Abstract
Background
We tested if rates of brain atrophy accelerate in individuals with amnestic mild cognitive impairment (aMCI) as they progress to typical late onset Alzheimer's Disease (AD). We included comparisons to aMCI subjects who did not progress (labeled aMCI-S) and also to cognitively normal elderly subjects (CN).
Methods
We studied 46 aMCI subjects who progressed to AD (labeled aMCI-P), 46 CN, and 23 aMCI-S. All subjects must have had three or more serial MRI scans. Rates of brain shrinkage and ventricular expansion were measured across all available serial MRI scans in each subject. Change in volumes relative to the point at which subjects progressed to a clinical diagnosis of AD (the index date) was modeled in aMCI-P. Change in volumes relative to age was modeled in all three clinical groups.
Results
In aMCI-P the change in pre to post index rate (i.e. acceleration) of ventricular expansion was 1.7 cm3/yr, and acceleration in brain shrinkage was 5.3 cm3/yr. Brain volume declined and ventricular volume increased in all three groups with age. Volume changes decelerated with increasing age in aMCI-P, and to a lesser extent aMCI-S, but were linear in the matched CN. Among all aMCI subjects, rates of atrophy were greater in apolipoprotein E ε4 carriers than non-carriers.
Conclusions
Rates of atrophy accelerate as individuals progress from aMCI to typical late onset AD. Rates of atrophy are greater in younger than older aMCI-P and aMCI-S subjects. We did not find that atrophy rates varied with age in 70 – 90 year old CN subjects.
Alzheimer's Disease (AD) is characterized by progressive deterioration of the neuropil (1-3) which is detected macroscopically as cerebral atrophy on magnetic resonance imaging (MRI). Progressive atrophy both precedes and parallels the observed clinical decline in affected individuals and brain shrinkage over time can be detected by serial MRI studies (4-7). Cross-sectional MRI studies of individuals spanning various age ranges have been performed to evaluate age effects on brain morphometry. However, this approach suffers from cohort effects and other biases. Serial measurements acquired in the same individuals are needed to assess atrophy rates. The limited number of longitudinal MRI studies published in normal aging, mild cognitive impairment (MCI), or AD typically employ two scans acquired at different time points from which a rate of change is calculated. These studies have provided estimates of the rates of morphometric change over time and demonstrated increased rates among AD and MCI relative to similarly aged cognitively normal elderly subjects (8-19), and increased rates in MCI subjects who progress to AD relative to MCI subjects who do not (11, 12). However, by sampling two time points per subject, characterization of longitudinal morphometric trajectory on a per subject basis is linear by default. In order to more directly assess a change in rate with time in individual subjects - i.e. acceleration or deceleration - three or more time points must be sampled in each subject. To our knowledge, this phenomenon has been studied only in young individuals with familial AD (20, 21). The purpose of our study was to test if the rates of whole brain shrinkage and ventricular expansion accelerate in elderly subjects with amnestic mild cognitive impairment (aMCI). We were particularly interested to see if rates of atrophy accelerate as individuals with aMCI progress to AD. MCI progressors were contrasted with a group of aMCI subjects that did not progress to dementia. Comparison to a group of cognitively normal elderly subjects was included for reference purposes.
Methods
Subjects
All subjects were identified from the Mayo Alzheimer's Disease Research Center or Alzheimer's Disease Patient Registry (22). These are longitudinal studies of aging and dementia which include serial clinical and cognitive assessments. At baseline all subjects met criteria either for cognitively normal (CN), or aMCI. Categorization into diagnostic groups was made on a clinical basis at consensus conferences involving neurologists, neuropsychologists, a neuropsychiatrist and study coordinators. Criteria for the diagnosis of CN were 1) no active neurological or psychiatric disorders; 2) some subjects may have had ongoing medical problems yet the illnesses or their treatments did not interfere with cognitive function; 3) normal neurological exam; 4) were independently functioning community dwellers. Potential aMCI subjects were identified as having possible memory impairment during routine outpatient general medical visits – ie these subjects were not recruited from memory clinic referrals. Criteria for the diagnosis of aMCI were those of Petersen et al. (23): 1) memory impairment documented by the patient and collateral source; 2) relatively normal general cognition; 3) normal activities of daily living; 4) not demented (DSM-3-R); 5) memory impaired for age and education. In general, the aMCI determination is made when the memory measures fall -1.0 to -1.5 SD below the means for age and education appropriate individuals in our community (24). The memory measures used include the Wechsler Memory Scale Revised Logical Memory and Visual Reproductions subtests (25), the Auditory Verbal Learning Test (AVLT) (26), and the Free and Cued Selective Reminding Test (27). The most salient measures are those involving delayed recall. For other cognitive domains, subtests from the Wechsler Adult Intelligence Scale Revised were used including Digit Span, Digit Symbol Substitution, Block Design, Picture Completion, Object Assembly, as well as other measures including the Boston Naming Test (28), category fluency, and Trailmaking A and B. Measures of global function were also used including the Clinical Dementia Rating (CDR) (29), Mini-Mental State Examination (MMSE) (30), Dementia Rating Scale (31), and the Short Test of Mental Status (32). Typically, aMCI subjects perform above -1 SD below the appropriate means on non-memory measures (23).
Inclusion Criteria and Longitudinal Characterization
We studied 46 individuals who began the study as aMCI and progressed to a clinical diagnosis of AD. These subjects are labeled aMCI-P from here onward. For inclusion, aMCI-P cases must have had a minimum of three MRI scans with a diagnostic sequence of aMCI, AD, and AD. That is, they progressed from aMCI to AD and had at least one more AD scan after the diagnosis of AD. The first scan with a diagnosis of AD is referred to as the index scan and the date of this scan is referred to as the index date. One or more pre- and one or more post – index scans must have occurred within 3 years of the index scan.
A group of 46 CN subjects were age and gender matched one to one to the aMCI-P subjects using the aMCI-P subjects' index date as the reference date for matching. In order to create a stable CN cohort with comparable inclusion criteria, we required that the timing of available scans match that used in assembling the aMCI-P cohort. That is, to be eligible for inclusion in the study, CN had to have three or more MRI scans each with a primary diagnosis of CN, and both the pre- and the post –reference scans must have occurred within 3 years of the reference scan used for age and gender matching to aMCI-P subjects. In addition CN subjects must have had no history of progression to a more impaired cognitive state for the entire available clinical longitudinal follow up after the series of MRI studies. The decision to not right censor CN subjects after the available series of MRI studies had ended but in whom additional clinical follow up was available insured that we assembled a group for reference purposes that was as “normal” as possible. Short of autopsy, the best way to obtain a group of elderly normal subjects that is least likely to be contaminated with sub-clinical pathology is to follow them as long a possible and exclude any who show significant cognitive decline. This approach has particular advantages in a study like this where the mean ages are near 80 and the probability of pre clinical AD pathology is high. To the extent possible, when matching subjects we also tried to minimize the calendar time between the two subjects' index dates. This was done to minimize any potential variation due to MRI hardware/software upgrades between matched pairs of subjects in the two different clinical groups.
We also analyzed aMCI subjects who remained clinically stable (i.e., did not convert to AD). These subjects are labeled aMCI-S from here onward. To be eligible for inclusion in the study, aMCI-S subjects had to have three or more MRI scans each with a diagnosis of aMCI and no history of progression to dementia for the entire available longitudinal clinical follow up after the series of MRI studies. As with the other two clinical groups, both the pre- and the post-reference scans must have occurred within 3 years of the reference scan. These criteria produced 23 available aMCI-S subjects in our data base.
Any potential subject was excluded from the study if they had secondary clinical diagnoses that could potentially interfere with the volumetric measurements process or that introduced ambiguity about the subject's clinical progression. For example, subjects on dialysis or with unstable congestive heart failure were excluded. Fig 1 is a flow diagram illustrating the derivation of the study samples of aMCI-P and aMCI-S subjects from the source population.
Figure 1.
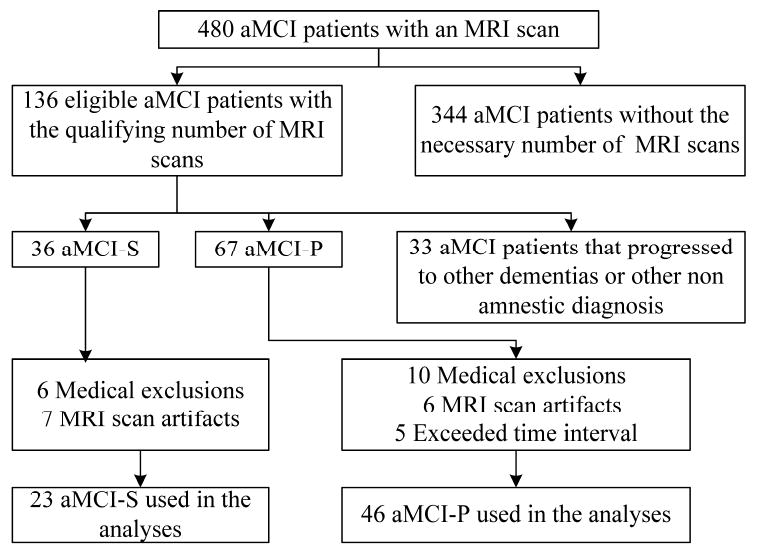
Flow diagram for the aMCI stable and aMCI progressor patients
MRI Methods
All MRI studies were performed with a standardized imaging protocol. T1-weighted 3D coronal volumetric SPGR images were used for all brain and ventricular volume measures with 124 contiguous partitions, and 1.6mm slice thickness, 22×16.5cm field of view, minimum full TE, TR of 23 msec, and 25° flip angle. The presence of cerebro vascular disease was assessed on Fluid Attenuated Inversion Recovery (FLAIR) scans acquired with the following parameters: TR=11,000 ms; TE=147ms; TI=2,250 ms; 3 mm interleaved images of the whole head (33, 34). Total intracranial volume (TIV) was measured from sagittal T1-spin echo images acquired with TR=500ms and TE =14msec. Different scanners were used, but all were GE Signa 1.5T with body resonance module gradients and transmit-receive single channel head coils. All scanners undergo a standardized quality control calibration procedure every morning which monitors geometric fidelity over a 200 mm volume along all 3 cardinal axes, signal to noise ratio, and transmit gain.
Image processing steps were performed by a research technician (MMS) who was blinded to all clinical information. Total intracranial volume (TIV) was measured by manually tracing the margin of the inner table of the skull on saggital T1 weighted spin echo scans as described previously (35, 36). Whole brain and ventricular atrophy rates were measured with the boundary shift integral (BSI) technique (15, 37). Differences were calculated in pair-wise fashion between the baseline scan, and each subsequent scan in the temporal series. Following spatial and intensity normalization of the nth scan in the series to the baseline scan, intensity differences between the two scans at the brain-CSF boundary are used to compute change in volume. The whole brain atrophy rate reflects shrinkage of the brain on scan n relative to the baseline scan from out to in at the cortical surface and from in to out at the ventricular surface. The ventricular atrophy rate was derived by creating a binary mask for each subject that selectively extracted ventricular change. The binary mask was an approximate area overlaying the ventricles within which the BSI was measured. Quality control testing in our laboratory shows the nonparametric intra-class correlation coefficient for test-retest reproducibility of rate measurements from serial MRI scans with the boundary shift integral method is 0.91 for ventricle and 0.89 for brain (13).
The presence of cerebro vascular disease was assessed semi quantitatively on FLAIR scans by an experienced research technician (MMS) who had no knowledge of the clinical information on cognitive status, gender, or age of the participant. The grading method was developed using a synthesis of published criteria (38-42). Scans were graded in two categories. The number of lacunar infarcts was counted in each subject. White matter hyper-intensity (WMH) load was graded with a visual analog scale. Each incoming FLAIR study was compared against a bank of example scans to assign it a WMH load in units of cm3. A bank of 10 example scans had been assembled from our database with increasing levels of WMH severity burden. WMH burden in units of cm3 had been determined quantitatively for each example case using an algorithm developed in our laboratory (43). The bank of example FLAIR studies was registered and resample in the space of a common template. Each new incoming FLAIR to be graded was likewise registered to the same common template. Therefore all scans were oriented in the same common template space for visual comparisons. The technician assigned each new incoming scan a WMH burden on a continuous scale (i.e. visual analog scale) relative to the bank of reference scans using an electronic slider bar. Quality control testing in our laboratory shows the intra-class correlation coefficient for inter-rater reliability of this visual analog WMH grading scale to be 0.96. The concordance correlation coefficient between quantitative measures and visual WMH grading is 0.93. The scans were viewed on a calibrated video monitor and entered into an electronic form linked to a database.
Statistical methods
We performed two types of analyses using linear mixed-effects models to estimate the longitudinal change in ventricular and brain volumes (44). The first analysis was among aMCI-P subjects only and assessed whether there was an atrophy rate increase or decrease after the subjects progressed to a clinical diagnosis of AD. The second analysis was among all three groups and estimated the change in volume as a function of age.
For the first analysis, we fit a piecewise-linear mixed effects model with the time component expressed as years from the index scan. The model specified a random intercept and included fixed effects for TIV, sex, age at index scan, pre-index slope, and post-index slope. The fixed effects part of the model specified that volume change was linear before and after the index scan but allowed for a change in slope at the time of the index scan. The difference between pre-index and post-index slopes represents the estimated rate change after progression to AD. We evaluated whether rates before or after the index scan depended on age by including age by pre-index slope and age by post-index interactions and tested whether these terms were both zero.
For the second analysis, we fit a model with a random intercept and the following fixed effects: TIV, sex, group, age, age2, group by age interaction, and the group by age2 interaction. Due to the interactions, this model allows for separate linear and quadratic age effects by group. We performed a 4 degree of freedom “global” test of group differences to evaluate whether there was a common age and age2 effect across groups. For each group, we also evaluated whether volume depended on age using a 2 degree of freedom test of whether the age and age2 terms were zero. Within group, we also tested the quadratic terms separately and whether volume changed with age after omitting the quadratic.
For comparison purposes, we examined cognitive decline over time by performing parallel analyses using the above-described mixed-effects model methods applied to three tests: AVLT summed learning over trials 1-5, CDR sum of boxes (omitting CN subjects), and MMSE. Sex and education were included as fixed effects for all cognitive models while age at index scan was also included as a fixed effect when modeling change before and after the index scan.
To examine the effect of apolipoprotein E (APOE) ε4 carrier status on volumes over time, we performed a similar age-based analysis among the combined aMCI groups (ie aMCI-P and aMCI-S). The model included a random intercept and fixed effects for TIV, sex, age, age2, and the interactions between APOE ε4 carrier (yes vs. no) and both age and age2.
For all mixed-model analyses we used restricted maximum likelihood estimation and modeled the within-subject correlation structure assuming Gaussian decay, such that two volume measures on the same subject x years apart would have a correlation of exp(-(x/a)2) where a is estimated from the data. This implies for large inter-scan durations the within-subject correlation is small while as the inter-scan duration approaches zero, the within-subject correlation approaches 1.00. In sensitivity analyses, the choice of within-subject correlation structure had little impact on estimates of the rate of volume change. In our modeling we included covariates such as TIV and sex based on their biologic importance and interest and did not remove terms from the models based on significance level. Data handling and analysis was performed with SAS version 9.1.3 (45) and R version 2.4.1 (46) with the mixed effects models fit using the nlme package in R (47). Further details on the mixed model specifications are presented as Supplementary Materials.
Results
Forty six aMCI-P, 23 aMCI-S, and 46 matched CN subjects are included in this analysis (Table 1). Volume measurements were derived from a total of 522 unique MRI scans with a median of 5 scans per aMCI-P, 3 per aMCI-S, and 4 per CN subject. The three groups did not differ on gender, age, or education (Table 1). The proportion of APOE ε4 carriers did not differ between the aMCI-P and aMCI-S groups (p=0.17), while CN had a lower proportion than either aMCI-P (p<0.001) and aMCI-S (p=0.008). At the time of the index/reference MRI scan, performance on MMSE and CDR sum of boxes (SOB) was worse in aMCI-P than in aMCI – S subjects (both p<0.001). Thirty seven of the 46 aMCI-P subjects (80%) and 7 of the 23 aMCI-S subjects (30%) were being treated with cholinesterase inhibitors during the period in which scans were acquired. Neither the prevalence of central grey lacunar infarctions (p = 0.57) nor the estimated white matter hyper intensity load (p= 0.78) differed among the three clinical groups.
Table 1.
Subject characteristics at index or match date.
| CN (n= 46) |
aMCI-S (n=23) |
aMCI-P (n= 46) |
P-value* | |
|---|---|---|---|---|
| No. of women (%) | 23 (50) | 10 (43) | 23 (50) | 0.86 |
| Mean (SD) age, y | 79.0 (5.7) | 80.0 (6.4) | 78.9 (6.3) | 0.94 |
| Mean (SD) number of years of education | 13.0 (2.6) | 14.2 (3.8) | 14.2 (3.3) | 0.14 |
| No. of APOE ε4 carriers (%) | 8 (17) | 11 (48) | 30 (65) | <0.001 |
| Total no. of MRI scans | 197 | 83 | 242 | ---- |
| Median (min., max.) no. of MRI scans per subject | 4 (3, 8) | 3 (3, 6) | 5 (3, 10) | <0.001 |
| Median (min., max.) time from first to last MRI scan, y | 5.5 (2.2, 11.0) | 2.8 (1.9, 7.6) | 4.7 (1.8, 10.4) | <0.001 |
| Median (min., max.) additional clinical follow-up time after last scan, y | 1.5 (0.0, 5.7) | 0.4 (0.0, 3.6) | 1.2 (0.0, 5.2) | 0.08 |
| Median (min. max.) white matter hyperintensity volume, cm3 | 10 (3, 77) | 14 (3, 55) | 10 (4, 61) | 0.78 |
| No. of subjects with any central grey lacunar infarctions (%) | 27 (59) | 11 (48) | 28 (61) | 0.57 |
| Median (min., max.) CDR sum of boxes* | 0.0 (0.0, 0.0) | 0.5 (0.5, 3.5) | 3.0 (0.5, 9.0) | <0.001 |
| Median (min., max.) MMSE* | 29 (25, 30) | 28 (23, 30) | 25 (16, 30) | <0.001 |
Based on 3-group comparisons except for cognitive tests which only compare aMCI - stables to aMCI - progressors.
The demographic information above is in reference to the time of the index/match MRI scan date. However, in order to more fully characterize the criteria used to establish a clinical diagnosis of aMCI subjects in this study, table 2 lists baseline cognitive data used to make the diagnosis of aMCI at the time the first MRI scan was obtained. At this baseline point in time, performance on AVLT summed learning over trials 1–5, CDR SOB, and WMS logical memory II, paragraph recall was better in aMCI-S than aMCI-P subjects. Performance on MMSE, WAIS-R block design, and WAIS-R picture completion was not significantly different between the groups.
Table 2.
Diagnostic characterization of aMCI subjects at earliest aMCI diagnosis with usable MRI scan
| aMCI combined (n= 69) |
aMCI - stable (n=23) |
aMCI - progressor (n= 46) |
P-value* | |
|---|---|---|---|---|
| Median (min., max.) time before matched scan, y | 1.7 (0.4, 8.1) | 1.5 (0.9, 3.7) | 1.8 (0.4, 8.1) | 0.26 |
| Median (min., max.) summed learning over trials 1–5 from AVLT | 28 (4, 43) | 30 (20, 38) | 25 (4, 43) | 0.004 |
| Median (min., max.) CDR sum of boxes | 0.5 (0.0, 3.5) | 0.5 (0.5, 3.0) | 1.0 (0.0, 3.5) | 0.005 |
| Median (min., max.) MMSE | 26 (18, 30) | 27 (23, 30) | 26 (18, 30) | 0.14 |
| Median (min., max.) WMS logical memory II, paragraph recall | 3 (0, 17) | 5 (0, 17) | 2 (0, 14) | 0.02 |
| Median (min., max) WAIS-R block design scaled score | 7 (1, 17) | 7 (4, 11) | 6 (1, 17) | 0.44 |
| Median (min., max) WAIS-R picture completion scaled score | 6 (1, 11) | 7 (2, 9) | 6 (1, 11) | 0.86 |
Based on comparisons between aMCI- stables and aMCI progressors.
In order to assess the representativeness of the aMCI subjects included in these analyses we compared demographic and cognitive characteristics of three groups of subjects: 1) the 344 aMCI subjects with at least one MRI study in the ADPR/ADRC data base who were not included in the study; 2) the 67 aMCI subjects with serial MRI who were eligible on the basis of having serial MRI but were excluded from the analysis because they later progressed to non amnestic diagnoses, had medical exclusions, or had MRI artifacts that precluded analysis of the MRI data; and 3) the 69 subjects who were included in the analyses. The following data is listed by group in supplementary Table E1; % men/women, age, educational attainment, % APOE 4 carrier, summed learning over AVLT trials 1-5, CDR - SOB, MMSE, WMS logical memory II paragraph recall, WAIS-R block design, and WAIS-R picture completion. These values were calculated in each subject at the time of the earliest MRI scan on record coupled with a clinical diagnosis of aMCI. There were no differences among the three groups on any of the preceding variables except: % APOE 4 carriers which was greater (p = 0.02) in the 69 subjects included in the analyses than the 67 excluded; the median WMS logical memory II, paragraph recall which was lower by one point on average (p=0.048) in aMCI subjects included in the analyses vs. subjects without a qualifying number of scans; and WAIS-R block design which was greater by one point on average (p= 0.04) in aMCI subjects in the analyses vs. subjects without a qualifying number of scans.
Rates of ventricular expansion and brain shrinkage in aMCI –P subjects from the piecewise-linear mixed effects model centered on index date are reported in units of cm3/yr in Table 3 and illustrated graphically in Fig 2. Ventricular rates are reported as positive values reflecting increase in volume over time while brain rates are reported as negative values reflecting loss of volume over time. The 95% confidence interval (CI) for both brain and ventricular rates before and after the index date did not include 0, indicating the presence of volume loss with time both before and after progression to AD. Rates of change after the index date were greater than before the index date for both ventricle and brain (both p < 0.001), indicating acceleration in atrophy rate as subjects progressed from aMCI to a clinical diagnosis of AD. The mean change in pre to post index date rate (ie acceleration) of ventricular atrophy was 1.7 cm3/yr, and of brain was 5.3 cm3/yr.
Table 3.
aMCI – progressors: estimates for pre-index rate, post-index rate, and volumetric rate increase based on a piecewise-linear mixed effects model
| Estimate (cm3/y) | 95% CI (cm3/y) | P-value* | |
|---|---|---|---|
| Ventricle rate of atrophy | |||
| Before AD diagnosis scan | 3.1 | 2.7 to 3.5 | <0.001 |
| After AD diagnosis scan | 4.8 | 4.2 to 5.3 | <0.001 |
| Rate increase | 1.7 | 0.9 to 2.5 | <0.001 |
| Whole brain rate of atrophy | |||
| Before AD diagnosis scan | −7.0 | −8.1 to −6.0 | <0.001 |
| After AD diagnosis scan | −12.3 | −13.7 to −10.9 | <0.001 |
| Rate increase | 5.3 | 3.3 to 7.4 | <0.001 |
Note: Estimates are adjusted for TIV, sex, and age at the time of the index scan
CI, confidence interval
Based on Wald test testing whether coefficient is zero
Figure 2.
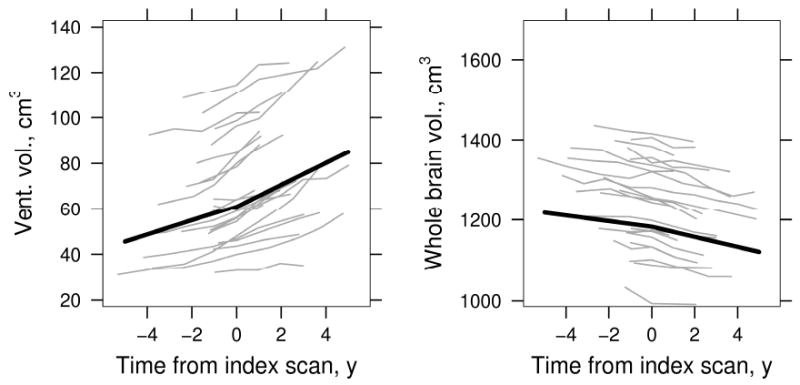
aMCI - progressors: ventricular and whole brain atrophy before and after AD diagnosis (index) scan. Thin gray lines represent a random sample of 23 individual subject volumes over time, thick black line indicates average volume. Average volumes are shown assuming a woman with a total intracranial volume of 1.4 L whose index scan is at age 79.
Using the same piecewise-linear mixed effects methods centered on index date, we estimated change with time of summed learning over trials 1-5 from the AVLT, CDR-SOB, and MMSE among aMCI-P subjects (Fig 3). We used this learning measure from the AVLT rather than a measure of delayed recall because of floor effects on the recall measure (i.e. a number of aMCI-P subjects had 0 recall in the latter stages of the testing series). There was no significant evidence for non-linearity – i.e., no significant change in the rate of decline pre to post index date in the AVLT, but rates of declining performance did accelerate for both the CDR-SOB (p < 0.001) and MMSE (p<0.001). Results of the piecewise-linear mixed effects modeling for AVLT, CDR-SOB, and MMSE for aMCI-P subjects are reported in online supplemental Table E2.
Figure 3.
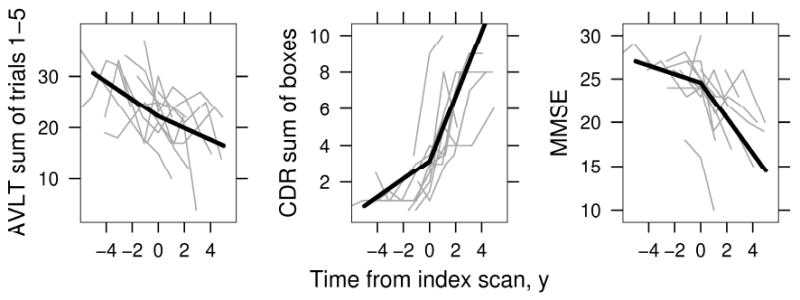
aMCI - Progressors. AVLT sum of words learned over trials 1 through 5, CDR sum of boxes, and MMSE before and after index date. Thin gray lines represent a random sample of 10 individual subject values over time, thick black line indicates estimated average values. Average values are shown assuming a woman with 12 years of education whose index scan is at age 79.
Results of the mixed model repeated measures analysis of volume by age for all three clinical groups are displayed graphically in Fig 4 for the ventricle and in Fig 5 for brain. Volume change with age differed by group for the ventricle and brain (P<0.001 for each). We report within-group tests of quadratic and linear age terms in Table 4. In Figs 4 and 5, the rate of ventricular expansion and brain shrinkage seems to slow with advancing age (i.e. atrophy rates are less in older subjects) in both aMCI-S and aMCI-P subjects. Deceleration in atrophy rates was highly significant in aMCI-P subjects with a trend in aMCI-S subjects (Table 4). In contrast, among CN subjects we did not detect a change with age in the rates of brain shrinkage and ventricular expansion– i.e. rates remained constant (Figs 4, and 5, and Table 4). Age based mixed model coefficients are reported in supplemental Tables E 3 and 4 for ventricular and brain volume.
Figure 4.
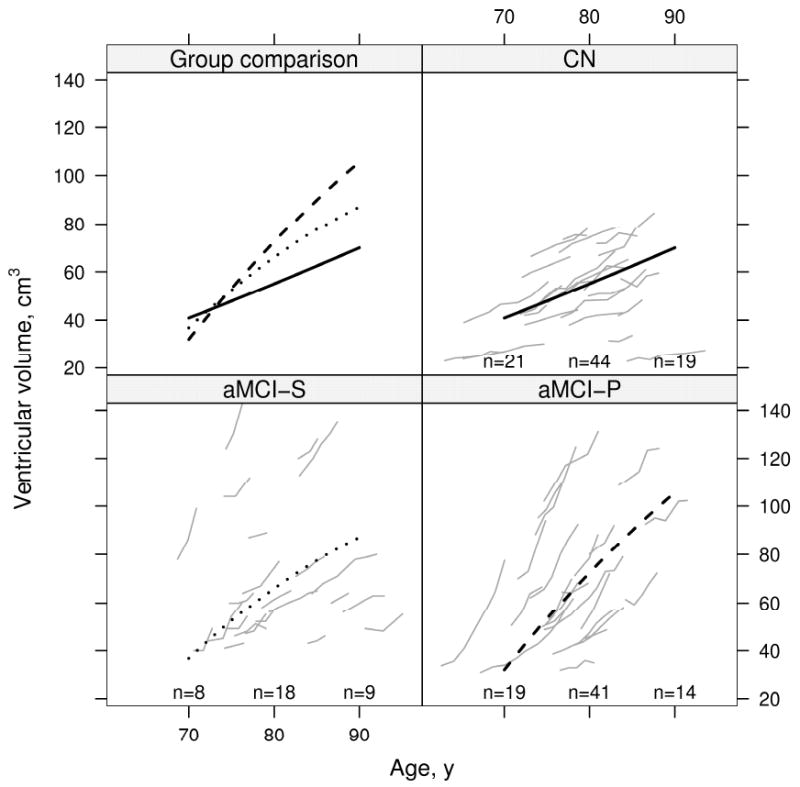
Change in ventricular volume with age by group. Thin gray lines represent individual subject volumes over time. The solid black line represents estimated average volume for CN, the dotted black line represents estimated average volume for aMCI-S, and the dashed black line represents estimated average volume for aMCI-P. Average volumes are shown assuming a woman with a total intracranial volume of 1.4 L. To better see individual trajectories, random subsets of 23 subjects in the CN and aMCI-P are shown. The number of subjects included in the analysis within 5 years of 70 years, 80 years, and 90 years are indicated within the panel.
Figure 5.
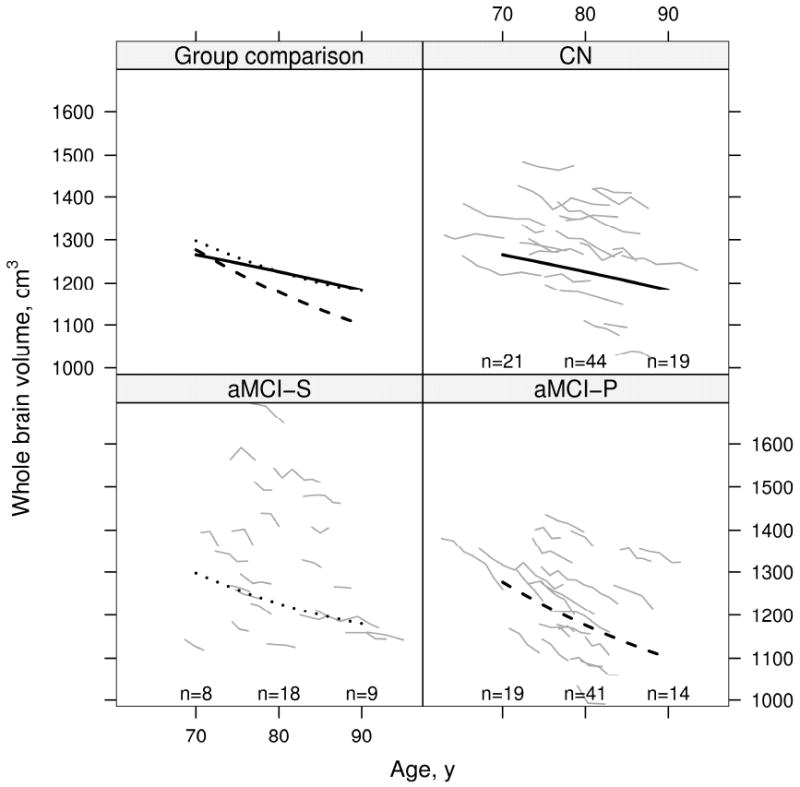
Change in brain volume with age by group. Thin gray lines represent individual subject volumes over time. The solid black line represents estimated average volume for CN, the dotted black line represents estimated average volume for aMCI-S, and the dashed black line represents estimated average volume for aMCI-P. Average volumes are calculated assuming a woman with a total intracranial volume of 1.4 L. To better see individual trajectories, random subsets of 23 subjects in the CN and aMCI-P are shown. The number of subjects included in the analysis within 5 years of 70 years, 80 years, and 90 years are indicated within the panel.
Table 4.
P-values for hypothesis tests of an overall age effect, a quadratic age effect, and a linear age effect by group
| Hypothesis tested | Ventricular vol. | Whole brain vol. |
|---|---|---|
| Cognitively normal | ||
| Volume associated with age* | <0.001 | <0.001 |
| Quadratic age effect† | 0.73 | 0.48 |
| Linear age effect‡ | <0.001 | <0.001 |
| aMCI stables | ||
| Volume associated with age* | <0.001 | <0.001 |
| Quadratic age effect† | 0.08 | 0.08 |
| Linear age effect‡ | <0.001 | <0.001 |
| aMCI - progressors | ||
| Volume associated with age* | <0.001 | <0.001 |
| Quadratic age effect† | <0.001 | <0.001 |
| Linear age effect‡ | <0.001 | <0.001 |
Two degree of freedom test of no association with age versus the quadratic model
Single degree of freedom test comparing linear and quadratic model
Single degree of freedom testing linear age effect when quadratic term omitted from the model
Results of the mixed model repeated measures analysis of cognitive test performance by age for all three clinical groups are displayed graphically for AVLT summed learning over trials 1-5, CDR-SOB, and MMSE. On the AVLT (Fig 6), CN subjects' performance improved slightly from baseline and then remained flat; aMCI-S subjects' performance was flat from 70 to 80 years and then declined slightly; aMCI-P subjects' performance declined starting at baseline. On the CDR-SOB (Fig 7) CN subjects' performance was flat as expected; aMCI-S subjects' performance declined minimally; and aMCI-P subjects' performance declined dramatically starting at baseline. On the MMSE (Fig 8) CN subjects' performance remained flat; aMCI-S subjects performance appears to have improved minimally; and aMCI-P subjects performance declined dramatically starting at baseline. There was evidence of accelerating declines in MMSE among aMCI-P subjects (p=0.006) but no evidence of accelerating declines on the other two measures. Age based mixed model coefficients are reported in online supplemental Tables E 5 – 7 for AVLT, CDR-SOB, and MMSE.
Figure 6.
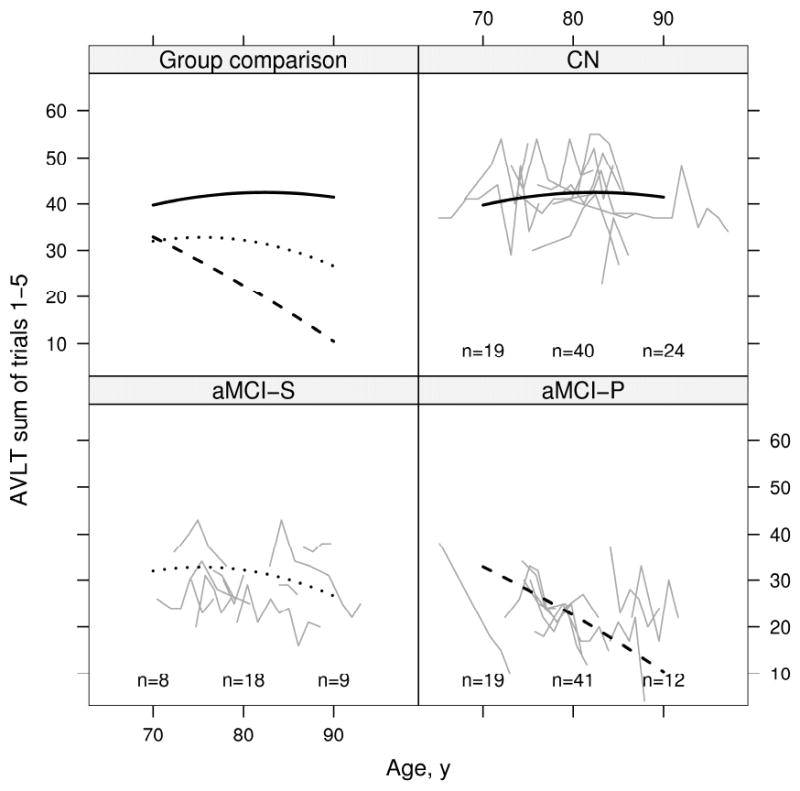
Change in AVLT sum of words learned on trials 1 to 5 with age by group. Thin gray lines represent individual subject scores over time. The solid black line represents the estimated average for CN, the dotted black line represents the estimated average for aMCI-S, and the dashed black line represents the estimated average for aMCI-P. Average scores are calculated assuming a woman with 12 years of education whose index scan is at age 79. To better see individual trajectories, random subsets of 10 subjects in each group are shown. The number of subjects included in the analysis within 5 years of 70 years, 80 years, and 90 years are indicated within the panel.
Figure 7.
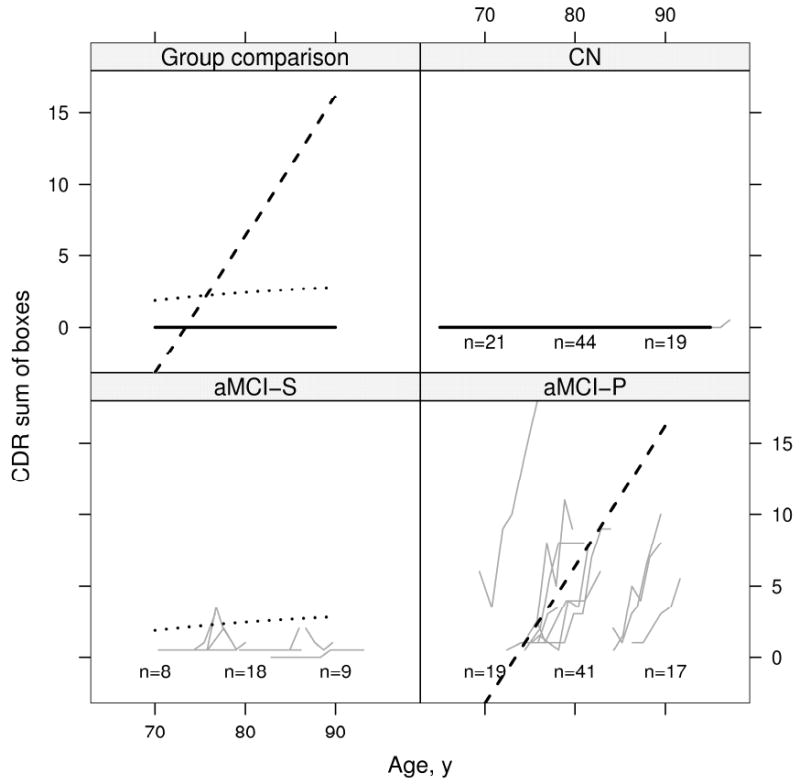
Change in CDR sum of boxes with age by group. Thin gray lines represent individual subject scores over time. The solid black line represents the estimated average for CN (zero by definition), the dotted black line represents the estimated average for aMCI-S, and the dashed black line represents the estimated average for aMCI-P. Average scores are calculated assuming a woman with 12 years of education whose index scan is at age 79. To better see individual trajectories, random subsets of 10 subjects in each group are shown. The number of subjects included in the analysis within 5 years of 70 years, 80 years, and 90 years are indicated within the panel.
Figure 8.
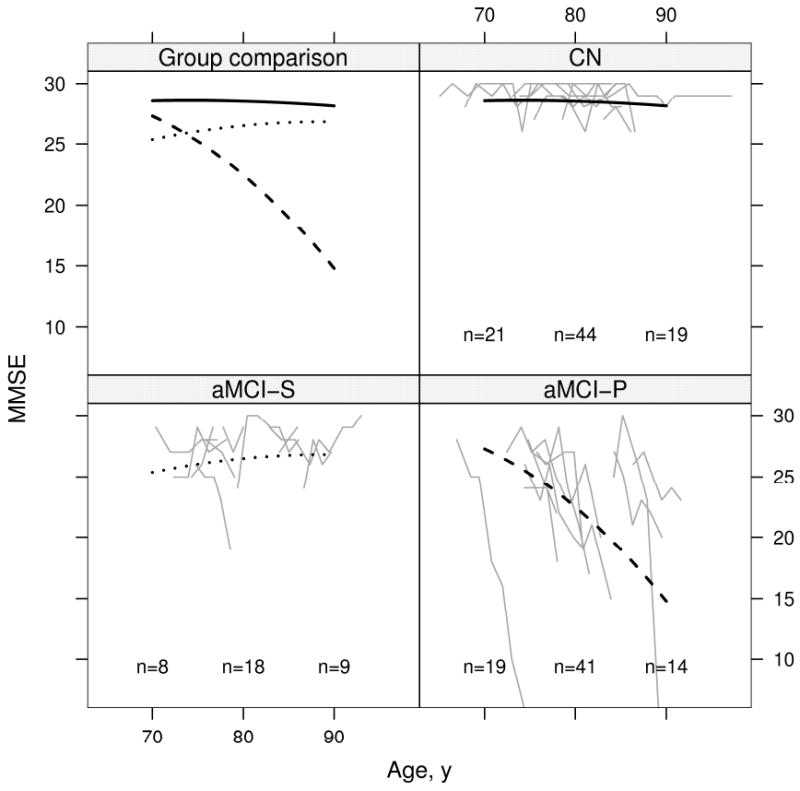
Change in MMSE with age by group. Thin gray lines represent individual subject scores over time. The solid black line represents the estimated average for CN, the dotted black line represents the estimated average for aMCI-S, and the dashed black line represents the estimated average for aMCI-P. Average scores are calculated assuming a woman with 12 years of education whose index scan is at age 79. To better see individual trajectories, random subsets of 10 subjects in each group are shown. The number of subjects included in the analysis within 5 years of 70 years, 80 years, and 90 years are indicated within the panel.
The effect of APOE ε4 carrier status on ventricular and brain rates by age was assessed in aMCI subjects (aMCI-P and aMCI-S combined). These data are displayed graphically in Fig 9. Volume change with age depended on APOE genotype in ventricle (p=0.005) and brain (p=0.015) with rates of both brain shrinkage and ventricular expansion overall greater in aMCI APOE ε4 carriers than non-carriers. Among APOE ε4 carriers we observed significant deceleration in rates with increasing age in ventricular volume (p<0.001) and brain (p<0.001). Among non-carriers, we observed deceleration for the brain (p=0.013) but not the ventricle (p=0.19)
Figure 9.
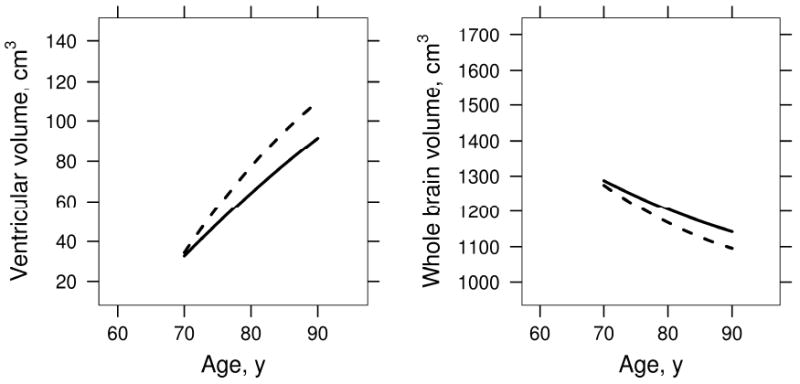
Effect of APOE ε4 on rates in aMCI subjects. Estimated volume by age for APOE ε4 non-carriers (solid line) vs. carriers (dashed line) among aMCI-S and aMCI-P subjects combined.
Discussion
Due to its progressive nature, a comprehensive picture of AD is best obtained through longitudinal observation. The rate at which various features of the disease progress with time is a topic of considerable research interest. Progression to a diagnosis of AD is a meaningful clinical event shared by all aMCI-P subjects in this study. Therefore, analyzing brain and ventricular volumes relative to this index point is a logical way to anchor the series of serial MRI studies in aMCI-P subjects to a common clinically definable point in the natural history of the disease. A major objective of our study was to ascertain if rates of brain shrinkage and ventricular expansion change as individuals progressed from aMCI to a clinical diagnosis of AD. We found that both brain and ventricular rates accelerate during this portion of the disease course. This observation fits with some clinical observations of acceleration in the rate of cognitive decline in AD as the disease progresses (48-50). As noted in (20), the anatomic substrate of acceleration in atrophy rates could be due to true acceleration in already affected areas, involvement of new areas, or both.
To our knowledge, previous studies examining rate acceleration with three or more serial scans per subject have evaluated subjects with early onset AD, most of whom had known pathogenic mutations (20, 21). One study (20) evaluated rates of brain loss with the boundary shift integral in 12 patients and another (21) from the same center modeled rates of brain and hippocampal atrophy in 9 patients. Both hippocampal and brain atrophy rates accelerated relative to index points defined using either a fixed MMSE score or a diagnosis of dementia (20, 21). While the mean ages of subjects in these studies (47 years and 43 years) (20, 21) were substantially younger than our aMCI-P subjects (79 years), the finding of acceleration in rates as subjects progressed to dementia was similar. However, linear rate estimates in terms of percentage brain volume lost per year are considerably higher in familiar young onset cases – on the order of 2% to 3% of brain volume lost per year in (20). In contrast, annualized rates of atrophy in elderly aMCI-P subjects from our center are 0.8% per year (12, 13). Comparing the data in young familial onset disease (20, 21) with our data in late onset cases leads to the conclusion that the qualitative nature of the disease in the window of time where subjects progress to dementia is similar regardless of age of onset. But, the quantitative nature of disease (ie absolute linear rates) is more aggressive in younger onset disease.
As with MRI data, rates of decline in CDR-SOB and MMSE performance accelerated as aMCI subjects progressed to AD, while rates of decline on AVLT summed learning did not accelerate significantly. In addition, if one compares the individual trajectories in cognitive performance vs time relative to index scan in Fig 3 with MRI volumes in Fig 2, it appears as though there is greater random variation in the cognitive measures compared to the MRI measures, particularly for the AVLT, although we did not explicitly compare stability of the downward trend in MRI vs. cognitive tests.
Analysis of volume vs time relative to the index point at which aMCI subjects progress to a diagnosis of AD is sensible in aMCI-P subjects, however there is no similar common clinical event with which to anchor CN or aMCI-S subjects in time. Hence in a second analysis we estimated change in brain and ventricular volume as a function of age in all three clinical groups. Among all three clinical groups, aMCI-P, aMCI-S, and CN, ventricular volume increased and brain volume decreased with advancing age (Figs 4 and 5; Tables 4 and 5). This result was expected and is consistent with earlier longitudinal and cross sectional imaging studies. However, the rate of ventricular expansion and brain shrinkage slowed with advancing age – i.e. atrophy rates were less in older aMCI-P subjects with a similar trend in aMCI –S subjects. This presents an apparent paradox. From the volume vs index date modeling we conclude that rates of atrophy accelerate with time as individual subjects progress from aMCI to AD. From the volume vs age modeling we conclude that rates of atrophy are less in older than younger aMCI-P subjects. One possible explanation is that this is the result of selection bias, as these subjects were not drawn randomly from an epidemiologically defined sample. However we do note that demographic and cognitive performance differences between aMCI subjects analyzed in this study and the larger pool of all aMCI subjects with at least one MRI study in the ADPR/ADRC data base were minimal. Another way to resolve this apparent paradox is to propose that our younger aMCI-P subjects on average had a more biologically aggressive disease course than our older aMCI-P subjects. This is consistent with observations of some clinicians that younger onset subjects have a more aggressive clinical course. As noted in the paragraph above, if we compare rates of percentage brain volume lost per year in our 79 year old aMCI-P subjects to young onset familial AD subjects in (20, 21) we find faster rates in the younger AD subjects. The trend toward slower rates in older aMCI-S subjects in our study could be attributed to the same phenomenon – ie more aggressive disease in younger onset subjects. This assumption implies that at least a proportion of aMCI subjects who did not progress were actually progressing and at some point a proportion of the aMCI-S group will progress to AD. Further longitudinal follow up is necessary to confirm this however. It is important to note that in labeling subjects aMCI-S, we do not imply that these subjects will not develop AD in their lifetimes, rather that they did not over the entire observation period currently available in each.
Mixed model repeated measures analysis of cognitive test performance by age was qualitatively similar for AVLT summed learning, CDR-SOB, and MMSE. CN and aMCI-S subjects' performance remained relatively flat while aMCI-P subjects' performance displayed a noticeable but decline starting at baseline. It is interesting that while the rate of brain and ventricular atrophy slows in older aMCI-P subjects, no slowing with age is seen in either AVLT or CDR-SOB and the rate of decline in MMSE accelerates slightly. We note that in contrast to the volumetric measurements, subjects' cognitive performance was used to assign clinical diagnosis. Therefore, it is not surprising that the longitudinal pattern on cognitive tests differs markedly between aMCI-S and aMCI-P subjects.
The number of CN subjects in our study is too small to draw sweeping conclusions about normal aging. However, among CN subjects the rates of brain shrinkage and ventricular expansion did not change with age (as indicated by the linear volume change over time). The relationship between brain size and age in non-demented individuals has been addressed through cross sectional studies of individuals spanning various age ranges. Many cross sectional studies of aging find decreasing volumes of brain, ventricle, or specific regions-of-interest with advancing age (41, 51-54). The effect of age on brain size has also been addressed in studies which employ pairs of scans, from which linear rates are measured, acquired in individuals spanning various age ranges (55-58). Some of these studies indicate a linear decline in brain, ventricle, or regional volume with age in non-demented subjects while others indicate acceleration with advancing age, often at an inflection point in middle or older age. Interaction of rates of atrophy with risk factors, such as hypertension, has also been described (55). Although, there is no evidence that differences in rates among groups in our study are due to differences in prevalence of lacunar infarcts or white matter hyper intensity load.
A strength of this study was the fact that we were able to acquire serial MRI data using consistent methods for a period extending over a decade. This same feature however also imposes a potential limitation. Over 10 years, multiple software upgrades are unavoidable. And the scanners used in this study were no exception. Fortunately, both the gradient hardware and transmit-receive head coils used on the scanners in this study were unchanged throughout the duration of the data collection. In addition, a rigorous daily quality control procedure was in place throughout the study. This consisted of a standardized daily calibration procedure which monitors geometric fidelity over a 200 mm volume along all 3 cardinal axes, signal to noise ratio, and transmit gain. Nonetheless, we acknowledge the inevitable noise which must have been introduced into our data given the long time period over which the MRI studies were obtained.
The proportion of APOE ε4 carriers was greatest in our aMCI-P and least in CN subjects as would be expected. This is consistent with the well established fact that AOPE 4 increases the risk of developing AD (59). Had we examined rates of atrophy as a function of APOE ε4 carrier status across all clinical groups combined, the results would have been a forgone conclusion since both the proportion of APOE ε4 carries and atrophy rates were highest among MCI-P and lowest among CN. We therefore analyzed the effect of APOE ε4 carrier status within the aMCI subjects (aMCI-P and aMCI-S) combined. The rational for this is that at the beginning of any longitudinal study, the eventual clinical outcome (progressor vs. stable) of individual aMCI subjects is unknown. Findings in our study on the relationship between MRI rates and APOE ε4 status are relevant to planning of future studies (both therapeutic and observational) that employ brain volumes as an outcome measure. Some implications are that studies should be powered based on an anticipated APOE ε4 effect on atrophy rates; therapeutic studies using MRI as an outcome measures could be designed to balance APOE ε4 status across treatment groups; and a non-linear effect of APOE ε4 on brain rates might be anticipated. Our finding of higher rates of atrophy in APOE ε4 carriers is consistent with some literature (60). However the literature itself is not consistent. Some reports indicate that APOE ε4 increases risk of AD but not the rate of clinical progression (61-65). In fact some publications indicate that the rate of clinical progression is slower in APOE ε4 carriers than non-carriers (66, 67).
Brain atrophy measures from serial MRI studies have most commonly been treated as a linear function in past studies - i.e. a line is fit to scans acquired at two time points. However, our data indicate that while the deviation from linearity is relatively minor, rates of both brain shrinkage and ventricular expansion do accelerate in individual aMCI subjects who progress to AD. Using a linear estimate for a nonlinear function will tend to be invalid when comparing rates of change across subjects who either have different inter-scan intervals, different intrinsic rates of change, or both. Our data indicate that such errors should be fairly minor in studies of elderly aMCI subjects when the inter-scan interval is limited to a year or so. In future studies however it might be useful to incorporate non-linearity in models of longitudinal morphometric change, especially when modeling change over periods exceeding 1-2 years. We do note that AD is a disease which evolves over decades. Conclusions drawn from our analyses are relevant only to the relatively narrow window in the overall progression of the disease that we examined. And our conclusions about rates and change in rate with time should not be extrapolated outside the range that we examined in the overall course of the disease.
Supplementary Material
Acknowledgments
The National Institute on Aging - AG11378, AG16574, AG06786, and The Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program
References
- 1.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 2.Hyman B. The neuropathological diagnosis of Alzheimer's disease: clinical-pathological studies. Neurobiology of Aging. 1997;18(4 Suppl):S27–32. doi: 10.1016/s0197-4580(97)00066-3. [DOI] [PubMed] [Google Scholar]
- 3.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 4.Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 1996;119:2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 5.Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999;52:1687–1689. doi: 10.1212/wnl.52.8.1687. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Petersen RC, Xu Y, O'Brien PC, Smith Ge, Ivnik RJ, et al. Rates of Hippocampal Atrophy in Normal Aging, Mild Cognitive Impairment, and Alzheimer's Disease. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teipel SJ, Bayer W, Alexander GE, et al. Progression of corpus callosum atrophy in Alzheimers disease. Arch Neurol. 2002;59:243–248. doi: 10.1001/archneur.59.2.243. [DOI] [PubMed] [Google Scholar]
- 9.Du AT, Schuff N, Kramer JH, et al. Higher atrophy rate of entorhinal cortex than hippocampus in AD. Neurology. 2003;60:481–486. doi: 10.1212/01.wnl.0000106462.72282.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du AT, Schuff N, Zhu XP, et al. Atrophy rates of entorhinal cortex in AD and normal aging. Neurology. 2003;60:481–486. doi: 10.1212/01.wnl.0000044400.11317.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR, Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, et al. The rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack CR, Jr, Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR, Jr, Shiung MM, Weignad SD, O'Brien PC, Gunter JL, Boever BF, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer's disease. Journal of Magnetic Resonance Imaging. 1997;7:1069–75. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- 15.Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans on Medical Imaging. 1997;15:623–629. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- 16.Laakso MP, Lehtovirta M, Partanen K, Riekkinen PJ, Soininen H. Hippocampus in Alzheimer's disease: A 3-year followup MRI study. Biol Psychiatry. 2000;47:557–561. doi: 10.1016/s0006-3223(99)00167-5. [DOI] [PubMed] [Google Scholar]
- 17.Kaye JA, Swihart T, Howieson D, Dame A. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 18.Kaye JA, Mueller EA, Moore MM, et al. Healthy elderly up to the tenth decade of life lose little brain volume when assessed longitudinally. Neurology. 1998;50(4) 4:A438. [Google Scholar]
- 19.Thompson PM, Hayashi KM, de Zubicaray G, et al. Dynamics of gray matter loss in Alzheimer's disease. Journal of Neuroscience. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan D, Janssen JC, Whitwell JL, Watt HC, Jenkins R, Frost C, et al. et al. Change in rates of cerebral atrophy over time in early-onset Alzheimer's disease: longitudinal MRI study. The Lancet. 2003;362:1121–22. doi: 10.1016/S0140-6736(03)14469-8. [DOI] [PubMed] [Google Scholar]
- 21.Ridha BH, Barnes J, Bartlett J, Godbolt AK, Pepple T, Rossor MN, et al. Tracking atrophy progression in familial Alzheimer's disease: a serial MRI study. Lancet Neurol. 2006;5:828–34. doi: 10.1016/S1474-4422(06)70550-6. [DOI] [PubMed] [Google Scholar]
- 22.Petersen RC, Kokmen E, Tangalos EG, et al. Mayo Clinic Alzheimer's Disease Patient Registry. Aging. 1990;2:408–415. doi: 10.1007/BF03323961. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 24.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's older Americans normative studies: WAIS-R, WMS-R, and AVLT norms for ages 56 through 97. The Clinical Neuropsychologist. 1992;6:1–104. [Google Scholar]
- 25.Wechsler D, editor. Wechsler Memory Scale-Revised. Psychological Corp; Harcourt Brace Janvanovich, Inc.; New York, NY: 1987. [Google Scholar]
- 26.Rey A. L'examen Clinique en Psychologie. Paris: Presses Universitaires de Frances; 1964. [Google Scholar]
- 27.Grober E, Buschke H. Genuine memory deficits in dementia. Developmental Neuropsychology. 1987;3:13–36. [Google Scholar]
- 28.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 29.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini Mental State”: A practical method for grading the cognitive state of patients for the clinician”. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Mattis S. Mental Status Examination for Organic Mental Syndromes in the Elderly Patient. In: Karasu TE, Bellak l, editors. Geriatric Psychiatry. Grune and Stratton; New York: 1976. [Google Scholar]
- 32.Kokmen E, Smith GE, Petersen RC, et al. The short test of mental status: correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 33.Rydberg JN, Hammond CA, Grimm RC, et al. Initial clinical experience in MR imaging of the brain with a fast fluid-attenuated inversion-recovery pulse sequence. Radiology. 1994;193:173–180. doi: 10.1148/radiology.193.1.8090888. [DOI] [PubMed] [Google Scholar]
- 34.Rydberg JN, Riederer SJ, Rydberg CH, et al. Contrast optimization of fluid-attenuated inversion recovery (FLAIR) imaging. MRM. 1995;34:868–877. doi: 10.1002/mrm.1910340612. [DOI] [PubMed] [Google Scholar]
- 35.Jack CR, Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements for MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- 36.Jack CR, Jr, Petersen RC, O'Brien PC. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 37.Gunter JL, Shiung MM, Manduca A, Jack CR., Jr Methodological considerations for measuring rates of brain atrophy. JMRI. 2003;18:16–24. doi: 10.1002/jmri.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 39.Prins ND, van Straaten ECW, van Dijk EJ, Simoni M, van Schijndel RA, Vrooman HA, et al. Measuring progression of cerebral white matter lesions on MRI: Visual rating and volumetrics. Neurology. 2004;62:1533–1539. doi: 10.1212/01.wnl.0000123264.40498.b6. [DOI] [PubMed] [Google Scholar]
- 40.Scheltens P, Barkhof F, Leys D, Pruvo JP, P NJJ, Vermersch P, et al. A semiquantitive rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. Journal of the Neurological Sciences. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- 41.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Bryan RN, Manolio TA, Schertz LD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the Cardiovascular Health Study. AJNR. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 43.Jack CR, Jr, Rettman DW, O'Brien PC, Shiung MM, Xu Y, Muthupillai R, et al. FLAIR-histogram-segmentation for measurement of leukoaraiosis volume. JMRI. 2001;14:668–676. doi: 10.1002/jmri.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, NJ: Wiley-Interscience; 2004. [Google Scholar]
- 45.Help and Documentation. Editor. 2004, SAS Institute Inc.: Cary, NC. p. SAS 9.1.3.
- 46.Development Core Team, editor. A language and environment for statistical computing. Foundation for Statistical Computing; Vienna, Austria: 2006. [Google Scholar]
- 47.Pinheiro JC, Bates DM, DebRoy S, Sarkar D. nlme: Linear and nonlinear mixed effected models. 2006:1–78. [Google Scholar]
- 48.Teri L, McCurry SM, Edland SD, Kukull WA, Larson EB. Cognitive decline in Alzheimer's Disease: A longitudinal investigation of risk factors for accelerated decline. Journal of Gerontology: Medical Sciences. 1995;50A(1):M49–M55. doi: 10.1093/gerona/50a.1.m49. [DOI] [PubMed] [Google Scholar]
- 49.Storandt M, Grant EA, Miller P, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer's disease. Neurology. 2002;59:1034–1041. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]
- 50.Doody RS, Massman P, Dunn JK. A method for estimating progression rates in Alzheimer Disease. Arch Neurol. 2001;58:449–454. doi: 10.1001/archneur.58.3.449. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiology of Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- 52.Blatter DD, Bigler ED, Gale SD, et al. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- 53.Riello R, Sabattoli F, Beltramello A, et al. Brain volumes in healthy adults aged 40 years and over: a voxel-based morphometry study. Aging Clin Exp Res. 2005;17:329–336. doi: 10.1007/BF03324618. [DOI] [PubMed] [Google Scholar]
- 54.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DA, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 56.Resnick SM, Goldszal AF, Davatziko C, Golski S, Kraut MA, Metter EJ, et al. One-year age changes in MRI brain volumes in older adults. Cerebral Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 57.Liu RSN, Lemieux L, Bell Gs, Sisodiya SM, Shorvon SD, S SJWA, et al. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage. 2003;20:22–33. doi: 10.1016/s1053-8119(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 58.Mueller EA, Moore MM, Kerr DCR, Sexon G, Camicioli R, Howieson DB, et al. Brain volume preserved in healthy elderly through the eleventh decade. Neurology. 1998;51(6):1555–1562. doi: 10.1212/wnl.51.6.1555. [DOI] [PubMed] [Google Scholar]
- 59.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high acidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer's disease. Proc Natl Aca Sci. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adak S, Illouz K, Gorman W, Tandon R, Zimmerman EA, Moore MM, et al. Predicting the rate of cognitive decline in aging and early Alzheimer disease. Neurology. 2004;63:108–114. doi: 10.1212/01.wnl.0000132520.69612.ab. [DOI] [PubMed] [Google Scholar]
- 61.Gomez-Isla T, West HL, Rebeck GW, et al. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer's disease. Ann Neurol. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- 62.Growdon J, Locasio J, Corkin S, et al. Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer's disease. Neurology. 1996;47:444–448. doi: 10.1212/wnl.47.2.444. [DOI] [PubMed] [Google Scholar]
- 63.DeCarli C, Mungas D, Reed HB, Weiner M, Chui HC, Jagust W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Convit A, de Asis J, de Leon MJ, Tarshish CY, De Santi S, Rusinek H. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer's disease. Neurobiology of Aging. 2000;21:19–26. doi: 10.1016/s0197-4580(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 65.Marquis S, Moore MM, Howieson DB, Sexton G, Payami H, Kaye JA, et al. Independent predictors of cognitive decline inhealthy elderly persons. Arch Neurol. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 66.Stern Y, Brandt J, Albert M, et al. The absence of an apolipoprotein epsilon4 allele is associated with a more aggressive form of Alzheimer's disease. Annals of Neurology. 1997;41:615–20. doi: 10.1002/ana.410410510. [DOI] [PubMed] [Google Scholar]
- 67.Frisoni GA, Govoni S, Geroldi C, Bianchetti A, Calabresi L, Franceschini G, et al. Gene dose of the E4 allele of apolipoprotein E and disease progression in sporadic late-onset Alzheimer's disease. Ann Neurol. 1995;37:596–604. doi: 10.1002/ana.410370509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


