Abstract
We used Western blot analysis to examine the effect of dietary K intake on the expression of serine/threonine protein phosphatase in the kidney. K restriction significantly decreased the expression of catalytic subunit of protein phosphatase (PP)2B but increased the expression of PP2B regulatory subunit in both rat and mouse kidney. However, K depletion did not affect the expression of PP1 and PP2A. Treatment of M-1 cells, mouse cortical collecting duct (CCD) cells, or 293T cells with glucose oxidase (GO), which generates superoxide anions through glucose metabolism, mimicked the effect of K restriction on PP2B expression and significantly decreased expression of PP2B catalytic subunits. However, GO treatment increased expression of regulatory subunit of PP2B and had no effect on expression of PP1, PP2A, and protein tyrosine phosphatase 1D. Moreover, deletion of gp91-containing NADPH oxidase abolished the effect of K depletion on PP2B. Thus superoxide anions or related products may mediate the inhibitory effect of K restriction on the expression of PP2B catalytic subunit. We also used patch-clamp technique to study the effect of inhibiting PP2B on renal outer medullary K (ROMK) channels in the CCD. Application of cyclosporin A or FK506, inhibitors of PP2B, significantly decreased ROMK channels, and the effect of PP2B inhibitors was abolished by blocking p38 mitogen-activated protein kinase (MAPK) and ERK. Furthermore, Western blot demonstrated that inhibition of PP2B with cyclosporin A or small interfering RNA increased the phosphorylation of ERK and p38 MAPK. We conclude that K restriction suppresses the expression of PP2B catalytic subunits and that inhibition of PP2B decreases ROMK channel activity through stimulation of MAPK in the CCD.
Keywords: potassium depletion, p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2, potassium secretion
THE KIDNEY PLAYS A KEY ROLE in maintaining plasma K level in a normal range by secretion of K to match the dietary intake (16). Renal K secretion mainly takes place in the late distal tubule, the connecting tubule, and the cortical collecting duct (CCD) by a two-step mechanism (16, 35): K enters the cell through Na-K-ATPase at the basolateral membrane and leaves the cell through apical K channels. Although a Ca2+-activated big-conductance K (BK) channel has been shown to be involved in K secretion during high K intake or increasing tubule flow rate (6, 36, 43), renal outer medullary K (ROMK)-like small-conductance K (SK) channels play a major role in K secretion under normal K intake.
Dietary K intake is an important factor regulating renal K secretion (13, 19, 33): increasing dietary K intake stimulates whereas decreasing K intake suppresses renal K secretion. However, the signaling pathway by which low K intake suppresses renal K secretion is not completely understood. We (3, 4) and others (24) have demonstrated that protein tyrosine kinases (PTK) and serine/threonine protein kinases are involved in mediating the effect of K restriction on SK channels. In addition, we previously demonstrated (3, 4) that K restriction increases superoxide anion production, presumably by stimulating NADPH oxidase, and that superoxide anions are at least partially responsible for inhibition of renal K secretion and downregulation of ROMK channels. The mechanism by which superoxide anions inhibit ROMK channels is not completely understood. Superoxide anions and related products such as H2O2 have been shown to regulate the activity of protein phosphatases (8, 21, 25). Because protein phosphatases play an important role in the regulation of protein phosphorylation (7, 11, 22), it is conceivable that alteration of protein phosphatase activity induced by superoxide anions should affect ROMK channels through interaction between protein phosphatase and kinases. Therefore, the aim of the present study was to explore the role of serine/threonine protein phosphatases in mediating the effect of K restriction on ROMK-like SK channels.
METHODS
Tissue preparation
Sprague-Dawley rats (5- 6 wk, either sex) were purchased from Taconic Farms (Germantown, NY), and male gp91phox(-/-) and C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME). Rats were maintained on a normal-K (1.1%) diet, a K-deficient (KD) diet, or a high-K (10%) diet (Harlan Teklad, Madison, WI), while mice were kept on a normal-K or KD diet for 7 days. Animals were killed by cervical dislocation (body wt of rats <90 g), and kidneys were removed immediately. The protocol was reviewed and approved by an independent animal use committee at New York Medical College. The renal cortex and the outer medulla were separated under a dissecting microscope and suspended in RIPA buffer solution (1:8, wt/vol) containing 1× phosphate-buffered saline (PBS), 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS. Ten microliters of PMSF (10 mg/ml stock solution in isopropanol) and ten microliters of a cocktail of protease inhibitors (Sigma, St. Louis, MO) were added per milliliter of buffer at the time of lysis. The samples were homogenized on ice for 15 min with a mortar and pestle. The suspension was incubated at 4°C for 1 h, followed by centrifugation at 12,000 rpm for 10 min, and the resultant supernatant was collected. Protein concentrations were measured in duplicate with a Bio-Rad Dc protein assay kit.
Preparation of 293T and M-1 cells
M-1 cells, a mouse CCD line, and 293T cells were purchased from the American Type Culture Collection (Manassas, VA). M-1 cells were maintained in a mixture containing (1:1) Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12 medium with 0.005 mM dexamethasome and 5% fetal bovine serum (FBS), and 293T cells were cultured in DMEM with 10% FBS. Glucose oxidase (GO) or H2O2 (100 μM) was directly added to the corresponding culture medium for 60-120 min. To study the phosphorylation of MAPK, M-1 cells were treated with cyclosporin A for 60 min, followed by washing with ice-cold PBS twice and incubation for 30 min in RIPA lysis buffer. We also transfected 293T cells with small interfering RNA (siRNA) of protein phosphatase (PP)2B and harvested cells to study the phosphorylation of MAPK 24 h after transfection. PP2B siRNA sequence, on-target plus smart pool L-008300-00-0005, human PPP3CA, and NM_000944 were purchased from Dharmacon (Chicago, IL). Lipofectamine 2000 (Invitrogen) transfection reagent was used to carry out transfection according to the product sheet.
Western blot
Proteins homogenized from renal tissue or M-1 and 293T cells were separated by electrophoresis on 8-10% SDS-polyacrylamide gels and transferred to Immuno-Blot polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). The membrane was blocked with Odyssey blocking buffer for fluorescence Western blotting (Rockland, Gilbertsville, PA) and incubated with the primary antibody at 4°C for 12 h. The membrane was washed four times (each 5 min) with PBS containing 0.1% Tween 20, followed by incubation with the secondary antibody for an additional 30 min. The membrane was then washed three times (10 min for each wash) with PBS and scanned by an Odyssey infrared imaging system (LI-COR, Lincoln, NB) at a wavelength of 680 or 800 nm.
Preparation of CCDs for patch-clamping
We used rats either on a normal-K diet or on a high-K diet for 7 days for the patch-clamp experiments. Single CCDs were isolated, placed on a 5 × 5-mm cover glass coated with polylysine, and transferred to a chamber (1,000 μl) mounted on an inverted Nikon microscope. The CCDs were superfused with HEPES-buffered NaCl solution containing (in mM) 140 NaCl, 5 KCl, 1.8 CaCl2, 1.8 MgCl2, and 5 HEPES (pH 7.4). The pipette solution was composed of (in mM) 140 KCl, 1.8 MgCl2, and 5 HEPES (pH 7.4). The temperature of the chamber was maintained at 37 ± 1°C by circulating warm water around the chamber. The CCD was cut open with a sharpened micropipette to expose the apical membrane.
Patch-clamp technique
Patch-clamp electrodes were made with a Narishige (P-81) puller and thick-wall glass capillaries (Degan, Minneapolis, MN) and had a resistance of 4-6 MΩ when filled with 140 mM KCl. The pipettes were then fire-polished with a homemade polisher. An Axon200A patch-clamp amplifier was used to record channel current. The current was low-pass filtered at 1 KHz by an eight-pole Bessel filter (902LPF, Frequency Devices, Haverhill, MA) and digitized with Axon interface (Digidata 1200). Data were analyzed with pCLAMP software system 6.04 (Axon Instruments, Burlingame, CA). Channel activity was defined as NPo (where N is number of channels and Po is open probability), which was calculated from data samples of 60-s duration in the steady state as follows: NPo = Σ (t1 + t2 +… ti), where ti is the fractional open time spent at each of the observed current levels.
Experimental materials and statistics
Protein of PP2B catalytic subunit purified from mouse brain and antibodies to phospho-p38, p38, phospho-ERK, ERK, PP1, PP2A, PP2B, and protein tyrosine phosphatase (PTP)1D were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Cyclosporin A, FK506, SB-202190, and PD-098059 were purchased from Biomol. The data are presented as means ± SE. We used paired Student's t-test to determine the statistical significance. If a P value is <0.05, the difference is considered to be significant.
RESULTS
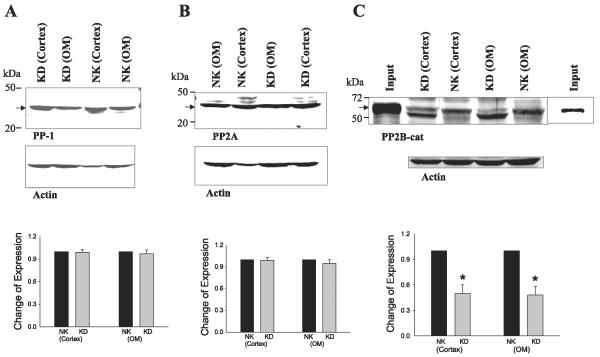
We first examined the expression of PP1, PP2A, and PP2B in the renal cortex and outer medulla with antibodies that recognize the catalytic subunit of these enzymes. From inspection of Fig. 1, it is apparent that PP1 (36 kDa), PP2A (36 kDa), and PP2B catalytic subunit (PP2B-cat; 62 kDa) are highly expressed in the kidney. We next examined the effect of dietary K intake on the expression of PP1, PP2A, and PP2B-cat in the renal cortex and outer medulla. K restriction had no effect on the expression of PP1 (n = 3; Fig. 1A) and PP2A (n = 3; Fig. 1B), but it significantly decreased the expression of PP2B-cat (n = 5) by 50 ± 10% in both cortex and outer medulla (Fig. 1C). From inspection of Fig. 1C, it is apparent that K restriction decreased density of 62-kDa protein band but increased the density of a protein band with low molecular size compared with those in rats on a normal-K diet. The 62-kDa protein band is the intact PP2B-cat, as confirmed by the positive control (input of purified PP2B-cat), while the protein band with a low molecular mass could be due to proteolysis of PP2B-cat. It was recently demonstrated that H2O2 stimulates the proteolytic cleavage of PP2B-cat and inactivates the phosphatase (25). H2O2 may activate the chloroquine-sensitive lysosomal proteases that cleave PP2B-cat within the catalytic domain. Because K restriction increased superoxide anions (4), we speculate that enhanced generation of superoxide anions induced by low K intake causes the cleavage of PP2B-cat and inhibits the activity of PP2B.
Fig. 1.
Effect of dietary K intake on the expression of protein phosphatase (PP)1 (n = 3 rats; A), PP2A (n = 3 rats; B), and PP2B catalytic subunit (PP2B-cat, 5 rats; C). Top: corresponding Western blots. Bottom: summary of data. Data are normalized according to corresponding control. Arrow indicates the position of each corresponding phosphatase that is quantified and presented in the bar graph. PP2B-cat protein purified from mouse brain (input) was used as a positive control. NK, normal K diet; KD, K-deficient diet; OM, outer medulla. *Significant difference (P < 0.05).
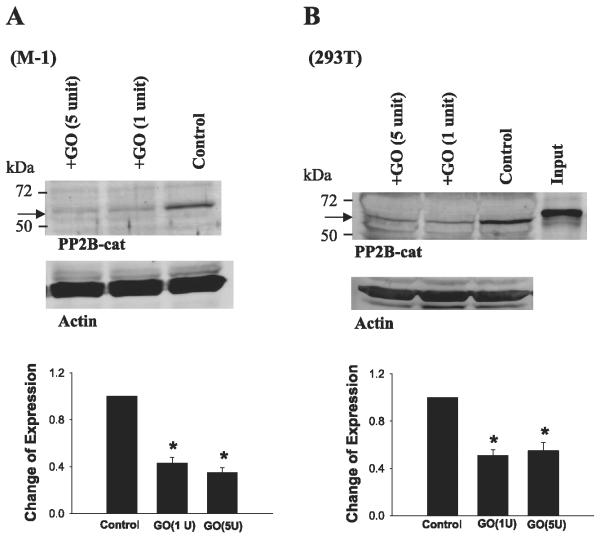
Thus we explored the role of superoxide anions and related products in the regulation of expression of PP2B-cat. We treated M-1 cells or 293T cells for 15 min with GO (1 U/ml), which is able to generate superoxide anions or related products in the presence of glucose (12, 14, 38). Figure 2 shows a Western blot demonstrating that GO (1 U/ml) treatment decreased the protein level of 62-kDa PP2B-cat by 60 ± 10% in M-1 cells (n = 4; Fig. 2A) and by 50 ± 10% in 293T cells (n = 4; Fig. 2B). We also examined the effect of 100 μMH2O2 on the expression of PP2B-cat in both M-1 and 293T cells. H2O2 mimicked the effect of GO and decreased the expression of 62-kDa PP2B-cat (data not shown). Although treatment of 293T and M-1 cells with GO or H2O2 mimicked the effect of K restriction on PP2B-cat expression, the low-molecularsize band was less obvious in both cells than that in the kidney. We speculate that protease activity was higher in both 293T and M-1 cells under our experimental conditions than that in the kidney from K-restricted animals. Thus the cleaved PP2B-cat protein was further degraded by protease. Since the response of PP2B-cat to GO treatment was the same in M-1 and 293T cells, we performed experiments in 293T cells in other series of studies.
Fig. 2.
Effect of glucose oxidase (GO) treatment on the expression of PP2B-cat in M-1 cells (A) and in 293T cells (B). Top: corresponding Western blots. Bottom: summary of data. Data are normalized according to corresponding control. Cells were treated with GO (1 or 5 U/ml) for 15 min, followed by homogenization. Arrow indicates the position of PP2B-cat. *Significant difference (P < 0.05).
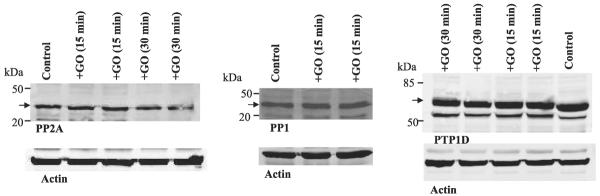
To determine whether the GO treatment-induced decrease in expression of PP2B-cat was specific, we examined the effect of GO treatment on the expression of PP2A and PTP1D. Figure 3 shows a typical Western blot demonstrating that treatment of 293T cells with GO (1 U/ml) for 15 min did not alter the expression of both PP1 and PP2A (n = 16). Moreover, GO treatment did not alter the expression of PTP1D (n = 4). Also, prolonged incubation (30 min) of the cells with GO did not change the protein level of both PP2A and PTP1D. Thus superoxide anions specifically decreased the expression of PP2B-cat. This notion is further confirmed by examining the expression of PP2B regulatory subunit in response to K restriction and GO treatment. In sharp contrast to the catalytic subunit of PP2B, the Western blot in Fig. 4A demonstrates that K restriction increased the expression of the regulatory subunit of PP2B by 100 ± 25% (n = 3). Also, treatment of 293T cells with GO (1 U/ml) for 15 min increased rather than decreased the protein level of PP2B regulatory subunit by 90 ± 15% (n = 3; data not shown).
Fig. 3.
Effect of GO treatment (1 U/ml) on the expression of PP2A (left), PP1 (center), and protein tyrosine phosphatase (PTP)1D (right) in 293 T cells. Cells were treated with GO for 15 or 30 min. Arrow indicates the position of each corresponding phosphatase.
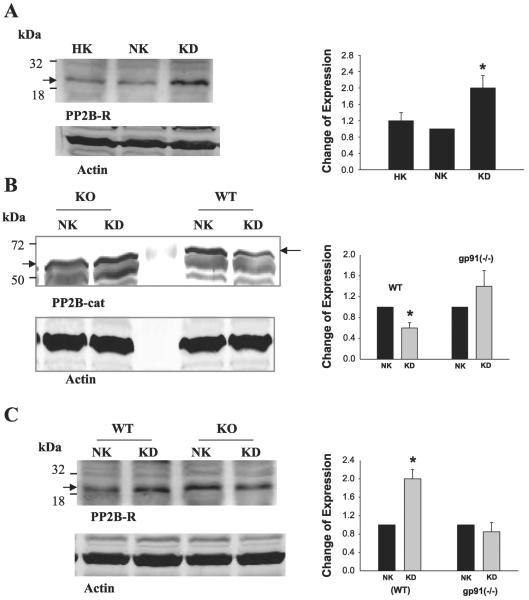
Fig. 4.
A: effect of K restriction on the expression of PP2B regulatory subunit (PP2B-R) in rat. HK, High K diet. B: effect of K restriction on the expression of PP2B-cat in wild-type (WT) mice or in gp91phox(-/-) (KO) mice. C: effect of K restriction on the expression of PP2B-R in WT and gp91phox(-/-) mice. Arrow indicates the position of PP2B-cat or PP2B-R. Right: summary of results for each corresponding experiment. *Significant difference (P < 0.05).
To further demonstrate that superoxide anions and related products are involved in mediating the effect of low K intake on the expression of PP2B-cat, we examined the effect of K restriction on PP2B expression in gp91phox(-/-) and wild-type (WT) mice. It is well established that gp91Phox-containing NADPH oxidase is expressed and is responsible for generating superoxide anions in the kidney (10, 18). We previously demonstrated (5) that production of superoxide anions was diminished in gp91phox(-/-) mice. Figure 4B shows a Western blot demonstrating that K restriction decreased the expression of PP2B-cat by 50 ± 11% in WT mice (n = 3) but had no effect in gp91phox(-/-) mice. Similar to rats, K depletion increased the expression of PP2B regulatory subunit by 90 ± 20% (n = 3) in WT mice but had no effect in gp91phox(-/-) mice (Fig. 4C). Therefore, superoxide anions or related products are responsible for mediating the inhibitory effect of K restriction on PP2B.
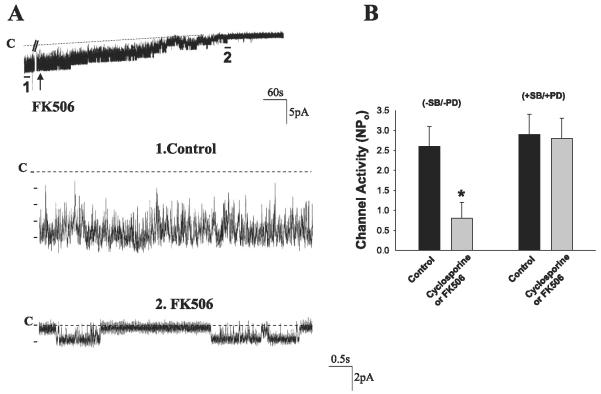
It is well established that both catalytic and regulatory subunits are required for a full activation of PP2B (29). Thus the decrease in PP2B-cat induced by K restriction is expected to decrease the enzymatic activity of PP2B. It has been reported that alteration of PP2B activity is related to changing MAPK activity such as p38 (27) and ERK (37) in cardiac myocytes and in sensory neurons of Aplysia, respectively. We previously demonstrated (3) that p38 and ERK inhibit the ROMK channels in the CCD. Moreover, PP2B has been shown to be expressed in the CCD (40). Thus it is possible that PP2B may regulate the ROMK channels in the CCD. The hypothesis was tested by examining the effect of inhibiting PP2B on the SK channels with either FK506 or cyclosporin A in isolated CCDs with a patch-clamp technique. FK506 and cyclosporin A have been shown to be specific inhibitors of PP2B phosphatase (32). Figure 5A is a channel recording demonstrating that inhibition of PP2B with 5 μM FK506 decreases SK channel activity. The same results were observed when cyclosporin A was used to inhibit PP2B. Data summarized in Fig. 5B show that inhibition of PP2B with either cyclosporin A or FK506 (5 μM) decreased NPo from 2.5 ± 0.5 to 0.8 ± 0.4 (n = 7, P < 0.05). In contrast, inhibition of p38 and ERK with 5 μM PD-098059 and 50 μM SB-202190 abolished the effect of FK506 because NPo is almost the same (control 2.9 ± 0.5, PP2B inhibitor 2.8 ± 0.5) (Fig. 5B). NPo of ROMK-like SK channels in the presence of MAPK inhibitors was not significantly higher than that in the absence of MAPK inhibitors, although a previous study had shown that inhibition of MAPK increased ROMK-like SK channels. This is due to the fact that we selected the patches that had channel activity in the presence of MAPK inhibitors similar to those without inhibitors. Thus the present study suggests that inhibition of PP2B-induced decrease in ROMK channel activity may be the result of stimulation of p38 and ERK MAPK.
Fig. 5.
Effect of FK506 (5 μM) on the renal outer medullary K (ROMK) channels in the cortical collecting duct (CCD). A: top trace shows the time course of the experiments, and 2 parts of the recording indicated with bars and numbers are extended to show the fast time resolution. The channel close level is indicated by a dotted line and C. The gap in the top trace is 300 s. The pipette hold potential was 0 mV. B: effect of FK506/cyclosporin A on ROMK channels in CCDs, which were treated with or without 5 μM SB-202190 (SB) + 50 μM PD-098059 (PD). Experiments were performed in cell-attached patches. *Significant difference (P < 0.05).
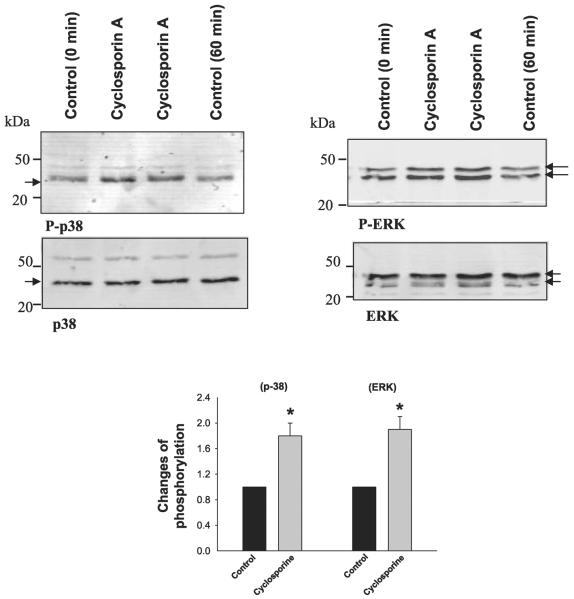
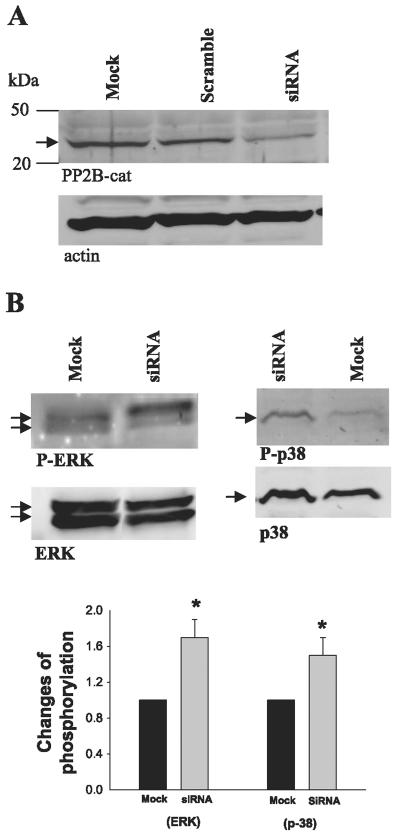
To further determine the role of MAPK in mediating the effect of PP2B inhibitor on ROMK channels, we examined the effect of cyclosporin A on the phosphorylation of p38 and ERK in M-1 cells. We treated M-1 cells with 5 μM cyclosporin A for 60 min, followed by harvesting the cells to study the effect of inhibiting PP2B on the phosphorylation of MAPKs with antibodies that recognize phosphorylated p38 and ERK. Figure 6 shows a Western blot demonstrating that inhibition of PP2B significantly increased the phosphorylation of p38 (80 ± 20%, n = 5; P < 0.05) and ERK (90 ± 20%, n = 5; P < 0.05) in M-1 cells without changing the protein expression of ERK and p38. The notion that inhibition of PP2B stimulates the phosphorylation of MAPK was also supported by experiments in which siRNA of PP2B was used. We transfected 293T cells with siRNA of PP2B (100 μM) and examined the phosphorylation of MAPK 24 h after the transfection. Figure 7 shows that treatment of 293T cells with siRNA significantly decreased the expression of PP2B-cat (50 ± 10% of the control) compared with mock treatment or treatment with scrambled RNA and increased the phosphorylation of p38 and ERK by 50 ± 10% and 70 ± 15% (n = 4, P < 0.05), respectively. Thus our results strongly suggest a reciprocal relationship between expression of PP2B-cat and phosphorylation of MAPK.
Fig. 6.
Top: Western blot showing the effect of cyclosporin A (5 μM) treatment (60 min) on the phosphorylation of ERK and p38 detected with phospho-ERK and phospho-p38 antibodies. Control cells were treated with vehicles for 0 or 60 min. Bottom: effect of cyclosporin A (5 μM) on the phosphorylation of p38 and ERK. Arrow indicates the position of p38 and ERK, which is quantified and presented in the corresponding bar graph. The phosphorylation level of ERK and p38 at the 60-min band was used as control value. *Significant difference (P < 0.05).
Fig. 7.
Effect of PP2B small interfering (si)RNA on the expression of PP2B-cat and actin (A) and the phosphorylation of ERK and p38 in 293T cells that were transfected with siRNA or mock-transfected for 24 h (B). Bottom: summary of results of the experiments. *Significant difference (P < 0.05).
DISCUSSION
ROMK channels have at least three PKA and two PKC phosphorylation sites (20, 28) and are regulated by serine/threonine protein kinases (2, 28, 31, 44, 45). Because protein phosphatases are also involved in the regulation of protein phosphorylation, serine/threonine protein phosphatases should also play an important role in the regulation of ROMK channels. For instance, we previously demonstrated (23) that PKA-induced stimulation of ROMK channel activity was completely abolished by addition of PP2A, suggesting that PP2A may cause dephosphorylation of ROMK channels induced by PKA. The present study has further indicated that PP2B is also involved in the regulation of ROMK channels in the CCD. However, two lines of evidence indicate that the effect of PP2B on ROMK channels is indirect: 1) A previous study demonstrated that addition of exogenous PP2B catalytic subunits had no effect on ROMK channels in excised patches (23). 2) Inhibition of p38 and ERK abolished the inhibitory effect of FK506/ cyclosporin A on ROMK channels. Thus PP2B regulates ROMK channels via alteration of MAPK activity rather than a direct dephosphorylation of ROMK. The finding that inhibiting PP2B decreased SK channels suggests that PP2B has a basal activity under physiological cell Ca2+ in the CCD. A similar finding has been reported in a study in which FK506- or cyclosporin A-sensitive PP2B activity was detected in microdissected CCD tubules (40). Thus it is possible that PP2B has a basal activity under physiological conditions. This notion is also supported by a report that the association of PP2B-cat and PP2B regulatory subunit gives the enzyme a basal activity, although Ca2+ is required for maximal stimulation of PP2B activity (29). A previous study demonstrated that increasing cell Ca2+ stimulates PKC, which inhibits the SK channels (41). However, Ca2+ also stimulates PP2B, which should decrease MAPK activity and increase SK channel activity. This seeming discrepancy is not likely to occur because increasing Ca2+ is most likely compartmentalized and only affects the targeted proteins under physiological conditions.
PP2B has been shown to play an important role in the regulation of renal function and development (17). The observation that K restriction decreased the expression of PP2B-cat strongly suggests that PP2B is involved in mediating the effect of low K intake on ROMK channels and renal K secretion. Although the mechanism by which K restriction decreases the expression of PP2B-cat is not completely understood, it is unlikely that decreased expression of PP2B-cat is due to changes in protein transcription. This notion is supported by two lines of evidence: 1) Treatment of M-1 and 293T cells with GO for as little as 15 min decreased expression of PP2B-cat. 2) K restriction did not alter the mRNA level of PP2B-cat detected with semiquantitative RT-PCR (unpublished observation). Also, the observation that expression of PP2B regulatory subunit increased in response to K restriction suggests that decreased expression of PP2B-cat was not the result of inhibition of protein translation. It is possible that the reduced protein level of PP2B-cat induced by K restriction was the result of a posttranslation modification. A large body of studies has documented that superoxide anions inhibit the activity of PP2B (9, 15, 25, 34, 42). The effect of superoxide anions on PP2B-cat is specific because neither low K intake nor GO treatment affects the expression of PP2A and PTP1D.
Superoxide anions have been shown to be involved in mediating the effect of low K intake on ROMK channels (4, 5). We have demonstrated (3, 26) that superoxide anions and related products stimulate the activity of Src family PTK and p38/ERK MAPKs. p38 and ERK MAPK have been shown to be involved in mediating the effect of low K intake on ROMK channels and BK channels in the CCD. We previously demonstrated that phosphorylation of p38 and ERK increased significantly 24 h after K depletion. Furthermore, we showed (5) that superoxide anions and related products are responsible for mediating the effect of K restriction on the phosphorylation of p38 and ERK because suppression of superoxide production abolished the effect of K depletion on the phosphorylation of MAPK. Thus we hypothesize that K restriction stimulates the activity of NADPH oxidase and increases the generation of superoxide anions and related products, which stimulate the phosphorylation of MAPK and the expression of Src family PTK. As a consequence, ROMK channels are inhibited and renal K secretion is diminished.
Although the role of superoxide anions in stimulating the phosphorylation of p38 and ERK is established, it is not completely understood which signal molecule is involved in mediating the effect of superoxide anions on MAPK. A previous study provided evidence supporting the idea that the tumor-suppressing protein inhibitor of growth 4 (ING4) is involved in mediating the effect of superoxide on MAPK in the kidney (46). First, low K intake increased ING4 expression, and suppression of superoxide abolished the effect of K restriction on ING4 expression. Second, treatment of mouse CCD cells with H2O2 stimulated the expression of ING4. Third, increasing expression of ING4 augmented while suppression of ING4 expression diminished the phosphorylation of p38 and ERK. The present study has further suggested that PP2B may also be involved in mediating the effect of superoxide anions on ERK and p38 phosphorylation: 1) treatment of M-1 cells or 293T cells with GO decreased the expression of PP2B-cat, and 2) inhibition of PP2B significantly stimulated the phosphorylation of p38 and ERK. The idea that the effect of K restriction on PP2B-cat is mediated by superoxide anions is also supported by the observation that K restriction did not decrease the level of PP2B-cat in gp91phox(-/-) mice, which have been shown to produce less superoxide anion in the kidney than WT mice (46). However, further experiments are required to explore the relationship between PP2B and ING4 in mediating the effect of low K intake or superoxide anions on MAPK. That inhibition of PP2B leads to a significant MAPK activation has also been reported in sensory neurons of the pleural ganglia of Aplysia (37). The mechanism by which PP2B regulates the phosphorylation of ERK and p38 is not clear. It has been reported that activation of PP2B enhances expression of MAPK phosphatase-1, which dephosphorylates p38 MAPK in cardiac myocytes (27).
Treatment with an immunosuppressive agent such as cyclosporin A in patients has been shown to cause sustained hyperkalemia (1). However, the mechanism by which cyclosporin A causes sustained hyperkalemia is not completely understood. It has been shown that cyclosporin A inhibits K channels in cultured rabbit CCD cells (30) and Na-K-ATPase in several nephron segments including CCD (39). Here we demonstrate that cyclosporin A inhibits ROMK channels. ROMK channels are responsible for K secretion under normal conditions. Thus cyclosporin A-induced inhibition of ROMK channels is expected to decrease renal K secretion in the connecting tubule and the CCD. In addition, stimulation of p38 and ERK MAPK has also been shown to inhibit Ca2+-activated BK channels. Thus it is conceivable that cyclosporin A may also inhibit BK channels, which are involved in K secretion especially when dietary K intake is increased or tubule flow rate is high. Therefore, application of cyclosporin A is expected to inhibit BK and ROMK channels in the CCD. This mechanism may contribute to hyperkalemia induced by application of cyclosporin A.
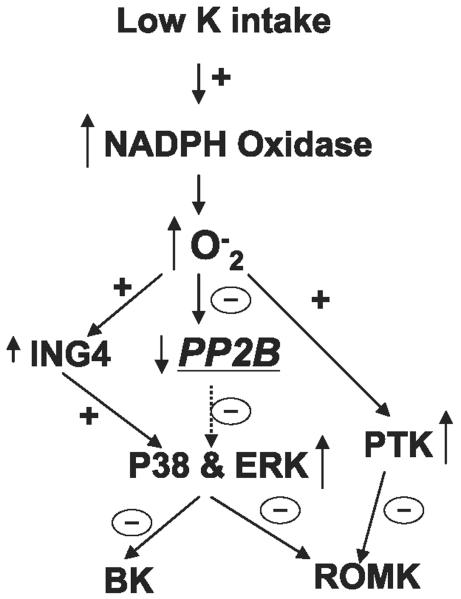
Figure 8 is a cell scheme illustrating the role of PP2B in mediating the effect of low K intake on apical K channels in the CCD. Low K intake stimulates NADPH oxidase and increases superoxide anion production, which stimulates ING4 expression and decreases PP2B activity. As a consequence, low K intake increases the phosphorylation of p38 and ERK, which inhibit both ROMK-like SK channels and BK channels in the CCD. In addition, the increase in superoxide anion production induced by K restriction also augments PTK activity, which inhibits ROMK channels in the CCD. Thus activation of MAPK and PTK suppresses renal K secretion in the CCD.
Fig. 8.
Scheme illustrating the possible role of PP2B in mediating the effect of low K intake on apical small-conductance K (SK) and big conductance K (BK) channels in the CCD. A dotted arrow indicates a diminished effect. ING4, inhibitor of growth 4; PTK, protein tyrosine kinases.
In conclusion, K restriction decreased the protein level of PP2B-cat and the activity of PP2B, and the effect of K restriction on PP2B-cat is mediated by increasing superoxide anions. Inhibition of PP2B activity stimulates the activity of p38 and ERK, which decrease the activity of apical K secretory channels and impair renal K secretion.
Acknowledgments
GRANTS This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-47402.
REFERENCES
- 1.Adu D, Turney J, Michael J, McMaster P. Hyperkalaemia in cyclosporin-treated renal allograft recipients. Lancet. 1983;2:370–372. doi: 10.1016/s0140-6736(83)90345-8. [DOI] [PubMed] [Google Scholar]
- 2.Ali S, Chen X, Lu M, Xu JZ, Lerea KM, Herbert SC, Wang WH. The A kinase anchoring protein (AKAP) is required for mediating the effect of protein kinase A on ROMK1 channels. Proc Natl Acad Sci USA. 1998;95:10274–10278. doi: 10.1073/pnas.95.17.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babilonia E, Li D, Wang Z, Sun P, Lin DH, Wang WH. Mitogenactivated protein kinases inhibit the ROMK (Kir 1.1)-like small conductance K channels in the cortical collecting duct. J Am Soc Nephrol. 2006;17:2687–2696. doi: 10.1681/ASN.2006050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babilonia E, Wei Y, Sterling H, Kaminski P, Wolin M, Wang WH. Superoxide anions are involved in mediating the effect of low K intake on c-Src expression and renal K secretion in the cortical collecting duct. J Biol Chem. 2005;280:10790–10796. doi: 10.1074/jbc.M414610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babilonia E, Lin D, Zhang Y, Wei Y, Yue P, Wang WH. Role of gp91phox-containing NADPH oxidase in mediating the effect of K restriction on ROMK channels and renal K excretion. J Am Soc Nephrol. 2007;18:2037–2045. doi: 10.1681/ASN.2006121333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey MA, Cantone A, Yan QS, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of type II Bartter's syndrome and in adaptation to a high K diet. Kidney Int. 2006;70:51–59. doi: 10.1038/sj.ki.5000388. [DOI] [PubMed] [Google Scholar]
- 7.Barford D, Jia ZC, Tonks NK. Protein tyrosine phosphatases take off. Nat Struct Biol. 1995;2:1043–1053. doi: 10.1038/nsb1295-1043. [DOI] [PubMed] [Google Scholar]
- 8.Barrett DM, Black SM, Todor T, Schmidt-Ullrich RK, Dawson KS, Mikkelsen RB. Inhibition of protein-tyrosine phosphatases by mild oxidative stresses is dependent on S-nitrosylation. J Biol Chem. 2005;280:14453–14461. doi: 10.1074/jbc.M411523200. [DOI] [PubMed] [Google Scholar]
- 9.Carballo M, Marquez G, Conde M, Martin-Nieto J, Monteseirin J, Conde J, Pintado E, Sobrino F. Characterization of calcineurin in human neutrophils. Inhibitory effect of hydrogen peroxide on its enzyme activity and on NF-kappaB DNA binding. J Biol Chem. 1999;274:93–100. doi: 10.1074/jbc.274.1.93. [DOI] [PubMed] [Google Scholar]
- 10.Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita A, Welch WJ, Wilcox CS. Expression and cellular location of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension. 2002;39:269–274. doi: 10.1161/hy0202.103264. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P, Cohen PT. Protein phosphatases come of age. J Biol Chem. 1989;264:21435–21438. [PubMed] [Google Scholar]
- 12.Das A, Hazra TK, Boldogh I, Mitra S, Bhakat KK. Induction of the human oxidized base-specific DNA glycosylase NEIL1 by reactive oxygen species. J Biol Chem. 2005;280:35272–35280. doi: 10.1074/jbc.M505526200. [DOI] [PubMed] [Google Scholar]
- 13.Field MJ, Stanton BA, Giebisch GH. Differential acute effects of aldosterone, dexamethasone, and hyperkalemia on distal tubular potassium secretion in the rat kidney. J Clin Invest. 1984;74:1792–1802. doi: 10.1172/JCI111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frederick KR, Tung J, Emerick RS, Masiarz FR, Chamberlain SH, Vasavada A, Rosenberg S, Chakraborty S, Schopfer LM, Schopter LM. Glucose oxidase from Aspergillus niger. Cloning, gene sequence, secretion from Saccharomyces cerevisiae and kinetic analysis of a yeast-derived enzyme. J Biol Chem. 1990;265:3793–3802. Erratum. J Biol Chem 265 (Jul 5): 11405, 1990. [PubMed] [Google Scholar]
- 15.Furuke K, Shiraishi M, Mostowski HS, Bloom ET. Fas ligand induction in human NK cells is regulated by redox through a calcineurin-nuclear factors of activated T cell-dependent pathway. J Immunol. 1999;162:1988–1993. [PubMed] [Google Scholar]
- 16.Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol Renal Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 17.Gooch JL. An emerging role for calcineurin Aα in the development and function of the kidney. Am J Physiol Renal Physiol. 2006;290:F769–F776. doi: 10.1152/ajprenal.00281.2005. [DOI] [PubMed] [Google Scholar]
- 18.Haque MZ, Majid DSA. Assessment of renal functional phenotype in mice lacking pp91Phox subunit of NAD(P)H oxidase. Hypertension. 2004;43:335–340. doi: 10.1161/01.HYP.0000111137.15873.4a. [DOI] [PubMed] [Google Scholar]
- 19.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 21.Howe CJ, LaHair MM, McCubrey JA, Franklin RA. Redox regulation of the calcium/calmodulin-dependent protein kinases. J Biol Chem. 2004;279:44573–44581. doi: 10.1074/jbc.M404175200. [DOI] [PubMed] [Google Scholar]
- 22.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 23.Kubokawa M, McNicholas CM, Higgins MA, Wang WH, Giebisch G. Regulation of ATP-sensitive K+ channel by membrane-bound protein phosphatases in rat principal tubule cell. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;269:F355–F362. doi: 10.1152/ajprenal.1995.269.3.F355. [DOI] [PubMed] [Google Scholar]
- 24.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA. 2006;103:1615–1620. doi: 10.1073/pnas.0510609103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JE, Kim H, Jang H, Cho EJ, Youn HD. Hydrogen peroxide triggers the proteolytic cleavage and the inactivation of calcineurin. J Neurochem. 2007;100:1703–1712. doi: 10.1111/j.1471-4159.2006.04340.x. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of MAPK stimulates the Ca2+-dependent big-conductance K channels in cortical collecting duct. Proc Natl Acad Sci USA. 2006;103:19569–19574. doi: 10.1073/pnas.0609555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim HW, New L, Han J, Molkentin JD. Calcineurin enhances MAPK phosphatase-1 expression and p38 MAPK inactivation in cardiac myocytes. J Biol Chem. 2001;276:15913–15919. doi: 10.1074/jbc.M100452200. [DOI] [PubMed] [Google Scholar]
- 28.Lin D, Sterling H, Lerea KM, Giebisch G, Wang WH. Protein kinase C (PKC)-induced phosphorylation of ROMK1 is essential for the surface expression of ROMK1 channels. J Biol Chem. 2002;277:44332–44338. doi: 10.1074/jbc.M203702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin X, Sikkink RA, Rusnak F, Barber DL. Inhibition of calcineurin phosphatase activity by a calcineurin B homologous protein. J Biol Chem. 1999;274:36125–36131. doi: 10.1074/jbc.274.51.36125. [DOI] [PubMed] [Google Scholar]
- 30.Ling BN, Eaton DC. Cyclosporin A inhibits apical secretory K+ channels in rabbit cortical collecting tubule principal cells. Kidney Int. 1993;44:974–984. doi: 10.1038/ki.1993.339. [DOI] [PubMed] [Google Scholar]
- 31.Liou HH, Zhou SS, Huang CL. Regulation of ROMK1 channel by protein kinase A via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. Proc Natl Acad Sci USA. 1999;96:5820–5825. doi: 10.1073/pnas.96.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Farmer JDJ, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBPFK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 33.Muto S, Sansom S, Giebisch G. Effects of a high potassium diet on electrical properties of cortical collecting duct from adrenalectomized rabbits. J Clin Invest. 1988;81:376–380. doi: 10.1172/JCI113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namgaladze D, Hofer HW, Ullrich V. Redox control of calcineurin by targeting the binuclear Fe2+-Zn2+ center at the enzyme active site. J Biol Chem. 2002;277:5962–5969. doi: 10.1074/jbc.M111268200. [DOI] [PubMed] [Google Scholar]
- 35.Palmer LG. Potassium secretion and the regulation of distal nephron K channels. Am J Physiol Renal Physiol. 1999;277:F821–F825. doi: 10.1152/ajprenal.1999.277.6.F821. [DOI] [PubMed] [Google Scholar]
- 36.Satlin LM. Developmental regulation of expression of renal potassium secretory channels. Curr Opin Nephrol Hypertens. 2004;13:445–450. doi: 10.1097/01.mnh.0000133979.17311.21. [DOI] [PubMed] [Google Scholar]
- 37.Sharma SK, Bagnall MW, Sutton MA, Carew TJ. Inhibition of calcineurin facilitates the induction of memory for sensitization in Aplysia: requirement of mitogen-activated protein kinase. Proc Natl Acad Sci USA. 2003;100:4861–4866. doi: 10.1073/pnas.0830994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tirosh A, Potashnik R, Bashan N, Rudich A. Oxidative stress disrupts insulin-induced cellular redistribution of insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. A putative cellular mechanism for impaired protein kinase B activation and GLUT4 translocation. J Biol Chem. 1999;274:10595–10602. doi: 10.1074/jbc.274.15.10595. [DOI] [PubMed] [Google Scholar]
- 39.Tumlin JA, Sands JM. Nephron segment-specific inhibition of Na+/K+-ATPase activity by cyclosporin A. Kidney Int. 1993;43:246–251. doi: 10.1038/ki.1993.38. [DOI] [PubMed] [Google Scholar]
- 40.Tumlin JA, Someren JT, Swanson CE, Lea JP. Expression of calcineurin activity and α-subunit isoforms in specific segments of the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;269:F558–F563. doi: 10.1152/ajprenal.1995.269.4.F558. [DOI] [PubMed] [Google Scholar]
- 41.Wang WH, Geibel J, Giebisch G. Mechanism of apical K+ channel modulation in principal renal tubule cells. Effect of inhibition of basolateral Na+-K+-ATPase. J Gen Physiol. 1993;101:673–694. doi: 10.1085/jgp.101.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Culotta VC, Klee CB. Superoxide dismutase protects calcineurin from inactivation. Nature. 1996;383:434–437. doi: 10.1038/383434a0. [DOI] [PubMed] [Google Scholar]
- 43.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol. 2001;280:F786–F793. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 44.Xu ZC, Yang Y, Hebert SC. Phosphorylation of the ATP-sensitive, inwardly rectifying K+ channel, ROMK, by cyclic AMP-dependent protein kinase. J Biol Chem. 1996;271:9313–9319. doi: 10.1074/jbc.271.16.9313. [DOI] [PubMed] [Google Scholar]
- 45.Yoo D, Flagg TP, Olsen O, Raghuram V, Foskett JK, Welling PA. Assembly and trafficking of a multiprotein ROMK (Kir1.1) channel complex by PDZ interactions. J Biol Chem. 2004;279:6863–6873. doi: 10.1074/jbc.M311599200. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Lin DH, Jin Y, Wang KS, Zhang Y, Babilonia E, Wang Z, Wang Z, Giebisch G, Han ZG, Wang WH. Inhibitor of growth 4 (ING4) is up-regulated by a low K intake and suppresses renal outer medullary K channels (ROMK) by MAPK stimulation. Proc Natl Acad Sci USA. 2007;104:9517–9522. doi: 10.1073/pnas.0703383104. [DOI] [PMC free article] [PubMed] [Google Scholar]