Abstract
Most peptide hormone genes are, in addition to endocrine cells, also expressed in neurons. The peptide hormone cholecystokinin (CCK) is expressed in different molecular forms in cerebral neurons and intestinal endocrine cells. To understand this difference, we examined the roles of the neuroendocrine prohormone convertases (PC) 1/3, PC2, and PC5/6 by measurement of proCCK, processing intermediates and bioactive, α-amidated, and O-sulfated CCK peptides in cerebral and jejunal extracts of null mice, controls, and in the PC5/6-expressing SK-N-MC cell-line. In PC1/3 null mice, the synthesis of bioactive CCK peptide in the gut was reduced to 3% of the translational product, all of which was in the form of α-amidated and tyrosine O-sulfated CCK-22, whereas the neuronal synthesis in the brain was largely unaffected. This is opposite to the PC2 null mice in which only the cerebral synthesis was affected. SK-N-MC cells, which express neither PC1/3 nor PC2, synthesized alone the processing intermediate, glycine-extended CCK-22. Immunocytochemistry confirmed that intestinal endocrine CCK cells in wild-type mice express PC1/3 but not PC2. In contrast, cerebral CCK neurons contain PC2 and only little, if any, PC1/3. Taken together, the data indicate that PC1/3 governs the endocrine and PC2 the neuronal processing of proCCK, whereas PC5/6 contributes only to a modest endocrine synthesis of CCK-22. The results suggest that the different peptide patterns in the brain and the gut are due to different expression of PCs.
PEPTIDE HORMONES ARE at various stages of development expressed in both endocrine cells and neurons. The processing of the prohormones, however, often differs considerably between neurons and endocrine cells (1). The peptide hormone cholecystokinin (CCK) illustrates the difference. CCK was originally discovered as a gut hormone that regulates gallbladder emptying, pancreatic enzyme secretion, and intestinal motility (for review, see Ref. 2). CCK is, however, also synthesized as a potent transmitter in cerebral neurons in quantities that make CCK the most abundant neuropeptide in the vertebrate brain (3,4,5).
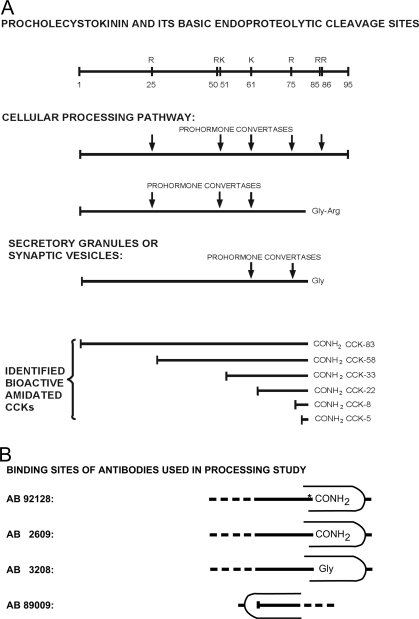
ProCCK harbors several endoproteolytic processing sites of which one dibasic (R85R86) and four monobasic (R25, R50, K61, and R75) are known to be cleaved (Fig. 1). The processing is cell specific in the sense that the pattern of bioactive CCK peptides in gut endocrine cells and cerebral neurons varies in an evolutionary conserved manner (for review, see Ref. 6). Hence, the endocrine intestinal I cells contain CCK peptides of varying length and bioactivity (CCK-58, CCK-33, CCK-22, and CCK-8) of which CCK-33 and/or CCK-22 predominate plasma of most mammals (7,8,9,10). In contrast, the octapeptide, CCK-8, is by far the most abundant form of CCK in vertebrate brains (3,5,11,12,13). The molecular mechanisms behind the cell-specific processing pattern have so far been an unsettled question.
Figure 1.
A, Diagram of the co- and posttranslational modifications of proCCK. Activation of the CCK amidation site (FGRR, sequence 83–86 of proCCK) occurs via a series of carboxyterminal cleavages and modifications. An early modification is tyrosyl O-sulfation by sulfotransferases of Y77, Y91, and Y93. In addition to ensuring binding to the CCK-A receptor, tyrosyl sulfation also increases endoproteolytic cleavage (39). Endoproteolytic cleavage by a PC (PC1/3, PC2, PC5/6, or other PCs) produces the carboxypeptidase E substrate. Carboxypeptidase E then acts to remove the C-terminal arginyl residue yielding glycine-extended CCK, which by peptidylglycine α-amidating monooxygenase (PAM) results in the production of bioactive CCK (F-NH2). Concomitant N-terminal cleavages by PCs produce bioactive CCKs of varying length. In combination with in vitro endoprotease treatment (trypsinization), a library of sequence-specific antibodies was used to measure bioactive CCK and precursor peptides, as described in the text. Although the text enlists only CCK-58, -33, -22, and -8 as bioactive CCKs, small amounts of CCK-83 and -5 may also be synthesized in the brain and gut (12). B, Binding sites of antibodies (AB) for the study of endoproteolytic proCCK processing: AB 92128 binds proCCK sequence 76–83 in its derivatized form, i.e. Tyr76 is O sulfated (marked with an asterisk) and Phe83 carboxyamidated. This structure constitutes the C terminus of the bioactive cholecystokinins (CCK-83,-58,-33,-22, and -8). AB 2609 binds the same sequence as AB 92128 but is independent of tyrosyl O-sulfation. Consequently, it binds both sulfated and nonsulfated CCK-peptides. AB 3208 binds proCCK sequence 76–84, i.e. the glycine-extended forms of CCK that constitute the immediate precursors of the bioactive carboxyamidated CCKs. AB 89009 binds proCCK sequence 62–68, corresponding to the N terminus of human CCK-22. Consequently, AB 89009 binds both CCK-22 and C-terminal extended forms of CCK-22 such as glycine-extended CCK-22. Further details of the epitope specificity of the antibodies are given elsewhere (8,25,26,27).
Endoproteolytic processing of prohormones requires prohormone convertases (PCs). Mammals are known to express seven subtilisin-like PCs, of which PC1/3 and PC2 are assumed to be responsible for most of the endoproteolytic processing of neuroendocrine proproteins (for reviews, see Refs. 14,15,16,17). PC5/6, however, has also been shown to be expressed in cerebral neurons and gut endocrine cells (18,19). The convertases typically cleave dibasic sites, although they are capable of cleaving also monobasic sites. Processing of the R85R86 site in proCCK determines the availability of the glycine-extended CCK precursor for the F83 α-amidation, a modification that is necessary for the binding of CCK peptides to specific receptors. Subsequent cleavage at the C terminus of R25, R50, K61, and R75 then determines the length of the N-terminal extensions and hence whether the α-amidated and O-sulfated fragments become CCK-8, CCK-22, CCK-33, or CCK-58 (Fig. 1).
To determine the role of PCs for the cell-specific expression of CCK peptides, we have now examined the cerebral and intestinal processing of proCCK in PC1/3 null mice and compared the results with those obtained from examination of PC2 null mice (20). Moreover, we examined the processing in SK-N-MC cells that express proCCK and PC5/6 but neither PC1/3 nor PC2 (21). Finally, the expression of PCs in endocrine CCK cells and CCK neurons was investigated by immunocytochemistry and in situ hybridization.
Materials and Methods
Experimental animals
PC1/3 knockout (−/−) and corresponding wild-type (+/+) mice of the 129 sv strain were generated by intercrossing heterozygotes and genotyping the offspring. The strain was the same as that used in the study of PC2 knockout mice (20). The PC1/3 knockouts (−/−) and littermate controls (22) were housed on a 12-h light, 12-h dark cycle and freely fed. All experiments were approved by the Animal Ethics Committees of the local institutions.
Tissue isolation and extraction
Groups of four mice 3 months of age were killed by CO2 inhalation. The whole brain and small intestinal (jejunal) tissue were rapidly dissected and frozen on dry ice. The tissue was gently cleaned in PBS on ice before freezing and otherwise kept at −80 C until analysis. The brain and jejunal tissue samples from PC1/3 knockout and corresponding wild-type mice were collected in Chicago (IL) and sent frozen by courier mail on abundant dry ice to Copenhagen (Denmark). Extraction of all tissues was performed at Rigshospitalet in Copenhagen, using the same procedure as previously described (12,23). Briefly, frozen tissues were boiled in water (1 ml/mg) for 20 min, homogenized (polytron), and centrifuged for 30 min at 15,000 × g. The supernatants were removed and the pellets reextracted in 0.5 m CH3COOH (1 ml/mg), rehomogenized, incubated at room temperature for 30 min, and centrifuged. The neutral water and acid supernatants were stored separately at −20 C until RIA analysis.
Cell line
The CCK-expressing human neuroepithelioma cell line, SK-N-MC, was obtained from the American Type Culture Collection (Manassas, VA) and maintained as previously described (24). The cells were grown in Falcon flasks in Eagle’s medium supplemented with 10% fetal calf serum (Seromed, Berlin, Germany); 1.76 g NaHCO3 per liter; 2.38 g HEPES per liter; 0.29 g l-glutamine per liter; and 10 ml penicillin-streptomycin per liter (10,000 U and 10 mg/ml) at 37 C under 5% carbon dioxide. The cells were grown to confluence and harvested before peptide extraction.
Chromatography
One or two milliliters of either neutral water or acetic extracts were applied to Sephadex G-50 superfine columns (10 × 1000 mm), which were eluted at 4 C with 0.02 m barbital buffer (pH 8.4), containing 0.1% BSA. Fractions of 1.0 ml were collected at a rate of 4.0 ml/h. The columns were calibrated with [125I]albumin (void volume), CCK-33, CCK-22, and CCK-8 and with 22NaCl (total volume). The elutions were monitored with sequence-specific RIAs using antisera nos. 92128, 3208, 89009, and 2609 as described below. Four independent water or acid extracts from each of the four groups of mice (PC1/3 knockouts and corresponding wild-type controls) were subjected to gel chromatography. The individual immunoreactive peak in each chromatographic curve was measured in percentage of area under the curve (AUC) by planimetry and the mean ± sem subsequently calculated for statistical comparison of proCCK products in PC1/3 knockouts and wild-type controls.
RIAs
A unique library of sequence-specific, high-affinity antibodies against different proCCK epitopes was used to measure proCCK, processing intermediates, and carboxyamidated forms of CCK (Fig. 1). The carboxyamidated and tyrosyl O-sulfated CCK peptides were measured using the CCK-specific antiserum no. 92128 (8). Antibody no. 92128 binds all the carboxyamidated and O-sulfated forms of CCK with equimolar affinity irrespective of size. It displays no cross-reactivity with homologous gastrin peptides. The sum of sulfated and nonsulfated carboxyamidated CCK peptides was measured in parallel using antibody no. 2609 (25). Glycine-extended processing intermediates of CCK were measured using antiserum no. 3208 with [125I]glycine-extended CCK-8 as tracer and glycine-extended CCK-8 as standard. Glycine-arginine-extended intermediate precursors were also measured using antiserum no. 3208 but after enzymatic pretreatment with carboxypeptidase (CP) B. To measure all CCK-precursor forms, samples were pretreated with both trypsin and CPB followed by RIA using antiserum no. 3208, as previously described (26). CPB mimics the effect of CPE, whereas trypsin cleaves at basic cleavage sites like PCs. The N-terminal sequence of human CCK-22 was measured using antiserum no. 89009 and the [125I]tyrosylated N-terminal decapeptide fragment of CCK-22 as tracer and human CCK-22 as standard (27). Antiserum no. 89009 is specific for the human sequence. It cannot bind mouse CCK-22 and was consequently used only for measurement in SK-N-MC cell extracts and media. Because the SK-N-MC cells may also express the homologous prohormone progastrin and some of its processing intermediates, the specificity of the measurements of cell line extracts was controlled with a RIA using antiserum no. 5284, [125I]glycine-extended gastrin-13 as tracer and glycine-extended gastrin-17 as standard. Antiserum no. 5284 binds only glycine-extended gastrins and no glycine-extended CCKs (26).
Immunocytochemistry
To examine the cellular colocalization between neuronal CCK and PC1/3 or PC2, six adult wild-type mice aged 3–4 months were transcardially perfusion fixed, with normal saline followed by 4% paraformaldehyde in buffered sodium phosphate (pH 7). Cerebral tissue was postfixed at least 24 h in the same solution, frozen, and cut in a microtome at 40-μm-thick sections. To examine the cellular colocalization between CCK and PC1/3 or PC2 in brain sections, the tissue was inmmunostained using well-characterized antibodies against PC1/3, PC2 (8,28,29), and CCK. Because both the CCK and the PC1/3 and PC2 antisera were raised in rabbits, we visualized colocalization by the tyramide amplification technique, as has been described by others (30). Sections were incubated overnight with CCK antiserum no. 92128 [1:10,000 (8)] followed by biotinylated donkey antirabbit antiserum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and visualized by biotinylated tyramide (tyramide system amplification; DuPont NEN Life Science Products, Boston, MA) and streptavidin conjugated Texas Red (Amersham Bioscience, Buckinghamshire, UK). After washing and blocking in 1% H2O2 for 10 min, the sections were incubated overnight with anti-PC1/3 (1:500) or anti-PC2 antiserum (1:500). On the third day, the PC1/3 or PC2 antibody was visualized using a CY2-conjugated donkey antirabbit antiserum (Jackson ImmunoResearch Laboratories). To examine the cellular colocalization between intestinal CCK and PC1/3 or PC2, the small intestines from three wild-type mice were removed, cut open, washed, and immersion fixed overnight in 4% paraformaldehyde. Six-micrometer traverse paraffin sections were cut, mounted on SuperFrosted glass slides, and baked overnight at 60 C. Antigen retrieval was performed by boiling the slides in a microwave oven for 3 min at 800 W, followed by 3 × 5 min at 400 W in a 10 mm Tris-buffered EGTA solution (pH 9.0). Preblocking was performed in 10% ovine serum in Tris-buffered saline for 20 min The sections were incubated with specific PC1/3 or PC2 antisera, respectively (28,29), both diluted 1:10,000 overnight at 4 C. The primary antiserum was detected with Envision+ (Dako, Glostrup, Denmark) for 1 h followed by incubation with a Cy3-conjugated antihorseradish peroxidase antibody (Jackson ImmunoResearch) diluted 1:400 for 1 h. Bioactive amidated CCK was detected with overnight incubation using antiserum no. 92128 (dilution 1:5000) at 4 C (8) and subsequently reacted to Alexa 488 conjugated donkey-antirabbit F(ab)-fragments (dilution 1:1000; Molecular Probes, Inc., Eugene, OR). Coverslips were mounted with aquamount (Dako). Analysis of the sections was performed with an LSM510 confocal microscope (Zeiss, Oberkochen, Germany).
In situ hybridization
To examine the cellular coexpression of endocrine CCK and PC5/6, serial sections were probed to the PC5/6 antisense probe, CCK antiserum no. 92128, and PC5/6 sense probe. In situ hybridization was performed as described previously (31). In brief, mouse jejunal mucosa was fixed in 4% paraformaldehyde; 8-μm sections were placed on SuperFrostRPlus slides (Menzel GmbH, Braunschweig Germany), deparaffinized, and paraformaldehyde (4%) fixed, and treated with proteinase K (P-2308; Sigma, St. Louis MO); postfixed (4% paraformaldehyde); prehybridized for 1 h at 50 C; and incubated overnight with the labeled PC5/6 probe (50 C) specific for nucleotide. Visualization was made with anti-digoxigenin-AP (Roche Diagnostics GmbH, Mannheim, Germany) and the chromogens 5-bromo-4-chloro-3-indoyl-phosphate, 4-toluidine salt (Sigma; B-8503) and 4-nitro blue tetrazolium chloride (Sigma; N6876).
Statistics
All peptide concentrations are expressed in picomoles per gram tissue as means ± sem. Statistical analysis was carried out using an unpaired t test. Only P < 0.05 was considered significant.
Results
As shown in Table 1, lack of PC1/3 almost blocked the maturation of proCCK in the small intestine. The total translational product (proCCK, processing intermediates plus α-amidated products) increased from 87 pmol/g in the wild-type jejunum to 216 pmol/g in the knockout jejunum; but the increase was almost entirely due to a 40-fold enhanced accumulation of proCCK. The concentrations of the processing intermediates (CCK-G-R and CCK-G) were also increased but in absolute amounts only moderately. In contrast, the concentration of the bioactive, amidated CCK was reduced to a near block of synthesis. The reduction also contrasts with the entirely normal expression and processing of proCCK in the small intestine of PC2 knockout mice (Table 1). Notably, identical extraction techniques and methods of measurement were used in the present study of PC1/3 and the previous study of PC2 null mice (20).
Table 1.
Intestinal proCCK and its products in PC knockout mice and corresponding wild-type controls [picomoles per gram tissue (mean ± sem)]a
| Knockout | Wild type | P value | |
|---|---|---|---|
| PC1/3 (n = 4) | |||
| ProCCK | 187.3 ± 40.3 | 4.8 ± 1.5 | <0.001 |
| CCK-Gly-Arg | 5.6 ± 1.4 | <0.1 | <0.001 |
| CCK-Gly | 16.5 ± 6.0 | 7.0 ± 1.6 | <0.02 |
| CCK-amide | 6.3 ± 0.6 | 75.3 ± 6.3 | <0.001 |
| PC 2 (n = 8) | |||
| ProCCK | 7.4 ± 1.2 | 7.3 ± 1.1 | >0.05 |
| CCK-Gly-Arg | 4.0 ± 0.8 | 5.6 ± 0.8 | >0.05 |
| CCK-Gly | 4.0 ± 0.5 | 3.1 ± 0.4 | >0.05 |
| CCK-amide | 64.3 ± 9.5 | 57.3 ± 9.8 | >0.05 |
The PC 2 knockout results shown for comparison are published elsewhere (20).
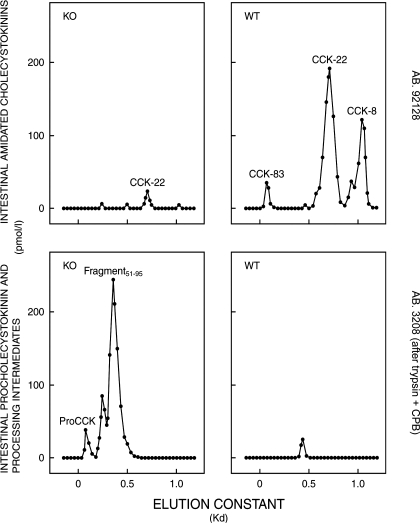
The chromatographic examination of intestinal extracts showed that the trace of α-amidated and O-sulfated CCK in the PC1/3 knockouts was CCK-22 (Fig. 2, upper left panel), which is also the predominant form of intestinal CCK in wild-type mice (Fig. 2, upper right panel). The high amount of precursors in the small intestines of the knockouts eluted like intact proCCK and a large fragment putatively containing both the CCK-33 sequence, the amidation site (G84, R85, R86), and the C-terminal flanking fragment (Fig. 2, lower left panel). The precursor pattern in PC1/3 knockouts deviated substantially from the wild types that expressed only traces of an intermediate size fragment corresponding to C-terminally extended CCK-33 (Fig. 2, lower right panel, and Table 2, upper part).
Figure 2.
Gel chromatography on Sephadex G-50 superfine columns of neutral water extracts from the small intestine of PC1/3 knockout mice (KO; left panels) and from corresponding wild-type mice (WT; right panels). The chromatographic elutions of carboxyamidated and tyrosylsulfated CCKs were monitored by a RIA using the CCK-specific antibody no. 92128 (upper panels), whereas the elutions of proCCK fragments were monitored after trypsin-CPB treatment by a RIA using antibody no. 3208 specific for glycine-extended CCK (lower panels). Extracts from four different KO and WT mice small intestines, respectively, were subjected to chromatography. Those shown are characteristic of each group. The peptide concentrations in acetic acid extracts were too low for chromatography.
Table 2.
Fractional AUC of proCCK and its products calculated from chromatographic curves of tissue extracts from PC1/3 knockout mice and corresponding wild-type controls [AUC (percentage of individual chromatographic curve (mean ± sem; n = 4])]
| PC1/3 knockout | Wild type | P value | |
|---|---|---|---|
| Intestinal proCCK and its products (see Fig. 2) | |||
| ProCCK | 14 ± 6 | <5 | <0.001 |
| CCK-Gly-(Arg) | 83 ± 27 | 100 | >0.05 |
| CCK-83a | <5 | 8 ± 3 | <0.02 |
| CCK-22a | 100 | 57 ± 21 | <0.05 |
| CCK-8a | <5 | 32 ± 10 | <0.001 |
| Cerebral proCCK and its products (see Fig. 3) | |||
| ProCCK | <5 | <5 | >0.05 |
| CCK-Gly-(Arg) I | 42 ± 19 | 10 ± 4 | <0.001 |
| CCK-Gly-(Arg) II | 16 ± 5 | 55 ± 19 | <0.001 |
| CCK-Gly-(Arg) III | 35 ± 14 | <5 | <0.001 |
| CCK-Gly-(Arg) IV | <5 | 34 ± 13 | <0.001 |
| CCK-83a | 12 ± 5 | <5 | <0.01 |
| CCK-8a | 86 ± 14 | 100 | >0.05 |
Carboxyamidated CCK.
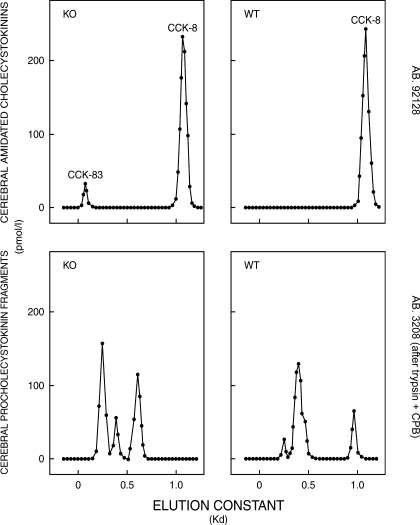
As shown in Table 3, the lack of PC1/3 was without significant effect on the neuronal maturation of proCCK. Hence, there is a remarkable similarity in the concentration of transmitter active, α-amidated CCK and its immediate precursor in PC1/3 knockout and the corresponding wild-type brains. This similarity contrasts with that found in PC2 knockout mice (Table 3), in which the cerebral processing of proCCK was grossly disturbed with a 9-fold increase in the proCCK concentration and a significant reduction in the concentration of α-amidated CCK (20). The gel chromatography revealed that, apart from a small amount of CCK-83-like immunoreactivity in the PC1/3 knockout brains, neuronal α-amidated CCK is almost entirely of octapeptide size as in normal mice (Fig. 3, upper panels, and Table 2, lower part). Despite almost similar concentrations, the size chromatography, however, showed that the CCK precursors in PC1/3 knockout brains are generally larger and hence to a lesser degree cleaved to CCK-8-like size (Fig. 3, lower panels, and Table 2, lower part). Thus, PC1/3 appears to have a minor effect on cerebral proCCK processing that influences the size of the processing intermediates.
Table 3.
Cerebral proCCK and its products in PC knockout mice and corresponding wild-type controls [picomoles per gram tissue (mean ± sem)]a
| Knockout | Wild type | P value | |
|---|---|---|---|
| PC1/3 (n = 4) | |||
| ProCCK | 16.8 ± 4.6 | 10.5 ± 2.2 | >0.05 |
| CCK-Gly-Arg | <0.1 | <0.1 | >0.05 |
| CCK-Gly | 26.2 ± 3.6 | 24.0 ± 3.0 | >0.05 |
| CCK-amide | 98.5 ± 4.6 | 97.1 ± 9.0 | >0.05 |
| PC 2 (n = 8) | |||
| ProCCK | 97.1 ± 16.5 | 11.3 ± 1.5 | <0.001 |
| CCK-Gly-Arg | 1.5 ± 0.4 | 3.0 ± 0.5 | >0.05 |
| CCK-Gly | 48.8 ± 6.3 | 28.4 ± 1.7 | <0.001 |
| CCK-amide | 88.0 ± 5.5 | 118.2 ± 8.1 | <0.01 |
The PC 2 knockout results shown for comparison are published elsewhere (20).
Figure 3.
Gel chromatography on Sephadex G-50 superfine columns of neutral water extracts from the whole brain of PC1/3 knockout mice (KO; left panels) and from corresponding wild-type mice (WT; right panels). The chromatographic elutions of carboxyamidated and tyrosylsulfated CCKs were monitored by a RIA using the CCK-specific antibody no. 92128 (upper panels), whereas the elutions of proCCK fragments were monitored after trypsin-CPB treatment by a RIA using antibody no. 3208 specific for glycine-extended CCK (lower panels). Extracts from four different KO and WT mice brains, respectively, were subjected to chromatography. Those shown are characteristic of each group. The peptide concentrations in acetic acid extracts were too low for chromatography. Although extracts of whole brain were used, it should be noted that nearly all CCK in the mammalian central nervous system is expressed in the cerebrum (3,4,12).
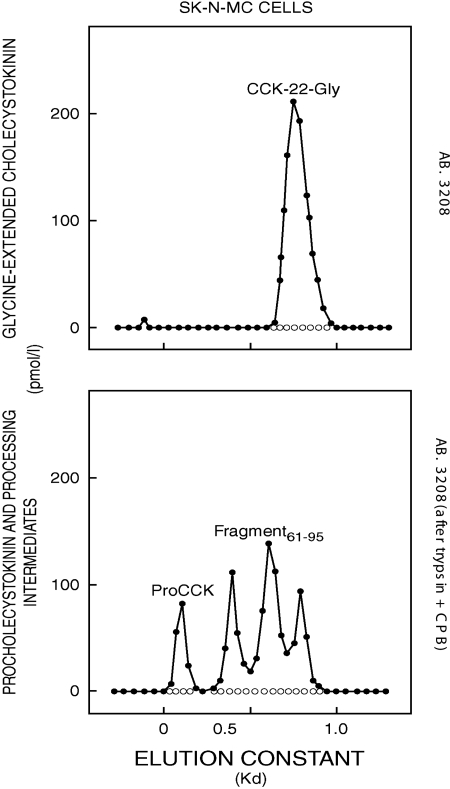
In the neuroepithelioma cell line, SK-N-MC, which expresses the CCK gene, however, proCCK hardly matured to bioactive, α-amidated peptides (Table 4) but was instead processed mainly to a peptide corresponding to glycine-extended CCK-22 in size and immunoreactivity (Fig. 4, upper panel) and larger processing intermediates extended beyond G84 at the C terminus (Fig. 4, lower panel). The cells also release substantial amounts of proCCK and the processing intermediates to the medium (Table 4). Gel chromatography revealed the same molecular forms in cell extracts and medium.
Table 4.
ProCCK and its products in the SK-N-MC cell line and its media [picomoles per gram and picomoles per liter, respectively (mean ± sem)]a
| SK-N-MC cells (n = 4) | SK-N-MC medium (n = 4) | |
|---|---|---|
| ProCCK | 19.4 ± 8.3 | 1724 ± 326 |
| CCK-Gly-Arg | 3.3 ± 1.6 | 74 ± 21 |
| CCK-Gly | 12.3 ± 5.8 | 180 ± 42 |
| CCK-amide | 1.2 ± 0.6 | 44 ± 16 |
SK-N-MC is a human neuroepithelioma cell line.
Figure 4.
Gel chromatography on Sephadex G-50 superfine columns of a neutral water extract from SK-N-MC cells that express the CCK gene. The chromatographic fractions were monitored by RIAs using antibody (AB) no. 3208 specific for glycine-extended CCK and confirmed with antibody no. 89009 specific for the N terminus of human CCK-22 (upper panel) or after trypsin-CPB treatment using the same RIAs (lower panel).
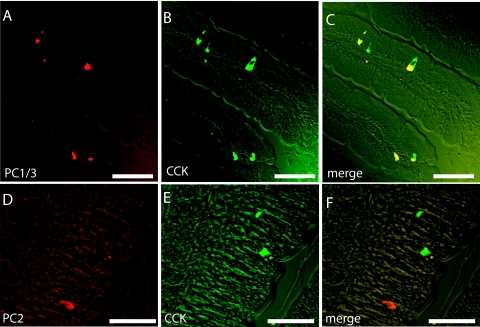
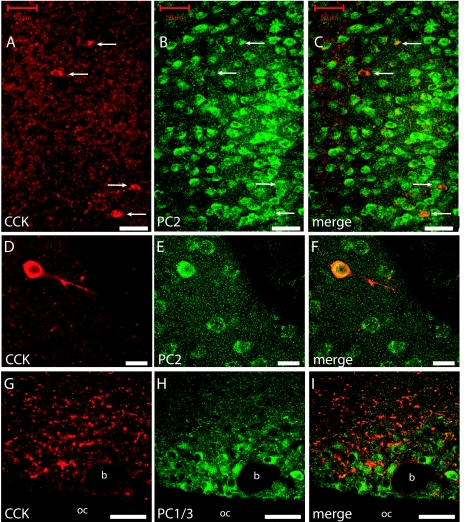
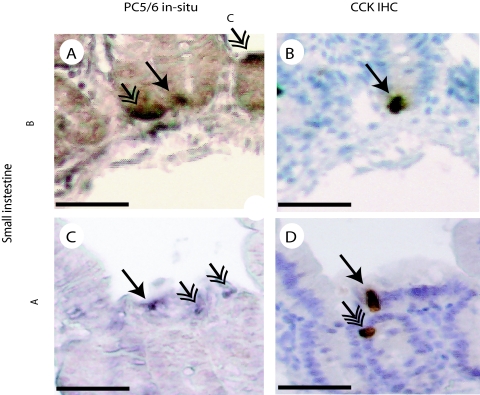
Immunocytochemistry supported the decisive role of PC1/3 in the intestinal CCK or I cells because the I cells in normal mice express PC1/3 (Fig. 5, A–C) but not PC2 (Fig. 5, D–F). On the other hand, cerebral CCK neurons express plenty of PC2 (Fig. 6, A–F), but almost no PC1/3 (Fig. 6, G–I). PC1/3-expressing neurons were found in several hypothalamic nuclei including the supraoptic nucleus. These neurons express no CCK, but CCK nerve fibers seem to innervate PC1/3-containing neurons (Fig. 6, G–I). The in situ hybridization showed that normal jejunal I or CCK cells coexpress PC5/6 (Fig. 7). Hence, the results indicate that PC5/6 may also play a minor role in the endocrine maturation of proCCK as seen in the SK-N-MC cells.
Figure 5.
Immunhistochemical visualization of CCK (green) and PC1/3 (red) (A–C) in I cells of the mouse small intestine. Note the complete colocalization of PC1/3 and CCK in these cells (A–C). In contrast, CCK (green) and PC2 were found in different cells of the of the small intestine (D–F). Scale bars, 50 μm (A-C); 25 μm (D–F).
Figure 6.
Immunohistochemical visualization of CCK (red) and PC2 (green) in the mouse cerebral cortex (A–F) and CCK (red) and PC1/3 (green) in the hypothalamic supraoptic nucleus (G–I). Note that all CCK-expressing neurons also express PC2 (arrows in A–C), whereas no colocalization were found between CCK and PC1/3 (G–I). Scale bars, 50 μm (A-C); 20 μm (D–F).
Figure 7.
Small intestinal colocalization of PC5/6 and CCK is shown in two different small intestinal samples. CCK-expressing cells were identified by immunohistochemistry (IHC) (B and D), whereas PC5/6-expressing cells were identified by in situ hybridization (A and C). The CCK-expressing cells that also express PC5/6 are indicated by an arrow with one arrowhead. Endocrine cells that express PC5/6 but not CCK are indicated by an arrow with two arrowheads. Lastly, a single cell that expresses CCK but not PC5/6 was also found and is indicated by an arrow with three arrowheads.
Discussion
This study has shown that the subtilisin-like prohormone convertase, PC1/3, is of decisive significance for the synthesis of bioactive CCK in intestinal endocrine cells. Hence, α-amidated and O-sulfated CCK peptides (the ligands for the CCK-A receptor) constituted only 2.9% of the total CCK mRNA translation product in the intestine of PC1/3 null mice, whereas the corresponding fraction in wild-type mice was 86.5% (Table 1). The significance of PC1/3 in the intestinal endocrine cells contrasts markedly with that observed in cerebral CCK neurons, in which the synthesis of amidated and sulfated CCK is essentially unaffected by the lack of PC1/3 (Table 2). Chromatography did, however, reveal small effects on the processing intermediates in the brain (Fig. 3). Consequently, the results indicate that small amounts of PC1/3 may be active in cerebral CCK neurons, as indicated also in a recent in situ hybridization study of rat brains (32) and by examination of the hippocampus, amygdala, and pons-medulla in mouse brains (33). But PC1/3 hardly plays a role of functional significance for the neuronal synthesis of transmitter-active CCK, i.e. carboxyamidated CCK.
The endocrine-specific significance of PC1/3 is opposite to that of the other neuroendocrine prohormone convertase, PC2. Hence, PC2 is not expressed in the intestinal endocrine I cells (Fig. 5) and therefore without effect on the endocrine proCCK processing, but PC2 displays major effects on the neuronal processing of proCCK (20,34). However, cerebral CCK neurons still synthesize amidated and transmitter-active CCK peptides in amounts that may be sufficient to ensure proper synaptic function in the PC2 knockouts. The negligible effect of PC1/3 and the moderate effect of PC2 on neuronal proCCK processing suggest either that the PC1/3 activity is up-regulated in CCK neurons of PC2 knockout mice or that other prohormone convertases contribute to the neuronal synthesis of transmitter-active CCK-8. One such candidate is PC5/6 that has been reported to be present in cerebral neurons (19), not the least in CCK neurons (32). PC5/6 has also been reported to cleave proCCK in neuronal cell lines at K61, R71, and R85R86 (21). However, in the present study using the PC5/6-containing SK-N-MC cells, we could not detect cleavage of the R71 site to generate CCK-12. This is in accordance with the fact that a CCK-12 peptide has not been purified and structurally identified from any tissue or cell line so far. Admittedly, comparison of processing patterns are hampered by the poor storage of neuroendocrine peptides in the de-differentiated SK-N-MC cells, which also renders additional cleavage at R75 in proCCK less likely. In any case, PC5/6 apparently does not cleave the monobasic R75 site, a prerequisite for release of CCK-8 (3). Additional convertases may therefore be necessary to explain the neuronal synthesis of CCK-8.
The tissue-specific CCK patterns in the gut vs. the brain are difficult to explain fully in terms of different PC activity only. Differences between endocrine cells and neurons in terms of granule and vesicle cycles probably also contribute to the observed patterns. Thus, the long-lived neurons allow a more complete processing toward the phenotypic endproduct of CCK gene expression (i.e. α-amidated and O-sulfated CCK-8), whereas the short-lived mucosal I cells in the gut may be shed off to the intestinal lumen before the endoproteolytic processing of proCCK has reached completion. Moreover, a different level or different kinetics of the cellular release may also influence the degree of prohormone processing (35,36). Nevertheless, the incomplete processing of endocrine proCCK in the gut may be physiologically expedient because CCK in plasma originates almost exclusively from the gut and not the brain. Hence, the food induces intestinal release to blood of a mixture of large- and intermediate-sized CCKs (CCK-58, CCK-33, and CCK-22) with a considerably slower metabolic clearance from plasma than CCK-8 (37). The longer CCK peptides may consequently ensure adequate stimulation of digestive functions during the entire period required for digestion (up to hours). Conversely, it is expedient that the CCK peptide required for synaptic transmission is as short and easily degraded as CCK-8.
As shown in Fig. 2, the only detectable form of amidated CCK in the gut of PC1/3 null mice was CCK-22. It is noteworthy because the synthesis of CCK-22 requires cleavage at the K61 residue, and Lys residues sometimes appear to be preferred for cleavage by PC2 rather than by PC1/3 (14,15,16,38), although single Lys cleavage sites may be too rare for such statement. On the other hand, CCK-22 is the most abundant form of CCK in the mouse intestine (Fig. 2). It is therefore possible that the small peak of CCK-22 simply reflects the presence of a very low level of PC2 undetectable by immunocytochemistry or the presence of other convertases in the I cells. One such possibility is that the I cells indeed contain PC5/6 (Fig. 7), which, as shown in the SK-N-MC cells, processes proCCK to glycine-extended CCK-22 (Fig. 4) that may subsequently be carboxyamidated by the amidation enzyme complex, peptidylglycine α-amidating monooxygenase (PAM). Whatever the mechanism, the tiny amount of bioactive CCK in the small intestine of PC1/3 null mice is hardly sufficient to ensure adequate hormonal CCK regulation. Shortage of CCK may therefore contribute to the significant intestinal and pancreatic deficiencies in PC1/3 knockouts (22).
Recently by in situ hybridization mapping, Cain et al. (32) showed that cerebral CCK neurons in the rat often express mRNA for PC1/3, PC2, and PC5/6. There was some regional variation in the coexpression pattern, but generally PC2 was the most abundant PC in terms of intensity and number of cells labeled. The results are in accordance with the present data from PC1/3 and those of PC2 knockout brains (20) as well as the immunocytochemical data from wild-type mice (Fig. 6). The results are also in good agreement with a recent study on regional CCK expression in the brain of PC2 knockout mice (34). Hence, PC2 plays a significantly larger role in cerebral proCCK maturation than PC1/3 and PC5/6. Further studies will be required to determine whether the endoproteolytic processing of proCCK requires additional prohormone convertases.
Acknowledgments
The skillful technical and secretarial assistance of Rikke Krøncke, Alice von der Lieth, Bo Lindberg, and Diana Skovgaard is gratefully acknowledged.
Footnotes
The work in the laboratory of J.F.R, was supported by grants from the Danish Medical Research Council and the Danish Biotechnology Program for Peptide Research and Cellular Communication. The work in the laboratory of D.F.S. was supported by National Institutes of Health Grants DK-13914 and DK-20595 and the Howard Hughes Medical Institute.
Author Disclosure Summary: The authors have nothing to declare.
First Published Online December 20, 2007
Abbreviations: AUC, Area under the curve; CCK, cholecystokinin; CP, carboxypeptidase; PC, prohormone convertase.
References
- Docherty K, Steiner DF 1982 Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol 44:625–638 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF 2004 Cholecystokinin. Best Pract Res Clin Endocrinol Metab 18:569–586 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF 1978 Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem 253:4022–4030 [PubMed] [Google Scholar]
- Crawley JN 1985 Comparative distribution of cholecystokinin and other neuropeptides. Why is this peptide different from all other peptides? Ann NY Acad Sci 448:1–8 [DOI] [PubMed] [Google Scholar]
- Goltermann N, Rehfeld JF, Petersen NR 1980 In vivo biosynthesis of cholecystokinin in rat cerebral cortex. J Biol Chem 255:6181–6185 [PubMed] [Google Scholar]
- Johnsen AH 1998 Phylogeny of the cholecystokinin/gastrin family. Front Neuroendocrinol 19:73–99 [DOI] [PubMed] [Google Scholar]
- Mutt V, Jorpes JE 1968 Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem 6:156–162 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF 1998 Accurate measurement of cholecystokinin in plasma. Clin Chem 44:991–1001 [PubMed] [Google Scholar]
- Bonetto V, Jörnvall H, Anderson M, Renlund S, Mutt V, Sillard R 1999 Isolation and characterization of sulphated and nonsulphated forms of cholecystokinin-58 and their action on gallbladder contraction. Eur J Biochem 264:336–340 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF, Sun G, Christensen T, Hillingsø JG 2001 The predominant cholecystokinin in human plasma and intestine is cholecystokinin-33. J Clin Endocrinol Metab 86:251–258 [DOI] [PubMed] [Google Scholar]
- Dockray GJ, Gregory RA, Hutchinson JB, Harris JI, Runswick MJ 1978 Isolation, structure and biological activity of two cholecystokinin octapeptides from sheep brain. Nature 274:711–713 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF, Hansen HF 1986 Characterization of preprocholecystokinin products in the porcine cerebral cortex. Evidence of different processing pathways. J Biol Chem 261:5832–5840 [PubMed] [Google Scholar]
- Lindefors N, Lindén A, Brené S, Sedvall G, Persson H 1993 CCK peptides and mRNA in the human brain. Prog Neurobiol 40:671–690 [DOI] [PubMed] [Google Scholar]
- Rouillé Y, Duguay SJ, Lund K, Furuta M, Gong Q, Lipkind G, Olivia AA, Chan SJ, Steiner DF 1995 Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Front Neuroendocrinol 16:322–361 [DOI] [PubMed] [Google Scholar]
- Steiner DF 1998 The proprotein convertases. Curr Opin Chem Biol 2:31–39 [DOI] [PubMed] [Google Scholar]
- Müller L, Lindberg I 2000 The cell biology of the prohormone convertases PC1 and PC2. Prog Nucleic Acids Res Mol Biol 63:69–108 [DOI] [PubMed] [Google Scholar]
- Taylor NA, van de Ven WJM, Creemers JWM 2003 Curbing activation: proprotein convertases in homeostasis and pathology. FASEB J 17:1215–1227 [DOI] [PubMed] [Google Scholar]
- Lusson J, Vieau D, Hamelin J, Day R, Chretién M, Seidah NG 1993 cDNA structure of the mouse and rat subtilisin/kexin-like PC5: a candidate proprotein convertase expressed in endocrine and nonendocrine cells. Proc Natl Acad Sci USA 90:6691–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve P, Seidah NG, Beaudet A 1999 Immunohistochemical distribution of the prohormone convertase PC5-A in rat brain. Neuroscience 92:641–654 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF, Lindberg I, Friis-Hansen L 2002 Increased synthesis but decreased processing of neuronal proCCK in prohormone convertase 2 and 7B2 knockout animals. J Neurochem 83:1329–1337 [DOI] [PubMed] [Google Scholar]
- Cain BM, Vishnuvardhan D, Beinfeld MC 2001 Neuronal cell lines expressing PC5, but not PC1 or PC2, process Pro-CCK into glycine-extended CCK 12 and 22. Peptides 22:1271–1277 [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhou A, Day A, Norrbom C, Caroll R, Zhang C, Laurent V, Lindberg I, Ugleholdt R, Holst JJ, Steiner DF 2002 Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci USA 99:10293–10298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourse KA, Friis-Hansen L, Samuelson LC, Rehfeld JF 1998 Altered processing of procholecystokinin in carboxypeptidase E-deficient fat mice: differential synthesis in neurons and endocrine cells. FEBS Lett 436:61–66 [DOI] [PubMed] [Google Scholar]
- Geijer T, Folkesson R, Rehfeld JF, Monstein HJ 1990 Expression of the cholecystokinin gene in a human (small-cell) lung carcinoma cell-line. FEBS Lett 270:30–32 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF 1978 Immunochemical studies on cholecystokinin. I. Development of sequence-specific radioimmunoassays for porcine triacontatriapeptide cholecystokinin. J Biol Chem 253:4016–4021 [PubMed] [Google Scholar]
- Hilsted L, Rehfeld JF 1986 Measurement of precursors for α-amidated hormones by radioimmunoassay of glycine-extended peptides after trypsin-carboxypeptidase B cleavage. Anal Biochem 152:119–126 [DOI] [PubMed] [Google Scholar]
- Paloheimo LI, Rehfeld JF 1994 A processing-independent assay for human procholecystokinin and its products. Clin Chim Acta 229:49–65 [DOI] [PubMed] [Google Scholar]
- Vindrola O, Lindberg I 1992 Biosynthesis of the prohormone convertase mPC1 in AtT-20 cells. Mol Endocrinol 6:1088–1094 [DOI] [PubMed] [Google Scholar]
- Müller L, Zhu X, Lindberg I 1997 Mechanism of the facilitation of PC2 maturation by 7B2: involvement in ProPC2 transport and activation but not folding. J Cell Biol 139:625–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler KS, Roth KA 1996 Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. J Histochem Cytochem 44:1331–1335 [DOI] [PubMed] [Google Scholar]
- Nielsen JE, Hansen MA, Jørgensen M, Tanaka M, Almstrup K, Skakkebæk NE, Leffers H 2003 Germ cell differentiation-dependent and stage-specific expression of LANCL1 in rodent testis. Eur J Histochem 47:215–222 [DOI] [PubMed] [Google Scholar]
- Cain B M, Connolly K, Blum A, Vishnuvardhan D, Marchand JE, Beinfeld MC 2003 Distribution and colocalization of cholecystokinin with the prohormone convertase enzymes PC1, PC2, and PC5 in rat brain. J Comp Neurol 467:307–325 [DOI] [PubMed] [Google Scholar]
- Cain BM, Connolly K, Blum AC, Vishnuvardan D, Marchand JE, Zhu X, Steiner DF, Beinfeld MC 2004 Genetic inactivation of prohormone convertase (PC1) causes a reduction in cholecystokinin (CCK) levels in the hippocampus, amygdala, pons and medulla in mouse brain that correlates with the degree of colocalization of PC1 and CCK mRNA in these structures in rat brain. J Neurochem 89:307–313 [DOI] [PubMed] [Google Scholar]
- Beinfeld MC, Blum A, Vishnuvardhan D, Fanous S, Marchand JE 2005 Cholecystokinin levels in prohormone convertase 2 knock-out mouse brain regions reveal a complex phenotype of region-specific alterations. J Biol Chem 280:38410–38415 [DOI] [PubMed] [Google Scholar]
- Jensen S, Borch K, Hilsted L, Rehfeld JF 1989 Progastrin processing during antral G-cell hypersecretion in humans. Gastroenterology 96:1063–1070 [DOI] [PubMed] [Google Scholar]
- Alarcón C, Leahy JL, Schuppin GT, Rhodes CJ 1995 Increased secretory demand rather than a defect in the proinsulin conversion mechanism causes hyperproinsulinemia in a glucose-infusion rat model of non-insulin-dependent diabetes mellitus. J Clin Invest 95:1032–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Fujimura M, Newman J, Zhu X-G, Greeley GH, Thompson JC 1985 Comparison of hepatic elimination of different forms of cholecystokinin in dogs. Bioassay and radioimmunoassay comparisons of cholecystokinin-8-sulfate and -33-sulfate. J Clin Invest 75:280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld JF, Lindberg I, Friis-Hansen L 2002 Progastrin processing differs in 7B2 and PC2 knockout animals: a role for 7B2 independent of action on PC2. FEBS Lett 510:89–93 [DOI] [PubMed] [Google Scholar]
- Bundgaard JR, Vuust J, Rehfeld JF 1995 Tyrosine O-sulfation promotes proteolytic processing of progastrin. EMBO J 14:3073–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]