Abstract
Based on the crystal structure of human DNA ligase I complexed with nicked DNA, computer-aided drug design was used to identify compounds in a database of 1.5 million commercially available low molecular weight chemicals that were predicted to bind to a DNA binding pocket within the DNA binding domain of DNA ligase I, thereby inhibiting DNA joining. Ten of 192 candidates specifically inhibited purified human DNA ligase I. Notably, a subset of these compounds were also active against the other human DNA ligases. Three compounds that differed in their specificity for the three human DNA ligases were analyzed further. L82 inhibited DNA ligase I, L67 inhibited DNA ligases I and III, and L189 inhibited DNA ligases I, III and IV in DNA joining assays with purified proteins and in cell extract assays of DNA replication, base excision repair and non-homologous end joinging. L67 and L189 are simple competitive inhibitors with respect to nicked DNA whereas L82 is an uncompetitive inhibitor that stabilized complex formation between DNA ligase I and nicked DNA. In cell culture assays, L82 was cytostatic whereas L67 and L189 were cytotoxic. Concordant with their ability to inhibit DNA repair in vitro, subtoxic concentrations of L67 and L189 significantly increased the cytotoxicity of DNA damaging agents. Interestingly, the ligase inhibitors specifically sensitized cancer cells to DNA damage. Thus, these novel human DNA ligase inhibitors will not only provide insights into the cellular function of these enzymes but also serve as lead compounds for the development of anti-cancer agents.
Keywords: Computer-aided drug design, DNA replication, DNA repair, DNA ligation, DNA ligase inhibitors
INTRODUCTION
Under normal circumstances, the genome is propagated and maintained by the combination of a highly accurate DNA replication machinery and a network of DNA repair pathways. The increased incidence of cancer associated with DNA repair-deficient human syndromes illustrates the role of these pathways in protecting against deleterious genetic changes that contribute to cancer formation. Conversely, many cancer therapeutic agents exert their cytotoxic effects by damaging DNA. Unfortunately these agents also kill normal cells, thereby limiting their utility. There is growing interest in the identification of DNA repair inhibitors that will enhance the cytotoxicity of DNA damaging agents because combinations of DNA damaging agents and DNA repair inhibitors have the potential to concomitantly increase killing of cancer cells and reduce damage to normal tissues and cells if either the damaging agent or the inhibitor can be selectively delivered to the cancer cells (1).
Since DNA ligation is required during replication and is the last step of almost all DNA repair pathways, DNA ligase-deficient cell lines exhibit sensitivity to a wide range of DNA damaging agents (2). Thus, DNA ligase inhibitors are predicted to have pleiotropic effects on cell proliferation and sensitivity to DNA damage. Human cells contain multiple species of ATP-dependent DNA ligase encoded by three genes, LIG1, LIG3 and LIG4 (2). Although these enzymes have a conserved catalytic domain and utilize the same reaction mechanism, they are directed to participate in different DNA transactions by specific protein-protein interactions (2). To date, experimental screening of a synthetic chemical collection and a natural product library has led to the identification of several compounds that inhibit human DNA ligase I (hLigI) in vitro although these compounds have not been fully characterized in terms of their specificity and mechanism of action (3, 4).
A problem with the screening of random chemical libraries for DNA ligase inhibitors is that many of the hits are likely to be non-specific inhibitors that either bind to the DNA substrate or are nucleotide analogs that inhibit a large number of ATP-dependent enzymes. Recently, a crystal structure of hLigI complexed with nicked DNA substrate was determined (5). Notably, this structure revealed three domains of hLigI that encircle and contact the nicked DNA. In addition to the adenylation (AdD) and OB-fold (OBD) domains that constitute the catalytic core of DNA and RNA ligases as well as other nucleotidyl transferases, hLigI has a DNA binding domain (DBD) located N-terminal to the catalytic core that is a conserved feature of eukaryotic DNA ligases (5).
Using the atomic resolution structure of hLig1 complexed with nicked DNA (5), a rational approach employing computer-aided drug design (CADD) was taken to identify potential inhibitors of hLigI by virtual screening of a database of commercially available, low molecular weight chemicals. Subsequent experimental evaluation of the candidate inhibitors led to the identification and characterization of novel inhibitors with different specificities for human DNA ligases I, III and IV.
MATERIALS AND METHODS
CADD screening
A DNA binding pocket between residues Gly448, Arg451 and Ala455 of the hLigI DBD (5) was chosen as the target for CADD (6–10). Details of the in silico screening will be described elsewhere. A total of 233 compounds were selected for biochemical and biological assays.
Chemicals
Compounds identified by CADD screening were purchased from Chembridge, Chemdiv, Maybridge, MDD, Nanosyn, Specs, Timtec, and Tripos. L189 was from Specs and L82 and L67 from Chemdiv. 10 mM stocks were prepared in DMSO and stored at −20° C. The molecular mass and purity of L67, L82 and L189 were confirmed by mass spectrometry in the University of Maryland School of Pharmacy facility.
Proteins
Purification of human DNA ligases is described in Supplementary Material. T4 DNA ligase was purchased from NEB.
DNA joining assays
Candidate ligase inhibitors identified by CADD were assayed for their ability to inhibit hLigI and T4 DNA ligase using a high throughput, fluorescence energy transfer-based DNA joining assay (11). Duplicate reactions (30 μl) containing 10 pmol of nicked DNA substrate and either 0.25 pmol hLigI or 10 units T4 DNA ligase were incubated in the presence or absence of 100 μM of the putative inhibitor.
A radioactive-gel-based DNA ligation assay was performed as previously described (11). A 25-mer (5′-CGC CAG GGT TTT CCC AGT CAC GAC C - 3′), and a 5′ [32P] end-labeled 18-mer (5′-GTA AAA CGA CGG CCA GTG - 3′) were annealed to a complementary 44-mer oligonucleotide, generating a linear duplex with a central nick. DNA joining reactions (30 μl) containing 0.5 pmol of labeled DNA substrate, and hLigI (0.02 pmol), hLigIIIβ (0.02 pmol), hLigIV/XRCC4 (0.1 pmol) or T4 DNA ligase (0.02 pmol) in ligation buffer were incubated in the absence or presence of ligase inhibitors at 25°C for 30 min.
Assays for steps 2 and 3 of the ligation reaction
To analyze step 2 of the ligation reaction, labeled ligase-AMP intermediates (11) (10 pmol) were incubated overnight at 25°C with an unlabeled non-ligatable version (dideoxy residue at the 3′ terminus of the nick) of the DNA oligonucleotide substrate (10 pmol), either in the presence or absence of the ligase inhibitors (100 μM).
To analyze step 3 of the ligation reaction, an adenylated labeled version of the 18 mer was prepared as described (12). The DNA substrate containing a pre-adenylated nick (0.5 pmol) and hLigI (0.05 pmol), hLigIIIβ (0.05 pmol), hLigIV/XRCC4 (0.1 pmol) or T4 DNA ligase (0.05 pmol) were incubated in ligation buffer without ATP either in the presence or absence of the ligase inhibitors (100 μM). Reactions were stopped by the addition of an equal volume of gel loading dye (95% formamide, 0.05% bromophenol blue and 0.05% xylene cyanol). After heating at 95 C for 5 min, DNA was separated by denaturing polyacrylamde gel electrophoresis. Labeled oligonucleotides were detected and quantitated in the dried gel by phosphorImager analysis (Molecular Dynamics).
Kinetic analysis of ligase inhibitors
To measure the initial rates of ligation, hLigI (0.05 pmol) was incubated with 0.5 to 100 pmol of the fluorescent, nicked DNA substrate and various concentrations of the ligase inhibitors. Ki values were obtained from Lineweaver-Burk double reciprocal plots and curve fitting using PRISM v3.03 (GraphPad).
Electrophoretic mobility shift assay
A labeled linear duplex with a non-ligatable nick was incubated with hLig1 in ligation buffer (30 μl total volume) with or without ligase inhibitors for 120 min at 25°C. After the addition of an equal volume of native gel buffer (160 mM Tris-HCl, pH 6.8, 20% glycerol, 1.4 M 2-mercaptoethanol, 0.05% bromophenol blue), samples were separated by electrophoresis through a 12% native polyacrylamide gel and detected in the dried gel by phosphorImager analysis.
Cell extract assay of DNA replication and repair
Extracts were prepared from HeLa cells as described previously (13, 14). For BER assays, the extraction buffer contained 100 mM KCl whereas for NHEJ assays, extraction buffer contained 400 mM KCl. Where indicated, DNA ligases were immunodepleted from the extracts as described (15) using protein A or G Sepharose beads (GE healthcare) and anti-Lig1, anti-LigIII (GeneTex), or anti-LigIV (ABCAM) antibodies. Depletion was confirmed by immunoblotting.
A labeled 5′ flap substrate (0.1 pmol) (14) was incubated with 20 μg of extract in the absence or presence of ligase inhibitors (100 μM), at 25°C for 5 min in ligation buffer (final vol. 50 μl). For short patch BER, a linear duplex containing a single uracil residue was pre-incised by treatment with uracil DNA glycosylase (NEB) and APE1 (NEB) to generate a strand break with 3′ hydroxyl and 5′ deoxyribose phosphate termini. Reactions (50 μl), containing 0.3 pmol of the incised DNA substrate, 10 μCi of [α32P]-dTTP and 20 μg of extract either in the absence or presence of ligase inhibitors (100 μM) were incubated at 25°C for 2 min in ligation buffer. After separation by denaturing polyacrylamde gel electrophoresis, labeled oligonucleotides were detected in the dried gel by phosphorImager analysis (Molecular Dynamics).
To assay non-homologous end joining (NHEJ) (13), a 1 kb end-labeled BamHI fragment (0.1 pmol) (16). and 20 μg of extract were incubated in ligation buffer (final vol. 20 μl), for 120 min at 25°C either in the presence or absence of ligase inhibitors (100 μM). DNA fragments were resolved by separation through a 0.8% agarose gel. Labeled DNA fragments were detected in the dried gel by phosphorImager analysis (Molecular Dynamics)
Cell culture assays
The culture conditions for normal human breast epithelial MCF10A cells, human colon cancer HCT116 cells, human cervical cancer HeLa cells and human breast cancer MCF7 cells are described in Supplementary Materials. Assays to measure cell proliferation and survival and the effect of the ligase inhibitors on cell cycle progression assays were carried out as described in Supplementary Material.
Immunocytochemistry
The effect of ligase inhibitors on the subcellular distribution of tubulin was examined by fluorescence microscopy as described in Supplementary Material.
RESULTS
In silico Screening for Putative DNA Ligase Inhibitiors
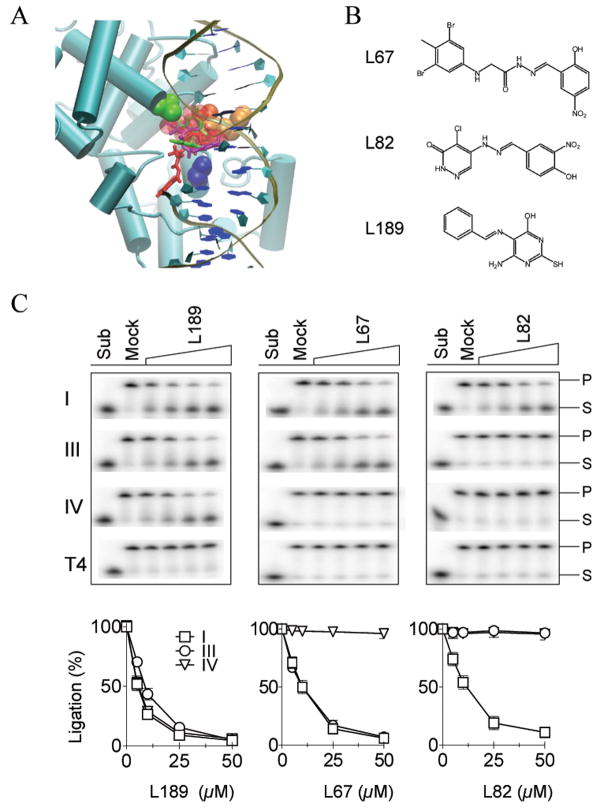
Since the DBD is the predominant DNA binding activity within hLigI (5) and both the AdD and OBD are likely to undergo significant conformational changes during the ligation reaction (2), we chose a DNA binding pocket between residues Gly448, Arg451 and Ala455 of the DBD (Fig. 1A) for the initial CADD screen. A database of 1.5 million commercially available, low molecular weight chemicals was subjected to an in silico screen for molecules that may bind within the DNA binding pocket using the program DOCK (6–10). From this virtual screen, a total of 233 compounds were selected for biochemical and biological assays.
Figure 1. Small molecule inhibitors of human DNA ligases identified by CADD.
A Key residues in the DNA binding pocket, Gly448 (green) Arg451 (orange) and Ala455 (blue), within the hLigI DBD (aqua ribbon format) are shown in VDW representation with the nicked DNA in cartoon format. The sphere set used to direct the docking of small molecules is indicated by red transparent spheres. Docked orientations of the three characterized compounds, L67 (purple), L82 (red), and L189 (green). B. Chemical structures of L67, L82 and L189. C. Representative gels of DNA ligation assays. The results of three independent experiments are shown graphically. For clarity, the data for T4 DNA ligase, which was not significantly inhibited, has been omitted (hLigI, □; hLigIIIβ, ○; hLigIV/XRCC4, ▽).
In vitro Screening of Putative hLigI Inhibitors
Of the 233 compounds selected from the in silico screen, 192 compounds were available from commercial sources. Using a fluorescence-based DNA joining assay (11), these compounds were assayed at 100 μM for their ability to inhibit purified hLigI (Fig. S1). To eliminate compounds that non-specifically inhibit DNA joining, the same 192 compounds were also assayed for their ability to inhibit T4 DNA ligase, an enzyme that utilizes the same reaction mechanism as hLigI, has similar AdD and OBDs but lacks a DBD (2, 5). Under these conditions, 10 compounds inhibited hLigI activity by >50% but had no significant effect on T4 DNA ligase. Thus, the overall hit rate for specific inhibitors of hLigI from the in silico database screen was about 5%.
Effect of hLigI Inhibitors on DNA Joining by Human DNA Ligases III and IV
Since the DNA binding pocket within the DBD of hLigI that was used as the target for CADD is likely to be conserved in human DNA ligases III (hLigIII) and IV (hLigIV), we determined whether the inhibitors of hLigI also inhibit DNA joining by purified hLigIIIβ and hLigIV/XRCC4 (Fig. S1). Based on the inhibition profile of each compound for the three human DNA ligases, the 10 compounds originally identified as inhibitors of hLigI were divided into three groups, and one member of each group was chosen for further analysis. The chemical structures and predicted binding of the three chosen compounds, L67, L82 and L189 to the DNA binding pocket within the DBD of hLigI are shown in Figure 1. L189 inhibited hLigI, hLigIIIβ, and hLigIV/XRCC4, L67 inhibited hLigI and hLigIIIβ, and L82 only inhibited hLigI (Fig. 1C). The IC50 values of L67, L82 and L189 for the three human DNA ligases and T4 DNA ligase are shown in Table S1.
Effect of the Ligase Inhibitors on the Three Steps of the Ligation Reaction
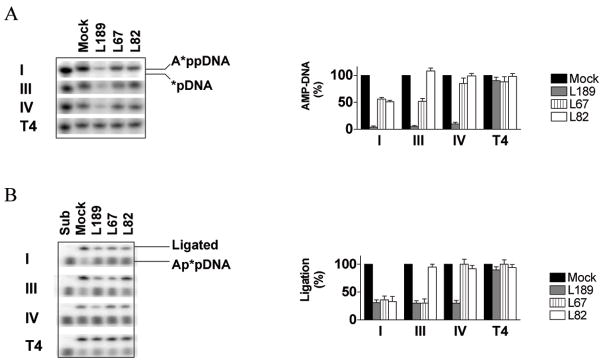
The ligase inhibitors had no detectable effect on formation of the covalent enzyme-AMP intermediate, which occurs independently of the DNA substrate and involves the active site lysine residue within the AdD of DNA ligases (17), by the three human DNA ligases (Fig. S2). To examine the second step of the ligation reaction, DNA ligases with a covalently-linked labeled AMP moiety were incubated with an unlabeled linear DNA substrate containing a single non-ligatable nick (Fig. 2A). Transfer of the labeled AMP moiety to the 5′ phosphate terminus of the nick results in the formation of a labeled DNA-adenylate intermediate. In accord with the results of the DNA joining assays (Fig. 1C), L189 inhibited the step 2 reaction catalyzed by all three human DNA ligases by at least 90% but had only a minor effect on T4 DNA ligase. DNA-adenylate formation by hLigI was inhibited by L189 in a concentration-dependent manner with an estimated IC50 of ~7 μM (Fig. S3). Although less effective than L189, L67 and L82 also inhibited the step 2 reaction and re-iterated the specificity for the human DNA ligases observed in the DNA joining assays (Fig. 1C). L67, which inhibited DNA joining by hLigI and hLigIIIβ but not hLig IV/XRCC4 (Fig. 1C), was more effective at inhibiting DNA-adenylate formation by hLigI and hLigIIIβ compared with hLig IV/XRCC4 or T4 DNA ligase (Fig. 2A). Similarly, L82, which specifically inhibited DNA joining by hLigI, was more effective at inhibiting DNA-adenylate formation by this enzyme compared with hLigIII, hLigIV, and T4 DNA ligase (Fig. 2A).
Figure 2. Effect of the ligase inhibitors on the second and third steps of the ligation reaction.
A Formation of the DNA adenylate reaction intermediate. Labeled ligase-adenylate forms of hLigI (I), hLigIIIβ (III), hLigIV (IV) and T4 (T4) DNA ligase were incubated with a linear DNA substrate containing a single non-ligatable nick in the absence or presence of L67, L82 and L189. The positions of the labeled 19 nucleotide DNA-adenylate (A*ppDNA) and a 5′ end–labeled 18 mer oligonucleotide (*pDNA) are indicated (left panel). The results of 2 independent experiments are shown graphically (right panel). Formation of DNA-adenylate is expressed as a percentage of DNA-adenylate formed by the DNA ligase in the absence of inhibitor. B. Phosphodiester bond formation. hLigI (I), hLigIIIβ (III), hLigIV (IV) and T4 (T4) DNA ligase were incubated with labeled linear DNA molecule containing a single ligatable nick with 3′ hydroxyl and 5′ adenylate termini in the absence (Mock) or presence of L67, L82 and L189. The postions of the labeled 19 nucleotide DNA-adenylate (Ap*pDNA) and labeled ligated product (43 mer) are indciated (left panel). The results of 2 independent experiments are shown graphically (right panel). Ligation is expressed as a percentage of DNA joining by the DNA ligase in the absence of inhibitor.
In the third and final step of the ligation reaction, the non-adenylated enzyme catalyzes phosphodiester bond formation, releasing AMP. Similar to step 2, L67, L82 and L189 generated the same pattern of inhibition of the third step of the ligation reaction catalyzed by the three human DNA ligases, and T4 DNA ligase (Fig. 2B) as was observed in the DNA joining assays (Fig. 1C). L67 and L82 were more effective inhibitors of step 3 than step 2 whereas the opposite was the case for L189. Thus, as expected, the inhibitors target the steps of the ligation reaction in which the enzyme interacts with nicked DNA.
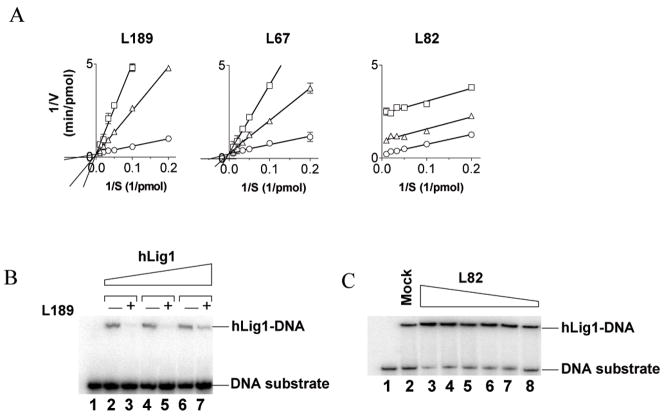
Kinetic Analysis of the Inhibition of DNA Joining: Effect of the inhibitors on binding to nicked DNA
To further characterize the inhibition of hLigI, we measured the kinetics of DNA joining in the absence or the presence of L67, L82 and L189. For L67 (Fig. 3A, middle panel) and L189 (Fig. 3A, left panel). The resulting Lineweaver-Burk plots indicate that these compounds are simple competitive inhibitors with respect to the DNA substrate, with Ki values of 10 and 5 μM, respectively. Since a DNA binding pocket within the DBD of hLigI was used for the in silico modeling, it appears likely that L67 and L189 compete with DNA for binding to this site. To provide direct evidence for this, we performed electrophoretic mobility shift assays using a linear DNA molecule containing a non-ligatable nick. As predicted, the addition of L189 (Fig. 3B) reduced the amount of hLigI-DNA complex. Similar results were obtained with the DBD of hLigI (Fig. S4). L67 was much less effective than L189 at inhibiting formation of the hLigI-DNA complex (data not shown). This may reflect the fact that L189, which is a better inhibitor of step 2 than step 3 of the ligation reaction (Fig. 2), acts at an earlier stage in the interaction with DNA than L67, which is a better inhibitor of step 3 than step 2. The inhibitory effect of L67 on step 3 of the ligation reaction was reduced by increasing the concentration of DNA-adenylate, confirming that L67 is a competitive inhibitor (Fig. S5)
Figure 3. Michaelis-Menten analysis of ligase inhibitors: Effect of ligase inhibitors on DNA-protein complex formation by hLigI.
A hLig1 (0.05 pmol) was incubated in the absence (○) and presence of L189 (left panel), L67 (middle panel) and L82 (right panel) at 25 (△) and 50 μM (□) with increasing amounts of a linear nicked DNA substrate. Lineweaver-Burk double reciprocal plots of initial reaction velocity (1/V) versus substrate concentration (1/S) are shown. B. A labeled linear substrate with a single non-ligatable nick (1 pmol) was incubated with; lane 1, no addition; lanes 2 and 3, 0.25 pmol hLigI; lanes 4 and 5, 0.5 pmol hLigI; lanes 6 and 7, 1 pmol hLigI in the absence (−) or presence (+) of 100 μM L189. C. A labeled linear substrate with a single non-ligatable nick (1 pmol) and hLigI (3 pmol) were incubated with either no additon (lane 2) or L82 at; 100 μM (lane 3); 60 μM (lane 4); 50 μM (lane 5); 30 μM (lane 6); 20 μM (lane 7); 10μM (lane 8). Lane 1, 1 pmol of DNA substrate alone. The positions of the labeled DNA substrate (DNA) and DNA-protein complexes (Lig-DNA) are indicated.
The Lineweaver-Burk plots obtained with L82 (Fig. 3A, right panel) are strikingly different than those obtained with L67 (Fig. 3A, middle panel) and L189 (Fig. 3A, left panel), and indicate that this compound acts uncompetitively. In accord with the prediction that L82 binds specifically to the enzyme-substrate complex, L82 increased the amount of hLigI-nicked DNA complex formed in a concentration-dependent manner (Fig. 3C). These results provide evidence that the inhibitors interact with the DBD and inhibit ligation either by blocking DNA binding (L67 and L189) or stabilizing a reaction intermediate (L82).
Effect of Ligase Inhibitors on Cell Extract Assays of DNA Replication and Repair
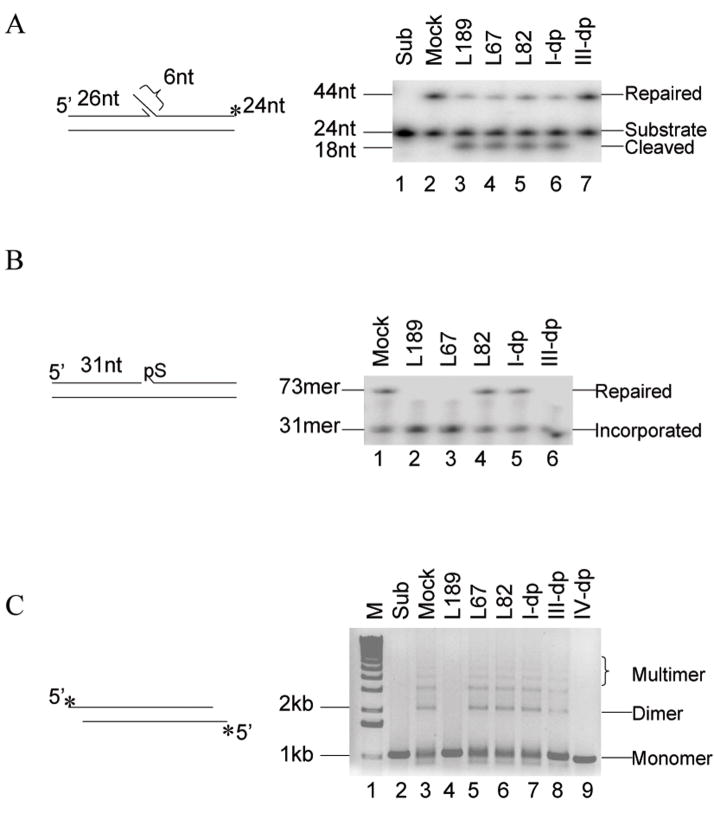
Cell extract assays for DNA replication and various DNA repair pathways have been developed and used to identify and purify the protein factors involved in these DNA transactions (13, 18–20). To determine whether L67, L82 and L189 retain their activity and specificity in cell extracts, we examined DNA replication and repair pathways for which there is an established cell extract assay (13, 21–23) and the DNA ligase involved has been identified by complementary genetic and biochemical evidence (13, 21, 24–27). During Okazaki fragment processing and the long patch subpathway of BER, short 5′ single strand flaps are removed and the resulting nicks ligated by the sequential action of FEN-1 and hLigI. In Figure 4A, we show the processing and joining of a DNA flap substrate, which mimics a common pathway intermediate, by a HeLa cell extract. As expected, immunodepletion of hLigI but not hLigIIIα reduced the amount of ligated product (Fig. 4A, compare lanes 2, 6 and 7). Addition of L67, L82 and L189, each of which inhibits purified hLigI (Fig. 1C), reduced the amount of ligated product (Fig. 4A, lane 3–5). Notably, an intermediate generated by flap removal accumulated in these reactions and the reaction with the extract immunodepleted for hLigI, indicating that the ligase inhibitors do not inhibit FEN-1.
Figure 4. Effect of ligase inhibitors on replication and repair reactions catalyzed by human cell extracts.
A The flap substrate (0.1 pmol) was incubated with cell extract (20 μg) in the absence (lane 2, Mock) or presence of 100 μM of; lane 3, L189; lane 4, L67; lane 5, L82. hLigI (lane 6, I-dp) and hLigIIIα (lane 7, III-dp) were immunodepeleted from the cell extracts prior to incubation with the DNA substrate. Lane 1, DNA substrate alone (Sub). The positions of the DNA substrate (24 mer), cleaved product (18 mer) and fully repaired product (43 mer) are shown. B. The linear DNA substrate with an incised AP site (0.3 pmol) was incubated with a cell extract (20 μg) and [α32P]dTTP in the absence (lane l, Mock) or presence of 100 μM of; lane 2, L189; lane 3, L67; lane 4, L82. hLigI (lane 5, I-dp) and hLigIIIα (lane 6, III-dp) were immunodepleted from the cell extracts prior to incubation with the DNA substrate. The positions of the single nucleotide insertion reaction intermediate (31 mer, Incorporated) and the ligated product (73 mer, Repaired) are indicated. C. A 1 kb fragment with cohesive ends (0.1 pmol) was incubated with cell extract (20 μg) in the absence (lane 3, Mock) or presence of 100 μM of; lane 4, L189; lane 5, L67; lane 6, L82. hLigI (lane 7, I-dp) hLigIIIα (lane 8, III-dp) and hLigIV (lane 9, IV-dp) were immunodepleted from the cell extracts prior to incubation with the DNA substrate. Lane 1, molecular mass standard (M). Lane 2, DNA substrate alone (Sub). The positions of the DNA substrate and dimers and multimers of the substrate are indicated.
The DNA ligase IIIα/XRCC1 complex is the predominant DNA ligase activity responsible for completing the short patch subpathway of BER following insertion of a single nucleotide and removal of the 5′ deoxyribose phosphate by DNA pol β (27). In Figure 4B, we show the processing and joining of a pathway intermediate, a DNA duplex with an incised abasic site, by a HeLa extract. As expected, immunodepletion of hLigIIIα but not hLigI reduced the amount of the ligated product (Fig. 4B, lanes 1, 5 and 6). L67 and L189, that are active against hLigIII, inhibited the formation of the repaired product (Fig. 4B, lanes 2 and 3), whereas, L82, the hLigI specific inhibitor, had no effect (Fig. 4B, lane 4). The ligase inhibitors had no detectable effect on gap-filling synthesis by Pol β that generates a labeled 31 mer (Fig. 4B). Similar results were obtained with DNA substrates containing larger gaps that are filled by DNA pol δ and/or ε (data not shown). Thus, the ligase inhibitors do not negatively impact gap-filling synthesis by the major DNA polymerases involved in DNA replication and excision repair.
NHEJ is the major pathway for the repair of DNA double strand breaks in human cells. In a cell extract assay that measures joining of cohesive-ended linear DNA fragments and is dependent upon the key NHEJ factors, Ku, DNA-PKcs, and hLigIV/XRCC4 (13), L189, the only inhibitor with activity against hLigIV (Fig. 1C), was the only compound to inhibit end joining by the HeLa cell extract (Fig. 4C, compare lane 4 with lanes 5 and 6). As expected, immunodedepletion of hLigIV abolished end joining (Fig. 4C, lane 9) whereas immunodepletion of either hLigI or hLigIIIα had no significant effect (Fig. 4C, lanes 7 and 8), confirming that the majority of DNA joining events were catalyzed by hLigIV/XRCC4. Together these results demonstrate that the ligase inhibitors retain their specificity for the different species of DNA ligases in cell extracts and thus can be used to determine the contributions of individual human DNA ligase(s) to DNA repair pathways in cell extracts or partially purified fractions. This utility further highlights the cellular function of those inhibitors in potential cancer therapeutics.
Effects of Ligase Inhibitors on Cultured Human Cells
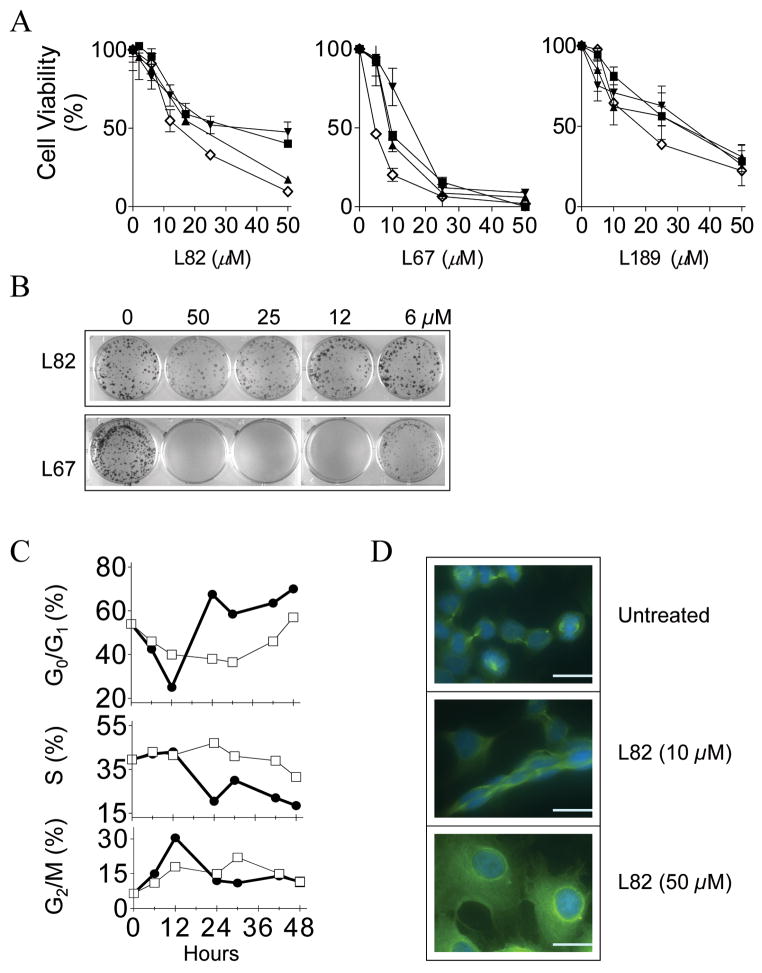
If L67, L82 and L189 enter human cells, it is likely that they will inhibit proliferation and may be cytotoxic because they all inhibit hLigI, the major replicative DNA ligase. As shown in Figure 5A, each of the compounds reduced the proliferation and/or viability of four human cell lines, including a normal breast epithelial cell line MCF10A and the cancer cell lines MCF7, HeLa and HCT116 established from breast, cervical and colon cancers, respectively, in a concentration-dependent manner. In colony forming assays, L67 and L189 were cytotoxic (Fig. 5B and 6A) whereas L82 was cytostatic, reducing the size but not the number of colonies formed by MCF7 (Fig. 5B) and the other cell lines (data not shown).
Figure 5. Characterization of the cytostatic effect of L82.
A MCF10A (■), MCF7 (◇), HCT116 (▲) and HeLa (▼;) cells were plated in the absence or presence of L82 (left panel), L67 (middle panel) and L189 (right panel). After 6 days, cell viability was measured and is expressed as a percentage of the value obtained with untreated cells. B. MCF7 cells were plated out in the absence or presence of L82 (upper panel) and L67 (lower panel) at the indicated concentrations. After two weeks, colonies were stained with crystal violet. C. After serum starvation for 4 days, MCF 7 cells were returned to serum-containing media either without (□) or with 50 μM L82 (●). The cell cycle distribution at various time intervals was determined by FACS. D. Asynchronous populations of MCF cells were either untreated (upper panel) or treated with L82 at 10 μM (middle panel) and 50 μM (lower panel). After 3 days, tubulin and DNA were visualized by fluorescence microscopy (Scale bars, 0.5 mm).
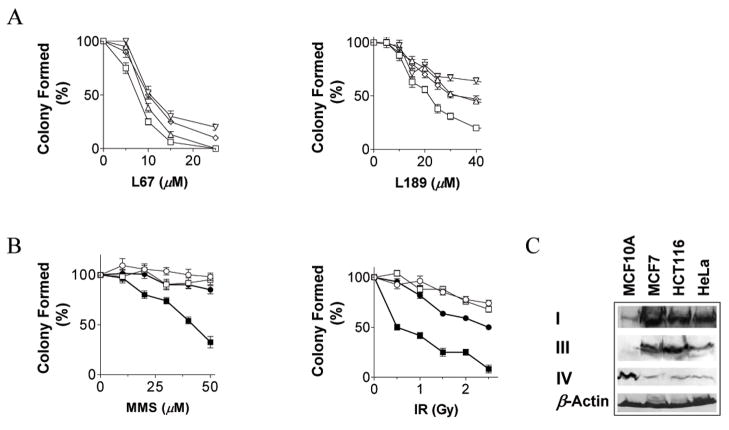
Figure 6. L67 and L189 are cytotoxic and potentiate the cytotoxic effects of DNA damaging agents; altered levels of DNA ligase in cancer cells.
A. Effect of L67 (left panel) and L189 (right panel) on the survival of MCF7 (△), HCT116 (□) and HeLa (◇) and MCF10A (▽) cells. B. Normal breast epithelial MCF10A cells (open symbols) and breast cancer MCF7 cells (filled symbols) in the absence (circles) or presence of 3 μM L67 (squares) were exposed to increasing concentrations of MMS (left panel). Normal breast epithelial MCF10A cells (open symbols) and colon cancer HCT116 cells (filled symbols) in the absence (circles) or presence of 20 μM L189 (squares) were exposed to increasing doses of ionizing radiation (right panel). C. hLigI (I), hLigIIIα (III) and hLigIV (IV) were detected in extracts (400 μg) of the indicated cell lines by immunoblotting. To control for extract loading, β-actin was also detected by immunoblotting.
To determine the effects of L82 on cell cycle progression, asynchronous MCF7 cells were enriched for the G0/G1 phase by serum starvation and then released into serum-containing media either without or with 50 μM L82. In the presence of L82, there was a transient accumulation of cells at G2/M after 12 h followed by an accumulation at G0/G1 that peaked after 24 h and then remained relatively stable (Fig. 5C). Since the increase in the G0/G1 fraction occurred concomitantly with a decrease in the S phase cell fraction (Fig. 5C), it appears that the cytostatic activity of L82 is primarily due to activation of the G1/S checkpoint. In accord with this conclusion, more than 80% of cells treated with 50 μM L82 had enlarged nuclei and a single centrosome, morphological characteristics of G0/G1 cells (Fig. 5D, compare upper and lower panels). Moreover, deformed nuclei and abnormal spindle separation occurred in cells treated with 10 μM L82 (Fig. 5D, middle panel) even though this lower concentration had much less of an effect on cell cycle distribution (data not shown).
In contrast to the cytostatic activity of L82, treatment of normal and cancer cells with either L67 or L189 (Fig. 6A) resulted in a concentration-dependent reduction in cell survival. Cells with a DNA content of less than 2n, indicative of apoptosis, were detected by FACS after treatment with either L67 or L189. This was confirmed by the detection of the chromosome fragmentation pattern characteristic of apoptotic cell death (data not shown).
Since DNA ligase-deficient mammalian cells exhibit increased sensitivity to DNA damage (2), we determined whether exposure to sub-toxic concentrations of ligase inhibitors potentiate the cytotoxic effects of DNA damaging agents. L67 markedly increased the killing of MCF7 breast cancer cells by the DNA alkylating agent, MMS (Fig. 6B, left panel). Similar results were obtained with the other cancer cell lines, HCT116 and HeLa (data not shown). Notably, the presence of L67 had no obvious effect on the sensitivity of normal breast epithelial MCF10A cells to DNA alkylation (Fig. 6B, left panel). L189 also enhanced killing of the cancer cell lines but not the normal cell line by MMS (data not shown). Furthermore, L189 markedly increased killing of HCT116 colon cancer cells (Fig. 6B, right panel) and the other cancer cell lines (data not shown) by ionizing radiation. Once again, the ligase inhibitor had very little effect on the sensitivity of normal breast epithelial MCF10A cells to DNA damage induced by ionizing radiation (Fig. 6B, right panel). Similarly, L67 enhanced the killing of the cancer cell lines but not the normal cell line by ionizing radiation (data not shown).
The ability of ligase inhibitors to specifically sensitize cancer cells to DNA damage prompted us to compare the levels of DNA ligases in the normal and cancer cell lines. As expected (28), the level of hLigI was elevated in the cancer cell lines compared with the normal cell line (Fig. 6C). Interestingly, there were changes in the levels of hLigIIIα and hLigIV in the cancer cell lines compared with the normal cell line (Fig. 6C). Specifically, the levels of hLigIIIα were elevated whereas the levels of hLigIV were markedly reduced in the three cancer cell lines, suggesting that these reciprocal changes in the levels of hLigIIIα and hLigIV may be a characteristic feature of cancer cells.
DISCUSSION
There is emerging interest in the development and use of therapeutics that target DNA repair pathways in the treatment of cancer (1). Since almost all DNA repair pathways are completed by a ligation event, the DNA ligases encoded by the three human LIG genes are attractive therapeutic targets. In this study, we have identified novel small molecule inhibitors of the human DNA ligases by CADD. Specifically, 1.5 million commercially available low molecular weight compounds were screened in silico for their ability to potentially bind to a DNA binding pocket within the DBD domain of hLigI (5). Notably, 5% of the compounds identified by the CADD screen inhibited DNA joining by hLigI but not T4 DNA ligase, confirming the utility of this structure-based approach.
In accord with the selection of the DBD of hLigI as the target for CADD, the three compounds that have been most extensively characterized inhibit the second and third steps of the ligation reaction in which the enzyme interacts with nicked DNA. Interestingly, L189 preferentially inhibits step 2 whereas the other two compounds, L67 and L82, preferentially inhibit step 3. This may reflect differences in the conformation of the DBD and/or the position of the DNA substrate during steps 2 and 3 of the ligation reaction. Moreover, these results raise the possibility of generating step 2- and step 3-specific inhibitors that could be used to gain more detailed insights into the ligation reaction. As expected, two of the compounds, L67 and L189, are competitive inhibitors with respect to the nicked DNA substrate, indicating that their binding to the DBD of hLigI prevents interaction with nicked DNA. In contrast, L82 is an uncompetitive inhibitor, analogous to the prototypic topoisomerase I (topo I) inhibitor camptothecin (29, 30). Camptothecin and other topo I inhibitors function as uncompetitive inhibitors by stabilizing the covalent linkage of topo I with the cleaved DNA, simultaneously binding to topo I and stacking within DNA base pairs adjacent to the cleavage site (31, 32). Since, both human topo I and hLigI encircle and interact with nicked DNA (33), it is possible that L82 simultaneously contacts the DNA and DBD within the context of the ring structure formed by hLigI on nicked DNA.
Because the DBD is conserved among human DNA ligases, we examined whether the hLigI inhibitors were also active against hLigIII and hLigIV. Examples of compounds that were either specific for hLigI (L82), specific for hLigI and hLigIII (L67) or inhibited all the human DNA ligases (L189) were identified. These results raise the possibility that the CADD approach may identify compounds that are specific for hLigIII and hLigIV. The availability of inhibitors that target each of the human DNA ligases will facilitate studies to identify the DNA ligase(s) in cell extracts participating in different DNA transactions. This has been problematic because of the larger repertoire of DNA ligases in mammals compared with lower eukaryotes, in particular the multiple isoforms encoded by the LIG3 gene (2), and the absence of viable lig3 mutant cell lines (34). Indeed, recent studies have implicated DNA ligase IIIα in nucleotide excision repair (35) and an alternative NHEJ pathway (36), in addition to its previously known roles in the repair of DNA single strand breaks and the short patch subpathway of BER (37).
Inhibitors that block ligation in vivo will be valuable reagents for elucidating the cellular functions of human DNA ligases. L67, L82 and L189, each of which inhibit hLig1, the replicative DNA ligase, all reduced cell proliferation and viability, indicating that they cross the cell membrane. Surprisingly, while L67 and L189 were cytotoxic, L82 was cytostatic. Since L82 is specific for hLigI, it is possible that hLigIIIα, which is also inhibited by L67 and L189, is required for cell viability either in the presence of normal levels of hLigI or specifically when hLigI activity is reduced. Alternatively, the differing effects of the inhibitors in vivo may reflect the uncompetitive mode of inhibition by L82 compared with L67 and L189, which are competitive inhibitors.
The hypersensitivity of DNA ligase-deficient cell lines to DNA damage (2) and the ability of the ligase inhibitors to inhibit BER and NHEJ in vitro suggested that sub-toxic levels of DNA ligase inhibitors may significantly increase cell killing by DNA damaging agents. In support of this idea, L67 and L189 increased DNA damage-induced cytotoxicity. Strikingly, the increased cytotoxicity occurred in the cancer cell lines but not in a cell line established from normal breast epithelium. Differences in the level of DNA ligases may underlie the selective effect of the ligase inhibitors on the cancer cell lines. Because unregulated proliferation is a characteristic of cancer, it was not surprising that the level of the replicative DNA ligase, hLigI, was significantly higher in all three cancer cell lines compared with the normal breast epithelial cell line. Notably, the cancer cell lines also had elevated levels of hLigIIIα but reduced levels of hLigIV. It is not known which, if any of these changes, is responsible for the specific sensitization of the cancer cells to DNA damage in the presence of a ligase inhibitor.
The reciprocal change in the levels of hLigIIIα and IV was also observed in cell lines with the bcr-abl translocation established from acute myelogenous leukemias but not in comparable normal cells (Rassool, F and AET, unpublished result) suggesting that this may be a characteristic feature of malignant cells. Since NHEJ is a major pathway for repairing DNA double strand breaks, the reduced levels of hLigIV may explain, at least in part, the increased sensitivity of cancer cells to ionizing radiation and suggest that cancer cell lines may be more susceptible to radiosensitization by a hLigIV inhibitor. In addition, the reduced levels of DNA ligase IV may result in more DNA double strand breaks being repaired by an error-prone DNA ligase III-dependent back-up NHEJ pathway, contributing to the increased genome instability that is a hallmark of cancer cells (38).
Differences in the network of pathways that maintain genome stability between cancer and normal cells constitute an opportunity to develop therapies that specifically target cancer cells. For example, loss of the function of either the BRCA1 or BRCA2 tumor suppressor genes results in defective homologous recombination (39–41). As a consequence of this abnormality, brca cancer cell lines are hypersensitive to killing by inhibitors of poly (ADP-ribose) polymerase (42, 43). Thus, the availability of a repertoire of inhibitors targeting different DNA repair pathways is likely to lead to the development of novel combinations of DNA damaging agents and DNA repair inhibitors that exploit differences in the DNA repair properties of normal and cancer cells. Because of the multiplicity of human DNA ligases and the almost ubiquitous requirement for DNA joining to complete DNA repair, DNA ligase inhibitors have the potential to target one or different combinations of DNA repair pathways.
In conclusion, we have used an in silico screening approach based on the structure of hLigI complexed with nicked DNA to identify low molecular weight inhibitors of human DNA ligases that specifically block functional interactions between these enzymes and nicked DNA. This is not only the first example of this type of inhibitor but also the first characterization of a set of inhibitors with different specificities for the three human DNA ligases that can be used to identify the DNA ligase(s) acting in extract-based assays of replication and repair. In addition to their in vitro activities, the selected ligase inhibitors inhibit cell proliferation and, at sub-toxic concentrations, they specifically potentiate the killing of cancer cells by DNA damaging agents. Thus, these inhibitors are promising lead compounds for the development of novel therapeutic agents to treat human cancer.
Supplementary Material
Acknowledgments
This work was supported by the US National Institutes of Heath (grants GM47251, ES12512 and GM57479 to AET, CA102428 to GMW, GM52504 to TE, a Structural Cell Biology of DNA Repair Program grant CA92584 to AET and TE) and the University of Maryland, School of Pharmacy Computer-Aided Drug Design Center (ADM).
References
- 1.Madhusudan S, Hickson ID. DNA repair inhibition: a selective tumour targeting strategy. Trends Mol Med. 2005;11:503–11. doi: 10.1016/j.molmed.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Tomkinson AE, Vijayakumar S, Pascal JM, Ellenberger T. DNA ligases: structure, reaction mechanism, and function. Chem Rev. 2006;106:687–99. doi: 10.1021/cr040498d. [DOI] [PubMed] [Google Scholar]
- 3.Sun D, Urrabaz R. Development of non-electrophoretic assay method for DNA ligases and its application to screening of chemical inhibitors of DNA ligase I. J Biochem Biophys Methods. 2004;59:49–59. doi: 10.1016/S0165-022X(02)00071-4. [DOI] [PubMed] [Google Scholar]
- 4.Tan GT, Lee S, Lee IS, et al. Natural-product inhibitors of human DNA ligase I. Biochem J. 1996;314:993–1000. doi: 10.1042/bj3140993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pascal JM, O’Brien PJ, Tomkinson AE, Ellenberger T. Human DNA ligase I completely encircles and partially unwinds nicked DNA. Nature. 2004;432:473–8. doi: 10.1038/nature03082. [DOI] [PubMed] [Google Scholar]
- 6.Ewing TJ, Makino S, Skillman AG, Kuntz ID. DOCK 4.0: search strategies for automated molecular docking of flexible molecule databases. J Comput Aided Mol Des. 2001;15:411–28. doi: 10.1023/a:1011115820450. [DOI] [PubMed] [Google Scholar]
- 7.Hancock CN, Macias A, Lee EK, Yu SY, Mackerell AD, Jr, Shapiro P. Identification of novel extracellular signal-regulated kinase docking domain inhibitors. J Med Chem. 2005;48:4586–95. doi: 10.1021/jm0501174. [DOI] [PubMed] [Google Scholar]
- 8.Huang N, Nagarsekar A, Xia G, Hayashi J, MacKerell AD., Jr Identification of non-phosphate-containing small molecular weight inhibitors of the tyrosine kinase p56 Lck SH2 domain via in silico screening against the pY + 3 binding site. J Med Chem. 2004;47:3502–11. doi: 10.1021/jm030470e. [DOI] [PubMed] [Google Scholar]
- 9.Kuntz ID, Blaney JM, Oatley SJ, Langridge R, Ferrin TE. A geometric approach to macromolecule-ligand interactions. J Mol Biol. 1982;161:269–88. doi: 10.1016/0022-2836(82)90153-x. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz J, Chen I, Gitti R, et al. Identification and characterization of small molecule inhibitors of the calcium-dependent S100B-p53 tumor suppressor interaction. J Med Chem. 2004;47:5085–93. doi: 10.1021/jm0497038. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Pascal J, Vijayakumar S, Wilson GM, Ellenberger T, Tomkinson AE. Human DNA ligases I, III, and IV-purification and new specific assays for these enzymes. Methods Enzymol. 2006;409:39–52. doi: 10.1016/S0076-6879(05)09003-8. [DOI] [PubMed] [Google Scholar]
- 12.Ahel I, Rass U, El-Khamisy SF, et al. The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature. 2006;443:713–6. doi: 10.1038/nature05164. [DOI] [PubMed] [Google Scholar]
- 13.Baumann P, West SC. DNA end-joining catalyzed by human cell-free extracts. Proc Natl Acad Sci U S A. 1998;95:14066–70. doi: 10.1073/pnas.95.24.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Bambara RA. Human Bloom protein stimulates flap endonuclease 1 activity by resolving DNA secondary structure. J Biol Chem. 2005;280:5391–9. doi: 10.1074/jbc.M412359200. [DOI] [PubMed] [Google Scholar]
- 15.Di Virgilio M, Gautier J. Repair of double-strand breaks by non-homologous end joining in the absence of Mre11. J Cell Biol. 2005;171:765–71. doi: 10.1083/jcb.200506029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Trujillo K, Ramos W, Sung P, Tomkinson AE. Promotion of Dnl4-catalyzed DNA end-joining by the Rad50/Mre11/Xrs2 and Hdf1/Hdf2 complexes. Mol Cell. 2001;8:1105–15. doi: 10.1016/s1097-2765(01)00388-4. [DOI] [PubMed] [Google Scholar]
- 17.Tomkinson AE, Totty NF, Ginsburg M, Lindahl T. Location of the active site for enzyme-adenylate formation in DNA ligases. Proc Natl Acad Sci U S A. 1991;88:400–4. doi: 10.1073/pnas.88.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JJ, Kelly TJ. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984;81:6973–7. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood RD, Robins P, Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988;53:97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- 20.Levin DS, Bai W, Yao N, O’Donnell M, Tomkinson AE. An interaction between DNA ligase I and proliferating cell nuclear antigen: implications for Okazaki fragment synthesis and joining. Proc Natl Acad Sci U S A. 1997;94:12863–8. doi: 10.1073/pnas.94.24.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin DS, McKenna AE, Motycka TA, Matsumoto Y, Tomkinson AE. Interaction between PCNA and DNA ligase I is critical for joining of Okazaki fragments and long-patch base-excision repair. Curr Biol. 2000;10:919–22. doi: 10.1016/s0960-9822(00)00619-9. [DOI] [PubMed] [Google Scholar]
- 22.Zheng L, Dai H, Qiu J, Huang Q, Shen B. Disruption of the FEN-1/PCNA Interaction Results in DNA Replication Defects, Pulmonary Hypoplasia, Pancytopenia, and Newborn Lethality in Mice. Mol Cell Biol. 2007;27:3176–86. doi: 10.1128/MCB.01652-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frosina G, Fortini P, Rossi O, et al. Two pathways for base excision repair in mammalian cells. J Biol Chem. 1996;271:9573–8. doi: 10.1074/jbc.271.16.9573. [DOI] [PubMed] [Google Scholar]
- 24.Barnes DE, Tomkinson AE, Lehmann AR, Webster AD, Lindahl T. Mutations in the DNA ligase I gene of an individual with immunodeficiencies and cellular hypersensitivity to DNA-damaging agents. Cell. 1992;69:495–503. doi: 10.1016/0092-8674(92)90450-q. [DOI] [PubMed] [Google Scholar]
- 25.Caldecott KW, Tucker JD, Stanker LH, Thompson LH. Characterization of the XRCC1-DNA ligase III complex in vitro and its absence from mutant hamster cells. Nucleic Acids Res. 1995;23:4836–43. doi: 10.1093/nar/23.23.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grawunder U, Zimmer D, Fugmann S, Schwarz K, Lieber MR. DNA ligase IV is essential for V(D)J recombination and DNA double-strand break repair in human precursor lymphocytes. Mol Cell. 1998;2:477–84. doi: 10.1016/s1097-2765(00)80147-1. [DOI] [PubMed] [Google Scholar]
- 27.Cappelli E, Taylor R, Cevasco M, Abbondandolo A, Caldecott K, Frosina G. Involvement of XRCC1 and DNA ligase III gene products in DNA base excision repair. J Biol Chem. 1997;272:23970–5. doi: 10.1074/jbc.272.38.23970. [DOI] [PubMed] [Google Scholar]
- 28.Sun D, Urrabaz R, Nguyen M, et al. Elevated expression of DNA ligase I in human cancers. Clin Cancer Res. 2001;7:4143–8. [PubMed] [Google Scholar]
- 29.Hertzberg RP, Caranfa MJ, Hecht SM. On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex. Biochemistry. 1989;28:4629–38. doi: 10.1021/bi00437a018. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz SB, Chang CK, Grollman AP. Studies on camptothecin. I. Effects of nucleic acid and protein synthesis. Mol Pharmacol. 1971;7:632–44. [PubMed] [Google Scholar]
- 31.Staker BL, Feese MD, Cushman M, et al. Structures of three classes of anticancer agents bound to the human topoisomerase I-DNA covalent complex. J Med Chem. 2005;48:2336–45. doi: 10.1021/jm049146p. [DOI] [PubMed] [Google Scholar]
- 32.Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Jr, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc Natl Acad Sci U S A. 2002;99:15387–92. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WG. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science. 1998;279:1504–13. doi: 10.1126/science.279.5356.1504. [DOI] [PubMed] [Google Scholar]
- 34.Puebla-Osorio N, Lacey DB, Alt FW, Zhu C. Early embryonic lethality due to targeted inactivation of DNA ligase III. Mol Cell Biol. 2006;26:3935–41. doi: 10.1128/MCB.26.10.3935-3941.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moser J, Kool H, Giakzidis I, Caldecott K, Mullenders LH, Fousteri MI. Sealing of chromosomal DNA nicks during nucleotide excision repair requires XRCC1 and DNA ligase III alpha in a cell-cycle-specific manner. Mol Cell. 2007;27:311–23. doi: 10.1016/j.molcel.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Rosidi B, Perrault R, et al. DNA ligase III as a candidate component of backup pathways of nonhomologous end joining. Cancer Res. 2005;65:4020–30. doi: 10.1158/0008-5472.CAN-04-3055. [DOI] [PubMed] [Google Scholar]
- 37.Caldecott KW, McKeown CK, Tucker JD, Ljungquist S, Thompson LH. An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol Cell Biol. 1994;14:68–76. doi: 10.1128/mcb.14.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bielas JH, Loeb KR, Rubin BP, True LD, Loeb LA. Human cancers express a mutator phenotype. Proc Natl Acad Sci U S A. 2006;103:18238–42. doi: 10.1073/pnas.0607057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–8. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 40.Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–72. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 41.Tutt A, Bertwistle D, Valentine J, et al. Mutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. Embo J. 2001;20:4704–16. doi: 10.1093/emboj/20.17.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 43.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.