18-pS K channel was specific since EETs other than 11,12-EET had no effect on the basolateral K channels. Because 11,12-EET accounts for >60% of the total renal EET (12) and is formed in the rat CCD (31), 11,12-EET should play a role in the regulation of membrane transport in the CCD. The mechanism by which 11,12-EET inhibits the 18-pS K channel is not clear. It has been demonstrated that EET may exert its biological effect through binding to a G protein-like receptor in the membrane (33). We need further experiments to explore the role of G protein in mediating the effect of 11,12-EET on the 18-pS K channel.
CYP epoxygenases responsible for converting AA to EETs in the rat kidney include the CYP2C11, 2C12, 2C23, 2C24, and 2J family (20). We have previously demonstrated that CYP2C23 is highly expressed in the CCD (31). Moreover, the expression of CYP2C23 is regulated by dietary Na intake such that high Na intake increases, whereas low Na intake decreases, the expression of CYP2C23 and EET formation (19). Inhibition of CYP epoxygenase causes hypertension in rats on a high-Na diet, and removal of epoxygenase inhibition decreases the blood pressure of rats maintained on a high-Na diet (17). The role of CYP2C23 in the regulation of renal Na transport has been best demonstrated in CYP4A-10 null mice (19). It has been demonstrated that deletion of CYP4A-10 gene impairs the expression of CYP2C44 in mouse kidney (an analog of rat CYP2C23). As a consequence, the renal EET level decreased significantly in CYP4A-10 null mice, and they were hypertensive even when fed with normal Na diet. The patch-clamp experiments have further revealed that AA failed to inhibit ENaC in the CCD from CYP4A-10 null mice, whereas 11,12-EET was still able to inhibit ENaC. This suggests that the defective regulation of ENaC by AA was because AA could not be converted to 11,12-EET in the CCD from CYP4A-10 null mice. Thus CYP2C23-epoxygenase plays an important role in the regulation of Na transport in the CCD in response to dietary Na intake. Although further experiments are needed to determine the role of CYP2C23 in mediating the effect of AA on the basolateral 18-pS K channels, it is safe to conclude that CYP epoxygenase-dependent AA metabolism plays a role in regulating basolateral K channels.
Inhibition of basolateral K channels by 11,12-EET has a physiological significance because the basolateral K channels participate in generating the cell membrane potential. The transepithelial Na transport in the CCD is an electrogenic process such that hyperpolarization should stimulate while depolarization of cell membrane should reduce the Na transport. We speculate that 11,12-EET should decrease the basolateral membrane potential and diminish the driving force for Na entry across the apical membrane in the CCD. Thus inhibition of Na transport induced by stimulating CYP epoxygenase-dependent AA metabolism is the result of both blocking basolateral K channels and suppressing the apical ENaC in the CCD. We conclude that AA inhibits basolateral 18-pS K channels by a CYP epoxygenase-dependent pathway and that 11,12-EET mediates the effect of AA on the basolateral K channels.
Acknowledgments
GRANTS The work is supported by National Institutes of Health Grants HL-34300 (W. H. Wang) and DK-38226 (J. R. Falck) and by the Robert A. Welch Foundation.
REFERENCES
- 1.Chen JK, Capdevila JH, Zeldin DC, Rosenberg RL. Inhibition of cardiac L-type calcium channels by epoxyeicosatrienoic acids. Mol Pharmacol. 1999;55:288–295. doi: 10.1124/mol.55.2.288. [DOI] [PubMed] [Google Scholar]
- 2.Chen JK, Capdevila J, Harris RC. Overexpression of C-terminal Src kinase blocks 14,15-epoxyeicosatrienoic acid-induced tyrosine phosphorylation and mitogenesis. J Biol Chem. 2000;275:13789–13792. doi: 10.1074/jbc.275.18.13789. [DOI] [PubMed] [Google Scholar]
- 3.Culpepper RM, Andreoli TE. Interactions among prostaglandin E2, antidiuretic hormone, and cyclic adenosine monophosphate in modulating Cl- absorption in single mouse medullary thick ascending limbs of Henle. J Clin Invest. 1983;71:1588–1601. doi: 10.1172/JCI110915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escalante B, Erlij D, Falck JR, McGiff JC. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science. 1991;251:799–802. doi: 10.1126/science.1846705. [DOI] [PubMed] [Google Scholar]
- 5.Good DW. PGE2 reverses AVP inhibition of absorption in rat mTAL by activation of protein kinase C. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;270:F978–F985. doi: 10.1152/ajprenal.1996.270.6.F978. [DOI] [PubMed] [Google Scholar]
- 6.Gray DA, Frindt G, Zhang YY, Palmer LG. Basolateral K+ conductance in principal cells of rat CCD. Am J Physiol Renal Physiol. 2005;288:F493–F504. doi: 10.1152/ajprenal.00301.2004. [DOI] [PubMed] [Google Scholar]
- 7.Gu RM, Wei Y, Jiang H, Balazy M, Wang WH. The role of 20-HETE in mediating the effect of dietary K intake on the apical K channels in the mTAL. Am J Physiol Renal Physiol. 2001;280:F223–F230. doi: 10.1152/ajprenal.2001.280.2.F223. [DOI] [PubMed] [Google Scholar]
- 8.Hebert RJ, Jacobson HR, Breyer MD. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin lnvest. 1991;87:1992–1998. doi: 10.1172/JCI115227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch J, Schlatter E. K+ channels in the basolateral membrane of rat cortical collecting duct are regulated by a cGMP-dependent protein kinase. Pflugers Arch Eur J Physiol. 1995;429:338–344. doi: 10.1007/BF00374148. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch JR, Schlatter E. K channels in the basolateral membrane of rat cortical collecting duct. Pflügers Arch. 1993;424:470–477. doi: 10.1007/BF00374910. [DOI] [PubMed] [Google Scholar]
- 11.Hirt DL, Capdevila J, Falck JR, Breyer MD, Jacobson HR. Cytochrome P450 metabolites of arachidonic acid are potent inhibitors of vasopressin action on rabbit cortical collecting duct. J Clin Invest. 1989;84:1805–1812. doi: 10.1172/JCI114365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holla VR, Makita K, Zaphiropoulos PG, Capdevila JH. The kidney cytochrome P450 2C23 arachidonic acid epoxygenase is upregulated during dietary salt loading. J Clin Invest. 1999;104:751–760. doi: 10.1172/JCI7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koeppen BM, Biagi BA, Giebisch GH. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol. 1983;244:F35–F47. doi: 10.1152/ajprenal.1983.244.1.F35. [DOI] [PubMed] [Google Scholar]
- 14.Liu HJ, Wei Y, Ferreri N, Nasjletti A, Wang WH. Vasopressin and PGE2 regulate the apical 70 pS K channel in the thick ascending limb of rat kidney. Am J Phyiol Cell Physiol. 2000;278:C905–C913. doi: 10.1152/ajpcell.2000.278.5.C905. [DOI] [PubMed] [Google Scholar]
- 15.Lu M, Wang WH. Nitric oxide regulates the low-conductance K+ channel in basolateral membrane of cortical collecting duct. Am J Physiol Cell Physiol. 1996;270:C1336–C1342. doi: 10.1152/ajpcell.1996.270.5.C1336. [DOI] [PubMed] [Google Scholar]
- 16.Ma YH, Schwartzman ML, Roman RJ. Altered renal P-450 metabolism of arachidonic acid in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 1994;267:R579–R589. doi: 10.1152/ajpregu.1994.267.2.R579. [DOI] [PubMed] [Google Scholar]
- 17.Makita K, Takahashi K, Kerara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest. 1994;94:2414–2420. doi: 10.1172/JCI117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muto S, Asano Y, Wang WH, Seldin D, Giebisch G. Activity of the basolateral K channels is coupled to the Na-K-ATPase in the cortical collecting duct. Am J Physiol Renal Physiol. 2003;284:F945–F954. doi: 10.1152/ajprenal.00081.2003. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price EJ, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt sensitive hypertension is associated with a dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roman RJ. P450 metaboltes of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2004;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 21.Sakairi Y, Jacobson HR, Noland TD, Capdevila JH, Falck JR, Breyer MD. 5,6-EET inhibits ion transport in collecting duct by stimulating endogenous prostaglandin synthesis. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;268:F931–F939. doi: 10.1152/ajprenal.1995.268.5.F931. [DOI] [PubMed] [Google Scholar]
- 22.Sansom SC, O'Neil RG. Mineralocorticoid regulation of apical cell membrane Na and K transport of the cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol. 1985;248:F858–F868. doi: 10.1152/ajprenal.1985.248.6.F858. [DOI] [PubMed] [Google Scholar]
- 23.Schafer JA, Troutman SL. Potassium transport in cortical collecting tubules from mineralocorticoid-treated rat. Am J Physiol Renal Fluid Electrolyte Physiol. 1987;253:F76–F88. doi: 10.1152/ajprenal.1987.253.1.F76. [DOI] [PubMed] [Google Scholar]
- 24.Schlatter E, Schafer JA. Electrophysiological studies in principal cells of rat cortical collecting tubules. Pflügers Arch. 1987;409:81–92. doi: 10.1007/BF00584753. [DOI] [PubMed] [Google Scholar]
- 25.Stokes JB, Kokko JP. Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J Clin Invest. 1977;59:1099–1104. doi: 10.1172/JCI108733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther. 1998;284:966–973. [PubMed] [Google Scholar]
- 27.Wang WH. The cGMP-dependent protein kinase stimulates the basolateral 18-pS K channel of the rat CCD. Am J Physiol Cell Physiol. 2000;278:C1212–C1217. doi: 10.1152/ajpcell.2000.278.6.C1212. [DOI] [PubMed] [Google Scholar]
- 28.Wang WH, Hebert SC, Giebisch G. Renal K channels: structure and function. Ann Rev Physiol. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- 29.Wang WH, Lu M, Hebert SC. Cytochrome P-450 metabolites mediate extracellular Ca2+-induced inhibition of apical K channels in the TAL. Am J Physiol Cell Physiol. 1996;270:C103–C111. doi: 10.1152/ajpcell.1996.271.1.C103. [DOI] [PubMed] [Google Scholar]
- 30.Wang WH, McNicholas CM, Segal AS, Giebisch G. A novel approach allows identification of K channels in the lateral membrane of rat CCD. Am J Physiol Renal Fluid Electrolyte Physiol. 1994;266:F813–F822. doi: 10.1152/ajprenal.1994.266.5.F813. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y, Lin DH, Kemp R, Yaddanapudi GSS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol. 2004;124:719–727. doi: 10.1085/jgp.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei Y, Sun P, Wang ZJ, Yang BF, Wang WH. Adenosine inhibits epithelial Na channels (ENaC) by cytochrome P-450 (CYP)-epoxygenase-dependent metabolites of arachidonic acid. Am J Physiol Renal Physiol. 2006;290:F1163–F1168. doi: 10.1152/ajprenal.00301.2005. [DOI] [PubMed] [Google Scholar]
- 33.Yang W, Tuniki VR, Anjaiah S, Falck JR, Hillard CJ, Campbell WB. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20-125I-14,15-epoxyeicosa-8(Z)-enoic acid. J Pharmacol Exp Ther. 2008;324:1019–1027. doi: 10.1124/jpet.107.129577. [DOI] [PubMed] [Google Scholar]
- 34.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K+-channel activity. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;270:F822–F832. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]