Abstract
The aim was to identify an appropriate infant pneumococcal vaccination strategy for resource poor countries. Fijian infants received 0, 1, 2, or 3 doses of 7-valent pneumococcal conjugate vaccine (PCV) in early infancy. Following 3 PCV doses, geometric mean concentration (GMC) to all 7 serotypes were ≥ 1.0μg/mL, and >85% of children achieved antibody levels ≥0.35μg/mL at 18 weeks. Following 2 doses, GMC were lower for 6B, 14, and 23F, but higher for 19F compared with 3 doses. Following a single dose, significant responses were seen for all serotypes post primary series compared with the unvaccinated. By 12 months, differences between 2 and 3 doses persisted for serotype 14 only. Although GMC following 3 doses are higher than after 2 doses, the differences were small. A single dose may offer some protection for most serotypes.
Keywords: Pneumococcal conjugate vaccine
Introduction
Streptococcus pneumoniae (pneumococcus) is the most common cause of bacterial pneumonia in children worldwide. It is the leading vaccine preventable cause of serious infection in infants [1]. An estimated 1.6 million deaths are attributable to pneumococcal disease each year with the majority of these deaths occurring in low income countries primarily in children and the elderly [2]. The case fatality rate is particularly high in infants less than 6 months old [3]. Over 40 serogroups comprising of over ninety serotypes of pneumococcus have been identified [4]. Within serogroups, serotypes cross-react immunologically, and in some cases this translates into cross-protection. The association of particular serotypes with disease varies according to age, geography, and clinical site. Serotypes 6B, 14, and 19F are important worldwide, while serotype 5 is mainly found in low income countries and 18C is more common in affluent countries [5]. In general, the range of serotypes causing disease in affluent countries like the United States and in Europe are relatively narrow and largely confined to the serotypes found in the 7-valent pneumococcal conjugate vaccine (PCV, Prevnar®, Wyeth Vaccines). PCV includes only serotypes 4, 6A, 9V, 14, 18C, 19F, 23F. In contrast, the range of serotypes causing disease in low income countries is wider. Therefore PCV covers a smaller proportion of the pneumococcal serotypes causing disease in children in low income countries compared to more affluent countries.
In the USA, PCV has been shown to be safe and efficacious in a 3 dose primary series with a booster in the second year of life [6]. Following the introduction of PCV into the US national immunisation schedule in 2000 there has been a significant decline in vaccine type (VT) invasive pneumococcal disease (IPD) in all ages [7]. Replacement disease, particularly due to serotype 19A has developed due to capsular switching and clonal expansion [8]. A study from the United Kingdom trialled alternative schedules with the aim of reducing the number of PCV doses in the primary series and administering an earlier booster at 12 months of age (“2+1” schedule) [9]. The UK subsequently introduced a “2+1” schedule in 2007, in which children receive PCV at 2, 4 and 13 months. In some Scandinavian countries and Italy, routine immunisations are given in a 2 dose primary series with a booster at or before the end of the first year of life. When the introduction of PCV into the USA national immunisation schedule was met with a global shortage of vaccine, many children received fewer than the recommended 4 doses of vaccine. A case control study documenting the impact of this on IPD due to vaccine serotypes showed that one and 2 dose schedules given to infants less than 7 months of age had an effectiveness of 73% (95%CI 43–87%) and 96% (95%CI 88–99%) respectively [10].
From seroprevalence data collected in Fiji, the 7 serotypes included in PCV would cover 55% of episodes of IPD in children aged under 5 [11]. This would potentially increase to 83% if the 23-valent polysaccharide vaccine (23vPPS) was used, and due to the high prevalence of serotype 6A which is not included in 23vPPS, this would increase to 87% if the new 13-valent pneumococcal conjugate vaccine produced by Wyeth Vaccines (which includes serotypes 1, 3, 5, 6A, 7F and 19A) was used [11]. The aim of this study was to find a vaccination strategy for resource poor countries in terms of serotype coverage, flexibility, and affordability. To address these issues, we undertook a Phase II vaccine trial in Fiji to document the safety, immunogenicity and impact on pneumococcal carriage of various pneumococcal vaccination regimens combining 1, 2, or 3 doses of PCV in infancy. In order to broaden the serotype coverage, the additional benefit of a booster of 23-valent PS vaccine at 12 months of age was also assessed. This paper compares the geometric mean serotype specific IgG antibody concentrations (GMC) following the different PCV primary series, and up to 12 months of age.
Materials and Methods
1. Study participants
The study was a single blind, open-label randomized Phase II vaccine trial in Suva, Fiji. Healthy infants aged between 6 and 8 weeks were eligible for enrolment if they had no significant maternal or perinatal disease history; they resided within 30 minutes of one of the three participating health centres; and the family anticipated living in the study area for 2 years. Infants were excluded if they had: a known allergy to any component of the vaccine; an allergic reaction or anaphylactoid reaction with previous vaccines; a known immunodeficiency disorder; a HIV positive mother; known thrombocytopenia or coagulation disorder; were on immunosuppressive medication; received any blood product since birth; a severe congenital anomaly; a chronic or progressive disease; a seizure disorder; or a history of invasive pneumococcal, meningococcal, or Haemophilus influenzae diseases prior to study entry.
The study was conducted and monitored according to Good Clinical Practice. It was jointly approved by the Fiji National Research Ethics Review Committee and the University of Melbourne Human Research Ethics Committee. Written, informed consent was sought from families of children eligible to join the study according to methods approved by the overseeing ethics committees.
2. Study procedures and vaccines
Infants were enrolled at the time they presented to any one of 3 participating health centres to receive their first dose of the combined Diphtheria-Tetanus-whole cell Pertussis-H. influenzae type b-Hepatitis B vaccine (Hiberix® containing 10μg of purified Hib capsular polysaccharide covalently bound to approximately 30μg tetanus toxoid mixed with Tritanrix-HepB® containing not less than 30 IU of adsorbed D toxoid, not less than 60 IU of adsorbed T toxoid, not less than 4 IU of wP, and 10μg of recombinant HBsAg protein, GlaxoSmithKline) at 6 weeks of age. Randomisation lists were produced by the study statistician and group allocation was concealed in opaque envelopes which study nurses removed sequentially from a box. Eligible infants were stratified by ethnicity and randomised using a computer-generated list of random numbers in blocks of variable size to one of 8 groups to receive 0, 1, 2, or 3 doses of PCV. The 7-valent CRM197 protein-polysaccharide conjugate vaccine containing polysaccharide antigen from pneumococcal serotypes 4, 6B, 9V, 14, 18C, 19F, 23F (Prevnar ®, Wyeth Vaccines) was used. The vaccine contains 2 μg/serotype, except serotype 6B which is 4μg. The 3 dose group received PCV at 6 weeks (window 6–8 weeks of age), 10 weeks (window 8–12 weeks of age) and 14 weeks of age (window 12–16 weeks of age). The 2 dose group received PCV at 6 and 14 weeks of age and the one dose group received PCV at 14 weeks of age. Vaccines were given a minimum of 25 days apart. Routine vaccines (Hiberix® mixed with Tritanrix-HepB®) and oral polio were given at 6, 10, and 14 weeks of age. Hiberix®/Tritanrix-HepB® and PCV were given in the right and left anterolateral thigh respectively. The children in all primary series groups were further randomized to receive 23vPPS (Pneumovax®, Merck Sharpe Dohme, which consists of a purified mixture of 25μg of capsular polysaccharide from each 23 pneumococcal serotypes) or no booster at 12 months of age. All children received Measles-Rubella vaccine at 12 months of age. Responses to the 12 month 23vPPS vaccination will be presented elsewhere.
3. Laboratory procedures
All children had blood taken at 18 weeks and 12 months of age. Those children that were not randomized to receive the 12 month 23vPPS additionally had blood taken at 9 months of age. The 12 month blood sample was taken prior to the administration of the 23vPPS, so that the results presented in this manuscript are from the 7 groups that had blood taken in the first year of life. Blood was separated in the health centre, kept chilled and transported to the Colonial War Memorial Hospital laboratory where it was divided into aliquots and stored at −70°C or −20°C on the same day, until transported to the Pneumococcal Laboratory, Murdoch Childrens Research Institute, Melbourne on dry ice for analysis.
Anticapsular pneumococcal antibody levels were assayed for all serotypes in PCV at 18 weeks, 9 and 12 months of age, using a modified WHO ELISA method [12]. In brief, microtitre wells were coated with pneumococcal polysaccharide diluted in phosphate buffered saline by incubating at room temperature overnight. To neutralise non-specific cell wall polysaccharide (CPS) antibodies, serum samples were diluted 1/100 in pre-absorption buffer containing CPS (10μg/mL) and serotype 22F (30μg/mL) and incubated overnight at 4°C. The reference serum standard 89-SF (Food and Drug Administration, Bethesda MD) was pre-absorbed with CPS at 10μg/mL and incubated overnight at 4°C. Horseradish peroxidase conjugated anti-human IgG and a TMB(3.3′, 5.5′-tetramethylbenzidine) substrate solution were used for detection. A high, medium, and low control serum were used on each plate to assess assay performance and inter-assay variation. Results from an inter-laboratory correlation between Wyeth Vaccines and the KTL Finland laboratory demonstrated a good correlation in serotype specific antibody concentrations [12]. Laboratory staff were blinded to the group allocation of each serum sample.
4. Statistical analysis
This manuscript reports analytic results concerning the secondary purpose of the trial. All case reporting forms were monitored prior to double data entry into Epidata version 3.1 (Centers for Disease Control, Atlanta, GA, USA). Cleaned data were exported to Stata version 9.0 (Stata Corporation, College Station, Texas) for analysis. Serotype specific antibody concentrations by ELISA were log (base e) transformed to calculate GMC. Pair-wise comparisons of serotype specific GMC between 0–3 dose PCV groups were performed using a two sample t-test. Comparisons of the proportion of infants between groups with serotype specific antibody concentrations ≥0.35 and ≥1μg/mL for 0–3 dose PCV groups were performed using Fisher’s exact test. A p-value of <0.01 was considered statistically significant due to the multiple comparisons.
Results
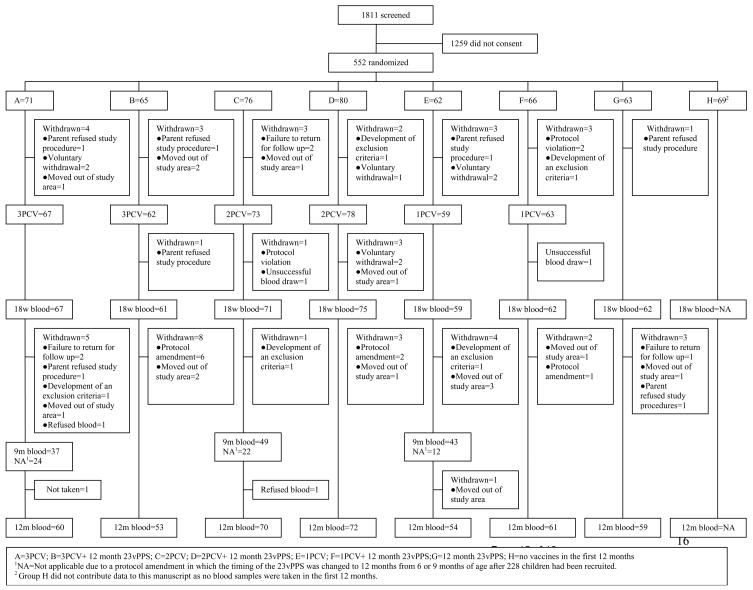
There were 552 children enrolled in the study (Figure 1). Characteristics and the number of children randomized to groups 1–7 are shown in Table 1. The consent rate was 30.5%. At 12 months of age the withdrawal rate among children who had consented to join the study was 10%. No participant was withdrawn due to a reaction to any of the vaccines.
Figure 1.
CONSORT chart of the screened and enrolled children to 12 months of age
Table 1.
Baseline characteristics of infants at enrolment and randomized to the 7 different PCV groups
| Characteristic | 3 PCV (n=136) | 2 PCV (n=156) | 1 PCV (n=128) | 0 PCV (n=63) |
|---|---|---|---|---|
| Gender | ||||
| Male | 71 (52%) | 70 (45%) | 59 (46%) | 32 (51%) |
| Female | 65 (48%) | 86 (55%) | 69 (54%) | 31 (49%) |
| Median age in weeks | 6.7 | 6.4 | 6.5 | 6.5 |
| Ethnicity | ||||
| Indigenous Fijian | 82 (60%) | 106 (68%) | 83 (65%) | 35 (55%) |
| Indo-Fijian | 46 (34%) | 45 (29%) | 39 (31%) | 20 (32%) |
| Other | 8 (6%) | 5 (3%) | 6 (4%) | 8 (13%) |
| Median weight in grams | 4900 | 4800 | 4825 | 4750 |
At 18 weeks of age, GMC in the 2 dose group were significantly lower (each p<0.001) for 3/7 serotypes (6B, 14, 23F), while the GMC for serotype 19F was significantly higher (p=0.003) than in the 3 dose group (Table 2). Similarly, the proportions of infants with antibody concentrations ≥0.35μg/mL were significantly lower for serotypes 14 (p=0.001) and 23F (p=0.003) when comparing the 2 and 3 dose groups. The proportions of infants with antibody concentrations ≥1μg/mL were significantly lower for serotypes 6B (p<0.001), 14 (p=0.004), and 23F (p<0.001) in the 2 dose group when compared to the 3 dose group (Table 3).
Table 2.
Geometric mean concentrations (GMC) of serotype specific IgG titres taken 4 weeks following the PCV‡ primary series, and at 9 and 12 months of age, by number of PCV doses administered in the primary series
| Serotypes | 3 PCV (n=1251) | 2 PCV (n=146) | 1 PCV (n=121) | 0 PCV 3,4 (n=62) | ||
|---|---|---|---|---|---|---|
| GMC (95%CI) | GMC (95%CI) | 3 vs 2 p value2 | GMC (95%CI) | 3 vs 1 p value2 | GMC (95%CI) | |
| 4 weeks post primary series | ||||||
| 4 | 5.47 (4.84–6.19) | 5.23 (4.46–6.13) | 0.661 | 2.20 (1.80–2.70) | <0.001 | 0.04 (0.03–0.04) |
| 6B | 1.66 (1.33–2.07) | 0.86 (0.70–1.07) | <0.001 | 0.19 (0.16–0.22) | <0.001 | 0.11 (0.10–0.13) |
| 9V | 4.76 (4.19–5.40) | 4.71 (3.88–5.71) | 0.933 | 0.90 (0.74–1.09) | <0.001 | 0.07 (0.06–0.08) |
| 14 | 5.51 (4.50–6.76) | 3.12 (2.42–4.03) | <0.001 | 1.07 (0.89–1.27) | <0.001 | 0.34 (0.27–0.43) |
| 18C | 3.20 (2.66–3.86) | 2.67 (2.16–3.31) | 0.22 | 0.58 (0.45–0.74) | <0.001 | 0.06 (0.05–0.07) |
| 19F | 5.52 (4.79–6.36) | 7.99 (6.62–9.64) | 0.003 | 0.84 (0.70–1.00) | <0.001 | 0.25 (0.21–0.30) |
| 23F | 2.93 (2.39–3.59) | 1.65 (1.29–2.11) | <0.001 | 0.23 (0.20–0.27) | <0.001 | 0.11 (0.10–0.14) |
| 9 months | (n=37) | (n=49) | (n=43) | |||
| 4 | 0.79 (0.55–1.14) | 0.86 (0.67–1.12) | 0.682 | 0.60 (0.42–0.85) | 0.277 | NA |
| 6B | 0.82 (0.58–1.17) | 0.81 (0.59–1.12) | 0.949 | 0.39 (0.29–0.52) | 0.001 | NA |
| 9V | 0.91 (0.71–1.16) | 1.00 (0.72–1.38) | 0.661 | 0.56 (0.40–0.77) | 0.021 | NA |
| 14 | 3.99 (2.86–5.57) | 1.93 (1.20–3.09) | 0.02 | 1.11 (0.79–1.57) | <0.001 | NA |
| 18C | 0.49 (0.37–0.65) | 0.41 (0.33–0.53) | 0.353 | 0.18 (0.14–0.24) | <0.001 | NA |
| 19F | 1.04 (0.70–1.54) | 1.40 (1.05–1.86) | 0.207 | 0.89 (0.61–1.29) | 0.568 | NA |
| 23F | 0.65 (0.46–0.94) | 0.44 (0.33–0.60) | 0.094 | 0.24 (0.18–0.32) | <0.001 | NA |
| 12 months | (n=113) | (n=142) | (n=1145) | (n=59) | ||
| 4 | 0.48 (0.41–0.57) | 0.47 (0.40–0.54) | 0.73 | 0.63 (0.50–0.81) | 0.066 | 0.07 (0.06–0.09) |
| 6B | 0.86 (0.72–1.03) | 0.76 (0.63–0.92) | 0.356 | 0.57 (0.46–0.71) | 0.005 | 0.14 (0.12–0.17) |
| 9V | 0.59 (0.51–0.67) | 0.62 (0.54–0.71) | 0.522 | 0.50 (0.41–0.62) | 0.221 | 0.09 (0.07–0.11) |
| 14 | 2.38 (1.98–2.86) | 1.52 (1.26–1.84) | 0.001 | 1.16 (0.94–1.44) | <0.001 | 0.19 (0.16–0.24) |
| 18C | 0.32 (0.27–0.38) | 0.24 (0.21–0.28) | 0.011 | 0.17 (0.15–0.20) | <0.001 | 0.06 (0.05–0.08) |
| 19F | 1.05 (0.83–1.34) | 1.14 (0.95–1.36) | 0.592 | 0.93 (0.76–1.15) | 0.462 | 0.46 (0.35–0.59) |
| 23F | 0.54 (0.44–0.66) | 0.42 (0.35–0.50) | 0.07 | 0.26 (0.21–0.31) | <0.001 | 0.07 (0.06–0.09) |
Three bloods not available for testing
Two sample t test was applied to compare GMC following 2 doses or a single dose of PCV with the GMC following 3 doses of PCV
Two sample t test was applied to compare GMC following a single dose of PCV with the GMC following 0 doses of PCV. For all serotypes the p-value was <0.001 except for serotype 23F at 12 months of age whereby the p-value was 0.042.
Two sample t test was applied to compare GMC following 0 doses of PCV with the GMC following 3 doses of PCV. For all serotypes and all time points the p-value was <0.001.
One blood not available for testing
NA Not applicable
Table 3.
Proportion of infants with antibody concentrations ≥0.35 and ≥1μg/mL at 4 weeks post primary series, and at 9 and 12 months of age, by number of PCV doses administered in the primary series
| ≥0.35μg/mL | ≥1μg/mL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotypes | 3 PCV | 2 PCV | 3 vs 2 p value | 1 PCV | 3 vs 1 p value | 0 PCV± | 1 vs 0 p value | 3 PCV | 2 PCV | 3 vs 2 p value | 1 PCV | 3 vs 1 p value | 0 PCV1 | 1 vs 0 p value |
| 4 weeks post primary series | ||||||||||||||
| (n=1252) | (n=146) | (n=121) | (n=62) | (n=1252) | (n=146) | (n=121) | (n=62) | |||||||
| 4 | 100 | 98.6 | 0.501 | 95.9 | 0.028 | 0 | <0.001 | 97.6 | 93.8 | 0.152 | 76.9 | <0.001 | 0 | <0.001 |
| 6B | 87.2 | 77.4 | 0.04 | 15.7 | <0.001 | 6.5 | 0.099 | 66.4 | 39.0 | <0.001 | 3.3 | <0.001 | 0 | 0.301 |
| 9V | 100 | 95.2 | 0.016 | 84.3 | <0.001 | 3.2 | <0.001 | 96.8 | 90.4 | 0.049 | 43.8 | <0.001 | 1.6 | <0.001 |
| 14 | 99.2 | 90.4 | 0.001 | 86.8 | <0.001 | 53.2 | <0.001 | 87.2 | 72.6 | 0.004 | 52.9 | <0.001 | 12.9 | <0.001 |
| 18C | 93.6 | 90.4 | 0.379 | 61.2 | <0.001 | 3.2 | <0.001 | 86.4 | 79.5 | 0.149 | 42.1 | <0.001 | 0 | <0.001 |
| 19F | 99.2 | 98.6 | 1.000 | 81.8 | <0.001 | 33.9 | <0.001 | 97.6 | 93.2 | 0.152 | 33.1 | <0.001 | 4.8 | <0.001 |
| 23F | 94.4 | 82.2 | 0.003 | 29.8 | <0.001 | 9.7 | 0.003 | 80.8 | 60.9 | <0.001 | 4.1 | <0.001 | 0 | 0.169 |
| 9 months | (n=37) | (n=49) | (n=43) | (n=37) | (n=49) | (n=43) | ||||||||
| 4 | 89.2 | 85.7 | 0.751 | 67.4 | 0.031 | NA | NA | 35.1 | 42.9 | 0.511 | 30.2 | 0.811 | NA | NA |
| 6B | 75.7 | 71.4 | 0.806 | 53.5 | 0.061 | NA | NA | 43.2 | 44.9 | 1.000 | 13.9 | 0.005 | NA | NA |
| 9V | 91.9 | 85.7 | 0.504 | 69.8 | 0.023 | NA | NA | 48.6 | 57.1 | 0.514 | 32.6 | 0.173 | NA | NA |
| 14 | 100 | 79.6 | 0.004 | 86.0 | 0.028 | NA | NA | 89.2 | 67.3 | 0.021 | 53.5 | 0.001 | NA | NA |
| 18C | 70.3 | 61.2 | 0.494 | 25.6 | <0.001 | NA | NA | 18.9 | 10.2 | 0.348 | 4.7 | 0.073 | NA | NA |
| 19F | 89.2 | 95.9 | 0.395 | 76.7 | 0.237 | NA | NA | 40.5 | 59.2 | 0.127 | 34.9 | 0.648 | NA | NA |
| 23F | 73.0 | 55.1 | 0.116 | 25.6 | <0.001 | NA | NA | 35.1 | 20.4 | 0.146 | 6.9 | 0.002 | NA | NA |
| 12 months | (n=113) | (n=142) | (n=1143) | (n=59) | (n=113) | (n=142) | (n=1143) | (n=59) | ||||||
| 4 | 64.6 | 66.2 | 0.793 | 63.2 | 0.89 | 6.8 | <0.001 | 16.8 | 18.3 | 0.869 | 32.5 | 0.009 | 0 | <0.001 |
| 6B | 82.3 | 73.2 | 0.099 | 64.9 | 0.004 | 11.9 | <0.001 | 48.7 | 34.5 | 0.029 | 27.2 | 0.001 | 3.4 | <0.001 |
| 9V | 72.6 | 81.0 | 0.133 | 67.5 | 0.469 | 8.5 | <0.001 | 25.7 | 28.9 | 0.672 | 18.4 | 0.203 | 1.7 | 0.001 |
| 14 | 95.6 | 88.0 | 0.042 | 86.0 | 0.02 | 11.9 | <0.001 | 83.2 | 69.0 | 0.013 | 57.9 | <0.001 | 8.5 | <0.001 |
| 18C | 41.6 | 31.7 | 0.116 | 19.3 | <0.001 | 5.1 | 0.012 | 10.6 | 4.9 | 0.097 | 1.8 | 0.006 | 0 | 0.548 |
| 19F | 90.3 | 88.7 | 0.838 | 80.7 | 0.059 | 54.2 | <0.001 | 41.6 | 57.0 | 0.017 | 44.7 | 0.688 | 23.7 | 0.008 |
| 23F | 61.9 | 57.0 | 0.444 | 30.7 | <0.001 | 20.3 | 0.155 | 29.2 | 17.6 | 0.035 | 9.6 | <0.001 | 5.1 | 0.386 |
Fisher’s exact test was applied to compare 3 PCV doses with no dose. For all serotypes the p-value was <0.001 except serotypes 18C (p=0.009) and 19F (p=0.029) for the ≥1μg/mL comparison at 12 months of age.
Three bloods not available for testing
One blood not available for testing NA Not applicable
By 9 months of age there were minimal differences between antibody levels in the 2 and 3 dose groups. At 12 months of age antibody levels against serotype 14 were still significantly lower (p=0.001) in the 2 dose group, with serotype 18C also being lower (p=0.011) (Table 2). The proportions of children with antibody concentrations ≥0.35μg/mL and ≥1μg/mL were not significantly different in the 2 dose group compared to the 3 dose group for any serotype (Table 3).
Discussion
Our study has shown that a 3 dose PCV schedule is more immunogenic than alternative schedules with one or 2 PCV doses. Despite a difference in post primary GMC between the 3 and 2 dose group, the proportions of infants with antibody concentrations above the estimated protective level of 0.35μg/mL 4 weeks after the primary series was >90% for all serotypes in the 2 dose group except 6B (77.4%) and 23F (82.2%). However the differences between the 3 and 2 PCV dose groups were minimal by 12 months of age. There was a natural decline in levels of circulating serotype specific antibodies from 18 weeks to 12 months of age in the 3 and 2 dose groups with no significant difference between the 2 groups in the proportion of children with antibodies ≥0.35μg/mL by 12 months of age.
The immunogenicity of 3 versus a 2 dose pneumococcal primary series with different coadministered vaccines, has shown different results in different settings [9,13–17]. Following 3 doses of the 9-valent pneumococcal conjugate vaccine in Icelandic infants, 7 out of the 9 serotypes had significantly higher post primary antibody levels compared to the 2 dose group [15]. However the proportion of infants in the 2 dose group with antibody levels >0.35μg/mL was only significantly lower compared to the 3 dose group for serotype 6B [15]. A study using an 11-valent pneumococcal conjugate vaccine in Israeli infants showed a 2 dose schedule was less immunogenic than a 3 dose post primary series for serotypes 6B, 14, 18C, and 23F with a significantly lower proportion of infants with antibody levels ≥0.35μg/mL for serotypes 6B, 18C, and 23F. This study used an unlicensed 11-valent pneumococcal conjugate vaccine conjugated to diphtheria and tetanus carrier proteins [16]. PCV given to US infants showed 3 doses were needed to achieve an immunological response for serotype 6B but 2 doses were sufficient for the other 6 PCV serotypes [17]. PCV was less immunogenic for serotype 6B in Italian infants after 2 PCV doses than described in the US and Finland, but similar for the other PCV serotypes [13,18,19]. In contrast, other studies have found minimal immunological differences between a 3 or 2 PCV dose primary series [9,13]. A study in UK infants showed no significant difference in GMC following a 3 or 2 dose schedule [9]. There was no significant difference in the proportion of infants achieving antibody concentrations >0.35μg/mL except for serotype 14 for which there was a higher proportion of infants in the 3 dose group [9]. Nevertheless, 2 doses may well be sufficient to protect against most PCV serotypes as early effectiveness data from the UK using the “2+1” schedule has shown a reduction in all infant IPD [20]. However 3 doses may be required to protect against serotype 6B as breakthrough cases of 6B have occurred [20]. Ongoing surveillance will determine whether these breakthrough cases will be a significant and consistent finding.
Whilst it is difficult to directly compare our immunogenicity results with other studies using different conjugate and co-administered vaccines in different ethnic groups, our data show at least comparable GMC results post primary series for all serotypes except serotype 6B and 23F following a 2 dose primary series compared with immunogenicity data from a USA trial [19]. In addition, our results for both the 3 and 2 dose groups were similar to those of Gambian infants who received 3 doses of an unlicensed 9-valent PCV also produced by Wyeth Vaccines [21]. The only exception was that a lower proportion of Fijian children who received 2 doses had antibody concentrations ≥0.35μg/mL for serotype 23F compared with the Gambian infants [21]. Our GMC were, in general, similar following 2 or 3 PCV doses compared with the GMC from South African infants, except serotype 6B was less immunogenic in the Fijian 2 dose group [22]. Immunogenicity results for a 2 dose primary series from this study suggests similar protection may be obtained for IPD in the first 12 months of life as achieved in vaccine effectiveness and clinical trials [7,23,24], except perhaps for serotype 6B.
Comparisons of immunogenicity results between studies is also difficult as laboratory methods may differ. Our laboratory’s results showed excellent inter-laboratory correlation between the Finnish KTL laboratory and Wyeth laboratories [12]. Dissimilar results between studies may also be influenced by the common circulating serotypes at different study sites, which provide natural exposure and boosting [25]. However in this study, the poorer immunogenicity of serotypes 6B and 23F is most likely due to these serotypes being inherently less immunogenic. It is noteworthy that the GMC for serotype 14 in this study is better maintained over time for all schedules. This serotype is the commonest cause (33%) of IPD in children under 5 years old in Fiji and as such may have provided natural boosting [11].
Our study has shown that a single PCV dose administered at 14 weeks of age induces a significant immunological response and is likely to offer some protection for most serotypes. This is consistent with findings from a South African trial which showed a significant and potentially protective antibody response to most serotypes following a single dose at 6 weeks of age with at least 70% of infants producing antibody concentrations >0.15μg/mL after a single PCV dose and at least 95% doing so after 2 doses [26]. A case control study in the USA similarly documented that a single dose schedule administered to infants less than 7 months of age had an effectiveness of 73% (95%CI 43–87%) on VT IPD [10]. Models however have predicted that a single PCV dose given between 5–10 months of age could prevent a significant amount of VT IPD [27]. However, it is unlikely that significant protection, from a single PCV dose administered early in infancy, would persist for children throughout the highest risk period for IPD and pneumonia and an early booster at 6 or 9 months of age (“1+1” schedule) is worthy of further investigation for use in developing countries. Indeed our study has shown that booster responses tend to be stronger after a single dose of PCV rather than 2 or 3 doses (unpublished data). This may be particularly relevant for low income countries where access to immunisation services is irregular, and middle income countries who have a significant burden of disease and are unable to pay affluent country vaccine prices but who do not benefit from immunisation financing mechanisms such as the Advanced Market Commitment or the International Finance Facility for Immunisation.
When interpreting our study it is important to note that two factors may have increased the immunogenicity of the one and 2 dose groups. Firstly, the co-administration of Diphtheria-Tetanus-whole cell Pertussis (DTwP) may have enhanced the immune response due to its potential adjuvant effect [28]. Although there are no studies in the literature directly comparing immunogenicity of PCV with Diphtheria-Tetanus-acellular Pertussis (DTaP) and DTwP, studies with co-administered DTwP tend to have stronger immune responses than those in which PCV is co-administered with DTaP. Furthermore, studies from Israel of an unlicenced 11-valent pneumococcal conjugate vaccine showed that co-administration with DTaP resulted in substantially lower immune responses compared to co-administration with DTwP [28]. Thus, in the present study, co-administration with DTwP may have augmented the immunogenicity of PCV.
Secondly, the carrier protein in PCV is CRM197, a modified diphtheria toxin. Experience with Hib vaccines has shown that prior administration of DTwP may augment responses to the CRM197 based conjugate vaccines [29,30]. Thus, the groups that received one or two doses of PCV may have had augmented responses to their 14 week dose of PCV as a result of extra doses of DTwP. Therefore, care should be taken before using these data to support a two dose schedule in which only 2 doses of either DTwP or DTaP are given.
Based on re-analysis of existing phase 3 efficacy trials, it has been estimated that 0.35μg/mL represents a protective level above which an individual is protected against IPD, particularly meningitis [31]. While the level required to protect children against pneumonia may be higher, it is unlikely that such an absolute level exists, and more likely that higher levels may be more helpful where other risk factors are present. Our results show that the proportion of infants with antibody concentrations ≥1μg/mL is significantly higher at 18 weeks of age in the 3 dose group compared with the 2 dose group for 3/7 PCV serotypes. However by 9 and 12 months of age the proportion of infants achieving this level is higher in the 2 dose group as compared to the 3 dose group for 4/7 and 3/7 serotypes, respectively. Hence infants may be protected from most VT IPD and severe pneumococcal disease but be less protected from non-bacteraemic pneumonia and otitis media in the first 9 months of life with a 2 dose schedule. For countries with high mortality, a one or 2 dose primary series may be sufficient to reduce mortality and the burden of severe pneumococcal disease.
In summary, the immunogenicity of 3 PCV doses is better than 2 doses. However it is likely that a 2 dose PCV primary series would offer similar protection as provided by 3 doses for most serotypes except possibly 6B. One PCV dose is likely to offer some protection for many serotypes. Further results documenting the avidity and opsonophagocytic activity post primary series, the responses post booster, and the impact on nasopharyngeal carriage will follow.
Acknowledgments
The authors wish to sincerely thank all the FiPP staff and families participating in the study, and the many other people who contributed to the study including: Amanda O’Brien, Kathryn Bright, Samantha Colqhoun, Amy Bin Chen, Timothy Gemetzis, Amy Auge, Katherine Gilbert, Evan Willis, Philip Greenwood, Beth Temple, Vanessa Johnston, Porter Anderson, Brian Greenwood, George Siber, David Klein, Elizabeth Horigan, and Farukh Khambaty. Funding was provided by NIAID and the National Health and Medical Research Council. Pneumovax® was kindly donated by the Commonwealth Serum Laboratories, Australia. The co-administered Tritanrix-HepB® and Hiberix® vaccines were kindly donated by GlaxoSmithKline.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The WHO Young Infants Study Group. Conclusions from the WHO multicenter study of serious infections in young infants. Pediatric Infect Dis J. 1999;18:S32–4. doi: 10.1097/00006454-199910001-00006. [DOI] [PubMed] [Google Scholar]
- 2.Pneumococcal Conjugate Vaccine for childhood immunization-WHO position paper. World Health Organization Weekly Epidemiological Record No. 12. 2007;82:93–104. [PubMed] [Google Scholar]
- 3.Denny FW, Loda FA. Acute respiratory infections are the leading cause of death in children in developing countries. American Journal of Tropical Medicine & Hygiene. 1986;35(1):1–2. doi: 10.4269/ajtmh.1986.35.1. [DOI] [PubMed] [Google Scholar]
- 4.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007;45(4):1225–33. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hausdorrf WP, Bryant J, Kloek C, Paradiso PR, Siber GR. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. CID. 2000;30(1):122–40. doi: 10.1086/313609. [DOI] [PubMed] [Google Scholar]
- 6.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19(3):187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 8.Moore MR, Gertz RE, Jr, Woodbury RL, Barkocy-Gallagher GA, Schaffner W, Lexau C, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197(7):1016–27. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 9.Goldblatt D, Southern J, Ashton L, Richmond P, Burbidge P, Tasevska J, et al. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 2006;25(4):312–9. doi: 10.1097/01.inf.0000207483.60267.e7. [DOI] [PubMed] [Google Scholar]
- 10.Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368(9546) doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 11.Russell FM, Chandra R, Carapetis JR, Seduadua A, Tikoduadua L, Buadromo E, et al. Epidemiology and Serotypes of Invasive Pneumococcal Disease in all ages in Fiji. 6th International Symposium of Pneumococci and Pneumococal Diseases; Reykjavik, Iceland. June 8–12; 2008. Abstract P2–038. [Google Scholar]
- 12.Balloch A, Mininni T, Nurkka A, Mackenzie G, Leach A, Kayhty H, et al. Interlaboratory comparison of the specific IgG response to serotypes in Prevenar. 5th International Symposium on Pneumococci and Pneumococcal Diseases; Alice Springs, Australia. April 2–6; 2006. Abstract PODT-31. [Google Scholar]
- 13.Esposito S, Pugni L, Bosis S, Proto A, Cesati L, Bianchi C, et al. Immunogenicity, safety and tolerability of heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 11 months post-natally to pre- and full-term infants. Vaccine. 2005;23(14):1703–8. doi: 10.1016/j.vaccine.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Käyhty H, Ahman H, Eriksson K, Sörberg M, Nilsson L. Immunogenicity and tolerability of a heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 12 months of age. Pediatr Infect Dis J. 2005;24(2):108–14. doi: 10.1097/01.inf.0000151022.92222.be. [DOI] [PubMed] [Google Scholar]
- 15.Sigurdardottir ST, Davidsdottir K, Arason VA, Jonsdottir O, Laudat F, Gruber WC, Jonsdottir I. Safety and immunogenicity of CRM197-conjugated pneumococcal-meningococcal C combination vaccine (9vPnC-MnCC) whether given in two or three primary doses. Vaccine. 2008;26(33):4178–86. doi: 10.1016/j.vaccine.2008.05.072. [DOI] [PubMed] [Google Scholar]
- 16.Dagan R, Givon-Lavi N, Chimney S, Jancu J, Greenberg D. Immunogenicity of CRM-Conjugated 7-Valent Pneumococcal Vaccine (PCV7) Administered at a Licensed 3-dose primary Schedule (3+1) Compared to a reduced 2-Dose Primary Schedule (2+1)- A Randomized Study. 45th meeting of IDSA; October 4–7; San Diego. 2007. Abstract 189. [Google Scholar]
- 17.Rennels MB, Edwards KM, Keyserling HL, Reisinger KS, Hogerman DA, Madore DV, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatics. 1998;101(4):604–11. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 18.Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, et al. Finnish Otitis Media Study Group. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344(6):403–9. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 19.Shinefield HR, Black S, Ray P, Chang I, Lewis N, Fireman B, et al. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 1999;18(9):757–63. doi: 10.1097/00006454-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Goldblatt D. Immunology and Impact of Reduced and Alternative Schedules of Pneumococcal Conjugate Vaccination. 6th International Symposium of Pneumococci and Pneumococal Diseases; June; Reykjavik, Iceland. 2008. [Google Scholar]
- 21.Saaka M, Okoko BJ, Kohberger RC, Jaffar S, Enwere G, Biney EE, et al. Immunogenicity and serotype-specific efficacy of a 9-valent pneumococcal conjugate vaccine (PCV-9) determined during an efficacy trial in The Gambia. Vaccine. 2008;26(29–30):3719–26. doi: 10.1016/j.vaccine.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 22.Madhi SA, Kuwanda L, Cutland C, Holm A, Käyhty H, Klugman KP. Quantitative and qualitative antibody response to pneumococcal conjugate vaccine among African human immunodeficiency virus-infected and uninfected children. Pediatr Infect Dis J. 2005;24(5):410–6. doi: 10.1097/01.inf.0000160942.84169.14. [DOI] [PubMed] [Google Scholar]
- 23.Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, et al. Gambian Pneumococcal Vaccine Trial Group. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365(9465):1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 24.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N Vaccine Trialists Group. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 349(14):1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 25.Puumalainen T, Dagan R, Wuorimaa T, Zeta-Capeding R, Lucero M, Ollgren J, Käyhty H, Nohynek H. Greater antibody responses to an eleven valent mixed carrier diphtheria- or tetanus-conjugated pneumococcal vaccine in Filipino than in Finnish or Israeli infants. Pediatr Infect Dis J. 2003;22(2):141–9. doi: 10.1097/01.inf.0000050459.74134.d5. [DOI] [PubMed] [Google Scholar]
- 26.Huebner RE, Mbelle N, Forrest B, Madore DV, Klugman KP. Immunogenicity after one, two or three doses and impact on the antibody response to coadministered antigens of a nonavalent pneumococcal conjugate vaccine in infants of Soweto, South Africa. Pediatr Infect Dis J. 2002;21(11):1004–7. doi: 10.1097/00006454-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Barzilay EJ, O’Brien KL, Kwok YS, Hoekstra RM, Zell ER, Reid R, et al. Could a single dose of pneumococcal conjugate vaccine in children be effective? Modeling the optimal age of vaccination. Vaccine. 2006;24(7):904–13. doi: 10.1016/j.vaccine.2005.08.092. [DOI] [PubMed] [Google Scholar]
- 28.Dagan R, Goldblatt D, Maleckar J, Yaich M, Eskola J. Reduction of antibody response to an 11-valent pneumococcal vaccine coadministered with a vaccine containing acellular pertussis components. Infect Immun. 2004;72(9):5383–91. doi: 10.1128/IAI.72.9.5383-5391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell F, Heath P, Shackley F, MacLennan J, Shearstone N, Diggle L, et al. Effect of combination with an acellular pertussis, diphtheria, tetanus vaccine on antibody response to Hib vaccine (PRP-T) Vaccine. 1998;16(6):637–42. doi: 10.1016/s0264-410x(97)84511-2. [DOI] [PubMed] [Google Scholar]
- 30.Eskola J, Ward J, Dagan R, Goldblatt D, Zepp F, Siegrist C-A. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet. 1999;354(9195):2063–8. doi: 10.1016/S0140-6736(99)04377-9. [DOI] [PubMed] [Google Scholar]
- 31.Jódar L, Butler J, Carlone G, Dagan R, Goldblatt D, Käyhty H, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine. 2003;21(23):3265–72. doi: 10.1016/s0264-410x(03)00230-5. [DOI] [PubMed] [Google Scholar]