Abstract
Background
Major depressive disorder is a leading debilitating disease known to occur at a two-fold higher rate in women than in men. The neurotrophic hypothesis of depression suggests that loss of brain-derived neurotrophic factor (BDNF) may increase susceptibility for depression-like behavior, although direct evidence is lacking.
Methods
Using the chronic unpredictable stress paradigm (CUS), we investigated whether male and female mice with inducible BDNF deletion in the forebrain were more susceptible to depression-related behavior.
Results
We demonstrate that in certain behavioral measures the loss of BDNF lowers the threshold for females studied at random throughout estrus to display anxiogenic and anhedonic behaviors after chronic stress compared to wild type females. However, the loss of BDNF in forebrain does not increase the susceptibility to depression-like behavior in males.
Conclusions
These gender differences suggest a role for BDNF in mediating some aspects of depression-related behavior in females.
Keywords: BDNF, stress, behavior, animal model, gender, depression
Introduction
Major depressive disorder (MDD) is a leading debilitating disease in the U.S. and affects about 14.8 million Americans over 18 each year. The clinical presentation of MDD has a spectrum of symptoms including anxiety, anhedonia, loss of appetite, and sleep disturbances as set forth in the Diagnostic and Statistical Manual (1). Notably, MDD occurs twice as often in women than in men though the cause is currently unknown (2, 3).
Recent work suggests an important role for neurotrophins in psychiatric diseases including MDD (4, 5). Brain-derived neurotrophic factor (BDNF), the most prevalent growth factor in the brain, may underlie depression-related behavior and mediate the therapeutic action of antidepressants. The neurotrophic hypothesis of depression suggests that loss of BDNF from hippocampus contributes to neuroanatomical and functional alterations that underlie aspects of depression-related behavior, while antidepressants may mediate therapeutic effects in part by increasing levels of BDNF in this brain region (6). Recent studies demonstrate that BDNF heterozygous mice, and mice with inducible BDNF deletion in forebrain (inducible knockouts), and conditional BDNF knockouts, display attenuated responses to antidepressants in forced swim test (7–9), a paradigm that predicts antidepressant efficacy and by analogy ‘depression-related’ behavior (10, 11). Indeed, we have recently extended these findings to show that BDNF in dentate gyrus of hippocampus is required for antidepressant efficacy in this paradigm (12).
While these studies demonstrated that loss of BDNF produces alterations in antidepressant responses, BDNF heterozygous mice, inducible BDNF knockouts (KOs), and dentate gyrus specific BDNF KOs were indistinguishable from wild type littermate control mice in ‘baseline’ depression-related behavior; this suggests that loss of BDNF per se is not sufficient to mediate ‘depression-like’ behavior (8, 12, 13). However, it is possible that loss of BDNF may increase vulnerability to particular chronic perturbations.
To investigate this possibility, we exposed BDNF inducible KOs to a chronic unpredictable stress (CUS) paradigm that is known to induce alterations in depression-related behaviors in rodents. We examined BDNF inducible KOs, since this line has a regionally restricted forebrain specific deletion of BDNF compared to our conditional line, to gain a direct assessment of the neurotrophic hypothesis of depression. Since previous work demonstrated that loss of BDNF produces gender specific effects, we examined both male and female inducible KO mice in depression-related behavior following CUS.
Materials and Methods
Mice
The inducible BDNF knockout mice were generated from a trigenic cross of NSE-tTA, TetOp-Cre, and floxed BDNF mice as previously described (8). For all behavior testing, male and female mice were age (three to six months) and weight matched and groups were balanced by genotype. Eight experimental groups of 7–14 animals were tested; male and female BDNF knockouts (KOs) or wild type littermates (CTLs), nonstressed or stressed (Supplement 1 - Table S1). The order of behavior tests was performed from least to most stressful and blind to group and genotype (Supplement 2 -Figure S1A). For more information, refer to supplemental methods (Supplement 3 –Materials and Methods).
Chronic unpredictable stress model
Our CUS model was adapted from Muscat et al. (14) and Monleon et al. (15). Mice were exposed to one or two stressors for a period of 4–12 hours during each 24 hour period over 52 days, though animals were not stressed within eight hours of behavioral testing. Stressors consisted of food or water deprivation, periods of overnight illumination, 45° cage tilt, single housing, and bedding soiled with water or rat feces (Table 1).
Table 1.
Stressor type coding is as follows: (A) water deprivation, (B) 45° cage tilt; (C), food deprivation; (D), rat feces in bedding; (E), single housing; (F), soiled bedding; (G), overnight illumination. Stressor period coding is as follows: (1) four hours, (2) seven hours, (3) twelve hours, (4) fourteen hours, (5) seventeen hours.
| Day | M | T | W | Th | F | Sa | Su |
|---|---|---|---|---|---|---|---|
| Week | |||||||
| 1 | A3 | B1, F2 | C1, G | D1, B3 | E | E | E, G |
| 2 | C1 | B3, F1 | B2 | A2 | D2 | E | B3, E |
| 3 | D2, G | B3 | C1 | A2, B3 | D2 | E | E |
| 4 | A2 | C1 | F1 | C1 | F1 | A2 | G |
| 5 | B3 | C1, F2 | A1, A4 | A5, B1 | A4, F1 | B3 | B2 |
| 6 | C1, G | B3 | F2 | D2 | A4, B1 | E | C3, E |
| 7 | B1, G | D2 | C1, G | D2, F2 | B3 | A2, D2 | C2 |
| 8 | G | B1, E |
Locomotor Activity
Mice were placed in cages and locomotor activity was recorded for 2 hours under red light by photocell beams linked to computer acquisition software (San Diego Instruments, San Diego, CA).
Open field
Mice were assessed for activity in a 72×72 cm open field (OF) arena at 40 lux for 5 minutes. Movement was tracked by video (Ethovision3.0 Noldus, Leesburg, Virginia) for time spent in center (14×14 cm) and peripheral zones (5 cm around perimeter).
Fur state assessment
Mouse fur state was rated on a 4-point scale with another point each for either hunched posture or redness around eyes (6 points total). The fur scoring scale (Supplement 3 – Materials and Methods) was adapted from Mineur et al. (16).
Sucrose consumption test
Sucrose consumption test (SCT) protocol was adapted from Gourley et al. (17). Mice were habituated to 1% sucrose solution and water deprivation periods followed by 1 hour of sucrose access. On test day, mice accessed sucrose solution for 1 hour and the following day accessed water. We measured percent sucrose intake compared to total volume consumed in both trials. For more information, refer to Supplement 3 – Materials and Methods.
Novelty suppressed feeding
The novelty suppressed feeding (NSF) task was performed as previously described (18). Detailed methods listed in Supplement 3.
Tail suspension test
The tail suspension test (TST) was performed as previously described (19) and detailed methods are listed in Supplement 3.
Forced swim test
The forced swim test (FST) was performed as previously described (10) and detailed methods are available in supplemental methods (Supplement 3 – Materials and Methods).
Corticosterone measure
Blood serum was isolated from trunk blood samples by centrifugation. A high sensitivity corticosterone (CORT) enzyme immunoassay (EIA) was performed according to manufacturer’s instruction (Immunodiagnostic Systems Ltd., Fountain Hills, AZ).
Quantitative RT-PCR
Fresh frozen whole hippocampi were dissected and total RNA was extracted using Trizol reagent (Invitrogen) according to manufacturer’s instruction. Conditions for cDNA synthesis, amplification, and primer sequences were described previously (20). Fold change in BDNF expression is normalized to GAPDH.
Statistical analysis
Weight and locomotor data were analyzed with repeated measures ANOVA using SAS software to determine statistical significance (p<0.05). The fur score data were analyzed by logistical regression analysis followed with a Mantel-Haenszel test for frequency comparison between groups. Anxiety data, SCT, NSF, FST, TST, CORT, and BDNF expression data were analyzed by a 2-way ANOVA followed with multiple comparisons using a Bonferroni t-test to assess the difference among groups. Data are presented as mean ± SEM.
Results
Weight
Mouse weights were monitored at an early, mid, and late time point during the time period of CUS (days 5, 21 and 40)(Supplement 2 - Figure S1B). In females, there was a significant stress effect (p<0.0001, F1,11=36.42), while there was no significant knockout effect (p=0.2722,F1,11=1.34) or interaction effect (p=0.2075,F1,10=1.82). For males, there was a significant stress effect (p=0.0135, F1,8=9.96), while there was no significant knockout effect (p=0.3016, F1,7=1.24) or interaction effect (p=0.3256,F1,7=1.12). Inducible BDNF KO mice have normal weight compared to littermate CTLs, similar to data previously reported (9). Our CUS paradigm was found to significantly impact weight of the animals over the course of the experiment. However, loss of BDNF in either sex did not further contribute to a change in weight following CUS.
Locomotor Activity
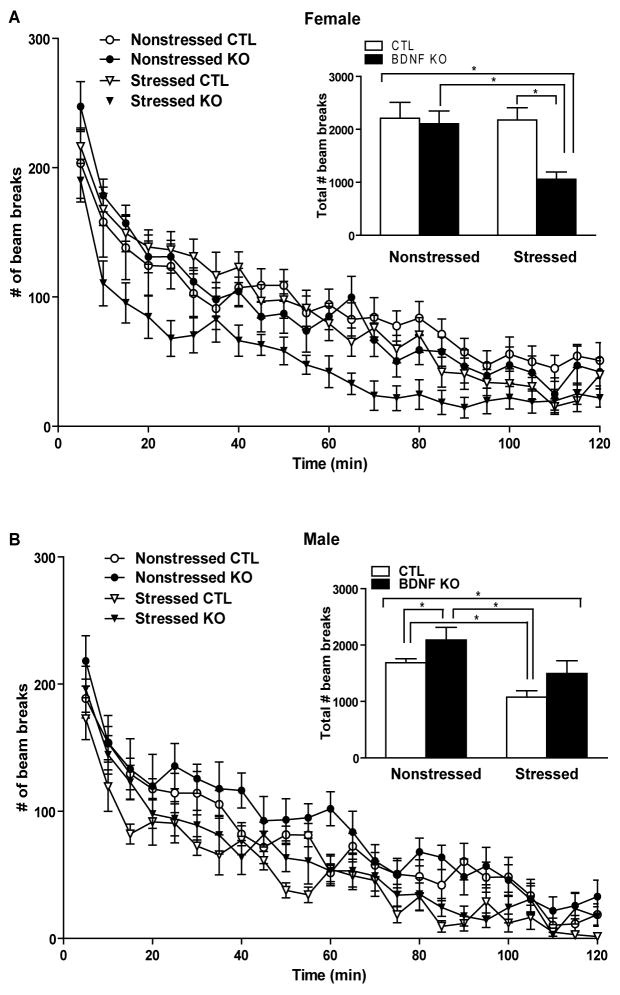
Previous studies showed that a reduction in locomotor activity after CUS correlates to depression-like behaviors (21). Examining two hour locomotor activity in females following CUS revealed a significant stress effect (p=0.0335,F1,42=4.83) and knockout effect (p=0.0461,F1,42=4.22) while there was no significant interaction effect (p=0.1104,F1,42=2.66). Multiple comparisons using a Bonferroni t-test indicated that stressed KOs were significantly hypoactive compared to the other groups (*p<0.05)(insert, Figure 1A). To gain a better understanding of this difference in females, data were analyzed in 5-minute epochs (Figure 1A); there was a significant main effect of stress (p<0.0001, F1,11 =62.00) and a significant main effect of genotype (p<0.0001, F1,9 =57.91) and the number of beam breaks significantly decreased over time (p<0.0001, F23,299 =52.56) with a significant interaction between stress and genotype (p<0.0001, F1,9 =50.76) while there were no other significant interaction effects (* p<0.05). Total locomotor activity in males during a two hour period following CUS revealed a significant stress effect (p=0.0092,F1,30=7.75), with no significant knockout effect (p=0.1796,F1,30=1.89) or interaction effect (p=0.9385,F1,30=0.01). Multiple comparisons using a Bonferroni t-test indicated that under nonstressed conditions BDNF KOs are significantly hyperactive compared with wild type mice and that following stress CTLs show a significant decrease in locomotor activity compared to nonstressed CTLs (*p<0.05)(insert, Figure 1B). For males, locomotor data were analyzed in 5-minute epochs (Figure 1B); there was a significant main effect of stress (p<0.0001,F1,8 =86.32), a significant main effect of genotype (p=0.0046,F1,7=16.73), and the number of beam breaks significantly decreased over time (p<0.0001,F23,208 =50.57) while there was no significant interaction effect (*p<0. 05).
Figure 1.
CUS produces significant effects on locomotor activity. (A) CUS produced a significant stress (F1,42 =4.83, p<0.05) and genotype effect (F1,42 =4.22, p<0.05) on locomotor activity in females. The female stressed BDNF KOs were significant hypoactivity compared to stressed CTLs and nonstressed BDNF KOs (*p<0.05). (B) CUS produced a significant stress effect (F1,30 =7.75, p<0.01) on locomotor activity in males. Male BDNF KO mice were hyperactive compared to CTL mice at baseline (*p<0.05). After CUS, male mice (*p<0.05) were significantly less active than their nonstressed cohorts while no significant effect was observed in nonstressed KOs compared to stressed KOs (p>0.05).
Anxiety-related behavior
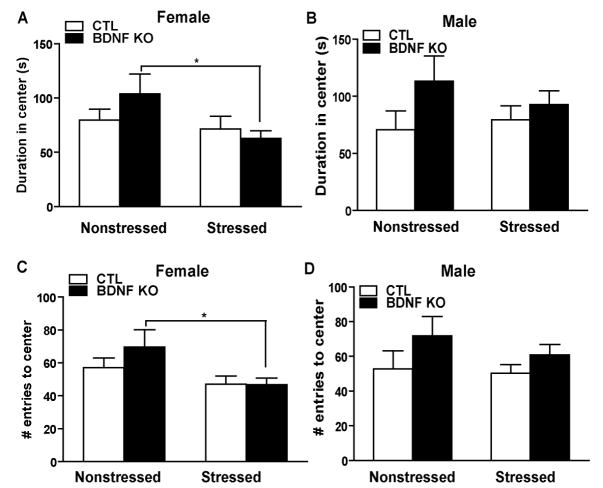
To determine the effect of CUS on anxiety-like measures, we assessed open field behavior. In this paradigm a decrease in duration of time in the center or a decrease in the number of entries to center is suggestive of an increase in anxiety related behavior (22). For females, examining duration in the center of the open field revealed a significant stress effect (p=0.0499,F1,45=4.06) while there is no significant knockout effect (p=0.5305,F1,45=0.40) or interaction effect (p=0.1829,F1,45=1.83). Multiple comparisons using a Bonferroni t-test indicated that stressed KOs spend significantly less time in the center compared to nonstressed KOs suggestive of an increase in anxiety (* p<0.05)(Figure 3A). In females, we examined the number of entries to the center and found a significant stress effect (p=0.0155,F1,45=6.33) while there was no significant knockout effect (p=0.3545,F1,45=0.88) or interaction effect (p=0.3239,F1,45=0.99). Multiple comparisons using a Bonferroni t-test indicated that stressed KOs have a significant decrease in the number of entries in the center of the open field compared to nonstressed KOs (*p<0. 05)(Figure 2C). We examined total distance traveled and during this test, like the initial five minute data point for locomotor activity, we did not observe any significant effect of stress (p=0.0854,F1,44=3.10), knockout (p=0.4798,F1,44=0.51) or interaction (p=0.8529,F1,44=0.03) for female mice and no significant difference among groups (p>0.05)(Supplement 4 - Figure S2A).
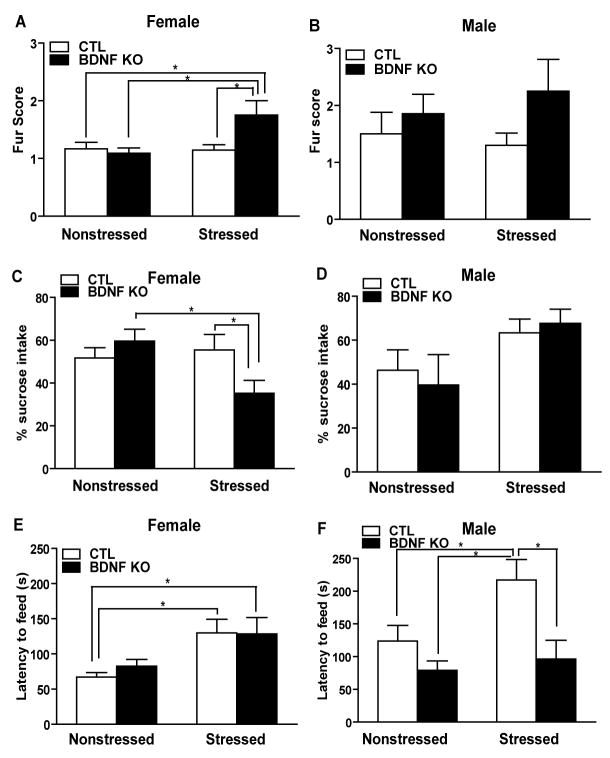
Figure 3.
CUS increases certain depression-like behaviors in females. (A–B) Females, but not males, show a poorer fur state after CUS and there is an additional effect of genotype. (C–D) CUS results in significant alterations in sucrose consumption in female and male mice. Female stressed KOs consumed significantly less sucrose than both nonstressed KOs and stressed CTLs (* p<0.05). (E–F) In the novelty suppressed feeding task, stress significantly increased latency to feed in female mice (F1,43 =8.79, p<0.005) but no effect in male behavior, and both stressed CTL and KO females took significantly longer to feed than their unstressed cohorts (p<0.05). However, there was a significant difference in male feeding behavior after stress in CTL mice compared to both nonstressed CTLs and stressed KOs(p<0.05).
Figure 2.
Females displayed heightened anxiety following CUS as assessed in the open field test. (A, C) In females, CUS produced a significant stress effect in the duration of time in the open field (F1,45 =4.06, p<0.05) as well as in the frequency to enter the center area (F1,45 =6.33, p<0.05). CUS in the BDNF KOs resulted in a significant decrease in duration of time in the center (p<0.05) and in the number of entries in the center (p<0.05) compared to nonstressed BDNF KOs. (B, D) Male mice did not differ in their anxiety behavior before or after stress regardless of genotype.
Measures of duration in the center of the open field for males revealed no significant stress effect (p=0.7146,F1,31=0.14), knockout effect (p=0.0884,F1,31=3.09) or interaction effect (p=0.3666,F1,31=0.84)(Figure 2B). In males, we examined the number of entries in the center and found no significant stress effect (p=0.4262,F1,31=0.65), knockout effect (p=0.0873,F1,31=3.12) or interaction effect (p=0.6111,F1,31=0.26)(Figure 2D). For total distance traveled, we did not observe any significant effect of stress (p=0.8213,F1,29=0.05), knockout (p=0.8842,F1,29=0.02) or interaction (p=0.5427,F1,29=0.5427) for male mice and no significant difference among groups (*p>0.05)(Supplement 4 - Figure S2B).
Depression-like behavior
To address the impact of CUS on depression-like measures, we examined fur state, sucrose intake, and feeding in a novel environment. In examining fur state we were interested whether CUS resulted in more of an ‘unkept’ appearance, as observed by an increase in fur score as a measure of depression-like behavior. There was no significant main stress and main genotype effect and interaction effect from Logistic regression analysis (*p>0.05). However, in females we noted significantly poorer fur state in stressed BDNF KO mice as assessed by a higher fur score with respect to the other groups of animals (*p<0.05)(Figure 3A). In contrast, in males we generally noted a poorer fur state than in females, however there was no significant difference between groups (p>0.05)(Figure 3B). SCT is a paradigm used to measure an animal’s responsiveness to a natural reward (23). A loss of sensitivity to reward has been suggested as a measure of anhedonia, an important feature of major depression. In females, there was a significant genotype × stress interaction effect (p=0.0278,F1,45=5.17). Multiple comparisons using a Bonferroni t-test indicated that stressed KOs had a significant decrease in percent sucrose intake compared to nonstressed KOs or stressed CTLs (*p<0.05)(Figure 3C). Importantly, there was no significant stress effect (p=0.0546,F1,45=3.89), knockout effect (p=0.7688,F1,45=0.09) or interaction effect (p=0.8213,F1,45=0.05) on water intake (*p>0.05)(Supplement 5 - Figure S3A). For males, there was a significant stress effect (p=0.0199,F1,31=6.02), while there was no effect of knockout (p=0.8997,F1,31=0.02) nor an interaction effect (p=0.5528,F1,31=0.36). Multiple comparisons using a Bonferroni t-test indicated no significant differences between groups (*p>0.05)(Figure 3D). The amount of water consumed was not significantly affected by stress (p=0.3893,F1,31=0.76), knockout (p=0.6705,F1,31=0.18) or interaction (p=0.9741,F1,31=0.00)(*p>0. 05)(Supplement 5 - Figure S3B). In NSF testing, an increase in the latency to feed suggests an increase in anxiety (18). In females, there was a significant stress effect (p=0.0049,F1,43=8.79) while there was no significant knockout effect (p=0.6509,F1,43=0.21) or interaction effect (p=0.5913,F1,43=0.29). Multiple comparisons using a Bonferroni t-test indicated that stress significantly increased the latency to feed in the CTLs (*p<0.05), with a similar trend observed in KOs (Figure 3E). For males, there was a significant knockout effect (p=0.0052,F1,29=9.16), while there was no effect of stress (p= .0532,F1,29=4.06) nor an interaction effect (p=0.1754,F1,29=1.93). Multiple comparisons using a Bonferroni t-test indicated that stress significantly increased latency to feed in CTLs compared to nonstressed CTLs and stressed KOs (*p<0.05)(Figure 3F).
We performed FST and TST, paradigms that are commonly referred to as depression-like tests and which have been shown to increase immobility in mice after CUS in other studies (24). An increase in immobility time is suggestive of an increase in depression-like behavior. For females in FST, there was no significant stress effect (p=0.9963,F1,45=0.00), knockout effect (p=0.4718,F1,45=0.53) or interaction effect (p=0.0931,F1,45=2.94)(Supplement 6 - Figure S4A). For males in FST, there was no significant stress effect (p=0.5919,F1,29=0.29), knockout effect (p=0.2265,F1,29=1.53) or interaction effect (p=0.9603,F1,29=0.00)(*p>0.05)(Supplement 6 - Figure S4B). Examining females in TST, there was no significant stress effect (p=0.7447,F1,31=0.11), knockout effect (p=0.6301,F1,31=0.24) or interaction effect (p=0.8980, F1,31=0.02)(*p>0.05)(Supplement 6 - Figure S4C). Males tested in TST displayed no significant stress effect (p=0.2959,F1,30=1.13), knockout effect (p=0.4904,F1,30=0.49) or interaction effect (p=0.2051,F1,30=1.68)(*p>0.05)(Supplement 6 - Figure S4D).
Corticosterone levels
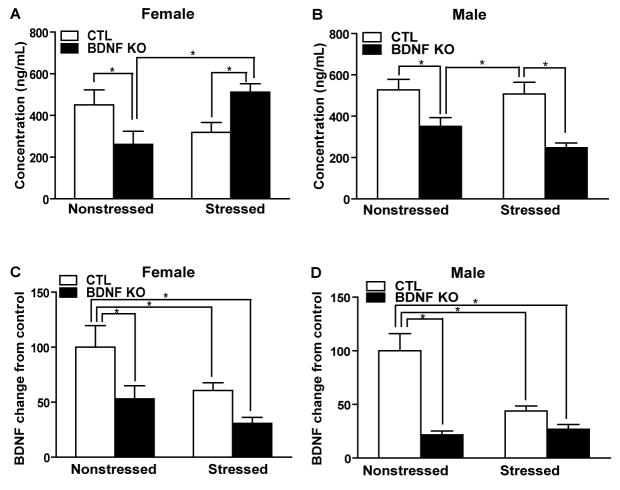
To assess the effect of stress on CORT levels in CTL and BDNF KO mice, we collected trunk blood 10–15 minutes after FST, an acute swim stress. We analyzed sera from these samples for CORT concentration by EIA. For female CORT concentrations, there is a significant genotype × stress interaction effect (p=0.0013,F1,42=11.92). Multiple comparisons using a Bonferroni t-test indicated that nonstressed KOs had a significant decrease in CORT levels compared to nonstressed CTLs and that stress significantly increases CORT levels in BDNF KOs compared to either nonstressed KOs or stressed CTLs (*p<0.05)(Figure 4A). For male CORT concentration, there is a significant knockout effect (p=0.0001, F1,24=20.25) while there is no significant stress effect (p=0.2152, F1,24=1.62) or interaction effect (p=0.3988,F1,24=0.74). Multiple comparisons using a Bonferroni t-test indicated that nonstressed KOs had a significant decrease in CORT levels compared to nonstressed CTLs and that stressed KOs had a significant decrease in CORT levels compared to stressed CTLs (*p<0.05)(Figure 4B).
Figure 4.
Corticosterone and BDNF levels following chronic stress. (A–B) Stress did not have a main effect on CORT levels in male or female mice. However, in females, stress and genotype have significant interaction on CORT measures (F1,42 =11.92, p<0.05). In both sexes, CORT levels are significantly lower in BDNF KOs under non stress conditions (p<0.05). In females, BDNF KO mice show heightened CORT levels after stress compared both to nonstressed KOs and stressed CTLs (p<0.05). However, in males, BDNF KOs displayed significantly lower CORT levels after stress compared to stressed CTLs (p<0.05). (C–D) BDNF levels following chronic stress. For female BDNF levels, there is a significant knockout effect (p=0.0040,F1,34=9.51) and stress effect(p=0.0185,F1,34=6.12) while there is no significant interaction effect (p=0.4947,F1,34=0.48). Multiple comparisons using a Bonferroni t-test indicated that the nonstressed CTLs are significantly different from other three groups (*p<0.05). For male BDNF levels, there is a significant knockout × genotype interaction effect (p=0.0040,F1,25=10.04). Multiple comparisons using a Bonferroni t-test indicated that, similarly to the females, the nonstressed CTLs are significantly different from the other three groups (* p<0.05).
BDNF levels
We quantified expression of BDNF in hippocampus, a region of interest in stress-response and a well-characterized site of BDNF knockdown in this mouse line (9), to determine the gender-specific impact of stress on BDNF mRNA levels. We collected whole hippocampi from all mice subjected to behavioral testing and used quantitative PCR to analyze BDNF mRNA levels. For female BDNF levels, there is a significant knockout effect (p=0.0040,F1,34=9.51) and stress effect (p=0.0185,F1,34=6.12) while there is no significant interaction effect (p=0.4947,F1,34=0.48). Multiple comparisons using a Bonferroni t-test indicated that nonstressed CTLs are significantly different from the other three groups (*p<0.05)(Figure 4C). For male BDNF levels, there is a significant knockout genotype interaction effect (p=0.0040,F1,25=10.04). Multiple comparisons using a Bonferroni t-test indicated that, similarly to females, nonstressed CTLs are significantly different from all other groups (*p<0.05)(Figure 4D).
Discussion
Results of this study demonstrate that in several behavioral paradigms female mice are more vulnerable to CUS than males. We found that loss of BDNF makes female mice more sensitive to some measures of anxiety and particular features of depression-like behaviors following CUS compared to littermate CTLs. In contrast, loss of BDNF in males fails to increase most measures of anxiety, anhedonia and depression-like behavior following CUS compared to wild type CTLs. This is not to say that there were no genotype effects observed following CUS in males, but rather that the effects of stress and genotype were more extensive in females than males. Collectively, these data suggest that loss of BDNF does not result in greater susceptibility to depression-related behavior per se, but rather is linked to expression of these behaviors in a gender-specific manner in response to stress. CUS paradigms have produced alterations in locomotor activity, anxiety-like behavior, fur state, sucrose consumption, forced swim test, tail suspension tests, and corticosterone levels in rodents (25, 26). However, most robust CUS effects on these measures are in rats and effects in mice have been more difficult to ascertain suggesting that mice may be more resilient to chronic stress (25). Most CUS studies have relied solely on males and our data would largely support the resiliency of male mice to stress in many of these behavioral paradigms. However, our data with female mice suggests they may have an increased vulnerability in some behavioral measures following CUS.
In females, we found that stress produced a significant decrease in total locomotor activity of inducible KOs compared to nonstressed CTLs, nonstressed KOs, and stressed CTLs suggesting that loss of BDNF in females exacerbated locomotor deficits. In males, we found that nonstressed inducible KOs were significantly hyperactive compared to nonstressed CTLs in agreement with previous findings (9). Following CUS, male CTLs displayed significant hypoactivity compared to nonstressed CTLs while a similar trend, although not significant, was observed in BDNF KOs.
We assessed anxiety-related behavior using the OF test. In females, we found that CUS in BDNF KOs significantly reduced the time in the center of the arena and the number of entries to the center, indicative of an increase in anxiety-like behavior, compared to nonstressed KOs. In males, loss of BDNF did not alter anxiety related behavior following CUS. Our data is in contrast to previous findings of anxiolytic like effects of CUS in elevated plus maze (27), however other reports utilizing chronic variable stress report anxiogenic like effects in this test (28) and further studies suggest that these differences may be accounted for by the length of time between stress exposure and testing (29).
We examined the effects of CUS in inducible KOs in paradigms that provide measures of depression-like behavior. We examined the fur state of animals to assess grooming behavior, SCT as a measure of anhedonia, and latency to feed in the NSF test in CTL and BDNF KO animals following CUS. In females, we found that in nonstressed conditions loss of BDNF did not alter fur score, sucrose intake or latency to feed in the NSF test compared to CTLs. Following CUS, we found that female BDNF KOs had a significantly poorer fur score and were more anhedonic than nonstressed KOs or stressed CTLs and displaying a strong trend towards an increase in latency to feed compared to nonstressed KOs. In male KOs, CUS did not produce significant differences in fur score or sucrose consumption compared to other groups, and in the NSF test BDNF KOs appeared less anxious following CUS than CTLs. Collectively, this data suggests that loss of BDNF in CUS females may increase anxiety related behavior and some measures of depression related behavior, however these effects are gender specific since similar effects were not observed in males.
We examined mice in the FST and TST, tests that are commonly used to assess antidepressant efficacy and, by extension, depression (30). Surprisingly, we did not observe increased depression-like behavior as assessed by increased immobility in either FST or TST following CUS in either females or males independent of genotype. The FST and TST are often associated with depressive phenotypes in rodents following acute stress (24). Our lack of a change in immobility in these paradigms may be due to the adaptive aspect of stress responses to CUS over time (31). Recent interest has focused on uncovering the genetic mechanism behind the consistent observation that some animals display resilience to stress (32, 33). To this end, neither susceptible nor resilient mice display differences in the FST or TST after chronic social defeat stress (33), although this finding is not unequivocal(34). It is possible that the lack of a change in depression-like behavior as determined here could be due to the fact these tests are designed to predict antidepressant efficacy rather than as measures indicative of depression-like behavior (10, 35).
As a physiological measure of stress to support our behavioral findings, we assessed CORT concentration. Rather surprisingly, we found that under nonstressed conditions loss of BDNF is associated with decreased CORT levels in both males and females. Furthermore, we found that susceptibility for developing depression-related behaviors in female BDNF KO mice after CUS was correlated to a significant increase in CORT levels compared to nonstressed KOs and stressed CTLs. In contrast, in males we found that CUS did not significantly alter CORT levels compared to baseline levels of CORT in nonstressed CTLs and KOs. These findings suggest that under nonstressed conditions there is an interaction between BDNF and CORT in that loss of BDNF significantly reduced plasma CORT levels although this was not significantly correlated with decreased anxiety or depression like behavior. Following CUS, we found gender specific effects of the interaction between BDNF and CORT. Interestingly, a significant increase in CORT levels following CUS was observed in female BDNF KOs, the animals with the most pronounced anxiety and in some measures depression like behavior here.
We examined BDNF mRNA expression in all female and male groups. We found that under nonstressed conditions, both female and male KOs had a significant reduction in BDNF in hippocampus in agreement with previous data (9). Following CUS, we found that both male and female CTLs showed a significant reduction in the amount of BDNF in hippocampus. Rather surprisingly, CUS did not further reduce BDNF levels in KOs compared to nonstressed conditions. This data suggests that there is a floor effect in the amount of BDNF reduction in hippocampus. Thus while there were significant behavioral differences in KOs following CUS, this was not directly correlated with the amount of BDNF mRNA.
Interestingly, studies examining the effect of gender on stress responses have shown that BDNF levels in dentate gyrus are reduced in females but not males after restraint stress in rats (36), contrary to what we observed. Instead, our data suggests that dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis stress response between males and females may account for the gender differences in agreement with previous literature (37, 38). Recent studies have suggested that depression based gender differences may be the result of alterations in hormonal levels (39–41) or in neuroanatomical differences between males and females (42). It is for this reason that many laboratories utilizing female mice control for estrus cycle. However, in this study, we did not control for the estrous stage of females. While there may be concern that female mice cycling at different stages could impact the behavioral data and result in inconclusive data, we were able to observe significant behavioral effects even in spite of this concern. Our very large number of females in each group, which likely represents a sampling across all stages of cycling, may well have contributed to our ability to observe significant behavioral effects.
Intriguingly, a recent study has shown that in conditional BDNF KO mice there is evidence of increased depression-like behavior in females BDNF KOs but not males (9), however it is difficult to make direct correlations with the data presented here and the previous study as the pattern of BDNF deletion in these various BDNF lines is quite different suggesting that regional pattern of BDNF deletion may influence depression based behavior. Future studies will be necessary to examine the mechanistic link between BDNF, gender differences, and the regional effect of BDNF in susceptibility to depression-related behavior.
Our findings suggest that loss of BDNF in forebrain contributes to some aspects of depression-like behavior in a complex manner with gender. The finding that BDNF deletion in males was not sufficient to produce alterations in many behaviors examined suggests that the neurotrophic hypothesis related to depression is more complicated than simply that loss of the gene triggers depression. The loss of forebrain BDNF increased vulnerability in aspects of depression-related behaviors in females after CUS suggesting a role for BDNF in mediating features of depression-related behavior in females.
Supplementary Material
Acknowledgments
We appreciate the excellent assistance of Nicole Buzin and Jennifer Winn. We thank Dr. Waseem Akhtar for helpful discussions. This work was supported by grant MH070727 (L.M.M) as well as the Division of Basic Sciences Training Program at UT Southwestern Medical Center T32 DA07290 (A.E.A).
Footnotes
Financial Disclosure
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diagnostic and Statistical Manual IV. Washington D.C: American Psychiatric Press; 2000. [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 3.Desai HD, Jann MW. Major depression in women: a review of the literature. J Am Pharm Assoc (Wash) 2000;40:525–537. [PubMed] [Google Scholar]
- 4.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 7.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 11.Dalvi A, Lucki I. Murine models of depression. Psychopharmacology (Berl) 1999;147:14–16. doi: 10.1007/s002130051131. [DOI] [PubMed] [Google Scholar]
- 12.Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective Loss of Brain-Derived Neurotrophic Factor in the Dentate Gyrus Attenuates Antidepressant Efficacy. Biol Psychiatry. 2008;63:642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezchlibnyk YB, Wang JF, McQueen GM, Young LT. Gene expression differences in bipolar disorder revealed by cDNA array analysis of post-mortem frontal cortex. J Neurochem. 2001;79:826–834. doi: 10.1046/j.1471-4159.2001.00628.x. [DOI] [PubMed] [Google Scholar]
- 14.Muscat R, Willner P. Suppression of sucrose drinking by chronic mild unpredictable stress: a methodological analysis. Neurosci Biobehav Rev. 1992;16:507–517. doi: 10.1016/s0149-7634(05)80192-7. [DOI] [PubMed] [Google Scholar]
- 15.Monleon S, D’Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl) 1995;117:453–457. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- 16.Mineur YS, Prasol DJ, Belzung C, Crusio WE. Agonistic behavior and unpredictable chronic mild stress in mice. Behav Genet. 2003;33:513–519. doi: 10.1023/a:1025770616068. [DOI] [PubMed] [Google Scholar]
- 17.Gourley SL, Wu FJ, Taylor JR. Corticosterone regulates pERK1/2 map kinase in a chronic depression model. Ann N Y Acad Sci. 2008;1148:509–514. doi: 10.1196/annals.1410.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiatry. 2001;49:575–581. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- 20.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 21.Pardon M, Perez-Diaz F, Joubert C, Cohen-Salmon C. Age-dependent effects of a chronic ultramild stress procedure on open-field behaviour in B6D2F1 female mice. Physiol Behav. 2000;70:7–13. doi: 10.1016/s0031-9384(00)00216-x. [DOI] [PubMed] [Google Scholar]
- 22.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 23.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 26.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 27.D’Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–867. doi: 10.1016/0031-9384(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 28.Zurita A, Martijena I, Cuadra G, Brandao ML, Molina V. Early exposure to chronic variable stress facilitates the occurrence of anhedonia and enhanced emotional reactions to novel stressors: reversal by naltrexone pretreatment. Behav Brain Res. 2000;117:163–171. doi: 10.1016/s0166-4328(00)00302-8. [DOI] [PubMed] [Google Scholar]
- 29.Matuszewich L, Karney JJ, Carter SR, Janasik SP, O’Brien JL, Friedman RD. The delayed effects of chronic unpredictable stress on anxiety measures. Physiol Behav. 2007;90:674–681. doi: 10.1016/j.physbeh.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cryan JF, Slattery DA. Animal models of mood disorders: Recent developments. Curr Opin Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- 31.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 32.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- 35.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 36.Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology. 2006;31:38–48. doi: 10.1016/j.psyneuen.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Young EA. The role of gonadal steroids in hypothalamic-pituitary-adrenal axis regulation. Crit Rev Neurobiol. 1995;9:371–381. [PubMed] [Google Scholar]
- 38.Young EA. Sex differences and the HPA axis: implications for psychiatric disease. J Gend Specif Med. 1998;1:21–27. [PubMed] [Google Scholar]
- 39.Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- 40.Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- 41.Rubinow DR, Roca CA, Schmidt PJ, Danaceau MA, Putnam K, Cizza G, et al. Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology. 2005;30:1906–1912. doi: 10.1038/sj.npp.1300742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raisman G, Field PM. Sexual dimorphism in the preoptic area of the rat. Science. 1971;173:731–733. doi: 10.1126/science.173.3998.731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.