Abstract
Foreign Ag-specific TCR-transgenic (Tg) mice contain a small fraction of T cells bearing the endogenous Vβ and Vα chains as well as a population expressing an intermediate level of Tg TCR. Importantly, these minor nonclonotypic populations contain ≥99% of the CD4+Foxp3+ regulatory T cells (Treg) and, despite low overall Treg expression, peripheral tolerance is maintained. In the OT-II TCR (OVA-specific, Vβ5highVα2high) Tg scurfy (Sf) mice (OT-II Sf) that lack Treg, nonclonotypic T cells markedly expanded in the periphery but not in the thymus. Expanded T cells expressed memory/effector phenotype and were enriched in blood and inflamed lungs. In contrast, Vβ5highVα2high clonotypic T cells were not expanded, displayed the naive phenotype, and found mainly in the lymph nodes. Importantly, V β5neg T cells were able to transfer multiorgan inflammation in Rag1−/− recipients. T cells bearing dual TCR (dual Vβ or dual Vα) were demonstrated frequently in the Vβ5int and Vα2int populations. Our study demonstrated that in the absence of Treg, the lack of peripheral expansion of clonotypic T cells is due to the absence of its high-affinity Ag OVA. Thus, the rapid expansion of nonclonotypic T cells in OT-II Sf mice must require Ag (self and foreign) with sufficient affinity. Our study has implications with respect to the roles of Ag and dual TCR in the selection and regulation of Treg and Treg-controlled Ag-dependent T cell expansion in TCR Tg and TCR Tg Sf mice, respectively.
T cell receptor-transgenic (Tg)4 mice have been widely used to study various aspects of Ag-specific response, ranging from T cell development to the expression of effector functions. The great majority of T cells in the TCR-Tg mice generated with a foreign Ag-specific TCRαβ pair strongly express the Tg TCR (1, 2). Despite the overwhelming power of the Tg TCRβ to freeze endogenous Vβ gene rearrangement, a small fraction containing endogenous TCRβ paired with Tg TCRα and/or endogenous TCRα invariably exists (1–3). T cells expressing a low level of the Tg TCR are also observed. T cells with dual TCR are often found in the latter population (4). These TCR are not Tg artifact because T cells with dual TCR were found in normal hosts (5–10). It has been estimated that 15–30% of T cells in normal mice bear dual Vα TCR and 1% of human T cells bear dual Vβ TCR (6, 7, 9, 10).
In the present study, T cells expressing the high Tg TCRαβ pair are referred to as “clonotypic,” whereas all other populations are grouped as “nonclonotypic” for clarity and simplicity purposes. Both OT-II-specific clonotypic mAb and tetramer are unavailable. T cells expressing Vβ5highVα2high are considered clonotypic in this study because such cells are highly expressed in OT-II mice but scarcely present in normal B6 mice. The immunological and functional properties of the nonclonotypic population have not been thoroughly studied because this is a minor population in the Tg mice. However, the dominant presence of clonotypic T cells often waned in the old TCR Tg mice (11, 12). An unusual TCR revision mechanism in the periphery and a defect in the longevity of clonotypic T cells have been proposed to explain this observation (11–13). We consider the fact that the clonotypic T cells are specific to one epitope, whereas the nonclonotypic T cells have the potential to respond to the large host Ag repertoire. Host Ag used here is defined as both self-Ag and foreign Ag that are present in the host. We hypothesize that the nonclonotypic T cells are selectively expanded by the vast repertoire of host Ag as the mice grow, resulting in the loss of clonotypic dominance. We further propose that this population contains naturally occurring CD4+ Foxp3+ regulatory T cells (Treg) and under the condition in which Treg are absent, the nonclonotypic T cells will rapidly expand within 1 wk after birth. Understanding the regulation and functions of these cells is important because T cells from TCR Tg mice containing Treg with dual TCR are widely used, but to use it as pure clonotypic T cells, either purification or breeding Tg TCR into Rag1−/− or Rag2−/− mice is required (4, 14, 15).
When introduced into mice with an autoimmune-prone background, foreign Ag-specific TCR Tg often inhibits or retards the autoimmune response (16). This could be due to the dilution of potential autoimmune T cells by the dominant clonotypic T cells. Additionally, the self-tolerance is maintained by Treg that are present in the nonclonotypic T cell population in the Tg mice. When bred TCR Tg into scurfy (Sf) mice that totally lack Treg, the short life span associated with Sf mice was prolonged but the multiorgan inflammation characteristic of Sf mice eventually developed (17). We hypothesize that the multiorgan inflammation is induced by the host Ag through the expansion of nonclonotypic T cells. Indeed, when compared with OT-II (OVA-specific, I-Ab-restricted) TCR Tg mice (18), we found that OT-II Sf mice displayed a massive increase in the T cell populations that either lacked or expressed a reduced level of the Tg TCR pair, i.e., expansion of the nonclonotypic T cells. Dual Vα TCR cells in the former population were derived from various endogenous Vβ families, whereas T cells bearing dual Vβ or dual Vα were observed in the latter. In sharp contrast, the clonotypic OT-II T cells that were dominantly expressed in the thymus were not expanded in the periphery. These findings demonstrate that the repertoire of nonclonotypic T cells in OT-II mice is large and widespread across the Vβ and Vα families and their expansion in the OT-II Sf mice requires the presence of Ag with sufficient affinity. The expanded population contains inflammation-inducing T cells because the nonclonotypic population lacking Vβ5 could transfer the multiorgan inflammation to Rag1−/− recipients. Our study demonstrated the roles of host Ag in the thymic selection and peripheral expansion of these T cells. Even in the absence of Treg, host Ag with sufficient affinity is required for the nonclonotypic T cells in the development of multiorgan inflammatory response.
Materials and Methods
Mice
C57BL/6 (B6), B6.Cg-Foxp3sf/x/J, B6-Tg (TcrαTcrβ)425Cbn/J (OT-II B6), and B6.129S7-Rag1tm/Mom/J (Rag1−/−) mice were obtained from The Jackson Laboratory. B6.Cg-Foxp3sf/+/J mice were bred with male B6 mice to produce Sf mice (Foxp3sf/Y). OT-II Sf mice were generated by breeding OT-II B6 males with B6.Cg-Foxp3sf/+/J mice. The presence of the Foxp3sf mutation was confirmed by PCR as described in The Jackson Laboratory’s web site. The presence of OT-II Tg was confirmed by staining with anti-Vβ5 and anti-Vα2 mAb and by PCR. Mice were examined twice weekly for clinical signs of the immune dysfunction, including skin inflammation, body weight loss, wasting, etc.
Flow cytometry
Thymus or axillary, brachial, inguinal, cervical, and facial lymph nodes from sex- and age-matched B6, Sf, OT-II B6, and OT-II Sf mice were isolated and pooled, and single-cell suspensions were prepared in PBS. Leukocytes in blood and inflamed lungs were prepared as previously described (19, 20). Cells (106) were suspended in 0.1 ml of PBS solution containing 4 mg of BSA and 1 μg of anti-FcγR mAb 2.4G2 and incubated with 0.2 μg of various fluorescent mAb for 30 min at 4°C. For multicolor staining, FITC-, PE-, PE-Cy5-, allophycocyanin-, or allophycocyanin-Alexa Fluor 750-conjugated anti-CD4, anti-CD8, anti-CD44, anti-CD62L, anti-CD69, and various anti-Vβ and anti-Vα mAb (BD Pharmingen or eBioscience) were used in various combinations. Since OT-II-specific clonotypic mAb and tetramer are not available, we used anti-Vβ5.1/5.2 (referred to as anti-Vβ5 in the text for simplicity purpose) and anti-Vα2 mAb to determine the expression of clonotypic OT-II cells. This is valid only because the expression of Vβ5highVα2high in normal B6 is much lower than that in OT-II mice (see Results). Staining for CD4+Foxp3+ T cells was conducted using a commercial kit (eBioscience). At least 104 stained cells were analyzed using a FACScan equipped with CellQuest (BD Biosciences). Postacquisition analyses were conducted using FlowJo software (Tree Star).
Anti-CD3 treatment
Adult B6 male mice were treated with 10 μg of 145.2C11 anti-CD3ε mAb (i.p.) twice, 1 wk apart. Two weeks later, they were treated again with 80 μg of anti-CD3 mAb (i.p.). Various organs/tissues were sectioned and stained 3 days after. In addition, spleen and lymph node cells were determined for cell number and activation status. This protocol resulted in strong polyclonal activation as indicated by a substantial increase in T cell activation marker (66% CD4+CD44high and 50% CD8+CD44high). The treatment resulted in 50% reduction of lymph node cells and 60% reduction of spleen cells as compared with untreated normal B6 adult mice.
Cell sorting and adoptive transfer
Lymph node cells of OT-II Sf mice were first depleted of non-T cells using the pan-T cell isolation kit (Miltenyi Biotec). Unbound cells were then used to purify Vβ5neg using PE-anti-Vβ5 mAb followed by depletion with anti-PE-magnetic beads. This step was repeated twice and depletion was verified by flow cytometry to be 99%. Purified cells (6 × 106) were transferred to individual adult Rag1−/− recipients. Mice were examined twice weekly for clinical signs of immune dysfunction, including skin inflammation, prolapse, body weight loss, wasting, etc. At 12 wk after transfer, various tissues/organs were fixed with 10% neutral-buffered formalin and sections of paraffin-embedded samples were stained with H&E and examined under a microscope.
Results
OT-II TCR Tg delayed the onset of multiorgan inflammation and prolonged the life span of Sf mice
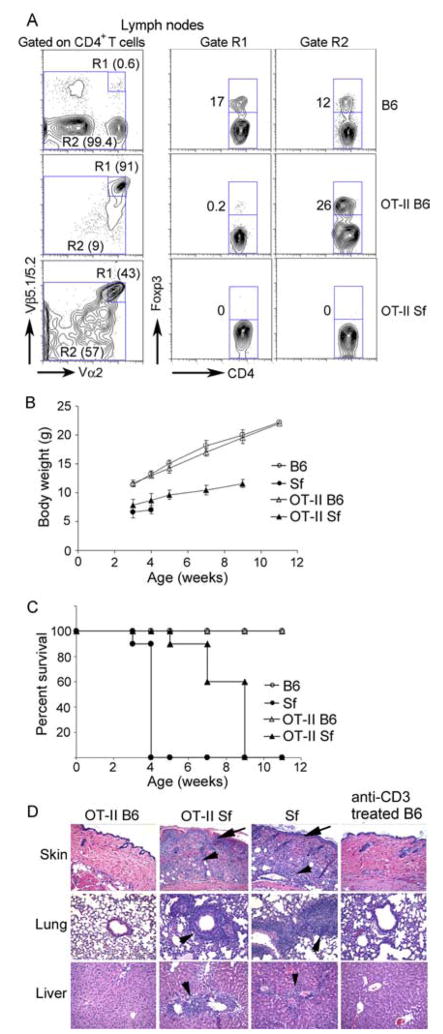
As a result of totally lacking Treg, Sf mice developed severe multiorgan inflammation and died around 4 wk after birth. Although the Treg level in the TCR Tg OT-II B6 mice was significantly reduced to 3% of CD4+ T cells as compared with 12% in B6 mice, multiorgan inflammation was not observed (Fig. 1). The great majority of the CD4+FoxP3+ Treg were found in the nonclonotypic T cells in OT-II mice (Fig. 1A). As reported for other TCR Tg Sf mice, breeding OT-II TCR into Sf mice delayed but did not prevent the fatal disease (16, 17). Their body weight was increased slightly and their life span was prolonged as compared with Sf mice (Fig. 1, B and C). The 2-to 3-wk delay of disease onset probably reflects the dilution effect of the initial dominant expression of Tg T cells and the time required for the expansion of nonclonotypic, inflammation-inducing T cells (see Fig. 2). Nevertheless, strong inflammation was observed in skin, lung, and liver (Fig. 1D). Moreover, leukocyte infiltration in these organs was not observed in B6 mice treated with anti-CD3 mAb that induced polyclonal T cell activation. These observations suggest that multiorgan inflammation was not caused simply by polyclonal T cell activation. Since the OT-II TCR is specific to a foreign Ag and not autoreactive, the inflammatory T cell response must be caused by non-Tg TCR, which may include the Tg TCRβ paired with non-Tg TCRα, the non-Tg TCRβ paired with the Tg TCRα, and the non-Tg TCRβ paired with the non-Tg TCRα.
FIGURE 1.
Comparison of Treg expression, multiorgan inflammation, and disease manifestation among OT-II, Sf, and OT-II Sf mice. Male B6, male OT-II, Sf, and OT-II Sf mice were examined for CD4+Foxp3+ Treg (A; n = 3, 3–4 wk old), body weight gain (B; n = 6), survival rate (C; n = 6), and multiorgan inflammation (D; n = 5). As compared with Sf mice, OT-II Sf mice (5–6 wk old) had increased body weight gain and prolonged life span that resulted in inflammation in multiple organs examined (D). To determine whether polyclonal-activated T cells induced multiorgan inflammation, adult B6 mice (n = 3, 9 wk old) were treated with anti-CD3 mAb as described in Materials and Methods and various organs were examined (D).
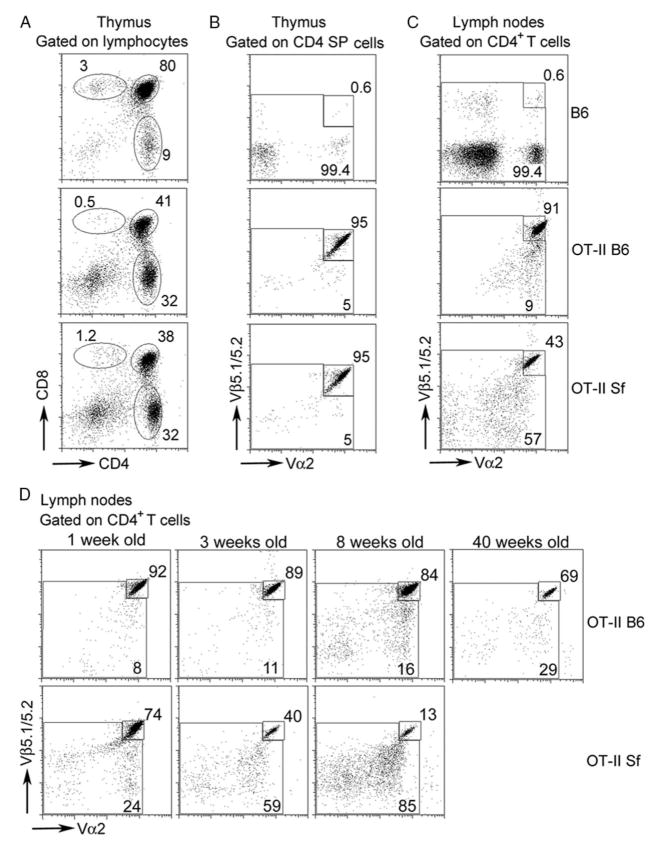
FIGURE 2.
Rapid expansion of nonclonotypic T cells in OT-II Sf mice occurs in the periphery but not thymus. Single-cell suspensions were prepared from thymus or pooled lymph nodes from B6, OT-II B6, and OT-II Sf mice (A–C; 3–4 wk old) or lymph node cells of OT-II Sf mice of various ages (D). Cells were stained with a mixture containing anti-Thy-1, anti-CD4 mAb, anti-CD8, anti-Vβ5, and anti-Vα2 mAb. Expression was analyzed on T cells (A) and CD4-gated cells (B–D). High expression of clonotypic T cells was observed in the thymus and lymph nodes of OT-II mice but only in the thymus of OT-II Sf mice. Expansion of nonclonotypic Vβ5int/neg and Vα2int/neg T cells was observed in the lymph nodes of OT-II Sf mice (C). A significant expansion of the nonclonotypic T cells was detected as early as 1 wk after birth (D). The data presented are one of at least two experiments for each study. Two or more individual mice were used for each experiment.
Rapid expansion of nonclonotypic T cells in OT-II Sf mice occurs in the periphery
We compared the distribution of OT-II clonotypic T cells (based on Vβ5highVα2high) and nonclonotypic T cells between thymus and lymph nodes among B6, OT-II B6, and OT-II Sf mice (age and sex matched) by four-color staining using anti-CD4, anti-CD8, anti-Vβ5, and anti-Vα2 mAb (Fig. 2). As with many class II-restricted TCR Tg mice, CD4+ (single- positive) T cells were enriched in the thymus (Fig. 2A). The great majority (95%) of CD4+ (single-positive) thymocytes of OT-II B6 and OT-II Sf expressed the clonotypic TCR as compared with B6 that normally expressed few Vβ5highVα2high T cells (0.6%; Fig. 2B). Moreover, clonotypic T cells were high and nonclonotypic T cells were low in the lymph nodes of OT-II B6 mice (Fig. 2C), whereas the reverse was true for OT-II Sf mice (Figs. 1A and 2C). The data indicate that the expansion of nonclonotypic T cells in the OT-II Sf mice did not occur during the thymic selection process but happened in the periphery due to the absence of Treg.
To determine how fast the expansion of nonclonotypic T cells occurs in OT-II Sf mice, we examined the proportion of Vβ5highVα2high CD4+ T cells in the pooled lymph node cells of OT-II Sf mice of various ages. As shown in Fig. 2D, the percentage of nonclonotypic CD4+ T cell populations was already increased at 1 wk after birth and continued thereafter to a remarkable 87% in just 8 wk after birth. By contrast, the nonclonotypic CD4+ T cells in 40-wk-old OT-II mice remained at a level lower than that observed in the 3-wk-old OT-II Sf mice. Thus, the kinetic study indicates a rapid expansion of nonclonotypic T cells preceding the development of multiorgan inflammation in OT-II Sf mice.
Comparison of T cell surface phenotypes between OT-II B6 and OT-II Sf mice and their tissue distribution
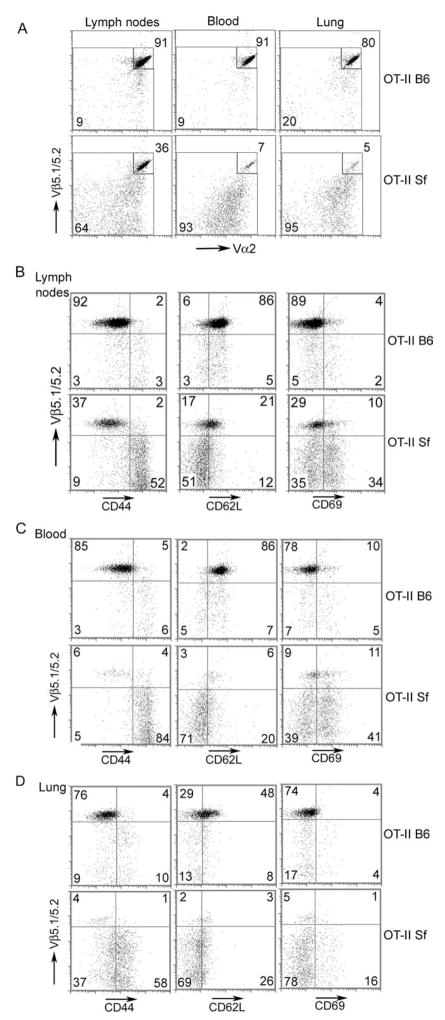
Spontaneous T cell activation response in Sf mice invariably results in the formation of T cells with the memory/effector (TM/TE) cell surface phenotype (21). The TM/TE cells tend to leave the lymph nodes and circulate through blood to reach their target organs, whereas the naive T (TN) cells sojourned in the lymph nodes more often. Therefore, we compared the cell surface phenotypes among various CD4+ T cell populations present in the lymph nodes, blood, and the inflamed lungs of OT-II Sf mice and correlated their phenotypes with Vβ5 expression (Fig. 3A). The clonotypic T cells that were reduced in the lymph nodes of OT-II Sf mice (36%) as compared with OT-II mice (91%) were reduced even more in the blood (7%) and inflamed lungs (5%) (Fig. 3A). Based on CD44 expression, the great majority (92%) of the OT-II B6 CD4+Vβ5+ T cells displayed the TN phenotype (CD44low), whereas a significant fraction (52%) of the OT-II Sf mice exhibited the TM/TE phenotypes (CD44high) (Fig. 3B). Cells that expressed the TM/TE phenotypes resided in the Vβ5neg and Vβ5int T cell populations, indicating that OT-II clonotypic T cells were not expanded since Vβ5high included the great majority of the clonotypic T cells. This interpretation is supported by the analysis of CD62L and CD69, markers for TN cells, and recently activated T cells, respectively (Fig. 3B). Dominant expression of TM/TE nonclonotypic T cells was also observed in the blood and inflamed lungs of OT-II Sf mice as compared with OT-II B6 controls (Fig. 3, C and D). The data correlated well with the interpretation that nonclonotypic T cells were expanded by host Ag and traveled to target organs through blood, whereas clonotypic T cells were not stimulated and tend to stay in the lymph nodes.
FIGURE 3.
Tissue distribution and cell surface phenotype of T cell populations compared between OT-II B6 and OT-II Sf mice. Single-cell suspensions were prepared from pooled lymph nodes, blood, and lungs from 4-wk-old OT-II B6 and OT-II Sf mice as previously described (19). Cells were doubly stained with anti-Vβ5 and anti-Vα2 mAb (A). Vβ5highVα2high expression was reduced from 91 to 36% for lymph nodes, 91 to 7% for blood, and 80 to 5% for lung. Cells were also stained with a mixture containing allophycocyanin-Alexa Fluor750-anti-CD4 mAb, PE-anti-Vβ5, and various FITC-mAb including CD44, CD69, and CD62L (B–D). Expression of cell surface markers was analyzed on CD4-gated cells. Clonotypic T cells as defined by Vβ5high displayed the TN phenotype and were present more in the lymph nodes, whereas TM/TE cells (CD44highCD62Llow) that were significantly increased in the Vβ5int/neg T cells in the OT-II Sf mice were more frequently observed in the blood and inflamed lungs. The data shown are representative of two experiments. At least two mice in each group were examined for each experiment.
Pervasive use of endogenous Vβ genes in nonclonotypic T cells
Because OT-II B6 expressed a small fraction of T cells bearing the non-Tg TCR, the expansion of these cells allows us to determine what Vβ genes were used and to what extent these Vβ families were expanded. We examined the Vβ families of the OT-II Sf mice by staining the CD4+ T cells with anti-Vα2 in combination with individual anti-Vβ family mAb (Table I). The Vα2 expression could be grouped into three levels, Vα2high, Vα2int, and Vα2neg. As compared with OT-II B6 mice, the expansion of endogenous Vβ T cells was observed in all of the Vβ families examined, indicating the pervasive and widespread nature of the expanded T cell repertoire (Table I and Ref. 21). The expansion was more evident in the populations that were Vα2int and Vα2neg, suggesting that endogenous Vβ paired with Tg Vα2high were less likely to form T cells that were selectively expanded by the host Ag in the OT-II Sf mice. Although we examined only 14 Vβ families (mouse contains ~50 Vβ genes), it is somewhat surprising that the sum of the fraction of the 14 Vβ CD4+ T cells exceeded 100% of the total CD4+ T cells even when one takes into consideration that dual TCR T cells were counted twice in this analysis (see below).
TABLE I.
Pervasive and stochastic expansion of nonclonotypic T cells involves many endogenous Vβ familiesa
| Vβ Family | OT-II B6 |
OT-II Sf |
||||||
|---|---|---|---|---|---|---|---|---|
| Vα2 Expression in Each Vβ Family | ||||||||
| Vα2high | Vα2int | Vα2neg | Total | Vα2high | Vα2int | Vα2neg | Totalb | |
| Vβ2 | 1.3 | 1.0 | 0.0 | 2.3 | 1.2 | 3.3 | 0.0 | 4.5 |
| Vβ3 | 0.8 | 0.0 | 0.0 | 0.8 | 0.8 | 1.9 | 0.0 | 2.7 |
| Vβ4 | 0.6 | 0.0 | 0.1 | 0.7 | 0.9 | 1.9 | 0.0 | 2.8 |
| Vβ5.1/5.2 | 91.0 | 3.3 | 0.0 | 94.3 | 45.0 | 12.0 | 1.1 | 58.1 |
| Vβ6 | 0.5 | 0.0 | 0.0 | 0.5 | 2.2 | 3.6 | 0.7 | 6.5 |
| Vβ7 | 1.1 | 0.0 | 0.0 | 1.1 | 0.7 | 1.2 | 0.0 | 1.9 |
| Vβ8.1 | 0.9 | 0.0 | 0.1 | 0.9 | 1.4 | 3.3 | 2.1 | 6.8 |
| Vβ8.3 | 0.5 | 0.0 | 0.0 | 0.5 | 0.6 | 2.2 | 0.0 | 2.8 |
| Vβ9 | 0.1 | 0.0 | 0.0 | 0.1 | 0.3 | 1.0 | 0.0 | 1.3 |
| Vβ10 | 0.1 | 0.0 | 0.0 | 0.1 | 0.5 | 2.0 | 0.0 | 2.5 |
| Vβ11 | 0.4 | 0.0 | 0.1 | 0.5 | 0.4 | 1.6 | 0.0 | 2.0 |
| Vβ12 | 0.7 | 0.0 | 0.0 | 0.7 | 0.6 | 1.9 | 0.0 | 2.5 |
| Vβ13 | 0.4 | 0.0 | 0.0 | 0.4 | 0.6 | 1.9 | 0.0 | 2.5 |
| Vβ14 | 1.1 | 0.0 | 0.0 | 1.1 | 1.4 | 4.6 | 0.7 | 6.7 |
| Total | 99.5 | 4.3 | 0.3 | 104.0 | 56.6 | 42.4 | 4.6 | 103.6 |
The data are derived from data obtained from flow cytometry staining using anti-CD4, anti-Vα2, and various anti-Vβ family mAb. Numbers represent the percentage of cells expressing the indicated phenotypes in the CD4+ T cell population. High, intermediate, and low expression levels of Vα2 are indicated as Vα2high, Vα2int, and Vα2neg, respectively. Three mouse samples were studied and a representative one is shown.
Comparing the expression levels of each Vβ family member between OT-II B6 and OT-II Sf and between OT-II Sf with B6 (Ref. 21 and data not shown) indicated the stochastic nature of the expansion of the endogenous Vβ families.
Expression of endogenous Vβ and Vα families and presence of dual TCR in the expanded CD4+ T cells
Because the expression of Tg Vβ5 and Vα2 is forced, a T cell expressing Vβ5neg and Vα2int pair is more likely to express two kinds of TCR, one by the endogenous Vβ paired with Vα2int and one by the endogenous V β paired with an endogenous V α. Similarly, a T cell expressing Vβ5int could coexpress an endogenous Vβ. To demonstrate the presence of dual TCR on single T cells and the pervasiveness of such repertoire with respect to Vβ and Vα gene utilization, we stained various T cell populations in the lymph nodes or inflamed lung for T cells expressing dual TCR (Fig. 4). The anti-Vα mAb used are the only four reagents currently available for staining among ~70 Vα families in mouse T cells. The anti-Vβ used were four that are the more frequently used Vβ in normal B6 mice.
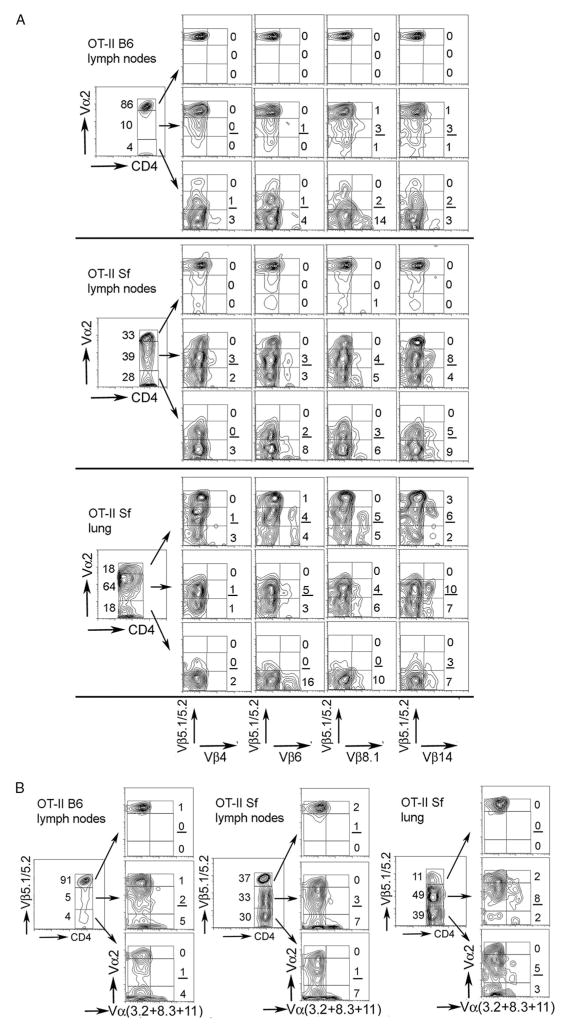
FIGURE 4.
Dual TCR T cells were observed in the expanded populations. Lymph node cells or leukocytes from inflamed lungs from 4-wk-old OT-II B6 and OT-II Sf mice were stained with a mixture containing anti-CD4 and anti-Vα2 mAb and the gated populations were stained for coexpression of Vβ5 and Vβ4 (or Vβ6, Vβ8.1, Vβ14; A) or for coexpression of Vα2 with a mixture containing FITC-anti-Vα3.1, anti-Vα8, and anti-Vα11 mAb (B). Lymph node cells from OT-II B6 mice were used for comparison. The number of cells with dual Vβ or dual Vα TCR were low in OT-II mice but were significantly increased in OT-II Sf mice. Note: please consider both the percentile of the gated population and the percentile of the dual TCR in the gated population. The data are representative of one of three experiments.
When CD4+ T cells were gated for Vα2 expression levels followed by costaining for Vβ5 vs Vβ4, Vβ6, Vβ8.1, and Vβ14, dual Vβ expression was observed in the Vα2int (underlined in Fig. 4) and Vα2neg populations but not in the Vα2high population. This is true for both OT-II B6 and OT-II Sf lymph node cells and the frequency tends to be higher in the latter samples (Fig. 4A). T cells with Vβ5Vβ4 dual TCR appeared less frequently but the reason is unclear. In the inflamed lung of OT-II Sf mice, T cells with dual Vβ TCR were frequently observed in the Vα2high and Vα2int populations, suggesting that they may play a role for the lung inflammatory response (Fig. 4Alower panels).
We also determined the expression of dual Vα TCR. We gated CD4+ T cells for Vβ5 expression levels and then costained each population with anti-Vα2 vs a pooled reagent containing mAb against Vα3.2, Vα8.3, and Vα11 (Fig. 4B). T cells with dual Vα TCR were more often found in the Vβ5int and Vβ5neg populations but infrequently observed in the Vβ5high population. The frequency of dual Vα TCR was quite variable and was not particularly increased in the OT-II Sf mice. This is because in these mice, the Vβ5int and Vβ5neg, regardless whether they are single TCR or dual TCR, were expanded as a result of lacking Treg. Interestingly, T cells with dual Vα TCR were enriched in the Vβ5int and Vβ5neg populations in the lung (Fig. 4B).
Vβ5neg T cells transfer multiorgan inflammation in Rag1−/− recipients
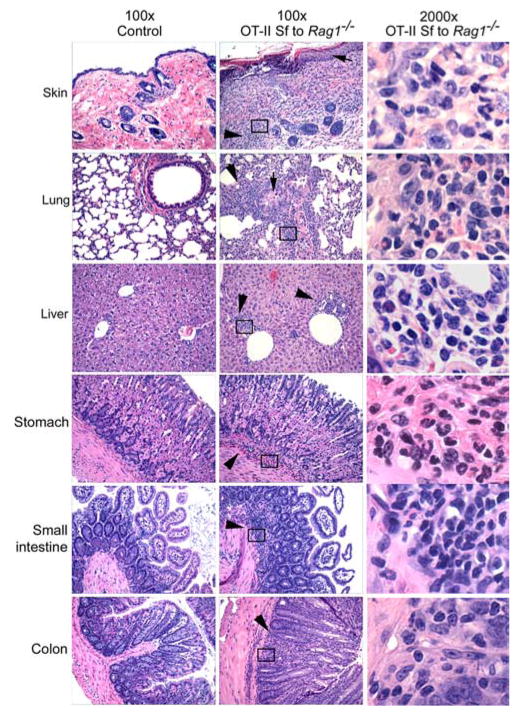
To determine whether the expanded populations that bear the non-Tg TCR indeed contain inflammation-inducing T cells, we purified the Vβ5neg population and transferred 6 × 106 of the purified cells into individual Rag1 −/− recipients to determine whether they could induce the multiorgan inflammation like Sf lymph node cells did (22). Multiorgan inflammation was observed in mice transferred with the Vβ5neg population when analyzed at 12 wk after transfer. Inflammation was observed in skin, lung, and liver, ear, stomach, intestine, and colon. Neutrophils and lymphocytes were present in the inflamed sites as revealed by ×2000 magnification. Thus, the expanded population bearing the non-Tg TCR in the OT-II Sf mice indeed contains inflammation-inducing T cells (Fig. 5).
FIGURE 5.
Vβ5neg T cells from OT-II Sf mice transferred multiorgan inflammation in Rag1−/− recipients. Vβ5neg T cells were prepared from OT-II Sf mice as described in Materials and Methods. Rag1−/− recipients (n = 3, 6 wk old) were transferred (i.v.) with 6 × 106 cells of the purified population. Inflammation in various organs/tissues was determined 12 wk after transfer by examination under a microscope of H&E-stained tissue sections (middle column; original magnification, ×100; right column; original magnification, ×2000). Untreated Rag1−/− mice were used as control (left column; original magnification, ×100). The right column panels demonstrate the presence of mononuclear and polymorphonuclear cells in the boxes in the middle column. The arrows indicate thickened skin and bronchial epithelium. The arrowheads indicate regions of leukocytic infiltration.
Because CD4+Foxp3+ Treg control the T cell response to foreign and self-Ag, lymphocytes in mice bearing the Sf mutation will undergo polyclonal activation. The data presented herein indicate that this polyclonal expansion in OT-II Sf mice still depends on Ag-specific stimulation, because these mice apparently lacked OVA and OT-II clonotypic T cells were neither stimulated nor expanded. Because the Ag repertoire in the Sf mice contains both foreign and self-Ag, the challenging issues are 1) to define the Ag responsible for the expansion of the inflammation-inducing T cells and 2) to isolate the Ag-specific T cells capable of inducing inflammation in specific organs/tissues.
Discussion
A major force in the maintenance of peripheral tolerance is mediated by Treg. Treg-controlled conventional T cells have not been thoroughly studied, in part because their expression is suppressed by the Treg. Of particular interest is the role of Ag in the selection and expansion of these T cells in the absence of Treg. To accentuate the host Ag specificity and the extent of Treg-controlled T cell repertoire, we used Sf mice with OVA-specific TCR Tg so that the role of Ag in the expansion of the Treg-controlled conventional T cell repertoire can be compared between the clonotypic and non-clonotypic populations.
Several major points were obtained from this study. First, the expanded repertoire in OT-II Sf mice is mainly derived from the nonclonotypic T cell population in which the endogenous Vβ and Vα genes were used pervasively. Second, Tg does not prevent the nonclonotypic T cells from expression and functioning. Third, host Ag selectively expanded nonclonotypic T cells, resulting in the formation of nonclonotypic T cells expressing the TM/TE phenotype and clonotypic T cells with the TN phenotype. Fourth, the expanded T cell population contains functional T cells capable of transferring multiorgan inflammation in Rag1−/− recipients. Fifth, polyclonal-activated T cells of normal mice failed to induce multiorgan inflammation, raising the possibility that the multiorgan inflammation in OT-II Sf mice contains an autoimmune element. Sixth, a significant fraction of the expanded T cells contain dual TCR T cells bearing two different Vα or two different Vβ chains. Finally, our findings also have implication in thymic selection, peripheral regulation, and expansion of T cells with respect to the affinity requirements (or threshold of activation) of the responsible Ag. In other words, Ag with sufficient affinity is required for the expansion of the nonclonotypic T cells even in the absence of Treg.
The presence of ~39% of expanded T cells bearing the Vα2int (Fig. 4A) and ~33% bearing the Vβint (Fig. 4B) in the OT-II Sf lymph nodes implies that the two populations contain individual T cells that express, respectively, an additional non-Vα2 chain and non-Vβ5 chain, i.e., they are dual TCR cells. Indeed, dual TCR cells were observed in the lymph nodes and inflamed lung (Fig. 4). These observations suggest that the dual TCR T cells were functional in OT-II Sf mice and were capable of recognizing specific Ag and expanded. There is also a significant fraction (~30%) of the spontaneously expanded Vβ5neg T cells (Fig. 4B) that are apparently derived from various members of the endogenous Vβ families. Importantly, this Vβ5neg population of T cells is sufficient to transfer multiorgan inflammation in Rag1−/− recipients, demonstrating that the inflammation-inducing T cells are present in this population and that they are derived without the participation of the Tg TCR.
It should be cautioned that the spontaneous expansion of non-clonotypic T cells over clonotypic T cells described here covers only one foreign Ag epitope in the TCR Tg mice. In the vast repertoire of foreign Ag-specific T cells in normal mice, a single T cell reactive with two different epitopes is not uncommon. This has been well appreciated in the alloreactive T cell clones of which some have specificities against foreign Ag such as OVA (8). In one such OVA-specific, alloreactive clone examined, it was demonstrated that the different epitopes reacted with only one TCR of the clone, presumably through molecular mimicking (8). In another report studying human collagen type IV α2 chain-specific TCR Tg mice, dual Vα TCR cells were identified. The role of one TCR was found to help positive selection in the thymus, whereas the “non-selected” TCR was responsible for collagen type IV α2 chain reactivity (23). Thus, Tg TCR may or may not function in dual TCR clones. Since only a limited number of cases were examined, it remains possible that there are dual TCR T cells and each TCR of the cell could react with a different epitope in the periphery. Such T cell clones were artificially generated from double TCR Tg mice and each TCR was found responsive to its respective Ag (24). The single TCR Tg OT-II Sf mice offer a good source to determine whether such T cells exist since dual TCR T cells have been greatly expanded. A study is in progress to determine whether the Vβ5intVα2int population in OT-II Sf mice could be expanded by OVA and what is the consequence of such activation on the inflammation potential of the population.
Thymic selection for Treg requires high-affinity TCR-ligand interaction that induces deletion of non-Treg (25, 26). In the absence of such a ligand such as in the foreign Ag-specific Tg mice, Treg are enriched in nonclonotypic T cells in which the clonotypic TCR are down-regulated and often coexpressed with nonclonotypic TCR. The role of dual TCR Treg in the regulation of a specific immune response, including autoimmune response, has been implicated (27–33). In the DO11.10 Tg mice, a substantial portion of the CD25+ Treg was found in the KJI-26int and expressed dual Vα TCR (4). It is known that positive selection of DO11.10 Treg (KJI-26high) is significantly increased in the presence of OVA, whereas non-Treg DO11.10 (KJI-26high) will be negatively selected under the same condition (15, 31). Thus, the possibility that this dual TCR Treg population (KJI-26int) was selected and maintained by host Ag but not by OVA is high. Importantly, Treg expressing dual TCR prevented T cell-mediated colitis against unrelated enteric bacterial Ag through OVA-induced tolerance (4). In addition, Treg isolated from Ag-specific TCR Tg mice (dual TCR) have been shown to suppress Ag-specific responses, suggesting the suppressive function of Treg can be induced through the down-regulated clonotypic TCR (29, 32, 33). In this regard, our study suggests that Treg in OT-II mice are able to inhibit the multiorgan inflammation apparently without involving clonotypic TCR (Vβ5highVα2high) that are OVA specific.
It is known for conventional T cells (non-Treg) that high-affinity Ag (or nominal Ag) induces clonal deletion in the thymus, whereas positive selection often occurs in the absence of the known nominal Ag, presumably low-affinity Ag analogs are sufficient for positive selection but such analogs do not induce clonal expansion in the periphery (34, 35). Our data indicate that the Ag analogs capable of positively selecting clonotypic T cells in the thymus are not able to induce clonotypic T cell expansion in the periphery even in the absence of Treg. This interpretation has a significant implication in the control of potential autoreactive T cells in the OT-II Sf mice. Since negative selection is normal in Sf mice (17), the potential autoreactive T cells in the periphery should have low affinity for self-Ag below the threshold of activation (36, 37). However, whether the threshold of activation could be reached in the periphery in the absence of Treg remains unknown. Our study based on the OT-II Sf mice suggests that the specific Ag for their expansion cannot be the Ag analogs responsible for the positive selection, but rather an Ag with sufficient affinity that is either absent during thymic selection or acquired later in the host. An issue critical to further understanding how autoimmunity is regulated is to identify the host Ag responsible for the expansion of autoreactive T cells in Sf mice.
Acknowledgments
The technical assistance of Christian E. Abaya and Alexandra Norcott is acknowledged.
Footnotes
This work was supported by National Institutes of Health Grants DE-017579 and AR-051203 (to S.-T.J.) and AR-047988 and AR-049449 (to S.M.F.) and a Grant-in-Aid from the Beirne B. Carter Center of Immunology (to R.S.).
Abbreviations used in this paper: Tg, transgenic; Sf, scurfy; Treg, regulatory T cell; TM/TE, memory T/effector T; TN, naive T; int, intermediate; neg, negative.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Uematsu Y, Ryser S, Dembic Z, Borgulya P, Krimpenfort P, Berns A, von Boehmer H, Steinmetz M. In transgenic mice the introduced functional T cell receptor β gene prevents expression of endogenous β genes. Cell. 1988;52:831– 841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- 2.Aifantis I, Buer J, von Boehmer H, Azogui O. Essential role of the pre-T cell receptor in allelic exclusion of the T cell receptor β locus. Immunity. 1997;7:60I–607. doi: 10.1016/s1074-7613(00)80381-7. [DOI] [PubMed] [Google Scholar]
- 3.Malissen M, Trucy J, Jouvin-Marche E, Cazenave P-A, Scollay R, Malissen B. Regulation of TCR α and β gene allelic exclusion during T-cell development. Immunol Today. 1992;13:315–322. doi: 10.1016/0167-5699(92)90044-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhou P, Borojevic R, Streutker C, Snider D, Liang H, Croitoru K. Expression of dual TCR on DO11.10 T cells allows for ovalbumin-induced oral tolerance to prevent T cell-mediated colitis directed against unrelated enteric bacterial antigens. J Immunol. 2004;172:1515–1523. doi: 10.4049/jimmunol.172.3.1515. [DOI] [PubMed] [Google Scholar]
- 5.Schittek B, Unkelbach E, Rajewsky K. Violation of allelic exclusion of the T cell receptor β genes in a helper T cell clone. Int Immunol. 1989;1:273–280. doi: 10.1093/intimm/1.3.273. [DOI] [PubMed] [Google Scholar]
- 6.Davodeau F, Peyrat M-A, Romagne F, Necker A, Haller M-M, Vie H, Bonneville M. Dual T cell receptor β chain expression on human T lymphocytes. J Exp Med. 1995;181:1391–1398. doi: 10.1084/jem.181.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padovan E, Giachino C, Cella M, Valitutti S, Acuto O, Lanzavecchia A. Normal T lymphocytes can express two different T cell receptor β chains: Implications for the mechanism of allelic exclusion. J Exp Med. 1995;181:1587–1591. doi: 10.1084/jem.181.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malissen M, Trucy J, Letourneur F, Rebai N, Dunn DE, Fitch FW, Hood L, Malissen B. A T cell clone expresses two T cell receptor α genes but uses one αβ heterodimer for allorecognition and self MHC-restricted antigen recognition. Cell. 1988;55:49 –59. doi: 10.1016/0092-8674(88)90008-6. [DOI] [PubMed] [Google Scholar]
- 9.Heath WR, Carbone FR, Bertolino P, Kelly J, Cose S, Miller JFAP. Expression of two T cell receptor α chains on the surface of normal murine T cells. Eur J Immunol. 1995;25:1617–1623. doi: 10.1002/eji.1830250622. [DOI] [PubMed] [Google Scholar]
- 10.Mason D. Allelic exclusion of α chains in TCR. Int Immunol. 1994;6:881– 885. doi: 10.1093/intimm/6.6.881. [DOI] [PubMed] [Google Scholar]
- 11.Jones SC, Clise-Dwyer K, Huston G, Dibble J, Eaton S, Haynes L, Swain SL. Impact of post-thymic cellular longevity on the development of age-associated CD4+ T cell defects. J Immunol. 2008;180:4465– 4475. doi: 10.4049/jimmunol.180.7.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink PJ, Swan K, Turk G, Moore MW, Carbone FR. Both intrathymic and peripheral selection modulate the differential expression of Vβ5 among CD4+ and CD8+ T cells. J Exp Med. 1992;176:1733–1738. doi: 10.1084/jem.176.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahan CJ, Fink PJ. Receptor revision in peripheral T cells creates a diverse Vβ repertoire. J Immunol. 2000;165:6902– 6907. doi: 10.4049/jimmunol.165.12.6902. [DOI] [PubMed] [Google Scholar]
- 14.Lohr J, Knoechel B, Kahn EC, Abbas AK. Role of B7 in T cell tolerance. J Immunol. 2004;173:5028 –5035. doi: 10.4049/jimmunol.173.8.5028. [DOI] [PubMed] [Google Scholar]
- 15.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahorsky-Reeves JL, Wilkinson JE. The murine mutation scurfy (sf) results in an antigen-dependent lymphoproliferative disease with altered T cell sensitivity. Eur J Immunol. 2001;31:196 –204. doi: 10.1002/1521-4141(200101)31:1<196::AID-IMMU196>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Benoist C, Mathis D. How defects in central tolerance impinge on a deficiency in regulatory T cells. Proc Natl Acad Sci USA. 2005;102:14735–14740. doi: 10.1073/pnas.0507014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323–329 epitope. J Immunol. 2000;164:4706 – 4712. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- 19.Sharma R, Bagavant H, Jarjour WN, Sung S-S, Ju S-T. The role of Fas in the immune system biology of IL-2Rα knockout mice: interplay among regulatory T cells, inflammation, hemopoiesis, and apoptosis. J Immunol. 2005;175:1965–1973. doi: 10.4049/jimmunol.175.3.1965. [DOI] [PubMed] [Google Scholar]
- 20.Sharma R, Zheng L, Deshmukh US, Jarjour WN, Sung S-S, Fu SM, Ju S-T. Cutting edge: a regulatory T cell-dependent novel function of CD25 (IL-2Rα) controlling memory CD8+ T cell homeostasis. J Immunol. 2007;178:1251–1255. doi: 10.4049/jimmunol.178.3.1251. [DOI] [PubMed] [Google Scholar]
- 21.Zheng L, Sharma R, Kung JT, Deshmukh US, Jarjour WN, Fu SM, Ju S-T. Pervasive and stochastic changes in the TCR repertoire of regulatory T-cell-deficient mice. Int Immunol. 2008;20:517–523. doi: 10.1093/intimm/dxn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma R, Jarjour WN, Zheng L, Gaskin F, Fu SM, Ju S-T. Large functional repertoire of regulatory T cell suppressible autoimmune T cells in scurfy mice. J Autoimm. 2007;29:10 –19. doi: 10.1016/j.jaut.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He X, Janeway CA, Jr, Levine M, Robinson E, Preston-Hurlburt P, Viret C, Bottomly K. Dual receptor T cells extend the immune repertoire for foreign antigens. Nat Immunol. 2002;3:127–134. doi: 10.1038/ni751. [DOI] [PubMed] [Google Scholar]
- 24.Dittel BN, Stefanova I, Germain RN, Janeway CA., Jr Cross-antagonism of a T cell clone expressing two distinct T cell receptors. Immunity. 1999;11:289 –298. doi: 10.1016/s1074-7613(00)80104-1. [DOI] [PubMed] [Google Scholar]
- 25.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 26.Ribot J, Enault G, Pilipenko S, Huchenq A, Calise M, Hudrisier D, Romagnoli P, van Meerwijk JP. Shaping of the autoreactive regulatory T cell repertoire by thymic cortical positive selection. J Immunol. 2007;179:6741– 6748. doi: 10.4049/jimmunol.179.10.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 28.Suto A, Nakajima H, Ikeda K, Kubo S, Nakayama T, Taniguchi M, Saito Y, Iwamoto I. CD4+CD25+ T-cell development is regulated by at least 2 distinct mechanisms. Blood. 2002;99:555–560. doi: 10.1182/blood.v99.2.555. [DOI] [PubMed] [Google Scholar]
- 29.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc Natl Acad Sci USA. 2002;99:8213– 8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larkin J, III, Rankin AL, Picca CC, Riley MP, Jenks SA, Sant AJ, Caton AJ. CD4+CD25+ regulatory T cell repertoire formation shaped by differential presentation of peptides from a self-antigen. J Immunol. 2008;180:2149 –2157. doi: 10.4049/jimmunol.180.4.2149. [DOI] [PubMed] [Google Scholar]
- 31.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, Yamamoto K. Generation of CD4+CD25+ regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399 – 4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969 –1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 33.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 34.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 35.Barnden MJ, Heath WR, Rodda S, Carbone FR. Peptide antagonists that promote positive selection are inefficient at T cell activation and thymocyte deletion. Eur J Immunol. 1994;24:2452–2456. doi: 10.1002/eji.1830241029. [DOI] [PubMed] [Google Scholar]
- 36.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 37.Lee DS, Aim C, Ernst B, Sprent J, Surh CD. Thymic selection by single MHC-peptide ligand: autoreactive T cells are low-affinity cells. Immunity. 1999;10:83–92. doi: 10.1016/s1074-7613(00)80009-6. [DOI] [PubMed] [Google Scholar]