Abstract
Chronic marijuana use during adolescence is frequently comorbid with heavy alcohol consumption and associated with CNS alterations, yet the influence of early cannabis and alcohol use on microstructural white matter integrity is unclear. Building on evidence that cannabinoid receptors are present in myelin precursors and affect glial cell processing, and that excessive ethanol exposure is associated with persistently impaired myelination, we used diffusion tensor imaging (DTI) to characterize white matter integrity in heavy substance using and non-using adolescents. We evaluated 36 marijuana and alcohol-using (MJ+ALC) adolescents (ages 16-19) and 36 demographically similar non-using controls with DTI. Diffusion parameters fractional anisotropy (FA) and mean diffusivity (MD) were subjected to whole-brain voxelwise group comparisons using tract-based spatial statistics (Smith et al., 2006). MJ+ALC teens had significantly lower FA than controls in 10 regions, including left superior longitudinal fasciculus (SLF), left postcentral gyrus, bilateral crus cerebri, and inferior frontal and temporal white matter tracts. These diminutions occurred in the context of increased FA in right occipital, internal capsule, and SLF regions. Changes in MD were less distributed, but increased MD was evident in the right occipital lobe, whereas the left inferior longitudinal fasciculus showed lower MD in MJ+ALC users. Findings suggest that fronto-parietal circuitry may be particularly impacted in adolescent users of the most prevalent intoxicants: marijuana and alcohol. Disruptions to white matter in this young group could indicate aberrant axonal and myelin maturation with resultant compromise of fiber integrity. Findings of increased anisotropic diffusion in alternate brain regions suggests possible neuroadaptive processes and can be examined in future studies of connectivity to determine how aberrancies in specific tracts might influence efficient cognitive processing.
Keywords: Marijuana, Alcohol, DTI, Adolescence, White matter
1. Introduction
Cannabis is the most widely used illicit substance among adolescents in the U.S. Of 2.1 million recent initiates, 63% were younger than 18 when they first used (SAMSHA, 2007). Early onset use is associated with dependence among many users, with youths ages 12-17 constituting the majority of admissions to treatment facilities for cannabis abuse (Chen et al., 2004; Hartman et al., 2008). Excessive alcohol consumption is also prevalent among adolescents, as close to a third of 16-17 year-olds report drinking in the past month with 20% transitioning to chronic use by age 20 (SAMSHA, 2007). Despite the frequent comorbidity of marijuana and alcohol use (MJ+ALC) in adolescence (Schweinsburg et al., 2008a), it is unclear how protracted use may affect brain structure and function during this period of continued neuromaturation (Giedd, 2004; Giedd et al., 1999; Gogtay et al., 2004; Hasan et al., 2007; Lenroot and Giedd, 2006; Paus, 2005; Paus et al., 1999).
Studies examining brain morphology in marijuana users, particularly within white matter, offer equivocal findings. Although some report no changes in white matter volume and composition in adult users (Block et al., 2000; Gruber and Yurgelun-Todd, 2005), reduced white matter volumes in the left parietal lobe and increased tissue density surrounding the left parahippocampal and fusiform gyri have been documented (Matochik et al., 2005). Whether the reported changes in brain structure among marijuana using adolescents persist into adulthood remains tentative. Increased mean diffusivity in the prefrontal fiber bundles of the corpus callosum in adults who initiated use during early adolescence suggests long-term changes to white matter quality as a result of adolescent marijuana use (Arnone et al., 2008). In conflict with these findings, a DTI study using whole-brain voxelwise analysis of 10 young adults who used moderately as adolescents suggested no loss of white matter integrity relative to non-users (Delisi et al., 2006). Still, another report shows higher fractional anisotropy in the rostral body and isthmus of the corpus callosum in adolescent substance users compared to controls (De Bellis et al., 2008).
Studies of grey matter show more consistent differences, with evidence for bilateral hippocampus and amygdala volume reductions in adults with history of long-term cannabis use, where left hippocampal volume was inversely related to chronicity of exposure to cannabis (Yucel et al., 2008). In addition, a voxel-based morphometry study in young adults with first episode schizophrenia and history of adolescent marijuana use showed more prominent gray matter density and volume reduction in the right posterior cingulate cortex compared to their non-using counterparts (Bangalore et al., 2008). These changes may be influenced by ongoing neurodevelopment, particularly when exposure begins early. The functional implications of these differences appear disadvantageous, as marijuana-using teens show an increased susceptibility to depressive symptoms (Medina et al., 2007b) and poorer performance than non-users on neuropsychological tests of psychomotor speed, complex attention, story memory, planning, and sequencing ability, even after a month of sustained abstinence (Medina et al., 2007a).
Converging evidence from functional neuroimaging studies of chronic marijuana using adolescents reveal deviations from the typical neural networks subserving these cognitive tasks. Brain response patterns in marijuana-using teens consistently indicate increased utilization of alternate brain networks. One of the first studies to demonstrate this profile administered a spatial working memory task to 15-17 year-old MJ+ALC-using teens. Relative to controls, users demonstrated increased blood oxygen level dependent (BOLD) response in right superior frontal cortices yet decreased activation in right inferior frontal and temporal regions (Schweinsburg et al., 2005). Tasks of verbal working memory (Jacobsen et al., 2007) and response inhibition (Tapert et al., 2007) elicit similar findings in marijuana-using teens, where greater activation was seen in frontal, parietal, and mid-cingulate regions as compared to performance-matched controls. Together, these data coincide with patterns of altered brain functioning found in marijuana-using adults (Eldreth et al., 2004; Kanayama et al., 2004), suggesting that users are less able to recruit the typical neural networks underlying complex cognitive functions and may require more neural resources to perform adequately. It is possible that diminutions in white matter caliber may be associated with this atypical processing tendency. Fiber pathways within and connecting superior medial and inferior frontal areas (Gruber and Yurgelun-Todd, 2005; Kanayama et al., 2004), temporal and parietal lobes (Grant et al., 2003), and the cerebellum including the tonsil (Chang et al., 2006) are implicated by structural and functional findings to show vulnerability to marijuana use.
Animal studies examining the mechanisms of physiological change induced by cannabis provide insight as to the probable source of CNS susceptibility, documenting the existence of cannabinoid (CB-1) receptors in numerous neuronal substrates and in cell processes that represent precursors to myelin. Present in astrocytes (Bouaboula et al., 1995; Sanchez et al., 1998), microglia (Moldrich and Wenger, 2000; Rodriguez et al., 2001; Waksman et al., 1999; Walter et al., 2003), and oligodendrocytes (Molina-Holgado et al., 2002a), CB-1 receptors are known to affect glial cell functions including migration toward sites of injury (Walter et al., 2003). These processes may be adversely impacted by early marijuana use and result in an altered trajectory of white matter development.
As an equally consumed intoxicant among adolescents, comorbid alcohol use is common among chronic marijuana users (Medina et al., 2007c). Heavy alcohol use is associated with a wide range of neural consequences in adults (Estruch et al., 1997; Nicolas et al., 2000; Pfefferbaum et al., 2006a; Pfefferbaum et al., 2006b; Pfefferbaum and Sullivan, 2005) and similar sequelae are implicated in adolescent users. Prefrontal white matter volumes appear smaller in heavy alcohol using adolescents (De Bellis et al., 2005; Medina et al., 2008). In addition, atypical brain response during spatial working memory (Tapert et al., 2004), and deficits on neuropsychological measures of attention (Tapert and Brown, 1999), retrieval (Brown et al., 2000), and visuospatial functioning (Tapert et al., 2002) suggest functional consequences of adolescent heavy drinking with sustained effects through adulthood (Brown et al., 2008). A detailed characterization of white matter is thus essential for understanding the influence of combined marijuana and alcohol use on the developing adolescent brain.
Here, we employ diffusion tensor imaging (DTI), a non-invasive technique for discerning microstructural white matter integrity in vivo. This technique is sensitive to variations in random motions, or diffusion, of water in neural tissues. In highly oriented and coherent brain tissue such as white matter fiber tracts, diffusion is anisotropic and greater along rather than perpendicular to axonal fibers (Le Bihan et al., 2001). Through acquisition of multiple images along different directions, one can quantify the directional dependence of diffusion and, from this, infer structural characteristics of the local tissue environment. Two primary scalar measures can be derived from DTI data: 1) fractional anisotropy (FA), a measurement of the directional variance of diffusional motion and 2) mean diffusivity (MD), measuring the overall magnitude of diffusional motion within a given voxel (Moritani et al., 2005). These measures index relationships between signal intensity changes and underlying structure, and are used in tandem to assess white matter quality (Conturo et al., 1999; Pierpaoli and Basser, 1996; Shimony et al., 1999).
Diffusion characteristics across typical adolescent development show a linear increase in FA and decrease in MD (Barnea-Goraly et al., 2005; Giorgio et al., 2008; Snook et al., 2005). These changes parallel the establishment of new cortical connections and growth of axons that continue at least through the second decade of life (Barnea-Goraly et al., 2005; Paus et al., 1999). Efficient organization of white matter fibers is linked to optimal cognitive performance. Anisotropic diffusion in the left parietal, right frontal, and corpus callosum regions correlate most with intellectual functioning in youth (Fryer et al., 2008; Schmithorst et al., 2005) and correspond to regional white matter maturation (Bonekamp et al., 2007).
In the current study, we examined the integrity of neuroanatomical pathways in adolescent marijuana users with concomitant alcohol use through DTI analysis. Based on findings of altered neural networks in marijuana-using teens, neurobiological evidence of cannabis receptors within myelin precursors, and persistent impairing effects of alcohol on myelination, we predicted that chronic MJ+ALC users would show poorer white matter integrity than non-users. Considering activation differences in fMRI studies in alcohol and marijuana users (Jacobsen et al., 2007; Rangaswamy et al., 2004; Schweinsburg et al., 2008b; Tapert et al., 2004; Tapert et al., 2007), we hypothesized white matter fiber integrity to be altered in fronto-parietal circuits.
2. Methods
2. 1. Participants
Seventy-two adolescents ranging in age from 16 through 19 years participated; 36 were heavy MJ+ALC users (180-1844 lifetime occurrences of marijuana use and 25-736 lifetime alcoholic drinks), and 36 were demographically similar non-using controls (see Table 1). Participants were recruited from local high schools with comparable numbers of each group recruited from each participating school as part of an ongoing adolescent brain imaging project (e.g., Tapert et al., 2007). Participants and their parents/legal guardians were screened with separate, private interviews to ascertain eligibility. Exclusionary criteria were: DSM-IV Axis I disorder other than alcohol or marijuana use disorder; nicotine dependence (Fagerstrom Test for Nicotine Dependence (FTND) score ≥ 3), use of psychoactive medications; history of neurological disorder, head trauma with loss of consciousness >2 minutes, learning disability, chronic health problem, or complicated/premature birth (<33 weeks gestation); parental history of bipolar I or psychotic disorder; any evidence of maternal drinking (>7 drinks in a week or >4 drinks in a day) or illicit drug use during pregnancy; left handedness; MRI contraindications; and clinically abnormal brain anatomy. Participants abstained from substance use for at least 24 hours before imaging, to minimize the confound of acute or withdrawal effects during scanning, verified by urine toxicology and Breathalyzer. The most recent marijuana use occurred 24 hours prior to imaging and last heavy alcohol use (4 or 5 alcoholic beverages in one sitting for females and males, respectively) was 3 days prior (see Table 1). Informed assent and consent were obtained from participants and their parents/legal guardians in accordance with UCSD Human Research Protections Program procedures.
Table 1.
Demographic and substance use characteristics of participants.
| MJ+ALC (n = 36) |
Controls (n = 36) |
|
|---|---|---|
| M (SD) or % | M (SD) or % | |
| Years of age (range 16.3-19.0) | 17.9 (0.9) | 17.8 (0.8) |
| Female | 27.8 % | 27.8 % |
| Caucasian | 61.1 % | 62.9 % |
| Annual household income (thousands) | 141.7 (128.3) | 115.9 (60.2) |
| Hollingshead socioeconomic level | 28.8 (13.7) | 30.4 (16.2) |
| Parental history of a substance use disorder*† | 41.7 % | 11.4 % |
| WASI Vocabulary T-score‡ | 58.4 (8.7) | 58.7 (8.7) |
| WRAT-3 Reading standard score§ | 106.7 (8.0) | 109.7 (6.6) |
| Spielberger State Anxiety T-score | 39.5 (6.7) | 37.7 (7.0) |
| Beck Depression Inventory Total | 3.2 (3.2) | 2.6 (2.7) |
| Child Behavior Checklist Internalizing T-score | 46.1 (10.0) | 44.8 (7.9) |
| Child Behavior Checklist Externalizing T-score | 49.7 (9.4) | 45.2 (10.3) |
| Age of first marijuana use* | 13.9 (2.0) | 15.4 (1.7) ‖ |
| Age of first weekly marijuana use | 14.7 (3.1) | - |
| Lifetime marijuana use episodes** | 551.7 (481.2) | 1.4 (2.3) |
| Marijuana use days per month (past 3 months)** | 11.6 (8.4) | 0.1 (0.3) |
| Days between last marijuana use and DTI study** | 52.1 (69.6) | 355.4 (330.3) ‖ |
| Age of first alcohol use* | 13.1 (1.9) | 14.6 (1.7) ‖ |
| Age of first weekly alcohol use | 15.5 (1.7) | 16.0 a |
| Lifetime alcohol drinks** | 195.3 (152.7) | 25.0 (38.6) |
| Drinks per month (past 3 months)** | 52.9 (52.4) | 8.2 (12.4) |
| Days between last alcohol use and DTI study | 43.0 (68.6) | 86.9 (118.2) ‖ |
| Cigarettes per smoking day* | 1.2 (3.8) | 0.0 (0.2) |
| Fagerstrom Test for Nicotine Dependence score* | 0.3 (0.7) | 0.0 (0.0) |
| Lifetime other drug use instances** | 10.5 (11.2) | 0.5 (2.2) |
P<0.05
P<0.001
Parent with DSM-IV alcohol or drug abuse or dependence
From Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)
From Wechsler Wide Range Achievement Test (Wilkinson, 1993)
For controls with history of any marijuana (n=14) or alcohol (n=12) use
(n=1)
MJ+ALC = Marijuana and alcohol user
2.2. Measures
2.2.1. Substance use
The Customary Drinking and Drug use Record (Brown et al., 1998) collected from the teen detailed information on quantity and frequency of lifetime and past 3-month alcohol, marijuana and other drug use (including misuse of prescription and over-the-counter medications), as well as abuse/dependence, withdrawal, and negative consequences. The Timeline Followback (Sobell and Sobell, 1992) assessed the pattern of substance use during the 28 days preceding the scan session using the youth and parent report (see Tapert et al., 2007). The FTND (Heatherton et al., 1991) indicated that no participant was dependent on nicotine.
2.2.2. Mood and psychopathological syndromes
The Beck Depression Inventory (Beck, 1978) and Spielberger State Trait Anxiety Inventory (Spielberger et al., 1970) assessed mood prior to scanning. The Child Behavior Checklist (Achenbach and Rescorla, 2001) was completed by parents to assay internalizing and externalizing psychopathological syndromes.
2.2.3. Cognition
As part of a larger neuropsychological battery, participants were administered the Wechsler Abbreviated Scale of Intelligence Vocabulary subtest (Wechsler, 1999) and Wide Range Achievement Test – 3 Reading test (Wilkinson, 1993) as estimates of premorbid intellectual functioning.
2.2.4. Family history
History of alcohol or drug use disorder in participants' biological parents was assessed by parent interview using the Family History Assessment Module (FHAM; (Rice et al., 1995). Parents were interviewed by a different psychometrist than who assessed the adolescent.
2.3. Procedures
2.3.1 MR acquisition
Participants were imaged in a 3T General Electric Excite MR system with an 8-channel phase-array head coil (General Electric Medical System, Milwaukee, WI, USA). A scout scan ensured good head placement and whole-brain coverage. Diffusion-weighted images were collected along 15 noncollinear directions determined by the electrostatic repulsion model which minimizes bias in measurements by sampling with approximately uniform distribution on a sphere (Jones et al., 1999), in addition to a reference image with no diffusion weighting (b=0). The diffusion encoding scheme consisted of a single-shot dual spin echo excitation optimized for minimum TE and reduction of eddy current artifacts (Reese et al., 2003). The following sequence parameters were applied and averaged over four volumes: TE/TR=93/12,000 ms, FOV=240 mm, matrix =128×128, 36 contiguous slices, 3 mm slice thickness, b-value=2000 s/mm2. Two field maps were collected for unwarping (TE/TR=3.8/1,000 ms) to correct for signal loss and geometric distortion due to B0 field inhomogeneities (Andersson and Skare, 2002; Jezzard and Balaban, 1995).
2.4. Data Analysis
2.4.1. Image pre-processing
First, datasets were visually inspected slice-by-slice for each subject. One control was excluded due to severe motion artifact, and 5 participants (1 MJ+ALC and 4 controls) were excluded due to technical problems during scanning (final N=72). Second, datasets were corrected for head motion, eddy current distortion, and signal loss using FSL tools (FMRIB Software Library, Oxford, United Kingdom; (Smith et al., 2004)). Specifically, image acquisitions for each direction were merged into a single 4D file and aligned to the first volume using affine registration with six degrees of freedom and Fourier interpolation to correct for motion (FLIRT-FMRIB's Linear Image Registration Tool; (Jenkinson et al., 2002)). Each of the 15 direction files were then registered to the B0 image using a six-parameter registration in 2D to minimize eddy current distortions (FDT-FMRIB's Diffusion Toolbox 2.0; (Behrens et al., 2003)). Next, phase unwrapping (PRELUDE-Phase Region Expanding Labeler for Unwrapping Discrete Estimates; (Jenkinson, 2003)) and regularization (FUGUE-FMRIB's Utility for Geometrically Unwarping EPIs; (Jenkinson and Smith, 2001)) of field maps were conducted for quantifying field distortions. Resulting measurements were translated into voxel shifts, effectively assigning image intensities to correct voxel locations.
2.4.2. DTI quantification
Pre-processed images were subjected to tensor decomposition to derive scalar diffusion indices, FA and MD (Le Bihan et al., 2001). This computation was performed in native coordinate space using Analysis of Functional NeuroImages' (Cox, 1996) diffusion plug-in routine, 3dDWItoDT (Cox and Glen, 2006). This algorithm uses a non-linear estimation method that guarantees that the diffusion tensor is positive definite, and provides estimates of FA and MD that are robust to increasing noise at high b-values (Jones and Basser, 2004; Skare et al., 2000). FA and MD were examined with whole-brain voxelwise analysis using Tract-Based Spatial Statistics (TBSS; (Smith et al., 2006)), which is optimized for multi-subject comparison and localized analysis of diffusion measurements, addressing the limitations of standard tools for voxelwise analysis where inaccurate registration and smoothing can lead to ambiguity in data interpretation.
TBSS analyses involved the following steps: To achieve initial alignment, FA maps were registered to an averaged FA template (FMRIB-58) in MNI-152 standard space using an affine-only registration. This was followed by a non-linear transformation into 1 mm cubic voxel dimensions using Image Registration Toolkit (Rueckert et al., 1999). Data were examined for laterality, orientation, and cross-subject anatomical alignment. Next, transformed images were averaged across subjects to create a mean diffusion image (FA), from which a white matter skeleton was derived, representing tracts common to all subjects. Individual transformed FA images were then projected onto the skeleton. To minimize partial-volume effects and areas of high inter-subject variability, values were thresholded at FA >0.2. FA values from individuals' nearest relevant tract center were assigned to the skeleton via a perpendicular search for the maximum FA value within local skeleton structure. This process accounts for residual misalignments between subjects after the initial registration and minimizes systematic differences in tract location between groups of subjects. These data formed the basis of voxelwise statistical comparisons. MD data were processed using the same nonlinear transformation, skeleton, and skeleton-projection vectors derived from the FA analysis (Smith et al., 2007).
2.4.3. Statistical analyses
Voxelwise statistics on the skeleton space FA and MD data were carried out in AFNI using independent sample t-tests. A combination of individual voxel probability and cluster size thresholding using Monte Carlo simulation (Ward, 2000) for multiple comparison correction was employed for Type I error control. Under this criteria, clusters ≥ 54 μl (54 contiguous 1×1×1 voxels) with an individual voxel probability threshold of P<0.05, yielding a brain-wise P<0.01 of finding such a cluster under the null hypothesis, were interpreted. Cohen's d effect sizes (Cohen, 1988) were computed from the average t-value within each significant cluster. In addition to being subjected to a whole-brain analysis, MD values were examined in regions of significant between-group FA differences, to assess the contribution of water diffusion to structural differences. Anatomical identification of tract structures was confirmed using a white matter atlas (Wakana et al., 2004).
2.4.4. Follow-up analyses
Distributions were examined for skewness and kurtosis; seven variables were found to deviate from normality (marijuana hits smoked per month, days since last marijuana use, years of regular drinking, days since last alcohol use, lifetime other drug use, cigarettes smoked per day, and FTND score) and were log transformed (Tabachnick and Fidell, 2007). Clusters showing significant group differences in FA or MD were examined in a repeated-measures analysis of covariance (ANCOVA) to control for familial and substance use variables that differed between groups. Regression analyses assessed the unique contributions of substance use and parental history of substance use disorder (SUD) on FA and MD within significant clusters; P-values of <0.004, corrected for multiple comparisons using Bonferroni adjustments, were considered significant. In follow-up exploratory analyses, mean FA and MD values in significant clusters were correlated with substance use variables in both groups using Pearson's r correlation coefficients (α=.05).
3. Results
Groups did not differ on demographic variables including age, gender distribution, ethnic composition, and socioeconomic status (Hollingshead, 1965) (see Table 1). Measures of emotional functioning and psychopathology were similar between groups and fell within normal limits. Estimated premorbid IQ and academic reading achievement were also comparable between groups, typically falling in the average to high average range. MJ+ALC youths were more likely to have a parental history of SUD (P<0.05), and reported more nicotine (P<0.05) and other drug (P<0.001) use than controls, so these variables were included as covariates in statistical analyses.
Independent samples t-tests, corrected with intensity and cluster-based thresholding, revealed 10 clusters (≥ 54 μl) in which MJ+ALC teens showed significantly lower FA than controls (see Table 2). The most prominent areas of diminished FA were in the left SLF, left postcentral sensory gyrus, and bilateral crus cerebri (P<0.001, see Figure 1). Temporal regions including the right superior temporal gyrus (P<0.001), projection fibers of the left temporo-thalamic tract (P<0.01), and right inferior longitudinal fasciculus (P<0.01) showed FA diminutions in MJ+ALC users, as did association fibers in right inferior frontal (P<0.001), left occipital-frontal (P<0.01), and splenium (P<0.01) regions (p-values refer to difference in the average FA value within each cluster). Interestingly, in three right hemisphere clusters, MJ+ALC users had higher FA than controls (P<0.001): the cuneus region of the occipital lobe, anterior limb of the internal capsule, and arcuate portion of the right SLF (see Table 2).
Table 2.
Clusters showing significant fractional anisotropy (FA) and mean diffusivity (MD) difference between marijuana+alcohol users and controls
| Anatomic region | Cluster Size (voxels) |
MNI Coordinate* | Effect Size (Cohen's d)† |
Group Difference |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| FA | ||||||
| Superior longitudinal fasciculus L | 212 | 33.2 | 42.4 | 33.5 | 0.75 | MJ+ALC < CT |
| Crus cerebri L | 152 | 13.9 | 18.0 | -13.2 | 0.72 | MJ+ALC < CT |
| Postcentral gyrus L | 109 | 22.8 | 34.3 | 57.8 | 0.89 | MJ+ALC < CT |
| Occipital-cuneus R | 95 | -17.5 | 86.3 | 16.2 | -1.00 | MJ+ALC > CT |
| Inferior longitudinal fasciculus R | 91 | -40.7 | 36.0 | -8.5 | 0.54 | MJ+ALC < CT |
| Superior longitudinal fasciculus (arcuate) R | 83 | -39.7 | -11.3 | 16.8 | -0.57 | MJ+ALC > CT |
| Superior temporal gyrus R | 80 | -37.6 | 8.7 | -15.7 | 0.69 | MJ+ALC < CT |
| Crus cerebri R | 80 | -15.1 | 18.0 | -12.4 | 0.83 | MJ+ALC < CT |
| Anterior limb internal capsule R | 74 | -18.2 | -15.7 | 3.1 | -0.50 | MJ+ALC > CT |
| Corpus callosum (splenium) R | 71 | -17.4 | 44.6 | 13.5 | 0.55 | MJ+ALC < CT |
| Inferior frontal gyrus (opercular/insular) R | 66 | -40.0 | -4.2 | 21.9 | 0.56 | MJ+ALC < CT |
| Temporo-thalamic tract L | 63 | 19.4 | 24.1 | -0.8 | 0.58 | MJ+ALC < CT |
| Occipto-frontal tract L | 60 | 27.1 | 2.2 | 21.4 | 0.48 | MJ+ALC < CT |
| MD | ||||||
| Inferior longitudinal fasciculus L | 57 | 49.2 | 41.3 | -5.9 | 0.57 | MJ+ALC < CT |
| Occipital-lingual R | 54 | -15.1 | 88.3 | 0.5 | -0.66 | MJ+ALC > CT |
Coordinates of the center of mass in significant clusters
Effect size from the average t-value for each cluster
L=Left; R=Right; MJ+ALC=marijuana+alcohol user; CT=Control
Figure 1.
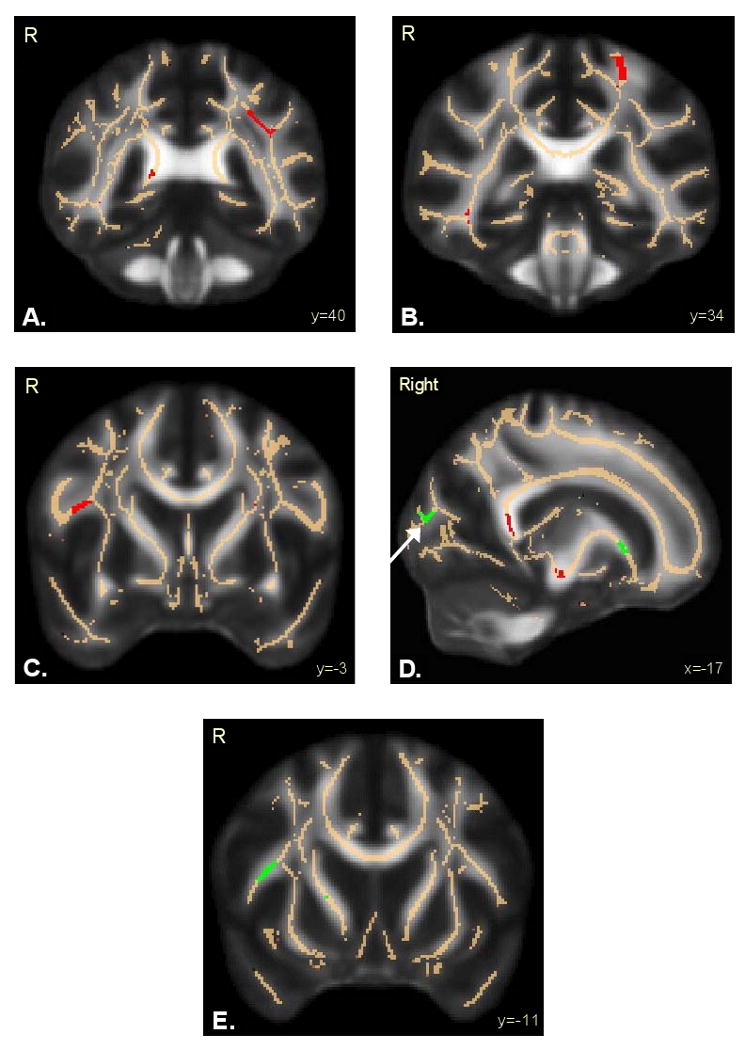
Regions of altered fractional anisotropy in adolescent marijuana+alcohol users (n=36) relative to controls (n=36). Results are superimposed on the fiber skeleton (beige) and overlaid on a standardized FA template. Red indicates decreased FA in marijuana+alcohol users in: A) left superior longitudinal fasciculus; B) postcentral gyrus; and C) inferior frontal gyrus. Green indicates increased FA in marijuana+alcohol users in: D) occipital lobe-cuneus (white arrow); and E) right superior longitudinal fasciculus – arcuate. R=Right.
Using the same model, analysis of MD within clusters of significant FA discrepancy yielded no differences between groups (see Figure 2). However, a whole-brain analysis of MD revealed small but significant differences in two areas. Inferior to the right occipital-cuneus and adjacent the lingual gyrus, MJ+ALC users had higher MD than controls (P<0.01). Contrary to hypotheses, users showed lower MD than controls in the left inferior longitudinal fasciculus (P<0.01).
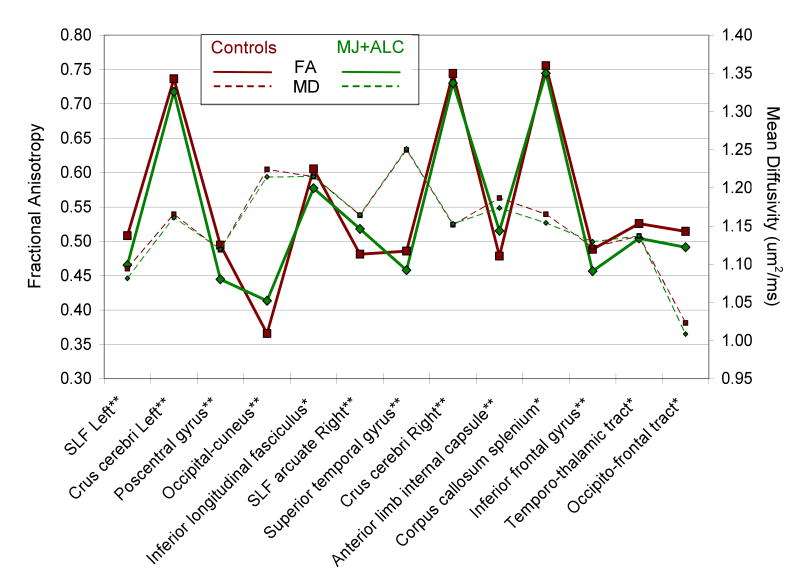
Figure 2.
Fractional anisotropy (FA) and mean diffusivity (MD) in areas of significant FA difference between adolescent marijuana+alcohol users (n=36) and controls (n=36). Relative to controls, users showed significantly lower FA in 10 regions and higher FA in 3 regions; within these 13 regions, MD did not differ between groups.
*P<0.01
**P<0.001
SLF=Superior longitudinal fasciculus
To evaluate the influence of potential confounds, group differences were examined in an ANCOVA (N = 72). Specifically, cigarettes smoked, FTND score, lifetime other drug use, and parental history of SUD were greater in MJ+ALC users than controls. ANCOVAs indicated that the group differences in FA and MD reported above persisted after controlling for these variables (F(12,54) = 6.50, P<0.001). Further, to see if parental SUD history might interact with adolescent substance use in accounting for variability in white mater integrity, follow-up hierarchical regressions (N = 72) entered parental SUD history on step 1, substance use group on step 2, and their interaction on step 3. Positive parental SUD history predicted lower FA in the right crus cerebri, but not other regions, across users and controls (β = -.37, P = 0.001), yet substance group status continued to predict FA above and beyond parental history (β = -.33, P = 0.003). The parent history × use group interaction did not predict FA.
Follow-up analyses examined relationships between substance use variables and FA in regions that differed between groups. The range of alcohol use in both groups provided the opportunity to examine correlations between lifetime alcohol use and FA in the full sample (N = 72). Results revealed positive relationships in the three clusters where users had higher FA than controls (r = 0.30 to 0.33, P = 0.005 to 0.01), and negative relationships in regions where users had lower FA than controls (right and left crus cerebri, corpus callosum splenium, right inferior frontal gyrus, and left postcentral gyrus; r = -0.24 to -0.40, P = 0.0004 to 0.036).
Correlations within the user group (n = 36) between FA values and alcohol and marijuana intensity, frequency, and duration were nonsignificant at P<0.004, but a few interesting trends emerged. Specifically, in three regions where users had lower FA than controls, FA was linked to use indices: right inferior frontal gyrus FA negatively correlated with years of regular drinking (r = -0.34, P = 0.04); but left SLF FA positively correlated with recent marijuana use days per month (r = 0.36 P = 0.03); and left occipito-frontal tract FA positively correlated with lifetime marijuana use (r = 0.39, P = 0.02), lifetime alcohol use (r = 0.38, p = 0.02), and years of regular drinking (r = 0.42, P = 0.01). In the left inferior longitudinal fasciculus, where users unexpectedly had lower MD than controls, lower MD related to more marijuana hits per month in the past 3 months (r = -0.35, P = 0.039) and fewer days since last alcohol use (r = 0.76, P = 0.03). For particularly heavy users (lifetime marijuana use ≥ 350 and lifetime alcohol drinks ≥165; n = 9), splenium FA negatively correlated with lifetime marijuana use (r = -0.67, P = 0.04), and FA in the anterior limb of the internal capsule (where users had higher FA than controls) positively correlated with lifetime alcohol use (r = 0.67, P = 0.04). Mean FA and MD did not relate to estimated premorbid IQ in either group, and no gender main effects or gender by group interactions were seen.
4. Discussion
This study investigated the integrity of white matter microstructure in adolescent marijuana and alcohol users. Using tract-based spatial analysis, we compared FA and MD within white matter structures throughout the brain. Based on neurobiological evidence of cannabis receptors within myelin precursors, persistently impaired myelination in alcoholism, and findings of altered neural networks in MJ+ALC-using teens, we predicted that substance users would show lower FA than non-users with predominant alterations in fronto-parietal white matter pathways. Consistent with our hypothesis, users evidenced diminutions in mean FA relative to controls, notable in frontal-parietal circuitry comprising fibers of the inferior frontal region, splenium of the corpus callosum, postcentral gyrus, and left SLF. Contrary to expectations, areas of increased FA were observed among MJ+ALC teens in the occipital lobe, internal capsule, and arcuate portion of the right SLF. MD was similar between groups within the regions of significant FA discrepancy. However, white matter adjacent the lingual gyrus showed higher MD among MJ+ALC users, whereas the posterior aspect of the left inferior longitudinal fasciculus demonstrated lower MD in users than controls. Together, these findings suggest that selective aberrancies in cerebral white matter are evident in early-onset adolescent marijuana and alcohol use.
Among affected tracts within fronto-parietal networks, the left SLF showed prominently decreased FA and the greatest volume of anisotropic differences. As the SLF encompasses projections from parietal to dorsomedial and dorsolateral prefrontal cortices (Makris et al., 2005), this anatomic finding bears a potential association with the increased activation in right prefrontal and parietal areas evident during inhibitory processing (Tapert et al., 2007) and with the increased right parietal BOLD response observed during spatial working memory (Schweinsburg et al., 2008b) among marijuana users. Greater reliance on right frontal and cerebellar regions to adequately learn novel verbal material has also been documented in marijuana-using adolescents (Jacobsen et al., 2007) and speaks to the broad range of functional impact that may be associated with white matter deficits within the left SLF.
Widespread supratentorial diminutions in FA (Pfefferbaum et al., 2006b) as well as reduced tract integrity in the genu and splenium of the corpus callosum and centrum semiovale (Pfefferbaum et al., 2006a; Pfefferbaum and Sullivan, 2005) are evident in adult alcoholism, consistent with splenium FA deficits among users here. Right inferior frontal FA deficits in MJ+ALC users also coincide with the reduced fiber coherence observed in prefrontal and orbitofrontal white matter of adult alcoholics (Harris et al., 2008). The strong negative relationship between lifetime alcohol consumption and FA in this region may indicate a common frontal vulnerability among adolescent and adult users.
White matter fibers within the bilateral crus cerebri also showed diminished FA. As important structural constituents of the fronto-cortico-striatal and corticopontocerebellar circuits, prior studies show a uniform decrease in these regions in disorders of attention (Ashtari et al., 2005) and first episode schizophrenia (Cheung et al., 2008). Although the internal capsule has shown corresponding diminutions in these populations, MJ+ALC users showed a departure from this trend, evidencing increased FA in the anterior limb. This aspect of the internal capsule contains both direct and indirect fronto-thalamic projections. Collectively, changes in the crus cerebri, internal capsule, and inferior frontal tracts may be consistent with aberrant corticothalamic and frontostriatal connectivity, and fit with previous reports of volumetric increases in the right thalamus and greater striatal activation during working memory tasks (Kanayama et al., 2004; Matochik et al., 2005; Padula et al., 2007).
Complex cognition such as inhibitory processing relies on the communication of prefrontal cortices, basal ganglia, and the thalamus to mediate input and selectively engage related circuits (Stevens et al., 2007). The current findings suggest that the integration of these processes may be significantly different in MJ+ALC users and may be a corollary of both positive and negative changes in white matter microstructure. Observed in three distinct regions, areas of increased FA in MJ+ALC users implicate possible signaling of alternate pathways as the result of aberrant fiber structure in dedicated areas. Within this context, increased FA in the arcuate portion of the SLF is particularly striking considering that this region shows reduced density in typically developing adolescents (Paus et al., 1999) and no age-dependent FA changes among male teens (Ashtari et al., 2007).
The intensity of substance use in relation to diffusion indices also revealed unexpected trends. More years of regular drinking was linked to lower FA in the right inferior frontal gyrus, whereas greater marijuana and alcohol use were linked to higher left SLF and occipito-frontal FA. Low FA values in the left SLF and occipito-frontal fasciculi are found to be associated with complex mental illness such as schizophrenia (Cheung et al., 2008; Karlsgodt et al., 2008; Xu et al., 2008), where altered fronto-parietal connectivity is also evident. While it is surprising that increased duration and intensity of use was associated with higher FA in these regions, it is possible that these differences may have predated the onset of use, and may be linked to increased risk for use or altered attentional processes that lead to more frequent use. Importantly, this finding did not hold in very heavy substance users, suggesting that differences in white matter composition may be dependent on the extent of use. Among heavy MJ+ALC users, greater lifetime alcohol use was linked to higher FA in the anterior limb of the internal capsule, where users showed greater FA than controls (i.e., greater divergence from controls for the heaviest drinkers). We speculate that these changes may reflect compensatory responses that are more likely to occur with increasing use, as also observed in studies of functional activation (Tapert et al., 2004; Tapert et al., 2007). Although further examination is needed, these findings highlight the complex interaction between neurotoxic substance effects and concomitant neurodevelopmental changes that take place across brain regions.
Given that the SLF and similar association tracts myelinate into late adolescence (Sowell et al., 1999), early MJ+ALC use may interfere with neuromaturation. The influence of CB-1 receptors on oligodendroglial development (Rodriguez de Fonseca et al., 1993) suggests that cannabis may impact cell differentiation and migratory processes. Poor differentiation of oligodendrocytes or death of oligodendroglia can lead to reductions in myelin initiation, deposition, compaction, and maintenance (Davis et al., 2003). Although cannabis can prevent oligodendrocyte death (Molina-Holgado et al., 2002b), early or chronic cannabis exposure may cause a down-regulation of CB-1 receptors and suppress oligodendrocyte function during neurodevelopment. Cannabis-induced alterations in myelin proteolipid protein could also affect the expression of this gene, particularly with long-term exposure (Grigorenko et al., 2002). Down-regulation of myelin-related genes (Lewohl et al., 2000; Mayfield et al., 2002) and deficits in oligodendrocyte myelin glycoprotein (Okamoto et al., 2006) found in chronic alcoholism may further interact with altered processes associated with cannabis exposure. Focal areas of fiber disintegrity, as suggested by the current study, may result from any of these neurodevelopmental processes.
Recent DTI work in young adults with history of cannabis dependence shows reduced FA, increased radial diffusivity, and increased MD in the bilateral posterior internal capsule/thalamic radiation, left middle temporal gyrus and right superior temporal gyrus (Ashtari et al., 2009). Vulnerability in fronto-temporal areas bears resemblance to the current findings, though predominance of parietal changes in our group may reflect more heterogeneous use of both cannabis and alcohol in our group. Another study of adults who used moderately as adolescents suggests no changes in white matter structure relative to non-users (Delisi et al., 2006). Although this study may indicate a reversibility of white matter changes with long-term abstinence, current alcohol use in 20% of their sample suggests the possibility that methodological factors may contribute to inconsistencies with the current findings. Our diffusion protocol, employing comparatively higher angular resolution and increased image resolution may yield increased sensitivity to white matter alterations. Similarly, differences in power and sample characteristics (e.g, chronicity of substance use) could impact detection of significant effects. Also diverging from the present study is a recent report of higher FA and lower MD in adolescent substance users compared to controls (De Bellis et al., 2008). Comorbid mood disorders, polysubstance dependence, and prenatal substance exposure in this sample may account for the incongruity with current findings, and underscores the challenge of minimizing confounding variables in substance use studies (Pope, 2002).
The findings from this study should be interpreted while considering the following limitations. Abstinence periods prior to imaging were variable, and although duration of abstinence did not correlate with FA or MD, delineation of the acute and chronic effects of marijuana and alcohol on white matter microstructure is of interest in follow-up studies. As alcohol use is so common among marijuana users, the specific effects of marijuana cannot be easily differentiated. Groups with singular substance use are difficult to obtain in naturalistic studies, but future work is needed to decipher the effects due to marijuana use alone versus combined MJ+ALC use on white matter alterations. Differences in diffusion properties between MJ+ALC users and non-users do not appear to be associated with premorbid functioning, as stable indicators of cognition were equal between groups and were unrelated to FA or MD. Despite this, pre-onset use white matter integrity in MJ+ALC users is unknown, and pre-existing vulnerabilities including increased incidence of behavioral dysregulation (Kirisci et al., 2004; Tarter et al., 2003) and negative affectivity (Chassin et al., 2004) may be associated with neurological differences that predispose individuals in this group to substance use. Further, MJ+ALC users were more likely to have a parental history of substance use disorders. Considering that white matter changes in adolescent MJ+ALC users may have predated the onset of use, future follow-ups of this cohort will help determine whether increases in substance use result in greater deviations in white matter microstructure. Assessments prior to MJ+ALC use onset will provide important information on the relative influence of premorbid characteristics and initiation of heavy substance use.
Determining the structural integrity of white matter from diffusion weighted data is complicated by the existence of multiple fiber orientations within a voxel (Pierpaoli et al., 1996). To resolve regions of complex fiber distributions in characterizing local diffusion, measurements at higher angular resolution, in conjunction with higher order tensor analysis (Frank, 2002), will be considered in future work. The macrostructural correlates of increased and decreased diffusion anisotropy are the subject of ongoing research combining DTI with structural techniques. In addition, studies incorporating functional data will determine how indices of white matter compromise are linked to atypical neural networks and to what extent deviations in diffusion anisotropy contribute to altered connectivity. Integration of neuropsychological data will inform how changes in FA are associated with tasks involving different cognitive demands. These investigations are best conducted within a longitudinal framework where both the persistent and potentially reversible effects of marijuana and alcohol use over adolescence can be comprehensively assessed.
In conclusion, this study provides new information with the use of DTI to elucidate changes in white matter microstructure associated with adolescent MJ+ALC use. Findings reveal prominent aberrancies in fronto-parietal networks and fiber projections within circuits responsible for modulating complex cognitive, motor, and sensory processing. Findings implicate possible neuroadaptive anatomic changes that can be applied to future studies of connectivity to determine how alterations in specific tracts might influence efficient cognitive processing.
Acknowledgments
This research was supported by grant R01 DA021182 to S.F. Tapert and grant R01 MH64729 to L.R. Frank. We extend our appreciation to participants and their families, as well as to Dr. Sandra Brown, Dr. Greg Balls, Dr. MJ Meloy, Jennifer Winward, Joanna Jacobus, Ann Park, Christina Burke, and Claudia Padula whose support was vital to the completion of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T, Rescorla L. Manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Andersson JL, Skare S. A model-based method for retrospective correction of geometric distortions in diffusion-weighted EPI. Neuroimage. 2002;16:177–199. doi: 10.1006/nimg.2001.1039. [DOI] [PubMed] [Google Scholar]
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage. 2008;41:1067–1074. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatry Research. 2009;43:189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szeszko PR, Kumra S. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biological Psychiatry. 2005;57:448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Bangalore SS, Prasad KM, Montrose DM, Goradia DD, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia--a region of interest, voxel based morphometric study. Schizophrenia Research. 2008;99:1–6. doi: 10.1016/j.schres.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Beck A. Beck Depression Inventory (BDI) San Antonio, TX, USA: Psychological Corporation; 1978. [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Block RI, O'Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–496. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, Mori S, Horska A. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34:733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Bourrie B, Rinaldi-Carmona M, Shire D, Le Fur G, Casellas P. Stimulation of cannabinoid receptor CB1 induces krox-24 expression in human astrocytoma cells. The Journal of Biological Chemistry. 1995;270:13973–13980. doi: 10.1074/jbc.270.23.13973. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, Winters KC, Lowman C, Murphy S. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121 4:S290–310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Chassin L, Fora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: the effects of familial alcoholism and personality. Journal of Abnormal Psychology. 2004;113:483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Chen K, Sheth AJ, Elliott DK, Yeager A. Prevalence and correlates of past-year substance use, abuse, and dependence in a suburban community sample of high-school students. Addictive Behaviors. 2004;29:413–423. doi: 10.1016/j.addbeh.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychological Medicine. 2008;38:877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R, Glen D. Efficient, Robust, Nonlinear, and Guaranteed Positive Definite Diffusion Tensor Estimation. Proceedings of the International Society of Magnetic Resonance in Medicine, 14th Scientific Meeting.2006. [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Archives of General Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, Payne ME, MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2008;32:395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduction Journal J. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Estruch R, Nicolas JM, Salamero M, Aragon C, Sacanella E, Fernandez-Sola J, Urbano-Marquez A. Atrophy of the corpus callosum in chronic alcoholism. Journal of Neurological Sciences. 1997;146:145–151. doi: 10.1016/s0022-510x(96)00298-5. [DOI] [PubMed] [Google Scholar]
- Frank LR. Characterization of anisotropy in high angular resolution diffusion-weighted MRI. Magnetic Resonance in Medicine. 2002;47:1083–1099. doi: 10.1002/mrm.10156. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, Tapert SF. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain and Cognition. 2008;67:225–233. doi: 10.1016/j.bandc.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. Journal of the International Neuropsychological Society. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Grigorenko E, Kittler J, Clayton C, Wallace D, Zhuang S, Bridges D, Bundey S, Boon A, Pagget C, Hayashizaki S, Lowe G, Hampson R, Deadwyler S. Assessment of cannabinoid induced gene changes: tolerance and neuroprotection. Chemistry and Physics of Lipids. 2002;121:257–266. doi: 10.1016/s0009-3084(02)00161-5. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Research. Cognitive Brain Research. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, Papadimitriou GM, Makris N, Oscar-Berman M. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcoholism: Clinical and Experimental Research. 2008;32:1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman CA, Gelhorn H, Crowley TJ, Sakai JT, Stallings M, Young SE, Rhee SH, Corley R, Hewitt JK, Hopfer CJ. Item response theory analysis of DSM-IV cannabis abuse and dependence criteria in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:165–173. doi: 10.1097/chi.0b013e31815cd9f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Sankar A, Halphen C, Kramer LA, Brandt ME, Juranek J, Cirino PT, Fletcher JM, Papanicolaou AC, Ewing-Cobbs L. Development and organization of the human brain tissue compartments across the lifespan using diffusion tensor imaging. Neuroreport. 2007;18:1735–1739. doi: 10.1097/WNR.0b013e3282f0d40c. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two-factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magnetic Resonance in Medicine. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magnetic Resonance in Medicine. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Jones DK, Basser PJ. “Squashing peanuts and smashing pumpkins”: how noise distorts diffusion-weighted MR data. Magnetic Resonance in Medicine. 2004;52:979–993. doi: 10.1002/mrm.20283. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magnetic Resonance in Medicine. 1999;42:515–525. [PubMed] [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176:239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biological Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Vanyukov M, Reynolds M, Habeych M. Relation between cognitive distortions and neurobehavior disinhibition on the development of substance use during adolescence and substance use disorder by young adulthood: a prospective study. Drug and Alcohol Dependence. 2004;76:125–133. doi: 10.1016/j.drugalcdep.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience Biobehavior Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcoholism: Clinical and Experimental Research. 2000;24:1873–1882. [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. Journal of Neurochemistry. 2002;81:802–813. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007a;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcoholism: Clinical and Experimental Research. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. Journal of Child Psychology and Psychiatry. 2007b;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007c;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Borrell J, Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. Journal of Neuroscience. 2002a;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado F, Molina-Holgado E, Guaza C, Rothwell NJ. Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocyte cultures. Journal of Neuroscience Research. 2002b;67:829–836. doi: 10.1002/jnr.10165. [DOI] [PubMed] [Google Scholar]
- Moritani T, Ekholm S, Westesson P. Diffusion-weighted MR imaging of the brain. Berlin: Springer; 2005. [Google Scholar]
- Nicolas JM, Fernandez-Sola J, Robert J, Antunez E, Cofan M, Cardenal C, Sacanella E, Estruch R, Urbano-Marquez A. High ethanol intake and malnutrition in alcoholic cerebellar shrinkage. QJM: Monthly Journal of the Association of Physicians. 2000;93:449–456. doi: 10.1093/qjmed/93.7.449. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Miki T, Lee KY, Yokoyama T, Kuma H, Wang ZY, Gu H, Li HP, Matsumoto Y, Irawan S, Bedi KS, Nakamura Y, Takeuchi Y. Oligodendrocyte myelin glycoprotein (OMgp) in rat hippocampus is depleted by chronic ethanol consumption. Neuroscience Letters. 2006;406:76–80. doi: 10.1016/j.neulet.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of Addiction Behaviors. 2007;21:478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Science. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of Aging. 2006a;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biological Psychiatry. 2006b;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Pope HG., Jr Cannabis, cognition, and residual confounding. Journal of the American Medical Association. 2002;287:1172–1174. doi: 10.1001/jama.287.9.1172. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, Lim KO, Begleiter H. A functional MRI study of visual oddball: evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage. 2004;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magnetic Resonance in Medicine. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. Journal of Neuroscience. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert D, Sonoda L, Hayes C, Hill D, Leach M, Hawkes D. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Transactions of Medical Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- SAMSHA 2007. Results from the 2006 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies, DHHS; [Google Scholar]
- Sanchez C, Galve-Roperh I, Canova C, Brachet P, Guzman M. Delta9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 1998;436:6–10. doi: 10.1016/s0014-5793(98)01085-0. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Human Brain Mapping. 2005 doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Reviews. 2008a;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research: Neuroimaging. 2008b doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimony JS, McKinstry RC, Akbudak E, Aronovitz JA, Snyder AZ, Lori NF, Cull TS, Conturo TE. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 1999;212:770–784. doi: 10.1148/radiology.212.3.r99au51770. [DOI] [PubMed] [Google Scholar]
- Skare S, Li T, Nordell B, Ingvar M. Noise considerations in the determination of diffusion tensor anisotropy. Magnetic Resonance Imaging. 2000;18:659–669. doi: 10.1016/s0730-725x(00)00153-3. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 1:S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TE. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature Protocols. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the state-trait anxiety inventory. Palo Alto, CA, USA: Consulting Psychologists Press; 1970. [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behavioral Brain Research. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick B, Fidell L. Using Multivariate Statistics. 5th. Pearson; Boston: 2007. [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four-year outcomes. Journal of the International Neuropsychological Society. 1999;5:481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. American Journal of Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Waksman Y, Olson JM, Carlisle SJ, Cabral GA. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. Journal of Pharmacology and Experimental Therapeutics. 1999;288:1357–1366. [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. Journal of Neuroscience. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DB. Simultaneous Inference for fMRI Data. Biophysics Research Institute, Medical College of Wisconsin; 2000. [Google Scholar]
- Wechsler D. Manual for the Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wilkinson G. The wide range achievement test-3 administration manual. Wilmington, DE, USA: Jastak Associates; 1993. [Google Scholar]
- Xu L, Pearlson G, Calhoun VD. Joint source based morphometry identifies linked gray and white matter group differences. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Archives of General Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]