Abstract
Estradiol-17β (E2) induces rodent hippocampal neural progenitor cell (NPC) proliferation in vitro, in vivo, and after brain injury. The purpose of the present investigation was to determine whether E2-induced proliferation observed in rodent model systems generalized to cells of human neural origin and the signaling pathway by which E2 promotes mitosis of human NPCs (hNPCs). Results of these analyses indicate that E2 induced a significant increase in hNPC proliferation in a time- and dose-dependent manner. E2-induced hNPC DNA replication was paralleled by elevated cell cycle protein expression and centrosome amplification, which was associated with augmentation of total cell number. To determine whether estrogen receptor (ER) and which ER subtype were required for E2-induced hNPC proliferation, ER expression was first determined by real-time RT-PCR, followed by Western blot analysis, and subsequently verified pharmacologically using ERα or β-selective ligands. Results of these analyses indicated that ERβ expression was predominant relative to ERα, which was barely detectable in hNPCs. Activation of ERβ by the ERβ-selective ligand, diarylpropionitrile, led to an increase in phosphorylated extracellular signal-regulated kinase, and subsequent centrosome amplification and hNPC proliferation, which were blocked by the MEKK antagonist, UO126, but not its inactive analog, UO124. These findings, for the first time, demonstrate the molecular cascade and related cell biology events involved in E2-induced hNPC proliferation in vitro. Therapeutic implications of these findings relevant to hormone therapy and prevention of neurodegenerative disease are discussed.
ESTROGENS REGULATE THE development, maturation, survival, and function of multiple types of neurons in multiple brain regions (1,2,3,4,5,6). A recent advance in our understanding of estrogen action in brain is that estradiol-17β (E2) can promote neurogenesis in rat brain in vivo and proliferation of neural progenitor cells (NPCs) in vitro (7,8,9,10). Studies using rat embryonic neural stem cells isolated from brain striatal tissue demonstrated that E2 increased rat embryonic neural stem cell proliferation, and this response is estrogen receptor (ER) dependent (8). Removal of circulating E2 by ovariectomy resulted in a significant decrease in the proliferation of hippocampal granule cell precursors, which could be reversed by E2 replacement in ovariectomized (OVX) rats (9,10).
These findings derived from the rodent served as the foundation for our analyses to determine the proliferative action of E2 in human NPCs (hNPCs) and to determine the molecular mechanisms required for E2-induced proliferation of hNPCs. The hNPC used in the current analyses was derived from birth-defected fetal cortex, and cultured for extended periods of time in the presence of basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), and leukemia inhibitory factor by Svendsen et al. (11). The phenotypic homogeneity of these cells has been demonstrated by immunoreactivity for the progenitor cell marker nestin and the neural progenitor/neuronal marker Tuj1 (12,13). Furthermore, these cells respond to a range of mitogenic and growth factors, including fibroblast growth factor 2, vascular endothelial growth factor, and nerve growth factor (13). We have recently demonstrated that allopregnanolone, a progesterone metabolite, promotes proliferation of these cells (12). Given that these hNPCs display response features consistent with neural progenitor phenotype, we used these cells as an in vitro model relevant to the human brain to determine the efficacy and molecular mechanism underlying estrogen-induced NPC proliferation.
Thus far, two types of ERs, ERα and ERβ, are well characterized. ERα and ERβ share the common feature of the nuclear receptor structure but are encoded by different genes located on different chromosomes, and, in addition, they exhibit different brain distribution profiles (for review, see Refs. 4 and 14,15,16). The brain region-specific distribution for these receptors is linked to functional distinctions between ERα and ERβ for estrogen-induced neuroprotection, neurotrophic and neurogenic activities (17,18,19,20,21). E2-induced neuroprotection and neurotrophism are regulated through a coordinated signaling cascade that involves ER protein interaction with the regulatory subunit (p85) of phosphatidylinositol 3-kinase, which upon activation, serves as the initiation mechanism for activation of downstream signaling cascades, including Akt (22,23,24,25) and phosphorylated ERK (pERK) (26). Other intracellular signal transduction cascades, including protein kinase C (27,28,29) activated Src and pERK, have also been proposed (30). Until now, the intracellular signaling pathway required for E2-induced neurogenic activity had not yet been determined.
Numerous observations have demonstrated the role of the ERK signaling cascade in regulating cell cycle progression (31,32,33,34). The ERK1/2 cascade is predominantly involved in the control of cell proliferation (32,35) and differentiation (31). Activation of ERK1/2 can promote cell cycle reentry (32,33,35), whereas inhibition of the ERK pathway results in G1 arrest in a variety of cell types (32,33,35,36). pERK1/2 translocates to the nucleus (37) to phosphorylate nuclear transcription factors (e.g. Jun, Myc, Tal1, and Elk1) (37), whereupon these factors promote transcription of cyclins and cyclin-dependent kinases (CDKs) to initiate G1/S phase transition (32). Activated ERK1/2 also translocates to the cytosolic located centrosomes to phosphorylate centrosome kinases, such as Aurora kinase A, to initiate centrosome amplification (32,38).
Given that E2 can promote neural progenitor proliferation in rodent brain in vivo (9,10) and rodent (8) and hNPCs in vitro (18,19), we sought to determine whether E2 would promote human neural progenitor proliferation and the mechanism underlying this response. Thus, we investigated: 1) the efficacy of E2 to promote hNPC proliferation by determining bromodeoxyuridine (BrdU) incorporation, total cell number, and expression of cell cycle markers; 2) ERα and ERβ expression in hNPC by real-time RT-PCR, Western blot, and immunocytochemistry (ICC); 3) the role of ERα and ERβ in E2-induced proliferation using receptor-selective ligands, propyl pyrazole triol (PPT) (an ERα-selective ligand) and diarylpropionitrile (DPN) (an ERβ-selective ligand); and 4) the intracellular signal cascades and the cellular events involved in E2-induced hNPC proliferation.
Materials and Methods
Culture of the human cerebral cortical neural stem cells
The use of the human fetal cerebral cortical progenitor cells (gift of Dr. Svendsen, Departments of Anatomy and Neurology and the Waisman Center, University of Wisconsin, Madison, WI) was approved through the institutional review board at the University of Southern California. NPCs were cultured as previously described by Svendsen et al. (11) and our group (12). Briefly, cells were thawed in DMEM/Ham’s F-12 (7:3) medium containing penicillin/streptomycin/amphotericin B (1%), supplemented with B27 (2%; Life Technologies, Inc., Gaithersburg, MD), EGF (20 ng/ml; Life Technologies, Inc.), fibroblast growth factor 2 (20 ng/ml; Life Technologies, Inc.), heparin (5 μg/ml; Sigma-Aldrich, St. Louis), and 10 ng/ml leukemia inhibitory factor (CHEMICON Intl., Inc., Temecula, CA) in a humidified incubator (37 C and 5% CO2), and half the growth medium was replenished every 3–4 d. Neurospheres were then mechanically triturated into single cells with flame polished Pasteur pipettes and plated onto T75 flasks coated with laminin (MP Biomedicals, Solon, OH) at a density equivalent to 2 × 106 cells per flask and passaged every 14 d. For BrdU assays, cells were plated onto laminin-coated 96-well plates at a density of 7.5 × 104 per well or chamber slides at a density of 2 − 4 × 104 per cm2. For biochemical assays and cell number counting, cells were seeded onto 60 or 30-mm Petri dishes at a density of 1 × 105 per cm2. The cells were treated with 17β-estradiol (E2), the specific ERα agonist PPT, or the selective ERβ agonist, DPN, UO126 [1,4-diamino-2,3- dicyano-1,4-bis(o-aminophenylmercapto)butadiene], UO124 [1,4-diamino-2,3-dicyano-1,4-bis(methylthio) butadiene] as indicated in Results.
Real-time RT-PCR
Total RNA was extracted using TRIzol (Invitrogen Corp., Carlsbad, CA) as described by the manufacturer. cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen) and oligo(dT) primer in accordance with the manufacturer’s protocols. The expression of related genes was quantified using the SYBR green reagent (2× SYBR Green Supermix; Bio-Rad Laboratories, Hercules, CA) following the manufacturer’s instructions on a Bio-Rad iCycler. PCR was performed in multiple replicates under optimized conditions [95 C denaturation for 3 min, followed by 40 cycles of 45 sec at 94 C, 45 sec at 55 C, and 45 sec at 72 C using the following primers: ERα (accession no. NM_000125), forward 5′-tggagatcttcgacatgctg-3′, reverse 5′-tccagagacttcagggtgct-3′; and ERβ (accession no. NM_001437.2), forward 5′-gtgtatgacctgctgctgga-3′, reverse 5′-tcagcttgtgacctctgtgg-3′]. No other products were amplified because melting curves showed only one peak in each primer pair, and only one specific product was observed on agarose-ethidium bromide gel for each primer pair. Fluorescence signals were measured over 40 PCR cycles. The cycle number (Ct) was recorded when the signals crossed a threshold set within the logarithmic phase. For quantitation, we evaluated the difference in cycle threshold (ΔCt) between ER isoforms in nontreated hNPCs and MCF7 cells, which serve as a positive control. The efficiency of amplification of each pair of primers was determined by serial dilutions of cDNA templates, and all were larger than 0.9. Each sample was normalized with loading references, β-actin and 18s rRNA. Ct values were calculated as the means of triplicates. Experiments were repeated at least three times and represented as folds of expression. Differences in expression of ERα and ERβ in hNPCs or MCF7 cells were determined using one-way ANOVA, followed by a Newman-Keuls post hoc analysis.
Western blot
Western blot was used to measure the protein expression of ERα and ERβ in hNPCs, to evaluate the cell proliferation effects of E2, PPT, and DPN by determining the protein expression of proliferating cell nuclear antigen (PCNA) and CDK1/cdc2, two known cell proliferation markers, and an centrosome marker, γ-tubulin, and to estimate the activation of ERK1/2 by test of the pERK expression. hNPCs were treated in the presence or absence of ligands for 1 d and lysed using ice-cold lysis buffer as described previously (12). Whole cell lysates from nontreated hNPCs and MCF7 cells were used as controls. Twenty to 40 μg whole cell lysates protein was separated under reducing and denaturing conditions on 12% SDS-PAGE, and was electrotransferred to a polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). Nonspecific binding sites were blocked with 5% skim milk in Tris-buffered saline containing 0.05% Tween 20. The expression of ER in hNPCs was determined using antibodies for ERα (1:25, 6F11; Novocastra Laboratories Ltd., Newcastle upon Tyne, UK) and ERβ (1:200, PA1-310; Affinity BioReagents, Inc., Golden, CO). To observe the effects of E2 and ER isoform-selective ligands on the cell cycle protein expression, membranes were blotted using a monoclonal antibody for PCNA (1: 300, clone PC10; Zymed Laboratories Inc., San Francisco, CA), a polyclonal antibody for the carboxy-terminal domain of CDK1/cdc2/p34 (1:500; Novus Biologicals Inc., Littleton, CO), or a polyclonal antibody against human γ-tubulin (1:500; Abcam, Inc., Cambridge, MA). For measuring the pERK and ERK expression, an anti-pERK1/2 antibody (1:750 in PBS-Tween/1% horse serum; Cell Signaling Technologies, Beverly, MA) and a total ERK1/2 antibody (C-14) (1:5000 in PBS-Tween/1% horse serum; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were used. Membranes were then incubated in horseradish peroxidase-conjugated goat antirabbit or horse antimouse IgG (1:6000), and results were visualized by the TMB Peroxidase Substrate Kit (Vector Laboratories, Inc., Burlingame, CA). The blots were then quantified by optical density analysis using UnScanIt gel software (Silk Scientific Co., Orem, UT). The data were then statistically analyzed using one-way ANOVA, followed by a Newman-Keuls post hoc analysis. The data are displayed as means ± sem of three independent experiments.
BrdU incorporation and cell number counting
hNPC proliferation was initially evaluated by measuring incorporation of BrdU using BrdU chemiluminescence immunoassay kits purchased from Roche (Penzberg, Germany) and further confirmed by standard Trypan blue cell counting. After overnight adhesion and then a 4- to 6-h starvation (medium without supplements), hNPCs were incubated with different concentrations of E2, PPT, and DPN as indicated, or b-FGF-heparin (20 ng/ml, positive control) in the starvation medium, or switched back to complete medium after starvation (another positive control) for 1 d and pulse loaded with 10 μm BrdU for 2 h. hNPCs were then incubated with anti-BrdU-peroxidase for 90 min and further developed with substrate solution for 3 min. The plates were read with an Lmax microplate luminometer (Molecular Devices, Sunnyvale, CA). After subtracting the value of the blank (without BrdU loading), the results were analyzed using a one-way ANOVA, followed by a Newman-Keuls post hoc test, and presented as percent (%) increase vs. control.
ICC
hNPCs were plated on poly-d-lysine- and laminin-coated chamber slides. After fixation with 4% paraformaldehyde, cells were incubated overnight with the following primary antibodies: monoclonal antibody for ERα (1:25; Novocastra); polyclonal antibody for ERβ (1:500; Affinity Bioreagents); monoclonal antibody for nestin (1:5000, stem cell marker; CHEMICON); monoclonal antibody for neuronal class III β-tubulin (Tuj1, 1:500, NPC marker; Covance, Berkeley, CA); anti-pERK1/2 antibody (1:300; Cell Signaling Technologies); and anti-total ERK1/2 antibody (C-14) (1:1000; Santa Cruz Biotechnology). After PBS washes, cells were incubated for 30 min with secondary antibodies containing antimouse, or antirabbit IgG conjugated with FITC or Taxes-Red (1: 250; Vector Laboratories). The centrosomes were labeled by a Cy3-conjugated polyclonal antibody against γ-tubulin (1;500; Abcam). Cells were mounted under coverslips with 4′,6′-diamidino-2-phenylindole (DAPI)-containing mounting medium (Vector Laboratories). Labeled cells were observed by a Zeiss Axiovert ×200 fluorescent microscope (Carl Zeiss, Inc., Thornwood, NY), and images were captured by SlideBook software (Intelligent Imaging Innovations Inc., San Diego, CA).
Results
E2 increases BrdU incorporation in hNPC in a time- and dose-dependent manner
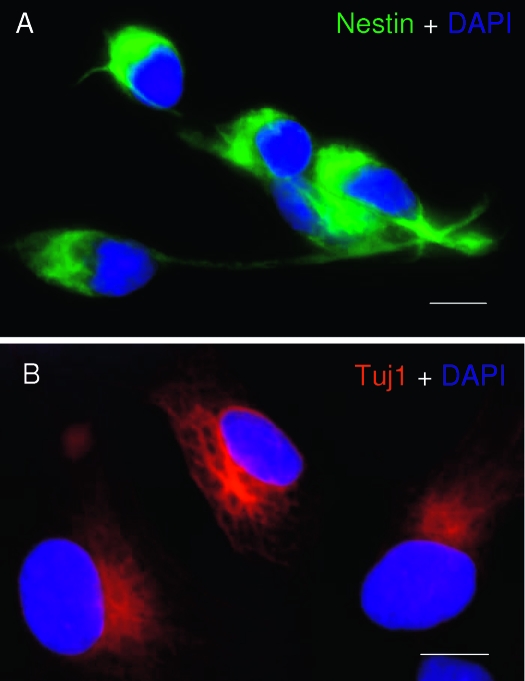
The neural progenitor phenotype of hNPCs was first verified immunocytochemically by labeling for stem/progenitor cell marker, nestin (a class VI large intermediate filament protein), and a neural progenitor/neuronal marker, Tuj1 (a neuron-specific βIII-tubulin). Expression of both nestin (Fig. 1A) and Tuj1 (Fig. 1B) in these cells confirmed their neural progenitor phenotype under our culture conditions and is consistent with the description by Svendsen et al. (11).
Figure 1.
Characterization of hNPC phenotype. Human cerebral cortex NPCs (hNPC) were cultured in poly-d-lysine and laminin-coated chamber slides and fixed by 4% paraformaldehyde for 20 min at room temperature. The NPC phenotype was detected by ICC with two well-known NPC markers, nestin (A) (green fluorescence) and Tuj1 (B) (red fluorescence). Cell nuclei were counterstained with DAPI. Scale bars, 10 μm.
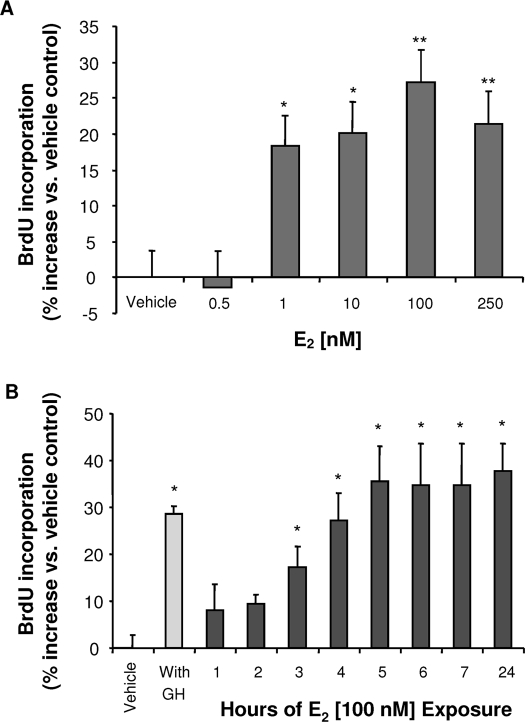
Proliferation of hNPCs was determined by BrdU chemiluminescence ELISA. To eliminate contributions of growth factors in the culture medium, hNPCs were growth factor deprived for 4–6 h before introduction of E2, vehicle, or other agents. hNPCs were exposed to differing concentrations of E2 for 24 h, followed by BrdU ELISA. Results of these analyses indicated that E2 induced a dose-dependent BrdU incorporation in hNPCs. E2 was ineffective at 0.5 nm, minimally effective at 1 nm (18 ± 3.5%), and maximally effective at 100 nm (27 ± 3.8%) (Fig. 2A). The decrement in BrdU incorporation at 250 nm suggests that the efficacy of E2 on proliferation is dose sensitive, and the optimal concentration is 100 nm. The efficacy of E2 as a neurogenic factor in hNPC, at optimal concentration, was comparable to that induced by bFGF (20 ng/ml) + heparin (5 μg/ml) treatment (30 ± 9% increase vs. control) (Fig. 2B).
Figure 2.
E2 promotes hNPC proliferation in a dose- and time-dependent manner. hNPCs were incubated in a starvation condition (absence of GH and B27 supplement) for 4–6 h, followed by exposure to different concentrations of E2 in the starvation medium for indicated time periods. hNPCs were pulse loaded with BrdU for 2 h, and BrdU incorporation was subsequently measured by chemiluminescence BrdU ELISA. Data were derived from at least three independent experiments conducted with eight replicates. Results were plotted as percentage of vehicle control (mean ± sem). *, P < 0.05. **, P < 0.01. A, E2 induced a dose-dependent increase in BrdU incorporation. The minimally effective concentration was 1 nm E2 (18 ± 10% increase), with the maximal proliferative effect achieved at 100 nm E2 (27 ± 3% increase). B, E2-induced hNPC BrdU incorporation was time dependent. Significant BrdU incorporation was evident at 3 h (17 ± 4%), reached maximum at 5 h (35 ± 8% vs. vehicle control), at which time BrdU incorporation plateaued and was sustained for 24 h. The positive control, complete medium with GH and B27 supplement, induced a 29 ± 2% increase in BrdU incorporation vs. control.
An analysis of the time course for E2-induced BrdU incorporation into hNPCs is shown Fig. 2B. Overall, E2 induced a linear increase in BrdU incorporation that was first evident at 1 h, significant by 3 h, and asymptotic at 5 h. The sustained level of BrdU incorporation between 5 and 24 h is consistent with a cessation of S-phase within 5 h and that additional BrdU was not incorporated beyond the 5-h time point.
Human cerebral cortical stem cells predominantly express ERβ
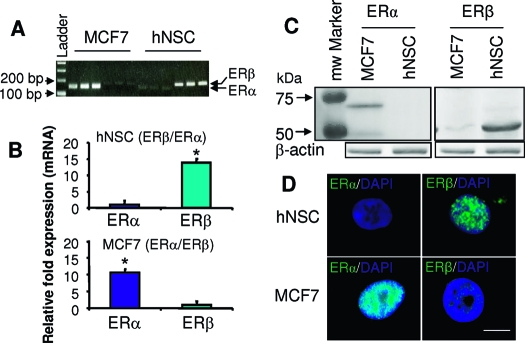
To determine the ER subtype mediating the proliferative action of E2, we first investigated the expression level of ER isoforms in hNPCs by performing quantitative real-time RT-PCR. Primers amplifying the cDNA encoding fragments of the ligand binding domain of ERα and ERβ revealed a 13.87 ± 1.15-fold increase in expression of ERβ relative to ERα in hNPC (Fig. 3, A and B). In contrast, the same primers used at the identical PCR conditions amplified a 10.50 ± 1.03-fold higher expression of ERα than ERβ in cDNA derived from MCF7 cells (an ER positive breast cancer cell line).
Figure 3.
hNPCs predominantly express ERβ. The expression of ERα and ERβ at the mRNA level in hNPCs was determined by real-time RT-PCR using specific primers for the ligand binding domain of human ERα and ERβ. A, A representative ethidium bromide agarose gel predicts the single band of each real-time RT-PCR product and the accurate size of the PCR product, ERα (143 bp) and ERβ (179 bp). B, The Ct values of the real-time RT-PCR were calculated by the (2*efficiency)ΔΔCt method, and normalized by the value of the internal control β-actin and 18s rRNA. Data are presented as mean of fold change ± sem vs. control and are derived from at least three independent experiments (*, P < 0.01). C, The expression of ERα and ERβ protein content in hNPCs was determined by Western blot using antibodies against ERα (6F11) and ERβ (PA1–310). In hNPCs, ERβ protein (54 kD) was highly expressed, whereas the full-length ERα (67 kD) was minimally expressed. MCF7 cells expressed abundant ERα protein but minimal ERβ, protein. Loading control, β-actin (*, P < 0.01). D, Localization of ERα and ERβ in single hNPCs was determined by ICC using antibodies as described in C. In hNPCs, ERβ was highly expressed, whereas ERα was hardly observed. In contrast to hNPCs, ERα immunoreactivity was observed in MCF7 cells, whereas ERβ was almost undetectable. Both ERα and ERβ were localized to the nucleus. Scale bar, 10 μm.
To validate the observation derived from mRNA analysis, ERα and ERβ protein expression levels were assessed in whole cell lysates of hNPC by Western blot. In the blot for ERα, a single dense band at 67 kD was detected in positive control MCF7 cells (Fig. 3C). However, ERα immunoreactivity was barely detectible in hNPCs. The opposite expression pattern was detected in the Western blot for ERβ, in which a single dense band of ERβ protein (55 kD) was detected in hNPCs, whereas a weakly immunoreactive (IR) band of ERβ appeared in MCF7 cells (Fig. 3C). These data are consistent with results obtained by real-time RT-PCR and support the observation that ERβ is the predominant ER in hNPCs (Fig. 3A).
To visually determine ER isoform expression in individual hNPCs, ICC was conducted using antibodies specific for ERα and ERβ. The specificity of these antibodies has been determined and used for immunocytochemical labeling of ER in brain by others (39,40) and ourselves (41). The monoclonal antibody, 6F11 (immunizing antigen corresponds to full-length recombinant ERα), and the polyclonal antibody, PA1–310B (immunizing peptides corresponds to amino acid residues 467–485 from rat ERβ, specifically react to human and rat), have been successfully used for Western blot and immunocytochemical labeling. By Western blot, the former detects a 67-kDa protein representing ERα both in rat brain and breast extracts, and the latter detects an approximate 55-kDa protein representing ERβ from rat brain and rat breast homogenate. Immunocytochemical labeling of ERα and ERβ by these two antibodies in neural cells results in nuclear staining (also see Refs. 39 and 40). Immunocytochemical labeling of hNPCs revealed that hNPCs were IR for ERβ, whereas they were devoid of ERα IR signal (Fig. 3D). ERβ IR mainly localized to the hNPC nucleus with minimal detection in the cytoplasm. In contrast, MCF7 cells were IR for ERα and devoid of ERβ immunoreactivity. ERα localized to the nucleus of MCF7 cells with no apparent cytoplasmic labeling. The negative control using nonimmune serum exhibited no labeling (data not shown). These results are consistent with data derived from real-time RT-PCR and Western blot, and are also in agreement with the reports from others that ERα is the predominant ER isoform in breast carcinoma cells (42,43). Together, these data indicate that ERβ is the predominant ER in hNPCs.
ERβ in hNPCs is functional and specifically regulates the E2-induced hNPC proliferation
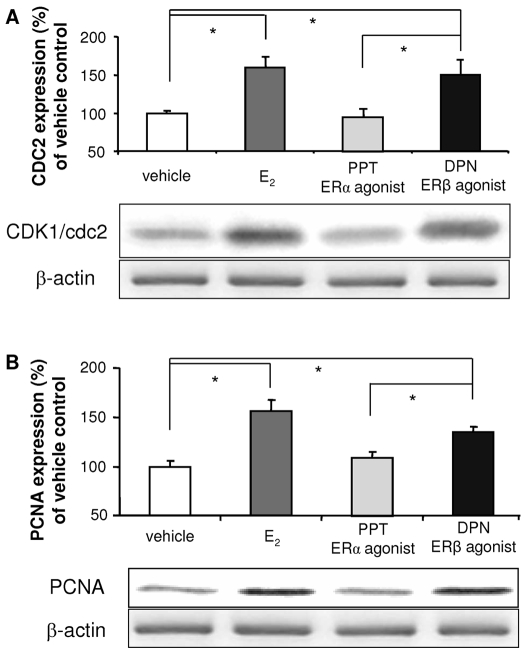
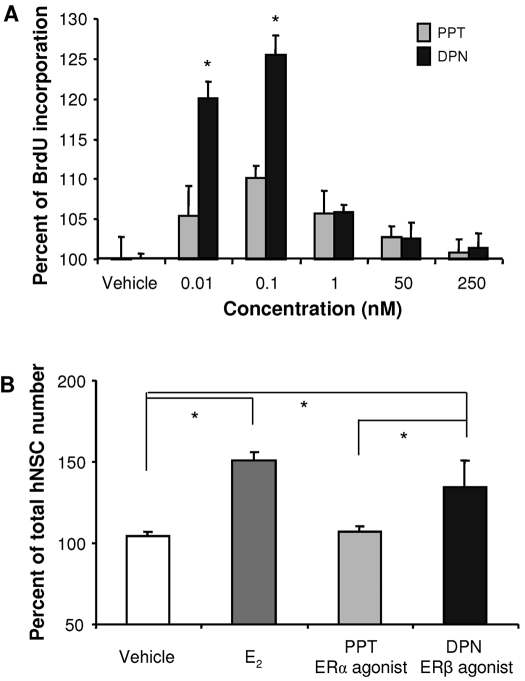
To evaluate the contribution of ERα and ERβ to E2-induced hNPC proliferation, the effect of either the mixed ERα/ERβ agonist E2, the specific ERα agonist PPT, or the selective ERβ agonist, DPN on expression of two well-defined cell proliferating markers, CDK1/cdc2 (Fig. 4A) and PCNA (Fig. 4B), were analyzed by Western blot using whole cell lysates from hNPCs exposed to 100 nm E2, 0.5 nm PPT (an ERα-selective agonist), or 0.3 nm DPN (an ERβ-selective agonist) for 24 h. As indicated in Fig. 4, E2 significantly increased expression of CDK1 (155.6 ± 11.8%; P < 0.01) and PCNA (159.3 ± 3.3%; P < 0.001). The ERβ selective agonist DPN increased expression of CDK1 (134.6 ± 5.4%; P < 0.01) and PCNA (162.7 ± 17.2%; P < 0.001). In contrast, the ERα-specific agonist PPT had no effect on CDK1 (97.3 ± 5.4%; P = 0.57) or PCNA (99.6 ± 6.5%; P = 0.47 vs. vehicle) protein expression. In addition, the difference between PPT and DPN was statistically significant for both PCNA and CDK1 (P < 0.001), but not between DPN and E2 treatment (for PCNA, P = 0.25; for CDK1, P = 0.13), indicating that the promotion of cell cycle entry by E2 is via activation of ERβ.
Figure 4.
Activation of ERβ, not ERα, promotes the mitotic markers, CDK1/cdc2 and PCNA expression in hNPCs. Cultures of hNPCs were incubated in the presence or absence of E2, PPT (an ERα-selective ligand, 0.5 nm), or DPN (an ERβ-selective ligand, 0.3 nm) for 24 h. Western blot analysis was conducted to determine protein expression of CDK1/cdc2/p34 (A) and PCNA (B). E2 and ERβ-selective agonist, DPN, significantly increased CDK1 and PCNA expression, whereas the ERα-specific ligand, PPT, did not. Western blot data, normalized by β-actin, are presented as mean ± sem from three independent experiments. *, P < 0.05.
To confirm that E2 and DPN increases in cell cycle proteins are associated with completely traversing the cell cycle, BrdU incorporation and quantitation of total cell number were conducted. DPN, in a dose-dependent manner, significantly increased hNPC BrdU incorporation at 10 and 100 pm. Higher concentrations were without significant effect (Fig. 5A). These data indicate that the effective concentration of DPN for hNPC proliferation is more than 1000 times more potent than E2 (compare Figs. 2A and 5A), which is consistent with the concentrations of DPN that induced neuroprotection and regulation apolipoprotein E expression in cultured neurons from rat hippocampus (41,44,45) and in vivo in rat hippocampus (41). The ERα-specific agonist PPT had no significant effect on hNPC BrdU incorporation. The increase in BrdU uptake was paralleled by a significant increase in total hNPC number induced by both E2 and DPN. E2 (100 nm) significantly increased total hNPC number by 45 ± 3%, and DPN (0.3 nm) increased hNPC number by 38 ± 15% vs. vehicle control. Total hNPC numbers induced by E2 and DPN were statistically equivalent (no statistically significant difference between the groups). Total hNPC number in PPT condition was not statistically different from vehicle control groups (Fig. 5B).
Figure 5.
E2-induced hNPC proliferation is mediated by ERβ. Cultures of hNPCs were incubated in the presence or absence of E2, PPT (0.5 nm), or DPN (0.3 nm) for 24 h. Proliferation was determined by BrdU ELISA (A) and total cell number (B). E2 and ERβ-selective agonist, DPN, significantly increased BrdU incorporation and total cell number, whereas the ERα-specific ligand, PPT, did not. Data are presented as mean ± sem from three independent experiments. *, P < 0.05 vs. control or as indicated.
E2 and DPN increase the expression of phospho-ERK in hNPCs
E2 activates a myriad of signaling cascades in neurons, including MAPK (23,29,46). Activation of ERK cascade by E2 has been linked to the neuroprotection against β-amyloid and glutamate toxicity (23,29,46), as well as E2 activation of phospho-cAMP response element-binding protein required for E2-inducible neurotrophism and expression of the antiapoptotic Bcl2 family of proteins (20,21,47). To determine whether E2-induced hNPC proliferation is dependent upon activation of ERK pathway, the effect of E2, PPT, and DPN on pERK expression was investigated by ICC and Western blot.
As demonstrated in Fig. 6A, both E2 and DPN increased the intensity and number of pERK IR positive cells as detected by fluorescent microscopy, whereas PPT was comparable to vehicle control. To further quantitatively measure the impact of E2 and ER-selective ligands on pERK expression in hNPC, Western blots were performed using whole cell lysates from hNPCs. Consistent with the ICC observations, E2 and DPN respectively induced a 38 and 45% increase in pERK as quantitated by optical density of Western blot bands normalized to β-actin. The ICC and Western blot data demonstrated that E2 and DPN, but not PPT, activated the pERK1/2 signaling pathway in hNPCs.
Figure 6.
E2 and DPN increase the expression of phospho-ERK in hNPCs. The hNPCs were incubated in the presence or absence of E2, PPT, or DPN for 24 h. Expression of pERK is shown in representative ICC images in A and Western blot quantitation in B. E2 and DPN significantly increased pERK expression in hNPCs, whereas PPT did not. Normalized Western blot data are presented as mean ± sem from three independent experiments. *, P < 0.05 vs. control or as indicated. Scale bar, 100 μm in A.
ERβ-mediated hNPC proliferation is regulated by the activation of pERK
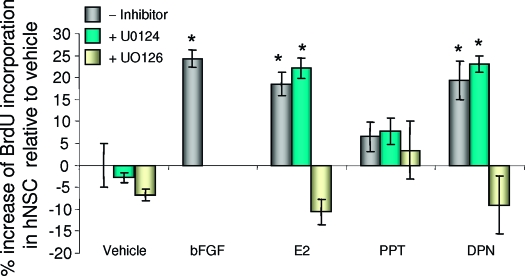
To determine whether phosphorylation of ERK was required for transduction of signals from ERβ activation through hNPC proliferation, hNPCs were treated with the MAPK pathway inhibitor, UO126 (20 μm), or the inactive isomer UO124 (20 μm), as a negative control, alone or in combination with E2 (100 nm), or PPT or DPN for 24 h. The concentration of the MAPK inhibitors was based on previous analyses from our group (20,23,48) Results of these analyses indicated that the MAPK kinase (MEK) 1/2 inhibitor UO126 completely abolished DPN-induced cell proliferation, whereas the inactive analog UO124 had no effect on DPN-induced hNPC proliferation (Fig. 7). UO126 inhibition of E2 and DPN-induced BrdU incorporation was selective for ER inducible proliferation because there was no statistically significant difference between BrdU incorporation under E2 + UO126 or DPN + UO126 conditions and vehicle control or UO126 alone. These results indicate that activation of the pERK cascade is a key signaling pathway required for ERβ-regulated E2-induced hNPC proliferation. These results are consistent with the report that UO126 completely inhibited MEK1/2 activity and resulted in G1 arrest in fibroblast cells via inactivation of ERK1/2, but not ERK5 (49). Both U0126 and U0124 showed no effects in PPT treated samples, which further supports that ERβ is the predominant receptor subtype regulating E2-induced hNPC proliferation.
Figure 7.
E2-induced hNPC proliferation is mediated by pERK. The hNPCs were treated with E2, DPN, or DPN plus the MEK1/2 inhibitor UO126, or its inactive analog, UO124. BrdU incorporation was measured by BrdU ELISA. Results indicated that UO126 abolished the promotion effects of DPN on hNPC proliferation, whereas the inactive isomer UO124 did not. Data are presented as mean ± sem from three independent experiments. *, P < 0.05 vs. control or as indicated. There were no statistical differences of E2 vs. DPN, or PPT vs. vehicle treatment.
pERK located in the M phase cell centrosomes
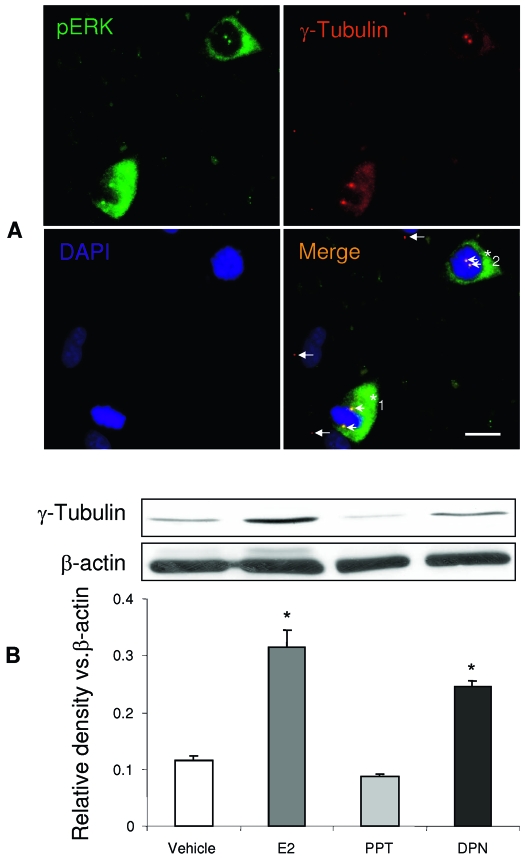
As an initial determination of the target for ERβ-inducible pERK, we investigated pERK localization in hNPCs. As demonstrated in Fig. 6A, pERK was observed in a subpopulation of the hNPCs. In these cells, pERK showed a predominantly cytoplasmic localization. The pERK positive cells exhibited a nuclear structure with condensed chromosomes (blue) containing two centrosomes, indicating that these cells are in mitotic stage (Fig. 8A). pERK IR was not observed in nondividing cells, which did not exhibit condensed chromosomal arrangement and contained only one centrosome. The centrosome was labeled by a centrosome marker, γ-tubulin (Fig. 8A, red, white arrows). The two centrosomes in the dividing cells are either apparent at both sides of metaphase plate (Fig. 8A, *1, the metaphase) or at one side of condensed chromosomes (*2, the prophase). Importantly, pERK IR (Fig. 8A, green) was only observed in the centrosomes of the dividing cells. In colocalization with γ-tubulin (Fig. 8A, red), the pERK positive centrosomes (green) appear yellow (arrowheads) in the merged image, but not in the centrosome of the nondividing cells (arrows). To identify further the relation of E2 effects and centrosome amplification, the γ-tubulin expression level was measured in E2, PPT, and DPN treated hNPCs by Western blot. Results of these analyses demonstrated an increase of γ-tubulin expression in E2 and DPN treated hNPCs but not in those treated with PPT treated cells vs. control (Fig. 8B). These data, coincident with the effects of E2 and DPN on pERK expression, indicated a close correlation between pERK and centrosome amplification, and demonstrate that E2 and DPN promoted centrosome amplification. Therefore, these data further support the discovery that activation of ERβ in hNPCs by either E2 or DPN increased proliferation.
Figure 8.
E2-induced ERK phosphorylation promotes centrosomes amplification. A. pERK immunoreactivity was observed in the dividing cell nuclear and cytosol located centrosome as demonstrated by double immunostaining of pERK (green) and centrosome marker, γ-tubulin (red). Basic nucleotides were counterstained with DAPI (blue). Dividing cells (*) are pERK positive and display two centrosomes (orange, indicated by arrowheads), indicating the colocalization of pERK (green) and γ-tubulin (red). In contrast, nondividing cells are pERK negative and exhibit only one centrosome (red, labeled only by γ-tubulin, white arrows). hNPC*1 hNPC, two centrosomes are located on both sides of the metaphase plate indicating entry into metaphase. hNPC*2 cell exhibited two centrosomes located to one side of the condensed chromosome indicating that this cell is likely in prophase. B, The centrosome marker protein, γ-tubulin, expression was detected in hNPCs by Western blot. Both E2 and DPN increased γ-tubulin levels in hNPCs, whereas PPT did not. Activation of ERβ either by E2 or DPN increased γ-tubulin protein levels in hNPCs and is indicative of amplification of centrosomes. Loading control, β-actin, normalized data are presented as mean ± sem from three independent experiments. *, P < 0.05 vs. control or as indicated.
Discussion
The present study demonstrates that E2 increased the proliferation of hNPCs in a dose- and time-dependant manner. Furthermore, the data indicate that ERβ is the predominant ER in this type of hNPC and is functionally competent. Importantly, the predominant expression of ERβ mediates the neurogenic effect of E2 in hNPCs as the proliferative effect of E2 was reproduced by the ERβ-selective ligand, DPN, but not by the ERα-selective ligand, PPT. Furthermore, results of the mechanistic analyses indicate that E2-induced hNPC proliferation is mediated by ERβ-activated pERK, which initiates the DNA replication and also triggers the centrosome amplification.
The effect of E2 on neurogenesis is supported by in vitro and in vivo animal studies under experimental and physiological conditions. E2 increased the proliferation of NPCs in the dentate gyrus subgranular layer zone of OVX rats, either with a single injection (10) or with a chronic E2 treatment (9). Under physiological conditions, cell proliferation in the dentate gyrus increases during proestrus, when ovarian hormone levels are highest, compared with estrus and diestrus (10). Augmentation of neural progenitor proliferation results in a transient increase in the number of new granular neurons (50,51). Further support comes from the observation that more new cells are found in the male dentate gyrus during the breeding season, when estradiol levels are high, than during the nonbreeding season when estradiol levels are low (52,53).
The magnitude of E2-induced proliferation reported here was an increase of 18–35% of BrdU incorporation in cultured hNPCs. The efficacy of E2 as a neurogenic factor was comparable to that induced by bFGF + heparin from our own study (30%) (12) and is also in agreement with previously published results by others. For example, bFGF induced a 40% increase of BrdU incorporation in cultured rat brain-derived progenitor cells after 3-d treatment (54) and a 25% increase of BrdU incorporation in 3-month-old rat brain subgranular zone in vivo (55).
The concentrations of E2 required to induce hNPC proliferation in vitro, a minimally effective dose of 1 nm (18% increase) and a maximally effective dose at 100 nm (35% increase), are comparable to those achieved in rat brain after peripheral E2 administration (9,10). A single 10 μg/rat E2 injection, which reflects an E2 concentration of about 100–120 nm in the brain as we measured by RIA, induced a 40% increase in BrdU incorporation in OVX adult female rat dentate gyrus. E2 appears to be a more potent neurogenic factor for hNPCs inducing a 35% increase in proliferation than in rat embryonic NPCs, in which 10 nm E2 induced a 7% increase in BrdU incorporation (8). We recently observed a 17% significant increase of BrdU positive cells in 14-d OVX rat subgranular zone (data not shown), which is lower than what was reported by Tanapat et al. (10) (40%). The discrepancy of a lower BrdU incorporation may due to the longer OVX time (14 vs. 6 d) before the E2 treatment (56).
E2-induced mitogenesis in hNPCs was a time-dependent process. E2 induced a significant increase in hNPC BrdU incorporation after 3-h E2 exposure, reached maximum by 5 h, and was sustained at a comparable magnitude for 24 h. These results are consistent with the reports by Tanapat et al. (9,10), showing that, in vivo in rodents, E2-induced stem cell proliferation reached a peak at 4 h after injection of E2. In our analyses, hNPCs were growth factor deprived for 4–6 h, shifting the cells into quiescence, which was the point at which E2 was added to the cultures. E2 induced a linear increase in BrdU incorporation within the first 5-h E2 exposure. The increase in BrdU incorporation was apparent within 1–2 h, significant at 3 h, and asymptotic at 5 h. Because BrdU is only incorporated during the DNA replication of S phase, the increase during the 1- to 5-h E2 exposure and the sustained level of BrdU incorporation between 6 and 24 h indicate that the duration of S phase of E2-treated hNPCs was about 3–4 h. The 3- to 4-h S-phase time frame is consistent with the report by Ostenfeld and Svendsen (57), who reported a 2.6- to 3.7-h S-phase duration when hNPCs were treated with FGF or EGF, respectively. It should be noted that the total time to traverse the cell cycle is 50–58 h in these cells. Thus, one would not expect another round of BrdU incorporation to occur within 24 h after exposure to E2. Therefore, the data indicate that the total amount of BrdU incorporation into DNA was achieved within the first 5-h E2 exposure and that this amount of BrdU remained within the cells for the 6- to 24-h time points.
It is interesting to note that the binding affinity of E2 is 10 times greater than DPN to purified ERβ, and the effective concentration of E2 in ERβ promoter driven luciferase report gene transcription is 10 times lower than that of DPN (58), but the concentration of DPN required to induce hNPC proliferation is much lower (in the 100-pm range) than that of E2 (in the nm range). This discrepancy has not been fully addressed but has also been reported in other studies by different groups (44,45). In these reports the effective neuroprotection concentration of E2 was in the nm range, but for PPT and DPN, the effective neuroprotection concentrations were in the pm range (23,45). These findings are consistent with results of the current study showing that the effective concentration of DPN for hNPC proliferation is more than 10 times potent than that of E2. Similar examples can be found for PPT, an ERα selective ligand, and E2 on ERα. The affinity of E2 on purified ERα is 10 times greater, and the effective concentration on ERα promoter regulated gene transcription is 10 times lower than that of PPT (59). However, the acute effect on vascular contractility, which is mainly triggered by a membrane-initiated signal (60,61), the effective concentration of PPT is 10 times lower than E2 (61). These similarities of the PPT on ERα-induced vascular contractility and the DPN on ERβ-mediated hNPC proliferation and neuroprotection (44,45) also suggest that these effects are mediated by membrane ERβ.
Both ERα and ERβ have been observed in rat NPCs and human embryonic NPCs (8,62,63), but ERβ is expressed at a higher level in hNPCs (8,63), and the expression is increased by E2 treatment (63). Results of the present study indicate that ERβ is the major receptor in the fetal cerebral cortical hNPC and that E2-induced proliferation is mediated by ERβ. In hNPCs, ERβ mRNA expression was more than 10-fold higher than ERα. Results of real-time RT-PCR analysis of mRNA level for ERβ was confirmed by a predominant ERβ protein expression in hNPCs and by predominant immunocytochemical detection of ERβ in hNPCs. The use of MCF7 cells, which predominantly express ERα, provided a positive control for detecting ERα expression and is consistent with that reported in the breast cancer, indicating that ERα is the predominant ER isoform in breast carcinoma cells (42,43). Our findings of ERβ expression in NPCs are supported by a recent study indicating that human embryonic stem cells express 5-fold higher ERβ than ERα (63). In short, the predominant ER isoform in hNPCs derived from cerebral cortex is ERβ, whereas ERα is barely detectable.
In this study we demonstrated that ERβ is predominantly expressed in hNPCs, and that ERβ is functional and activated by both E2 and an ERβ-selective ligand. Activation of ERβ resulted in an increase in hNPC proliferation, as evidenced by BrdU incorporation, total cell number, and the expression of mitotic markers, PCNA and CDK1/cdc2. Selective activation of ERα in hNPCs was without effect on either hNPC proliferation or on cell cycle protein expression.
Both PCNA and cdc2 are well defined and commonly used markers for cell proliferation (12). PCNA is a marker for cells in early G1 phase and S phase of the cell cycle, and acts as a homotrimer to increase the processing of leading strand synthesis during DNA replication. CDK1/cdc2 is the catalytic subunit of the protein kinase complex M-phase promoting factor and is universal among eukaryotes. CDK1 exists as a CDK1/cyclin B complex that is required for the G2 to M phase transition (64). In this study we demonstrated that both E2 and ERβ-selective ligand, DPN, increased the expression of PCNA and CDK1 expression, whereas PPT, an ERα-selective ligand, did not. These data indicate that activation of ERβ in hNPCs is sufficient to promote E2-induced hNPC proliferation.
Our findings of ERβ-mediated proliferation of human cortical NPCs are relevant to earlier findings of Gustafsson and colleagues in ERβ knockout mice (65). ERβ appears to play a major role in brain development and neurogenesis (62,63,65). In ERβ knockout mice, brain size was smaller, and fewer neurons were observed (65). Expression of ERβ in human embryonic brain cells suggests a comparable role of ERβ in human brain development (62,63). ERβ-mediated proliferation of hNPCs suggests that ERβ is a significant regulator of cortical development in the human, comparable to that observed in the ERβ knockout mouse.
Recently, Suzuki et al. (66) reported that E2 enhanced neurogenesis in ischemic model mice; both ERα and ERβ contributed almost equally as demonstrated by ischemic ERα and ERβ knockout mice and their background. Interestingly, in normal rodents, ERβ is a major mediator for E2-induced BrdU incorporation increase in dentate gyrus (67). The higher contribution of ERα on E2-induced dentate cell proliferation in ischemic rodents than in normal rats may explained by the higher level of ERα than ERβ expression in the ischemia model, in which there is a 2- to 3-fold increase of ERα and a decrease trend of ERβ expression (68). Together, these data suggest that under normal conditions, ERβ is the major estrogen mediator to enhance the dentate NPC proliferation.
Recent studies have demonstrated that neurogenesis may serve as a neural basis for antianxiety drugs (69,70,71). Handa and colleagues (72) demonstrated that the ERβ-selective ligand, DPN, showed significantly decreased anxiety related behaviors. The current findings that ERβ is predominantly expressed in hNPCs and DPN enhances NPC proliferation may provide a cellular mechanism to Handa and his colleagues’ discoveries.
Parallel to the promotion of hNPC proliferation, activation of hNPC ER by either E2 or DPN also increased the pERK positive cell number and intensity in the culture of hNPC, which strongly suggests that the ERβ-regulated hNPC proliferation process is mediated via the phosphorylation of ERK, an active intracellular signal that can regulate the G1/S transition in many cell models (31,32,33,49,73). Furthermore, the MEK inhibitor UO126 abolished DPN-induced hNPC proliferation.
p-ERK is known to translocate into the nucleus to promote transcription of cell cycle genes, such as cyclin D1 and CDK expression, and subsequently activate the cyclin-dependent protein kinase CDK4/6, which promotes cell cycle entry by phosphorylating the retinoblastoma tumor suppressor, leading to the release of the transcriptional factor E2F (74). Although this pathway was proposed in cancer cell models, recent analyses indicated that, in breast cancer cells, estrogen-induced cell cycle progression was not sensitive to the inhibition of ERK-regulating kinases MEK1 and 2 (34), suggesting that estrogens may elicit a distinct pattern of early and delayed activation of ERK in breast cancer cells (34). In hNPCs, the DPN-induced proliferation was completely abolished by inhibition of the ERK-regulating kinase MEK1/2. The distinction between ERα and ERβ regulation of proliferation in MCF-7 breast cancer cells and hNPCs, respectively, suggests the intriguing possibility that ERβ-selective hNPC proliferation may specifically increase neurogenesis while potentially reducing the risk of proliferation of ERα-sensitive tumors (42,43,75,76). In support of this hypothesis, overexpression of ERβ by ectopic transfection in MCF7 cells inhibited E2-induced breast cancer cell proliferation (77).
In addition to initiating entry into the cell cycle, phospho-ERK also triggers centrosome amplification (78). The amplified centrosomes then migrate to both sides of the metaphase plate and facilitate the spindle formation. These centrosome activities involve centrosome-related kinase phosphorylation and γ-tubulin assembly (38,79). In the current study, we found that pERK IR was only observed in dividing cells and also in the replicated centrosomes, but not in unreplicated centrosomes. Localization of the pERK signal to the centrosome indicates that pERK plays a key role in ERβ-inducible hNPC proliferation. Moreover, this finding further suggests that pERK regulates not only the intranuclear events for G1/S enter or reentry into the cell cycle (33), but also extranuclear events required for mitosis, such as centrosome assembly and amplification (78,80). In parallel, the increased expression of pERK induced by either E2 or DPN also increased the γ-tubulin protein levels in hNPC cultures, as demonstrated by Western blot.
γ-Tubulin combines with several other associated proteins to form a circular structure known as the γ-tubulin ring complex. This complex acts as a scaffold for α/β-tubulin dimers to begin polymerization. During cell division, centrosomes double, which would necessarily include γ-tubulin. Our data indicated that activation of ERβ by E2 or DPN promotes hNPC proliferation centrosome amplification and γ-tubulin expression. These findings are consistent with E2-induced cell proliferation and centrosome amplification in rat mammary gland by the increase of γ-tubulin expression in rat mammary gland (81,82).
In conclusion, the present study revealed that ERβ is the predominant ER isoform in hNPCs and mediates E2-induced hNPC proliferation. ERβ-induced proliferation was mediated through the pERK signaling cascade. Furthermore, ERβ activation led to a replication of the centrosome, consistent with entry into S phase of the cell cycle and DNA segregation, and was a phosphorylation target of pERK. This discovery has important implications for understanding the molecular mechanisms by which estrogens promote neurogenesis in humans. Moreover, this discovery suggests that ERβ, in hNPCs, may serve as a novel, safer, and efficacious therapeutic target to promote neurogenesis in vivo to sustain neurological function and renewal in postmenopausal women.
Acknowledgments
The authors thank Anna Tran, Tino Sanchez, Sean Iwamoto, and Eun Ji Chuang for their excellent technical assistance.
Footnotes
This work was supported by grants from the Alzheimer’s Association (to J.M.W.), and the National Institutes of Aging, the Kenneth T. and Eileen L. Norris Foundation, and the L. K. Whittier Foundation (to R.D.B.).
Disclosure Statement: The authors have no disclosures.
First Published Online October 25, 2007
Abbreviations: bFGF, Basic fibroblast growth factor; BrdU, bromodeoxyuridine; CDK, cyclin-dependent kinase; DAPI, 4′,6′-diamidino-2-phenylindole; DPN, diarylpropionitrile; EGF, epidermal growth factor; E2, estradiol-17β; ER, estrogen receptor; hNPC, human neural progenitor cell; ICC, immunocytochemistry; IR, immunoreactive; MEK, MAPK kinase; NPC, neural progenitor cell; OVX, ovariectomized; PCNA, proliferating cell nuclear antigen; pERK, phosphorylated ERK; PPT, propyl pyrazole triol.
References
- Brinton RD 2002 Selective estrogen receptor modulators (SERM) for the brain: recent advances and remaining challenges for developing a NeuroSERM. Drug Dev Res 56:380–392 [Google Scholar]
- Brinton RD 2004 Impact of estrogen therapy on Alzheimer’s disease: a fork in the road? CNS Drugs 18:405–422 [DOI] [PubMed] [Google Scholar]
- Finch CE 2003 The biology of aging in model organisms. Alzheimer Dis Assoc Disord 17(Suppl 2):S39–S41 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE 1999 Estrogen actions in the central nervous system. Endocr Rev 20:279–307 [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G 1997 Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer’s disease. Am J Med 103:19S–25S [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD 2000 Estrogen as a treatment for Alzheimer disease. JAMA 284:307–308 [PubMed] [Google Scholar]
- Brannvall K, Bogdanovic N, Korhonen L, Lindholm D 2005 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur J Neurosci 21:871–878 [DOI] [PubMed] [Google Scholar]
- Brannvall K, Korhonen L, Lindholm D 2002 Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci 21:512–520 [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E 2005 Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol 481:252–265 [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E 1999 Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19:5792–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, Caldwell MA 1998 A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods 85:141–152 [DOI] [PubMed] [Google Scholar]
- Wang JM, Johnston PB, Ball BG, Brinton RD 2005 The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci 25:4706–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LS, Li J, Caldwell MA, Wallace K, Johnson JA, Svendsen CN 2003 Gene expression in human neural stem cells: effects of leukemia inhibitory factor. J Neurochem 86:179–195 [DOI] [PubMed] [Google Scholar]
- Wang L, Andersson S, Warner M, Gustafsson JA 2002 Estrogen actions in the brain. Sci STKE 2002:PE29 [DOI] [PubMed] [Google Scholar]
- Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA 2005 Reflections on the discovery and significance of estrogen receptor β. Endocr Rev 26:465–478 [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA 2003 Estrogen signaling: a subtle balance between ER α and ER β. Mol Interv 3:281–292 [DOI] [PubMed] [Google Scholar]
- Struble RG, Rosario ER, Kircher ML, Ludwig SM, McAdamis PJ, Watabe K, McAsey ME, Cady C, Nathan BP 2003 Regionally specific modulation of brain apolipoprotein E in the mouse during the estrous cycle and by exogenous 17β estradiol. Exp Neurol 183:638–644 [DOI] [PubMed] [Google Scholar]
- Wang JM, Brinton RD, Estrogen-induced neurogenesis and activation of pERK is mediated by estrogen receptor β in human cerebral cortical neural stem cells. Society For Neuroscience Annual Meeting, Washington DC, 2005 (Abstract 633.3) [Google Scholar]
- Wang JM, Brinton RD, Estrogen receptor mediates estrogen-induced proliferation in human cerebral cortical neural stem cells. Program of the 88th Annual Meeting of The Endocrine Society, Boston, MA, 2006 (Abstract P1–125) [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD 2005 17β-Estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neurosciene 135:59–72 [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD 2005 Estrogen receptor β as a therapeutic target in brain for promoting neurogenesis and preventing neurodegeneration., estrogens: neurodegeneration and cytoprotection. In: Moos W, Dykens J, eds. Drug development research. Wilmington, DE: Wiley-Liss, Inc.; 1–15 [Google Scholar]
- Gatson JW, Kaur P, Singh M 2006 Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology 147:2028–2034 [DOI] [PubMed] [Google Scholar]
- Mannella P, Brinton RD 2006 Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci 26:9439–9447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M 2001 Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine 14:407–415 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS 2005 17α-Estradiol: a brain-active estrogen? Endocrinology 146:3843–3850 [DOI] [PubMed] [Google Scholar]
- Singh M, Dykens JA, Simpkins JW 2006 Novel mechanisms for estrogen-induced neuroprotection. Exp Biol Med (Maywood) 231:514–521 [DOI] [PubMed] [Google Scholar]
- Setalo Jr G, Singh M, Guan X, Toran-Allerand CD 2002 Estradiol-induced phosphorylation of ERK1/2 in explants of the mouse cerebral cortex: the roles of heat shock protein 90 (Hsp90) and MEK2. J Neurobiol 50:1–12 [DOI] [PubMed] [Google Scholar]
- Setalo Jr G, Singh M, Nethrapalli IS, Toran-Allerand CD 2005 Protein kinase C activity is necessary for estrogen-induced Erk phosphorylation in neocortical explants. Neurochem Res 30:779–790 [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo Jr G, Guan X, Frail DE, Toran-Allerand CD 2000 Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-α knock-out mice. J Neurosci 20:1694–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC 2006 Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci 26:10332–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Morooka T, Ogawa S, Nishida E 2001 ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol 3:453–459 [DOI] [PubMed] [Google Scholar]
- Chambard JC, Lefloch R, Pouyssegur J, Lenormand P 2007 ERK implication in cell cycle regulation. Biochim Biophys Acta 1773:1299–1310 [DOI] [PubMed] [Google Scholar]
- Ussar S, Voss T 2004 MEK1 and MEK2, different regulators of the G1/S transition. J Biol Chem 279:43861–43869 [DOI] [PubMed] [Google Scholar]
- Geffroy N, Guedin A, Dacquet C, Lefebvre P 2005 Cell cycle regulation of breast cancer cells through estrogen-induced activities of ERK and Akt protein kinases. Mol Cell Endocrinol 237:11–23 [DOI] [PubMed] [Google Scholar]
- Zeldich E, Koren R, Nemcovsky C, Weinreb M 2007 Enamel matrix derivative stimulates human gingival fibroblast proliferation via ERK. J Dent Res 86:41–46 [DOI] [PubMed] [Google Scholar]
- Lewis TS, Shapiro PS, Ahn NG 1998 Signal transduction through MAP kinase cascades. Adv Cancer Res 74:49–139 [DOI] [PubMed] [Google Scholar]
- Costa M, Marchi M, Cardarelli F, Roy A, Beltram F, Maffei L, Ratto GM 2006 Dynamic regulation of ERK2 nuclear translocation and mobility in living cells. J Cell Sci 119:4952–4963 [DOI] [PubMed] [Google Scholar]
- Furukawa T, Kanai N, Shiwaku HO, Soga N, Uehara A, Horii A 2006 AURKA is one of the downstream targets of MAPK1/ERK2 in pancreatic cancer. Oncogene 25:4831–4839 [DOI] [PubMed] [Google Scholar]
- Hu S, Lu SF, Kaplan JR, Adams MR, Simon NG 2005 ERβ protein expression in female cynomolgus monkey and CF-1 mouse brain: Western analysis. J Neurobiol 64:298–309 [DOI] [PubMed] [Google Scholar]
- Perez S, Sendera TJ, Kordower JH, Mufson EJ 2004 Estrogen receptor α containing neurons in the monkey forebrain: lack of association with calcium binding proteins and choline acetyltransferase. Brain Res 1019:55–63 [DOI] [PubMed] [Google Scholar]
- Wang JM, Irwin RW, Brinton RD 2006 Activation of estrogen receptor α increases and estrogen receptor β decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc Natl Acad Sci USA 103:16983–16988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi J, Otsuki T, Kunisue H, Tanaka K, Yamamoto S, Sonoo H 2000 Expression levels of estrogen receptor-α, estrogen receptor-β, coactivators, and corepressors in breast cancer. Clin Cancer Res 6:512–518 [PubMed] [Google Scholar]
- Iwao K, Miyoshi Y, Egawa C, Ikeda N, Tsukamoto F, Noguchi S 2000 Quantitative analysis of estrogen receptor-α and -β messenger RNA expression in breast carcinoma by real-time polymerase chain reaction. Cancer 89:1732–1738 [DOI] [PubMed] [Google Scholar]
- Cordey M, Pike CJ 2005 Neuroprotective properties of selective estrogen receptor agonists in cultured neurons. Brain Res 1045:217–223 [DOI] [PubMed] [Google Scholar]
- Zhao L, Wu TW, Brinton RD 2004 Estrogen receptor subtypes α and β contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res 1010:22–34 [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD 2003 Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci USA 100:10506–10511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chen S, Ming Wang J, Brinton RD 2005 17beta-estradiol induces Ca(2+) influx, dendritic and nuclear Ca(2+) rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience 132:299–311 [DOI] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Brinton RD 2002 Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res 930:216–234 [DOI] [PubMed] [Google Scholar]
- Squires MS, Nixon PM, Cook SJ 2002 Cell-cycle arrest by PD184352 requires inhibition of extracellular signal-regulated kinases (ERK) 1/2 but not ERK5/BMK1. Biochem J 366:673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA 2001 Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: a possible regulatory role for estradiol. Neuroscience 102:369–379 [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA 2003 Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol 55:247–260 [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS 1999 Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience 89:955–964 [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA 2003 Reproductive status influences the survival of new cells in the dentate gyrus of adult male meadow voles. Neurosci Lett 346:25–28 [DOI] [PubMed] [Google Scholar]
- Gago N, Avellana-Adalid V, Evercooren AB, Schumacher M 2003 Control of cell survival and proliferation of postnatal PSA-NCAM(+) progenitors. Mol Cell Neurosci 22:162–178 [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA 2003 Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell 2:175–183 [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL 2006 Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 147:607–614 [DOI] [PubMed] [Google Scholar]
- Ostenfeld T, Svendsen CN 2004 Requirement for neurogenesis to proceed through the division of neuronal progenitors following differentiation of epidermal growth factor and fibroblast growth factor-2-responsive human neural stem cells. Stem Cells 22:798–811 [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA 2001 Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44:4230–4251 [DOI] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS 2003 Activities of estrogen receptor α- and β-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol 206:13–22 [DOI] [PubMed] [Google Scholar]
- Al Zubair K, Razak A, Bexis S, Docherty JR 2005 Relaxations to oestrogen receptor subtype selective agonists in rat and mouse arteries. Eur J Pharmacol 513:101–108 [DOI] [PubMed] [Google Scholar]
- Montgomery S, Shaw L, Pantelides N, Taggart M, Austin C 2003 Acute effects of oestrogen receptor subtype-specific agonists on vascular contractility. Br J Pharmacol 139:1249–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried G, Andersson E, Csoregh L, Enmark E, Gustafsson JA, Aanesen A, Osterlund C 2004 Estrogen receptor β is expressed in human embryonic brain cells and is regulated by 17β-estradiol. Eur J Neurosci 20:2345–2354 [DOI] [PubMed] [Google Scholar]
- Hong SH, Nah HY, Lee YJ, Lee JW, Park JH, Kim SJ, Lee JB, Yoon HS, Kim CH 2004 Expression of estrogen receptor-α and -β, glucocorticoid receptor, and progesterone receptor genes in human embryonic stem cells and embryoid bodies. Mol Cells 18:320–325 [PubMed] [Google Scholar]
- Masui Y 2001 From oocyte maturation to the in vitro cell cycle: the history of discoveries of maturation-promoting factor (MPF) and cytostatic factor (CSF). Differentiation 69:1–17 [DOI] [PubMed] [Google Scholar]
- Wang L, Andersson S, Warner M, Gustafsson JA 2003 Estrogen receptor (ER)β knockout mice reveal a role for ERβ in migration of cortical neurons in the developing brain. Proc Natl Acad Sci USA 100:703–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM 2007 Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors α and β. J Comp Neurol 500:1064–1075 [DOI] [PubMed] [Google Scholar]
- Mazzucco CA, Lieblich SE, Bingham BI, Williamson MA, Viau V, Galea LA 2006 Both estrogen receptor α and estrogen receptor β agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience 141:1793–1800 [DOI] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM 2005 Estrogen can act via estrogen receptor α and β to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology 146:3070–3079 [DOI] [PubMed] [Google Scholar]
- Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, Luscher B 2007 GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci 27:3845–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM 2003 New molecular targets for antianxiety interventions. J Clin Psychiatry 64(Suppl 3):28–35 [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA 2007 Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci 27:2734–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ 2005 Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology 146:797–807 [DOI] [PubMed] [Google Scholar]
- Balmer NN, Richer JK, Spoelstra NS, Torkko KC, Lyle PL, Singh M 2006 Steroid receptor coactivator AIB1 in endometrial carcinoma, hyperplasia and normal endometrium: correlation with clinicopathologic parameters and biomarkers. Mod Pathol 19:1593–1605 [DOI] [PubMed] [Google Scholar]
- Sherr CJ, McCormick F 2002 The RB and p53 pathways in cancer. Cancer Cell 2:103–112 [DOI] [PubMed] [Google Scholar]
- Palmieri C, Cheng GJ, Saji S, Zelada-Hedman M, Warri A, Weihua Z, Van Noorden S, Wahlstrom T, Coombes RC, Warner M, Gustafsson JA 2002 Estrogen receptor β in breast cancer. Endocr Relat Cancer 9:1–13 [DOI] [PubMed] [Google Scholar]
- Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P 2004 Loss of ERβ expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer 11:537–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA 2004 Estrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA 101:1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C, Cho H, Kim SJ, Lee JH, Park SY, Chan GK, Cho H 2004 Mitotic aberration coupled with centrosome amplification is induced by hepatitis B virus X oncoprotein via the Ras-mitogen-activated protein/extracellular signal-regulated kinase-mitogen-activated protein pathway. Mol Cancer Res 2:159–169 [PubMed] [Google Scholar]
- Hata T, Furukawa T, Sunamura M, Egawa S, Motoi F, Ohmura N, Marumoto T, Saya H, Horii A 2005 RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res 65:2899–2905 [DOI] [PubMed] [Google Scholar]
- Chae S, Yun C, Um H, Lee JH, Cho H 2005 Centrosome amplification and multinuclear phenotypes are induced by hydrogen peroxide. Exp Mol Med 37:482–487 [DOI] [PubMed] [Google Scholar]
- Hontz AE, Li SA, Lingle WL, Negron V, Bruzek A, Salisbury JL, Li JJ 2007 Aurora a and B overexpression and centrosome amplification in early estrogen-induced tumor foci in the Syrian hamster kidney: implications for chromosomal instability, aneuploidy, and neoplasia. Cancer Res 67:2957–2963 [DOI] [PubMed] [Google Scholar]
- Li JJ, Weroha SJ, Lingle WL, Papa D, Salisbury JL, Li SA 2004 Estrogen mediates Aurora-A overexpression, centrosome amplification, chromosomal instability, and breast cancer in female ACI rats. Proc Natl Acad Sci USA 101:18123–18128 [DOI] [PMC free article] [PubMed] [Google Scholar]