Abstract
Subclinical inflammation is a recently discovered phenomenon in type 2 diabetes. Elevated cytokines impair β-cell function and survival. A recent clinical trial shows that blocking IL-1β signaling by IL-1 receptor antagonist (IL-1Ra) improves β-cell secretory function in patients with type 2 diabetes. In the present study, we provide further mechanisms of the protective role of IL-1Ra on the β-cell. IL-1Ra prevented diabetes in vivo in C57BL/6J mice fed a high-fat/high-sucrose diet (HFD) for 12 wk; it improved glucose tolerance and insulin secretion. High-fat diet treatment increased serum levels of free fatty acids and of the adipokines resistin and leptin, which were reduced by IL-1Ra treatment. In addition, IL-1Ra counteracted adiponectin levels, which were decreased by high-fat feeding. Studies on isolated islets revealed that IL-1Ra specifically acted on the β-cell. IL-1Ra protected islets from HFD treated animals from β-cell apoptosis, induced β-cell proliferation, and improved glucose-stimulated insulin secretion. Insulin mRNA was reduced in islets from mice fed a HFD but normalized in the IL-1Ra group. Our results show that IL-1Ra improves β-cell survival and function, and support the potential role for IL-1Ra in the treatment of diabetes.
OBESITY IS A risk factor for insulin resistance and type 2 diabetes (T2DM). Most obese individuals are insulin resistant but compensate by increasing insulin secretion to maintain normoglycemia. The factors determining the amount of insulin that can be secreted are β-cell function as well as β-cell mass. T2DM manifests when the β-cell fails to adaptively increase insulin secretion as a result of impaired β-cell function (1,2,3,4), as well as decreased β-cell mass (5,6,7). The decrease in β-cell mass in both type 1 diabetes and T2DM can be attributed to the increase in frequency of β-cell apoptosis (5,8,9).
Once hyperglycemia is present, the loss of β-cells accelerates, accompanied by further impairment of β-cell secretory function, both factors in the development of T2DM (10).
The mechanisms of β-cell destruction in a diabetic milieu are complex. Studies on isolated islets show the interplay of glucotoxicity and lipotoxicity (11). In addition, the β-cells are particularly prone to oxidative stress due to their low expression of antioxidant molecules. Reactive oxygen species cause direct cellular damage in the β-cell by oxidizing nucleic acids and proteins (12), and inactivating genes that are involved in cellular defense (13,14). Furthermore, the β-cell is especially sensitive to inflammatory attack, and in vitro studies have shown that proinflammatory cytokines induce β-cell apoptosis and impair function (15).
In human islets we have provided evidence that glucose-induced β-cell apoptosis and dysfunction are partly mediated by β-cell production and secretion of the proinflammatory cytokine IL-1β (16). After chronic exposure to high glucose, the β-cell itself produces IL-1β, followed by nuclear factor-κB activation, Fas up-regulation, DNA-fragmentation, and impaired β-cell function. IL-1β has contributed to β-cell destruction in type 1 diabetes (reviewed in Refs. 17 and 18) as well as in T2DM (reviewed in Ref. 10). In addition, in three animal models, the Psammomys obesus (16), the Goto-Kakizaki rat (19), and the human islet amyloid polypeptide transgenic rat (20) (Butler, P., personal communication), pancreatic β-cell expression of IL-1β under hyperglycemic conditions has been observed. IL-1β signal transduction is initiated by ligand binding to type 1 IL-1 receptor, allowing docking of the IL-1R accessory protein (reviewed in Ref. 21). This activates downstream effectors, which regulate β-cell survival and function (reviewed in Refs. 18 and 22). A promising target to block these deleterious effects of IL-1β (23,24) as well as of elevated glucose levels (16) is the use of IL-1 receptor antagonist (IL-1Ra). IL-1Ra is an antiinflammatory cytokine and naturally occurring antagonist of IL-1α and β (25,26,27). Four forms of IL-1Ra have been described, three of them are intracellular proteins (icIL-1Ra I, II, and III), and one is secreted (sIL-1Ra) (28). Similarly to IL-1β, IL-1Ra binds to type 1 and 2 IL-1 receptor but lacks a second binding domain. Therefore, IL-1Ra does not recruit IL-1R accessory protein, the second component of the receptor complex. Endogenous production and secretion of sIL-1Ra have been shown to limit inflammation and tissue damage, but the biological effects of icIL-1Ra remain unclear. Exogenous sIL-1Ra has protected against IL-1β induced β-cell damage (23,24), and counteracted both low-dose streptozotocin-induced diabetes (29) and autoimmune diabetes (30), as well as promoted graft survival (30,31,32). In addition, IL-1Ra protected from glucose as well as IL-1β induced apoptosis in human islets (16). Therefore, the balance of IL-1β and IL-1Ra may play a crucial role in the pathogenesis of diabetes. We have recently shown that IL-1Ra is secreted from β-cells and is expressed in β-cell granules (33). Inhibition of endogenous IL-1Ra by culturing the islets with small interfering RNAs to IL-1Ra, or with leptin for the long term, leads to β-cell apoptosis and impaired function, providing a link from obesity to diabetes. The possible crucial role of inflammatory cytokines in the pathogenesis of T2DM is underscored by several recent studies (34). Spranger et al. (35) observed that individuals with elevated levels of IL-1β and IL-6 have a significantly increased risk of developing T2DM, pointing to a possible role for IL-1Ra in the treatment of diabetes. Results from a recent clinical study in patients with T2DM showed that IL-1Ra improved glycemic control and β-cell function (36). In the present study, we show that IL-1Ra is able to protect from diabetes progression induced by a high-fat diet.
Materials and Methods
Animals
For IL-1Ra or vehicle injections, C57BL/6J wild-type mice were obtained from Jackson Laboratories (Bar Harbor, ME) at 4 wk of age. One of the four treatments was initiated in mice at 5 wk of age. Two groups were kept on a normal diet (ND) (Harlan Teklad Rodent Diet 8604 containing 12.2, 57.6, and 30.2% calories from fat, carbohydrate, and protein, respectively; Harlan Teklad, Madison, WI). Another two groups were fed a high-fat/high-sucrose diet (HFD) (“Surwit,” containing 58, 26, and 16% calories from fat, carbohydrate, and protein, respectively; Research Diets, Inc., New Brunswick, NJ) (37). Half of the animals from each diet group were treated daily with IL-1Ra (IL-1Ra ND and IL-1Ra HFD; Kineret, ip injection at a dose of 10 mg/kg body weight; Amgen, Thousand Oaks, CA) or with vehicle as a control (control ND and control HFD). Throughout the study period of 12 wk, food consumption and body weight were measured weekly. Four independent experiments with a total of 16 mice (four mice per cage) in each group were performed. Transgenic mice overexpressing IL-1Ra (IL-1Ra-OE) were kindly provided by Dr. Emmet Hirsch [Northwestern University, Evanston, IL (38)]. Beginning at 5 wk of age and continuing for 16 wk, transgenic animals as well as wild-type littermates were fed a ND or HFD as described previously. Body weight and food intake were measured weekly. Four independent experiments with a total of 16 mice per group, respectively, were performed. All animals were housed four per cage in a temperature-controlled room with a 12-h light, 12-h dark cycle, and were allowed free access to food and water according to the protocol approved by the University of California Los Angeles Chancellor’s Animal Research Committee in agreement with the National Institutes of Health animal care guidelines.
Intraperitoneal glucose and insulin tolerance tests
After 4, 8, and 12-wk diet and IL-1Ra treatment, all animals underwent in vivo studies. For ip glucose tolerance tests (IPGTTs), mice were fasted 12 h overnight and injected ip with glucose (40%; Phoenix Pharmaceuticals Inc., St. Josephs, MO) at a dose of 2 mg/g body weight. Blood samples were obtained at time points 0, 15, 30, 60, 90, and 120 min for glucose measurements using a Glucometer (Freestyle; TheraSense Inc., Alameda, CA), and at time points 0 and 30 min for measurement of serum insulin levels. For ip insulin tolerance tests, mice were injected ip with 0.75 U/kg body weight recombinant human insulin (Novolin, Novo Nordisk) after 5-h fasting, and glucose concentration was determined with the Glucometer.
Islet isolation and culture
After 12-wk diet and treatment, mice were killed, blood was taken by cardiac puncture, and serum stored at −80 C until further analysis. Thereafter, islets from all groups were isolated as described previously (39). In brief, pancreata were perifused with a collagenase solution (Collagenase type 4 according to the manufacturer’s instructions; Worthington, Lakewood, NJ) and digested in the same solution at 37 C, followed by washing and handpicking. The islets were then cultured in RPMI 1640 medium containing 11.1 mm glucose, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum (Invitrogen Corp., Carlsbad, CA), hereafter referred to as culture medium, in a humid environment containing 5% CO2 before performing experiments. Islets were kept for 24 h in culture medium in suspension dishes before harvesting for islet sections or RNA extraction.
Glucose stimulated insulin secretion
Islets used to perform glucose-stimulated insulin secretion experiments were kept in culture medium on matrix-coated plates derived from bovine corneal endothelial cells (Novamed Ltd., Jerusalem, Israel) for 4 d, allowing the cells to attach to the dishes and spread (40). These conditions allowed direct comparison to our previous studies in human islets pretreated with IL-1Ra in vitro (16). For acute insulin release in response to glucose, islets were washed and preincubated (30 min) in Krebs-Ringer bicarbonate buffer (KRB) containing 2.8 mm glucose and 0.5% BSA. KRB was then replaced by KRB 2.8 mm glucose for 1 h (basal), followed by an additional 1 h in KRB 16.7 mm glucose. Islets were extracted with 0.18 n HCl in 70% ethanol for determination of insulin content. Islet insulin was determined using mouse insulin ELISA (ALPCO Diagnostics, Salem, NH).
Serum analysis
Serum obtained by cardiac puncture at killing was analyzed for serum lipids by the University of California Los Angeles Lipid and Lipoprotein Laboratory as described (41). All lipid assays were performed in triplicate determinations. An external control sample with known analyte concentration was run for each assay to ensure accuracy. Free plasma glycerol concentrations were also determined and used to correct the triglyceride (TG) values. Leptin and resistin were measured using a mouse serum adipokine panel (LINCOplex; LINCO Research, Inc., St. Charles, MO). Serum adiponectin and insulin concentrations were determined using mouse adiponectin and insulin ultrasensitive ELISA (ALPCO Diagnostics). Serum IL-1Ra levels were determined using mouse IL-1Ra Quantikine ELISA (R&D, Inc., Minneapolis, MN).
Pancreatic insulin and glucagon content
To determine the total pancreatic insulin and glucagon content, approximately 30 mg pancreatic tissue from eight mice per treatment group, respectively, was homogenized in 1 ml 0.18 N HCl in 70% ethanol and incubated overnight at 4 C. After centrifugation, supernatants were collected and stored at −80 C. Insulin concentrations were measured using mouse insulin ELISA (ALPCO Diagnostics), and glucagon levels were determined by glucagon EIA (ALPCO Diagnostics).
Adipocyte size
Epididymal fat pads were dissected and fixed overnight at 4 C in 4% paraformaldehyde, followed by washing in 30% sucrose/PBS for 12 h at 4 C. After 30-min incubation in a 1:1 mixture of 30% sucrose and cryomedium OCT (Tissue Tek; Sakura Finetek, Inc., Torrance, CA), the tissue was placed in 100% OCT for 30 min, embedded in plastic molds, and frozen on dry ice before sectioning. To determine adipocyte size, sections were stained with hematoxylin and eosin. Cross-sectional adipocyte area was measured by manual tracing of 300 or more cells per mouse and four to eight animals per treatment group using an Olympus IX70 inverted system microscope (Olympus America, Melville, NY) and Image-Pro Plus software (Media Cybernetics, Silver Springs, MD).
β-Cell mass and histochemical analyses
After 12-wk diet and treatment, pancreata were weighed and fixed overnight in 4% paraformaldehyde at 4 C under continuous shaking, followed by paraffin embedding, orienting pancreata such that sections were cut along the head-tail axis. To obtain sections from isolated islets, islets were washed with PBS, fixed in Bouin’s solution for 15 min, and resuspended in 2% melted agarose in PBS, followed by short centrifugation and paraffin embedding. To determine β- and α-cell mass, 10 sections (spanning the width of the pancreas) were deparaffinized, rehydrated, and incubated overnight at 4 C with guinea pig anti-insulin antibody (Dako Corp., Carpinteria, CA), followed by detection with a fluorescein-conjugated donkey antiguinea pig antibody (Dako). Subsequently, the specimens were labeled for glucagon with rabbit antiglucagon (Dako), followed by detection with donkey antirabbit Cy3-conjugated antibody (Dako). An image of each slide was captured using Openlab (Improvision Inc., Lexington, MA) and ImageJ software (National Institutes of Health, Bethesda, MD). Tissue areas were determined by marking the image for total tissue and for β-cells (fluorescein labeled) or α-cells (Cy3 labeled), respectively. β-Cell and α-cell mass was analyzed using Openlab software. The relative area of β-cells (green fluorescence) or α-cells (red fluorescence) was determined by quantification of the cross-sectional β-cell or α-cell area divided by the cross-sectional area of total tissue, respectively. The cell mass per pancreas was estimated as the product of the relative cross-sectional area of β-cells or α-cells per total tissue and the weight of the pancreas. For analysis of β-cell proliferation, mouse pancreas sections were deparaffinized, rehydrated, and incubated overnight at 4 C with rat antimouse Ki-67 (Dako), followed by detection with donkey-antirat Cy3-conjugated antibody (Dako). For detection of β-cell apoptosis, sections were incubated with 20 μg/ml proteinase K (Roche Diagnostics, Indianapolis, IN) for 15 min at 37 C, and apoptosis was analyzed by the terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling (TUNEL) technique according to the manufacturer’s instructions (In Situ Cell Death Detection Kit, TMR red; Roche Diagnostics). Subsequently, all sections were double stained for insulin as described previously. Same staining was performed on sections of isolated islets.
RNA extraction and quantitative RT-PCR analysis
Total RNA of isolated islets was extracted after overnight culture as described previously (42). Total RNA from epididymal fat pads was isolated using the RNeasy Lipid Tissue Kit (QIAGEN, Inc., Valencia, CA). For quantitative analysis we used the LightCycler Quantitative PCR System (Roche Diagnostics) with a commercial kit (LightCycler FastStart DNA Master plus SYBR Green I; Roche Diagnostics). Mouse primers used were: 5′-ttcttctacacaccca-3′ and 5′-ctagttgcagtagttct-3′ (insulin); 5′-gtccatgccatcactgccac-3′ and 5′-cagcaccagtggatgcaggg-3′ [glyceraldehyde-3-phosphate dehydrogenase (GAPDH)]; 5′-gttggccaggctggtgtccag-3′ and 5′-ctgtgatgagctgctcagggtgg-3′ (tubulin); 5′-ctttggctatgggcttccagtc-3′ and 5′-gcaaggaggacagagtttatcgtg-3′ (F4/80); 5′-ctggatagcctttcttctgctg-3′ and 5′-gcacactgtgtccgaactc-3′ (CD11c); 5′-acggcatggatctcaaagac-3′ and 5′-agatagcaaatcggctgacg-3′ (TNFα), 5′-gaccttccaggatgaggaca-3′ and 5′-agctcatatgggtccgacag-3′ (IL-1β); and 5′-ccagctcattgctgggtact-3′ and 5′-cagctgactcaaagctggtg-3′ (IL-1Ra).
Statistical analysis
Samples were evaluated in a randomized manner by a single investigator (N.S.S.) who was blinded to the treatment conditions. Data are presented as means ± se and were analyzed by the paired, Student’s t test or by ANOVA with a Bonferroni correction for multiple group comparisons.
Results
IL-1Ra has no impact on weight gain or food intake in mice
To assess the effect of IL-1Ra on diet-induced diabetes, we injected C57BL/6J mice with IL-1Ra daily for 12 wk. Mice were fed a ND or a HFD. Mice fed the HFD gained more weight than the ND control group; this was not influenced by IL-1Ra treatment. After 12-wk treatment, the body weight of control mice was 35.6 ± 1.7 g on the HFD vs. 24.1 ± 0.5 g on the ND (P < 0.001), and of IL-1Ra treated mice 34.3 ± 1.3 g on the HFD vs. 25.4 ± 0.5 on the ND (P < 0.001). Average weekly food intake was stable throughout the study in both ND and HFD groups, and was not changed by IL-1Ra treatment (data not shown). Similar results were obtained from transgenic IL-1Ra-OE mice (data not shown).
IL-1Ra protects from diet-induced hyperglycemia and improves glucose tolerance in mice
Before and throughout the treatment period, we measured fasting and fed blood glucose every 4 wk. Fasted glucose levels before the HFD in the 5-wk-old mice were approximately 4 mm glucose and insulin levels approximately 0.4 μg/liter insulin, similar as shown from the control ND mice throughout the study. As shown in Fig. 1, neither fasting (Fig. 1A) nor fed (Fig. 1B) blood glucose concentrations changed during the 12-wk study in both control and IL-1Ra-treated mice on the ND. After 4-wk treatment, no differences in glucose levels were observed in all four treatment groups (Fig. 1, A and B). After 8 wk, fasting glucose was 1.9-fold increased in the HFD group compared with the ND group. This increase was prevented in animals from the HFD group that received IL-1Ra (Fig. 1A). After 12 wk, high-fat feeding increased both fasting and fed glucose levels (1.7- and 1.5-fold increase in fasting and fed glucose levels, respectively, in the HFD group, compared with the ND, Fig. 1, A and B; P < 0.001), whereas in the IL-1Ra-treated HFD group, glucose levels were significantly lower than in the untreated HFD group (1.2-fold reduction compared with the untreated HFD group in all conditions; P < 0.05). In parallel, glucose tolerance was impaired in the HFD group already after 4 wk (data not shown) and was further declined during the experiment. Figure 1C shows the response to an ip glucose challenge after 12-wk diet and treatment. High-fat feeding resulted in significantly higher glucose levels before (0 min), and 30, 60, 90, and 120 min after glucose injection (P < 0.05). IL-1Ra administration protected the HFD mice from this effect, resulting in blood glucose levels that were significantly lower compared with controls at all time points during the IPGTT (P < 0.05). Moreover, IL-1Ra treatment led to decreased peak glucose levels in the ND group compared with control animals, which reached significance at 15 min (1.3-fold reduction; P < 0.001; Fig. 1C).
Figure 1.
IL-1Ra protects from diet-induced diabetes and improves glucose tolerance. A and B, Blood glucose levels were measured after 4, 8, and 12-wk diet and IL-1Ra treatment after overnight fasting (A) or without fasting (B). Blood glucose (C) and plasma insulin (D) levels after ip injection of 2 g/kg body weight glucose in mice after 12-wk treatment. E, Blood glucose concentrations of mice after 12-wk treatment, undergoing an insulin tolerance test. Insulin was injected ip at a concentration of 0.75 U/kg body weight. F, Blood glucose levels during the IPGTT experiment as described previously in IL-1Ra-OE animals and their wild-type (WT) littermates after 16-wk ND or high-fat diet. Data show mean ± se. *, P < 0.05 on the HFD vs. ND. +, P < 0.05 on the HFD plus IL-1Ra vs. vehicle or wild-type same diet. #, P < 0.05 at stimulated vs. basal insulin secretion. Data were collected from 16 animals per group, performed in four separate experiments.
Figure 1D shows serum insulin levels during an IPGTT after 12-wk diet and treatment. As previously described (43), high-fat feeding resulted in hyperglycemia (Fig. 1, B and C) as well as hyperinsulinemia (Fig. 1D) compared with control diet mice. Compared with ND counterparts, fasted insulin levels were 2.9-fold higher in high-fat fed mice (P < 0.01). These mice also failed to further increase their insulin levels in response to a glucose challenge. HFD mice under IL-1Ra treatment exhibited significantly lower fasting insulin levels (1.7-fold; P < 0.01) and increased serum insulin concentrations during the IPGTT (2.5-fold). On the ND, IL1-Ra treatment resulted in a higher stimulatory index compared with untreated animals (1.9- vs. 4.2-fold; P < 0.05).
To determine insulin sensitivity, we performed an insulin tolerance test by measuring glucose concentrations after ip insulin injection of 0.75 U/kg body weight. No significant difference in insulin sensitivity was found among the animals of the ND group (Fig. 1E). As previously described, untreated HFD mice displayed impaired insulin sensitivity compared with ND mice. Animals that received IL-1Ra injections were protected against this high-fat diet-induced insulin resistance (Fig. 1E).
Similar results were obtained from the IL-1Ra-OE mice. After 16-wk HFD treatment, glucose tolerance was impaired showing increased glucose levels at all time points during the IPGTT as well as increased fasting glucose levels in the wild-type control mice, compared with the ND (P < 0.05; Fig. 1F). This was prevented in the IL-1Ra-OE mice, showing significantly reduced fasting glucose levels and improved glucose tolerance at all time points during the IPGTT in the HFD group (P < 0.05). In addition, IL-1Ra treatment led to decreased peak glucose levels in the ND group compared with wild-type control mice, which reached significance at 30 min (1.3-fold reduction; P < 0.001; Fig. 1F).
IL-1Ra treatment has no effect on β-cell mass but induces proliferation after 12 wk in mice on the ND
Pancreatic weight per body weight remained unchanged by diet and treatment. Immunohistochemical evaluation of pancreata of all four treatment groups after 12-wk diet and treatment revealed a normal islet structure (Fig. 2A). Islet β-cell mass was dramatically increased by high-fat feeding compared with the ND in control and in IL-1Ra-injected animals (2.3-fold increase in the control and 2.6-fold increase in the IL-1Ra-treated HFD group compared with the ND, respectively; P < 0.001; Fig. 2B). High-fat feeding also resulted in increased α-cell mass (2.1 and 1.8-fold increase in the control and the IL-1Ra-treated HFD group compared with the ND; respectively; P < 0.05, Fig. 2C). IL-1Ra treatment did not affect β-cell mass or α-cell mass in both ND and HFD groups. Consistent with an increase in β-cell mass, the pancreas of control and IL-1Ra-treated high-fat diet-mice contained 2.6- and 3.1-fold more insulin compared with their ND counterparts (P < 0.01; Fig. 2D). In addition, pancreatic glucagon content also increased by high-fat feeding (3.3- and 2.6-fold increase in the control and the IL-1Ra-treated HFD group compared with the respective ND group; P < 0.05; Fig. 2E).
Figure 2.
IL-1Ra treatment has no effect on β-cell mass but increases β-cell proliferation in animals on the ND. A and B, Double immunostaining for insulin in green and glucagon in red (magnification, ×125). β-Cell mass (B) and α-cell mass (C) in tissue sections spanning the width of the whole pancreas of vehicle-treated control mice or IL-1Ra-injected mice fed a ND or high-fat diet for 12 wk (n = 4 mice for each group). The β- and α-cell mass per pancreas was estimated as the product of the relative cross-sectional area of β-cells (determined by quantification of the cross-sectional area occupied by β-cells divided by the cross-sectional area of total tissue) and the weight of the pancreas. Pancreatic insulin (D) and glucagon (E) content in ng/mg pancreas of ND and high-fat fed mice injected with vehicle or IL-1Ra for 12 wk. F and G, Triple staining for proliferation by Ki-67 antibody in red, insulin in green, and 4′,6-diamidino-2-phenylindole in blue was performed on fixed, paraffin-embedded mouse pancreas sections (magnification, ×250). G, Results are expressed as percentage of Ki-67-positive β-cells ± se normalized to control ND islets (100%, in absolute values: 0.3 ± 0.1% Ki-67-positive cells in isolated islets from vehicle-treated mice on the ND). The mean number of β-cells scored was 4599 ± 643 for each treatment condition in four independent experiments. *, P < 0.05 on the HFD vs. ND same treatment. +, P < 0.05 on IL-1Ra vs. vehicle same diet.
In cultured human islets, IL-1Ra increased basal β-cell proliferation (44) and protected from hyperglycemia-induced β-cell apoptosis (16). Therefore, we investigated β-cell turnover in islets from all four groups after 12-wk diet and treatment. Staining of pancreatic sections for the replication marker Ki-67 revealed that IL-1Ra injections increased β-cell proliferation 2-fold (Fig. 2G; P < 0.05) in the ND treated mice. In contrast, HFD treatment in the control mice induced a 2.2-fold reduction in proliferation compared with ND mice (Fig. 2G), whereas IL-1Ra-treated mice on the HFD were protected against this decrease in proliferation. The same results were obtained from isolated islets. Proliferation was 2-fold increased (P < 0.05) in vitro in the IL-1Ra injected ND fed mice. In the HFD, IL-1Ra injected mice were protected against the decreased proliferation induced by the HFD in control mice.
IL-1Ra protects from β-cell apoptosis in cultured isolated islets
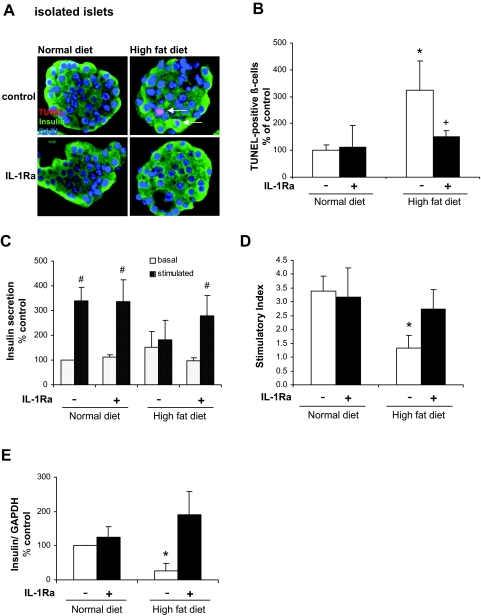
In addition to proliferation, we investigated β-cell apoptosis in pancreatic sections. Staining revealed no TUNEL-positive β-cells in sections from both ND groups and from the IL-1Ra-treated HFD group. We detected 0.016% TUNEL-positive β-cells/islet in sections from the vehicle-treated HFD group (one TUNEL-positive β-cell was found in 61 islets analyzed). Due to this low frequency of positive β-cells in pancreatic sections, we analyzed the occurrence of β-cell apoptosis in isolated islets (Fig. 3A). We found a 3.2-fold increase in β-cell apoptosis in islets derived from the high-fat diet group. Islets from high-fat fed-mice that were treated with IL-1Ra showed a rate of β-cell apoptosis similar to islets from the ND group (Fig. 3B).
Figure 3.
IL-1Ra protects from β-cell apoptosis and preserves β-cell function in cultured isolated islets of HFD mice. Islets were isolated after 12-wk diet and treatment. A, Triple staining for TUNEL in red, insulin in green, and 4′,6-diamidino-2-phenylindole in blue was performed on fixed, paraffin-embedded islet sections (magnification, ×750). B, Results are expressed as percentage of TUNEL-positive β-cells ± se normalized to control ND islets (100%, in absolute values: 1.02 ± 0.19% TUNEL-positive β-cells in isolated islets from vehicle-treated mice on the ND). The mean number of β-cells scored was 2169 ± 894 for each treatment condition from six individual mice. *, P < 0.05 on the HFD vs. ND same treatment. +, P < 0.05 on IL-1Ra vs. vehicle same diet. #, P < 0.05 on ND plus IL-1Ra vs. ND vehicle. C and D, For glucose-stimulated insulin secretion experiments, islets were cultured on extracellular matrix-coated dishes. C, Glucose-stimulated insulin secretion of islets. Basal and stimulated insulin secretion during successive 1-h incubations at 2.8 mm (basal) and 16.7 mm (stimulated) glucose. D, Stimulatory index denotes the ratio between stimulated and basal values of insulin secretion. Assay was performed in triplicate from four individual mice. E, Quantitative RT-PCR analysis of insulin expression relative to control ND conditions. In the LightCycler System, the levels of insulin expression were normalized to GAPDH and tubulin with the same result. Islets were isolated from six individual mice per treatment group Data are shown as mean ± se. *, P < 0.05 HFD compared with ND same treatment. #, P < 0.05 stimulated compared with basal insulin secretion. Analyses were performed in four independent experiments.
IL-1Ra treatment preserves β-cell function in high-fat fed mice
Because we observed a protective effect of IL-1Ra on blood glucose levels, glucose tolerance, as well as insulin secretion during the glucose tolerance test, we investigated if this effect can be explained by improved β-cell function. Islets were isolated from all four treatment groups, and insulin secretion into the culture medium was measured. Acute glucose-stimulated insulin secretion was completely abolished in islets derived from animals fed a high-fat diet (Fig. 3C). We found that islets from high-fat fed animals, which were treated with IL-1Ra, showed a normal response to a glucose challenge and a stimulatory index comparable to ND animals (Fig. 3D). In parallel, high-fat feeding induced a decrease in insulin mRNA in isolated islets, which was prevented by IL-1Ra administration (Fig. 3E).
IL-1Ra prevents HFD induced changes in serum adipokine and lipid levels
Epididymal fat pad mass was significantly increased in both HFD groups (3.7-fold in the control and 3.8-fold in the IL-1Ra-treated HFD group, compared with ND group, respectively; P < 0.01; data not shown). IL-1Ra injections did not affect epididymal fat mass in both diet groups.
At the end of the study, we measured serum adipokines, lipids, and cytokines known to affect insulin action and secretion. IL-1Ra levels were unchanged by the HFD. IL-1Ra levels in the IL-1Ra injected mice were increased in both diets (Fig. 4A; P < 0.05).
Figure 4.
IL-1Ra treatment prevents HFD-induced changes in serum adipokine levels. Serum levels of IL-1Ra (A), leptin (B), and resistin (C) in mice after 12-wk diet and treatment. Data show mean results from eight mice per treatment group from four independent experiments ± se. *, P < 0.05 on the HFD vs. ND. +, P < 0.05 on IL-1Ra vs. vehicle-treated same diet.
High-fat diet treatment has increased serum levels of lipids, and of the adipokines resistin and leptin (45). To confirm this and to test the hypothesis that IL-1Ra is able to prevent those changes despite the increase in fat mass, we analyzed serum from all mice at the end of the study.
Levels of the adipokines leptin and resistin were raised 22- and 1.8-fold in the vehicle-treated HFD group compared with the ND, respectively (Fig. 4, B and C; P < 0.05). IL-1Ra treatment led to a 3.9- and 1.6-fold reduction in leptin and resistin levels in the HFD group, respectively (P < 0.05).
Free fatty acid (FFA) levels were increased by 21% in vehicle-treated HFD animals compared with ND (P < 0.05, data not shown). In contrast, animals on the HFD that were given IL-1Ra did not show any significant increase in FFA levels compared with their ND fed counterparts. High-fat feeding also significantly increased TG and cholesterol (Chol) levels (TG, 1.4-fold increase; Chol, 2.1-fold increase; P < 0.05). IL-1Ra administration to HFD mice had the tendency to reduce TG and Chol levels compared with vehicle-treated animals, however, this reduction did not reach statistical significance (data not shown). Mice on the high-fat diet, which endogenously overexpress IL-1Ra, showed significantly reduced levels of FFA as well as TG and Chol (Fig. 5, A–C; P < 0.05). In addition, circulating adiponectin levels were reduced by 20% in the HFD mice compared with ND mice (P < 0.05; Fig. 5D). The HFD-induced reduction in adiponectin levels was prevented in IL-1Ra-OE mice (P < 0.01 compared with wild-type HFD mice). Because IL-1Ra-induced changes in adipokine and lipid levels occurred without changes in fat mass, we investigated the size of adipocytes in hematoxylin- and eosin-stained sections of epididymal fat pads. Measuring the cross-sectional area by manual tracing revealed a more than 3-fold high-fat diet-induced increase in adipocyte size in both wild-type and IL-1Ra-OE mice compared with their ND counterparts. IL-1Ra overexpression did not affect adipocyte size in either diet group (P < 0.005; Fig. 5E). To further elucidate the underlying mechanisms of the observed changes in serum adipokines and lipids, we isolated RNA from epididymal fat tissue and assessed expression of inflammatory genes by quantitative RT-PCR. While in wild-type mice, high-fat diet increased adipose tissue expression of F4/80, CD11c, and TNFα (P < 0.05), this induction was attenuated in IL-1Ra-OE mice (P < 0.05; Fig. 5F).
Figure 5.
IL-1Ra prevents HFD-induced changes in serum lipid levels. A–D, Serum levels of TGs, Chol, FFAs, and adiponectin in IL-1Ra-OE and their wild-type littermates (WT) fed a normal or high-fat diet for 16 wk (n = 8). E, Size of adipocytes in epididymal fat tissue of wild-type or IL-1Ra-OE mice on the ND or HFD. Sections were stained with hematoxylin and eosin, followed by measuring the cross-sectional area by manual tracing (n = 8). F, Quantitative RT-PCR of inflammatory gene expression in epididymal fat pads. Total RNA was isolated after 16-wk ND or high-fat diet, and gene expression was evaluated in the LightCycler System after normalization to tubulin. Results are expressed relative to mRNA levels in wild-type mice on the ND (n = 7). All data are shown as mean ± se. *, P < 0.05 on the HFD vs. ND. +, P < 0.05 on IL-1Ra-OE vs. wild type. Analyses were performed in four independent experiments.
In the ND groups, neither IL-1Ra injections nor overexpression changed lipid or adipokine levels.
Discussion
Elevated glucose concentrations impair β-cell function and induce apoptosis, and, thus, are implicated in accelerating the progression of diabetes. Strategies to block these deleterious effects on the β-cell are needed for a successful diabetes therapy. IL-1Ra has inhibited the deleterious effects of glucose on β-cells by blocking pro-inflammatory IL-1β signaling in vitro (16). Here, we show that IL-1Ra improved glucose tolerance, insulin secretion, and insulin sensitivity in C57BL/6J mice fed a HFD (Surwit), serving as an animal model of T2DM. Twelve weeks of high-fat feeding resulted in hyperglycemia accompanied by impaired glucose-stimulated insulin secretion. Interestingly, the changes induced by the HFD feeding strongly correlated with the individual weight gain of the mice. For instance, a mouse with a 1.15-fold higher body weight in our study (46 vs. 40 g) also had 1.2-fold higher glucose levels at all time points during the IPGTT (P < 0.05; data not shown). This strong association of glucose tolerance and body weight may explain the relatively large variation we observed throughout the study, as already reported by a similar diet-induced obesity model before (46).
Long-term high-fat diet in C57BL/6J mice is associated with an adaptive increase in β-cell number but an early functional abnormality (47). In the present study, β-cell mass increased over 2-fold during the 12-wk HFD. Despite this increase in β-cell mass, β-cell turnover was already impaired at that time point. We assume that the combination of high-fat and high-sucrose in the diet results relatively early in an impairment of β-cell turnover together with a loss of function. In line with this, massive reduction in β-cell replication after long-term high-fat/high carbohydrate diet (decreased to one third of the control group) was observed in mice before a significant decrease in β-cell mass (48). Consistent with our present study, approximately 0.3% proliferating β-cell were observed in the control mice (48), and low numbers as 0.025% proliferating β-cells were reported from human pancreatic sections from autopsy (5). Proliferation in adult β-cells is a rare event, but small changes in β-cell proliferation or apoptosis over the years can result in marked changes in β-cell mass and diabetes progression (5,48).
Previous studies observed that glucose induces increased proliferation in short-term culture but decreases proliferation after long-term exposure of isolated islets (49). We hypothesize that in our model a transient increase in diet-induced proliferation occurred earlier in the diet induced by mildly elevated glucose levels, proceeding to decreased proliferation measured at 12 wk, when severe hyperglycemia was present. IL-1Ra-treated mice were protected from this HFD-induced decrease in proliferation. On the ND, IL-1Ra injections resulted in increased β-cell proliferation after 12 wk, consistent with in vitro data on isolated human islets (44).
We measured only a very few apoptotic β-cells in vivo and could not quantify significant changes among the four treatment groups. It is noteworthy that isolated islets derived from the HFD group displayed a 3-fold induction of β-cell apoptosis compared with islets from the ND group. This suggests that high-fat feeding renders β-cells more susceptible to apoptosis induced by isolation procedures and/or culture. This increased sensitivity was not observed in islets from IL-1Ra-treated HFD mice.
The effect of IL-1Ra on improving glucose tolerance is probably a result of both, improved insulin action and secretion. Especially the effects of IL-1Ra on lowering serum lipids and adipokines would in turn improve insulin sensitivity as well as β-cell function. FFAs have directly mediated insulin resistance through activating pro-inflammatory Toll-like receptor 4 signaling (50). In addition, FFAs (39) and TGs (51) directly induce β-cell dysfunction and apoptosis. Our study provides more evidence for an existing link between inflammation, insulin resistance, and insulin secretion/β-cell survival. To analyze inflammatory markers in the serum of the mice, we measured IL-1 and IL-6 serum levels. Unfortunately, most of the values were under the detection levels of the assays. Interestingly, we got positive IL-1 and IL-6 readings in some but not all samples from the HFD fed control mice, but none in the ND mice or HFD IL-1Ra mice. However, data analysis and statistics were not possible. On the other hand, serum IL-1Ra levels were unchanged by the HFD after 12 wk. Therefore, we believe that indeed the ratio of IL-1 to IL-1Ra plays an important role in maintaining glucose homeostasis.
Amounts of secreted leptin and FFAs are dependent on fat mass (52), but also from the direct effect of IL-1β on the adipocytes (53). The fact that we do observe changes in leptin and FFA secretion in the IL-1Ra-treated mice without changes in fat mass and adipocyte size supports the IL-1 dependent effect. mRNA levels of inflammatory cytokines IL-1β, TNF-α, the macrophage marker F4/80, and proinflammatory CD11c are increased by the HFD in the control mice but reduced by IL-1Ra injections. Here, the proinflammatory effect by the HFD on the level of the adipocytes may be the reason for the increased leptin, FFA, Chol, and TG production in the HFD control mice. Even though IL-1Ra mRNA in adipocytes was also 7.4-fold increased by the HFD, this could likely not counteract the 2.7-fold HFD-induced increase of IL-1β because an excess of 10- to 1000-fold of IL-1Ra is needed to inhibit IL-1β effects, dependent on the exposure time (23). TNF-α and IL-1β expression in the epididymal fat pads could trigger HFD-induced insulin resistance (54), and also negatively act on the β-cell inducing β-cell dysfunction and apoptosis (55,56). Undoubtedly, the effect on the adipocytes participated in the protective role of IL-1Ra on the level of the β-cell.
In contrast, IL-1Ra serum levels are increased in humans with obesity and the strong correlation with the degree of insulin resistance (57) suggest that IL-1Ra induces rather than protects from insulin resistance. Moreover, a negative correlation of elevated IL-1Ra and whole body glucose uptake has been shown in the offspring of T2DM subjects. When normal rats are injected with IL-1Ra for 5 consecutive days, whole body glucose disposal is decreased due to a selective decrease in glucose uptake in the muscle (58).
In our study we have not assessed the short-term effects of IL-1Ra administration in mice. However, we had no indication that 12-wk administration of IL-1Ra negatively influences insulin sensitivity. IL-1Ra-treated mice showed similar insulin levels and insulin tolerance as the vehicle-treated controls. Moreover, on the high-fat diet, IL-1Ra mice had lower insulin levels than the control mice and improved insulin sensitivity. Possibly, the increased IL-1Ra serum levels in obese patients could be secondary in response to insulin resistance.
The fact that IL-1Ra potentially prevented hyperglycemia and improved β-cell function is in favor for the critical role of IL-1β signaling in the β-cell, not only in type 1 but also type 2 diabetic environments. Our data provide new insights into mechanisms of the protective effect of IL-1Ra on β-cell function and turnover, and support IL-1Ra as a potential therapy of diabetes.
Acknowledgments
We thank Heather Gerber, Rosemary Soliz, and Lendy N. Le for excellent technical assistance, and Drs. Marc Y. Donath and Aleksey Matveyenko for critical discussion.
Footnotes
This work was supported by the American Diabetes Association (Junior Faculty Grant 706JF41), the Larry L. Hillblom Research Foundation (Grant 2005 1C), and the National Institutes of Health/Older Americans Independence Center at the University of California, Los Angeles, CA (Older Americans Independence Center Career Development Award). N.S.S. and F.T.S. are recipients of the Swiss National Foundation Fellowship award.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 31, 2008
Abbreviations: Chol, Cholesterol; FFA, free fatty acid; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HFD, high-fat/high-sucrose diet; IL-1Ra, IL-1 receptor antagonist; IL-1Ra-OE, overexpressing IL-1 receptor antagonist; IPGTT, ip glucose tolerance test; KRB, Krebs-Ringer bicarbonate buffer; ND, normal diet; TG, triglyceride; TUNEL, terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling; T2DM, type 2 diabetes.
References
- Cerasi E, Luft R 1967 Insulin response to glucose infusion in diabetic and non-diabetic monozygotic twin pairs. Genetic control of insulin response? Acta Endocrinol (Copenh) 55:330–345 [DOI] [PubMed] [Google Scholar]
- Taylor SI, Accili D, Imai Y 1994 Insulin resistance or insulin deficiency. Which is the primary cause of NIDDM? Diabetes 43:735–740 [DOI] [PubMed] [Google Scholar]
- Gerich JE 2000 Insulin resistance is not necessarily an essential component of type 2 diabetes. J Clin Endocrinol Metab 85:2113–2115 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S 2000 Islet growth and development in the adult. J Mol Endocrinol 24:297–302 [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC 2003 β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 [DOI] [PubMed] [Google Scholar]
- Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S 2002 Reduced β-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese type II diabetic patients. Diabetologia 45:85–96 [DOI] [PubMed] [Google Scholar]
- Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S 2003 Selective β-cell loss and α-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 88:2300–2308 [DOI] [PubMed] [Google Scholar]
- Kurrer MO, Pakala SV, Hanson HL, Katz JD 1997 β cell apoptosis in T cell-mediated autoimmune diabetes. Proc Natl Acad Sci USA 94:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC 2005 Sustained β cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 48:2221–2228 [DOI] [PubMed] [Google Scholar]
- Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M 2005 Mechanisms of {beta}-cell death in type 2 diabetes. Diabetes 54(Suppl 2):S108–S113 [DOI] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ 2006 Islet β cell failure in type 2 diabetes. J Clin Invest 116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B 1994 Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344:721–724 [DOI] [PubMed] [Google Scholar]
- Rabinovitch A, Suarez WL, Power RF 1992 Combination therapy with an antioxidant and a corticosteroid prevents autoimmune diabetes in NOD mice. Life Sci 51:1937–1943 [DOI] [PubMed] [Google Scholar]
- Sumoski W, Baquerizo H, Rabinovitch A 1989 Oxygen free radical scavengers protect rat islet cells from damage by cytokines. Diabetologia 32:792–796 [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T 2001 β-cell apoptosis: stimuli and signaling. Diabetes 50(Suppl 1):S58–S63 [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY 2002 Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup-Poulsen T 1996 The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia 39:1005–1029 [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Mandrup-Poulsen T 2001 A choice of death–the signal-transduction of immune-mediated β-cell apoptosis. Diabetologia 44:2115–2133 [DOI] [PubMed] [Google Scholar]
- Mine T MK, Okutsu T, Mitsui A, Kitahara Y 2004 Gene expression profile in the pancreatic islets of Goto-Kakizaki (GK) rats with repeated postprandial hyperglycemia. Diabetes 53(Suppl 2):2475A [Google Scholar]
- Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC 2004 Diabetes due to a progressive defect in β-cell mass in rats transgenic for human islet amyloid polypeptide (HIP rat): a new model for type 2 diabetes. Diabetes 53:1509–1516 [DOI] [PubMed] [Google Scholar]
- Dinarello CA 1996 Biologic basis for interleukin-1 in disease. Blood 87:2095–2147 [PubMed] [Google Scholar]
- Donath MY, Storling J, Maedler K, Mandrup-Poulsen T 2003 Inflammatory mediators and islet β-cell failure: a link between type 1 and type 2 diabetes. J Mol Med 81:455–470 [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Tracey DE, Bendtzen K, Sandler S 1991 An interleukin-1 receptor antagonist protein protects insulin-producing β cells against suppressive effects of interleukin-1 β. Diabetologia 34:445–448 [DOI] [PubMed] [Google Scholar]
- Giannoukakis N, Rudert WA, Ghivizzani SC, Gambotto A, Ricordi C, Trucco M, Robbins PD 1999 Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents IL-1β-induced β-cell impairment and activation of islet cell apoptosis in vitro. Diabetes 48:1730–1736 [DOI] [PubMed] [Google Scholar]
- Dinarello CA 2000 The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med 343:732–734 [DOI] [PubMed] [Google Scholar]
- Seckinger P, Lowenthal JW, Williamson K, Dayer JM, MacDonald HR 1987 A urine inhibitor of interleukin 1 activity that blocks ligand binding. J Immunol 139:1546–1549 [PubMed] [Google Scholar]
- Seckinger P, Williamson K, Balavoine JF, Mach B, Mazzei G, Shaw A, Dayer JM 1987 A urine inhibitor of interleukin 1 activity affects both interleukin 1 α and 1 β but not tumor necrosis factor α. J Immunol 139:1541–1545 [PubMed] [Google Scholar]
- Arend WP, Guthridge CJ 2000 Biological role of interleukin 1 receptor antagonist isoforms. Ann Rheum Dis 59(Suppl 1):i60–i64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg JO, Andersson A, Eizirik DL, Sandler S 1994 Interleukin-1 receptor antagonist prevents low dose streptozotocin induced diabetes in mice. Biochem Biophys Res Commun 202:543–548 [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Di Marco R, Barcellini W, Magro G, Schorlemmer HU, Kurrle R, Lunetta M, Grasso S, Zaccone P, Meroni P 1994 Protection from experimental autoimmune diabetes in the non-obese diabetic mouse with soluble interleukin-1 receptor. Eur J Immunol 24:1843–1847 [DOI] [PubMed] [Google Scholar]
- Sandberg JO, Eizirik DL, Sandler S 1997 IL-1 receptor antagonist inhibits recurrence of disease after syngeneic pancreatic islet transplantation to spontaneously diabetic non-obese diabetic (NOD) mice. Clin Exp Immunol 108:314–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffels K, Gysemans C, Waer M, Laureys J, Bouillon R, Mathieu C 2002 Interleukin-1 receptor antagonist inhibits primary non-function and prolongs graft survival time of xenogeneic islets transplanted in spontaneously diabetic autoimmune NOD mice. Diabetologia 45(Suppl 2):424–424 [Google Scholar]
- Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY 2004 Leptin modulates β cell expression of IL-1 receptor antagonist and release of IL-1β in human islets. Proc Natl Acad Sci USA 101:8138–8143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Mandrup-Poulsen T 2005 An immune origin of type 2 diabetes? Diabetologia 48:1038–1050 [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF 2003 Inflammatory cytokines and the risk to develop type 2 diabetes: results of the Prospective Population-Based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52:812–817 [DOI] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY 2007 Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356:1517–1526 [DOI] [PubMed] [Google Scholar]
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN 1988 Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37:1163–1167 [DOI] [PubMed] [Google Scholar]
- Hirsch E, Irikura VM, Paul SM, Hirsh D 1996 Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci USA 93:11008–11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY 2001 Distinct effects of saturated and monounsaturated fatty acids on β-cell turnover and function. Diabetes 50:69–76 [DOI] [PubMed] [Google Scholar]
- Kaiser N, Corcos AP, Sarel I, Cerasi E 1991 Monolayer culture of adult rat pancreatic islets on extracellular matrix: modulation of B-cell function by chronic exposure to high glucose. Endocrinology 129:2067–2076 [DOI] [PubMed] [Google Scholar]
- Warnick GR 1986 Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol 129:101–123 [DOI] [PubMed] [Google Scholar]
- Maedler K, Fontana A, Ris F, Sergeev P, Toso C, Oberholzer J, Lehmann R, Bachmann F, Tasinato A, Spinas GA, Halban PA, and Donath MY 2002 FLIP switches Fas-mediated glucose signaling in human pancreatic β cells from apoptosis to cell replication. Proc Natl Acad Sci USA 99:8236–8241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M 1995 Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 44:645–651 [DOI] [PubMed] [Google Scholar]
- Maedler K, Schumann DM, Sauter N, Ellingsgaard H, Bosco D, Baertschiger R, Iwakura Y, Oberholzer J, Wollheim CB, Gauthier BR, Donath MY 2006 Low concentration of interleukin-1β induces FLICE-inhibitory protein-mediated β-cell proliferation in human pancreatic islets. Diabetes 55:2713–2722 [DOI] [PubMed] [Google Scholar]
- Koerner A, Kratzsch J, Kiess W 2005 Adipocytokines: leptin—the classical, resistin–the controversial, adiponectin–the promising, and more to come. Best Pract Res Clin Endocrinol Metab 19:525–546 [DOI] [PubMed] [Google Scholar]
- Reimer MK, Ahren B 2002 Altered beta-cell distribution of pdx-1 and GLUT-2 after a short-term challenge with a high-fat diet in C57BL/6J mice. Diabetes 51(Suppl 1):S138–S143 [DOI] [PubMed] [Google Scholar]
- Hull RL, Kodama K, Utzschneider KM, Carr DB, Prigeon RL, Kahn SE 2005 Dietary-fat-induced obesity in mice results in β cell hyperplasia but not increased insulin release: evidence for specificity of impaired β cell adaptation. Diabetologia 48:1350–1358 [DOI] [PubMed] [Google Scholar]
- Sone H, Kagawa Y 2005 Pancreatic β cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 48:58–67 [DOI] [PubMed] [Google Scholar]
- Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath MY 2001 Glucose induces beta-cell apoptosis via upregulation of the Fas-receptor in human islets. Diabetes 50:1683–1690 [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS 2006 TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrich ME, Mooser V, Lenain V, Herz J, Nimpf J, Azhar S, Bideau M, Capponi A, Nicod P, Haefliger JA, Waeber G 2003 Insulin-secreting β-cell dysfunction induced by human lipoproteins. J Biol Chem 278:18368–18375 [DOI] [PubMed] [Google Scholar]
- Sell H, Dietze-Schroeder D, Eckel J 2006 The adipocyte-myocyte axis in insulin resistance. Trends Endocrinol Metab 17:416–422 [DOI] [PubMed] [Google Scholar]
- Simons PJ, van den Pangaart PS, van Roomen CP, Aerts JM, Boon L 2005 Cytokine-mediated modulation of leptin and adiponectin secretion during in vitro adipogenesis: evidence that tumor necrosis factor-α- and interleukin-1β-treated human preadipocytes are potent leptin producers. Cytokine 32:94–103 [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Mangalat D, Korbut R 2006 Adipocytokines—novel link between inflammation and vascular function? J Physiol Pharmacol 57:505–528 [PubMed] [Google Scholar]
- Liuwantara D, Elliot M, Smith MW, Yam AO, Walters SN, Marino E, McShea A, Grey ST 2006 Nuclear factor-κB regulates β-cell death: a critical role for A20 in β-cell protection. Diabetes 55:2491–2501 [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T, Bendtzen K, Dinarello CA, Nerup J 1987 Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic β-cell cytotoxicity. J Immunol 139:4077–4082 [PubMed] [Google Scholar]
- Abbatecola AM, Ferrucci L, Grella R, Bandinelli S, Bonafe M, Barbieri M, Corsi AM, Lauretani F, Franceschi C, Paolisso G 2004 Diverse effect of inflammatory markers on insulin resistance and insulin-resistance syndrome in the elderly. J Am Geriatr Soc 52:399–404 [DOI] [PubMed] [Google Scholar]
- Somm E, Cettour-Rose P, Asensio C, Charollais A, Klein M, Theander-Carrillo C, Juge-Aubry CE, Dayer JM, Nicklin MJ, Meda P, Rohner-Jeanrenaud F, Meier CA 2006 Interleukin-1 receptor antagonist is upregulated during diet-induced obesity and regulates insulin sensitivity in rodents. Diabetologia 49:387–393 [DOI] [PubMed] [Google Scholar]