Abstract
Reciprocal chromosomal translocations at the MLL gene locus result in expression of novel fusion proteins such as MLL-ENL associated with leukemia. The three PHD finger cassette, one of the highly conserved domains in MLL, is absent in all fusion proteins. This domain has been shown to interact with Cyp33, a cyclophilin which enhances the recruitment of HDACs to the MLL repression domain and mediates HOX gene repression. Insertion of the third PHD finger of MLL, into MLL-ENL allows the recruitment of Cyp33, and subsequently HDAC1, to the fusion protein. Furthermore, expression of the fusion protein with the PHD finger insertion mediates the down-regulation of the HOXC8 gene expression in a Cyp33 dependent manner. Finally, the addition of the PHD finger domain or the 3rd PHD finger alone, into MLL-ENL, blocks the hematopoietic-stem-cell immortalization potential of the fusion protein in serial plating colony assays. Insertion of only the 1st and 2nd PHD fingers has no such effect. These data support the hypothesis that the binding of Cyp33 to the MLL 3rd PHD finger switches the MLL function from trans-activation to repression. In the immortalizing MLL fusion protein, the loss of the PHD fingers, in combination with the gain of the activation domain of ENL, or of other partner proteins, makes the fusion protein a constitutive trans-activator. This leads to constitutive over expression of MLL target genes that block stem cell commitment and promote stem cell renewal, probably the first step in MLL-related leukemogenesis.
Keywords: MLL, PHD finger, Cyp33, ENL, HDAC1, Hox, repression, myeloid differentiation, cyclophilin, stem cell, immortalization, progenitor cell
Introduction
The MLL (HTRX, HRX, ALL-1) gene is frequently rearranged in infant acute leukemia and both de novo, and therapy-related, leukemia in adults. Reciprocal translocations with a breakpoint at chromosomal band 11q23 generate various MLL fusion genes, encoding chimeric proteins in which MLL contributes its N-terminal DNA binding domains and its repression domain. In the translocations, MLL loses the sequences encoding its highly conserved PHD finger domain, activation domain, and SET domain, and fuses in frame with one of up to 40 different partner genes (1). The MLL fusion proteins are postulated to disrupt normal gene expression patterns maintained by wild type MLL, which contributes to malignancy.
MLL-ENL is a fusion protein encoded by the fusion gene generated by the translocation t(11;19)(q23;p13.3) (2). The fusion partner, ENL, has been shown to be a transcriptional activator in human lymphoid and myeloid cells (3). Transduction with retroviral MLL-ENL expression constructs immortalizes murine hematopoietic progenitor cells in vitro, and mice adoptively transferred with these immortalized cells develop myeloid leukemia (4, 5). The immortalization depends on the abnormal activation of Hoxa7, Hoxa9, Meis1 and c-Myc (6–8).
Soon after translation MLL is proteolytically split in two fragments, MLL-N and MLL-C, by the taspase enzyme (9, 10). MLL-N contains the DNA and chromatin targeting domains of MLL: the three AT hook motifs, several nuclear localization domains, and a repression domain (11) which recruits repression complexes both dependent and independent of histone deacetylase activity (12). MLL-C contains a CBP-binding activation domain, and the SET domain (13), which has histone methyltransferase activity with specificity for tri-methylation of lysine 4 of histone H3 (14). The two MLL fragments associate in complexes that include other proteins. The presence of both transcriptional repression and activation potentials within the same protein suggests that the normal MLL may switch between these two opposite functions in response to cellular signals.
The PHD finger cassette is one of the highly conserved domains in MLL and is located between the repression domain and the activation domain. PHD fingers, represented by a highly conserved Cys4HisCys3 zinc finger motif, have been found in greater than 300 eukaryotic nuclear proteins and are involved in protein-protein interaction and chromatin mediated transcriptional regulation (15) (16).
We have previously shown that the third PHD finger of MLL interacts with Cyp33/PPIE (17, 18), an RNA binding nuclear cyclophilin (19). Cyp33 enhances the recruitment of HDAC1 to the MLL repression domain and down-regulates expression of the HOXC8 gene in an MLL dependent manner(12, 17). This indicates that Cyp33 may contribute to the intrinsic repression potential of MLL. The MLL repression domain, Cyp33, and the PHD finger domain form a repression unit that contributes to the repression potential of MLL-N and balances the activation potential of MLL-C. In MLL-ENL, the loss of the PHD fingers disrupts this repression unit. Therefore, the intrinsic repression potential is compromised, and in the presence of an activation domain on the partner protein, the MLL-ENL fusion functions as a dominant constitutive activator of MLL target HOX genes.
To test the above hypothesis and also to investigate the function of the PHD finger domain in the context of an MLL fusion protein, the DNA sequences encoding either the third PHD finger or the first three PHD fingers of MLL were re-inserted into MLL-ENL. Then we tested the functionality of the restored “repression domain-Cyp33-PHD finger” repression unit, and the cellular effects (HOX gene regulation and progenitor cell immortalization) of expressing this modified fusion protein in human cell lines and mouse primary hematopoietic progenitor stem cells.
Materials & Methods
Constructs
The ePHDf3 (aa1531–1636) or PHDf1–3 (aa1405–1636) were amplified by PCR using full length MLL cDNA (pcDNA3-MLL-F from M. Seto) as the template, the common reverse primer R 5’-GCTAGCAAGGGCCAGTCGCCACTCTG-3’ with forward primers 5’-AATGTCGACGTTCGCTGTAAGAGCTGTGGA-3’ and 5’-AATGTCGACGTTCGCTGTAAGAGCTGTGGA-3’. ePHDf3 and PHDf1–3 PCR products were then subcloned into pGEMT vector and recovered as SalI-ePHDf3-NheI or SalI-PHDf1–3-NheI fragment. Both fragments were then inserted into SalI/XbaI sites after MLL exons 1–8 of the MSCVneo-MLL-ENL construct to generate the ePHDf3 insertion mutant (MSCVneo-MLL-ePHDf3-ENL) and the PHDf1–3 insertion mutant (MSCVneo-MLL-PHDf1–3-ENL) respectively. The MSCVneo-MLL-PHDf1–2-ENL construct was prepared with a reverse primer at the end of MLL exon 14.
To generate MSCVneo-MLL-ΔRD2-ePHDf3-ENL, the ePHDf3 sequence was amplified using the common reverse primer R and forward primer 5’-GAGTCGACTTTAAGGAGGATTGT-3’. The ePHDf3 sequence was then subcloned into the pGEMT vector and recovered as XbaI-ePHDf3-NheI fragment. This fragment was inserted into the XbaI site in MLL1–4.
All of the fusion proteins were tagged with a FLAG peptide sequence (DYKDDDDK) at the N-termini by exchanging the EcoRI/BglII fragment (cut and recovered from pcDNA3-MLL-F and containing FLAG-tagged 5’MLL sequence) with the 5’ sequence of the cDNA of all fusion proteins in the MSCVneo vector. The cDNAs of all fusion proteins were also subcloned into pCMV2 for transient expression.
Cell culture
HEK293 cells and ecotropic Phoenix cells were cultured at 37 °C, 5% CO2 in Dulbecco’s Modified Eagle’s medium (DMEM) with 10% fetal calf serum, 1% penicillin /streptomycin, and 2mM L-glutamine (Invitrogen).
FLAG-Cyp33 inducible HEK293 cells were maintained in the above DMEM medium supplemented with 200µg/ml Hygromycin (Calbiochem) and 10µg/ml Blasticidin (Invitrogen). To induce FLAG-Cyp33 expression, 1µg/ml tetracycline (Sigma) was added to the culture.
Coimmunoprecipitation & Western blot analysis
293 cells were transiently transfected using GenPorter (GST Inc) with 8µg pCMV-Flag-fusions and 4µg pHA-Cyp33 per 10 cm dish. 48 hrs post transfection, cell lysates were prepared in 1ml RIPA buffer (20) and subjected to the binding assay. 40–60µl anti-FLAG monoclonal antibody gel (Sigma) was used to pull down the fusion proteins according to the supplier’s instructions. The bound proteins were then separated by 10% SDS-PAGE gel for Cyp33 and HDAC1 detection or 5% SDS-PAGE gel for MLL fusion protein detection, transferred to immobilon-P membrane (Millipore) and subjected to western blot analysis with anti-HA polyclonal antibody (Sigma). The membranes were then re-blotted with anti-HDAC1 antibody (Santa Cruz). The immunoreactive bands were revealed using the ECL kit (Amersham) according to the manufacturer’s instructions.
Induction of Cyp33 & Enrichment of CD2+ cells
HEK293 cells were cotransfected using GenPorter reagent (GST Inc.) with 8µg pCMV-Flag-fusion constructs DNA and 4µg hCD2-pcDNA3 (a kind gift from Dr David Ron at New York University School of Medicine) per 10 cm dish. 24 hrs post transfection, 1µg/ml tetracycline (Sigma) was added to the Cyp33 induced group. 60 hrs post transfection, all samples were collected by mild trypsinization and the trypsin-EDTA (Invitrogen) was inactivated by added fresh medium. After the preparation of single cell suspension at a density of 107 cells per 100µl buffer (phosphate buffered saline PBS pH 7.2, supplemented with 0.5% BSA and 2mM EDTA and stored at 4 °C), the samples were subjected to magnetic labeling according to the manufacturer’s instruction (MACS).
Briefly, 10µl CD2-PE (monoclonal anti-human CD2 antibody conjugated to PE, MACS) was added per 107 cells and the mixture was incubated for 10 minutes in the dark at 4°C. Then cells were washed to remove unbound primary antibody by adding 1 ml buffer per 107 cells and centrifuged at 300g for 10 min. Cell pellets were resuspended in 80µl buffer per 107 cells. 20µl anti-PE MicroBeads (MACS) were added per 107 cells and the mixture was incubated for 15 min at 4°C. The washing step was repeated again and cells were resuspended in 500µl cold buffer. All samples were then subjected to magnetic separation by running through MS column (MACS) according to the manufacturer’s manual and CD2+ cells were collected and visualized by fluorescent microscopy. The majority of the cells (>70%) enriched by the magnetic column were CD2+. mRNA was extracted from one third of the total CD2+ cells for each sample and semi-quantitative RT-PCR was later performed to detect the endogenous HOXC8 expression level. The rest of the cells were then used for western blot analysis to detect the expression of FLAG-tagged Cyp33 and the fusion proteins using anti-FLAG monoclonal antibody (Sigma).
Semi-quantitative RT-PCR
RT-PCR was performed on all the samples as described in RT-PCR & PCR. PCR products were collected after 26, 28, 30 cycles for HOXC8 and after 16, 18, 20 cycles for GAPDH control. The PCR primers for HOXC8 and GAPDH are listed below:
HOXC8 forward primer: 5’-CCGCCAACACTAACAGTAGC-3’
HOXC8 reverse primer: 5’-CAGTCCCAGGGCATGAGAG-3’
GAPDH forward primer: 5’-GAAGGTGAAGGTCGGAGTC-3’
GAPDH reverse primer: 5’-GAAGATGGTGATGGGATTTC-3’
After electrophoresis and photography, the band intensity of HOXC8 and GAPDH was visualized with Kodak 1D Image Analysis Software and quantified using the region of interest (ROI) intensity measurement option. The band net intensity of HOXC8 (at cycle 28) was normalized to that of GAPDH (at cycle 18) to get HOXC8/GAPDH ratio for each sample. The relative level of HOXC8 mRNA in the sample was then calculated by comparing the HOXC8/GAPDH ratio of that sample to the ratio of the control i.e. Flag-Cyp33 uninduced 293 cells transfected with empty vector. Statistical significance was determined using the student T-test, set at a confidence level of 95%.
RT-PCR & PCR
1µg of total RNA, prepared using Trizol reagent (Invitrogen), was treated with DNase I (Invitrogen) and then reverse transcribed following Superscript First-strand synthesis instruction manual (Invitrogen) using oligo dT primer. PCR amplifications were performed with 2µl of cDNA in a 50µl reaction volume for appropriate cycle numbers. The prototype of the amplification cycle was (94°C for 1’, 50 to 65°C for 45”, 72°C for 45”) using the appropriate primers.
To detect the mRNA levels of MLL-ENL & its derivatives (nt3529/1289–1414), the forward primer for MLL-ΔRD2-ENL, MLL-ΔRD2-ePHDf3-ENL or for MLL-ENL, MLL-ePHDf3-ENL, and MLL-PHDf1–3-ENL is 5’-CCCAAGTTTGGTGGTCGCAATA and 5’-GGACTTTAAGGAGGATTG, respectively. The reverse primer is 5’-GCTAGCAAGGGCCAGTCGCCACTCTG.
The amplified products were separated on agarose gel of appropriate percentage and visualized under UV light.
Retroviral packaging
Ecotropic Phoenix packaging cells (ATCC) were transfected with retroviral vectors using GenePorter (GST Inc) according to the manufacturer’s instructions. 32 hrs post transfection, cells were incubated at 32 °C to generate virus particles. Supernatants were collected 72 hrs post transfection, filtered and used to infect the enriched hematopoietic progenitor cells. Viral titers were measured in NIH3T3 cells and determined by the number of neomycin resistant colonies after infection with retroviral stocks and 800µg/ml G418 selection (Invitrogen) for 9 days as described previously (4).
Enrichment and infection of murine hematopoietic progenitors
The enrichment and infection of murine hematopoietic progenitors and subsequent colony assay were performed as previously described (21).
Results
Insertion of the PHD finger sequence into the MLL-ENL fusion protein
MLL possesses four distinctive types of PHD finger conserved among different species (17). The third PHD finger binds Cyp33 (17, 18). Since the N terminal extension sequence of that PHD finger contains multiple cysteines that may influence the correct folding of the motif, we included this 5’-extension sequence as part of the third PHD finger domain (ePHDf3, aa1531–1636). The ePHDf3 encoding sequence was then inserted in frame into MLL-ENL to form the MLL-ENL insertion mutant (MLL-ePHDf3-ENL). We also inserted the DNA sequences encoding the first three PHD fingers (aa1405–1636) into MLL-ENL to form MLL-PHDf1–3-ENL (Figure 1).
Figure 1. Structures of MLL, MLL-ENL and its derivatives with or without PHD finger insertion.
Structure of the full length MLL and fusion proteins, highlighting the domains related to this study. The third PHD finger with the 5’- extension sequence is shown as ePHDf3. MLL-ENL contains 5’ MLL sequence (aa1-1444) and the C-terminal part of ENL (aa430-560). MLL ePHDf3 (aa1531-1636) or PHDf1-3 (aa1405-1636) sequence was inserted between 5’MLL and ENL to generate MLL-ePHDf3-ENL and MLL-PHDf1-3-ENL respectively. The ePHD finger (ePHDf3) sequence was also inserted into MLL-ΔRD2-ENL (an MLL-ENL mutant with a deletion of the repression domain from aa1213 to 1444 that includes part of RD1 and the complete RD2 domain sequence) to generate MLL-ΔRD2-ePHDf3-ENL. The construct expressing MLL AT-hook repeats (aa1-667, shown as MLL-AT hook), or empty vector were used as controls in the experiments performed in this study. All fusion proteins were tagged with the FLAG sequence for easy detection of the protein.
MLL-ENL with a PHD finger 3 insertion recruits both Cyp33 and HDAC1
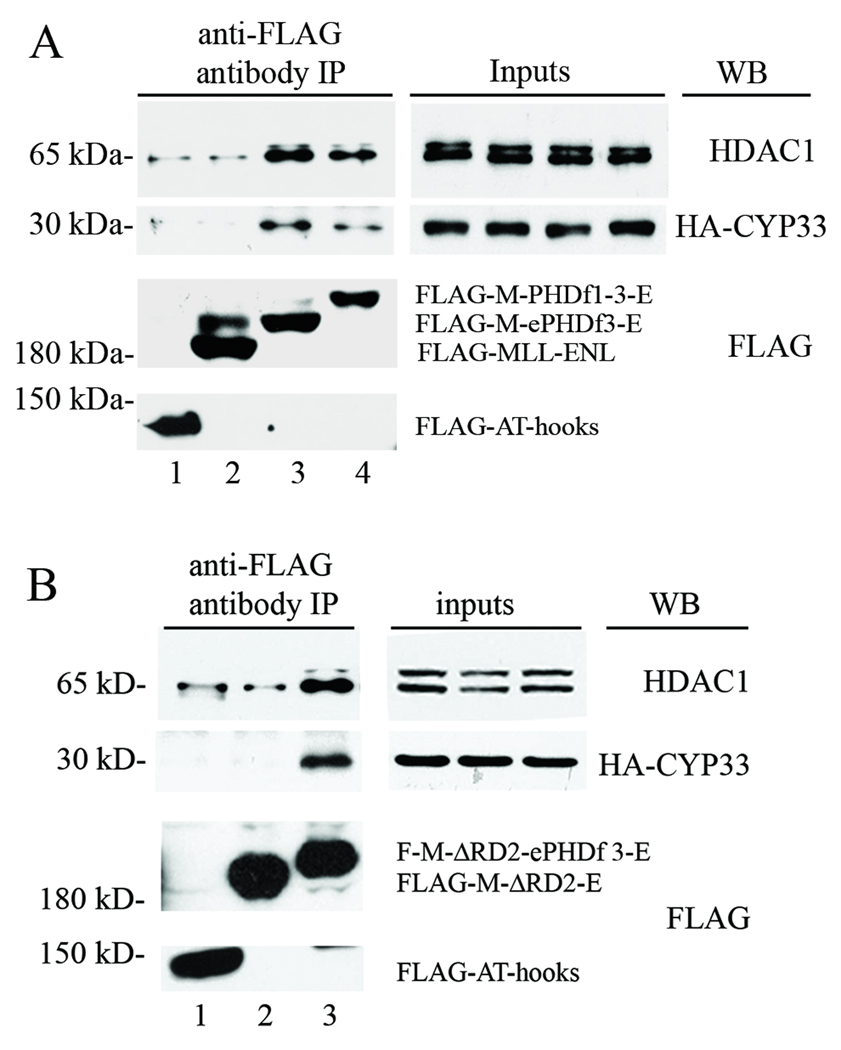
To determine if the insertion of the PHD finger sequence into the MLL-ENL fusion protein restores the interaction between the fusion protein and Cyp33, FLAG-tagged MLL-ePHDf3-ENL or FLAG-tagged MLL-PHDf1–3-ENL expression vectors were co-transfected with an HA-tagged Cyp33 expression construct into 293 cells. The fusion proteins were then precipitated with anti-FLAG antibody and the bound Cyp33 was detected by anti-HA antibody.
All the fusion proteins which included either ePHDf3 (Figure 2A, lane3) or PHDf1–3 (Figure 2A, lane4), were able to co-precipitate Cyp33 whereas their PHD finger-lacking counterpart, MLL-ENL could not (Figure 2A, lane2). A construct expressing a FLAG-tagged 5’ MLL fragment that contains N-terminal sequences including the AT-hooks, and nuclear localization signals but no PHD finger 3, was used as a negative control (Figure 2A, lane1). These data suggest that the insertion of the PHD finger sequence into MLL-ENL allows the latter to interact with Cyp33 similar to wild type MLL.
Figure 2. Insertion of the PHD fingers allows the recruitment of Cyp33 & HDAC1.
All fusion protein constructs in the pCMV2 vector were cotransfected with HA tagged Cyp33 construct into 293 cells. The fusion proteins were immunoprecipitated with anti-FLAG antibody and any bound HA-Cyp33 or endogenous HDAC1 was detected by western blot analysis using anti-HA antibody and anti-HDAC1 antibody respectively. 1% (v/v) whole cell lysate for each sample was used as input. The MLL-AT-hook construct was the negative control. MLL-ENL and MLL-ENL with the ePHDf3 or with PHDf1-3 were tested in (A) and MLL-ENL without the RD2 domain and its ePHDf3 insertion mutant were examined in (B). The data shown here is representative of the results in three independent experiments.
Because Cyp33 can enhance HDAC1 recruitment by the repression domain of MLL (15), insertion of the PHD finger into MLL-ENL may enhance HDAC1 binding to the fusion protein. To test this possibility, the level of endogenous HDAC1 bound to the fusion proteins was also determined. The results showed that MLL-ePHDf3-ENL or MLL-PHDf1–3-ENL co-precipitate more HDAC1 than MLL-ENL (Figure 2A, lane3, 4 versus lane 2). The 5’-MLL fragment also co-precipitated a small amount of HDAC1 (Figure 2A, lane1) similar to that co-precipitated by a FLAG-GFP protein (data not shown). It is noticeable that even though two forms of HDAC1 with different mobility were found in the input, the slower form was preferentially co-precipitated.
The MLL RD2 domain is not required for the recruitment of HDAC1 to MLL-ENL with PHD finger insertion
The repression domain of MLL can be split into two sub-domains, RD1 and RD2, each one of which can mediate repression of reporter genes (11). Both of these domains recruit HDACs (12). To investigate the role that the MLL RD1 and RD2 domains played in the above interactions, the RD2 domain was deleted from MLL-ENL to generate MLL-ΔRD2-ENL. The ePHDf3 was then introduced into this RD2-deleted MLL-ENL to form MLL-ΔRD2-ePHDf3-ENL (Figure 1).
As expected, MLL-ΔRD2-ENL did not interact with Cyp33, and could not recruit more HDAC1 than the background level (Figure 2B, lane2), but the insertion of ePHDf3 restored the interaction (Figure 2B, lane3). Addition of the ePHDf3 into MLL-ΔRD2-ENL enhanced HDAC1 recruitment (Figure 2B, lane3), suggesting that Cyp33 may facilitate the binding of HDAC1 to RD1 as well as to the complete RD1 + RD2 repression domain.
The PHD finger domain when inserted into MLL-ENL allows Cyp33-mediated HOXC8 gene repression in human cells
The next question we addressed was if the insertion of the PHD finger sequences into MLL-ENL has any effects on the regulation of a target gene of these fusion proteins. MLL fusion proteins have been proposed to act as transcription activators that constitutively activate MLL-target genes. This idea has been supported by the fact that HOX genes are abnormally up-regulated in primary leukemia cells carrying MLL translocations (22) (23, 24), in a leukemia cell line harboring MLL fusion proteins and in MLL-ENL transduced mouse bone marrow progenitor cells (7). The HOXC8 gene is one of the genes that have been thoroughly characterized as a direct target of MLL (14,25), MLL-AF9 directly activates the HOXC8 promoter in an in vitro reporter gene assay (14). We expected MLL-ENL to have the same effect as MLL-AF9 on the HOXC8 gene. MLL and Cyp33 bind the HOXC8 promoter in HEK293 cells (Supplementary Figure 1), and Cyp33 overexpression results in reduced acetylation of histones H3 and H4 at this promoter (Supplementary Figure 2).
High levels of Cyp33 down regulate HOXC8 gene expression in cells expressing only wild type MLL, but not in cells that express MLL-fusion proteins (17) indicating that Cyp33 may regulate HOXC8 gene expression indirectly through its interaction with MLL. The MLL-ENL protein with the PHD finger sequence insertion may recruit Cyp33 to the HOXC8 gene locus and restore this second layer of regulation.
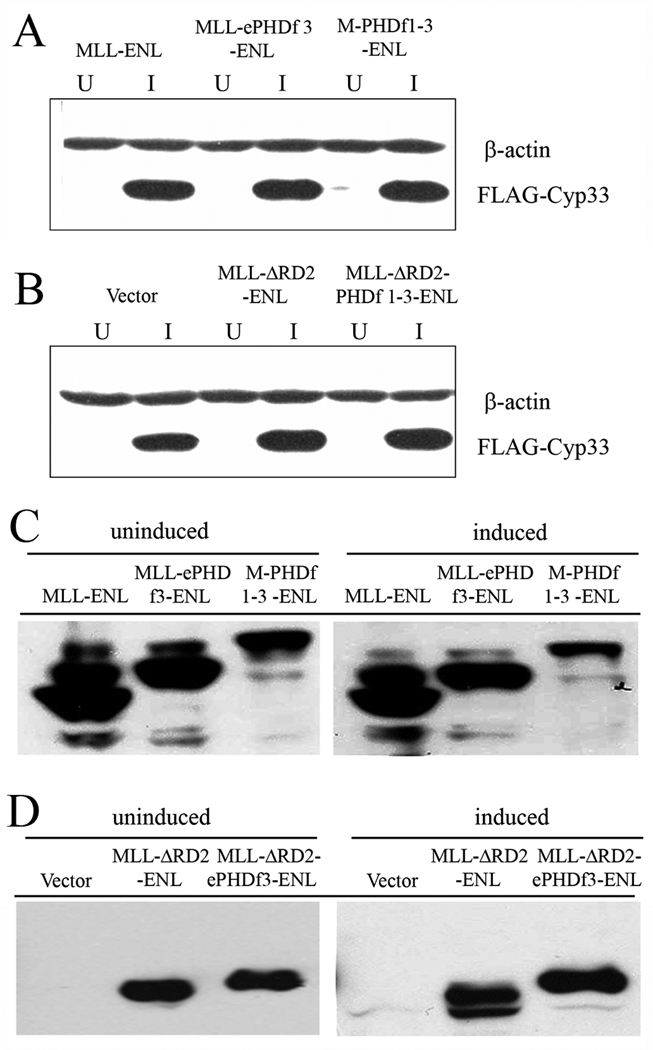
To test the effect of the different MLL-ENL constructs described above, on HOXC8 expression we used a Cyp33 inducible system in the HEK293 cell line. Different pCMV2 plasmid constructs expressing each one of the fusion proteins were introduced into the system and the expression of FLAG-Cyp33 (Figure 3 A and B) and of the FLAG-MLL-fusion proteins (Figure 3 C and D) was confirmed by western blot; the HOXC8 mRNA levels were compared in samples with or without Cyp33 induction, by RT-PCR (Figure 4). When Cyp33 is induced in HEK293 cells, the HOXC8 gene is down regulated as previously described for leukemia cells with wild type MLL(17). To minimize the variation in transfection efficiency in different experiments, a plasmid expressing a truncated human CD2 cell surface marker was co-transfected with the fusion protein constructs into Cyp33-induced or un-induced HEK293 cells. CD2+ cells, representing the cells that were transfected, were enriched by immunoselection using antibody conjugated magnetic beads. Twenty-four hours post co-transfection Cyp33 over-expression was induced by tetracycline for 36 hrs before the samples were harvested. In parallel, Cyp33-uninduced samples were collected 60 hrs after co-transfection.
Figure 3. Expression of Flag-Cyp33 and fusion proteins in tetracycline treated or untreated 293 cells.
Western blot analysis was performed on enriched CD2+ 293 cells transfected with fusion protein constructs. The induced Cyp33 (A and B) or the fusion protein (C and D), were detected using anti-flag antibody. β-actin was used as a loading control. U, uninduced; I, induced
Figure 4. Modulation of the HOXC8 gene expression by the fusion proteins with or without Cyp33 induction.
Primers specific to HOXC8 transcripts were used to amplify HOXC8 mRNA from different cell samples, and GAPDH was used as a loading control. A and C: Representative gel images of ethidium bromide stained gels after electrophoretic separation of the RT-PCR products (MLL and ENL are sometimes abbreviated as M and E respectively). (B and D) The relative expression level of HOXC8 in Cyp33 uninduced or induced 293 cells over expressing various fusion proteins as determined by RT-PCR and estimation of the PCR product amount from image analysis. The values are the mean of the samples in three independent experiments. The error bars represent standard deviations. All samples are compared to empty vector-transfected Cyp33-uninduced 293 cells.
Thirty six hours post addition of tetracycline, Cyp33 was expressed at high levels (Figure 3) and the HOXC8 gene was down regulated (Figure 4). Both un-induced and induced HEK293 cells expressed comparable levels of the fusion proteins and Flag-tagged Cyp33 was induced at similar levels in tetracycline treated HEK293 cells (Figure 3).
The HOXC8 mRNA level in all samples was measured using semi-quantitative RT-PCR. Different numbers of PCR cycles were used to insure that the signal was not saturated. The band intensity (at cycle 28 for HOXC8 signal) was quantified and normalized to that of the GAPDH control (at 18 cycles). All samples were then compared to the empty vector-transfected, Cyp33-uninduced, HEK293 cells.
In the un-induced HEK293 cells, the expression of all fusion proteins had little effect on the endogenous HOXC8 gene expression (Figure 4 A and B). MLL-ENL did not upregulate HOXC8 in the absence of Cyp33 and this result is consistent with what has been found in a MLL-ENL immortalized murine cell line(7). Upon induction of Cyp33 in the presence of the fusion protein without PHD finger insertion, a relatively high HOXC8 mRNA level equivalent to that in the un-induced 293 cells was observed (Figure 4 A and B). On the other hand, over expression of Cyp33, in the presence of the fusion proteins with PHD fingers resulted in down regulation of the HOXC8 mRNA level (Figure 4 A and B).
The RD2 domain of MLL is not necessary for repression of HOXC8 by Cyp33
We tested if the deletion of RD2 impairs the regulation of HOXC8 by Cyp33. Expression of the RD2 deleted MLL-ENL fusion protein did suppress the Cyp33-dependent down regulation of HOXC8 in HEK-293 cells. Expression of the RD2 deleted fusion protein with the included 3rd PHD finger instead did support repression of the HOXC8 gene after Cyp33 over-expression (Figure 4 C and D).
The insertion of PHD fingers into MLL-ENL suppresses MLL-ENL-induced immortalization of murine bone marrow progenitor cells
Reestablishment of the Cyp33-MLL interaction in the MLL fusion protein by introduction of the PHD fingers may antagonize the hematopoietic stem cell immortalization potential of the fusion protein. To test this hypothesis, we used the methylcellulose colony-forming assay (MCF assay) to evaluate the mouse hematopoietic progenitor immortalization potential of various MLL-ENL fusion proteins with or without PHD finger insertion.
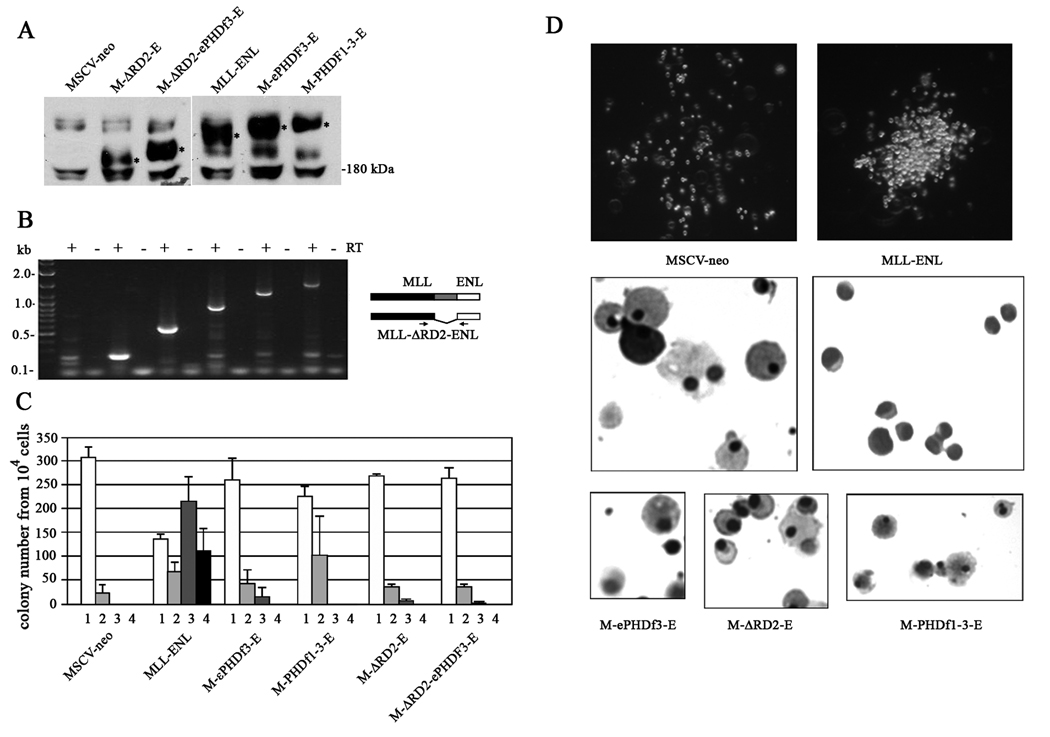
Each one of the fusion protein-coding genes with or without PHD and RD domain mutations (expressed from MSCVneo vectors) was introduced into enriched murine bone marrow progenitor cells by retroviral transduction. The expression level of these fusion proteins was monitored by western blot in the transiently transfected ecotropic packaging Phoenix cells (Figure 5 A). The re-plating potential of the infected progenitor cells was then evaluated by serial methylcellulose colony-forming assays. Figure 5 B shows the detection of the mRNA from various fusion genes by RT-PCR in the neomycin resistant bone marrow progenitor cells after the primary plating.
Figure 5. Serial colony assays of mouse bone marrow progenitor cells (BMPCs) transduced with retroviral constructs.
A: Expression of various fusion protein constructs (in MSCVneo vector) at the protein level in transiently transfected Phoenix cells detected by IP-western blot. B: Expression of the various fusion genes in the MSCVneo vector (in the same order as in A) at the mRNA level, detected by RT-PCR (+ RT) in BMPC after primary plating on methylcellulose medium supplemented with IL-3, IL-6, GM-CSF, SCF and G418. The primers used in the PCR reaction amplify the junction region between 5’ MLL and the ENL portion as shown in the diagram. C: Number of primary, secondary, tertiary and quaternary colonies generated per 104 plated cells following methylcellulose culture of BMPCs transduced with MLL-ENL and its PHD finger insertion mutants. The values are the mean of three independent assays and the error bars represent standard deviations. D: The colonies that appeared on the methylcellulose plates after the tertiary plating were photographed. Cells collected after the tertiary plating were prepared by cytospinnning, and stained with Wright-Giemsa (Hema3 Stain Set, Fisher Scientific).
Expression of the MLL-ENL fusion protein, as previously shown (4, 5), extended the proliferation potential of the progenitor cells and the cells continued forming colonies after the third serial plating (Figure 5 C). The MLL-ENL-infected cells formed big and compact colonies (Figure 5C) and maintained an immature phenotype (Figure 5 D). In contrast, empty vector infected cells, stopped proliferating after the second serial plating and generated small and dispersed cell clusters on the plate, indicating these cells are undergoing myeloid differentiation and become quiescent (Figure 5 D).
When the ePHDf3 or PHDf1–3 were inserted into MLL-ENL, the modified proteins could no longer immortalize murine bone marrow progenitor cells as indicated by the absence of colonies after the third or fourth serial plating; the few colonies formed at the tertiary plating were similar to the cell clusters formed by empty vector infected cells (Figure 5 D). In similar experiments in which the MLL PHD fingers 1 and 2 only, were inserted into the MLL-ENL fusion protein the re-plating potential was conserved through the third plating with average colony counts of 186 (sd = 64) in the third plating in four independent experiments; these colonies were of the same type observed in MLL-ENL transduced cells.
An MLL-ENL fusion protein without the RD2 domain loses the ability to immortalize hematopoietic progenitors
When we used a MLL-ENL fusion gene without the RD2 domain, expressed from a retroviral vector to transduce mouse bone marrow cells, and tested these in a serial plating colony essay, the cells behaved as wild type mouse cells, extinguishing their proliferation potential after two platings. This was independent of the inclusion or not of the 3rd PHD finger coding sequences in the construct. So, the RD2 domain is essential for HSC immortalization.
Discussion
The MLL fusion proteins associated with leukemia conserve the N-terminal sequences of MLL, and lose the C-terminal sequences including the highly conserved PHD- fingers, and SET domain, and an activation domain that interacts with the histone acetyltransferase CBP (1, 26). These C-terminal sequences are replaced by sequences from the partner protein that, in the case of ENL, as well as other partner proteins, has a transcription activation domain.
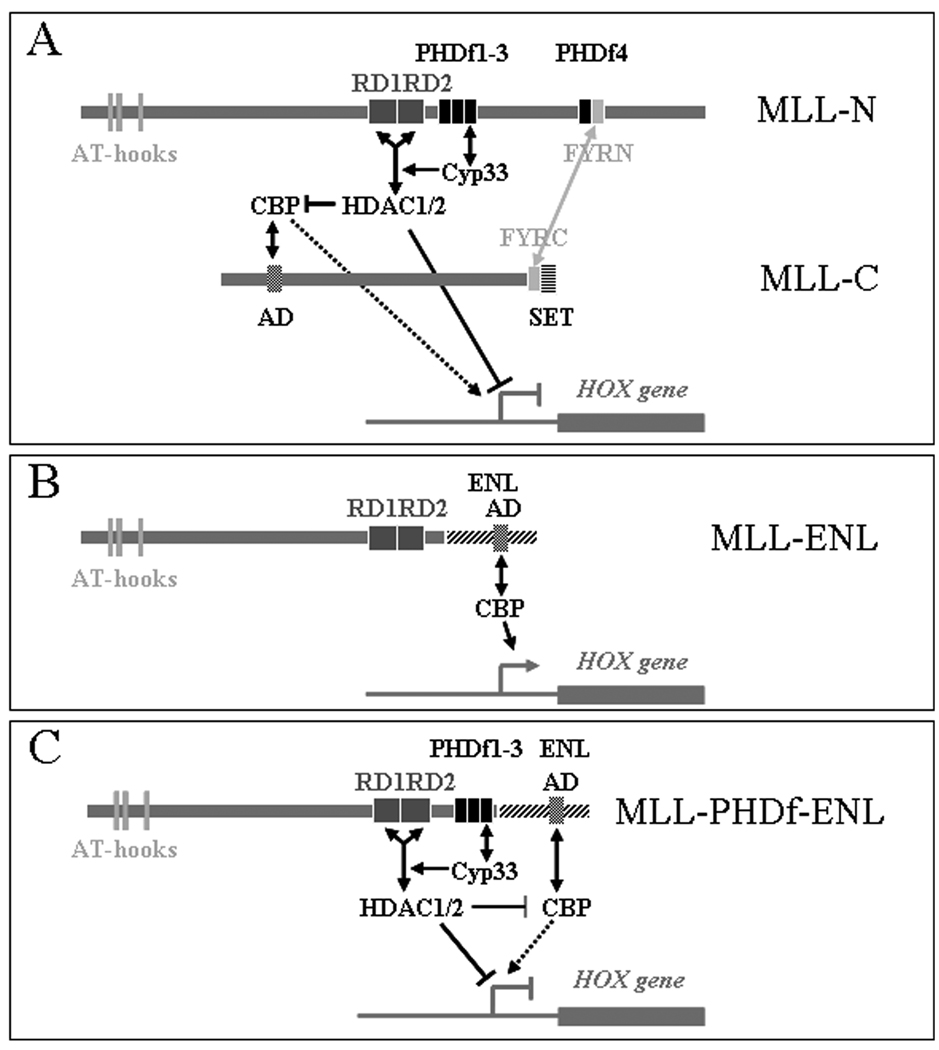
We have identified Cyp33 as a ligand of the third PHD finger of MLL (17). Upon binding to MLL, Cyp33 mediates a switch from transcriptional activation to repression. This switch to repression is mediated in part by an increased recruitment of histone deacetylases to the repression domain of MLL. Concordantly, the HDAC inhibitor trichostatin A inhibits the switching to repression function (12). The HDAC activity may directly antagonize histone acetylation by CBP, but it may also inhibit the CBP enzymatic activity that is dependent on autoacetylation (27) (Figure 6A). We postulate that loss of the third PHD finger of MLL from the MLL-fusion proteins associated with leukemia eliminates this switch to repression, converting the MLL fusion protein into a constitutive activator (Figure 6B).
Figure 6. Diagram of a hypothetical model of HOX gene regulation by MLL and MLL-fusion proteins.
A: Wild type MLL is proteolytically cleaved and the two fragments associate within a larger protein complex through interaction of their FYRN and FYRC domains. Cyp33 binding to the 3rd PHD finger enhances the recruitment of HDAC1 and 2, resulting in inhibition of the MLL-associated CBP histone acetyltransferase activity and in histone deacetylation at the promoter of the target HOX genes. Depending on the binding of Cyp33, wild type MLL can switch between activation and repressing functions. B: The MLL-ENL fusion protein which has an activation domain on the ENL component that may also bind CBP, lacks the 3rd PHD finger and cannot bind Cyp33; thus, it cannot recruit HDAC1 and 2 and functions as a dominant constitutive activator. C: Reinsertion of the 3rd PHD finger restores the regulation of the fusion protein by Cyp33-HDACs restoring its function to regulated activation/repression.
Reinsertion of the MLL PHD fingers 1 through 3 or of the 3rd PHD finger alone in an MLL-ENL fusion protein, and expression of this modified protein in HEK293 cells, resulted in recruitment of Cyp33, and increased recruitment of HDAC1 to the fusion protein upon Cyp33 over expression. These results confirm that the repression domain of MLL can recruit HDAC1 in the context of the fusion protein, as has been shown before for the wild type MLL protein (12), and shows that such recruitment depends on the presence of the 3rd PHD finger (Figure 6C).
We had previously shown that over expression of Cyp33 results in repression of the HOXC8 gene in human leukemia cells that have wild type MLL, but such repression did not occur in leukemia cells expressing MLL-fusion genes (MLL-AF9, or MLL-AF6), even in the presence of the wild type MLL allele (17). In our present experiments, the HOXC8 gene is repressed in HEK293 cells by over expression of Cyp33, but such repression is inhibited after expression of an MLL-ENL fusion protein. Expression of MLL-ENL alone does not increase the levels of HOXC8 expression in HEK293 cells (Figure 4A, B, second lane), because the gene is already maximally expressed in these cells. Nevertheless, expression of an MLL-ENL fusion protein with a reinserted 3rd PHD finger again supports HOXC8 repression in this system. These results support the hypotheses that repression of HOX genes upon over expression of Cyp33, is mediated by MLL, that the interaction of Cyp33 with the 3rd PHD finger is necessary for this repression, and that the MLL fusion proteins without the 3rd PHD finger are constitutive activators that cannot respond to Cyp33 (Figure 6).
The 3rd PHD finger of MLL also has all of the features necessary to interact with methylated lysines on histone tails (28). So, the presence or absence of this PHD finger may affect the targeting of MLL to chromatin bearing certain histone modifications. Thus, the absence of this PHD finger from the MLL-fusion proteins may release them from targeting constraints, allowing them to interact with chromatin that normally would not be bound by wild type MLL. The loss of these targeting constraints, with the loss of the PHD fingers, may contribute to the increased target gene activation by the MLL-fusion proteins, but additional experiments will be needed to clarify its role.
Hematopoietic progenitor cells in a serial plating colony assay become exhausted after one or two passages. It has been shown previously that expression of an MLL-ENL fusion protein in mouse bone marrow cells results in dominant immortalization of progenitors, such that they survive for multiple passages without losing their self renewal potential (5). MLL-ENL proteins with the reinserted 3rd PHD finger were not able to support hematopoietic progenitor immortalization. This suggests that the immortalization ability of the MLL fusion protein depends on its constitutive trans-activating activity, which is lost with the reinsertion of the 3rd PHD finger. This is not due simply to the separation of the MLL sequences from the ENL sequences by the PHD finger peptide, because a similar fusion with inserted PHD fingers 1 and 2 does immortalize hematopoietic progenitors.
The constitutive trans-activating activity of MLL fusion proteins is thus explained by two features of the fusion protein: a) the incorporation of a transcription activating domain from the fusion partner, and b) the loss of the 3rd PHD finger, that controls a repression function of MLL mediated by recruitment of HDACs to the repression domain (Figure 6).
Nevertheless this mechanism only applies to a subset of MLL fusion proteins. A different subset seems to transform through the acquisition of a dimerization domain, but lacks an activation domain(29, 30). It is possible that these MLL fusion proteins activate transcription through some other mechanism. We don’t know if the loss of the 3rd PHD finger is a relevant event for leukemogenicity in this subset of leukemia. Nevertheless, in the MLL partial tandem duplications, the 3rd PHD finger is conserved, but only in one copy, while the repression domain is present in two copies. It is possible that the presence of an extra repression domain that cannot be targeted by Cyp33 results in constitutive trans-activation function for the abnormal protein.
The MLL repression domain is contained in two subdomains each one of which can repress a reporter gene when attached to a promoter (11). We tested the role of the RD2 sub-domain by using MLL-ENL fusion proteins that had a deletion of this domain with or without the inclusion of the 3rd PHD finger. The results showed that the RD2 sub-domain is not necessary for the Cyp33 dependent recruitment of HDAC1, or for the Cyp33 dependent repression of the HOXC8 gene. This means that the RD1 is sufficient to mediate these two activities, and that it can be targeted by Cyp33. Nevertheless, we also found that the RD2 sub-domain is necessary for the immortalization activity of the MLL-ENL fusion protein, independently of the inclusion of the 3rd PHD finger in the protein. This observation suggests that RD2 has a function that is non-redundant with RD1, and is independent of Cyp33.
Supplementary Material
Acknowledgements
We appreciate the generous gifts from Dr Korsmeyer S.J. and Dr Seto M. for the FLAG-tagged full length MLL cDNA construct (pcDNA-MLL-F), and Dr David Ron for the hCD2-pcDNA3 plasmid. This work is supported by NIH grants PO1 CA105049, and PO1 CA40046.
References
- 1.Popovic R, Zeleznik L. MLL: how complex does it get? J Cell Biochem. 2005;95:234–242. doi: 10.1002/jcb.20430. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T, Alder H, Gu Y, Prasad R, et al. Genes on chromosomes 4, 9, and 19 involved in 11q23 abnormalities in acute leukemia share sequence homology and/or common motifs. Proc Natl.Acad.Sci.U.S.A. 1993;90:4631–4635. doi: 10.1073/pnas.90.10.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubnitz JE, Morrissey J, Savage PA, Cleary ML. ENL, the gene fused with HRX in t(11;19) leukemias, encodes a nuclear protein with transcriptional activation potential in lymphoid and myeloid cells. Blood. 1994;84:1747–1752. [PubMed] [Google Scholar]
- 4.Lavau C, Szilvassy SJ, Slany R, Cleary ML. Immortalization and leukemic transformation of a myelomonocytic precursor by retrovirally transduced HRX-ENL. EMBO J. 1997;16:4226–4237. doi: 10.1093/emboj/16.14.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slany RK, Lavau C, Cleary ML. The oncogenic capacity of HRX-ENL requires the transcriptional transactivation activity of ENL and the DNA binding motifs of HRX. Mol.Cell Biol. 1998;18:122–129. doi: 10.1128/mcb.18.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiner S, Birke M, Garcia-Cuellar MP, Zilles O, Greil J, Slany RK. MLL-ENL causes a reversible and myc-dependent block of myelomonocytic cell differentiation. Cancer Res. 2001;61:6480–6486. [PubMed] [Google Scholar]
- 7.Ayton PM, Cleary ML. Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev. 2003;17:2298–2307. doi: 10.1101/gad.1111603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeisig BB, Milne T, Garcia-Cuellar MP, et al. Hoxa9 and Meis1 are key targets for MLL-ENL-mediated cellular immortalization. Mol.Cell Biol. 2004;24:617–628. doi: 10.1128/MCB.24.2.617-628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh JJ, Cheng EH, Korsmeyer SJ. Taspase1: a threonine aspartase required for cleavage of MLL and proper HOX gene expression. Cell. 2003;115:293–303. doi: 10.1016/s0092-8674(03)00816-x. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh JJ, Ernst P, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Proteolytic cleavage of MLL generates a complex of N- and C-terminal fragments that confers protein stability and subnuclear localization. Mol.Cell Biol. 2003;23:186–194. doi: 10.1128/MCB.23.1.186-194.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeleznik L, Harden AM, Rowley JD. 11q23 translocations split the "AT-hook" cruciform DNA-binding region and the transcriptional repression domain from the activation domain of the mixed-lineage leukemia (MLL) gene. Proc Natl.Acad.Sci.U.S.A. 1994;91:10610–10614. doi: 10.1073/pnas.91.22.10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia ZB, Anderson M, Diaz MO, Zeleznik L. MLL repression domain interacts with histone deacetylases, the polycomb group proteins HPC2 and BMI-1, and the corepressor C-terminal-binding protein. Proc Natl.Acad.Sci.U.S.A. 2003;100:8342–8347. doi: 10.1073/pnas.1436338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst P, Wang J, Huang M, Goodman RH, Korsmeyer SJ. MLL and CREB bind cooperatively to the nuclear coactivator CREB-binding protein. Mol.Cell Biol. 2001;21:2249–2258. doi: 10.1128/MCB.21.7.2249-2258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milne TA, Briggs SD, Brock HW, et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol.Cell. 2002;10:1107–1117. doi: 10.1016/s1097-2765(02)00741-4. [DOI] [PubMed] [Google Scholar]
- 15.Aasland R, Gibson TJ, Stewart AF. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem.Sci. 1995;20:56–59. doi: 10.1016/s0968-0004(00)88957-4. [DOI] [PubMed] [Google Scholar]
- 16.Stassen MJ, Bailey D, Nelson S, Chinwalla V, Harte PJ. The Drosophila trithorax proteins contain a novel variant of the nuclear receptor type DNA binding domain and an ancient conserved motif found in other chromosomal proteins. Mech.Dev. 1995;52:209–223. doi: 10.1016/0925-4773(95)00402-m. [DOI] [PubMed] [Google Scholar]
- 17.Fair K, Anderson M, Bulanova E, Mi H, Tropschug M, Diaz MO. Protein interactions of the MLL PHD fingers modulate MLL target gene regulation in human cells. Mol.Cell Biol. 2001;21:3589–3597. doi: 10.1128/MCB.21.10.3589-3597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson M, Fair K, Amero S, Nelson S, Harte PJ, Diaz MO. A new family of cyclophilins with an RNA recognition motif that interact with members of the trx/MLL protein family in Drosophila and human cells. Dev.Genes Evol. 2002;212:107–113. doi: 10.1007/s00427-002-0213-8. [DOI] [PubMed] [Google Scholar]
- 19.Mi H, Kops O, Zimmermann E, Jaschke A, Tropschug M. A nuclear RNA-binding cyclophilin in human T cells. FEBS Lett. 1996;398:201–205. doi: 10.1016/s0014-5793(96)01248-3. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Gething MJ. Protein structure Chaperones, paperones. Nature. 1989;342:224–225. doi: 10.1038/342224a0. [DOI] [PubMed] [Google Scholar]
- 21.Santillan DA, Theisler CM, Ryan AS, et al. Bromodomain and histone acetyltransferase domain specificities control mixed lineage leukemia phenotype. Cancer Res. 2006;66:10032–10039. doi: 10.1158/0008-5472.CAN-06-2597. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong SA, Staunton JE, Silverman LB, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat.Genet. 2002;30:41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 23.Ferrando AA, Armstrong SA, Neuberg DS, et al. Gene expression signatures in MLL-rearranged T-lineage and B-precursor acute leukemias: dominance of HOX dysregulation. Blood. 2003;102:262–268. doi: 10.1182/blood-2002-10-3221. [DOI] [PubMed] [Google Scholar]
- 24.Bullinger L, Dohner K, Bair E, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N.Engl.J Med. 2004;350:1605–1616. doi: 10.1056/NEJMoa031046. [DOI] [PubMed] [Google Scholar]
- 25.Hanson RD, Hess JL, Yu BD, et al. Mammalian Trithorax and polycomb-group homologues are antagonistic regulators of homeotic development. Proc Natl.Acad.Sci.U.S.A. 1999;96:14372–14377. doi: 10.1073/pnas.96.25.14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daser A, Rabbitts TH. The versatile mixed lineage leukaemia gene MLL and its many associations in leukaemogenesis. Seminars in Cancer Biology. 2005;15:175–188. doi: 10.1016/j.semcancer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Stiehl DP, Fath DM, Liang D, Jiang Y, Sang N. Histone deacetylase inhibitors synergize p300 autoacetylation that regulates its transactivation activity and complex formation. Cancer Res. 2007;67:2256–2264. doi: 10.1158/0008-5472.CAN-06-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li HT, Ilin S, Wang WK, et al. Molecular basis for site-specific read-out of histone H3K4me3 by the BPTF PHD finger of NURF. Nature. 2006;442:91–95. doi: 10.1038/nature04802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.So CW, Lin M, Ayton PM, Chen EH, Cleary ML. Dimerization contributes to oncogenic activation of MLL chimeras in acute leukemias. Cancer Cell. 2003;4:99–110. doi: 10.1016/s1535-6108(03)00188-0. [DOI] [PubMed] [Google Scholar]
- 30.Martin ME, Milne TA, Bloyer S, et al. Dimerization of MLL fusion proteins immortalizes hematopoietic cells. Cancer Cell. 2003;4:197–207. doi: 10.1016/s1535-6108(03)00214-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.