Abstract
The transcription factor, ΔFosB, accumulates in a region-specific manner in brain in response to many types of chronic stimulation due to the unusual stability of the protein. The phosphorylation of Ser27 in ΔFosB has been shown to promote this stability in vitro. We show here that this phosphorylation reaction is also important for ΔFosB’s stability in the brain in vivo and for the unique behavioral plasticity mediated by this transcription factor.
Keywords: nucleus accumbens, cocaine, casein kinase 2, viral gene transfer
ΔFosB, a truncated product of the fosB gene, is a member of the Fos family of transcription factors. Unlike all other Fos family proteins, however, which are induced rapidly but transiently in brain in response to diverse types of stimuli (Morgan and Curran, 1995), stable isoforms of ΔFosB accumulate gradually during a course of chronic stimulation and then persist in brain for many weeks due to the unusual stability of these isoforms (Chen et al., 1997; Hiroi et al., 1997; Alibhai et al., 2007). This imbues ΔFosB with the unique ability to function as a sustained molecular switch, that is, to mediate transcriptional changes that occur gradually in response to some chronic stimulus and persist for relatively long periods of time after removal of the stimulus. Such a role for ΔFosB has been demonstrated for several types of stimuli, including drugs of abuse, stress, natural rewards, antipsychotic drugs, and neuronal lesions, to name a few (for review, see Cenci, 2002; McClung et al., 2004; Nestler, 2008). Recent work in cultured cells in vitro has suggested that the phosphorylation of ΔFosB at Ser27 by casein kinase 2 (ck2) may be one mechanism contributing to ΔFosB’s unique stability (Ulery et al., 2006), however, the relevance of this mechanism to the in vivo situation has remained unknown. We now provide direct evidence that phosphorylation of ΔFosB on Ser27 is important for the protein’s stability in vivo and, in turn, for its persistent behavioral effects.
EXPERIMENTAL PROCEDURES
To study the effect of Ser27 phosphorylation on the stability of ΔFosB within the brain in vivo, we generated herpes simplex virus (HSV) vectors encoding either wild-type ΔFosB (HSV-ΔFosB) or altered forms of ΔFosB with Ser to Ala [HSV-ΔFosB(Ser27Ala)] or Ser to Glu [HSV-ΔFosB(Ser27Glu)] mutations. The Ser to Ala mutation obliterates Ser27 phosphorylation, whereas the Ser to Glu mutation is a “phosphomimetic” alteration in that the negatively charged Glu residue can mimic a phospho-Ser residue as demonstrated for many phosphoproteins (Greengard, 2001). The HSV vectors were then injected into the nucleus accumbens of male C57Bl/6 mice (initial weight ~30 g), the brain region where drugs of abuse dramatically induce ΔFosB (McClung et al., 2004), and the animals were analyzed immunohistochemically 3, 6, 10, or 14 days thereafter for levels of ΔFosB in this brain region using published methods (Perrotti et al., 2008). HSV vectors are ideal for this type of in vivo time course experiment because the vectors infect neurons relatively quickly, with maximal levels of transgene expression seen within 12 h of injection, but transiently, with transgene levels reverting to normal within 5 days (Barrot et al., 2002).
RESULTS
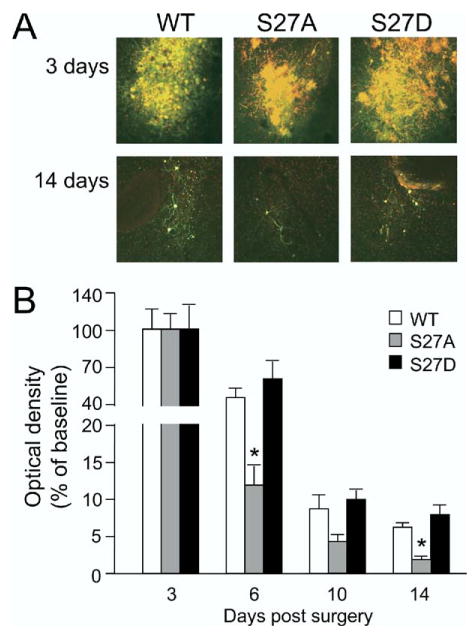
The question we first addressed was: Does mutation of Ser27 with Ala or Glu alter the persistence of ΔFosB in brain? As shown in Fig. 1, all three vectors induced roughly comparable levels of ΔFosB protein within the site of the injection as evaluated on day 3, when levels of transgene expression are still at maximal levels. By days 6–14, levels of ΔFosB expression decayed appreciably in all three groups. However, at these later time points, clear differences were observed in persisting expression of ΔFosB among the treatment groups. Wild-type ΔFosB showed significant levels of expression at days 6, 10, and 14. This contrasts dramatically with all other proteins expressed by these HSV vectors to date, all of which fully dissipate to non-detectable levels within 5–7 days (e.g. see Barrot et al., 2002; Bolaños et al., 2003; Zachariou et al., 2003; Green et al., 2006, 2008; Renthal et al., 2007; Russo et al., 2007). Since ΔFosB mRNA levels fully dissipate, like the other mRNAs, within 5 days (data not shown), this finding demonstrates that ΔFosB protein per se is more stable in brain than the other proteins investigated, which include numerous transcription factors: CREB (cAMP response element binding protein), CREMτ (cAMP response element modulator-τ), ICER (inducible cAMP early repressor), ATF2 (activating transcription factor-2), ATF3, ATF4, and JunD, as well as other signaling proteins, e.g. HDAC4 (histone deacetylase-4), HDAC5, HDAC9, RGS4 (regulator of G protein signaling-4), RGS9, PLCγ (phospholipase Cγ), ERK (extracellular signal regulated kinase), IRS2 (insulin receptor substrate-2), and AKT (akt thymoma viral oncogene).
Fig. 1.
Regulation of ΔFosB stability in vivo. HSV-ΔFosB (WT), HSV-ΔFosB(Ser27Ala) (S27A), or HSV-ΔFosB(Ser27Glu) (S27D) was injected bilaterally into the nucleus accumbens exactly as described (Barrot et al., 2002). Animals were perfused 3, 6, 10, or 14 days later, and fixed brains were sectioned and analyzed immunohistochemically for ΔFosB. Photomicrographs are representative findings for each vector; data in the bar graph are means±S.E.M. and expressed as % of day 3 (maximum) levels (n=4 animals in each group). * P<0.05 by t-test.
Interestingly, mutation of Ser27 to Ala decreased this persistent expression of ΔFosB, such that by day 6 (ANOVA; F=6.591, P<0.05) significantly less ΔFosB immunoreactivity was evident and this difference persisted through day 14 (ANOVA; F=5.618, P<0.05) after HSV-ΔFosB(Ser27Ala) injection (Fig. 1). In contrast, mutation of Ser27 to Glu did not affect ΔFosB’s stability in vivo, as levels of ΔFosB were comparable between HSV-ΔFosB-injected mice and HSV-ΔFosB(Ala27Glu)-injected mice. The reduced stability of ΔFosB in the Ser27Ala mutant provides the first evidence that Ser27 regulates ΔFosB’s stability in vivo. The lack of effect of the Ser to Glu mutant on ΔFosB stability indicates that mimicking phosphorylation at this site is sufficient to retain ΔFosB’s unique stability. The fact that the Ser27Glu mutant did not exhibit greater stability than wild-type ΔFosB suggests that phosphorylation of Ser27 may be saturating under normal conditions, consistent with the knowledge that ck2, which is responsible for Ser27 phosphorylation of ΔFosB (Ulery et al., 2006), is constitutively expressed at high levels in this brain region (Greengard, 2001). Future studies are needed to determine whether the Ser to Ala or Ser to Glu mutation, or the phosphorylation of Ser27 per se, changes the structural properties of the ΔFosB protein (Jorissen et al., 2007).
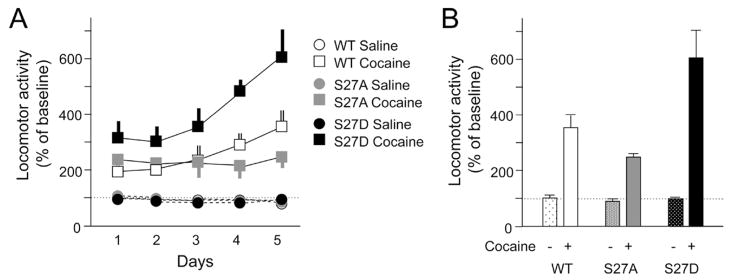
To study the functional significance of Ser27-mediated stability of ΔFosB, mice received bilateral intra-nucleus accumbens injections of HSV-ΔFosB, HSV-ΔFosB (Ser27Ala), or HSV-ΔFosB(Ser27Glu) and, beginning 5 days after surgery when transgene mRNA expression is back to baseline, mice were given daily i.p. injections of saline or cocaine (15 mg/kg) for 5 days (Fig. 2A, B). Mice overexpressing wild-type ΔFosB showed the expected phenotype: greater cocaine-induced locomotion after five daily doses of cocaine compared with responses after the first dose (ANOVA; F(4,24)=3.206, P<0.05). This reflects locomotor sensitization to repeated cocaine administration. In contrast, mice expressing the Ser to Ala mutant of ΔFosB showed no significant change between the first and last doses of cocaine (ANOVA; F(4,28)=1.229, P<0.1), reflecting the absence of locomotor sensitization. This loss of locomotor sensitization to cocaine in mice expressing a mutant form of ΔFosB which does not persist in brain (see Fig. 1) is consistent with prior observations that ΔFosB overexpression in this brain region of inducible bitransgenic mice increases locomotor sensitization (Kelz et al., 1999). In contrast to the Ser to Ala mutant, mice expressing the Ser to Glu mutant of ΔFosB exhibited significantly greater locomotor sensitization to cocaine compared with either wild-type or Ser27Ala ΔFosB. We observed a Drug×Mutation interaction (ANOVA=F(2,36)=57.95, P<0.05), and Newman Keuls post hoc analysis showed that Ser27Glu mice exhibited greater locomotor activity after the last dose of cocaine compared with all other groups (P<0.05). The observation that the Ser27Glu mutant form of ΔFosB exerted a greater effect on cocaine locomotor sensitization than wild-type ΔFosB, despite the fact that the two proteins are expressed at roughly comparable levels (Fig. 1), is consistent with recent findings in cell culture that phosphorylation of ΔFosB at Ser27 promotes the transcriptional activity of the protein at its AP-1 (activator protein-1) sites (Ulery and Nestler, 2007).
Fig. 2.
Regulation of the behavioral effects of cocaine by ΔFosB. Mice received bilateral intra-nucleus accumbens injections of HSV-ΔFosB (WT), HSV-ΔFosB(Ser27Ala) (S27A), or HSV-ΔFosB(Ser27Glu) (S27D) and, beginning 5 days after surgery, mice were given daily i.p. injections of saline or cocaine (15 mg/kg) and placed immediately in locomotor chambers with photocell beams for 5 days. Data are expressed as % of baseline beam breaks. (A) Locomotor responses over five consecutive days of cocaine exposure. (B) Locomotor responses on day 5. See text for statistical analysis (n=8 animals in each group).
DISCUSSION
The persistent expression of ΔFosB in the nucleus accumbens has been shown to be a crucial mechanism underlying sensitized responses to cocaine and other drugs of abuse (Kelz et al., 1999; Colby et al., 2003; McClung et al., 2004; Zachariou et al., 2006) as well as to natural rewards such as wheel running (Werme et al., 2002), food (Olausson et al., 2006; Wallace et al., 2008), and sex (Wallace et al., 2008). This persistent expression has been shown to correlate in previous studies with the enhanced stability of ΔFosB in cultured cells, however, the mechanism of ΔFosB’s stability in vivo has remained completely unknown. The results of the present study demonstrate that phosphorylation of ΔFosB at Ser27 is one important mechanism of this in vivo stability, which is critical for ΔFosB’s prolonged behavioral effects. These results raise the novel possibility that drugs aimed at blocking ΔFosB phosphorylation, for example, ck2 inhibitors, may be of use in the treatment of drug addiction or other compulsive disorders.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse and National Institute of Mental Health.
Abbreviations
- ATF
activating transcription factor
- ck2
casein kinase 2
- HDAC
histone deacetylase
- HSV
herpes simplex virus
References
- Alibhai IN, Green TA, Potashkin JA, Nestler EJ. Regulation of fosB and ΔfosB mRNA expression: in vivo and in vitro studies. Brain Res. 2007;1143:22–33. doi: 10.1016/j.brainres.2007.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Olivier JDA, Perrotti LI, Impey S, Storm DR, Neve RL, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños CA, Perrotti LI, Edwards S, Eisch AJ, Barrot M, Olson VG, Russell DS, Neve RL, Nestler EJ. Viral-mediated expression of phospholipase Cγ in distinct regions of the ventral tegmental area differentially modulates mood-related behaviors. J Neurosci. 2003;23:7569–7576. doi: 10.1523/JNEUROSCI.23-20-07569.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA. Transcription factors involved in the pathogenesis of L-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Amino Acids. 2002;23:105–109. doi: 10.1007/s00726-001-0116-4. [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic FRAs: Stable variants of ΔFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. ΔFosB enhances incentive for cocaine. J Neurosci. 2003;23:2488 –2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Hommel JD, DiLeone RJ, Kumar A, Theobald DE, Neve RL, Nestler EJ. Induction of ICER expression in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. J Neurosci. 2006;26:8235– 8242. doi: 10.1523/JNEUROSCI.0880-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Unterberg S, Neve RL, Ghose S, Tamminga CA, Nestler EJ. Induction of activating transcription factors ATF2, ATF3, and ATF4 in the nucleus accumbens and their regulation of emotional behavior. J Neurosci. 2008;28:2025–2032. doi: 10.1523/JNEUROSCI.5273-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Brown J, Haile C, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: Loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine’s psychomotor and rewarding effects. Proc Natl Acad Sci U S A. 1997;94:10397–10402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorissen H, Ulery P, Henry L, Gourneni S, Nestler EJ, Rudenko G. Dimerization and DNA-binding properties of the transcription factor deltaFosB. Biochemistry. 2007;46:8360–8372. doi: 10.1021/bi700494v. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang Y-J, Marotti L, Self DW, Tkatch R, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. ΔFosB: A molecular switch for long-term adaptation in the brain. Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Immediate-early genes: ten years on. Trends Neurosci. 1995;18:66–77. [PubMed] [Google Scholar]
- Nestler EJ. Transcriptional mechanisms of addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Tronson N, Neve R, Nestler EJ, Taylor FR. ΔFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196 –9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ. Distinct patterns of ΔFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, Xiao GH, Kumar A, Russo SJ, Graham A, Tsankova N, Kerstetter KA, Kippin TE, Neve RL, Haggarty SJ, McKinsey TA, Bassel-Duby R, Olson EN, Nestler EJ. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Bolaños CA, Theobald DE, DeCarolis NA, Renthal WR, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. The IRS2-Akt pathway in midbrain dopaminergic neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Ulery PG, Rudenko G, Nestler EJ. Regulation of ΔFosB stability by phosphorylation. J Neurosci. 2006;26:5131–5142. doi: 10.1523/JNEUROSCI.4970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulery PG, Nestler EJ. Regulation of ΔFosB transcriptional activity by ser27 phosphorylation. Eur J Neurosci. 2007;25:224–230. doi: 10.1111/j.1460-9568.2006.05262.x. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolaños CA. The influence of ΔFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thorén P, Nestler EJ, Brené S. ΔFosB regulates wheel running. J Neurosci. 2002;22:8133– 8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Georgescu D, Sanchez N, Rahman Z, DiLeone R, Berton O, Simon M, Neve RL, Sim-Selley LJ, Selley DE, Gold SJ, Nestler EJ. Essential role for RGS9 in opiate action. Proc Natl Acad Sci U S A. 2003;100:13656–13661. doi: 10.1073/pnas.2232594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, DiLeone RJ, Kumar A, Nestler EJ. ΔFosB: An essential role for ΔFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]