Abstract
Patients with schizophrenia show altered patterns of functional activation during working memory processing; specifically, high-performing patients appear to hyper-activate and low-performing patients appear to hypo-activate when compared with controls. It remains unclear how these individual differences in neurophysiological activation relate to the clinical presentation of the syndrome. In this study, this relationship is examined using partial least squares (PLS), a multivariate statistical technique that selects underlying latent variables based on the covariance between two sets of variables, in this case, clinical variables and regional fMRI activations during a verbal working memory task. The PLS analysis extracted two latent variables, and the significance of these associations was confirmed through permutation. Lower levels of activation during task performance across frontal and parietal regions of interest in the left hemisphere was found to covary with poorer role functioning and greater severity of negative and disorganized symptoms, while lower activation in right frontal and subcortical regions of interest was found to covary with better social functioning and fewer positive symptoms. These results suggest that appropriately lateralized patterns of functional activation during working memory processing are related to the severity of negative and disorganized symptoms and level of role and social functioning in schizophrenia.
Keywords: fMRI, partial least squares, verbal working memory
1. Introduction
Compared with healthy subjects, patients with schizophrenia show altered patterns of regional brain activation during cognitive tasks, particularly on tests of working memory (WM) (Callicott et al., 2003; Manoach et al., 2003; Cannon et al., 2005, Karlsgodt et al., 2007; Karlsgodt et al., in press). These differences may be related to the underlying neuropathology of schizophrenia and specifically to findings of reduced neuropil in the frontal cortex (Goldman-Rakic and Selemon, 1997; Selemon et al., 1998). Previous work has demonstrated that these physiological differences can take the form of either hypo- (Ragland et al., 1998; Stevens et al., 1998, Barch et al., 2003; Cannon et al., 2005) or hyper-activation (Manoach et al., 1999; Callicott et al., 2000; Manoach et al., 2000). Moreover, it has been suggested that the presence of hypo- vs. hyper-activation can be predicted based on WM performance, such that patients who perform well activate frontal regions more than similarly performing controls, while low-performing patients activate this region less than controls (Karlsgodt et al., 2007; Karlsgodt et al., in press).
One implication of this line of research that remains ambiguous is how this performance-activation relationship corresponds to the severity of the clinical presentation of schizophrenia. In other words, if physiological change during WM processing is a marker of potential pathophysiological mechanisms underlying schizophrenia, then the severity of clinical symptoms and level of functioning in these patients should be related to the measurable physiological indicators of this change (i.e., fMRI BOLD signal). The objective of this study was to examine whether the variability in regional brain physiology associated with verbal WM is related to the variability in clinical presentation of schizophrenia, as measured by positive symptoms, negative symptoms, and disorganized symptoms, as well as measures of social and role functioning. In order to control for level of performance, each subject’s WM capacity was calculated. In this way, a subjects’ physiological signal represents the point at which their WM system is maximally engaged, but not overtaxed. Establishing a relationship between the physiological basis of WM dysfunction and variation in the severity of symptom dimensions and day-to-day functioning would provide further support for considering the WM system as a primary target for interventions.
Some prior neuroimaging studies have examined how physiologic activity in the WM system relates to clinical symptomatology. For example, there is evidence that hypoactivation in the dorsolateral prefrontal cortex (DLPFC) during WM tasks is related to disorganized symptoms (Perlstein et al., 2001) and thought disturbance scores (Menon et al., 2001). Other work has demonstrated that DLPFC connectivity during tasks involving cognitive control correlates with disorganization and global impairment scores (Yoon et al., 2008). With respect to positive symptoms, some work has suggested a relationship between the presence of hallucinations and changes in activation during cognitive tasks in the temporal cortex (Whalley et al., 2007; Wible et al., 2008).
Attempts to relate physiological variables to clinical variables (such as positive, negative, and disorganized symptoms), however, are complicated by their multidimensional nature. Physiological activation tends to covary across regions, just as symptom clusters and functional variables may covary with one another. In fact, Yoon et al.’s study (2008) highlights the importance of connectivity between regions rather than the activation of a single region. This collinearity makes it very difficult for traditional univariate analyses to query how the activation across a physiological system might explain clinical variation across multiple dimensions. Because of this difficulty, most studies have tended to focus on a particular region (e.g., DLPFC) or symptom.
To overcome the statistical limitations just described, this study utilized partial least squares (PLS) analyses to reduce the data into latent variables that covary with one another (Nestor et al., 2002). PLS is an extension of multivariate linear regression that examines the covariance structure between sets of predictor and response variables. Given that there appears to be a positive relationship between performance and activation, such that greater activation in frontal regions corresponds to better performance in patients, it is hypothesized that less activation in the network at WM capacity corresponds to a greater disruption of underlying cellular integrity and connectivity, and therefore, greater severity in clinical presentation. More specifically, based on prior work (Green, 1996; Green and Braff, 2001; Green, 2007), both the severity of negative and disorganized symptoms, and the level of social and role functioning, are expected to systematically covary with physiological activation associated with WM, while the severity of positive symptoms is not. Because a verbal WM task is utilized, indicators of activation in left frontal cortex are expected to play a greater role in explaining these clinical correlates than the corresponding indicators in right frontal cortex.
2. Method
2.1 Participants
Thirteen patients with schizophrenia successfully completed a verbal WM task while undergoing fMRI (as described below). Table 1 shows demographic information for the sample. All subjects underwent a verbal and written informed consent process for the study, as approved by the UCLA IRB. Participants under the age of 18 signed assent forms, while a parent or guardian signed informed consent documents. Patients with schizophrenia were recruited through the Aftercare Research Program (for those 18 years or over) and Adolescent Brain-Behavior Research (ABBRC) Clinics at UCLA. To be included in this study, patients must have experienced recent onset of psychotic illness, with the start of the first major psychotic episode (characterized by psychotic symptoms lasting at least 2 weeks) occurring within the last 2 years, and must currently meet DSM-IV criteria for a diagnosis of schizophrenia, schizophreniform, or schizoaffective disorder - depressive subtype (American Psychological Association, 1994). Trained interviewers using the SCID gathered the diagnostic information from the patient and used all sources of information. The training program required interviewers to achieve a minimum overall kappa of .75 (minimum kappa sensitivity = .75 and specificity = .75) on symptom agreement and .85% accuracy on diagnosis (Ventura et al., 1998). Interviewers also were required to demonstrate “Good” or “Excellent” interviewer behaviors. Each SCID interviewer participated in an ongoing quality assurance program to minimize rater drift. Patients admitted to these two research programs are between 12–45 years of age; however, only participants aged 16–25 were included in this analysis to constitute a late-adolescent/young adult sample. Patients were excluded in the case of known neurological disorders, significant and habitual drug abuse or alcoholism in the last six months, mental retardation (i.e., premorbid IQ less than 70), or insufficient fluency in the English language. For each subject, every effort was made to minimize the time between appointments scheduled for neuroimaging and clinical assessment. The average time between the neuroimaging appointment and the appointment where the SANS, SAPS, SCOS, and SAS were administered was 1.5 months (standard deviation = 1.3). The average time between the neuroimaging appointment and the clinical appointment where the NAPLS role functioning and social functioning scales were administered was 1.7 months (standard deviation = 1.7). If a subject performed below chance on the WM paradigm, or if their neuroimaging data contained excessive artifacts, their data were excluded from further analyses.
Table 1.
Demographics
| Age (years ± stdev) | 19.85 ±3.24 |
| Gender (M/F) | 8/5 |
| Years of Education | 12.50 ± 2.83 |
| Handedness (R/L) | 13/0 |
| Age of Onset | 18.92 ± 3.32 |
| Diagnosis: | |
| Schizophrenia | 8 |
| Schizophreniform | 3 |
| Schizoaffective | 2 |
| Medication | (0/11/2) |
| (Typical/Atypical/Mood Stabilizer) |
The fMRI data collected during this study were also included in analyses for a patient-control study examining the relationship between WM performance and activation in the DLPFC (Karlsgodt et al., in press).
2.2 Clinical Measures
2.2.1 Clinical Symptoms
Positive and Negative Symptoms were assessed with the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984) and the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983). Since there is support across a variety of studies for a three-symptom dimension model of schizophrenia (Andreasen et al., 1995), the global scores from these two instruments were used to characterize three symptom dimensions: Positive symptoms (the sum of the global hallucinations and global delusions scores), Negative symptoms (the sum of the global affective flattening, global apathy, and global anhedonia scores), and Disorganization symptoms (the sum of the global logical thought disorder, global alogia, global inattention, inappropriate affect, and global bizarre behavior scores). Trained raters were used to make SANS and SAPS ratings. The training program required trainees to rate SANS and SAPS training videos, conduct “live” patient interviews, and achieve a median Intraclass Correlation Coefficient of .80 or higher across all items or on global ratings compared with criterion ratings (Ventura et al., 1993). Each SANS and SAPS rater participated in an ongoing Quality Assurance program designed to minimize rater drift.
2.2.2 Social and Role Functioning Measures
The Social Attainment Scale (SAS, Goldstein, 1978) was used to assess overall social functioning. This scale assesses the frequency and quality of social contacts and activities. The Global Scale of Functioning: Social (SFS, Auther, 2006) was also used to assess social functioning. This scale is a 10-point Likert scale developed for use in transitional aged psychiatric populations, which measures global social functioning.
The Global Scale of Functioning: Role (RFS, Niendam, 2006), a 10-point Likert scale that was developed for use in transitional-aged psychiatric populations, was used to assess functioning at school or work. In addition, the Strauss-Carpenter Outcome Rating Scale (SCORS, Strauss and Carpenter, 1972) was used as a more general measure of functioning. This scale assesses the frequency of recent hospitalizations, social contacts, and school/work activities.
2.3 Working Memory Task
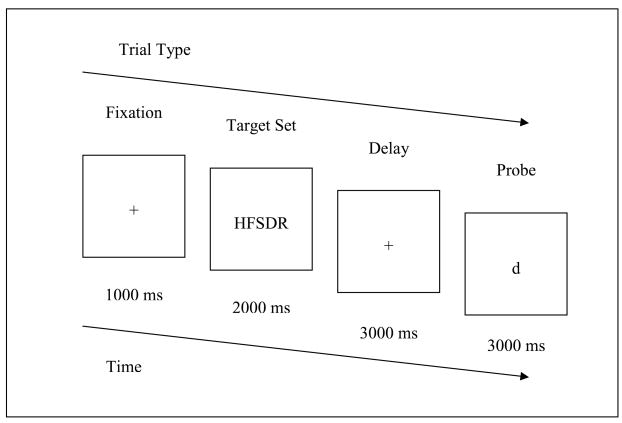
The verbal capacity task (VCAP) is a Sternberg-style verbal maintenance paradigm (Sternberg, 1966; Karlsgodt et al., 2007; Karlsgodt et al., in press). During each 9000ms trial, subjects are presented with a 1000 ms fixation followed by a set of 3, 5, 7, or 9 uppercase letters for 2000 ms. This is followed by a 3000 ms maintenance delay period. Then a lowercase probe letter is shown, and the subject is given 3000 ms to respond “1” if the letter was in the original array of letters, or “2” if it was not (see figure 1).
Figure 1.
Verbal Capacity Task (VCAP) – Task Design and Sample Stimuli. This figure illustrates sample stimuli for each trial, including the initial fixation point, the target set of letters to be remembered, the delay, and the probe.
Trials were blocked by load (3, 5, 7, or 9 letters), and each 18-second block contained 2 trials. There were 12 trials (i.e., 6 blocks) for each load type. Each block was followed by a 4 second rest/visual fixation period (4 total rest periods), and there were 6 additional 18s blocks of rest/visual fixation distributed throughout.
2.4 Scanning Parameters
Scanning was performed on a 3 Tesla Siemens scanner at the Ahmanson-Lovelace Brain Mapping Center at UCLA. A standard radiofrequency head coil was employed, and head motion was restricted with foam padding. Anatomical reference scans were acquired first and used to configure slice alignment. A coplanar T2 scan was then acquired for anatomical registration using an Echo Planar sequence (EPI) with a TR = 5 seconds, TE = 33ms, flip angle = 90 degrees. There were 33 slices, 3 mm thick with a slice gap of 1 mm, 128 × 128 matrix and 200 × 200 FOV, AC-PC aligned. Functional data was acquired using an EPI sequence with a TR/TE of 3s/45ms, and flip angle of 90 degrees. Functional slices were matched to those in the T2 weighted structural images. 33 functional slices, 3mm each with 1mm spacing were acquired in an interleaved pattern, with a 64 × 64 in plane matrix and 200mm FOV, AC-PC aligned. The task was 9 minutes in duration (180 scans). Stimuli were presented using a PC running E-Prime (Psychology Software Tools), and subjects viewed the stimuli via LCD goggles. Responses were collected using a button box.
2.5 FMRI data processing
Because subtle morphological differences are expected between patients and controls, the use of a standard space template based only on healthy brains may create unnecessary distortion when registering patients’ functional EPI data during spatial registration. To address this issue, a center-specific common brain was created from all available subjects’ T2 scans, including age matched control subjects who have participated in other studies for our group, using an automated image registration package, AIR (Woods et al., 1998). Each subject’s data were first registered to their own T2 image, and then to the common brain, before being entered in a group analysis.
Functional images were analyzed using FSL (FMRIB software library v 3.3, www.fmrib.ox.ac.uk/fsl (Smith et al., 2004). Motion in the EPI data was corrected to combat motion artifacts using a 6 parameter, rigid body 3D co-registration (FLIRT), which registered each BOLD image in the time series to a middle data point in the time series. Each subject’s EPI was then registered to their own T2-weighted structural image, then to the study-specific common brain (Jenkinson and Smith 2001; Jenkinson et al., 2002). Data were spatially smoothed with a 5mm Gaussian kernel and filtered with a non-linear high-pass filter with a cut off of 72 seconds to remove any low frequency artifacts. Individual subject analyses were carried out using FEAT (FMRI Expert Analysis Tool).
Time-series statistical analyses were carried out using FILM (FMRIB’S Improved Linear Model) with local autocorrelation correction (Woolrich et al., 2001). FILM applies the general linear model on a voxel-by-voxel basis such that each voxel’s time course is fit to the model, while local autocorrelation correction applied within tissue type improves the estimation of temporal smoothness (Woolrich et al., 2001; Smith et al., 2004). Goodness-of-fit of each voxel to the model is estimated, which results in parameter estimates that indicate the degree to which change in fMRI signal is explained by the model. Each WM load was modeled, and each subject’s motion parameters across the scan (calculated as translation and rotation parameters) were entered as covariates to reduce the effects of motion on the results. Subjects with greater than an average of 3mm of motion were excluded, as it was found that this degree of motion introduced prominent and non-correctible artifacts in the data.
Group analysis was carried out using FLAME (FMRIB’s Local Analysis of Mixed Effects) (Behrens et al., 2003; Smith et al., 2004). Resulting Z-statistic images were thresholded with clusters determined by Z>2.3 and a corrected cluster significance threshold of p=0.01 (Worsley et al., 1992; Friston, 1994; Forman et al., 1995). Cluster p-values were determined using a spatial smoothness estimation implemented in FEAT (Jenkinson, 2001) based on the methods of Forman (Forman et al., 1995) and Friston (Friston, 1994).
2.6 Region of Interest Definition
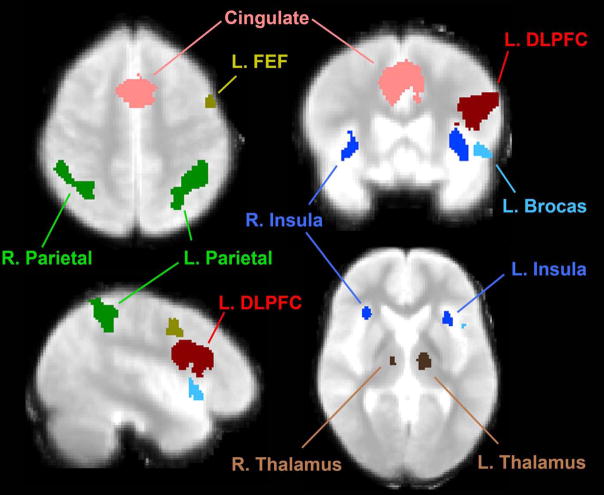
Functionally defined regions of interest (ROIs) were created in the space of the study-specific standard brain using the omnibus contrast (all subjects, all loads vs. rest/visual fixation contrast). This allows for equal contribution of all subjects across all working memory loads to the definition of ROIs. ROIs were defined from a thresholded t-statistic map (T12>4.2, p<0.001, 2-tailed). Within this thresholded map, clusters were defined by referring to Brodmann’s and anatomical landmarks, and including all contiguous voxels above the threshold in the ROI (see figure 2). Using these criteria, the regions of interest selected for this study included bilateral dorsolateral prefrontal cortex (RDLPFC and LDLPFC), bilateral frontal eye fields (RFEF and LFEF), bilateral parietal cortices (RPAR and LPAR), bilateral insular cortex (RINS and LINS), Left Inferior Frontal gyrus (Broca’s area), Cingulate gyrus, and bilateral thalamus (RTHAL and LTHAL).
Figure 2.
Regions of Interest from the Verbal Capacity Task.
The Featquery program (fmrib.ox.ac.uk/fsl/feat5/featquery.html) was used to warp each ROI back into each individual subject’s space by applying the inverse of the transformation matrix using during the initial registration. The motion corrected, smoothed, and filtered data were probed for their percent signal change from baseline for each ROI at each load.
2.7 Capacity Calculation
In order to characterize the WM system at a point where it is being maximally utilized but not overtaxed, each individual’s WM capacity was calculated. This also ensures that the signal represents the point at which each individual is at their own maximal behavioral output. Capacity was calculated for each individual at each WM load according to Cowan’s formula: k=n*(H+CR−1) (Cowan, 2001), where k=capacity, n= the number of items in the display, H= hit rate, and CR=correct rejection rate.
Once each individual’s WM capacity was determined, the average percent signal change from each ROI corresponding to the closest WM load to capacity for each individual was entered into subsequent analyses.
2.8 Partial Least Squares Analysis
To examine the relationship between regional brain activation across the WM system and symptom and functioning variables, a partial least squares analysis (PLS) was conducted. The statistical method is especially useful in small samples with multicollinear measures (Nestor et al., 2002). Functional activations, calculated as percent signal change based on contrast of the closest WM load to capacity, extracted from the 12 regions of interest in the verbal WM network specified above (right and left DLPFC, right and left FEF, right and left parietal, right and left thalamus, right and left insula, cingulate, and Broca’s area) were included as predictor variables, and seven clinical variables (RFS, SFS, SCORS, SAS, positive symptoms, negative symptoms, and disorganized symptoms) were included as response variables in the analyses. The PLS method extracts latent variables (LVs) by taking the singular value decomposition of the cross-correlation matrix composed of the lists of predictor and response variables. The procedure extracts as many LVs as there are measures in the smallest set of variables. The covariance between the LVs is represented by d, the singular value of the cross-correlation matrix, with each pair of LVs being orthogonal to previously extracted pairs. Because of this, successive pairs of LVs explain less of the covariance and have smaller d values. The ratio of each squared singular value as compared to the sum of all squared singular values provides the percent of the covariance explained by that LV pair. Each LV is composed of a set of ‘X’ coefficients or saliences, one for each ROI, and a set of ‘Y’ saliences, one for each clinical measure. Based on accepted practice, saliences above the cutoff of 0.30 indicate which measures are considered significant (McIntosh et al., 1996; Nestor et al., 2002).
The significance of the first pair of LVs was assessed using a permutation test. This procedure tests the central tendency of the LV pair when considered as a vector, and uses a Monte-Carlo-like procedure that randomly reorders the cross-correlation matrix and computes a singular value for each of the 10,000 permutations. In this way, the probability that the first d was obtained by chance can be calculated. In order to protect against over-fitting the model, only those pairs of LVs that explain 10% or more of the covariance were considered.
3. Results
Seven pairs of LVs were extracted, with 12 saliences corresponding to the functionally defined ROIs and 7 saliences corresponding to the clinical variables. The first pair of LVs explains 80.7% of the covariance between blocks (d1 = 3.03), and the second pair of LVs explains 10.3% of the covariance (d2 = 1.08). The remaining LVs did not account for a substantial proportion of covariance. Permutation tests indicated that the obtained model achieved a high level of significance (p<0.001).
For the first pair of LVs (table 2), the functional ROIs in the left DLPFC, left FEF, left insular cortex, Broca’s area, and left parietal cortex and the clinical variables role functioning (RFS and SCORS), negative symptoms, and disorganized symptoms achieved significant saliences of 0.30 or greater. The physiological measures covaried directly with role functioning, but inversely with symptom severity. Overall, this pattern indicates that during a verbal WM task in this sample of patients with recent-onset schizophrenia, relatively greater activation in most of the components of the WM network in the left hemisphere covaries with better role functioning and with lower severity of negative and disorganized symptoms.
Table 2.
Saliences for first pair of latent variables
| Regions of Interest | Salience | Clinical Variable | Salience |
|---|---|---|---|
| Left DLPFC | −0.39* | Negative Symptoms | 0.45* |
| Left Frontal Eye Fields | −0.36* | Disorganized Symptoms | 0.46* |
| Left Insula | −0.36* | Global Scale of Functioning: Role | −0.62* |
| Broca’s Area | −0.36* | Strauss-Carpenter Role Functioning | −0.40* |
| Left Parietal | −0.36* | Social Attainment Scale | 0.17 |
| Right Insula | −0.29 | Global Scale of Functioning: Social | −0.04 |
| Left Thalamus | −0.26 | ||
| Right Parietal | −0.24 | ||
| Cingulate | −0.22 | ||
| Right Frontal Eye Fields | −0.19 | ||
| Right DLPFC | −0.18 | ||
| Right Thalamus | −0.01 |
Indicates significant values.
The fMRI measures that achieved 0.30 or greater saliences for the second pair of LVs (table 3) included the right DLPFC, the right thalamus, both measures of social functioning (SCORS and SFS), and positive symptoms. In this case, however, the physiological measures were found to inversely covary with social functioning. There was also an inverse relationship with positive symptoms. This pattern suggests that during a verbal WM task in this sample of patients with recent-onset schizophrenia, relatively greater activation in right frontal-subcortical circuitry covaries with poorer social functioning, but with fewer positive symptoms.
Table 3.
Saliences for the second pair of latent variables
| Regions of Interest | Salience | Clinical Variable | Salience |
|---|---|---|---|
| Right DLPFC | −0.71* | Social Attainment Scale | 0.72* |
| Right Thalamus | −0.44* | Global Scale of Functioning: Social | 0.54* |
| Right Insula | 0.29 | Positive Symptoms | 0.31* |
| Right Parietal | −0.22 | Strauss-Carpenter Role Functioning | 0.25 |
| Left Insula | 0.21 | Disorganized Symptoms | 0.12 |
| Cingulate | −0.21 | Negative Symptoms | −0.10 |
| Right Frontal Eye Fields | 0.19 | Global Scale of Functioning: Role | 0.07 |
| Left DLPFC | 0.15 | ||
| Left Parietal | −0.10 | ||
| Left Frontal Eye Fields | 0.09 | ||
| Broca’s Area | −0.07 | ||
| Left Thalamus | 0.02 |
Indicates significant values
4. Discussion
The results of this analysis generally support the hypothesis that greater activation in the distributed verbal WM network is associated with better functioning and less severe clinical symptoms. Additionally, though not predicted by our initial hypotheses, these results suggest that appropriately lateralized regional brain activation during performance of a verbal WM task in patients with schizophrenia is related to the severity of clinical presentation. In other words, greater activation in right (as opposed to left) frontal-subcortical circuitry during verbal WM is related to poorer social functioning.
Deficits in the WM system are thought to be a core feature of schizophrenia, one that is tied closely to the pathophysiology of the syndrome. Specifically, findings of reduced synaptic connectivity, most prominently in the frontal lobes (Goldman-Rakic and Selemon, 1997; Selemon et al., 1998), and potentially in more widespread cortical areas (Selemon et al., 1995; Selemon and Goldman-Rakic, 1999) could potentially be at the root of reduced WM capacity in these patients. In addition, Selemon and Goldman-Rakic (1999) have suggested that even very subtle depletion in neuropil could have significant functional consequences because these connections form local and distributed circuits that are responsible for a wide variety of cognitive functions. Interpreted in the context of this prior work, our results might indicate that the degree or severity of the neuropathology that impacts with WM system (i.e., reduced synaptic connectivity) as indexed by fMRI, is related to functional and clinical status.
Role functioning and social functioning were found to covary with indices of the neural underpinnings of WM. This finding is consistent with - and extends upon - previous neuropsychological research suggesting that verbal WM function is linked to functional status (Green, 1996; Green and Braff, 2001; Green, 2007). It is also consistent with previous neuropsychological and neuroimaging studies, negative and disorganized symptoms were related to activation during the verbal WM task (Menon et al., 2001; Perlstein et al., 2001).
Interestingly, despite the fact that most previous research in this area has focused the DLPFC, these results do not imply that this area is uniquely related to clinical severity, as other frontal areas, the parietal lobes, and thalamus are also implicated. This pattern suggests that it is the distributed activity of the WM circuit, and not only the frontal lobe node of this circuit, that is tied to clinical severity, role, and social functioning. In addition, our findings suggest that better social functioning may be associated with appropriate lateralization of activity during this verbal task, since greater activation in right frontal and subcortical areas was associated with poorer social functioning. Unexpectedly, although representing a more minor component of the covariance pattern, greater activation in right frontal and subcortical areas was also related to less severe positive symptoms. This relationship is difficult to interpret, particularly because the structure of the latent variable suggests that fewer positive symptoms are associated with poorer social functioning, a pattern not typically observed in samples of schizophrenia patients. Previous work also has not typically found a relationship between positive symptoms and frontal cortical activation (Whalley et al., 2007; Wible et al., 2008). However, given that the significance of the second pair of LVs is also not confirmed by the PLS procedure, which performs a permutation test only for the first pair, these secondary results should be interpreted with caution. It is possible that, given a greater number of subjects, a more stable covariance structure would emerge to better explain the relationship between positive symptoms and regional brain activity.
These findings indicate a linear relationship between brain activation and clinical presentation. Rather than testing each individual’s activation based on within-subjects variation in task difficulty, this study controls for task difficulty by assessing each subject at their own WM capacity. This approach allows for a linear test of a between-subjects model by examining individual differences at the apex of each individual’s WM function, and improves on prior work by ensuring that performance is intact and controlled for. This linear relationship, in which greater activation in the left hemisphere during verbal WM processing is associated with better functioning, is consistent with and extends previous work demonstrating a similar relationship between activation and behavioral accuracy on WM tasks in patients (Karlsgodt et al., 2007; Karlsgodt et al., in press). Namely, high performing patients tend to activate DLPFC more than low performing patients. Taken together, these results appear to suggest that high performing patients show a compensatory increase in activation (when compared with controls) to achieve similar levels of performance, and that this compensatory increase is also linked to a less severe clinical presentation. Conversely, relative hypo-activation in the low performing subjects may represent greater levels of changes in cellular microcircuitry or disruption in glutamatergic/dopaminergic signaling, which preclude such compensatory activation. Interpreted within this framework, an absence of compensatory activation during WM would then be related to poorer functioning and greater severity of negative and disorganized symptoms.
The term “cortical inefficiency” has often been employed to describe findings of hyperactivation in patients with schizophrenia (e.g., Callicott et al., 2000). However, the results of this study also suggest that a single, simple linear increase in activation in a region of interest may not be the only definition of neural inefficiency. The lateralization findings suggest a form of “allocative” inefficiency, or a non-optimal distribution of resources. In other words, compensatory increases observed outside of the verbal WM network may also represent inefficient processes (Glahn et al., 2005). Indeed, recent studies have shown when receiving atypical neuroleptic medication, patients do not demonstrate appropriate lateralization of task activity during spatial and verbal WM tasks, even in the absence of hypofrontality when compared to controls (Walter et al., 2003). In this sense, hyper- and hypoactivavtion both indicate disrupted patterns of activation, with hypoactivation representing an inability to activate WM circuitry sufficiently and hyperactivation representing more functional but still faulty activation of the WM circuitry. The current study did not include controls because it would be unlikely to find a meaningful range of “clinical” symptoms or functional status in this group. It would be interesting, however, to investigate the possibility of different levels of activation in typically developing individuals, or in relatives of individuals with schizophrenia.
Overall, this study demonstrates a relationship between activation in the distributed WM system and clinical severity of schizophrenia. This finding expands on previous work showing relationships between activation in frontal regions and disorganized symptoms (Menon et al., 2001; Perlstein et al., 2001; Yoon et al., 2008) by demonstrating a wider pattern of covariance between clinical presentation and distributed functional activation. The mechanisms underlying the relationships between regional brain activity and clinical presentation remain to be determined, but reductions in neuropil, and thus functional connectivity within the WM circuitry, represent prominent theoretical suspects.
Acknowledgments
This research was supported by NIH Grants MH65079, MH066286, GM072978 and RR021992 to T.D.C, F31-MH068111-02 to K.H.K., NIMH P50 MH066286 and MH037705 to K.H.N., NARSAD (Young Investigator Award to C.E.B.) and a gift to the UCLA Foundation by Garen and Shari Staglin. The authors would like to thank Molly Hardt and Lara Zimmerman for their help with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. The scale for the assessment of negative symtpoms (SANS) University of Iowa; Iowa City: 1983. [Google Scholar]
- Andreasen NC. The scale for the assessment of positive symptoms (SAPS) University of Iowa; Iowa City: 1984. [Google Scholar]
- Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M. Symptoms of schizophrenia. Methods, meanings, and mechanisms. Archives of General Psychiatry. 1995;52:341–51. doi: 10.1001/archpsyc.1995.03950170015003. [DOI] [PubMed] [Google Scholar]
- Auther A, Smith C, Cornblatt B. Global Functioning Scale: Social (GFS: Social) Zucker Hillside Hospital; Glen Oaks, NY: 2006. [Google Scholar]
- Barch DM, Sheline YI, Csernansky JG, Snyder AZ. Working memory and prefrontal cortical dysfunction: specificity to schizophrenia compared with major depression. Biological Psychiatry. 2003;53:376–384. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6:750–7. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJP, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological Dysfunction of the Dorsolateral Prefrontal Cortex in Schizophrenia Revisited. Cerebral Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. American Journal of Psychiatry. 2003;160:2209–15. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Glahn DC, Kim J, van Erp TGM, Karlsgodt KH, Cohen MS, Nuechterlein KH, Bava S, Shirinyan D. Dorsolateral Prefrontal Cortex Activity During Maintenance and Manipulation of Information in Working Memory in Patients With Schizophrenia. Archives of General Psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frakowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: A quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Human Brain Mapping. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophrenia Bulletin. 1997;23:437–58. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- Goldstein MJ. Further data concerning the relation between premorbid adjustment and paranoid symptomatology. Schizophrenia Bulletin. 1978;4:236–43. doi: 10.1093/schbul/4.2.236. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Braff DL. Translating the basic and clinical cognitive neuroscience of schizophrenia to drug development and clinical trials of antipsychotic medications. Biological Psychiatry. 2001;49:374–384. doi: 10.1016/s0006-3223(00)01027-1. [DOI] [PubMed] [Google Scholar]
- Green MF. Stimulating the Development of Drug Treatments to Improve Cognition in Schizophrenia. Annual Review of Clinical Psychology. 2007;3:159–180. doi: 10.1146/annurev.clinpsy.3.022806.091529. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Estimation of smoothness from the residual field: FMRIB technical report TR00MJ3. 2001 http://www.fmrib.ox.ac.uk/analysis/techrep/tr00mj3/tr00mj3/
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Glahn DC, van Erp TGM, Therman S, Huttunen M, Manninen M, Kaprio J, Cohen MS, Lönnqvist J, Cannon TD. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophrenia Research. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Sanz JH, van Erp TGM, Bearden CE, Nuechterlein KH, Cannon TD. Schizophrenia Research. Evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task as measured by fMRI. Biological Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biological Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia Research. 2003;60:285–98. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–57. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- Menon V, Anagnoson RT, Mathalon DH, Glover GH, Pfefferbaum A. Functional neuroanatomy of auditory working memory in schizophrenia: relation to positive and negative symptoms. Neuroimage. 2001;13:433–46. doi: 10.1006/nimg.2000.0699. [DOI] [PubMed] [Google Scholar]
- Nestor PG, O’Donnell BF, McCarley RW, Niznikiewicz M, Barnard J, Jen Shen Z, Bookstein FL, Shenton ME. A new statistical method for testing hypotheses of neuropsychological/MRI relationships in schizophrenia: partial least squares analysis. Schizophrenia Research. 2002;53:57–66. doi: 10.1016/s0920-9964(00)00171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Johnson JK, Cannon TD. Global Functioning Scale: Role (GFS: Role) University of California; Los Angeles: 2006. [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. American Journal of Psychiatry. 2001;158:1105–13. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Glahn DC, Censits DM, Smith RJ, Lazarev MG, Alavi A, Gur RE. Frontotemporal cerebral blood flow change during executive and declarative memory tasks in schizophrenia: a positron emission tomography study. Neuropsychology. 1998;12:399–413. doi: 10.1037//0894-4105.12.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biological Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Archives of General Psychiatry. 1995;52:805–18. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. Journal of Comparative Neurology. 1998;392:402–12. [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Strauss JS, Carpenter WT. The prediction of outcome in schizophrenia. I. Characteristics of outcome. Archives of General Psychiatry. 1972;27:739–46. doi: 10.1001/archpsyc.1972.01750300011002. [DOI] [PubMed] [Google Scholar]
- Stevens AA, Goldman-Rakic PS, Gore JC, Fulbright RK, Wexler BE. Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Archives of General Psychiatry. 1998;55:1097–1103. doi: 10.1001/archpsyc.55.12.1097. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green M, Shaner A, Liberman RP. Training and quality assurance on the BPRS: “The Drift Busters. International Journal of Methods in Psychiatric Research. 1993;3:221–244. [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV I/P (SCID) Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Walter H, Wunderlich AP, Blakenhorn M, Schafer S, Tomczak R, Spitzer M, Gron G. No hypofrontality, but absence of prefrontal lateralization comparing verbal and spatial working memory in schizophrenia. Schizophreina Research. 2003;61:175–84. doi: 10.1016/s0920-9964(02)00225-6. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Gountouna VE, Hall J, McIntosh A, Whyte MC, Simonotto E, Job DE, Owens DGC, Johnstone EC, Lawrie SM. Correlations between fMRI activation and individual psychotic symptoms in un-medicated subjects at high genetic risk of schizophrenia. BMC Psychiatry. 2007;7:61–71. doi: 10.1186/1471-244X-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible CG, Lee K, Molina I, Hashimoto R, Preus AP, Roach BJ, Ford JM, Mathalon DH, McCarthey G, Turner JA, Potkin SG, O’Leary D, Belger A, Diaz M, Voyvodic J, Brown GG, Notestine R, Greve D, Lauriello J FBIRN. fMRI activity correlated with auditory hallucinations during performance of a working memory task: data from the FBIRN consortium study. Schizophrenia Bulletin. 2008 doi: 10.1093/schbul/sbn142. e-publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22:139–52. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Walters R, Wendelken C, Ragland JD, Carter CS. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. American Journal of Psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]