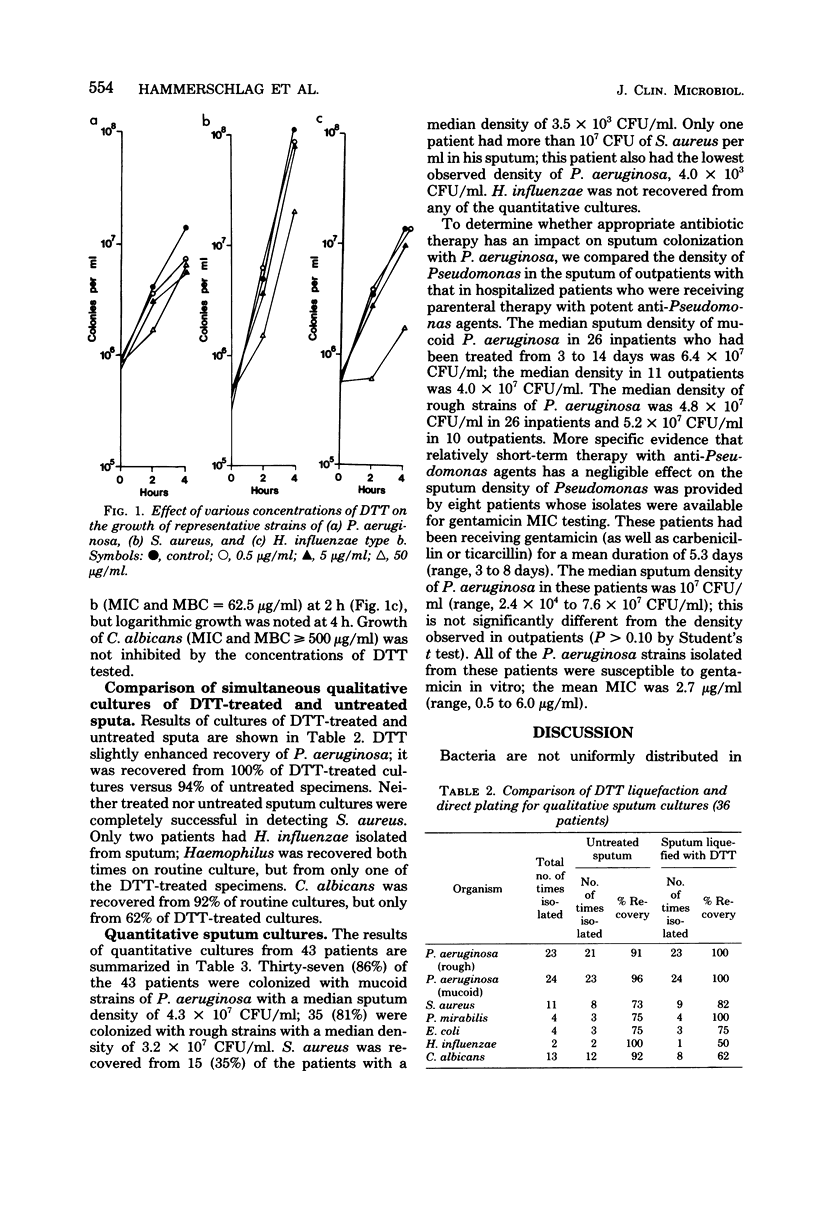
Abstract
Liquefaction and homogenization have been recommended to ensure accurate, representative sputum cultures. We evaluated dithiothreitol (DTT) as mucolytic agent for culturing sputum samples obtained from 79 cystic fibrosis (CF) patients. Liquefaction with DTT was not superior to direct plating of specimens for routine qualitative cultures. Unliquefied sputum cultures failed to direct 3 of 47 Pseudomonas aeruginosa isolates; DTT-treated specimens missed 5 of 13 Candida albicans isolates. Neither treated nor untreated sputum cultures were completely successful in detecting Staphylococcus aureus or Enterobacteriaceae. Since Haemophilus influenzae was recovered from only two qualitative cultures, we could not evaluate the effect of DTT on the receovery of this organism. However, 27 of 29 strains of H. influenzae were inhibited by concentrations of DTT near the recommended final working concentration of 50 micrograms/ml, suggesting that liquefaction might impair isolation of this organism. Liquefaction with DTT permitted quantitative cultures of CF sputum. The predominant pathogen in our CF population was P. aeruginosa; 37 of 43 (86%) patients were colonized with this organism. Median densities of rough and mucoid strains were 3.2 x 10(7) and 4.3 x 10(7) colony-forming units per ml, respectively. Previous oral antistaphylococcal therapy may have accounted for the observed low density of S. aureus (mean density, 3.5 x 10(3) colony-forming units per ml). We conclude that DTT treatment does not improve recovery of organisms from qualitative cultures but does facilitate quantitative studies of S. aureus and P. aeruginosa in CF sputum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J. G., Finegold S. M. Bacteriology of expectorated sputum with quantitative culture and wash technique compared to transtracheal aspirates. Am Rev Respir Dis. 1978 Jun;117(6):1019–1027. doi: 10.1164/arrd.1978.117.6.1019. [DOI] [PubMed] [Google Scholar]
- Crozier D. N., Khan S. R. Tobramycin in treatment of infections due to Pseudomonas aeruginosa in patients with cystic fibrosis. J Infect Dis. 1976 Aug;134 (Suppl):S187–S190. doi: 10.1093/infdis/134.supplement_1.s187. [DOI] [PubMed] [Google Scholar]
- Dixon J. M., Miller D. C. Value of dilute inocula in cultural examination of sputum. Lancet. 1965 Nov 20;2(7421):1046–1048. doi: 10.1016/s0140-6736(65)90572-6. [DOI] [PubMed] [Google Scholar]
- Hirsch S. R., Zastrow J. E., Kory R. C. Sputum liquefying agents: a comparative in vitro evaluation. J Lab Clin Med. 1969 Aug;74(2):346–353. [PubMed] [Google Scholar]
- Kilbourn J. P., Campbell R. A., Grach J. L., Willis M. D. Quantitative bacteriology of sputum. Am Rev Respir Dis. 1968 Nov;98(5):810–818. doi: 10.1164/arrd.1968.98.5.810. [DOI] [PubMed] [Google Scholar]
- LOURIA D. B., KAMINSKI T. The effects of four antimicrobial drug regimens on sputum superinfection in hospitalized patients. Am Rev Respir Dis. 1962 May;85:649–665. doi: 10.1164/arrd.1962.85.5.649. [DOI] [PubMed] [Google Scholar]
- Lorian V., Khavari P., Gray N. Quantitative bacteriological analysis of sputum as a test of antibiotic efficacy. Appl Microbiol. 1967 May;15(3):564–565. doi: 10.1128/am.15.3.564-565.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY J. R. The bacteriology of chronic bronchitis. Lancet. 1953 Sep 12;265(6785):534–537. doi: 10.1016/s0140-6736(53)90274-8. [DOI] [PubMed] [Google Scholar]
- MEAD G. R., WOODHAMS A. W. N-ACETYL-L-CYSTEINE AS LIQUEFYING AGENT IN THE BACTERIOLOGICAL EXAMINATION OF SPUTUM. Tubercle. 1964 Dec;45:370–373. doi: 10.1016/s0041-3879(64)80051-9. [DOI] [PubMed] [Google Scholar]
- Marks M. I., Prentice R., Swarson R., Cotton E. K., Eickhoff T. C. Carbenicillin and gentamicin: pharmacologic studies in patients with cystic fibrosis and pseudomonas pulmonary infections. J Pediatr. 1971 Nov;79(5):822–828. doi: 10.1016/s0022-3476(71)80401-8. [DOI] [PubMed] [Google Scholar]
- Monroe P. W., Muchmore H. G., Felton F. G., Pirtle J. K. Quantitation of microorganisms in sputum. Appl Microbiol. 1969 Aug;18(2):214–220. doi: 10.1128/am.18.2.214-220.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M. F., Neu H. C. Effect of N-acetylcysteine on antibiotic activity and bacterial growth in vitro. J Clin Microbiol. 1977 Jan;5(1):58–61. doi: 10.1128/jcm.5.1.58-61.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phair J. P., Tan J. S., Watanakunakorn C., Schwab L., Sanders L. W. Carbenicillin treatment of Pseudomonas pulmonary infection. Use in children with cystic fibrosis. Am J Dis Child. 1970 Jul;120(1):22–25. doi: 10.1001/archpedi.1970.02100060056006. [DOI] [PubMed] [Google Scholar]
- Pirtle J. K., Monroe P. W., Smalley T. K., Mohr J. A., Rhoades E. R. Diagnostic and therapeutic advantages of serial quantitative cultures of fresh sputum in acute bacterial pneumonia. Am Rev Respir Dis. 1969 Dec;100(6):831–838. doi: 10.1164/arrd.1969.100.6.831. [DOI] [PubMed] [Google Scholar]
- RAWLINS G. A. Liquefaction of sputum for bacteriological examination. Lancet. 1953 Sep 12;265(6785):538–539. doi: 10.1016/s0140-6736(53)90275-x. [DOI] [PubMed] [Google Scholar]
- Rawlins G. A. Use of a pancreatin-trypsin solution for the liquefaction of sputa for routine bacteriological examination. J Clin Pathol. 1968 Jul;21(4):531–532. doi: 10.1136/jcp.21.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., Schoenknecht F. D., Kenny M. A., Sherris J. C. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J Infect Dis. 1974 Nov;130(5):454–463. doi: 10.1093/infdis/130.5.454. [DOI] [PubMed] [Google Scholar]
- Wilson M. J., Martin D. E. Quantitative sputum culture as a means of excluding false positive reports in the routine microbiology laboratory. J Clin Pathol. 1972 Aug;25(8):697–700. doi: 10.1136/jcp.25.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]



