Summary
Insect pathogens and parasites often affect the growth and development of their hosts, but understanding of these processes is fragmentary. Among the most species-rich and important mortality agents of insects are parasitoid wasps that carry symbiotic polydnaviruses (PDVs). Like many PDV-carrying wasps, Microplitis demolitor inhibits growth and pupation of its lepidopteran host, Pseudoplusia includens, by causing host hemolymph juvenile hormone (JH) titers to remain elevated and preventing ecdysteroid titers from rising. Here we report these alterations only occurred if P. includens was parasitized prior to achieving critical weight, and were fully mimicked by infection with only M. demolitor bracovirus (MdBV). Metabolic assays revealed that MdBV infection of pre-critical weight larvae caused a rapid and persistent state of hyperglycemia and reduced nutrient stores. In vitro ecdysteroid assays further indicated that prothoracic glands from larvae infected prior to achieving critical weight remained in a refractory state of ecdysteroid release, whereas infection of post-critical weight larvae had little or no effect on ecdysteroid release by prothoracic glands. Taken together, our results suggest MdBV causes alterations in metabolic physiology, which prevent the host from achieving critical weight. This in turn inhibits the endocrine events that normally trigger metamorphosis.
Keywords: insect, infection, growth, molting, parasite
INTRODUCTION
All growth in insects occurs during the premetamorphic nymphal or larval stages, and is regulated by the interactions between nutrition, other environmental factors and hormones (Nijhout, 1994; Nijhout, 2003). Studies with Lepidoptera (moths and butterflies) and Diptera (flies) indicate the final size an insect achieves is a function of a species-specific threshold (critical weight) that must be attained during the final instar to molt to a pupa without developmental delay (Nijhout, 1975). In Lepidoptera, reaching critical weight triggers the release of the neuropeptide prothoracicotropic hormone (PTTH), which stimulates the prothoracic glands to synthesize and release ecdysteroids. This causes the larva to stop feeding and initiate premetamorphic behaviors like wandering and cocoon spinning, while a second release of ecdysteroids causes the larva to pupate (Riddiford, 1976; Gilbert et al., 2002). Less clear is how insects mechanistically assess when a size threshold like critical weight has been reached. Positive regulators include nutrient acquisition and metabolic activity mediated through the insulin and TOR signaling pathways, whereas juvenile hormone (JH), ecdysteroids and the glucagon-like adipokinetic hormones (AKHs) can repress growth under conditions of stress such as starvation (Takaki and Sakurai, 2003; Oldham and Hafen, 2003; Truman et al., 2006; Mirth and Riddiford, 2007; Baker and Thummel, 2007).
Infection by microbial pathogens and multicellular parasites represents another stress that can profoundly affect growth and metabolism (Andersen et al., 2004; Hotamisligil, 2006), yet we know little about the mechanisms underlying growth-related pathology in insects (Thompson, 1993; Dionne et al., 2006; Adamo, 2008). Polydnavirus (PDV)-carrying parasitoid wasps are among the most species-rich and important mortality agents of insects (Pennacchio and Strand, 2006). Most of these wasps parasitize larval stage Lepidoptera by laying one or more eggs into the hemocoel of the host, and their progeny develop by consuming host blood and/or tissues. Development also fully depends on infection of the host by the wasp's associated PDV (Webb and Strand, 2005). The Polydnaviridae are DNA viruses with large multipartite genomes that only exist in association with parasitoid wasps. Each PDV carried by a given wasp species is genetically unique and persists as a stably integrated provirus in both sexes. Transmission to the wasp's offspring is strictly vertical through the germ line, while replication occurs only in the reproductive tract of female wasps, who inject a quantity of virus when ovipositing in a host. Importantly, PDVs do not replicate in the wasp's host but expression of viral gene products causes or contributes to several physiological alterations including suppression of the immune response, which is essential for survival of the wasp's progeny (Kroemer and Webb, 2004; Webb and Strand, 2005; Strand, 2008).
Parasitized insects usually exhibit reduced hemolymph ecdysteroid titers that in the final instar are associated with altered growth and inhibition of pupation (Beckage and Gelman, 2004; Pennacchio and Strand, 2006). Most studies have focused on the hypothesis that the wasp or its associated PDV produce molecules which directly reduce ecdysteroid titers by altering processing or release of PTTH (Tanaka et al., 1987; Tanaka and Vinson, 1987; Zitnan et al., 1995; Kelly et al., 1998), disrupting ecdysteroid biosynthesis by prothoracic glands (Pennacchio et al., 1998; Pennacchio et al., 2001; Fallabella et al., 2003), or metabolizing ecdysteroids in circulation (Grossniklaus-Burgin et al., 1998; Coudron et al., 1990). An alternative hypothesis is that inhibition of pupation is an indirect response to virus- and/or parasitoid-induced alterations in host growth or nutritional physiology (Thompson, 1993; Pennacchio and Strand, 2006). Here, we conducted studies with the braconid wasp Microplitis demolitor, its associated PDV named M. demolitor bracovirus (MdBV) and its host, the soybean looper, Pseudoplusia includens (Lepidoptera: Noctuidae). Our results indicate that MdBV is primarily if not exclusively responsible for arresting host growth and pupation of P. includens, but these events only occur if hosts are infected prior to achieving critical weight.
MATERIALS AND METHODS
Insects and host staging
Pseudoplusia includens Walker 1858 larvae were reared as previously described on an agar-based artificial diet in plastic rearing cups at 27±1°C with a 16 h light (L):8 h dark (D) photoperiod (lights on 10:00 h, lights off 02:00 h) (Strand, 1990). Pseudoplusia includens larvae undergo five instars before pupation, and were physiologically staged using previously established morphological criteria (Strand, 1990). The point immediately following ecdysis to the fifth instar was designated as 0 h with subsequent events recorded as hours post-ecdysis to the fifth instar. For most experiments, larvae were inspected every 6 h. Pseudoplusia includens larvae that were left untreated (i.e. not parasitized or infected with virus) were referred to as normal. Microplitis demolitor Wilkinson 1934 is a solitary endoparasitoid that produces a single offspring per host and parasitizes all instars of P. includens. Wasps were reared as outlined previously (Strand et al., 1988). Hosts in which M. demolitor naturally oviposited were referred to as parasitized.
MdBV collection and host infection
MdBV was collected from wasps as previously described (Strand et al., 1992; Beck and Strand, 2003). For some experiments, MdBV was inactivated by treatment with psoralen and UV light before injection (Strand et al., 1992). As is convention in the PDV literature, the amount of MdBV collected from the reproductive tract of a single adult female was defined as one wasp equivalent. One wasp equivalent on average equals 1×1010 virions and wasps inject 0.1–0.01 wasp equivalents per host during oviposition (Beck et al., 2007). For this study, we injected 0.1 wasp equivalents of MdBV suspended in Grace's insect medium (Sigma, St Louis, MO, USA) into CO2-anesthetized P. includens fifth instars at specific times post-ecdysis to the fifth instar using a glass needle mounted on a micromanipulator (Beck et al., 2007). In this study, larvae injected with MdBV were referred to as infected, while larvae injected with Grace's medium alone were referred to as mock-infected controls.
Measurement of growth and determination of critical weight
All larvae used in the study were directly observed during apolysis of the fourth instar and molted to the fifth instar between 15:00 and 22:00 h. Normal, parasitized and MdBV-infected larvae were maintained individually in plastic rearing cups and fed ad libitum on artificial diet. Larvae were starved by transferring individuals to rearing cups containing only agar at particular times during the final instar. Wet masses of larvae after different treatments were measured at specific times using an analytical balance (Ohaus, Waukegan, IL, USA). The total wet mass of diet a larva consumed was indirectly estimated by collecting and weighing the fecal pellets (frass) every 24 h. All larvae were monitored until death, pupation or molting to a larval–pupal intermediate. Larvae from the above treatments were also collected at specific times to measure nutrient reserves or collect prothoracic glands for in vitro ecdysteroid assays.
Analysis of nutrient reserves
Hemolymph from CO2-anesthetized P. includens was collected from a cut proleg into anticoagulant buffer (98 mmol l–1 NaOH, 146 mmol l–1 NaCl, 17 mmol l–1 EDTA, 41 mmol l–1 citric acid; pH adjusted to 4.5; 1:1 v/v). Hemocytes were removed by centrifugation for 2 min at 200g. The supernatant (plasma) was boiled for 5 min, centrifuged for 15 min at 14,000g, and stored at –80°C. Hemolymph blood sugar titers (glucose+trehalose) were measured using a glucose oxidase assay (Sigma). In 96-well plates, boiled plasma samples (0.5–5μl) were combined with 50μl of porcine kidney trehalase (Sigma; 1:20 in PBS) and phosphate-buffered saline (PBS) to a total volume of 100μl at 37°C for 3 h. Aliquots of the above samples were then added to 200μl of glucose oxidase/peroxidase reagent (Sigma; 0.25μg ml–1 in DMSO) and 2μl of Amplex Red (Invitrogen, Carlsbad, CA, USA) in microfuge tubes for 30 min at room temperature. After transfer to a Costar 96-well plate, the fluorescence of each sample was measured at 530 nm excitation and 590 nm emission with a Biotek Synergy 4 plate reader (Winooski, VT, USA). Total glycogen and lipid (triglyceride) in larval tissues was measured as outlined by Telang and Wells (Telang and Wells, 2004). Briefly, larvae used for the hemolymph samples were dissected in PBS to remove the gut. The remaining tissues were placed in 400μl of saturated Na2SO4 and frozen (–80°C). Whole-body homogenates were then diluted so that glycogen and triglyceride amounts fell within the standard range of modified anthrone–sulfuric acid and vanillin–phosphoric acid assays, respectively.
Hormone titers and in vitro ecdysteroid secretion by prothoracic glands
Hemolymph ecdysteroid titers in non-infected and M. demolitor-parasitized fifth instar P. includens were determined by radioimmunoassay (RIA) as previously outlined (Strand et al., 1990). To measure ecdysteroid secretion by prothoracic glands in short-term primary culture, normal, MdBV-infected and starved larvae were dissected in PBS at specific times in the fifth stadium to remove each pair of prothoracic glands. A single gland was then placed in a 25 μl drop of Grace's insect medium for a 1 h preincubation period at 27°C. Ecdysteroid release was then measured by incubating each gland in a fresh 25 μl drop of Grace's medium for an additional 3 h at 27°C with or without the cell-permeable cAMP analog dibutyryl cyclic AMP (dbcAMP; 10 mmol l–1) or 5 equivalents of brain extract. dbcAMP (Sigma) was prepared as 100 mmol l–1 stock in water that was frozen at –80°C and diluted to a 10 mmol l–1 working solution in Grace's medium before use in assays. To prepare brain extracts, brains were collected from 20 late-feeding stage (52 h) fifth instars, washed three times in 1 ml Grace's medium and then homogenized on ice in 100 μl of medium. After filter sterilization using a 0.2 μm filter (Acrodisc, Pall Life Sciences, Ann Arbor, MI, USA), 5 brain equivalents of extract were used per bioassay.
Ecdysteroids were quantified in the incubation solution by RIA using the procedure of Sieglaff et al. (Sieglaff et al., 2005). Briefly, each experimental sample contained 5–10 μl of the incubation solution in a 300 μl total volume. Anti-ecdysteroid rabbit serum (AS 4919, a generous gift from P. Porcheron; Université P. et M. Curie, Paris, France) that recognizes ecdysone and 20-hydroxyecdysone equally was diluted to 1:25,000–1:35,000. Sample values reported for each treatment were means of three to five replicates. Because the anti-ecdysteroid antibody had non-specific binding to brain extract, appropriate dilutions of brain extract alone were assayed and their values subtracted from values obtained for prothoracic glands stimulated with brain extract. Prothoracic gland cell size was estimated by measuring the length and width of four randomly selected cells in the loop and body region of a gland using a Leica IRE2 inverted epifluorescence microscope (Bannockburn, IL, USA) fitted with a Hamamatsu digital camera (Bridgewater, NJ, USA) and SimplePCI software (Compix, Cranberry, PA, USA). Mean gland cell size was then recorded in μm2 (±s.e.).
Data analyses
All data were analyzed using JMP 7.0 software (SAS Institute, Cary, NC, USA). Comparisons of frass weight, nutrient stores and ecdysteroid release from prothoracic glands were made by one-way analysis of variance (ANOVA) and a post-hoc Tukey–Kramer multiple comparison procedure for data with uneven sample sizes. Data comparing ecdysteroid release by unstimulated and dbcAMP-stimulated prothoracic glands in control larvae were analyzed by Student's paired t-tests.
RESULTS
Parasitism suppresses weight gain and inhibits metamorphosis of pre-critical weight P. includens larvae
We compared the growth and associated endocrine events of normal P. includens during its final (fifth) instar with those of larvae parasitized by M. demolitor by combining previous results reporting staging characters and hemolymph JH titers (Strand, 1990; Balgopal et al., 1996) with data collected during the current study in which we determined size thresholds and hemolymph ecdysteroid titers. Following the criteria of Nijhout (Nijhout, 1975), we defined the threshold size for metamorphosis as the size a larva must equal or surpass in the penultimate instar in order to pupate after molting to the ultimate (final) instar, with failure to do so resulting in a supernumerary larval instar. In P. includens, all larvae with a head capsule width ≥1.18 mm and weighing more than 36 mg at the end of the fourth instar pupate after the fifth instar (Strand, 1990). Minimal viable weight was defined as the mass >90% of larvae must attain to survive and molt to a pupa albeit with developmental delay when compared with normally fed larvae. Critical weight was defined as the mass >90% of larvae must attain in the final instar before starvation to pupate without developmental delay when compared with larvae fed ad libitum. Below the minimal viable weight, larvae died or eventually molted to larval–pupal intermediates.
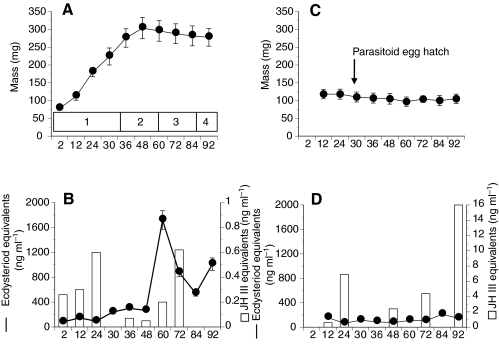
Starvation experiments established that P. includens attained a minimal viable weight averaging 223±21 mg (mean ± s.d., N=40) between 24 and 30 h, and critical weight (275±22 mg, N=40) by 36 h (Fig. 1A). Larvae ceased post-critical weight feeding between 48 and 56 h in association with a drop in hemolymph JH titers to near undetectable levels, and initiated wandering (60 h) and cocoon spinning behavior (66 h) in concert with a large rise in hemolymph ecdysteroid titer (Fig. 1B). A second rise in ecdysteroid titer then preceded the molt to a pupa between 88 and 92 h (Fig. 1B). In contrast, P. includens fifth instars parasitized by M. demolitor at 12 h subsequently gained almost no weight and never initiated wandering or other events associated with metamorphosis (Fig. 1C). Hemolymph JH titers remained elevated in parasitized larvae and no increase in hemolymph ecdysteroid titer occurred over 92 h (Fig. 1D). Over this same period, the M. demolitor egg hatched 26–28 h after oviposition (host age 38–40 h, Fig. 1C). The wasp thereafter undergoes three larval molts over 5 days while feeding exclusively on host hemolymph (Strand et al., 1988; Harvey and Strand, 2002). The fourth instar wasp larva then emerges from the host on day 6 (host age 144–156 h), spins a cocoon, and pupates on day 7.
Fig. 1.
Growth, ecdysteroid, and juvenile hormone (JH) titers in normal fifth instar P. includens larvae (A,B) and fifth instars parasitized at 12 h by M. demolitor (C,D). (A) The mean ± s.d. wet mass of P. includens is plotted in relation to time (hours) post-ecdysis and phase of growth as indicated in the box above the x-axis. Stages of growth are: 1, pre-critical weight feeding phase with minimum viable weight being achieved between 24 and 30 h and critical weight being achieved by 36 h; 2, post-critical weight feeding phase; 3, wandering and cocoon-spinning phase; and 4, pupation. (B) Hemolymph ecdysteroid titers are shown as a black line (± s.e.), while JH titers are shown as open bars. (C) The wet mass of parasitized P. includens is plotted in relation to time (hours) post-ecdysis. The timing of hatching of the M. demolitor egg is indicated. (D) ecdysteroid and JH titers are shown as described in B but note the difference in scaling of the y-axis for JH. Sources: staging characters for P. includens (Strand, 1990), hemolymph JH titers in normal and parasitized larvae (Balgopal et al., 1996). Critical sizes and hemolymph ecdysteroid titers were determined during the current study.
MdBV infection mimics the developmental alterations associated with parasitism
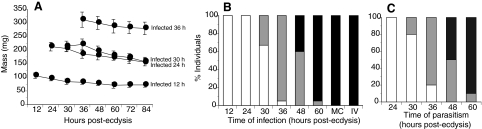
We next asked whether the effects of parasitism on host growth required the presence of the parasitoid's offspring or was mimicked by infection with only MdBV. We also asked how late into the fifth stadium infection or parasitism could occur and still prevent P. includens from pupating. Our results indicate that fifth instars infected with a physiological dose of MdBV at 12 h and 24 h post-ecdysis ceased gaining additional mass and died as larvae (Fig. 2) like hosts parasitized at the same period (Fig. 1C). Larvae infected or parasitized after attaining critical weight (36 h) also ceased gaining additional mass but most subsequently initiated cocoon spinning and molted to larval–pupal intermediates. Almost all larvae infected or parasitized at 60 h (wandering) pupated without developmental delay (Fig. 2B,C). Larvae infected at 60 h subsequently emerged as adults but hosts that were parasitized at 60 h often failed to emerge as adults and died as pupae (data not shown). Regardless of the timing of infection, all P. includens larvae injected with psoralen-inactivated MdBV pupated and emerged as normal adults (Fig. 2B). These results indicate that suppression of host growth depended primarily if not exclusively on MdBV infection, and that inhibition of pupation only occurred if hosts were infected prior to achieving critical weight.
Fig. 2.
Effects of infection by M. demolitor bracovirus (MdBV) and parasitism by M. demolitor on wet mass and metamorphosis of P. includens fifth instars. (A) Wet mass of larvae infected by MdBV at 12, 24, 30 or 36 h. N=30 larvae per treatment. See Fig. 1A,C for comparison with growth of normal larvae and larvae parasitized at 12 h. (B) Effect of MdBV infection at 12–60 h on metamorphosis. Shown are percentages of individuals that died as fifth instars (open bars), that molted to a larval–pupal intermediate (grey bars), or that molted to a pupa (black bars). MC indicates the response of mock-infected control larvae while IV indicates the response of larvae injected at 12 h with inactivated MdBV. N=20 larvae per treatment for a total of 180 individuals. (C) Effect of M. demolitor parasitism on metamorphosis of P. includens fifth instars. Hosts were parasitized at 24, 30, 36, 48 or 60 h. Bars indicating the percentage of individuals that died as larvae, molted to larval–pupal intermediates or molted to pupae are as in B. N=20 larvae per treatment for a total of 100 individuals.
MdBV infection rapidly induces hyperglycemia and depletes nutrient stores
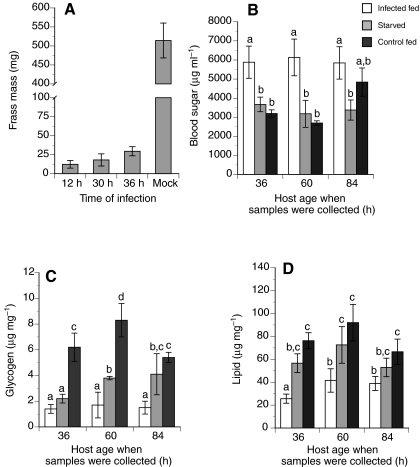
The significant reduction in mass gain that occurred after virus infection correlated with a similar decline in food consumption and frass mass when compared with mock-infected control larvae (Fig. 3A). This suggests that similar to starvation, infection of pre-critical weight larvae inhibited pupation by preventing larvae from attaining the nutrients necessary to achieve viable or critical weight. Yet measures of blood sugar titers and nutrient stores revealed that the effects of virus infection did not mirror the effects of starvation. Most notably, hosts infected with MdBV exhibited significantly higher blood sugar levels that resulted in a persistent hyperglycemic state, whereas starvation of non-infected larvae caused little change in blood sugar levels when compared with mock-infected control larvae fed ad libitum (Fig. 3B). Virus-infected larvae also exhibited reduced glycogen and lipid stores (Fig. 3C,D).
Fig. 3.
Total wet mass of frass produced and nutrient reserves of MdBV-infected, starved or mock-infected P. includens fifth instars. (A) Wet mass of frass produced by larvae infected with virus at 12, 30 or 36 h compared with controls injected with Grace's medium only at 12 h (Mock). N=20 larvae per treatment. Bars with different letters are significantly different from one another (Tukey–Kramer HSD, P≤0.05). (B) Blood sugar amounts (trehalose+glucose) from larvae infected with MdBV at 12 h (Infected fed), larvae starved beginning at 12 h (Starved), or mock-infected control larvae injected with Grace's medium at 12 h (Control fed). Samples were collected at 36, 60 and 84 h. (C,D) Glycogen and lipid amounts for the same treatments as in B. All values are means ± s.e. with a minimum of three replicates per treatment and time point. Bars with different letters are significantly different (Tukey–Kramer HSD, P≤0.05).
Prothoracic glands from pre-critical weight larvae release low amounts of ecdysteroids
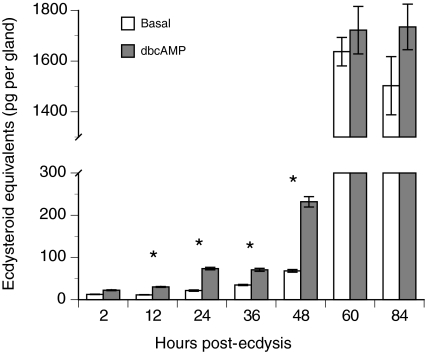
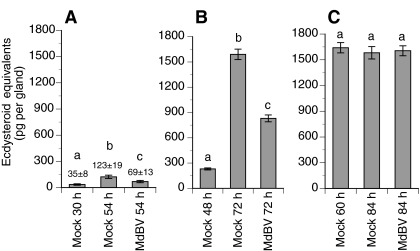
Studies of the silkmoth, Bombyx mori, indicate that prior to attaining critical weight, prothoracic glands from last instars release little or no ecdysteroids and are insensitive to stimulation by PTTH or cAMP analogs (Okuda et al., 1985; Gu et al., 1997; Gu et al., 2000; Takaki and Sakurai, 2003). After attaining critical weight, prothoracic glands become sensitive to PTTH or cAMP stimulation, and produce large amounts of ecdysteroids in the absence of stimulation. In vitro ecdysteroid assays with prothoracic glands from normal P. includens fifth instars revealed a similar pattern (Fig. 4). Prothoracic glands from 2–36 h old fifth instars (pre-critical weight) released low basal levels of ecdysteroids, and while treatment with dbcAMP significantly increased ecdysteroid release by 12, 24 and 36 h old glands, quantities were still very low (Fig. 4). Basal ecdysteroid release and the stimulatory effect of dbcAMP treatment were higher for prothoracic glands from early (48 h) post-critical weight larvae than observed for glands from pre-critical weight larvae (Fig. 4). In contrast, prothoracic glands from 60 h (wandering stage) and 84 h (late cocoon spinning) larvae released large quantities of ecdysteroids whether cultured in the presence of dbcAMP or not (Fig. 4). We then compared ecdysteroid release by prothoracic glands from virus-infected larvae with that by glands from mock-infected control larvae. In these assays, larvae were injected with virus or Grace's medium alone at 30 h (pre-critical weight), 48 h (early post-critical weight) or 60 h (late post-critical weight wandering stage), followed by collection and in vitro assay of the prothoracic glands immediately afterwards (30, 48 or 60 h respectively) or 24 h later (54 h, 72 h or 84 h, respectively). At the 30 h time point, prothoracic glands from mock-infected control larvae, as expected, released little ecdysteroid while at 54 h glands released more ecdysteroids than at the time of injection but amounts remained low (Fig. 5A). At 54 h, prothoracic glands from virus-infected larvae also released low amounts of ecdysteroids, intermediate between the 30 and 54 h control samples (Fig. 5A). Prothoracic glands from larvae infected with MdBV at 48 h and bioassayed at 72 h also released ecdysteroid amounts intermediate between the mock-infected control samples, but amounts were also much higher than those from glands of larvae infected with virus at 30 h (Fig. 5B). Prothoracic glands from larvae infected with MdBV at 60 h and bioassayed at 84 h produced the same large amounts of ecdysteroids as 60 and 84 h glands from mock-infected controls (Fig. 5C).
Fig. 4.
In vitro release of ecdysteroids (means ± s.e.) by prothoracic glands from P. includens fifth instars. Each pair of prothoracic glands was collected by dissecting cohorts of larvae from 2 to 84 h of the fifth instar. Basal secretion was determined for one prothoracic gland from a given larva by incubating in Grace's medium only for 3 h, while stimulated secretion was determined for the other prothoracic gland by incubating in Grace's medium plus 10 mmol l–1 dbcAMP for 3 h. A minimum of seven gland pairs were bioassayed per time point. Asterisk indicates a significant difference between the basal and stimulated treatment for a given time point (paired t-test, P≤0.05).
Fig. 5.
In vitro release of ecdysteroids (means ± s.e.) by prothoracic glands from MdBV- or mock-infected P. includens fifth instars. For each treatment, larvae were injected with MdBV or Grace's medium only (Mock) at 30 h (A), 48 h (B) or 60 h (C). Prothoracic glands were then collected immediately after injection [30 h (A), 48 h (B), or 60 h (C)] or 24 h after injection [54 h (A), 72 h (B) or 84 h (C)] and bioassayed for basal ecdysteroid production. A minimum of five glands were bioassayed per time point and treatment. Bars with different letters are significantly different (Tukey–Kramer HSD, P≤0.05).
Prothoracic glands from pre-critical weight infected larvae remain insensitive to stimulation
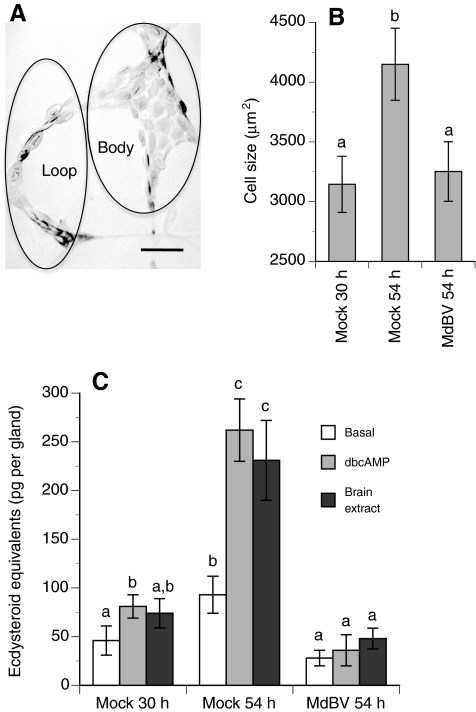
The preceding results indicate that prothoracic glands from virus-infected larvae released little ecdysteroid if the host was infected prior to achieving critical weight (30 h) but also indicate that infection of post-critical weight larvae had no effect (60 h) on ecdysteroid release. Given evidence from Drosophila suggesting that prothoracic gland cell size correlates with: (1) competency to respond to stimulation and (2) assessment of when a larva achieves critical weight (Mirth et al., 2005), we hypothesized that MdBV suppresses pupation of pre-critical weight larvae by preventing gland cells from growing to a size that is responsive to stimulation or capable of high level ecdysteroid production. Measurements indicated that prothoracic gland cells increased significantly in size between 30 h and 54 h in mock-infected control larvae, whereas cell size remained unchanged at 54 h in larvae that were infected at 30 h with MdBV (Fig. 6A,B). Subsequent measurements indicated that prothoracic gland cell size in infected larvae also remained unchanged at 84 h and 108 h (data not presented). Correspondingly, basal levels of ecdysteroid release by prothoracic glands from mock-infected control larvae at 30 h were low and only weakly stimulated by dbcAMP or brain extract, whereas prothoracic glands at 54 h produced higher basal levels of ecdysteroids and were more responsive to stimulation by dbcAMP and brain extract (Fig. 6C). In contrast, 54 h old prothoracic glands from larvae infected by MdBV at 30 h released low basal amounts of ecdysteroids akin to 30 h control glands and were insensitive to stimulation by dbcAMP or brain extract (Fig. 6C).
Fig. 6.
Prothoracic gland morphology and in vitro release of ecdysteroids (means ± s.e.) by glands from MdBV- or mock-infected P. includens fifth instars. (A) Light micrograph of a prothoracic gland from a 30 h fifth instar showing two domains designated as the loop and gland body region. Scale bar, 100 μm. (B) Estimated size of prothoracic gland cells. Fifth instars were injected with MdBV or Grace's medium only (Mock) at 30 h. Cell size was then determined for prothoracic glands from mock-infected larvae immediately after injection of medium only (30 h) or 24 h later (54 h). Cell size for prothoracic glands from MdBV-infected larvae was determined 24 h after infection (54 h). Cells from three prothoracic glands were measured per treatment. Bars with different letters are significantly different from one another (Tukey–Kramer HSD, P≤0.05). (C) In vitro release of ecdysteroids (means ± s.e.) by prothoracic glands from MdBV- or mock-infected fifth instars. Prothoracic glands were collected immediately after injection (30 h) or 24 h post-injection (54 h) as described in B. Basal, dbcAMP-stimulated and brain extract-stimulated levels of ecdysteroid released by glands were then determined. A minimum of four prothoracic glands were bioassayed per treatment and time point. Bars with different letters are significantly different from one another (Tukey–Kramer HSD, P≤0.05).
DISCUSSION
Like many hosts parasitized by PDV-carrying wasps (Beckage and Gelman, 2004; Pennacchio and Strand, 2006), P. includens exhibits a marked reduction in food consumption, static or declining weight, and a failure to pupate after parasitism by M. demolitor (Strand et al., 1988; Strand and Dover, 1991; Harvey and Strand, 2002). In this study, we have shown that last instar P. includens only exhibit these symptoms if parasitized or infected by MdBV prior to achieving critical weight. Our results also show that MdBV infection of pre-critical weight larvae induces a rapid and persistent state of hyperglycemia, reduces nutrient stores and causes prothoracic glands to remain in a persistently refractory state of basal ecdysteroid release. Taken together, these findings indicate that developmental arrest of parasitized hosts depends primarily on MdBV and does not require the presence of the M. demolitor larva or other factors (venom, teratocytes) the wasp introduces into the hemocoel when ovipositing. Our results also suggest a requirement for viral gene activity because hosts infected with inactivated MdBV pupate normally.
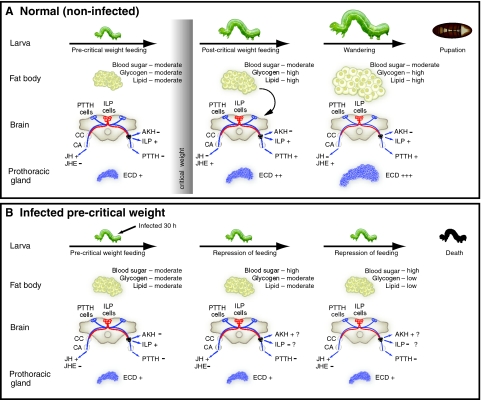
Two possibilities exist for why MdBV inhibits pupation of pre- but not post-critical weight larvae. The first is that post-critical weight larvae are refractory to infection or that insufficient time exists after infection of post-critical weight larvae for the effects of viral expression to block pupation. We think this unlikely given prior analyses of the fully sequenced MdBV genome which indicate transcription of most viral genes begins within 2 h of infection and continues near steady state for multiple days thereafter with no differences between last instar hosts infected pre- and post-critical weight (Strand et al., 1992; Strand, 1994; Webb et al., 2006; Pruijssers and Strand, 2007; Beck et al., 2007) (and M.R.S. and M. H. Beck, unpublished). Functional assays of immunosuppressive viral gene products also reveal similar activity in pre- and post-critical weight larvae (Beck and Strand, 2005; Beck and Strand, 2007; Lu et al., 2008). Taken together, these data indicate that MdBV rapidly infects and alters several physiological processes independent of when in an instar the host is infected. The second possibility then is that MdBV inhibits metamorphosis not by directly suppressing a process like ecdysteroid biosynthesis/release but by affecting other events essential for pupation that occur in pre-critical weight larvae. Our results more strongly support this option and in particular favor the hypothesis that virus-induced alterations in metabolic physiology prevent the host from achieving critical weight. This in turn prevents endocrine events that normally regulate metamorphosis from occurring. We summarize our hypothesis through a conceptual model presented in Fig. 7.
Fig. 7.
A model for MdBV-mediated suppression of host growth and pupation. (A) Growth and metamorphosis of normal (non-infected) P. includens larvae during the fifth instar. Growth of pre-critical weight larvae is nutrition dependent. Upon achieving critical weight, growth and differentiation become nutrition independent. Signaling from the fat body associated with accumulation of sufficient nutrient stores suppresses feeding, down regulates JH synthesis by the corpora allata (CA), and increases JH metabolism in the hemolymph by juvenile hormone esterase (JHE). An absence of JH stimulates release of prothoracicotropic hormone (PTTH) from the brain which increases ecdysteroid (ECD) production by the prothoracic glands. Increased size of prothoracic glands correlates with competency to respond to PTTH and high-level production of ecdysteroids necessary for pupation. The broader literature also indicates production of insulin-like peptides (ILPs) and repressed release of adipokinetic hormone (AKH) from the corpora cardiaca (CC) maintains blood sugar titers and nutrient stores (see text). (B) MdBV infection of pre-critical weight larvae results in arrested growth and inhibition of metamorphosis. As growth and development of pre-critical weight larvae are nutrition dependent, we hypothesize that MdBV arrests growth and development by disrupting feeding and metabolism. The rapid onset of hyperglycemia, progressive loss of nutrient stores, and arrested growth of tissues like the prothoracic glands also suggest MdBV could suppress ILP signaling or increase signaling by AKH. Infection could also block metamorphosis by elevating JH titers and preventing high level release of ecdysteroids by the prothoracic glands.
Normal growth of last instar P. includens, like other insects, occurs through intense feeding by pre-critical weight larvae. During this period the prothoracic glands are small, weakly responsive to stimulatory factors (cAMP analog, brain extract, and likely PTTH), and release low basal levels of ecdysteroids (Fig. 7A). An elevated hemolymph JH titer also likely suppresses both PTTH release by brain neurosecretory cells and ecdysteroids by prothoracic glands as shown for the silkmoth Bombyx mori and Manduca sexta (Rountree and Bollenbacher, 1986; Gilbert et al., 2002; Takaki and Sakurai, 2003; Truman et al., 2006). As feeding and growth continue, larvae exceed critical weight, which causes the hemolymph JH titer to decline and PTTH to be released. Prothoracic glands in post-critical weight larvae are also larger, which correlates with increased responsiveness to stimulation and greatly increased levels of ecdysteroid release (Fig. 7A). In contrast, MdBV infection of pre-critical weight P. includens causes larvae to quickly reduce their rate of food consumption, which results in failure to ever achieve critical weight (Fig. 7B). This correlates with the hemolymph JH titer remaining elevated and arrested growth of prothoracic glands that remain unresponsive to stimulation and release little ecdysteroid. Starvation of pre-critical weight P. includens also greatly delays or blocks pupation, which correlates with an elevated JH titer similar to starved M. sexta larvae (Cymborowski et al., 1982). But unlike infection by MdBV, starvation does not cause a rapid rise in blood sugar levels or decline in nutrient stores. In contrast, MdBV infection of post-critical weight larvae fails to inhibit pupation, because growth and development at this stage is nutrition independent.
Limited understanding of the mechanisms linking attainment of critical weight to the endocrine events regulating metamorphosis (see Mirth and Riddiford, 2007) makes it unclear how MdBV alters these processes but our results suggest two possibilities. The first is that host developmental arrest is due to the sustained high hemolymph JH titer and suppressed activity of JH esterase that occurs after infection by MdBV (Balgopal et al., 1996) (Fig. 7B). Parasitism by several other PDV-carrying wasps also causes host hemolymph JH titers to increase [summarized by Beckage and Gelman, and Pennacchio and Strand (Beckage and Gelman, 2004; Pennacchio and Strand, 2006)]. In contrast, an elevated JH titer could also reflect an indirect response to infection and associated changes in nutrient availability given that starvation induces similar changes. The second possibility is that MdBV alters nutrient metabolism and appetite feedback mechanisms required for growth. This hypothesis is based on studies with Drosophila and other insects indicating that insulin-like peptides (ILPs) are required for the maintenance of appropriate blood sugar and lipid levels and increasing metabolite stores, which results in growth. Reciprocally, starvation stimulates the release of AKHs that maintain blood sugar and lipid levels but at the expense of glycogen and lipid stores, which results in no growth [summarized by Wu and Brown, and Baker and Thummel (Wu and Brown, 2006; Baker and Thummel, 2007)]. The interplay between ILP and AKH signaling has also been implicated in the regulation of prothoracic gland size (Mirth et al., 2005; Colombani et al., 2005) and feeding behavior (Wu et al., 2005; Lee and Park, 2004; Isabel et al., 2005; Wicher, 2007). Thus, the alterations in metabolism and growth of P. includens associated with MdBV infection could reflect either reduced ILP activity or enhanced AKH-like effects (Fig. 7B).
We note that illness-induced anorexia has also been reported in response to infection by other pathogens in a diversity of other animals (Dantzer, 2004; Adamo et al., 2007; Adamo, 2008). Some studies suggest this effect is induced by the host's own immune system (Exton, 1997; Dantzer, 2004), while others suggest anorexia could be a response by the host to reallocation of energetic reserves to defense (Moret and Schmid-Hempel, 2000; Ahmed et al., 2002; Armitage et al., 2003; Dionne et al., 2006). As previously noted, we think it unlikely MdBV-induced alterations in feeding and metabolism reflect responses mediated by the host's immune system given: (1) the absence of pathology when hosts are infected with inactivated virus, and (2) how severely immunosuppressed hosts are when infected by normal, transcriptionally active virus (Webb and Strand, 2005; Strand, 2008). As MdBV does not replicate in P. includens, it is also unlikely the virus directly benefits from the metabolic changes it causes, although it could benefit development of the M. demolitor larva (Thompson, 1993; Vinson et al., 2001; Pennacchio and Strand, 2006).
In summary, our results argue that M. demolitor and likely several other PDV-carrying wasps inhibit host pupation by altering feeding behavior and metabolic physiology rather than directly altering hormone synthesis or release. MdBV encodes more than 60 predicted open reading frames which include a mixture of single copy genes and structurally related genes that form multimember families (Webb and Strand, 2005; Webb et al., 2006; Strand, 2008). A key priority is to determine whether any MdBV gene products function as growth inhibitors.
We thank J. A. Johnson and A. Robertson for assistance. This work was funded by a Sigma Xi grant in aid award to A.J.P., a visiting scholar award from the Ellison Medical Foundation to P.F., a post-doctoral fellowship from the Korea Research Foundation (KRF-2007-357-C00106) to J.H.E., and support from the National Science Foundation (IOS 0749450) and US Department of Agriculture National Research Initiative (2005-05382) to M.R.S. This work was supported by an NIH grant (NIH A103108). Deposited in PMC for release after 12 months.
References
- Adamo, S. A. (2008). Bidirectional connections between the immune system and the nervous system on insects. In Insect Immunity (ed. N. E. Beckage), pp. 129-150. San Diego, CA: Academic Press.
- Adamo, S. A., Fidler, T. L. and Forestell, C. A. (2007). Illness-induced anorexia and its possible function in the caterpillar, Manduca sexta. Brain Behav. Immun. 21, 292-300. [DOI] [PubMed] [Google Scholar]
- Ahmed, A. M., Baggott, S. L., Maingon, R. and Hurd, H. (2002). The costs of mounting an immune response are reflected in the reproductive fitness of the mosquito Anopheles gambiae. Oikos 97, 371-377. [Google Scholar]
- Andersen, S. K., Gjedsted, J., Christiansen, C. and Tonnesen, E. (2004). The roles of insulin and hyperglycemia in sepsis pathogenesis. J. Leukoc. Biol. 75, 413-421. [DOI] [PubMed] [Google Scholar]
- Armitage, S. A., Thompson, J. J., Rolff, J. and Siva-Jothy, M. T. (2003). Examining costs of induced and constitutive immune investment in Tenebrio molitor. J. Evol. Biol. 16, 1038-1044. [DOI] [PubMed] [Google Scholar]
- Baker, K. D. and Thummel, C. S. (2007). Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 6, 257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balgopal, M. M., Dover, B. A., Goodman, W. G. and Strand, M. R. (1996). Parasitism by Microplitis demoliitor induces alterations in the juvenile hormone titer of its host, Pseudoplusia includens. J. Insect Physiol. 42, 337-345. [Google Scholar]
- Beck, M. and Strand, M. R. (2005). Glc1.8 from Microplitis demolitor bracovirus induces a loss of adhesion and phagocytosis in insect high five and S2 cells. J. Virol. 79, 1861-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, M. H. and Strand, M. R. (2007). A novel polydnavirus protein inhibits the insect prophenoloxidase activation pathway. Proc. Natl. Acad. Sci. USA 104, 19267-19272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, M. H., Inman, R. B. and Strand, M. R. (2007). Microplitis demolitor bracovirus genome segments vary in abundance and are individually packaged in virions. Virology 359, 179-189. [DOI] [PubMed] [Google Scholar]
- Beckage, N. E. and Gelman, D. B. (2004). Wasp parasitoid disruption of host development: implications for new biologically based strategies for insect control. Annu. Rev. Entomol. 49, 299-330. [DOI] [PubMed] [Google Scholar]
- Colombani, J., Bianchini, L., Layelle, S., Pondeville, E., Dauphin-Villemant, C., Antoniewski, C., Carré, C., Noselli, S. and Léopold, P. M. (2005). Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310, 667-670. [DOI] [PubMed] [Google Scholar]
- Coudron, T. A., Kelly, T. J. and Puttler, B. (1990). Development responses of Trichoplusia ni (Lepidoptera: Noctuidae) to parasitism by the ectoparasite Euplectrus plathypenae (Hymenoptera: Eulophidae). Arch. Insect Biochem. Physiol. 13, 83-94. [Google Scholar]
- Cymborowski, B., Bogus, M., Beckage, N. E., Williams, C. M. and Riddiford, L. M. (1982). Juvenile hormone titers and metabolism during starvation-induced supernumerary larval moulting of the tobacco hornworm, Manduca sexta. J. Insect Physiol. 28, 129-135. [Google Scholar]
- Dantzer, R. (2004). Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 500, 399-411. [DOI] [PubMed] [Google Scholar]
- Dionne, M. S., Pham, L. N., Shirasu-Hiza, M. and Schneider, D. S. (2006). Akt and foxo dysregulation contribute to infection-induced wasting in Drosophila. Curr. Biol. 310, 1977-1985. [DOI] [PubMed] [Google Scholar]
- Exton, M. S. (1997). Infection-induced anorexia: active host defense strategy. Appetite 29, 369-383. [DOI] [PubMed] [Google Scholar]
- Falabella, P., Varricchio, P., Gigliotti, S., Tranfaglia, A., Pennacchio, F. and Malva, C. (2003). Toxoneuron nigriceps polydnavirus encodes a putative aspartyl protease highly expressed in parasitized host larvae. Insect Mol. Biol. 12, 9-17. [DOI] [PubMed] [Google Scholar]
- Gilbert, L. I., Rybczynski, R. and Warren, J. T. (2002). Control and biochemical nature of the ecdysteroidogenic pathway. Annu. Rev. Entomol. 47, 883-912. [DOI] [PubMed] [Google Scholar]
- Grossniklaus-Burgin, C., Pfister-Wilhelm, R., Meyer, V., Treiblmayr, K. and Lanzrein, B. (1998). Physiological and endocrine changes associated with polydnavirus/venom in the parasitoid-host system Chelonus inanitus-Spodoptera littoralis. J. Insect Physiol. 44, 305-321. [DOI] [PubMed] [Google Scholar]
- Gu, S. H., Chow, Y. S. and Yin, C. M. (1997). Involvement of juvenile hormone in regulation of prothoracicotropic hormone transduction during the early last larval instar of Bombyx mori. Mol. Cell Endocrinol. 127, 109-116. [DOI] [PubMed] [Google Scholar]
- Gu, S. H., Chow, Y. S. and Yin, C. M. (2000). Temporal analysis of ecdysteroidogenic activity of the prothoracic glands during fourth larval instar of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 30, 499-505. [DOI] [PubMed] [Google Scholar]
- Harvey, J. A. and Strand, M. R. (2002). The developmental strategies of endoparasitoid wasps vary with host feeding ecology. Ecology 83, 2439-2451. [Google Scholar]
- Hotamisligil, G. S. (2006). Inflammation and metabolic disorders. Nature 444, 860-867. [DOI] [PubMed] [Google Scholar]
- Isabel, G., Martin, J. R., Chidami, S., Veenstra, J. A. and Rosay, P. (2005). AKH-producing neuroendocrine cell ablation decreases trehalose and induces behavioral changes in Drosophila. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R531-R538. [DOI] [PubMed] [Google Scholar]
- Kelly, T. J., Gelman, D. B., Reed, D. A. and Beckage, N. E. (1998). Effects of parasitization by Cotesia congregata on the brain-prothoracic gland axis of its host, Manduca sexta. J. Insect Physiol. 44, 323-332. [DOI] [PubMed] [Google Scholar]
- Kroemer, J. A. and Webb, B. A. (2004). Polydnavirus genes and genomes: emerging gene families and new insights into polydnavirus replication. Annu. Rev. Entomol. 49, 431-456. [DOI] [PubMed] [Google Scholar]
- Lee, G. and Park, J. H. (2004). Hemolymph sugar homeostatsis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167, 311-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Z., Beck, M. H., Jiang, H., Wang, J. and Strand, M. R. (2008). The viral protein Egf1.0 is a dual activity inhibitor of prophenoloxidase activating proteinases 1 and 3 from Manduca sexta. J. Biol. Chem. 283, 21325-21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirth, C. K. and Riddiford, L. M. (2007). Size assessment and growth control: how adult size is determined in insects. BioEssays 29, 344-355. [DOI] [PubMed] [Google Scholar]
- Mirth, C. K., Truman, J. W. and Riddiford, L. M. (2005). The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr. Biol. 15, 1796-1807. [DOI] [PubMed] [Google Scholar]
- Moret, Y. and Schmid-Hempel, P. (2000). Survival and immunity: the price of immune system activation for bumblebee workers. Science 290, 1166-1168. [DOI] [PubMed] [Google Scholar]
- Nijhout, H. F. (1975). A threshold size for metamorphosis in the tobacco hornworm, Manduca sexta. Biol. Bull. 149, 214-225. [DOI] [PubMed] [Google Scholar]
- Nijhout, H. F. (1994). Insect Hormones. Princeton, NJ: Princeton University Press.
- Nijhout, H. F. (2003). The control of body size in insects. Dev. Biol. 261, 1-9. [DOI] [PubMed] [Google Scholar]
- Okuda, M., Sakurai, S. and Ohtaki, T. (1985). Activity of the prothoracic gland and its sensitivity to prothoracicotropic hormone in the penultimate and last-larval instar of Bombyx mori. J. Insect Physiol. 31, 455-461. [Google Scholar]
- Oldham, S. and Hafen, E. (2003). Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 13, 79-85. [DOI] [PubMed] [Google Scholar]
- Pennacchio, F. and Strand, M. R. (2006). Evolution of developmental strategies in parasitic Hymenoptera. Annu. Rev. Entomol. 51, 233-258. [DOI] [PubMed] [Google Scholar]
- Pennacchio, F., Falabella, P. and Vinson, S. B. (1998). Regulation of Heliothis virescens prothoracic glands by Cardiochiles nigriceps polydnavirus (CnPDV). Arch. Insect. Biochem. Physiol. 38, 1-10. [Google Scholar]
- Pennacchio, F., Malva, C., Vinson, S. B. (2001). Regulation of host endocrine system by the endophagous braconid Cardiochiles nigriceps and its polydnavirus. In Endocrine Interactions of Insect Parasites and Pathogens (ed. J. P. Edwards and R. J. Weaver) pp. 123-132. Oxford: BIOS.
- Pruijssers, A. J. and Strand, M. R. (2007). PTP-H2 and PTP-H3 from Microplitis demolitor bracovirus localize to focal adhesions and are antiphagocytic in insect immune cells. J. Virol. 81, 1209-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford, L. M. (1976). Hormonal control of insect epidermal cell commitment in vitro. Nature 259, 115-117. [DOI] [PubMed] [Google Scholar]
- Rountree, D. B. and Bollenbacher, W. E. (1986). The release of prothoracicotropic hormone in the tobacco hornworm, Manduca sexta, is controlled intrinsically by juvenile hormone. J. Exp. Biol. 120, 41-58. [DOI] [PubMed] [Google Scholar]
- Sieglaff, D. H., Duncan, K. A. and Brown, M. R. (2005). Expression of genes encoding proteins involved in ecdysteroidogenesis in the female mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 35, 471-490. [DOI] [PubMed] [Google Scholar]
- Strand, M. R. (1990). Characterization of larval development in Pseudoplusia includens (Walker) (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 83, 538-544. [Google Scholar]
- Strand, M. R. (1994). Microplitis demolitor polydnavirus infects and expresses in specific morphotypes of Pseudoplusia includens haemocytes. J. Gen. Virol. 75, 3007-3020. [DOI] [PubMed] [Google Scholar]
- Strand, M. R. (2008). Polydnaviruses: abrogation of the insect immune system. In Encyclopedia of Virology, vol. 4. (ed. B. M. J. Mahy and M. van Regenmortel), pp. 250-256. San Diego, CA: Elsevier. [Google Scholar]
- Strand, M. R. and Dover, B. A. (1991). Developmental disruption of Pseudoplusia includens and Heliothids virescens larvae by the polydnavirus and venom of Microplitis demolitor. Arch. Insect Biochem. Physiol. 18, 131-145. [DOI] [PubMed] [Google Scholar]
- Strand, M. R., Johnson, J. A. and Culin, J. D. (1988). Developmental interactions between the parasitoid Microplitis demolitor (Hymenoptera: Braconidae) and its host Heliothis virescens (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 81, 822-830. [Google Scholar]
- Strand, M. R., Johnson, J. A. and Dover, B. A. (1990). Ecdysteroid and juvenile hormone esterase profiles of Trichoplusia ni parasitized by the polyembryonic wasp Copidosoma floridanum. Arch. Insect Biochem. Physiol. 13, 41-51. [Google Scholar]
- Strand, M. R., McKenzie, D. I., Grassl, V., Dover, B. A. and Aiken, J. M. (1992). Persistence and expression of Microplitis demolitor polydnavirus in Pseudoplusia includens. J. Gen. Virol. 73, 1627-1635. [DOI] [PubMed] [Google Scholar]
- Takaki, K. and Sakurai, S. (2003). Regulation of prothoracic gland ecdysteroidogenic activity leading to pupal metamorphosis. Insect Biochem. Mol. Biol. 33, 1189-1199. [DOI] [PubMed] [Google Scholar]
- Tanaka, T. and Vinson, S. B. (1987). Depression of prothoracicotropic gland activity of Heliothis virescens by venom and calyx fluids from the parasitoid Cardiochiles nigriceps. J. Insect Physiol. 37, 139-144. [Google Scholar]
- Tanaka, T., Agui, N. and Hiruma, K. (1987). The parasitoid Apanteles kariyai inhibits pupation of its host, Pseudaletia separata, via disruption of prothoracicotropic hormone release. Gen. Comp. Endocrinol. 67, 364-374. [DOI] [PubMed] [Google Scholar]
- Telang, A. and Wells, M. A. (2004). The effect of larval and adult nutrition on successful autogenous egg production by a mosquito. J. Insect Physiol. 50, 677-685. [DOI] [PubMed] [Google Scholar]
- Thompson, S. N. (1993). Redirection of host metabolism and effects on parasite nutrition. In Parasites and Pathogens of Insects, vol. 1 (ed. N. E. Beckage, S. N. Thompson, and B. A. Federici), pp. 125-144. New York: Academic Press. [Google Scholar]
- Truman, J. W., Hiruma, K., Allee, J. P., Macwhinnie, S. G., Champlin, D. T. and Riddiford, L. M. (2006). Juvenile hormone is required to couple imaginal disc formation with nutrition in insects. Science 310, 1385-1388. [DOI] [PubMed] [Google Scholar]
- Vinson, S. B., Pennacchio, F. and Consoli, F. L. (2001). The parasitoid-host endocrine interaction from a nutritional perspective. In Endocrine Interactions of Insect Parasites and Pathogens (ed. J. P. Edwards and R. J. Weaver) pp. 187-205. Oxford: BIOS.
- Webb, B. A. and Strand, M. R. (2005). The biology and genomics of polydnaviruses. In Comprehensive Molecular Insect Science, vol. 6 (ed. L. I. Gilbert, K. Iatrou and S. Gill), pp. 260-323. San Diego, CA: Elsevier. [Google Scholar]
- Webb, B. A., Strand, M. R., Dickey, S. E., Beck, M. H., Hilgarth, R. S., Barney, W. E., Kadash, K., Kroemer, J. A., Lindstrom, K. G., Rattanadechakul, W. et al. (2006). Polydnavirus genomes reflect their dual roles as mutualists and pathogens. Virology 347, 160-174. [DOI] [PubMed] [Google Scholar]
- Wicher, D. (2007). Metabolic regulation and behavior: how hunger produces arousal-an insect study. Endocr. Metab. Immune Disord. Drug Targets 7, 304-310. [DOI] [PubMed] [Google Scholar]
- Wu, Q. and Brown, M. R. (2006). Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 51, 1-24. [DOI] [PubMed] [Google Scholar]
- Wu, Q., Zhang, Y., Xu, J. and Shen, P. (2005). Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc. Natl. Acad. Sci. USA 102, 13289-13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitnan, D., Kingan, T. G., Kramer, S. J. and Beckage, N. E. (1995). Accumulation of neuropeptides in the cerebral neurosecretory system of Manduca sexta larvae parasitized by the braconid wasp Cotesia congregata. J. Comp. Neurol. 356, 83-100. [DOI] [PubMed] [Google Scholar]