Abstract
Objective
To determine whether brain activation changes in clinically and neurocognitively normal human immunodeficiency virus (HIV)–infected and in HIV-seronegative control (SN) participants over a 1-year period.
Methods
Functional magnetic resonance imaging (fMRI) was performed in 32 SN and 31 HIV patients (all with stable combination antiretroviral treatment) at baseline and after 1 year. Each participant performed a set of visual attention tasks with increasing attentional load (from tracking two, three, or four balls). All HIV and SN participants had normal neuropsychological function at both examinations.
Results
Over 1 year, HIV patients showed no change in their neurocognitive status or in task performance during fMRI. However, HIV patients showed significant 1-year increases in fMRI signals in the prefrontal and posterior parietal cortices for the more difficult tasks, whereas SN control participants showed only decreases in brain activation in these regions. This resulted in significant interactions between HIV status and time of study in left insula, left parietal, left temporal, and several frontal regions (left and right middle frontal gyrus, and anterior cingulate).
Interpretation
Because fMRI task performance remained unchanged in both groups, the HIV patients appeared to maintain performance by increasing usage of the attention network, whereas the control participants reduced usage of the attention network after 1 year. These findings suggest improved efficiency or a practice effect in the SN participants but declined efficiency of the neural substrate in HIV patients, possibly because of ongoing brain injury associated with the HIV infection, despite their apparent stable clinical course.
Human immunodeficiency virus (HIV) can invade the brain and cause encephalitis, leukoencephalopathy, axonal damage, and variable neuronal loss.1 These neuropathological processes are accompanied by microglial and glial activation,1 which, in turn, may lead to the release of neurotoxic substances that may further contribute to neuronal apoptosis or neuronal dysfunction. These neuropathological alterations may ultimately lead to the clinical syndrome of HIV-associated dementia.2 In previous studies, the introduction of combination antiretroviral therapy (CART) dramatically improved the survival of HIV-infected individuals, and decreased the prevalence and the incidence of HIV-associated dementia.3–5 CART may also improve cognitive function, especially verbal memory, psychomotor speed, and executive functioning.6 However, despite these advances in HIV treatments, 20 to 40% of HIV-infected individuals on stable CART demonstrate HIV-associated neurocognitive disorder (HAND), which involves cognitive impairment in at least two of six cognitive domains commonly affected by HIV.7 One contributing factor to the progression of HIV brain disease, despite viral control, may be the interactive effect with aging because some cognitive domains, such as attention, are affected both by HIV8 and normal aging.9–11 Furthermore, ongoing neuroinflammation in both CART-treated HIV patients12 and older individuals may eventually lead to cognitive dysfunction. Detection of potential subclinical brain injury in HIV patients is important for guiding future preventive treatments.
Functional MRI (fMRI) using the blood oxygen level dependent (BOLD) contrast can assess brain activity, including subclinical alterations. Studies of normal aging have demonstrated task and region-dependent BOLD changes.13–19 fMRI studies involving attention or working memory generally have found greater load-dependent increases in activation in selected brain regions of HIV-positive patients with8,20 or without21 cognitive deficits relative to controls. These findings suggest that HIV patients utilize their “brain reserve” when performing working memory and attention tasks, to compensate for injury to the neural substrate.
This study extends prior work by evaluating whether performance and brain activation change after 1 year during a set of visual attention tasks. Based on prior cross-sectional results, and our expectation that ongoing neuroinflammation and infection with HIV would lead to chronic and progressive brain injury, we hypothesized that neuroasymptomatic, cognitively stable HIV patients would show load-dependent increases in BOLD signals after 1 year to compensate for decreased neural efficiency while maintaining task performance. In contrast, HIV-seronegative (SN) participants with relatively unchanged neural substrate would show no significant signal changes in fMRI signals and task performances, or possibly signal decreases caused by increased efficiency from practice effects.
Patients and Methods
Research Participants
Sixty-three individuals (30 male and 1 female HIV-infected patients, and 28 male and 4 female SN controls) completed the study after signing a written consent form approved by our institution and in accordance with the Declaration of Helsinki. Each participant completed detailed clinical assessments. SN controls were included if they fulfilled the following criteria: (1) age older than 18 years, and (2) SN for HIV type 1 and able to provide informed consent. HIV patients fulfilled the following criteria: (1) age older than 18 years; (2) positive for HIV infection, and stable on CART for at least 6 months; (3) neuroasymptomatic by clinical and neuropsychological testing (specifically, none had HIV-associated dementia or neurocognitive impairment/disorder7 at baseline and at 1 year); and (4) nadir CD4 count less than 500/mm3. Exclusion criteria for both groups were: (1) chronic medical or neuropsychiatric illnesses that might confound the study; (2) abnormal laboratory studies or electrocardiogram that might confound data (eg, hematocrit <34% for women, <42% for men, severe abnormalities on liver enzymes or renal function); (3) any medications that might affect brain function (except HIV medications); (4) head trauma with loss of consciousness longer than 30 minutes; (5) current, or history of, drug dependence or positive urine toxicology screen (for cocaine, amphetamine, methamphetamine, benzodiazepine, and opiates) that could not be explained by a medication; (6) any contraindications for magnetic resonance studies; (7) pregnant or breast-feeding (if female); and (8) less than eighth-grade-level English reading skills. These 63 participants belong to a cohort of 150 participants (75 seropositive and 75 SN) who may or may not have cognitive deficits and are being followed longitudinally over 5 years. This small longitudinal cohort was selected after screening more than 350 individuals.
Clinical and Neuropsychological Evaluations for Cognitive Function
At baseline, after 6 months, and after 1 year, all participants had detailed medical, psychiatric, and drug histories in a face-to-face interview, and physical and neurological examinations by a board-certified psychiatrist or neurologist. The participants performed a battery of neuropsychological tests (at baseline and 1 year) sensitive for detecting cognitive deficits in HIV-infected patients. The tests evaluated six cognitive domains: (1) visual memory: Rey–Osterrieth Complex Figure Immediate and Delayed Recall; (2) verbal memory: Rey Auditory Verbal Learning Test Trials 5 and 7 (20 minute-delay); (3) attention: Stroop color, Stroop word, and Trail-Making Tests A and B, Symbol Digit Modalities; (4) working memory: Wechsler Adult Intelligence Scale–III Forward, Backward, Arithmetic and Letter-Number Sequencing; (5) fluency: Ruff Figural Fluency and Verbal Fluency (FAS); and (6) motor function: Grooved Pegboard dominant and nondominant hands, California Computerized Assessment Package (CalCAP) Simple Reaction Time.
In addition, two mood assessments were administered: the Center for Epidemiological Studies Depression Scale (CES-D) and the Symptom Checklist (SCL-90). The New Adult Reading Test Revised (NART-R) was also assessed at the initial screening visit to estimate premorbid intellectual functioning.
An age-adjusted Z-score was derived for each cognitive domain, and a global Z-score was generated. None of the patients had HAND according to the “Updated Research Nosology for HIV-Associated Neurocognitive Disorders”7 at baseline or after 1 year. Specifically, no patient had impairment in two or more domains of one standard deviation less than the mean, or more than two standard deviations less than the mean on a single domain, or a cognitive impairment that interfered with everyday functioning, assessed by self-report, neurological evaluations, and by the Functional Activities Questionnaire22 and Karnofsky score.23
Magnetic Resonance Imaging and Functional Magnetic Resonance Imaging Data Acquisition
fMRI studies were performed on a 3-Tesla Siemens Trio scanner during a set of visual-attention tasks.24,25 All patients were trained outside the scanner to ensure sufficient task performance. Structural imaging included a sagittal magnetization-prepared rapid gradient-echo (TR/TE/TI = 2,200/4.91/1,000 milliseconds; 256 × 256 × 160 matrix) and an axial fluid-attenuated inversion recovery sequence (TR/TE = 10,000/85 milliseconds; 205 × 320 × 28 matrix) to rule out focal brain abnormalities. fMRI was performed using single-shot gradient-echo echoplanar imaging (TE/TR = 30/3,000 milliseconds, typically 42 axial 3mm slices, 642 matrix, 20cm field of view, 126 time points), using motion26 and distortion27 corrections. The stimulus display and magnetic resonance acquisitions were synchronized using a trigger pulse.
The fMRI tasks required mental tracking of multiple (2, 3, or 4) target balls that were highlighted briefly among 10 randomly moving, colliding balls. Every 10 seconds, the same or new target balls were highlighted briefly, and the participants pushed a response button if the balls were the same as the original targets (for monitoring reaction times and performance accuracy). Periods of tracking alternated with passive viewing of balls that moved in the same random motion without target highlighting (block design: 1 min/period × 2 periods × 3 blocks). Task performance during ball tracking and participant motion were monitored in real-time during fMRI, to assure task performance accuracy more than 80% and motion less than 1mm translations and less than 1-degree rotations.
Functional Magnetic Resonance Imaging Data Analyses
fMRI time series were analyzed with the Statistical Parametric Mapping software (SPM2; Welcome Department of Cognitive Neurology, London, United Kingdom). After spatial normalization and smoothing, maps of brain activation and 1-year changes in activation were calculated for each participant and task, using a fixed-effects model. In a subsequent random-effects analysis, group differences in the 1-year BOLD signal change were evaluated for each task (t test). Statistical significance was based on cluster-level significance (at p < 0.05 corrected for multiple comparisons), using a voxel-level threshold of p < 0.05 and a cluster size greater than 300 voxels. A customized program was used to extract regional percentage BOLD signals from cubic regions of interest (ROIs; 0.729cm3) centered at cluster maxima.
Statistical Analyses
Two-way repeated-measures analysis of variance were performed in SAS (SAS Institute, Cary, NC) to assess group differences between baseline and 1 year in clinical, neuropsychological, and ROI variables. Post hoc analyses using unpaired or paired t tests were performed to evaluate group differences in clinical variables. Statistical significance was defined as p < 0.05.
Results
Patient Characteristics and Task Performance
SN and HIV patients had similar ages (46.9 ± 2.3 vs 49.6 ± 1.5 years) and education (both 15.5 ± 0.5 years), but the estimated premorbid intelligence was slightly lower in HIV patients (Table 1). All of the patients in the HIV group were treated with CART at both time points. Over 1 year, the Log viral loads, and CD4 and nadir CD4 counts remained unchanged. Depressive symptom scores (CES-D) tended to be greater in HIV than SN participants, but were unchanged over 1 year, and did not exceed a level that suggested clinical depression (CES-D ≥ 16). Over the study period, HIV patients had stable HIV Dementia Scale, Memorial Sloan Kettering AIDS Dementia Complex, and Karnofsky scores.
Table 1. Characteristics of Seronegative Control and Human Immunodeficiency Virus–Positive Patients at Baseline and at 1 Year (Mean ± Standard Error).
| Characteristics | SN Control Subjects (n = 32) |
HIV Patients (n = 31) |
pa | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1 Year | Baseline | 1 Year | SN Controls (baseline vs 1 year) |
HIV Patients (baseline vs 1 year) |
Control vs HIV Patients at Baseline | Control vs HIV Patients at 1 Year | |
| Age, yr | 46.9 ± 2.3 | 48.0 ± 2.3 | 49.6 ± 1.5 | 50.6 ± 1.5 | — | — | 0.44 | 0.44 |
|
| ||||||||
| Education, yr | 15.5 ± 0.4 | 15.5 ± 0.4 | 15.5 ± 0.4 | 15.7 ± 0.4 | — | — | 0.94 | 0.73 |
|
| ||||||||
| Nadir CD4, number/mm3 | — | — | 152 ± 24 | 142 ± 25 | — | 0.14 | — | — |
|
| ||||||||
| CD4, number/mm3 | — | — | 415 ± 40.4 | 454 ± 42 | — | 0.06 | — | — |
|
| ||||||||
| Log viral load, copies/ml | — | — | 2.24 ± 0.20 | 2.08 ± 0.16 | — | 0.24 | — | — |
|
| ||||||||
| HIV Dementia Scale (0-16) | — | — | 14.6 ± 0.3 | 14.9 ± 0.3 | — | 0.41 | — | — |
|
| ||||||||
| Clinical Dementia Rating (0-3) | — | — | 0.27 ± 0.06 | 0.27 ± 0.06 | — | 1.0 | — | — |
|
| ||||||||
| Karnofsky score (0-100) | — | — | 93.5 ± 1.4 | 91.6 ± 1.5 | — | 0.11 | — | — |
|
| ||||||||
| CES-D | 6.0 ± 1.1 | 6.6 ± 1.0 | 9.0 ± 1.7 | 9.6 ± 1.2 | 0.56 | 0.78 | 0.14 | 0.07 |
|
| ||||||||
| Estimated verbal IQb | 114.0 ± 1.4 | — | 111.4 ± 1.3 | — | — | — | 0.17 | — |
p values are from post hoc analyses between any two groups as indicated.
Estimated premorbid verbal intelligence quotient [IQ] is derived from the National Adult Reading Test. SN = seronegative; HIV = human immunodeficiency virus; CES-D = Center for Epidemiological Studies Depression Scale.
For both SN and HIV participants, neuropsychological testing showed a significant practice effect (improvement over 1 year) in four of six cognitive domains and the global deficit score (Table 2). Compared with the SN group, the HIV patients showed poorer performance on verbal memory (p = 0.01; both time points combined). However, no significant interactions between HIV status and time of examination were observed in any cognitive domain.
Table 2. Cognitive Deficit Scores and Performance during Functional Magnetic Resonance Imaging in Study Participants at Baseline and 1 Year (Mean ± Standard Error).
| Characteristics | SN Control (n = 32) |
HIV Patients (n = 31) |
p | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 Year | Baseline | 1 Year | HIV Status Effect | Time Effect | HIV Status × Time | |
| Age, yr | 46.9 ± 2.3 | 48.0 ± 2.3 | 49.6 ± 1.5 | 50.6 ± 1.5 | 0.34 | <0.0001 | 0.81 |
|
| |||||||
| Education, yr | 15.5 ± 0.44 | 15.5 ± 0.4 | 15.5 ± 0.4 | 15.7 ± 0.4 | 0.84 | 0.07 | 0.18 |
|
| |||||||
| CES-D | 6.0 ± 1.1 | 6.6 ± 1.0 | 9.0 ± 1.7 | 9.6 ±1.2 | 0.03 | 0.61 | 0.99 |
|
| |||||||
| Cognitive Domains | |||||||
|
| |||||||
| Visual memory | 0.29 ± 0.14 | 0.80 ± 0.17 | 0.22 ± 0.17 | 0.82 ± 0.15 | 0.89 | <0.0001 | 0.70 |
|
| |||||||
| Verbal memory | 0.59 ± 0.13 | 0.59 ± 0.17 | 0.13 ± 0.11 | 0.13 ± 0.16 | 0.01 | 0.98 | 0.95 |
|
| |||||||
| Attention | −0.05 ± 0.12 | 0.43 ± 0.15 | 0.10 ± 0.10 | 0.47 ± 0.08 | 0.55 | <0.0001 | 0.32 |
|
| |||||||
| Working memory | 0.11 ± 0.13 | 0.11 ± 0.12 | −0.19 ± 0.15 | −0.13 ± 0.14 | 0.14 | 0.62 | 0.59 |
|
| |||||||
| Verbal fluency | 0.31 ± 0.10 | 0.61 ± 0.11 | 0.18 ± 0.12 | 0.56 ± 0.11 | 0.52 | <0.0001 | 0.54 |
|
| |||||||
| Motor | 0.02 ± 0.10 | 0.42 ± 0.14 | −0.08 ± 0.10 | 0.33 ± 0.09 | 0.53 | <0.0001 | 0.97 |
|
| |||||||
| Global deficit score | 0.21 ± 0.08 | 0.50 ± 0.11 | 0.06 ± 0.07 | 0.36 ± 0.08 | 0.23 | <0.0001 | 0.82 |
|
| |||||||
| Task Performance during fMRI | |||||||
|
| |||||||
| Tracking two balls | |||||||
|
| |||||||
| Accuracy, % | 95.1 ± 8.5 | 95.7 ± 9.9 | 96.4 ± 8.2 | 95.9 ± 6.5 | 0.66 | 0.97 | 0.71 |
|
| |||||||
| Reaction times, milliseconds | 850 ± 377 | 826 ± 362 | 807 ± 306 | 762 ± 370 | 0.41 | 0.60 | 0.87 |
|
| |||||||
| Tracking three balls | |||||||
|
| |||||||
| Accuracy, % | 92.5 ± 9.1 | 94.1 ± 8.4 | 93.3 ± 9.4 | 95.2 ± 6.2 | 0.57 | 0.19 | 0.95 |
|
| |||||||
| Reaction times, milliseconds | 867 ± 290 | 740 ± 297 | 803 ± 287 | 757 ± 331 | 0.64 | 0.14 | 0.48 |
|
| |||||||
| Tracking four balls | |||||||
|
| |||||||
| Accuracy, % | 93.9 ± 8.5 | 91.9 ± 10.7 | 93.5 ± 8.9 | 90.9 ± 11.4 | 0.74 | 0.14 | 0.87 |
|
| |||||||
| Reaction times, milliseconds | 872 ± 233 | 870 ± 406 | 830 ± 356 | 822 ±364 | 0.49 | 0.97 | 0.96 |
SN = seronegative; HIV = human immunodeficiency virus; CES-D = Center for Epidemiological Studies Depression Scale; fMRI = functional magnetic resonance imaging.
Functional Magnetic Resonance Imaging Findings
fMRI task performance did not differ between patient groups or time of examination, either on accuracy (>90% for all tasks) or reaction times (see Table 2). Activated brain regions were similar for HIV and SN participants, and involved various regions within the attention network (not shown), in agreement with previous studies at different magnetic field strengths and in different cohorts.8,25 At baseline, the HIV patients showed no significant differences in activation from SN participants on the two- or three-ball tasks, but greater BOLD activation on the four-ball task in the right prefrontal region [cluster maximum: medial frontal gyrus (18, 30, 27), p corrected < 0.0001 and cerebellar tonsil/occipital (36, −63, −33), p corrected < 0.02; see Figure 1 (left)].
Fig 1.
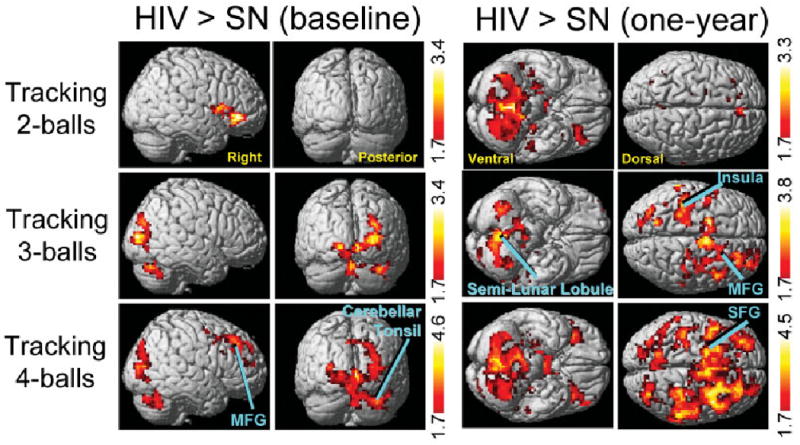
Statistical parametric maps showing significantly greater brain activation in human immunodeficiency virus (HIV) than seronegative (SN) participants (unpaired t test) for the two- (top), three- (middle), and four-ball tasks (bottom) at the baseline examination (left) and 1-year follow-up (right). Compared with SN participants, the HIV group showed significantly greater activation only with the 4-ball task at baseline [cluster maxima: medial frontal gyrus (MFG: 18, 30, 27), p corrected < 0.0001, 2,149 voxels; cerebellar tonsil/occipital (36, −63, −33), p corrected < 0.02, 1,446 voxels)], but with all 3 tasks at the 1-year time point [2 balls: large cluster with maximum at claustrum (−21, 24, 9), p corrected < 0.0001, 6,416 voxels (only cerebellar activation visible); 3 balls: MFG (6, −3, 60), p corrected < 0.002, 1,813 voxels; insula (−36, −27, 21), p corrected < 0.008, 1,403 voxels; and cerebellar semilunar lobule (0, −63, −36), p corrected < 0.0001, 1,894 voxels; 4 balls: large cluster with maximum at superior frontal gyrus (SFG, −9, 6, 69), p corrected < 0.0001, 16,612 voxels]. Z-scores shown in the red-yellow color bars indicate significance (p < 0.05) at the voxel level. Labeled clusters are significant after correction for multiple comparisons (cluster level, p < 0.05), whereas unlabeled clusters were significant at the voxel level (p < 0.05), all clusters greater than 300 voxels.
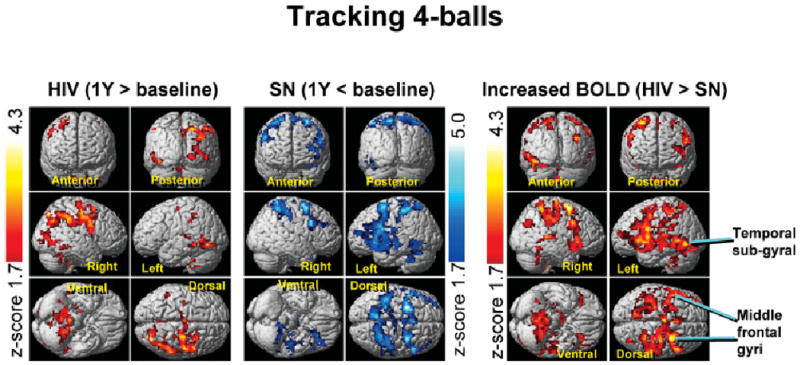
After 1 year, HIV patients showed further increases in BOLD signals for all three tasks, primarily in the right prefrontal and posterior parietal cortices, and the cerebellum bilaterally (Figs 2 and 3, left panels, and Supplementary Table 1). However, none of the regions within the attention network showed decreased brain activation in the HIV patients over this 1-year period.
Fig 2.
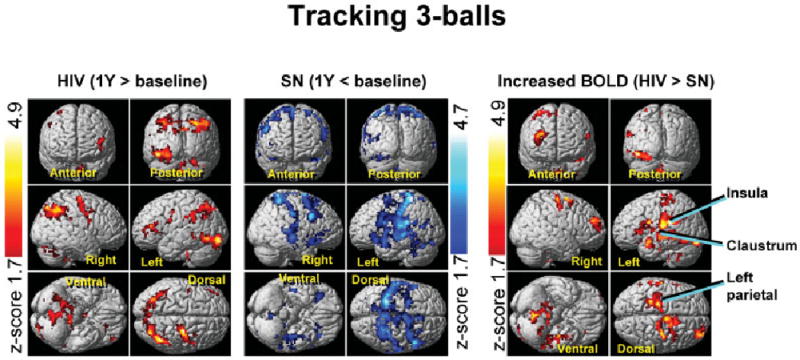
Statistical parametric maps of changes in brain activation over the course of 1 year for human immunodeficiency virus (HIV)–positive (left) and seronegative (SN) controls (center) for the three-ball tracking task. Significant blood oxygen level dependent (BOLD) signal increases are indicated by Z-scores shown in the red-yellow scale and decreases by Z-scores on the blue-white scale. The HIV group shows only increases in brain activation, whereas the controls show only signal decreases after 1 year. The opposite changes led to significant interactions in brain activation between HIV status and time, with greater 1-year BOLD signal increases in HIV patients compared with SN controls (right). These findings suggest a practice effect in the controls but neural compensation in the HIV group to maintain performance. Clusters with greater than 300 voxels and p corrected < 0.05 (voxel level) are shown.
Fig 3.
Statistical parametric maps of changes in brain activation over the course of 1 year for human immunodeficiency virus (HIV)–positive (left) and seronegative (SN) participants (center) for the four-ball tracking task. HIV patients showed greater BOLD signal increases at 1 year compared with SN participants (right). Clusters with greater than 300 voxels and p corrected < 0.05 (voxel level) are shown.
Conversely, SN participants showed only decreases in BOLD activation in bilateral prefrontal and parietal cortices over 1 year, both for the three- and four-ball tasks (see Figs 2 and 3, center panels). Hence, HIV patients had greater 1-year increases in brain activation than SN participants for both of these tasks (see Figs 2 and 3, right panels); this effect was more pronounced for the four-ball task. This opposite effect on brain activation changes in HIV and SN participants led to even greater group differences after 1 year (see Fig 1, right panel). Notably, none of the 1-year activation changes was greater in SN compared with HIV-positive patients.
ROI data from areas with group differences in the BOLD signal change (see right panels in Figs 2 and 3; cluster maxima in Supplementary Table 1) confirmed 1-year increases in BOLD signals in HIV-positive patients, but 1-year decreases in SN participants for the three- and four-ball tasks (Fig 4). Significant interactions (all p < 0.005) between HIV status and study time point were typically observed in regions that did not show greater activation in HIV than SN at baseline. Linear regressions between 1-year changes in ROI BOLD signals and changes in CES-D scores were not significant (p > 0.25; HIV group).
Fig 4.
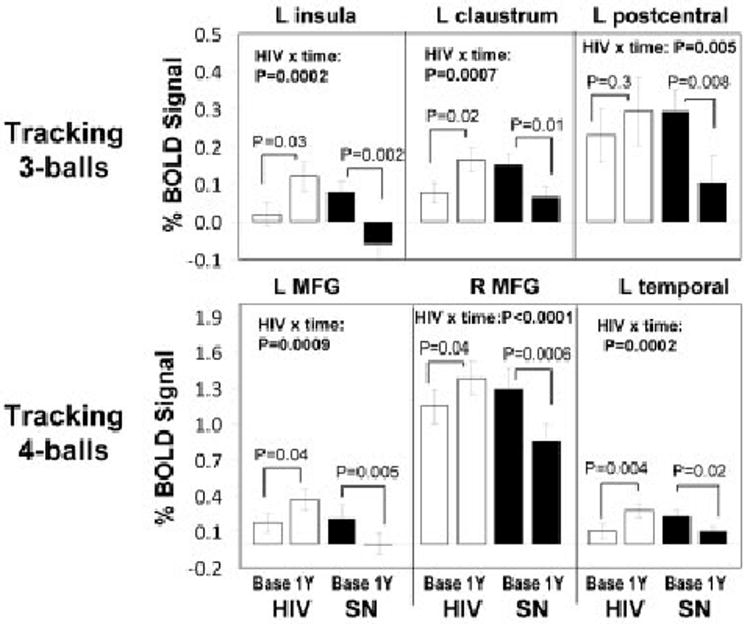
(top) Extracted blood oxygen level dependent (BOLD) signal amplitudes centered at 3 major cluster maxima that showed interaction effects for human immunodeficiency virus (HIV) status and time for the 3-ball tracking task: left insula (−36, −27, 21), left claustrum (−20, 3, 18), and left postcentral gyrus (−57, −21, 24). (bottom) Extracted BOLD signal amplitudes centered at three major cluster maxima that showed an interaction effect of HIV status and time for the 4-ball tracking task: left middle frontal gyrus (L MFG: −51, 30, 24), right middle frontal gyrus (R MFG: 33, 6, 63), and left temporal subgyral (−48, −48, −6). The extracted data confirm the findings of the Statistical Parametric Mapping analysis, with significant (p corrected < 0.005) HIV status × time interactions, using repeated-measure analysis of variance. Post hoc paired t tests were used to compare baseline and 1-year data in each group.
Discussion
At baseline, a group of clinically stable HIV patients with normal cognitive performance had greater brain activation in the occipital/cerebellar and right prefrontal regions than SN controls only on the task with the greatest attentional load. These findings are consistent with earlier and smaller studies of HIV patients with normal cognition21 or mild neurocognitive impairment.8 However, after 1 year, greater activation in HIV than SN participants was observed in many regions within the attention network across all three tasks, most prominently on the four-ball task. This pattern of load-dependent increases in brain activation suggests a compensatory response in the HIV patients that was more evident at 1-year follow-up. Furthermore, after 1 year, SN patients showed either no change or decreased activation within the attention network. Because fMRI task performance remained unchanged and all patients remained cognitively normal on neuropsychological testing, these findings suggest that the compensatory response enabled the HIV patients to maintain performance, whereas the SN controls appeared to be more efficient or show a practice effect (at the neural level) during the repeat fMRI studies. If differences in brain activation between the two groups continue to increase over longer periods, cognitive decline may occur once the need to further increase brain activation exceeds the available reserve capacity in the HIV patients.
More specifically, 1-year BOLD signal increases in the HIV patients commonly occurred in the prefrontal and posterior parietal cortices, regions without group differences at baseline. Also, these brain regions showed strong correlations between task difficulty and BOLD signals in control participants,25 suggesting that they provide the “reserve capacity” for visual attention.8 Therefore, these findings indicate that the HIV patients required recruitment of the previously unused reserve brain network to maintain normal performance 1 year later. The 1-year BOLD signal increases in the HIV group are not likely driven by changes in depressive symptoms or viral loads, because there was no association with these variables.
Conversely, the 1-year BOLD signal decreases in the same brain regions of the SN participants suggest they were more efficient during the repeat fMRI, probably because of a practice effect. Reduced BOLD activation with repeated tasks has been observed on many attention-requiring paradigms28,29 including the same visual attention task.30 However, prior fMRI studies typically involved relatively short training periods (days or less). A prior neuropsychological study showed practice effects after 1 year in asymptomatic HIV patients, but not in those who were symptomatic (with AIDS or ARC).31 Similarly, our cognitively normal participants in both groups demonstrated significant practice effects after 1 year on four of six cognitive domains. However, fMRI task performance did not improve over 1 year in either group, probably because of a “ceiling effect” from the practice trials before the fMRI scanning.
The brain activation in regions associated with load effects (tracking more balls) increased in the HIV patients over 1 year but was relatively unchanged or decreased in the SN participants, leading to even greater group differences at 1-year follow-up. This finding further supports the proposed model that the SN, but not the HIV, participants had more efficient neural processing and required less usage of the reserve capacity after 1 year.
The findings of this study also suggest premature aging of the HIV-infected brain. Although age-dependent decreases in brain activation were reported in many fMRI studies, including paradigms evaluating primary sensory13,32,33 motor (finger-tapping)14 working and episodic memory,15,16 greater brain activation was observed in older compared with younger individuals in the frontal and parietal regions on a visual-attention task,34 and in the hippocampi on attention-requiring working memory tasks.17,18 Furthermore, normal aging is generally associated with a shift of brain activity from posterior (occipital or parietal) to anterior (frontal) regions.35 Therefore, greater activation in our HIV patients at baseline and 1-year BOLD signal increases in brain regions with normal activation at baseline (especially prefrontal areas) are consistent with premature brain aging in the HIV patients.
Although the exact mechanisms underlying BOLD signal increases in the HIV patients are unclear, our findings suggest ongoing brain injury despite well-controlled viral load and CD4 counts. First, increased activation may have resulted from continued neuroinflammation, with glial activation, which may be present even in patients treated with CART for long periods and who had well-controlled systemic immune status, although some had comorbid disorders (eg, hepatitis C or drug abuse).36 In a prior study, we indeed observed greater BOLD activation in HIV patients with higher neurometabolites that are located primarily in glia.37 Second, increased BOLD signals may be mediated by ongoing injury to the dopaminergic system, which plays a major role in regulating attention and has been implicated in HIV-associated dementia.38,39 Patients with HIV-associated dementia had lower dopamine transporter availability,39 and those with lower dopamine transporters had poorer performance on several attention-requiring neuropsychological tests.40 The possible role of dopaminergic deficit–mediated increased BOLD signals is further supported by an fMRI study of Parkinson patients who showed greater activation of the working memory network during a hypodopaminergic state.41 Another pathway to brain injury with ongoing HIV infection is oxidative stress-mediated neuronal apoptosis or neurodegeneration,36,42 which could damage the attention network. Collectively, all of these mechanisms may progressively reduce the efficiency of the neural substrate, necessitating HIV-positive patients to compensate by increasing brain activation. With further progression of neuroinflammation and neurodegeneration (including dopaminergic neuronal injury), HIV-infected patients may ultimately become unable to compensate and manifest HAND, particularly if aging, illicit drug use, or other comorbidities are superimposed.
The longitudinal intrasubject design, the requirement that all participant had normal cognitive function and were on stable CART at both time points, and the relatively large sample size minimized various potential confounds that occur in cross-sectional studies, such as differences in patient characteristics, differences in viral strains, or the variable treatment responses across patients. We also carefully excluded participants with a history of illicit drug dependence, which can cause neuroinflammation, neurodegeneration,36 as well as BOLD signal abnormalities.43,44 However, because all of our HIV-positive patients were on CART, it is possible that fMRI changes were due to the HIV medications rather than the viral infection itself. Future longitudinal fMRI studies45 of larger groups of patients before and after CART may help to clarify this possibility.
In conclusion, temporally increased BOLD activation in HIV patients suggests decreased neural efficiency, possibly because of progressive injury to the neural substrate, despite their asymptomatic clinical and neuropsychological status. These findings may explain why many HIV-infected individuals ultimately experience development of cognitive and functional abnormalities even with continued antiretroviral treatment and viral control. With the longer survival of an increasing number of HIV patients, it is important to understand and detect such ongoing subclinical brain injury, and develop preventive treatments to minimize the number of patients who will experience development of HAND.
Supplementary Material
Acknowledgments
This work was supported by the NIH (National Institute of Mental Health, 2R01 MH61427, L.C., T.E., H.N., M.W.,; National Institute on Drug Abuse, K24-DA16170, L.C.; K02-DA16991, T.E.; Core support from the National Institute on Neurological Disorders and Strokes, U54NS56883, L.C., T.E., M.W.; National Center for Research Resources, G12 RR003061-21, T.E., 1P01 RR011091-11, L.C., T.E.) and the Office of National Drug Control Policy DABK39-03-C-0060, L.C., T.E.).
We thank other members of our research team for their assistance in data collection: Dr C. Cloak, L. Girton, D. Ramones, K. Taketa, T. Wu, and B. Tokeshi. We are also grateful to our research participants and for the patient referrals from local physicians, including Drs C. Goshima, D. Kovach, J. Franks, and A. Tice.
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Gray F, Adle-Biassette H, Chretien F, et al. Neuropathology and neurodegeneration in human immunodeficiency virus infection Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol. 2001;20:146–155. [PubMed] [Google Scholar]
- 2.Janssen RS, Cornblath DR, Epstein LG, et al. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Neurology. 1991;41:778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- 3.Dore GJ, McDonald A, Li Y, et al. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 2003;17:1539–1545. doi: 10.1097/00002030-200307040-00015. [DOI] [PubMed] [Google Scholar]
- 4.Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McArthur JC, Haughey N, Gartner S, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 6.Sacktor N, Nakasujja N, Skolasky R, et al. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology. 2006;67:311–314. doi: 10.1212/01.wnl.0000225183.74521.72. [DOI] [PubMed] [Google Scholar]
- 7.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang L, Tomasi D, Yakupov R, et al. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol. 2004;56:259–272. doi: 10.1002/ana.20190. [DOI] [PubMed] [Google Scholar]
- 9.Madden DJ. Aging and visual attention. Curr Dir Psychol Sci. 2007;16:70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Gerven PW, Meijer WA, Jolles J. Education does not protect against age-related decline of switching focal attention in working memory. Brain Cogn. 2007;64:158–163. doi: 10.1016/j.bandc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Corral M, Rodriguez M, Amenedo E, et al. Cognitive reserve, age, and neuropsychological performance in healthy participants. Dev Neuropsychol. 2006;29:479–491. doi: 10.1207/s15326942dn2903_6. [DOI] [PubMed] [Google Scholar]
- 12.Anthony IC, Ramage SN, Carnie FW, et al. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 13.Buckner R, Snyder A, Sanders A, et al. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;12:24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- 14.Hesselmann V, Zaro Weber O, Wedekind C, et al. Age related signal decrease in functional magnetic resonance imaging during motor stimulation in humans. Neurosci Lett. 2001;308:141–144. doi: 10.1016/s0304-3940(01)01920-6. [DOI] [PubMed] [Google Scholar]
- 15.Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- 16.Daselaar S, Veltman D, Rombouts S, et al. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell K, Johnson M, Raye C, D'Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain Res Cogn Brain Res. 2000;10:197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- 18.Adler C, Holland S, Enseleit S, Strakowski S. Age-related changes in regional activation during working memory in young adults: an fMRI study. Synapse. 2001;42:252–257. doi: 10.1002/syn.1111. [DOI] [PubMed] [Google Scholar]
- 19.Grossman M, Cooke A, DeVita C, et al. Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage. 2002;15:302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- 20.Chang L, Speck O, Miller E, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- 21.Ernst T, Chang L, Jovicich J, et al. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- 22.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 23.Karnofsky DA, Burchenal JH. In: The clinical evaluation of chemotherapeutic agents in cancer. MacLeod C, editor. New York: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- 24.Pylyshyn ZW, Storm RW. Tracking multiple independent targets: evidence for a parallel tracking mechanism. Spat Vis. 1988;3:179–197. doi: 10.1163/156856888x00122. [DOI] [PubMed] [Google Scholar]
- 25.Jovicich J, Peters RJ, Koch C, et al. Brain areas specific for attentional load in a motion tracking task. J Cogn Neurosci. 2001;13:1048–1058. doi: 10.1162/089892901753294347. [DOI] [PubMed] [Google Scholar]
- 26.Thesen S, Heid O, Mueller E, Schad L. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 27.Zaitsev M, Hennig J, Speck O. Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust, and flexible method for echo-planar imaging distortion correction. Magn Reson Med. 2004;52:1156–1166. doi: 10.1002/mrm.20261. [DOI] [PubMed] [Google Scholar]
- 28.Petersen SE, van Mier H, Piez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci U S A. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schumacher E, Hendricks M, D'Esposito M. Sustained involvement of a frontal-parietal network for spatial response selection with practice of a spatial choice-reaction task. Neuropsychologia. 2005;43:1444–1455. doi: 10.1016/j.neuropsychologia.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. NeuroImage. 2004;23:1414–1421. doi: 10.1016/j.neuroimage.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 31.Poutiainen E, Elovaara I, Raininko R, et al. Cognitive decline in patients with symptomatic HIV-1 infection No decline in asymptomatic infection. Acta Neurol Scand. 1996;93:421–427. doi: 10.1111/j.1600-0404.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 32.Ross MH, Yurgelun-Todd DA, Renshaw PF, et al. Age-related reduction in functional MRI response to photic stimulation. Neurology. 1997;48:173–176. doi: 10.1212/wnl.48.1.173. [DOI] [PubMed] [Google Scholar]
- 33.Yousem D, Maldjian J, Hummel T, et al. The effect of age on odor-stimulated functional MR imaging. Am J Neuroradiol. 1999;20:600–608. [PMC free article] [PubMed] [Google Scholar]
- 34.Madden DJ, Spaniol J, Whiting WL, et al. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis SW, Dennis NA, Daselaar SM, et al. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–191. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- 37.Ernst T, Chang L, Arnold S. Increased glial markers predict increased working memory network activation in HIV patients. Neuroimage. 2003;19:1686–1693. doi: 10.1016/s1053-8119(03)00232-5. [DOI] [PubMed] [Google Scholar]
- 38.Berger JR, Kumar M, Kumar A, et al. Cerebrospinal fluid dopamine in HIV-1 infection. AIDS. 1994;8:67–71. doi: 10.1097/00002030-199401000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Wang G, Chang L, Volkow N, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- 40.Chang L, Wang G, Volkow N, et al. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattay V, Tessitore A, Callicott J, et al. Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol. 2002;51:156–164. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- 42.Lipton S. Similarity of neuronal cell injury and death in AIDS dementia and focal cerebral ischemia: potential treatment with NMDA open-channel blockers and nitric oxide-related species. Brain Pathol. 1996;6:507–517. doi: 10.1111/j.1750-3639.1996.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 43.Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- 44.Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang L, Yakupov R, Nakama H, et al. Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. J Neuroimmune Pharmacol. 2008;3:95–104. doi: 10.1007/s11481-007-9092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


