Abstract
Objective
Thioredoxin-2 (Trx2),a major antioxidant protein in mitochondria, enhances nitric oxide bioavailability and inhibits ASK1-dependent apoptosis in endothelial cells (EC). However, the in vivo role of Trx2 in angiogenesis has not been defined. Here we used endothelial cell (EC)-specific transgenesis of Trx2 (Trx2-TG) in mice to determine the in vivo function of Trx2 in arteriogenesis and angiogenesis.
Methods and Results
In a femoral artery ligation model, Trx2-TG mice had enhanced capacity in limb perfusion recovery and ischemic reserve capacity compared to the non-transgenic littermates. Ischemia-initiated arteriogenesis in the upper limb was augmented in Trx2-TG mice. Trx2-TG mice also showed significantly enhanced capillary formation and maturation in the lower limb. In non-transgenic limb, ischemia specifically induced a downregulation of Trx2 protein, leading to increased oxidative stress, ASK1 activation and EC apoptosis. In contrast, Trx2-TG maintained a constitutive level of Trx2, reducing the ischemia-induced deleterious responses. We then defined the mechanism by which Trx2 increases angiogenesis using EC isolated from Trx2-TG mice. Trx2-TG EC showed increased NO and NO-dependent migration. In addition, these cells were more resistant to oxidative stress-induced activation of ASK1 signaling and apoptosis. Moreover, Trx2-augmented EC survival is NO-independent. To define the relative contributions of Trx2-increased NO and Trx2-reduced ASK1 apoptotic activity to angiogenesis in vivo, we examined Trx2 effects on ischemia-induced angiogenesis in eNOS-deficient mice. The eNOS deletion caused severe impairment in the functional flow recovery in response to ischemia. Trx2 expression in eNOS-KO mice still dramatically inhibited ischemia-induced ASK1 and EC apoptosis, leading to an enhanced functional flow recovery.
Conclusion
These in vivo and in vitro data support that Trx2 maintains EC function by two parallel pathways – scavenging ROS to increase NO bioavailability and inhibiting ASK1 activity to enhance EC survival, facilitating ischemia-mediated arteriogenesis and angiogenesis.
Keywords: Thioredoxin, Angiogenesis, Ischemia, Apoptosis
INTRODUCTION
Angiogenesis, a process of new blood vessel formation, contributes to various physiological processes and pathological settings1. While excessive angiogenesis links atherosclerosis, cancer and diabetic retinopathy, defects in angiogenesis directly contribute to myocardial infarction and peripheral arterial disease. Recent reports suggest that reactive oxygen species (ROS) can positively or negatively regulate angiogenesis. While physiological levels of ROS are required for angiogenesis, excess amount of ROS generated during inflammation and ischemic response may inhibit reparative vascular remodeling by inducing endothelial dysfunction and apoptosis. ROS-producing systems in vascular endothelial cells (EC) are numerous including various NADPH oxidases, xanthine oxidase, the uncoupling of NO synthase as well as mitochondria 2–5. The NADPH oxidases have been considered predominant sources of ROS in the pathogenesis of cardiovascular diseases5, 6. Recent data support that ROS generated from mitochondria significantly regulate EC function2, 3, 7, 8. Physiological ROS may function as a second messenger in signal transduction and regulate EC growth/proliferation, apoptosis, EC barrier function, vasorelaxation and vascular remodeling9–11. However, cardiovascular risk factors often induce mitochondria dysfunction, leading to overproduction of ROS. These excess ROS induce EC dysfunction by reducing NO bioavailability and activating apoptotic signaling.
The role of ROS as signaling molecules in growth factor-mediated angiogenesis has been investigated. One of the major sources of ROS in EC is NADPH oxidase which consists of Nox family proteins, p22phox, p47phox, p67phox and the small G protein Rac1 6, 12. The in vitro and in vivo function of NADPH oxidase in angiogenesis has been defined in several mouse models. Neovascularization in an ischemic hindlimb model is significantly impaired in gp91phox-KO mice, likely due to reduction of superoxide produced from inflammatory cells as well as neovasculature13. On the contrary, negative regulation of angiogenesis by ROS has also been reported from studies with ROS-scavenging enzymes. Mice lacking ecSOD (ecSOD-KO) have impaired ischemia-induced increase in collateral vessel formation, capillary density and blood flow recovery14. This impairment correlates with enhanced superoxide production, decreased NO activity and increased apoptotic cells in ecSOD-KO mice. Thus, angiogenesis is delicately balanced by both these cytosolic ROS-generating oxidases and ROS-scavenging enzymes.
However, the roles of mitochondria-derived ROS and mitochondrial anti-oxidant system in angiogenesis have not been addressed. The mitochondrial thioredoxin (Trx2) system appears to be a critical component to maintain mitochondrial normal function. Trx2-dependent peroxidase (Prx3) and reductase (TrxR2), together with MnSOD (SOD2) 15, provide a primary line of defense against ROS produced by the mitochondrial respiratory chain. In support of this notion, mice with a deletion of Trx2 or TrxR2 display embryonic lethality, likely due to increased oxidative stress 16, 17. Similarly, cells with deficiency or knockdown of Trx2 or Prx3 accumulate endogenous ROS and are highly sensitive to exogenous oxygen radicals 15, 18. However, little is known about the function of the Trx2 system in the vasculature.
Previously we have shown that EC-specific transgenesis of Trx2 (Trx2-TG) improves aortic EC function and reduces atherosclerotic lesions at aortic roots in the Apo E-deficiency mouse model by reducing oxidative stress 19. However, the role of Trx2 in microvessels has not been investigated. In the present study, we demonstrate a critical role of Trx2 in ischemia-mediated reparative arteriogenesis and angiogenesis in a femoral artery ligation model using the aforementioned Trx2-TG mice.
METHODS
The detailed materials and methods are provided in the online supplement. Most of the methods have been previously published, including generation of the EC-specific Trx2 transgenic mice 19, mouse hindlimb ischemic model, post-contraction hyperemia 20, 21, histology and immunohistochemistry, gene expression in ischemic muscle, cell culture and cytokines, EC migration assay and image analysis 20–22, immunoblotting, ASK1 and JNK kinase assays and quantification of apoptosis 22, 23.
RESULTS
Trx2-TG augments perfusion recovery in ischemic hindlimbs
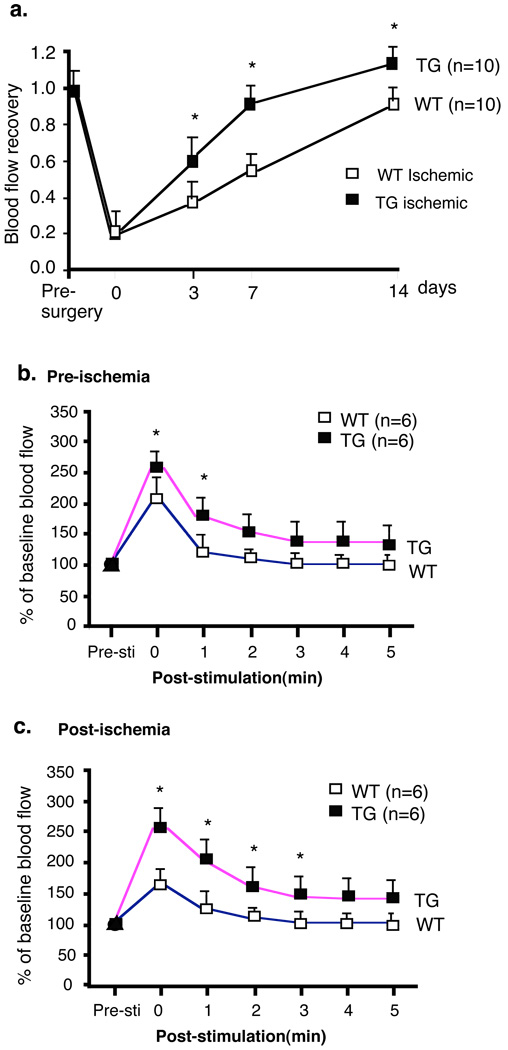
Non-transgenic littermates (WT) and Trx2-TG male mice were subjected to femoral artery ligation. Blood flow in ischemic and non-ischemic limb perfusion were measured pre-surgery and post-surgery as indicated (days 0 (30 min), 3, 7 and 14). Trx2-TG mice were higher in baseline blood flow (Supplemental Fig.I), correlating with a lower aortic pressure in Trx2-TG compared to WT mice as we observed previously 19. Before surgery, the ratio of left leg to right leg gastrocnemius blood flow was 1. 30 min post-surgery, flow dropped to 0.2 and returned to a ratio of 0.8 over 2 weeks in WT mice. Trx2-TG mice showed augmented recovery of hindlimb perfusion on days 3 and 7 and the limb flow returned to normal on day 14 (Fig.1a). To further define the functional defects in mice, we examined skeletal muscle contraction stimulated hyperemia in the gastrocnemius muscle in WT and Trx2-TG mice at baseline and after ischemia as we described recently for Bmx-KO and TNFR2-KO mice 20, 21. As seen in the representative traces in Fig.1b, electrical stimulation of the adductor muscle groups in the upper legs of WT and Trx2-TG mice results in a marked increase in peak blood flow [compare pre-stimulation (Pre-Sti) to the peak response of stimulation (time 0)]. Trx2-TG mice had greater blood flow compared to WT and a delayed return to baseline compared to WT mice, suggesting that Trx2-TG mice have reduced peripheral vascular resistance. These data are consistent with the high NO activity in Trx2-TG mice as NO is critical for maintaining vasodilation necessary for the gradual return of flow back to normal 19. Next, we measured the same physiological response after limb ischemia in WT and Trx2-TG mice at 2 weeks post-ischemia. Electrical stimulation of the adductor muscle groups in the upper leg of WT mice after 2 weeks surgery showed only 70% of the peak response measured in the gastrocnemius muscle group compared to pre-ischemia. However, Trx2-TG mice had a complete recovery in peak blood flow (Fig.1b pre-ischemia vs Fig.1c post-ischemia, 105±5%). These data show that Trx2-TG mice have augmented perfusion recovery in ischemic hindlimbs.
Fig.1. Critical roles of Trx2 in the recovery of hindlimb perfusion post-injury.
a. Trx2-TG mice show augmented recovery of limb perfusion compared to non-transgenic WT mice. Ischemic hindlimb model was performed, and blood flow of ischemic and non-ischemic limb were measured on gastrocnemius muscle at 30 min, 3, 7 and 14 days after surgery. Ratio of perfusion unit from non-ischemic (left) to ischemic limbs (right) are shown). N number for each strain is shown in parenthesis. Data are mean ± SEM, *, p <0.05. b–c. Trx2-TG mice show enhanced postcontraction hyperemia. Adductor muscle groupsof mice from pre-surgery (b) and 2 weeks post-surgery (c) were electro-stimulated, and gastronemius blood flow was recorded. Both baseline and stimulated lower leg perfusion in legs were measured as an index of the maximal vasodilatory capacity. Data are mean ± SEM, *, p<0.05 comparing Trx2-TG to WT.
Post-ischemic arteriogenesis and angiogenesis are enhanced in Trx2-TG mice
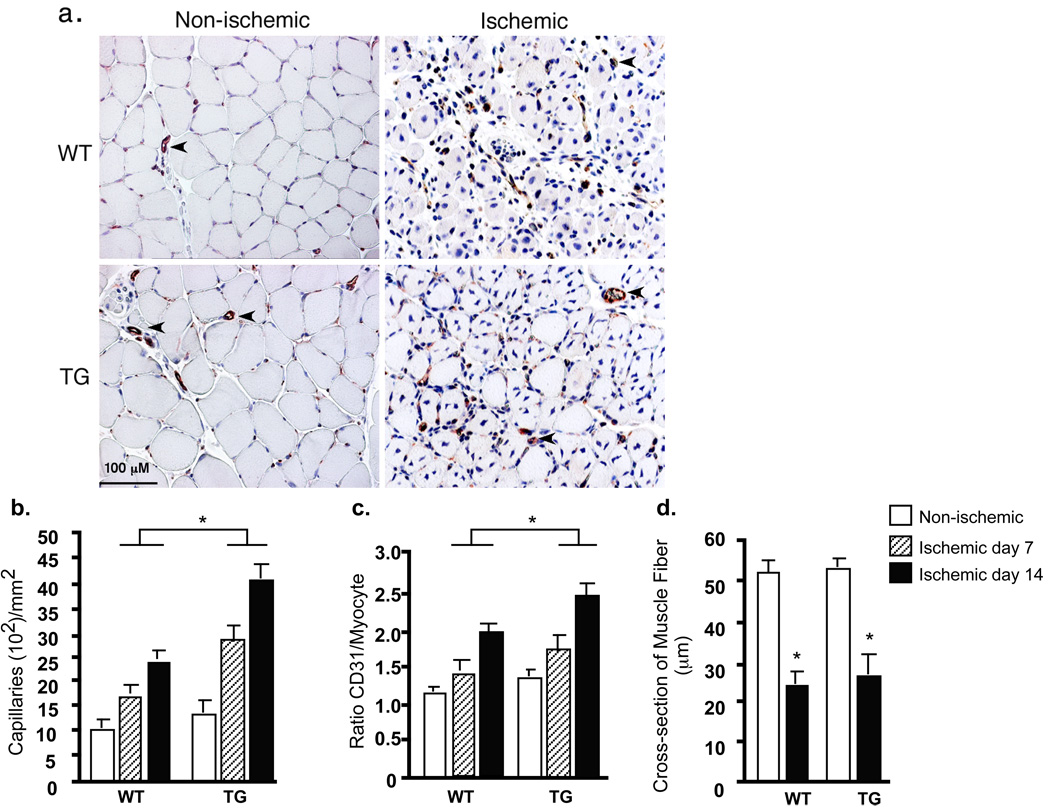
Enhanced limb perfusion could be due to increased arteriogenesis from existing vessels of the upper limb and increased neovascularization/vessel maturation in the lower limb. Previously we have visualized vascular architecture by Microfil casting to determine ischemia-initiated collateral artery growth (arteriogenesis) 20, 21. We compared the collateral growth in WT and Trx2-TG mice by the same approach. There was an enlargement of collaterals compared to the contralaterals in WT mice after 2 weeks of ischemia. Trx2-TG showed enhanced arterialization as determined by the ratio of vascular density in the left arteries vs the right arteries (Supplemental Fig.II). Interestingly, the Trx2-TG mice showed greatly enhanced vessel sprouting, consistent with the increased NO activity in these mice 19 which has been shown to mediate vessel branching and morphogenesis 24. To determine if neovascularization and vessel maturation in the lower limb are increased in Trx2-TG mice, we performed immunohistochemistry with EC- and pericyte-specific markers. After 7 and 14 days of ischemia, there was an increase in CD31-positive capillaries surrounding the skeletal muscle fibers in WT mice (Fig.2a for day 7 with quantification of the number of capillaries and capillary/fiber ratio in Fig.2b and 2c, respectively). Trx2 expression did not alter the cross-section of muscle fibers and muscle morphology (Fig.2d). However, Trx2-TG mice show increased numbers of dilated vessels as well as SMA-positive capillaries, consistent with the role of NO in vessel stabilization 24. Ischemic-induced vessel maturation as determined by smooth muscle α-actin (SMA) staining was also increased (Supplemental Fig.II for day 14 with quantification of SMA-positive capillaries/mm2). Consistent with the results that Trx2-TG mice showed much greater recovery in hindlimb perfusion compared to WT mice, CD31 positive capillaries surrounding the skeletal muscle fibers (neovascularization) and SMA-positive SMC (pericyte recruitment) were significantly increased in Trx2-TG mice compared to WT secondary to ischemia on both days 7 and 14 (Fig.2 and Supplemental Fig.II).
Fig.2. Critical roles of Trx2 in ischemia-induced angiogenesis.
7 days after femoral ligation, gastrocnemius muscles were harvested. Capillary density was immunostained with CD31 (an EC marker). a. Representative images of CD31 staining. Dilated vessels are shown by arrowheads. b–d. Quantification of capillaries (number/mm2 muscle area), ratio of CD31/muscle fiber and cross-section of muscle fibers (µm). Data from 3 sections of each mouse muscle tissue are shown in graphics and n=4 for each strain (total 12 sections). *, p<0.05.
Trx2 reduces oxidative response, ASK1-JNK activation and cellular apoptosis in ischemic tissue
To understand how Trx2-TG promotes angiogenesis, we first measured Trx2 mRNA (Supplemental Fig.III) and protein expression (Supplemental Fig.III) in response to ischemia. We found that endogenous Trx2, but not other anti-oxidant proteins Trx1, SOD1 or SOD2, was drastically reduced in non-transgenic mice in response to ischemia (Supplemental Fig.III). In contrast, Trx2 transgene was resistant from ischemia-induced downregulation. We measured ischemia-induced ROS production and activation of ASK1-JNK signaling and infiltration of leukocytes. Oxidative stress in muscle tissue was determined by an in situ detection of superoxide with dihydroethedium fluorescence (DHE) and by immunostaining with antibody against nitrotyrosine, an indicator of peroxynitrite-induced tyrosine nitrosylation. DHE intercalates into DNA and gives fluorescence primarily from the nucleus. Ischemia on day 7 strongly induced production of superoxide, apparently from vascular EC and infiltrated cells but not from myocytes (Fig.3a). However, basal and ischemia-induced oxidative stress were significantly reduced in Trx2-TG mice (Fig.3a–b). Similar results were obtained for nitrotyrosine staining (Supplemental Fig.IV). Inflammatory response was examined by leukocyte infiltration with anti-CD45 antibody, and Trx2-TG mice showed significantly reduction in leukocyte infiltration compared to WT mice (Supplemental Fig.IV). The reduction of leukocyte infiltration is consistent with the increased NO activity in Trx2-TG mice. It is also correlated with reduced ROS production and oxidative stress in Trx2-TG mice.
Fig.3. Trx2 reduces oxidative response and ASK1-JNK activation in ischemic tissue.
WT and Trx2-TG mice were subjected to ischemia ligation, and tissues were harvested on day 7. Tissue oxidative stress and inflammation in gastronemius were determined by immunohistochemistry with respective markers. a–b. Oxidative stress in muscle tissue was determined by an in situ detection of superoxide with dihydroethedium (DHE) fluorescence. DHE-positive nuclei of EC, myocyte and infiltrated cells are indicated by black arrows, white arrows and black arrowheads, respectively. DHE-positive cells were quantified as DHE-positive cells/PF (per field). Data from 5 sections of each mouse muscle tissue are shown in graphics and n=4 for each strain (total 20 sections). *, p<0.05. (b). c. Activation of ASK1-JNK/p38 MAPK and Akt. Activation ASK1-JNK/38 and Akt was determined by Western blot with phospho-specific antibodies. Relative ratios of p-ASK1/ASK1, p-p38/p38 and p-JNK/JNK as well as p-Akt/Akt are shown, with untreated WT as 1.0. Data from three independent experiments were subjected to statistic analysis, and p-ASK1, p-p38 and p-JNK as well as p-Akt are significantly reduced in Trx2-TG compared to WT.
ASK1-JNK/p38 MAPK signaling pathway can be activated by oxidative stress and inflammatory stimuli 25. Activation of ASK1-JNK/p38 was determined by Western blot with phospho-specific antibodies, and was strongly induced in ischemia hindlimb on day 7. However, Trx2 significantly blunted ischemia-induced activation of ASK1-JNK/p38 signaling (Fig.3c). We then measured tissue apoptosis by TUNEL assay and cellular proliferation by PCNA staining. Kinetics studies indicated that apoptosis peaked at day 7 post-surgery, suggesting that ischemia-induced apoptosis is an early event in the adaptive response 21. More importantly, ischemia-induced apoptosis was dramatically decreased in Trx2-TG mice compared to WT mice (Supplemental Fig.IV). Cellular proliferation started at day 7 and sustained until 4 wks (not shown, also see 21). Both capillaries and myocytes showed PCNA-positive staining (Supplemental Fig.IV, arrowheads and arrows, respectively). These data suggest that Trx2-TG reduces oxidative stress, leading to reduced ASK1-JNK/p38 signaling and apoptosis while yet enhancing cellular proliferation in ischemic tissue.
Trx2 expression increases EC migratory activity
To determine if the in vivo function of Trx2 in angiogenesis correlates to in vitro activities of Trx2 in EC, we examined the effects of Trx2 on EC migration and survival which are critical components of the angiogenesis process. Mouse microvessel EC (MEC) from lung were isolated from WT and Trx2-TG mice, and the effect of Trx2 on VEGF-induced EC migration was first determined in a monolayer injury model. Trx2-TG MEC showed increased migration compared to WT MEC (Fig.4a and Supplemental Fig.V). Consistent with our previous finding 19, Trx2 augments NO bioavailability in MEC by measuring nitrite release into the culture media using a NO-specific chemiluminescence (Fig.4b) without effects on eNOS phosphorylation. Addition of an eNOS inhibitor L-nitro arginine methyl ester (L-NAME, 100 µM) blocked NO release (Fig.4b) as well as EC migration in both WT and Trx2-TG cells (Fig.4a), suggesting that NO contributes to Trx2-augmented EC migration. To further Trx2 in angiogenic signaling, Trx2 expressed was suppressed by Trx2 siRNA as we described previously 23. Trx2 siRNA, but not a control siRNA, knocked down Trx2 expression by 90%. However, Trx2 knockdown had no effects on expression of Trx1 (Fig.4c) or other anti-oxidant proteins (SOD1 and SOD2, not shown). Trx2 knockdown significantly reduced NO bioavailability (Fig.4c) and EC migration under a normal EC culture condition (Fig.4d). To determine the effects of Trx2 on EC survival, WT and Trx2-TG MEC were treated with TNF (10 ng/ml) in the presence of cycloheximide (CHX, 10 µg/ml). TNF is known to induce superoxide in EC and mitochondria-dependent apoptosis 19, 23. Consistent with our previous findings that Trx2 protects EC from apoptosis by inhibiting ASK1 activity 23, TNF-induced ASK1 activity was significantly reduced in Trx2-TG MEC (Fig.4e). EC apoptosis was determined by FACS analysis with FITC-conjugated annexin V or DAPI staining for nuclear fragmentation. Trx2-TG MEC showed dramatically reduced apoptosis by annexin V-staining (Fig.4f and Supplemental Fig.V) and nuclear fragmentation assay (not shown). A similar inhibition of Trx2 on H2O2-induced EC death was observed (not shown).
Fig.4. Trx2 expression increases EC migration and EC survival.
a. Effects of Trx2 on EC migration. Mouse microvessel EC (MEC) from muscle and lung were isolated from WT and Trx2-TG mice, and the effect of Trx2 and L-NAME on VEGF-induced EC migration was first determined in a monolayer injury model. Data presented are means (±SEM) of the two triplicates from two independent experiments. *, p<0.05. b. Effects of Trx2 transgene on VEGF-induced NO bioavailability. MEC were cultured in media deprived EC growth factors overnight followed by treatment with VEGF (50 ng/ml) in the absence or presence of eNOS inhibitor L-NAME (100 µM) for 60 min. NO release was determined by measuring nitrite in the media. c–d. Trx2 siRNA on angiogenic signaling. WT MEC were transfected with control or Trx2 siRNA oligonucleotides. 72 h post-transfection, expression of Trx2 and Trx1 was determined by Western blot with respective antibodies (c). Basal NO bioavailability (c) and EC migration (d) were also determined. Data presented are means (±SEM) of the two triplicates from two independent experiments. *, p<0.05. e–f. Trx2 on TNF signaling and apoptosis in EC. In e, MEC were untreated or treated with TNF (10 ng/ml) for indicated times. Activation of ASK1-p38 MAPK was determined by Western blot with phospho-specific antibodies. In f, WT and Trx2-TG MEC were treated with TNF (10 ng/ml)+CHX (10 µg/ml) for 6 h. EC apoptosis was determined by FACS analysis with FITC-conjugated annexin V (X-axis) and propidium iodide (PI, Y-axis). Quantification of apoptotic (annexin V-positive, both upper and lower right quadruplets in FACS) are shown. *, p<0.05. Note that the necrotic cells (annexin V-negative/PI-positive, upper left) are similar between WT and Trx2-TG groups.
Both NO and ASK1 pathways contribute to ischemia-induced angiogenesis
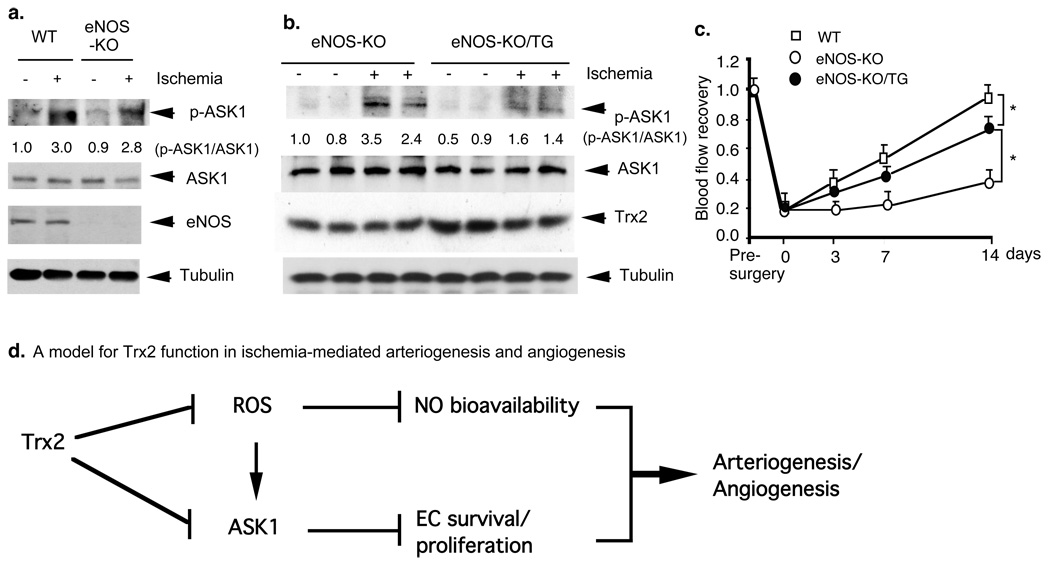
To define the relative contributions of Trx2-increased NO and Trx2-reduced ASK1 apoptotic activity to angiogenesis in vivo, we examined Trx2 effects on ischemia-induced angiogenesis in eNOS-deficient mice. The eNOS-KO/Trx2-TG mice were obtained by mating with Trx2-TG with eNOS-KO mice (both eNOS-KO and Trx2-TG mice are C57Bl/6 background) (Supplemental Fig.VI) and Trx2 protein expression in Trx2-TG mice was not altered after crossing with eNOS-KO mice (see Fig.5b). We have added the blot into the revised Fig.5b. eNOS-KO and eNOS-KO/Trx2-TG mice were subjected to femoral artery ligation, and activation of ASK1, functional blood flow, and morphological analysis were performed as described for WT and Trx2-TG mice. A deletion of NOS had no significant effects on ASK1 activity (Fig.5a). Trx2 expression in eNOS-KO mice significantly inhibited ischemia-induced ASK1 apoptotic signaling (Fig.5b). Consistent with previous findings 26, the eNOS deletion caused severe impairment in functional flow recovery in response to ischemia (Fig.5c). Similar to the results obtained from Trx2-TG (Fig.2), Trx2 expression in eNOS-KO mice also led to increased angiogenesis, numbers of dilated vessels (Supplemental Fig.VII) and flow recovery (Fig.5c). However, ischemia-induced angiogenesis and functional flow in eNOS-KO/Trx2-TG mice were still less than those observed in WT and Trx2-TG mice. These data strongly support that both Trx2-increased NO and Trx2-reduced ASK1 apoptotic activity contribute to in vivo angiogenesis.
Fig.5. Both NO and ASK1 pathways contribute to ischemia-induced angiogenesis.
a–b. eNOS deletion had no effects on ischemia-induced ASK1 activation. WT, eNOS-KO and eNOS-KO/Trx2-TG mice were subjected to ischemia ligation, tissues were harvested on day 7. Trx2 expression was determined by Western blot with anti-Trx2. Phospho- and total ASK1 were determined by Western blot with a phospho-specific antibody. Relative ratios of p-ASK1/ASK1 are shown, with untreated WT as 1.0. c. Effects of eNOS deletion on Trx2 augmented ischemia-induced flow recovery. WT, eNOS-KO and eNOS-KO/Trx2-TG mice were subjected to ischemia ligation. blood flow was measured as described in Fig.1. Data from different mice are shown in graphics and n=4 for each strain. *, p<0.05. d. A model for Trx2 function in ischemia-mediated angiogenesis (see text for details). Our previous and current data support that Trx2 maintains EC function by two parallel pathways – scavenging ROS to increase NO bioavailability and inhibiting ASK1 activity to enhance EC survival, leading to enhanced ischemia-mediated arteriogenesis and angiogenesis.
DISCUSSION
The most important finding of this study is that mitochondrial anti-oxidant protein Trx2 plays a critical role in ischemia-mediated arteriogenesis and angiogenesis, leading to an enhanced tissue reparative response. Specifically, EC-specific transgenesis of Trx2 enhances capacity in limb perfusion and ischemic reserve capacity compared to the non-transgenic wild-type mice. Immunohistochemical analyses indicate that Trx2-TG mice had increased ischemia-initiated EC proliferation, neovascularization and vessel maturation. In non-transgenic mice, the Trx2 protein (but not other anti-oxidant proteins such as Trx1, SOD1 or SOD2), is dramatically downregulated in response to ischemia. The reduction of Trx2 protein correlates with concomitant increases in oxidative stress, leukocyte infiltration, ASK1 activation and cellular apoptosis. Interestingly, EC-specific Trx2 transgenesis renders a constitutive level of Trx2 with blunted ischemia-induced oxidative responses. In vitro studies suggest that EC isolated from Trx2-TG mice show increased NO bioavailability and NO-dependent EC migration. These cells are also more resistant to oxidative stress-induced ASK1 signaling and apoptosis in a NO-independent manner. We further express Trx2 in eNOS-deficient mice to demonstrate that both Trx2-increased NO and Trx2-reduced ASK1 apoptotic activity contribute to in vivo angiogenesis. (Fig.5d, proposed model for Trx2 function). Our study provides the first evidence that a mitochondrial anti-oxidant protein plays a critical role in vascular remodeling.
Genetic deletion of Trx1, Trx2 or TrxR2 causes embryonic lethality due to overproduction of ROS 16, 17, 27, suggesting that thioredoxin system is the first defense mechanism against ROS. Therefore, we took a Trx2-transgenesis approach and demonstrate that Trx2-TG specifically expressed in EC strongly reduces ischemia-induced oxidative stress concomitant with increase in NO and decrease of EC apoptosis, leading to enhanced angiogenesis. Our Trx2-TG mice show a very similar phenotype as the EC-specific eNOS transgenic mice (eNOS-TG) in NO bioavailability and vessel function (previous study 19) as well as reparative angiogenesis (present study). Functionally, eNOS-TG and Trx2-TG mice have markedly ischemia-induced collateral vessel formation and increased recovery of blood perfusion in ischemic limbs 28. Mechanistically, both eNOS-TG and Trx2-TG mice show reduced ischemia-induced superoxide production, expression of proinflammatory molecules and leukocyte extravasation into tissue, consistent with the anti-inflammation of NO functions. Although VEGF-mediated signaling was not significantly altered in both Trx2-TG and eNOS-TG compared to wild-type mice, ischemia-induced tissue injuries were dramatically reduced in both Trx2-TG and eNOS-TG. We have further confirmed our studies by expressing Trx2 in eNOS-KO mice. Consistent with previous findings 26, endothelial cell-derived NO plays a critical role in arteriogenesis, pericyte recruitment and stabilization of angiogenic vessels. Trx2 expression in eNOS-KO mice only partially rescues angiogenic defects in eNOS-KO mice, suggesting that Trx2-augmented NO bioactivity contributes significantly to enhanced angiogenesis in Trx2-TG mice.
Anti-apoptotic activity is another important function of Trx2. We have previously demonstrated a critical role of ASK1 in TNF/ROS-induced mitochondria-dependent apoptotic signaling pathway. We showed that ASK1 is localized in the cytosol as well as in mitochondria. Trx2, like Trx1 29, directly binds to ASK1, and forms a complex with ASK1 in mitochondria, inhibiting ASK1 activity 23. Angiogenic factors such as bFGF can induce a translocation of survival protein Raf-1 to EC mitochondria where it also associates with and inhibits ASK1 activity 30. However, our data support that Trx2 functions as an endogenous inhibitor of ASK1 in mitochondria. In supporting this notion, knockdown of Trx2 in EC enhances ASK1 activation and EC apoptosis 23. Conversely, Trx2-TG microvessel EC show more resistance to oxidative stress-induced ASK1 activation and EC apoptosis (this study). Similarly, ischemia-induced downregulation of Trx2 resulted in increased ASK1 activation and apoptotic injury in hindlimb. In contrast, Trx2-TG mice show resistance to ischemia-induced activation of ASK1 and apoptosis. The role of NO in inhibition of ASK1 activity needs further investigation. eNOS deletion in Trx2-TG mice does not eliminate effects of Trx2 inhibition on ASK1 and tissue apoptosis in vivo. It has been shown that NO contributes to anti-apoptosis, in part, by directly nitrosylating and inhibiting proapoptotic proteins including capsases 31, ASK1 32 and Trx1 33. Although it is not known if ASK1 is modified by NO from eNOS in EC, our data suggest that NO does not significantly contribute to Trx2-enhanced anti-apoptotic activity in EC.
The mechanism for Trx2 downregulation by ischemia is not clear, although the regulation of Trx1 by ROS has been extensively investigated 34. It is shown that low doses of reactive oxygen species protect EC from apoptosis by increasing Trx1 expression. This upregulation appears to be at the transcriptional level. However, high levels of ROS can directly induce Trx1 protein degradation in a cathepsin-dependent manner. Cathepsin D is a lysosomal aspartic proteinase and plays an important role in the degradation of proteins and in apoptotic processes induced by oxidative stress and cytokines 34. Our results indicate that Trx2 is regulated by ROS in a different manner as Trx1. This is supported by our data that Trx2 but not Trx1 is downregulated by ischemia. Ischemia appears to regulate Trx2 at a transcriptional level. Further studies are required to define the mechanism for the Trx2 regulation in pathological settings.
In conclusion, our data strongly support that Trx2, by reducing oxidative stress, enhancing NO bioactivity and improving EC survival, plays a critical role in ischemia-mediated arteriogenesis and angiogenesis. Trx2 may be a novel target for the treatment of vascular diseases such as coronary artery disease and peripheral arterial disease.
Supplementary Material
ACKNOWLEDGEMENTS
a. Source of funding:
This work was supported by grants from NIH grants R01 HL-65978-8, R01 HL077357-1 and P01HL070295-6, R01 HL085789-01 and an Established Investigator Award from the American Heart Association (0440172N) and a National Nature Science Foundation of China (30828032) to WM, a National Cancer Institute (NCI) Ruth Kirschstein Predoctoral Fellowship Award (F31 CA 136316) to DJ and a Scientific Development Grant from the American Heart Association (0835544N) to HC.
Footnotes
b. Disclosure: none.
REFERENCES
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 4.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 5.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 6.Keaney JF., Jr Oxidative stress and the vascular wall: NADPH oxidases take center stage. Circulation. 2005;112:2585–2588. doi: 10.1161/CIRCULATIONAHA.105.578146. [DOI] [PubMed] [Google Scholar]
- 7.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran A, Levonen AL, Brookes PS, Ceaser E, Shiva S, Barone MC, Darley-Usmar V. Mitochondria, nitric oxide, and cardiovascular dysfunction. Free Radic Biol Med. 2002;33:1465–1474. doi: 10.1016/s0891-5849(02)01142-5. [DOI] [PubMed] [Google Scholar]
- 9.Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med. 1999;31:53–59. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- 10.Cai H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovascular Research. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, Aplin AE, Tai YT, Aguirre-Ghiso J, Flores SC, Melendez JA. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 12.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 14.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 15.Chang TS, Cho CS, Park S, Yu S, Kang SW, Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J Biol Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 16.Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad M, Jakupoglu C, Moreno SG, Lippl S, Banjac A, Schneider M, Beck H, Hatzopoulos AK, Just U, Sinowatz F, Schmahl W, Chien KR, Wurst W, Bornkamm GW, Brielmeier M. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka T, Hosoi F, Yamaguchi-Iwai Y, Nakamura H, Masutani H, Ueda S, Nishiyama A, Takeda S, Wada H, Spyrou G, Yodoi J. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. Embo J. 2002;21:1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Luo Y, Zhang W, He Y, Dai S, Zhang R, Huang Y, Bernatchez P, Giordano FJ, Shadel G, Sessa WC, Min W. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am J Pathol. 2007;170:1108–1120. doi: 10.2353/ajpath.2007.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, Luo Y, Tang S, Rajantie I, Salven P, Heil M, Zhang R, Luo D, Li X, Chi H, Yu J, Carmeliet P, Schaper W, Sinusas AJ, Sessa WC, Alitalo K, Min W. Critical function of Bmx/Etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest. 2006;116:2344–2355. doi: 10.1172/JCI28123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo D, Luo Y, He Y, Zhang H, Zhang R, Li X, Dobrucki WL, Sinusas AJ, Sessa WC, Min W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol. 2006;169:1886–1898. doi: 10.2353/ajpath.2006.060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo D, He Y, Zhang H, Yu L, Chen H, Xu Z, Tang S, Urano F, Min W. AIP1 is critical in transducing IRE1-mediated endoplasmic reticulum stress response. J Biol Chem. 2008 doi: 10.1074/jbc.M710557200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R, Al-Lamki R, Bai L, Streb JW, Miano JM, Bradley J, Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 24.Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest. 2005;115:1816–1827. doi: 10.1172/JCI24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pober JS, Min W. Endothelial cell dysfunction, injury and death. Handb Exp Pharmacol. 2006:135–156. doi: 10.1007/3-540-36028-x_5. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol. 1996;178:179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 28.Ozaki M, Kawashima S, Hirase T, Yamashita T, Namiki M, Inoue N, Hirata Ki K, Yokoyama M. Overexpression of endothelial nitric oxide synthase in endothelial cells is protective against ischemia-reperfusion injury in mouse skeletal muscle. Am J Pathol. 2002;160:1335–1344. doi: 10.1016/s0002-9440(10)62560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Cir. Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 30.Alavi AS, Acevedo L, Min W, Cheresh DA. Chemoresistance of endothelial cells induced by basic fibroblast growth factor depends on Raf-1-mediated inhibition of the proapoptotic kinase, ASK1. Cancer Res. 2007;67:2766–2772. doi: 10.1158/0008-5472.CAN-06-3648. [DOI] [PubMed] [Google Scholar]
- 31.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 32.Park HS, Yu JW, Cho JH, Kim MS, Huh SH, Ryoo K, Choi EJ. Inhibition of apoptosis signal-regulating kinase 1 by nitric oxide through a thiol redox mechanism. J Biol Chem. 2004;279:7584–7590. doi: 10.1074/jbc.M304183200. [DOI] [PubMed] [Google Scholar]
- 33.Haendeler J, Hoffmann J, Tischler V, Berk BC, Zeiher AM, Dimmeler S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat Cell Biol. 2002;16:16. doi: 10.1038/ncb851. [DOI] [PubMed] [Google Scholar]
- 34.Haendeler J. Thioredoxin-1 and posttranslational modifications. Antioxid Redox Signal. 2006;8:1723–1728. doi: 10.1089/ars.2006.8.1723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.