Abstract
Activation of Delta-Notch signaling pathway promotes the development of the vascular system in embryo, normal adult tissues, and cancerous lesions. Delta and Notch genes are known to be expressed in endothelial cells, and little is known of their expression beyond the vascular system. The purpose of this study was to investigate whether Delta gene would be expressed in cells of the uterine endometrium. In this study, we found that the human endometrial cells expressed one of the Delta ligands, Delta-like 4 protein (Dll4). Dll4 was expressed in human endometrium in a spatiotemporal fashion. Immunohistochemistry studies showed the cytoplasm as well as membrane staining with apical localization both in the luminal and glandular epithelium and moderate diffuse staining in the cytoplasm of the stromal cells. Western blot analysis showed that the size of the endometrial Dll4 was identical to that in the human umbilical endothelial cells. The expression of Dll4 mRNA in human endometrial cells was quantitatively determined by real-time PCR. Dll4 mRNA expressed in the glandular epithelium showed large variations, and it was significantly elevated in the mid and late proliferative and early secretory endometrium. Endometrial stromal cells contained less Dll4 mRNA and had no clear correlation with the menstrual cycle. The effect of hormones was studied in the primary culture of isolated glandular epithelial and stromal cells. In glandular cells, estradiol had little effect, and medroxyprogesterone acetate significantly reduced the mRNAs compared with that of control. Relaxin induced the Dll4 mRNA. In stromal cells, both estradiol and medroxyprogesterone acetate reduced the Dll4 mRNA. To our knowledge, this is the first report of the expression of Dll4 in the endometrium. We propose that endometrial Dll4 may enhance the development of the endometrial microvascular system and facilitate the implantation of blastocyst in a fertile cycle.
THE DELTA-NOTCH signaling pathway plays a pivotal role in determining the cell fate, proliferation, and apoptosis of the vascular system in embryo, adult tissue, and cancerous lesions. When the ligand, Delta-like or Jagged proteins, binds to the Notch receptor, it initiates the proteolytic cleavage to liberate the cytoplasmic domain of the receptor, which translocates into the nucleus to activate transcription of the target gene (1). As a consequence of both ligand and receptor being membrane bound, receptor-ligand binding requires direct cell to cell contact resulting in a directional specific activation of the Notch signaling pathway. Up to date, five Delta ligands have been shown to be expressed in the vascular endothelial cells of various types of organs (2), but little is known in other cell types.
We believe that the Delta-Notch system plays a role in development of the endometrium. Formation of the spiral arteries is one of the main morphogenetic events that occur in endometrium during the menstrual cycle that is distinctly different from the angiogenesis in other organs (3). We propose that Delta-Notch signaling promotes cell differentiation and formation of the unique structure of the endometrial arteries, even though it is well known that vascular endothelial growth factor (VEGF) enhances angiogenesis in the endometrium (4). We hypothesized that Delta ligand may be expressed in the endometrial cells, which would activate the Notch signaling pathway in the endothelial cells to direct the endometrial vasculature. To address this issue, our first approach was to investigate whether the Delta ligand would be present in the endometrial cells. In this study, we provide evidence that Delta-like 4 protein (Dll4) is expressed in human endometrial cells and is regulated by hormones.
Materials and Methods
Human endometrial specimens
Immunohistochemistry of Dll4 in human endometrium was carried out using the tissue blocks selected from the archived endometrial specimens at the Department of Pathology. The Human Subject Committee of Stony Brook University approved to use these materials for research. Viable human endometrial specimens were collected anonymously without any link to the living subject from a group of women in their reproductive ages excluding any abnormal or cancerous endometrium. Each specimen was individually approved by the attending pathologist after an adequate tissue sample for clinical diagnosis had been set aside. The specimens used in this study were the part of the tissue that otherwise were discarded. The Human Subject Committee of Stony Brook University approved the research protocol, and it is classified as nonhuman subject research according to the Federal/National Institutes of Health regulations governing expedited review procedures in accordance with U.S. Department of Health and Human Services.
Localization of Dll4 by immunohistochemistry in endometrium
The 4-μm tissue sections of the endometrial specimens were heated for 15 min for antigen retrieval. These sections were incubated with Dll4 polyclonal antibody (1:50 dilution; Abcam, Cambridge, MA) and followed by the secondary antibody (Super Sensitive Multilink; BioGenex, San Ramon, CA). Immunostains were visualized by streptavidin peroxidase labeling method (SS label; BioGenex). Appropriate control stains were run in parallel. All sections are shown at ×400 amplification.
Isolation and culture of endometrial glandular epithelial and stromal cells
Endometrial glandular and stromal cells were isolated by digesting the viable tissue fragments with collagenase as described previously (5,6,7). The purity of each preparation was estimated by examining the cell morphology (stromal cells, flat spindle monolayer, and glandular cells, polygonal shape) and exclusion of blood cells and endothelial cells confirmed by negative staining with anti-CD31 and -CD45 (8,9). Preparations with purity more than 95% were included in this study. Endometrial cells were cultured in RPMI 1640 medium with 2% charcoal-dextran-stripped fetal bovine serum. To study the effect of hormones, medium was supplemented with 100 nm estradiol (E2), medroxyprogesterone acetate (MPA) (Steraloids Inc., Newport, RI) or 20 ng/ml relaxin (RLX; National Hormone and Peptide Program, Torrance, CA) and cultured for 3 and 8 d for glandular and stromal cells, respectively. The human umbilical vascular endothelial cells (HUVECs) were isolated and cultured in medium with endothelial cell growth supplement (Sigma Chemical Co., St. Louis, MO) described previously (10).
Identification of Dll4 and Dll4 mRNA by Western blot and real-time PCR, respectively
Western blot was carried out to identify Dll4 in endometrial cells. Cell lysates were resolved on 8% SDS-PAGE and then blotted on polyvinylidene difluoride membrane. Polyclonal rabbit Dll4 antibody (1:500 dilution) was used to identify the Dll4 protein and detected by enhanced chemiluminescence. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal standard and identified by anti-GAPDH-horseradish peroxidase (Abcam). The intensities of the Dll4 and GAPDH were scanned on a Bio-Rad Image analysis system.
To determine the Dll4 mRNA, total RNA was isolated from the cell lysates using RNAqueous Kit (Ambion, Austin, TX) and reverse transcribed using random primers. Optimal primers for the Dll4 cDNAs were selected from NCBI GenBank, accession number NM_0190740 (631aactgcccttcaatttcacct 651 and 806gctggtttgctcatccaataa786, forward and reverse primers, respectively). The amplified PCR product was confirmed to be the Dll4 cDNA (176 bp) by DNA sequencing and was used as standard curve for real-time PCR using QuantiTec SYBR Green PCR Kit (204143; QIAGEN, Valencia, CA) and carried out in Opticon 2 (MJ Research Inc., Waltham, MA). Results were normalized by an internal standard of β-actin in each sample described in detail in a previous publication (11).
Statistical analysis
Student’s t test was used for statistical analysis to estimate the significance of Dll4 mRNA expression (P values) among various cell types, glandular cells at different stages of menstrual cycle, and effects of hormones on Dll4 in endometrial cells.
Results
Localization of Dll4 in the human endometrium by immunohistochemistry
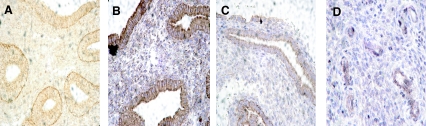
The subcellular localization of Dll4 was carried out in the tissue sections of human endometrium by immunohistochemistry. The corresponding tissue section was stained with hematoxylin and eosin (data not shown) for dating of the endometrium, proliferative, mid-secretory (d 22–23), and late secretory (d 26–28) (Fig. 1, A–C, respectively) and localization of the blood vessels (Fig. 1D). Immunohistochemistry studies showed the cytoplasm as well as membrane staining with apical localization both in the luminal and glandular epithelium (Fig. 1, A–C) and moderate diffuse staining in the cytoplasm of the stromal cells and blood vessels (Fig. 1D).
Figure 1.
Immunohistochemistry localization of Dll4 in human endometrium. Sections of the endometrium of proliferative (A), mid secretory (d 22–23) (B), and late secretory (d 26–27) (C) phase were incubated with Dll4 antibody following the procedure described in Materials and Methods. All sections are shown at ×400 amplification. A–C, Cytoplasm and membrane staining with apical localization both in the luminal and glandular epithelium and moderate diffuse stains in the cytoplasm of stromal cells; D, immunostains in blood vessels of the mid secretory specimen.
Western blot of Dll4 in the endometrial cells
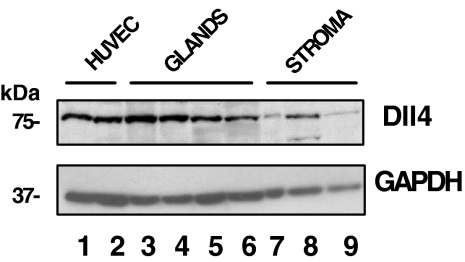
Figure 2 shows a set of data of Western blot of Dll4 protein in endometrial cells. For comparison, HUVEC lysates were run in parallel. Endometrial glandular and stromal cells expressed Dll4 (lanes 3–9) with the size corresponding to that of HUVECs (lanes 1 and 2), similar to the molecular weight described in the other vascular systems (12,13,14). In comparison, the Dll4 in the glandular cells of proliferative and early secretory phase were higher than that of HUVECs (∼2-fold increase; lanes 3 and 4 vs. 1 and 2). Dll4 contents in stromal cells were either similar or less than those in HUVECs and glandular cells.
Figure 2.
Western blot analysis of Dll4 in HUVECs and in human endometrial cells. Total protein (100 μg) from cell lysates was applied to each lane: HUVECs (lanes 1 and 2), glandular cells (proliferative, early, and two late secretory phase, lanes 3–6), and stromal cells (proliferative and mid and late secretory phase, lanes 7–9, respectively). The upper and lower panels show the intensity of Dll4 and GAPDH bands, respectively. The relative intensities of Dll4 bands, normalized by GADPH, were 1.1 (HUVEC); 2.7, 1.7, 0.9, and 0.6 (glandular cells); and 0.3, 1.0, and 0.4 (stromal cells), respectively.
Expression of Dll4 mRNA in endometrial cells
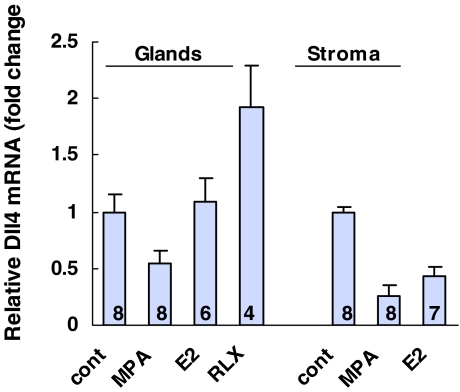
Total RNA was isolated from the tissue fragments of the endometrium and in isolated endometrial glandular, stromal cells, and HUVECs. The content of Dll4 mRNA determined in the intact tissue fragments and in HUVECs was 1.2 ± 0.2 (n = 4) and 8.2 ± 2 (n = 3) (mean ± sem, amol/10 μg RNA), respectively. Figure 3 shows the relative expression of Dll4 mRNA in endometrial glandular cells at various stages of the menstrual cycle and in stromal cells. Dll4 mRNA levels in glandular cells of mid-late proliferative and early secretory endometrium were significantly higher than that of the early proliferative and mid and late secretory endometrium (P < 0.05). Endometrial stromal cells contained a relatively low amount of Dll4 mRNA, with no clear correlation with stages of the menstrual cycle. The difference of Dll4 mRNA content between two types of cells, stromal cells (mean of n = 11) and glandular cells (mean of n = 25), was significant (P < 0.01).
Figure 3.
Dll4 mRNA in human endometrial glandular and stromal cells. Dll4 mRNA contents were determined by real-time PCR, normalized by β-actin. The relative expression of Dll4 mRNA in glandular cells was presented with respect to the mean value of stromal cells, which was set as 1 (equivalent to ∼0.36 amol/10 μg RNA, mean ± sem). EP, Early proliferative; M/LP, mid/late proliferative; ES, early secretory; LS, late secretory; MS, mid secretory. Eleven endometrial stromal cell samples were EP (1), MP/LP (3), LS (2), MS (1), and LS (4). Statistical analysis: in glands vs. stroma (P < 0.01); in glandular cells, P or ES vs. EP, MS, and LS (P < 0.05) and MS vs. LS (P < 0.05).
Effects of hormones on Dll4 mRNA in endometrial cells
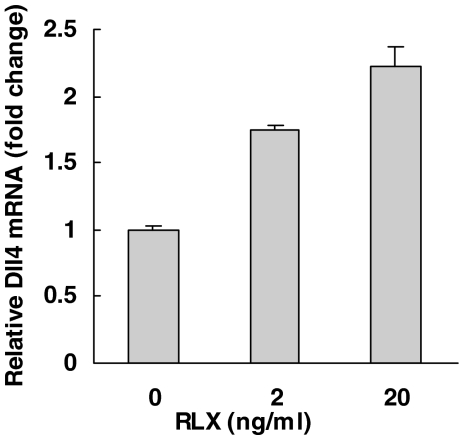
Figure 4 shows the effect of MPA, E2, and RLX on the Dll4 mRNA in the primary culture of endometrial glandular cells and stromal cells. MPA (100 nm) significantly reduced the Dll4 mRNA content in both types of endometrial cells (P < 0.05). MPA at 10 nm was also effective to reduce the mRNA (data not shown). E2 reduced the mRNA in stromal cells (P < 0.05) and had little effect in glandular cells. Relaxin (20 ng/ml) increased the Dll4 mRNA by 2-fold in glandular cells (P < 0.05), and it was also effective at the lower concentration of 2 ng/ml. A representative experiment of dose response is shown in Fig. 5.
Figure 4.
Effect of MPA, E2, and RLX on the Dll4 mRNA in human endometrial cells. Glandular cells (3-d incubation) and stromal cells (8-d incubation) were incubated with 100 nm MPA or E2 or 20 ng/ml RLX. Number of specimens determined in each culture condition are shown in the lane. Data were normalized by β-actin in each sample The effect of hormone was presented with respect to control as 1, mean ± sem. Statistical analysis: control vs. MPA (P < 0.05) in both types of cells, control vs. RLX (P < 0.05) in glandular cells, and control vs. E2 (P < 0.05) in stromal cells.
Figure 5.
Effect of relaxin on Dll4 mRNA in endometrial glandular cells. Glandular cells isolated from a proliferative endometrium were incubated with 0, 2, and 20 ng/ml of RLX for 3 d. Data were normalized by the β-actin in each sample and presented as fold increase with respect to control (1), mean ± sem of triplicate determinations.
Discussion
To our knowledge, this is the first report to show that Dll4 is present in endometrial cells. At the present time, we do not know whether other Delta ligands and Notch receptors are expressed in the endometrium, and it requires additional study.
The present study showed that Dll4 is expressed in the human endometrium in a spatiotemporal fashion. The intense expression of Dll4 mRNA in the mid/late proliferative and early secretory phase appears to correlate with the growth profile of the glandular epithelium during the menstrual cycle (Fig. 1), similar to the expression pattern in the developing vascular system (15); i.e. Dll4 expression is closely associated with rapid cell growth. Preliminary data show that Notch1 is expressed in endometrium, suggesting that Delta-Notch signaling may be involved in endometrial cell differentiation.
The intensified Dll4 antibody stains on the apical cell membrane of the luminal epithelium (Fig. 1) suggest that, in a fertile cycle, Dll4 may be accessible to bind to the Notch receptor of the implanting blastocyst. Such interaction can enhance the process of implantation and activation of the Notch signaling pathway in the embryo. Knockout of the Dll4 gene has proven to be embryo-lethal in the mouse (16,17). In vivo and in vitro, recombinant Dll4 significantly reduced the embryonic neural stem cell death (18). Thus, an extraembryonic source of Dll4, as seen in Fig. 1, may play a critical role to promote implantation and the development of blastocyst.
The weak stains of Dll4 in the stromal cells and blood vessel cells (Fig. 1D) may be a result of the activation of the Notch system in situ because Delta ligand undergoes proteolytic degradation after receptor binding (19). Diffuse staining appears to be common in the developing vascular system and in cancerous lesions when the cell contains both ligand and Notch receptor (14,20,21). Nevertheless, full-length Dll4 was detected in stromal cells (Fig. 2), suggesting that they are available for binding to the Notch receptor on the vascular system to enhance angiogenesis.
Figure 3 shows that the overall Dll4 mRNA content in endometrial stromal cells was significantly less than that in glandular cells and in HUVECs, yet Dll4 protein in stromal cells was comparable to that in HUVECs (Fig. 2). These data suggest that Dll4 synthesis may be differentially regulated in various cell types, and it appears to be more efficient in the endometrial cells, especially in the stromal cells.
Figure 4 shows that E2, a growth-promoting hormone, had little effect on Dll4 in glandular cells (Fig. 4), suggesting that the E2 does not account for the surge of Dll4 mRNA at mid menstrual cycle (Fig. 3). The suppressive effect of progestin in endometrial cells correlates with the lower levels of Dll4 mRNA in the secretory phase (Fig. 3). Although estrogen and progestin did not exert a direct stimulatory effect, endometrial cells, however, produce a variety of growth factors and peptide hormones under the influence of estrogen and progestin (22), which in turn, can up-regulate Dll4. We have shown that endometrium expresses RLX (23), and its action is mediated by its receptor, which is induced by progestin and RLX (11). As shown in Fig. 4, RLX induces Dll4 mRNA, which may counteract the inhibitory effect of progesterone in vivo, resulting in a gradual decrease of Dll4 mRNA in the secretory phase (Fig. 3).
Previous studies have shown that RLX induces VEGF in endometrial cells (23). Because VEGF induces Dll4 in HUVECs (14,20), the effect of RLX may be mediated through the VEGF. Alternatively, RLX may exert parallel effects on both VEGF and Dll4 through the activation of cAMP response element-binding protein in endometrial cells (9). The major regulating factor, however, remains unclear and requires additional study to reveal the cellular mechanism of the profound expression of Dll4 in the middle of the menstrual cycle.
In summary, we have shown that Dll4 is expressed in human endometrial cells. This finding opens a novel avenue to explore the physiology of the activation of the Delta-Notch signaling pathway in the endometrium.
Acknowledgments
We thank the clinicians in the Department of Obstetrics/Gynecology and Reproductive Medicine and Department of Pathology and SUNY-Stony Brook for providing us with endometrial specimens.
Footnotes
This work was supported by in part by National Institute of Child Health and Human Development Grant 19247.
Disclosure Statement: All authors have nothing to declare.
First Published Online October 4, 2007
Abbreviations: Dll4, Delta-like 4 protein; E2, estradiol; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HUVEC, human umbilical vascular endothelial cell; MPA, medroxyprogesterone acetate; RLX, relaxin; VEGF, vascular endothelial growth factor.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ 1999 Notch signaling: cell fate control and signal integration in development. Science 284:770–776 [DOI] [PubMed] [Google Scholar]
- Sparrow DB, Clements M, Withington SL, Scott AN, Novotny J, Sillence D, Kusumi K, Beddington RS, Dunwoodie SL 2002 Diverse requirements for Notch signalling in mammals. Int J Dev Biol 46:365–374 [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J 1975 Dating the endometrial biopsy. Am J Obstet Gynecol 122:262–263 [DOI] [PubMed] [Google Scholar]
- Bausero P, Cavaille F, Meduri G, Freitas S, Perrot-Applanat M 1998 Paracrine action of vascular endothelial growth factor in the human endometrium: production and target sites, and hormonal regulation. Angiogenesis 2:167–182 [DOI] [PubMed] [Google Scholar]
- Lane B, Oxberry W, Mazella J, Tseng L 1994 Decidualization of human endometrial stromal cells in vitro: effects of progestin and relaxin on the ultrastructure and production of decidual secretory proteins. Hum Reprod 9:259–266 [DOI] [PubMed] [Google Scholar]
- Liu HC, Tseng L 1979 Estradiol metabolism in isolated human endometrial epithelial glands and stromal cells. Endocrinology 104:1674–1681 [DOI] [PubMed] [Google Scholar]
- Zhang W, Mazella J, Kloosterboer HJ, Tseng L 2006 Effects of tibolone on nuclear receptors in human endometrial cells. Am J Obstet Gynecol 195:97–102 [DOI] [PubMed] [Google Scholar]
- Zhu HH, Huang JR, Mazella J, Rosenberg M, Tseng L 1990 Differential effects of progestin and relaxin on the synthesis and secretion of immunoreactive prolactin in long term culture of human endometrial stromal cells. J Clin Endocrinol Metab 71:889–899 [DOI] [PubMed] [Google Scholar]
- Tang M, Mazella J, Zhu HH, Tseng L 2005 Ligand activated relaxin receptor increases the transcription of IGFBP-1 and prolactin in human decidual and endometrial stromal cells. Mol Hum Reprod 11:237–243 [DOI] [PubMed] [Google Scholar]
- Tseng L, Zhang J, Peresleni T, Goligorsky MS 1996 Cyclic expression of endothelial nitric oxide synthase mRNA in the epithelial glands of human endometrium. J Soc Gynecol Investig 3:33–38 [DOI] [PubMed] [Google Scholar]
- Mazella J, Tang M, Tseng L 2004 Disparate effects of relaxin and TGFβ1: relaxin increases, but TGFβ1 inhibits, the relaxin receptor and the production of IGFBP-1 in human endometrial stromal/decidual cells. Hum Reprod 19:1513–1518 [DOI] [PubMed] [Google Scholar]
- Dorsch M, Zheng G, Yowe D, Rao P, Wang Y, Shen Q, Murphy C, Xiong X, Shi Q, Gutierrez-Ramos JC, Fraser C, Villeval JL 2002 Ectopic expression of Delta4 impairs hematopoietic development and leads to lymphoproliferative disease. Blood 100:2046–2055 [PubMed] [Google Scholar]
- Hainaud P, Contreres JO, Villemain A, Liu LX, Plouet J, Tobelem G, Dupuy E 2006 The role of the vascular endothelial growth factor-delta-like 4 ligand/Notch4-ephrin B2 cascade in tumor vessel remodeling and endothelial cell functions. Cancer Res 66:8501–8510 [DOI] [PubMed] [Google Scholar]
- Williams CK, Li JL, Murga M, Harris AL, Tosato G 2006 Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood 107:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J 2004 Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 18:2474–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD 2004 Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA 101:15949–15954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T 2004 Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 18:2469–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD 2006 Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442:823–826 [DOI] [PubMed] [Google Scholar]
- Bland CE, Kimberly P, Rand MD 2003 Notch-induced proteolysis and nuclear localization of the Delta ligand. J Biol Chem 278:13607–13610 [DOI] [PubMed] [Google Scholar]
- Patel NS, Li JL, Generali D, Poulsom R, Cranston DW, Harris AL 2005 Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res 65:8690–8697 [DOI] [PubMed] [Google Scholar]
- Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, Kintner CR, Stark KL 2000 Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev 14:1313–1318 [PMC free article] [PubMed] [Google Scholar]
- Tseng L, Mazella J 2002 Endometrial cell specific gene activation during implantation and early pregnancy. Front Biosci 7:d1566–dl1574 [DOI] [PubMed] [Google Scholar]
- Palejwala S, Tseng L, Wojtczuk A, Weiss G, Goldsmith LT 2002 Relaxin gene and protein expression and its regulation of procollagenase and vascular endothelial growth factor in human endometrial cells. Biol Reprod 66:1743–1748 [DOI] [PubMed] [Google Scholar]