Abstract
Allocation of attentional resources during early visual processing was investigated in schizophrenia. Pupillary responses were recorded during a backward masking task as an index of resource allocation in schizophrenia patients (n = 51) and nonpsychiatric controls (n = 51). Two time-linked components of pupillary response waveforms appeared to differentially index resource allocation to targets versus masks. Two patient subgroups were identified: One with normal overall pupillary responses (resource allocation), but greater allocation on mask relative to target components, and another with abnormally small overall pupillary responses and similar allocation between target and mask components. Thus, misallocation of resources to masks contributed to masking deficits in one subgroup, whereas reduced resource allocation contributed to deficits in the other. The nature of resource-related deficits can vary across schizophrenia subgroups.
Keywords: Backward masking, schizophrenia, pupillary responses, resource allocation
The visual backward masking task is an extensively studied measure of cognitive impairment in schizophrenia. The typical masking task procedure consists of a visually presented target stimulus (e.g., line or letter) that is rapidly followed and usually spatially covered by a mask (e.g., patterned lines) at varying stimulus onset asynchronies (SOAs) between the target and mask (e.g., 10 to 700 ms). The participant is typically asked to identify the target or its location. Successful target identification requires the transfer of target stimulus information from the sensory register to short-term memory before the mask disrupts information transfer. When the mask is of similar energy (e.g., duration and luminance) to the target and spatially overlapping the target location, studies have consistently reported impaired target detection at longer SOA intervals (e.g., > 120 ms) in medicated and unmedicated, acute and remitted people with schizophrenia (Balogh & Merrit, 1987; Braff & Saccuzzo, 1981; Green, Nuechterlein, & Mintz, 1994; Miller, Saccuzzo, & Braff, 1979; Rund, Landro, & Orbeck, 1993; Cadenhead, Serper, & Braff, 1998). This masking deficit has also been found in schizophrenia-spectrum participants with schizotypal or psychosis prone traits (Steronko & Woods, 1978; Balogh & Merrit, 1985; Saccuzzo & Schubert, 1981; Cadenhead, Perry, & Braff, 1996) and in unaffected siblings of people with schizophrenia (Green, Nuechterlein, & Breitmeyer, 1997). Masking impairment, therefore, may reflect vulnerability to schizophrenia, rather than an episodic feature of the illness.
Despite consistent findings of masking deficits in schizophrenia, the nature of this impairment remains unclear. Attentional shifting (or replacement) is one cognitive mechanism that has been proposed to underlie masking task performance at longer SOAs. The identification of stimulus meaning is thought to occur in short-term visual memory at SOAs of 100 ms or longer (Philips, 1974). At this time point, limited controlled processes must be shifted and shared between the target and mask to identify their stimulus meaning (Loftus, Hanna, & Lester, 1988; Michaels & Turvey, 1979; Phillips, 1974). Michaels and Turvey (1979) referred to this as “replacement,” whereby target information is replaced by mask information as the main focus of attention. At longer masking SOA’s, attentional mechanisms may contribute to performance impairments to a greater extent than at shorter SOA’s, when mechanisms influencing the quality of the percept, itself (e.g., integration and interruption; for a review, see Breitmeyer, 1984) may contribute more to performance. At longer SOA’s, target identification deficits occur because the target and mask icons compete for common stimulus identification algorithms, not because the quality of the target icon is degraded. Masking deficits on in schizophrenia commonly observed at SOAs greater than 100 ms, therefore may be due to dysfunctional attentional allocation mechanisms in short-term visual memory (Knight, 1992; 1993).
This type of masking deficit due to attentional misallocation may be related to defects involving the availability of information processing resources in people with schizophrenia (Gjerde, 1983; Granholm, 1992; Nuechterlein & Dawson, 1984). The resource limitations hypothesis proposes that people with schizophrenia have insufficient information processing resources available to cope with high, but not low, controlled processing demands, which results in reduced speed and efficiency of processing. Masking impairments at longer SOA intervals are consistent with this hypothesis, because at this SOA, limited controlled processing resources must be shared between target and mask to identify their stimulus meaning. Normal amounts of processing resources may not be available for target identification for several reasons, including a misallocation of limited resources to mask processing and/or an overall reduction in the actual pool of resources (Nuechterlein & Dawson, 1984). The first explanation refers to a wasteful allocation of resources (from an intact pool of resources) to mask processing, thus leaving fewer resources available for accurate target identification (Braff, Saccuzzo, & Geyer, 1991). The second suggests that additional resources necessary for successful completion of tasks with higher processing demands (e.g., SOA intervals greater than 100 ms) are unavailable because the actual pool (or pools) of resources in schizophrenia is smaller than nonpsychiatric comparisons. While both explanations predict a common pattern of performance (i.e., greater deficits at longer SOA intervals), they offer different, testable hypotheses about the nature of masking impairment commonly observed in schizophrenia.
Pupillometry methods can be used to examine these resource limitations hypotheses. Pupillary responses provide a reliable and sensitive psychophysiological index of the amount of processing resources allocated to cognitive task stimuli (Beatty, 1982; Beatty & Lucero-Wagoner, 2000; Kahneman, 1973). Increased pupil dilation during cognitive task performance reflects increased allocation of processing resources to the task. In numerous studies (for a review, see Beatty, 1982), increased task demands reliably evoked greater pupillary dilation, irrespective of the cognitive domain reflected by different tasks. For example, pupillary responses recorded during a perceptual discrimination task increased systematically in nonpsychiatric participants as the difference in pitch between comparison and standard tones decreased (Kahneman & Beatty, 1967). Additionally, several studies have found greater pupil dilation in nonpsychiatric participants and people with schizophrenia when identifying target letters in larger relative to smaller letter arrays during the span of apprehension task (Granholm, Morris, Asarnow, Chock, & Jeste, 2000).
In our previous research (Verney, Granholm, & Dionisio, 2001), task-evoked pupillary responses of healthy undergraduates were recorded during a visual backward masking task. Pupillary responses were compared in masked and no-mask conditions to index the relative processing loads associated with each condition. Pupil dilation in the no-mask condition reflected only target processing, while pupil dilation in the masking conditions reflected both target and mask processing. Pupillary responses were significantly greater in longer (> 134 ms) SOA conditions relative to the no-mask condition, suggesting that the mask demanded additional processing resources only when it was presented more than 100 ms after the target. This finding was consistent with masking models that predicted controlled processing demands are highest in longer SOA conditions (greater than 100 ms), when resources must be shifted and shared between targets and masks for identification of both stimuli in short-term visual memory (Loftus, Hanna, & Lester, 1988; Michaels & Turvey, 1979; Phillips, 1974).
A principal components analysis (PCA) of pupillary response waveforms was also computed to investigate the time course of resource-demanding target and mask processing (Verney, Granholm, & Marshall, 2001). Three time-linked factors were found in the waveform: Early (0 – 0.7 s), middle (0.7 – 1.55 s), and late (1.55 – 3.0 s) factors. Pupil dilation on the middle factor was greater in the no-mask condition (target processing only) relative to masking conditions (target and mask processing). In contrast, the opposite pattern of pupil dilation occurred on the late factor; dilation was greater in masking relative to no-mask conditions. This pattern of results suggested that the middle factor indexed allocation of resources to target processing, while the late factor indexed allocation to masks.
In a follow-up study (Granholm & Verney, 2004), task-evoked pupillary responses of people with schizophrenia and age- and education-comparable nonpsychiatric participants were recorded during performance of the same masking task. People with schizophrenia detected significantly fewer targets than nonpsychiatric participants only during the longest (317 ms) SOA interval. The previously found PCA factor structure was also replicated. Importantly, participants with schizophrenia showed more dilation on the late (allocation to mask) relative to middle (allocation to target) factor, whereas, nonpsychiatric participants showed less dilation on the late relative to middle factor. These findings suggested that masking deficits at longer SOA intervals in people with schizophrenia may be due to a misallocation of processing resources to masks, leaving fewer resources available for target processing. Alternatively, a smaller pool of resources may explain this masking impairment, because the patients showed significantly smaller overall pupil dilation relative to controls in both mask and no-mask conditions. Therefore, the extent to which a misallocation of attentional resources accounted for the observed masking impairment over and above impairment attributable to a depleted pool of resources remained unclear in that study.
In the present study, pupillary responses were recorded during a backward masking task in a larger sample of people with schizophrenia (n = 51) and age- and premorbid intelligence-comparable nonpsychiatric participants. Consistent with many previous studies using similar masking tasks, we predicted that people with schizophrenia would show target identification deficits only in longer SOA intervals. We also predicted that the PCA factor structure for pupillary response waveforms found in three prior studies (Verney, 2001; Verney, Granholm, & Marshall, 2004; Granholm & Verney, 2004) would be replicated in this larger sample. Consistent with a resource misallocation account of masking impairment in schizophrenia and our previous research (Granholm & Verney, 2004) using a smaller sample (n = 16), we predicted that people with schizophrenia would show significantly greater dilation on the late (mask) relative to middle (target) factor. In addition, to distinguish between resource misallocation and depleted resource pool explanations of masking impairment, we divided the schizophrenia sample into high versus low resource allocation subgroups based on a median split on overall pupillary response. Masking task performance deficits in the subgroup with high resource allocation (pupil dilation) cannot be attributed to a depleted resource pool, because high pupil dilation would confirm that resources were available and invested in the task.
Method
Participants
Participants from both groups were recruited from a larger longitudinal study of age-related change in pupillary responses and cognition in middle-aged and older people with schizophrenia. Fifty-one outpatients with a Diagnostic and Statistical Manual (Fourth Edition; American Psychiatric Association, 1994) diagnosis of schizophrenia or schizoaffective disorder were included. People with schizophrenia were recruited from the VA San Diego Healthcare System, University of California, San Diego, Outpatient Psychiatry Services, San Diego County Mental Health Services, local supported housing facilities, and private physicians. The Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1995) was administered by trained bachelor’s-level technicians and a final diagnosis was reached by a single licensed doctoral-level clinical psychologist based on the SCID and medical record review, when available.
Symptom severity ratings for the past two weeks were obtained by trained technicians using the Scale for the Assessment of Positive Symptoms (SAPS; Andreasan & Olsen, 1982) and the Scale for the Assessment of Negative Symptoms (SANS; Andreasan & Olsen, 1982). Inter-rater reliability (mean kappa = .88) for each technician was based upon ratings of 10 training tapes of middle-aged and older people with schizophrenia or schizoaffective disorders. Four symptom dimensions derived from factor analytic studies of the SAPS and SANS in schizophrenia were computed: positive symptoms, conceptual disorganization, diminished motivation and diminished expression (Sayers, Curran, & Mueser, 1996; Peralta & Cuesta, 1999; Blanchard & Cohen, 2006; for a meta-analytic review, see Grube, Bilder, & Goldman, 1998). The positive dimension was the average of SAPS Hallucination and Delusion global ratings [(SAPS items 7 + 20)/2], and the conceptual disorganization dimension was the average of SAPS Bizarre Behavior and Positive Formal Thought Disorder global ratings [(SAPS items 25 + 34)/2]. Diminished motivation was the average of SANS Avolition-Apathy and Anhedonia-Asociality global ratings [(SANS items 17 + 22)/2], and diminished expression was the Affective Flattening global rating (SANS item 8). Scores for each dimension ranged from 0 (none) to 5 (severe) and are shown in Table 1.
Table 1.
Participant Characteristics
| Schizophrenia |
||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Nonpsychiatric (n = 51) | Total (n = 51) | High pupil respondersa (n = 26) | Low pupil respondersa (n = 25) | ||||
| Age - M (SD) | 54.4 | (8.6) | 54.9 | (8.2) | 54.5 | (7.5) | 55.4 | (9.0) |
| Gender - % male | 51 | 61 | 65 | 56 | ||||
| Race - % Caucasian | 63 | 84 | 81 | 88 | ||||
| ANART - M (SD)b | 108.9 | (7.9) | 105.5 | (9.8) | 103.9 | (11.6) | 107.1 | (7.4) |
| No-mask accuracy - % | 89.9 | (8.8) | 86.4 | (11.9) | 86.6 | (12.5) | 86.2 | (11.6) |
| SAPS/SANS dimensions:c | ||||||||
| Positive Symptoms - M(SD) | --- | --- | 1.17 | (1.00) | 1.23 | (1.19) | 1.12 | (0.81) |
| Concept. Disorganization - M(SD) | --- | --- | 0.55 | (0.81) | 0.48 | (0.84) | 0.70 | (0.83) |
| Diminished Motivation - M(SD) | --- | --- | 1.45 | (0.99) | 1.48 | (1.05) | 1.48 | (0.95) |
| Diminished Expression - M(SD) | --- | --- | 1.41 | (1.15) | 1.42 | (1.23) | 1.48 | (1.05) |
Note. ANART = American National Adult Reading Test. SAPS = Scale for Assessment of Positive Symptoms. SANS = Scale for Assessment of Negative Symptoms.
The schizophrenia group was split into high versus low resource allocation subgroups based on a median split on overall peak-to-peak pupillary response during the backward masking task.
High pupil subgroup had a small but statistically significant difference (p <.05) in mean ANART score relative to nonpsychiatric controls. Groups did not differ significantly on any other variable.
Dimension scores ranged from 0 (none) to 5 (severe).
Forty-six patients were known to be taking antipsychotic medications (14 typical, 21 atypical, 7 both, 4 type unknown, and 5 none), 18 were taking anticholinergic medications (30 none; 3 unknown), and 23 were taking antidepressant or mood stabilizing medications (24 none; 4 unknown). The average daily dosage (mg) of antipsychotic medication was calculated as chlorpromazine equivalents (CPZE; Ereshefsky & Richards, 1990; Saddock & Saddock, 2000) for patients whose dosage could be verified through medical records or information from psychiatrists (M = 336.7, SD = 368.2, n = 36). The average daily dosage (mg) of anticholinergic medication was calculated as clinically recommended benztropine equivalents (AChE; Ereshefsky & Richards, 1990; Saddock & Saddock, 2000) for patients whose dosage could be verified through medical records or information from psychiatrists (M = 0.47, SD = 1.0, n = 40).
Fifty-one age- and pre-morbid verbal intelligence (American National Adult Reading Test, or ANART; Grober & Sliwinski, 1991) comparable nonpsychiatric participants with no DSM-IV diagnoses of lifetime past or current mood or psychotic disorders (based on the SCID-Nonpatient version; First, Spitzer, Gibbon, & Williams, 1995) were also recruited from the general community through local newspaper and flyer advertisements, as part of the larger longitudinal study of age-related change in middle-aged and older people with schizophrenia. Participants in both groups were excluded for the following: (1) age < 45 years old; (2) neurologic disorders (e.g., seizure disorder; head injury with loss of consciousness > 30 min); (3) alcohol or substance dependence diagnosis (DSM-IV criteria) other than nicotine or caffeine in the past year; (4) uncorrected/corrected visual acuity less than 20/40 based on a Snellen wall chart exam; (5) ocular medications, diseases or surgery that might affect pupil function; (6) near-chance performance (i.e., less than 65% accuracy) in the no-mask condition and (7) abnormal pupil measurements (resting diameter and/or pupillary response outliers greater than two standard deviations in all conditions or excessive artifacts in recording). Clinical and demographic data for both groups are presented in Table 1.
Apparatus
Pupillary responses were recorded from the left eye using a Micromeasurements System 1200 infrared corneal-reflection pupillometer (Micromeasurements, Inc.; out of business). A video camera sensitive to infrared light and an infrared light source were positioned 24 cm from the participant’s eye, below their field of view. Participants were seated comfortably in a lighted room (background illumination = 84.4 lux) with their head stabilized in a head/chin rest. The headrest was used to minimize movement artifact and maintain a distance of 77 cm between the participants’ eyes and the computer monitor. Pupil diameter was digitized at a 60-Hz sampling rate and saved for later analysis off-line. The resolution of the pupillometer was 0.05-mm diameter, but with signal averaging, differences on the order of 0.01 to 0.02-mm can be reliably detected. A two-button joystick was used to collect “Left” and “Right” responses. A PC-compatible microcomputer and standard 50-cm flat screen CRT color monitor was used to administer the visual backward masking task. This computer was networked with the pupillometer computer to ensure precise time locking between pupillary responses and backward masking stimulus/response events.
Procedure
On the backward masking task, target stimuli consisted of two vertical lines (dark lines on a white background) of different length (2 and 3 cm) that were presented side-by-side 3 cm apart randomly offset (i.e., not aligned at the top or bottom) in the center of the monitor. Participants were asked to identify the longer of the two lines (target) by pressing the right or left response button to indicate the horizontal position of target. The targets were randomly and equally distributed to the right and left side. Both detection accuracy and speed were emphasized with the instructions. During the masking condition, longer (4 cm) adjacent lines (masking stimuli) followed target stimuli in the same locations. The interval between the onset of the target and onset of the mask (stimulus onset asynchrony or SOA) was 67, 100, 134, 317, and 717 ms; thus, with the inclusion of a no-mask condition there were a total of 6 conditions. These SOA intervals were chosen to be similar to the range reported in previous studies, within the constraints of the video refresh rate of the monitor (about 17 ms or 60-Hz). Due to the video refresh rate limitations, targets and masks were each presented for 17 ms.
At the beginning of each trial, a green fixation square (0.85 × 0.85 cm, 0.63° of visual angle and 7 lux) was presented in the center of the monitor (against a black screen background) along with a high-pitched tone (1500 Hz for 500 ms). The square and tone functioned as visual and auditory cues to prepare the participant for the trial’s target stimulus. Three seconds after the onset of the target stimulus, a low-pitched tone (800 Hz for 500 ms) served as an auditory cue signaling the end of a trial. Participants were instructed to try to refrain from blinking during the trial period and encouraged to blink during the 3-s inter-trial interval.
Before the test phase of the task, participants were presented with 24 practice trials (4 per condition). Practice trials began with the easiest conditions, namely, two no-mask trials followed by two 317 ms SOA trials. The SOA durations of the remaining 20 practice trials were randomly presented. Computer-automated feedback was provided to indicate the correctness of participant’s response for the first 12 practice trials. Feedback was not provided during test trials. In the test phase, blocks of 20 test trials per condition were presented in randomized blocks, resulting in a total of 120 test trials. During test trials, a moment of rest was allowed after each presentation of six trials since the computer required time to periodically save strings of pupillary response data. Additionally, each participant was given a few minutes to rest at the halfway point of the test. The entire task (i.e., instructions, practice and test) typically required approximately 20 minutes to complete. The percentage of correct target identifications (detection accuracy) for the 20 trials for each condition were recorded.
Data Reduction
A trained technician first visually inspected graphic displays of the raw pupil diameter data and discarded trials with gross artifacts. A computer algorithm was then used to remove eye blinks and other minor artifacts from trials by linear interpolation. Artifacts and blinks were defined as large changes in dilation beyond the possible rate of change in pupil diameter. Trials in each condition were then discarded if more than 50% of the trial waveform was comprised of artifacts. A two-pass five-point digital smoothing filter (3.7 Hz) was then applied to the data, and valid trials were averaged for each condition. Participants with less than four valid trials in each condition were excluded, because visual inspection of the waveforms revealed instability when fewer than 20% of the trials were used. The task was originally administered to 101 people with schizophrenia in the larger longitudinal study. Pupil data were not recorded for 11 (9%) of these participants due to instrument malfunction. Twenty-four (27%) of the 90 people with schizophrenia with pupil data were excluded from further analysis for having near-chance behavioral task performance (i.e., less than 65% correct detection accuracy in the no-mask condition). Additionally, 15 (23%) of the remaining 66 people with schizophrenia were excluded for excessive pupil data artifacts, leaving 51 participants in the final patient sample. Excluded participants (n = 50) did not differ significantly from included participants on ANART intelligence estimates, or any demographic or symptom variable. Age- and ANART-comparable nonpsychiatric participants were then consecutively selected from the larger longitudinal study sample until fifty-one participants with adequate performance (no-mask detection accuracy greater than 65% correct) and pupillary responses (at least 4 artifact-free trials in each condition) were identified.
To remove individual differences in resting pupil size, each participant’s baseline pupil size for each condition waveform (i.e., average of five pupil diameter samples recorded 100 ms prior to onset of the target display) was subtracted from all subsequent samples for that waveform. Peak-to-peak dilation for each mean condition waveform was then determined by computing the difference in amplitude from peak constriction (smallest diameter between 100 to 500 ms post target display onset) to peak dilation (largest diameter between 500 to 2500 ms post target display onset). This peak-to-peak score was used to remove light reflex constriction responses to the visual displays from dilation responses. To objectively examine the pupillary response waveform across the 3-s trial, a PCA with varimax rotation was performed on 180 time-points (3 s) of the pupil response waveform time-locked to stimulus onset across the seven masked and no-mask conditions for all participants. Three prominent factors were expected based on our previous research (Verney, 2001; Granholm & Verney, 2004; Verney, Granholm, & Marshall, 2004).
Statistical Analyses
To examine the hypothesis that people with schizophrenia would demonstrate significantly greater masking impairment relative to nonpsychiatric participants in longer (317 and 717 ms), but not shorter, SOA conditions, planned group comparisons of detection accuracy within each masking condition were computed using Bonferroni-corrected, independent samples, two-tailed t-tests (p < .05/5 = .01). Pupillary responses were examined by computing separate 2 (groups) × 6 (conditions) split-plot ANOVAs, with group as a between-subjects factor and condition as a repeated-measures factor, for baseline pupil size and peak-to-peak dilation. In addition, a 2 (groups) × 6 (conditions) × 3 (PCA factors) split-plot ANOVA was computed for the factor scores from the pupil waveform PCA (described below). Greenhouse-Geisser correction was used when sphericity assumptions were violated. Dunnett’s procedure comparing each masked condition with the no-mask condition was used to follow-up significant interaction effects for each ANOVA. Based on our previous findings (Granholm & Verney, 2004), a planned 2 (groups) × 2 (middle vs. late PCA factor) contrast was computed for the factor scores from the pupil waveform PCA to test the hypothesis that people with schizophrenia would show less pupil dilation on the middle (target) relative to late (mask) factor, whereas, nonpsychiatric participants would demonstrate the opposite pattern. Finally, the schizophrenia sample was split into high and low resource allocation subgroups based on a median split on pupil dilation (mean peak-to-peak dilation across conditions). A split-plot 3 (groups: nonpsychiatric v. high dilation v. low dilation) × 2 (middle v. late PCA factors) ANOVA was computed to examine whether patients with high and low dilation (resource allocation) differed in relative allocation of resources between targets (middle factor) and masks (late factor).
Results
Task performance
Detection accuracy in each condition is shown for both groups in Table 2. As predicted, people with schizophrenia detected significantly fewer targets than nonpsychiatric participants in the 717 ms SOA condition, t(100) = 3.03, p < .01, d = .60. Significant group differences were also found in the 67 ms, t(100) = 2.91, p < .01, d = .58, and 134 ms, t(100) = 2.75, p < .01, d = .54, SOA conditions, but the groups did not differ significantly (Bonferroni-corrected p < .05/5 = .01) in any other SOA condition (100: t = 2.61, p = .01, d = .52; 317: t = 2.26, p = .03, d = .45). This study did not use a procedure to match groups on no-mask performance. Although groups did not differ significantly in no-mask condition performance, t(100) = 1.70, p = .09, d = .34, the medium group difference found in no-mask performance may have contributed to group differences in the masking conditions. Ratio scores, therefore, were computed for each masking condition relative to no-mask performance (i.e., masking/no-mask accuracy) to remove individual differences in general level of performance from masking effects. These ratio scores showed that the groups differed significantly (Bonferroni-corrected (p < .05/5 = .01) only in the 717 ms SOA condition, with effect sizes generally larger in longer SOA conditions (67: t = 1.75, p = .08, d = .33; 100: t = 1.10, p = .27, d = .21; 134: t = 2.12, p = .04, d = .45; 317: t = 1.77, p = .08, d = .35; 717: t = 3.03, p < .01, d = .60).
Table 2.
Performance and pupillary response measures for the two participant groups
| Condition |
||||||
|---|---|---|---|---|---|---|
| Dependent Measure | 67 ms | 100 ms | 117 ms | 317 ms | 717 ms | No mask |
| Schizophrenia (n = 51) | ||||||
| Detection accuracy (%) | 53.1 (11.4) | 56.4 (9.2) | 60.4 (15.4) | 73.0 (16.6) | 77.4 (18.7) | 86.4 (11.9) |
| Baseline pupil diameter (mm) | 3.24 (0.58) | 3.26 (0.62) | 3.33 (0.61) | 3.24 (0.61) | 3.27 (0.61) | 3.28 (0.60) |
| Peak-to-peak dilation (mm) | 0.09 (0.06) | 0.09 (0.05) | 0.10 (0.07) | 0.12 (0.07) | 0.11 (0.08) | 0.08 (0.05) |
| Early PCA factor | 0.29 (1.04) | 0.15 (0.98) | 0.04 (0.72) | 0.11 (0.85) | 0.08 (0.90) | 0.25 (1.04) |
| Middle PCA factor (target) | 0.21 (0.95) | 0.29 (0.97) | 0.44 (1.12) | 0.38 (1.04) | 0.44 (1.08) | 0.54 (0.97) |
| Late PCA factor (mask) | −0.02 (1.03) | −0.16 (1.29) | −0.05 (0.93) | 0.08 (1.13) | 0.33 (1.24) | 0.04 (1.04) |
|
| ||||||
| Nonpsychiatric (n = 51) | ||||||
| Detection accuracy (%) | 59.4 (10.4) | 62.2 (12.7) | 69.2 (17.0) | 80.3 (15.8) | 87.1 (13.5) | 89.9 (8.8) |
| Baseline pupil diameter (mm) | 3.39 (0.58) | 3.39 (0.60) | 3.43 (0.62) | 3.40 (0.59) | 3.40 (0.56) | 3.42 (0.61) |
| Peak-to-peak dilation (mm) | 0.10 (0.08) | 0.11 (0.08) | 0.12 (0.07) | 0.12 (0.08) | 0.14 (0.11) | 0.10 (0.08) |
| Early PCA factor | −0.10 (0.95) | −0.03 (0.82) | −0.39 (0.95) | −0.14 (0.70) | −0.02 (0.91) | −0.21 (1.04) |
| Middle PCA factor (target) | −0.36 (0.87) | −0.36 (0.75) | −0.27 (0.85) | −0.55 (0.81) | −0.54 (0.75) | −0.23 (0.85) |
| Late PCA factor (mask) | −0.12 (0.64) | −0.11 (0.67) | −0.11 (0.79) | 0.21 (1.03) | 0.17 (0.93) | −0.20 (0.64) |
Note. Values represent means (with SD) and percentages where indicated.
Pupillary responses
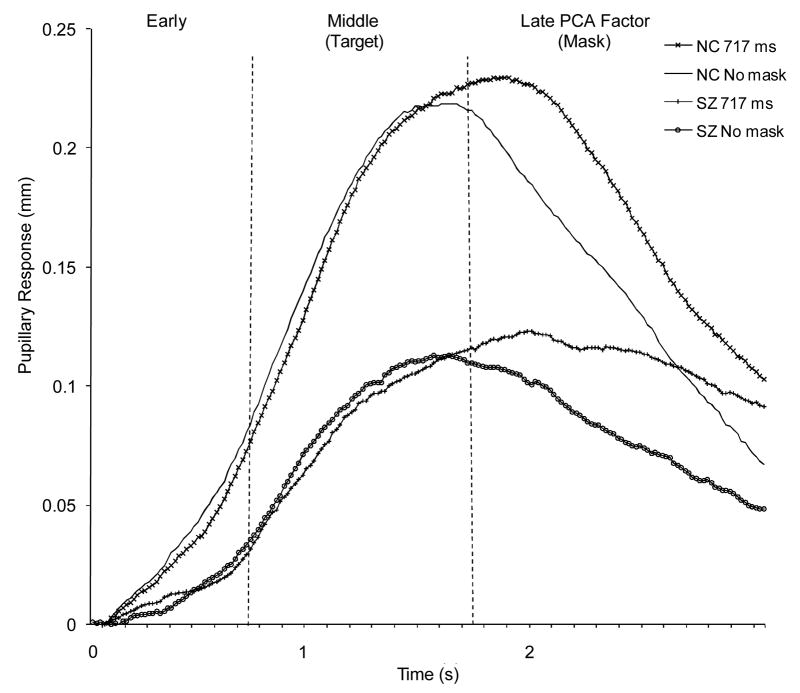
Baseline pupil size (Table 2) did not differ significantly between the groups, F (1,100) = 1.38, p = .24, η2 = .01, and the group by condition interaction was not significant, F (5,500) = 0.53, p = .75, η2 = .01. The groups, therefore, did not differ significantly in resting, tonic pupil size. Figure 1 illustrates the pupillary response waveforms in the 717 ms SOA and no-mask conditions for each group and Table 2 shows mean peak-to-peak values for each group across all conditions. Peak-to-peak dilations did not differ significantly between groups, F(1,100) = 1.79, p = .18, η2 = .02, and the group by condition interaction was not significant, F(5,500) = .94, p = .46, η2 = .01, but peak-to-peak dilations did differ significantly across conditions, F(5,500) = 8.74, p < .001, partial η2 = .08. Peak-to-peak dilation was significantly greater in the 134 ms, 317 ms and 717 ms SOA condition relative to the no-mask condition (Dunnett’s tests, p < .05), but peak-to-peak dilation in the remaining conditions did not differ significantly from the no-mask condition. This pattern replicates our previous research (Verney, Granholm, & Dionisio, 2001; Granholm & Verney, 2004) that found significantly greater pupil dilation (resource allocation) in longer, but not shorter, SOA intervals relative to the no-mask condition.
Figure 1.
PCA factors of pupillary response waveforms in the 717 ms SOA and no-mask conditions for each group. NC= nonpsychiatric controls; SZ= schizophrenia.
Pupillary response PCA factor structure
Similar to our previous findings (Verney, 2001; Granholm & Verney, 2004), three prominent PCA factors accounted for 92% of the variance in the pupillary response data for the total sample. The factor structure for each group was identical to that of the total sample. All factors were internally consistent and well defined by the data (the smallest squared multiple correlation for factors from the data was 0.89). Figure 1 shows the following linear time course of the three factors: (1) an early factor from 0 to 0.75 s (eigenvalue = 12.4); (2) a middle factor from 0.75 to 1.73 s (eigenvalue = 39.0) and (3) a late factor from 1.73 to 3.0 s (eigenvalue = 115.4). Table 2 presents PCA factor scores in each condition for each group.
The 2 (groups) × 6 (conditions) × 3 (PCA factors) split-plot ANOVA resulted in a significant group effect, F(1,100) = 19.29, p < .001, η2 = .16, condition by PCA factor interaction, F(10,1000) = 3.79, p < .001, η2 = .04, and group by PCA factor interaction, F(2,200) = 6.71, p < .01, η2 = .06. None of the other main effects or interactions were significant. The significant group effect indicated that nonpsychiatric participants had larger pupil dilations than patients with schizophrenia. The significant condition by PCA factor interaction was examined using Dunnett’s tests (p < .05) to compare each masking condition with the no-mask condition collapsed across groups. For the early factor, no masking condition differed significantly from the no-mask condition. For the middle factor (see Table 2), significantly less pupil dilation was found for all masking conditions relative to the no-mask condition (p < .05), with the exception of the 134 SOA condition. As target detection accuracy increased, therefore, middle factor dilation (target allocation) generally increased. In contrast, for the late factor, pupil dilation was greater in most masking conditions relative to the no-mask condition, but the only difference to reach statistical significance was the 717 ms SOA vs. no-mask comparison (p < .05; see Table 2). Therefore, as the masking stimulus became more salient in the longer SOA masking conditions, the late factor (mask allocation) showed greater dilation than when no masks were presented.
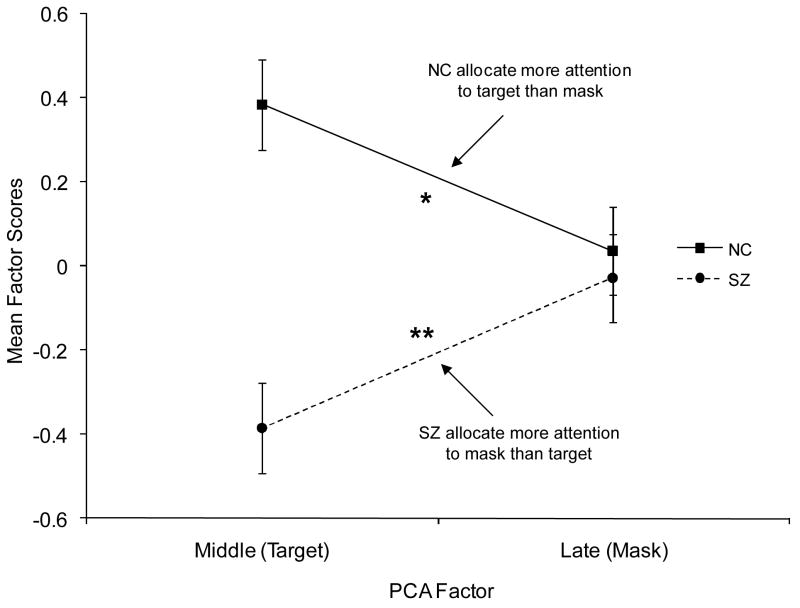
The significant group by PCA interaction reflected the opposite pattern of pupil dilation across middle and late factors shown by the two groups (Figure 2). Consistent with the study hypothesis and previous research (Granholm & Verney, 2004), a significant group × PCA factor (middle v. late) interaction was found, F(1,100) = 11.51, p < .01, η2 = .10, whereby people with schizophrenia showed significantly greater dilation on the late (mask) relative to middle (target) factor, t(50) = −2.79, p < .01, d = .58, while nonpsychiatric participants showed significantly greater dilation on the middle (target) relative to late (mask) factor, t(50) = 2.14, p < 0.05, d = .46.
Figure 2.
Pupillary responses on middle (target) and late (mask) PCA factors for each group. Error bars are ± 1 SE. *P < 0.05. **P < 0.01. NC= nonpsychiatric controls; SZ= schizophrenia.
Subgroup effects
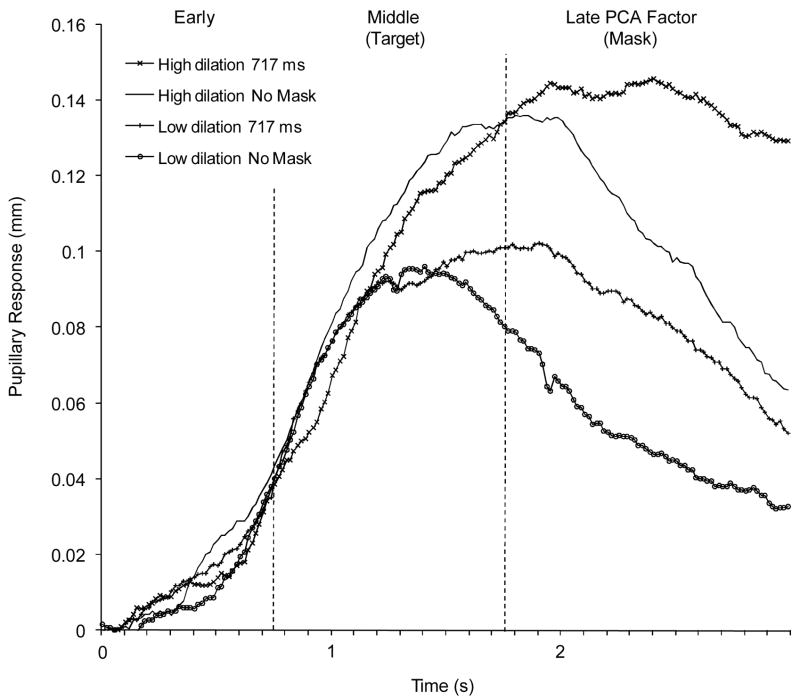
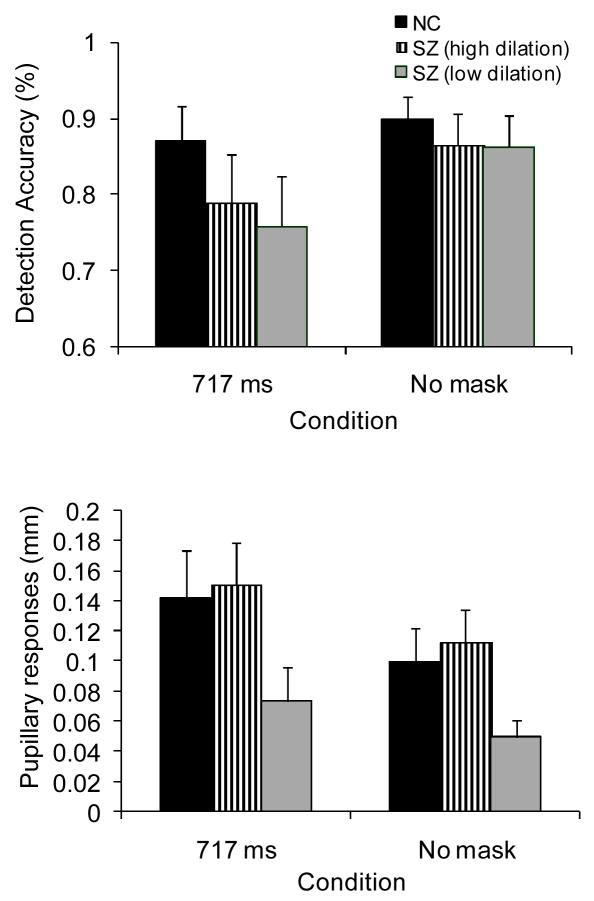
People with schizophrenia were divided into high and low pupil dilation (resource allocation) subgroups based on a median split on mean peak-to-peak dilation across conditions. Figure 3 shows pupillary response waveforms for both subgroups in the 717 ms and no-mask conditions, and Figure 4 shows detection accuracy and peak-to-peak dilation for the 717 ms SOA and no-mask conditions for both subgroups and nonpsychiatric participants. Inspection of Figures 3 and 4 suggest that pupillary responses were abnormally small and detection accuracy was impaired in the low dilation subgroup. In contrast, pupillary responses were normal in the high dilation subgroup, but performance remained impaired. These impressions were confirmed by 3 (group) × 2 (condition) split-plot ANOVA’s computed for detection accuracy and pupillary responses (peak-to-peak dilation). For detection accuracy, significant effects were found for condition, F(1,99) = 25.80, p < .001, η2 = .19, and group, F(2,99) = 3.97, p < .05, η2 = .07, and a trend was found for the group × condition interaction, F(2,99) = 3.07, p = .05, η2 = .04. The nonpsychiatric participants showed significantly greater detection accuracy in the 717 ms SOA, but not no-mask, condition relative to both high and low dilation subgroups (p < .05), which did not differ significantly from each other. For pupillary responses, significant effects were also found for condition, F(1,99) = 18.37, p < .001,η2 = .17, and group, F(2,99) = 8.04, p < .01, η2 = .14, but not for the group × condition interaction, F(2,99) = 0.50, p = .61, η2 = .01. The low dilation subgroup showed significantly smaller pupillary responses in both conditions relative to both other groups (p <.05), who showed comparable pupillary responses to each other.
Figure 3.
Pupillary response waveforms with PCA factor windows indicated in the 717 ms SOA and no-mask conditions for subgroups of high and low dilation subgroups (based on median split on peak-to-peak dilation) of participants with schizophrenia.
Figure 4.
Detection accuracy and pupillary responses for nonpsychiatric participants (NC) and schizophrenia (SZ) subgroups. Error bars are ± 1 SE.
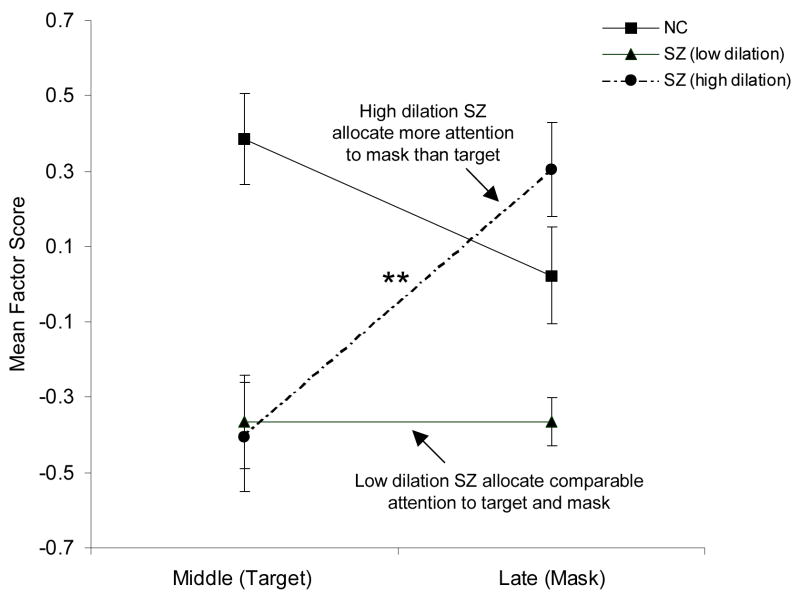
Figure 5 shows pupillary responses on middle (target) and late (mask) PCA factors for these subgroups and nonpsychiatric participants. Inspection of Figure 5 suggests that the high dilation subgroup showed smaller pupillary responses on the middle (target) relative to late (mask) factor; whereas, the nonpsychiatric participants showed the opposite pattern of greater dilation on the middle (target) relative to late (mask) factor. In contrast, the low dilation subgroup showed smaller pupillary responses on both factors. This pattern was confirmed by a 3 (group) × 2 (middle and late PCA factors) split-plot ANOVA computed for factor scores from the pupil waveform PCA, which revealed significant effects for group, F (2,99) = 9.18, p < .001, η2 = .16, and group × condition, F (2,99) = 8.91, p < .001, η2 = .15, but not for condition, F (1,99) = 1.12, p = .29, η2 = .01. The high dilation subgroup showed significantly greater pupillary responses on the late (mask) relative to middle (target) factor (p < .01), whereas the low dilation subgroup showed comparable pupil dilation in both the late and middle factors (p = .99). In sum, the low dilation subgroup demonstrated impaired detection accuracy relative to controls, low overall resource allocation (pupil responses) relative to both other groups, and comparable low allocation of resources across target and mask factors. In contrast, the high pupillary response subgroup showed impaired detection accuracy relative to controls, despite normal overall allocation of resources (pupillary responses), but showed misallocation of resources to mask rather than target factors.
Figure 5.
Pupillary responses on middle (target) and late (mask) PCA factors for nonpsychiatric (NC) participants and schizophrenia (SZ) subgroups. Error bars are ± 1 SE. **P < 0.01.
Medication and symptom effects
Comparisons (two-tailed t-tests) between patients receiving and not receiving anticholinergic medications and between patients receiving and not receiving mood medications showed no significant group differences for mean detection accuracy, mean baseline pupil size, mean peak-to-peak dilation or any mean PCA factor score across conditions. Additionally, current daily dosage of antipsychotic (CPZE) or anticholinergic (AChE) medication did not correlate significantly with any of these dependent variables.
As shown in Table 1, the high and low dilation subgroups did not differ significantly on any symptom dimension. Regression analyses were also explored within each subgroup to determine the extent to which the symptom dimensions predicted pupillary response variables. For the high pupil dilation subgroup, the combined model for the regression of symptom dimensions onto the mean middle (target) PCA factor did not explain a significant amount of variance, R2 = .19, F(4, 21) = 1.25, p = .32, and no predictor accounted for a significant amount of variance in the dependent variable. The combined model for the regression of symptom dimensions onto the mean late (mask) PCA factor did not explain a significant amount of variance, R2 = .31, F(4, 21) = 2.37, p = .09, but the positive dimension accounted for significant variance, β = −.27, t = −2.54, p < .05, which indicated that more severe positive symptoms were associated with less resource allocation to masks. For the low pupil dilation subgroup, the combined models for the regression of symptom dimensions onto the mean middle (target) and late (target) PCA factors did not explain a significant amount of variance, R2 = .15, F(4, 20) = .90, p = .49 and R2 = .19, F(4, 20) = 1.18, p = .35, respectively, and no predictor account for significant variance in either dependent variable.
Discussion
This study investigated the contribution of attentional mechanisms to backward masking task deficits in people with schizophrenia by recording pupillary responses as an index of resource allocation during a target-identification, high-energy mask version of the backward masking task. Consistent with our previous research with healthy undergraduates (Verney, Granholm, & Dionisio, 2001; Verney, 2001; Verney, Granholm, & Marshall, 2004) and people with schizophrenia (Granholm & Verney, 2004), pupil dilation responses were significantly greater in longer SOA conditions relative to the no-mask condition. This finding is consistent with masking models that predict attentional demands are highest in SOA conditions greater than 100–200 ms, because selective attention must be shifted and shared between targets and masks in order to identify both stimuli in short-term visual memory (Loftus, Hanna, & Lester, 1988; Michaels & Turvey, 1979; Phillips, 1974). Target identification accuracy was also significantly impaired in people with schizophrenia in the longest SOA condition. This finding of greater masking task impairment in the SOA condition with the highest processing load is consistent with the hypothesis that backward masking task impairments on similar types of tasks at longer SOA intervals may be due abnormalities in resource availability (Nuechterlein & Dawson, 1984; Granholm, 1992).
The present study replicated the PCA factor structure found for pupillary response waveforms in three previous masking studies (Verney, 2001; Verney, Granholm, & Marshall, 2004; Granholm & Verney, 2004). The middle factor occurred when the extent of task-evoked peak pupil dilation is typically found to reflect the processing load associated with key task operations (Beatty, 1982; Beatty & Lucero-Wagoner, 2000; Steinhauer & Hakerem, 1992). The late factor occurred after this task processing load factor. Importantly, the middle and late dilation factors showed the opposite pattern of results when masking and no-mask conditions were compared. Less dilation was found for masking relative to no-mask conditions for the middle factor, but greater dilation was found for masking relative to no-mask conditions for the late factor, especially in the longest SOA condition. An index of target processing should show greater processing load in the no-mask condition, where only targets are presented, while an index of mask processing should show greater processing load in masking conditions. This was exactly the pattern of results found for the middle and late factors, respectively. These findings suggest that the middle factor indexed target processing and the late factor indexed mask processing. We have previously shown that this pattern of PCA factor scores across conditions was not simply an artifact of presenting two stimuli (targets and masks) instead of one (targets only) or variations in response times (Granholm & Verney, 2004; Verney, Granholm, & Marshall, 2004).
An important question about schizophrenia that remains unanswered despite decades of neurocognitive research is whether cognitive impairments in these individuals are due to: 1) Deficit(s) in specific cognitive functions; or 2) generalized deficits related to insufficient effort and reduced general processing capacity. The difficulty distinguishing between these sources of impairment is a classic problem in schizophrenia research. One approach to partially examine this is to record pupillary responses or other psychophysiological measures of effortful resource allocation during cognitive tasks (Gopher, 1994; Kramer & Weber, 2000). One subgroup of patients in this study showed normal overall pupillary dilation responses, but unlike controls, showed greater resource allocation to masks than to targets on the PCA factor scores. The normal pupillary responses shown by this subgroup confirmed that resources were available and invested in the task. The performance impairment found in this subgroup, therefore, cannot be attributed insufficient effort or a depleted resource pool. Rather, a specific filtering deficit was identified in this subgroup of patients, who misallocated limited resources to masks leaving fewer resources spared for target processing. This finding is consistent with an attentional allocation problem that occurs in a later stage of processing (e.g., short-term visual memory) when inputs compete for stimulus identification resources (Knight, 1992; 1993), or an “attentional disengagement” deficit that occurs at longer SOAs (Green, Nuechterlein, & Breitmeyer, 1997). In contrast, another subgroup of patients showed significantly smaller pupillary dilation responses relative to controls, and showed similar low levels of allocation to targets and masks on the PCA factor scores. Poor effort or generalized capacity limitations cannot be ruled out in this subgroup. Nuechterlein and Dawson (1984) proposed that people with schizophrenia may perform poorly at higher processing loads due to limited availability of processing resources, and that reduced availability of resources might be due to misallocation of resources to task irrelevant operations and/or generally diminished resource pools. The findings in this study suggested that these different resource-limitation problems contributed to early visual processing impairments in different subgroups of people with schizophrenia.
Research on the nature of masking task deficits in schizophrenia has typically focused on two other masking mechanisms: Integration and interruption (or inhibition) and transient and sustained systems (Butler et al., 2001; Cadenhead, Serper, & Braff, 1998; Green, Nuechterlein, & Mintz, 1994), as well as aberrant gamma range activity in sustained channels (Green & Nuechterlein, 1999; Green et al., 2003; Green, Nuechterlein, & Breitmeyer, 1997; Kwon et al., 1999). The question of whether transient or sustained channel deficits or attentional resource allocation deficits better account for masking impairments centers on the question of whether input stages (e.g., quality of initial icon representation formation) and/or attentional mechanisms (e.g., competition for and allocation of higher cortical stimulus identification resources; top-down attentional modulation of early visual processing) can better account for deficits. Consistent with the findings in the present study, a number of top-down, attention manipulations have been found to modulate masking effects in healthy controls and people with schizophrenia (Ramachandran & Cobb, 1995; Havig, Breitmeyer, & Brown, 1998; Rassovsky, Green, Nuechterlein, Breitmeyer, & Mintz, 2005; Keri, Antal, Szekeres, Benedek, & Janka, 2000).
Bachmann (1994; 1997) described a neurophysiological masking model that emphasizes the role of attention and activation mediated by thalamic reticular systems (Bachmann, 1994; 1997). In this model, identification and conscious awareness of a stimulus requires both a specific input carrying content information (e.g., orientation, color, form, spatial frequency, etc.) through classic visual pathways to visual cortex and a nonspecific input from the thalamic reticular system. The nonspecific input is required to raise the level of activation of specific inputs to a level sufficient for awareness and identification. Because the nonspecific input is slower than the specific input, the timing of some masking intervals can lead to enhancement of mask specific inputs by target nonspecific inputs. Consistent with this model, several thalamic mechanisms are known to selectively enhance transfer and processing of sensory-perceptual data, such as sensory gating by the nucleus reticularis thalami (Scheibel, 1980; Crick, 1984) and cortical activity synchronization in cortico-thalamic loops (Alexander, DeLong, & Strick, 1986; Crick, 1984). Interestingly, gamma oscillations that may be involved in masking effects can be produced by stimulus-driven activation of cortico-thalamic loops (Tallon-Baudry, Bertrand, Delpuech, & Perneir, 1997).
It is possible that pupillary responses reflect these reticular-thalamic activation inputs. Pupil dilation is a function of the balance of activity between opposing sympathetic and parasympathetic systems. The brainstem nuclei driving these autonomic systems receive extensive inhibitory input from the reticular system and structures in cortico-thalamic loops. For example, electrical stimulation of the midbrain reticular formation in animals results in pupillary dilation (Loewenfeld, 1999). It is through these reticular connections that pupillary responses may provide an index of central brain systems that govern selective enhancement of sensory-perceptual inputs or attentional allocation (Beatty, 1986). In the present study, the reduced overall pupillary responses and abnormal distribution of pupillary responses between targets and masks found in patients with schizophrenia may implicate dysfunction in the general level of reticular system activation and/or the allocation of activation among inputs. Several investigators have emphasized thalamic mechanisms and cortico-thalamic loop dysfunction in schizophrenia (Andreasen et al., 1994; Carlsson & Carlsson, 1990; Swerdlow & Koob, 1987).
This study had several limitations. Participants were part of a larger longitudinal study of age-related decline in cognition in middle-aged and older people with schizophrenia, so the findings may not generalize to younger people. Decline in resource availability due to normal aging (Salthouse, 1988) may combine with reduced resource availability due to schizophrenia to produce greater impairment in older patients. In addition, a standard target exposure duration procedure was used in this study, because the limitations of our computer display did not permit matching participants for no-mask detection accuracy using a critical stimulus duration procedure. We attempted to correct for moderate group differences in no-mask performance by using ratio scores (masking/no-mask accuracy), and found more specific impairment in the longest SOA condition. However, masking effects are less ambiguous when no-mask performance is equated using a CSD or other procedure (e.g., graded contrast changes, as in Green, Nuechterlein, & Breitmeyer, 2002). Many participants (about half the patient sample) were also excluded either due to near-chance performance in this fixed-duration no-mask condition or excessive artifacts in pupil data. This amount of data loss may seriously impact the generalizability of findings, although included and excluded patients did not differ significantly on any demographic or symptom variable. While medication dosages were not correlated with performance or pupillary responses, there are many methodological problems with examining the relationships between psychophysiological and cognitive variables and medications expressed in terms of equivalent dosages (Spohn & Strauss, 1989). Estimates of drug equivalency are primarily based on clinical practice, so patients may have been treated with higher dosages because their symptoms are more severe. It may not be possible, therefore, to evaluate medication effects independent of symptom severity. Replication of these results in patients who are not taking medications is warranted, although difficult to accomplish. Finally, future studies should also further validate our interpretations of the pupil waveform PCA factors found in our masking studies. Additional evidence that the late factor reflects mask processing would be provided by manipulating the information value of the mask or using a procedure that required greater attention to masks and testing for increased dilation on the late factor with increased mask load. These studies are currently under way in our laboratory.
Acknowledgments
This research was supported by the National Institute of Mental Health (R01MH061381). We wish to thank the participants who volunteered for this research and Greg Siegle, Ph.D. for assistance with pupillary response data reduction software.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Reviews of Neuroscience. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Andreasen NC, Arndt S, Swayze V, Cizadlo T, Flaum M, O’Leary D, et al. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294 –298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Olsen S. Negative vs. positive schizophrenia: Definition and validation. Archives of General Psychiatry. 1982;39:789–94. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- Bachmann T. Psychophysiology of visual masking: The fine structure of conscious experience. Commack, NY: Nova Science Publishers, Inc; 1994. [Google Scholar]
- Bachmann T. Visibility of Brief Images: The Dual-Process Approach. Consciousness and Cognition. 1997;6:491–518. doi: 10.1006/ccog.1997.0320. [DOI] [PubMed] [Google Scholar]
- Balogh DW, Merritt RD. Susceptibility to type A backward pattern masking among hypothetically psychosis-prone college students. Journal of Abnormal Psychology. 1985;94:377–83. doi: 10.1037//0021-843x.94.3.377. [DOI] [PubMed] [Google Scholar]
- Balogh DW, Merritt RD. Visual masking and the schizophrenia spectrum: Interfacing clinical and experimental methods. Schizophrenia Bulletin. 1987;13:679–98. doi: 10.1093/schbul/13.4.679. [DOI] [PubMed] [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin. 1982;91:276–292. [PubMed] [Google Scholar]
- Beatty J. The pupillary system. In: Coles MGH, Donchin E, Porges SW, editors. Psychophysiology: Systems, Processes, and Applications. Amsterdam: Elsevier; 1986. pp. 43–50. [Google Scholar]
- Beatty J, Lucero-Wagoner B. The pupillary system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2. New York: Cambridge University Press; 2000. pp. 142–162. [Google Scholar]
- Blanchard JJ, Cohen AS. The structure of negative symptoms with schizophrenia: Implications for assessment. Schizophrenia Bulletin. 2006;32(2):238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Saccuzzo DP. Information processing dysfunction in paranoid schizophrenia: A two-factor deficit. American Journal of Psychiatry. 1981;138:1051–1056. doi: 10.1176/ajp.138.8.1051. [DOI] [PubMed] [Google Scholar]
- Braff DL, Saccuzzo DP, Geyer MA. Information processing dysfunctions in schizophrenia: Studies of visual backward masking, sensorimotor gating, and habituation. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of Schizophrenia. Vol. 5. New York: Elsevier; 1991. [Google Scholar]
- Breitmeyer BG. Visual masking: An integrative approach. New York: Oxford University Press; 1984. [Google Scholar]
- Breitmeyer BG, Ogmen H. Recent models and findings in visual backward masking: A comparison, review, and update. Perception and Psychophysics. 2000;62:1572–95. doi: 10.3758/bf03212157. [DOI] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, et al. Dysfunction of early-stage visual processing in schizophrenia. American Journal of Psychiatry. 2001;158:1126–33. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Perry W, Braff DL. The relationship of information-processing deficits and clinical symptoms in schizotypal personality disorder. Biological Psychiatry. 1996;40(9):853–858. doi: 10.1016/0006-3223(95)00547-1. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biological Psychiatry. 1998;43:132–8. doi: 10.1016/S0006-3223(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Schizophrenia: A subcortical neurotransmitter imbalance syndrome? Schizophrenia Bulletin. 1990;16:425–32. doi: 10.1093/schbul/16.3.425. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: The searchlight hypothesis. Proceedings of the National Academy of Sciences, U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereshefsky L, Richards A. Psychoses. In: Young LY, Koda-Kimble MA, editors. Applied Terapeutics: The Clinical Use of Drugs. Vancouver: Applied Therapeutics, Inc; 1990. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Gopher D. Analysis and measurement of mental load. In: Gery d’Y, Eelen P, Bertelson P., editors. International perspectives on psychological science: Vol. 2. The state of the art. Hillsdale, NJ, England: Lawrence Erlbaum Associates; 1994. [Google Scholar]
- Gjerde PF. Attentional capacity dysfunction and arousal in schizophrenia. Psychological Bulletin. 1983;93:57–72. [PubMed] [Google Scholar]
- Granholm E. Processing resource limitations in schizophrenia: Implication for predicting medication response and planning attentional training. In: Margolin DI, editor. Cognitive neuropsychology in clinical practice. New York: Oxford University Press; 1992. pp. 43–69. [Google Scholar]
- Granholm E, Chock D, Morris S. Pupillary responses evoked during verbal fluency tasks indicate semantic network dysfunction in schizophrenia. Journal of Clinical and Experimental Neuropsychology. 1998;20:1–17. doi: 10.1076/jcen.20.6.856.1107. [DOI] [PubMed] [Google Scholar]
- Granholm E, Verney SP. Pupillary responses and attentional allocational problems on the backward masking task in schizophrenia. International Journal of Psychophysiology. 2004;52:37–51. doi: 10.1016/j.ijpsycho.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Granholm E, Morris S, Asarnow RF, Chock D, Jeste DV. Accelerated age-related decline in processing resources in schizophrenia: Evidence from pupillary responses recorded during the span of apprehension task. Journal of the International Neuropsychological Society. 2000;6:30–43. doi: 10.1017/s1355617700611049. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. Cortical oscillations and schizophrenia: Timing is of the essence. Archives of General Psychiatry. 1999;56:1007–8. doi: 10.1001/archpsyc.56.11.1007. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenic patients: Evidence for a vulnerability indicator. Archives of General Psychiatry. 1997;54:465–72. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B. Development of a computerized assessment for visual masking. International Journal of Methods in Psychiatric Research. 2002;11:83–89. doi: 10.1002/mpr.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Mintz J, Salveson D, Nuechterlein KH, Breitmeyer B, Light GA, et al. Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biological Psychiatry. 2003;53:1113–1119. doi: 10.1016/s0006-3223(02)01813-9. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania: Specifying a mechanism. Archives of General Psychiatry. 1994;51:939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13(6):933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Grube BS, Bilder RM, Goldman RS. Meta-analysis of symptom factors in schizophrenia. Schizophrenia Research. 1998;31(2–3):113–120. doi: 10.1016/s0920-9964(98)00011-5. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Attention and Effort. Englewood Cliffs, N.J.: Prentice-Hall; 1973. [Google Scholar]
- Kahneman D, Beatty J. Pupillary responses in a pitch-discrimination task. Perception and Psychophysics. 1967;2(3):101–105. [Google Scholar]
- Havig PR, Breitmeyer BG, Brown VR. The effects of pre-cueing attention on metacontrast masking. Investigative Ophthalmology and Visual Science. 1998;39:S630. [Google Scholar]
- Keri S, Antal A, Szekeres G, Benedek G, Janka Z. Visual information processing in patients with schizophrenia: Evidence for the impairment of central mechanisms. Neuroscience Letters. 2000;293:69–71. doi: 10.1016/s0304-3940(00)01473-7. [DOI] [PubMed] [Google Scholar]
- Knight RA. Specifying cognitive deficiencies in premorbid schizophrenics. In: Walker EF, Dworkin BA, Cornblatt BA, editors. Progress in Experimental Personality and Psychopathology Research. Vol. 15. New York: Springer; 1992. pp. 253–289. [PubMed] [Google Scholar]
- Knight RA. Comparing cognitive models of schizophrenics’ input dysfunction. In: Cromwell RL, Snyder CR, editors. Schizophrenia: Origins, processes, treatment, and outcome. London: Oxford University Press; 1993. pp. 151–175. [Google Scholar]
- Kramer AF, Weber T. Applications of psychophysiology to human factors. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2. New York: Cambridge University Press; 2000. pp. 794–814. [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Archives of General Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenfeld IE. The pupil: Anatomy, Physiology, and Clinical Applications. Boston: Butterworth Heinemann; 1999. [Google Scholar]
- Loftus GR, Hanna AM, Lester L. Conceptual masking: How one picture captures attention from another picture. Cognitive Psychology. 1988;20:237–282. doi: 10.1016/0010-0285(88)90020-5. [DOI] [PubMed] [Google Scholar]
- Michaels CF, Turvey MT. Central sources of visual masking: Indexing structures supporting seeing at a single, brief glance. Psychological Research. 1979;41:1–61. doi: 10.1007/BF00309423. [DOI] [PubMed] [Google Scholar]
- Miller S, Saccuzzo DP, Braff D. Information processing deficits in remitted schizophrenics. Journal of Abnormal Psychology. 1979;88:448–451. [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorder. Schizophrenia Bulletin. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. Dimensional structure of psychotic symptoms: An item-level analysis of SAPS and SANS symptoms in psychotic disorders. Schizophrenia Research. 1999;38:13–26. doi: 10.1016/s0920-9964(99)00003-1. [DOI] [PubMed] [Google Scholar]
- Phillips WA. On the distinction between sensory storage and short-term visual memory. Perception and Psychophysics. 1974;16:283–290. [Google Scholar]
- Purushothaman G, Ogmen H, Bedell HE. Gamma-range oscillations in backward-masking functions and their putative neural correlates. Psychological Review. 2000;107:556–577. doi: 10.1037/0033-295x.107.3.556. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Cobb S. Visual attention modulates metacontrast masking. Nature. 1995;373:66–8. doi: 10.1038/373066a0. [DOI] [PubMed] [Google Scholar]
- Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Modulation of attention during visual masking in schizophrenia. American Journal of Psychiatry. 2005;162(8):1533–1535. doi: 10.1176/appi.ajp.162.8.1533. [DOI] [PubMed] [Google Scholar]
- Rund BR, Landro NI, Orbeck AL. Stability in backward masking performance in schizophrenics, affectively disturbed patients, and normal subjects. Journal of Nervous Mental Disorders. 1993;181:233–7. doi: 10.1097/00005053-199304000-00004. [DOI] [PubMed] [Google Scholar]
- Saccuzzo DP, Schubert DL. Backward masking as a measure of slow processing in schizophrenia spectrum disorders. Journal of Abnormal Psychology. 1981;90:305–12. doi: 10.1037//0021-843x.90.4.305. [DOI] [PubMed] [Google Scholar]
- Sadock BJ, Sadock VA, editors. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. Philadelphia, PA: Lippincott, Williams & Wilkins Publishers; 2000. [Google Scholar]
- Salthouse TA. Resource reduction interpretations of cognitive aging. Developmental Review. 1988;8:238–272. [Google Scholar]
- Sayers SL, Curran PJ, Muesser KT. Factor structure and construct validity of the Scale for the Assessment of Negative Symptoms. Psychological Assessment. 1996;8(3):269–280. [Google Scholar]
- Scheibel AB. Anatomical and physiological substrates of arousal: A view from the bridge. In: Hobson JA, Brazier MAB, editors. The Reticular Formation Revisited. Raven Press; 1980. pp. 55–66. [Google Scholar]
- Spohn HE, Strauss ME. Relation of Neuroleptic and Anticholinergic Medication to Cognitive Functions in Schizophrenia. Journal of Abnormal Psychology. 1989;98:367 –380. doi: 10.1037//0021-843x.98.4.367. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Hakerem G. The pupillary response in cognitve psychophysiology an schizophrenia. In: Friedman D, Bruder GE, editors. Psychophysiology and experimental psychopathology: A tribute to Samuel Sutton. New York, NY: New York Academy of Sciences; 1992. [DOI] [PubMed] [Google Scholar]
- Steronko RJ, Woods DJ. Impairment in early stages of visual information processing in nonpsychotic schizotypic individuals. Journal of Abnormal Psychology. 1978;87:481–90. doi: 10.1037//0021-843x.87.5.481. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF. Dopamine, schizophrenia, mania, and depression: Toward a unified hypothesis of cortico-striato-pallido-thalamic function. Behavioral and Brain Sciences. 1987;10:197–245. [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Perneir J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. Journal of Neuroscience. 1997;17:722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney SP. Pupillary responses index: Information processing efficiency across cultures. Dissertation Abstracts International: Section B: The Sciences and Engineering. 2001;61(11-B):6152. (UMI No. AA19992386) [Google Scholar]
- Verney SP, Granholm E, Marshall SP. Pupillary responses on the visual backward masking task reflect general cognitive ability. International Journal of Psychophysiology. 2004;52:23–36. doi: 10.1016/j.ijpsycho.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Verney SP, Granholm E, Dionisio DP. Pupillary responses and processing resources on the visual backward masking task. Psychophysiology. 2001;38:76–83. [PubMed] [Google Scholar]