Abstract
Mammalian central pattern generators producing rhythmic movements exhibit robust but flexible behavior. However, brainstem network architectures that enable these features are not well understood. Using precise sequential transections through the pons to medulla, it was observed that there was compartmentalization of distinct rhythmogenic mechanisms in the ponto-medullary respiratory network, which has rostro-caudal organization. The eupneic 3-phase respiratory pattern was transformed to a 2-phase and then to a 1-phase pattern as the network was physically reduced. The pons, the retrotrapezoid nucleus and glycine mediated inhibition are all essential for expression of the 3-phase rhythm. The 2-phase rhythm depends on inhibitory interactions (reciprocal) between Bötzinger and pre-Bötzinger complexes, whereas the 1-phase-pattern is generated within the pre-Bötzinger complex and is reliant on the persistent sodium current. In conditions of forced expiration, the RTN region was found to be essential for the expression of abdominal late expiratory activity. However, it is unknown whether the RTN generates or simply relays this activity. Entrained with the central respiratory network is the sympathetic nervous system, which exhibits patterns of discharge coupled with the respiratory cycle (in terms of both gain and phase of coupling) and dysfunctions in this coupling appear to underpin pathological conditions. In conclusion, the respiratory network has rhythmogenic capabilities at multiple levels of network organization, allowing expression of motor patterns specific for various physiological and pathophysiological respiratory behaviors.
Keywords: retrotrapezoid nuclei, Bötzinger Complex, abdominal nerve
1. Introduction
Breathing in mammals is a critical robust homeostatic process that ensures adequate levels of oxygen (O2) in blood and provides a means to exhaust carbon dioxide (CO2). Rhythmic respiratory movements must occur continuously throughout life so the control system has to be robust, yet flexible to account for integrated responses under different stressors. The mammalian respiratory oscillator originates from brainstem networks encompassing the ventrolateral medulla and pons and has evolved to generate rhythmic patterns of motor activity producing coordinated movements of both the respiratory pump muscles (diaphragm, thorax, abdomen), which bring about lung inflation and deflation, and upper airway muscles, controlling resistance to airflow. These rhythmic motor outflows must be coordinated temporally to allow efficient and optimal exchange and transport of O2 and CO2 thereby ensuring physiological homeostasis within the brain and the rest of the body. Clearly, the cardiovascular system plays an essential role for gas transport and distribution to meet demand. It is therefore not surprising that the respiratory and cardiovascular systems are tightly coupled via neural connections within the pons and medulla (Fig 1; See Dick et al. 2009 –this Special Issue).
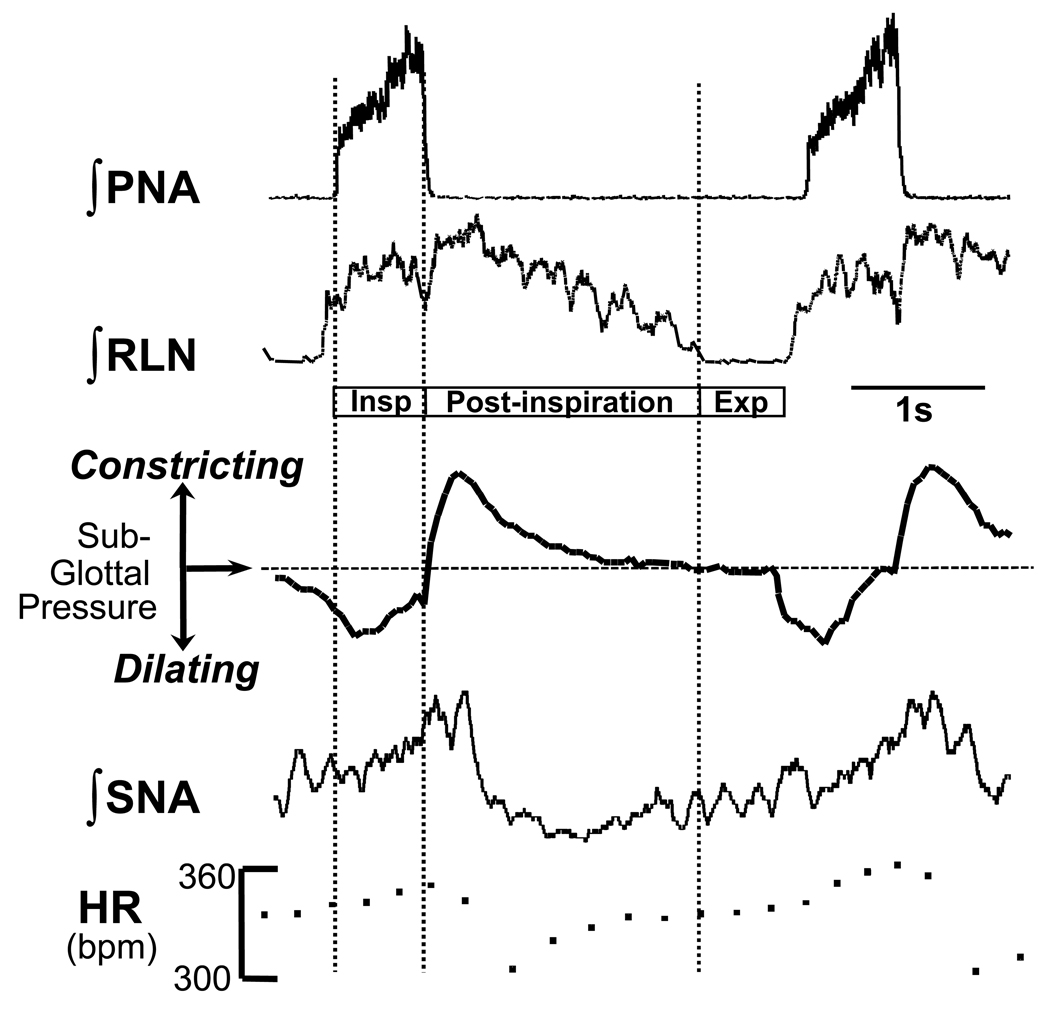
Figure 1. Cardio-laryngo-sympathetic-respiratory coupling recorded in situ.
A montage showing integrated activities of the phrenic nerve (PNA), recurrent laryngeal nerve (RLN) and sympathetic nerve activity (SNA; thoracic chain) to show coordination of cranial and spinal cardio-respiratory motor outflows in an arterially perfused rat preparation without pulmonary stretch receptor feedback. Note the three phase rhythm (inspiration, Insp; post-inspiration; expiration , Exp) corresponding to that defined by Richter (1982). During central inspiratory activity indexed by phrenic (Insp), heart rate (HR) increases (due to central synaptic inhibition of cardiac vagal motoneurons and increased sympathetic discharge) and the glottis dilates due to activation of laryngeal abductors. During early expirations (so called, post-inspiration), heart rate falls as the inspiratory related inhibition of the cardiac vagal motoneurons is removed. At this time the laryngeal adductors fire causing a transient constriction of the glottis; the latter stalls expiratory air flow so giving adequate time for gas exchange. Note, that the SNA is respiratory phase locked and peaks in the post-inspiratory phase. Modified from Paton & Nolan (2000).
This highly phase coordinated respiratory motor output relies on a combination of synaptic interactions among populations of respiratory neurons, and their electrophysiological properties. This network is highly dynamic, plastic and designed to be modulated to meet the demands of an ever-changing environment. It is, therefore, highly state-dependent and it is under control of various peripheral and central sensory inputs as well as all types of endogenous neuromodulatory signals. For example, alterations in the metabolic state of a mammal, or the O2 and CO2 levels in the blood, will modify the respiratory motor pattern and mechanisms of rhythm generation (Rybak et al. 2007; St-John et al. 2002; Smith et al. 2007; Abdala et al. 2009). State-dependent changes not only regulate the frequency and amplitude of the motor activity (i.e., rate and depth of breathing), but can dramatically reconfigure the network generating respiratory rhythm. This occurs rapidly and seamlessly and can result in short term and chronic responses (see below). This short review will summarise a number of recent studies that have described the hierarchical compartmental organization of the ponto-medullary network generating respiratory rhythm. Below highlights the transitions dependent on metabolic state and its essential coupling to the cardiovascular system. The latter is illustrated with reference to pathological conditions.
2. The three-phase rhythm of respiration: a unified starting point?
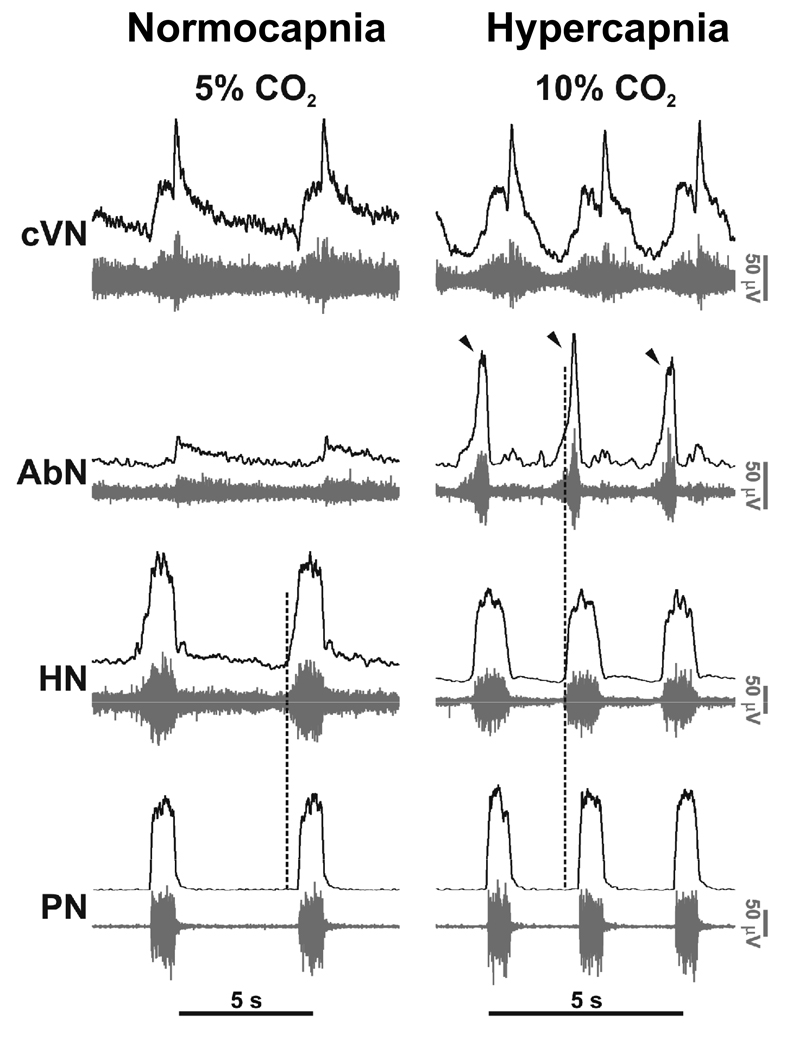
Richter (1982) emphasised the concept that the ‘normal’ or eupneic respiratory pattern comprised 3 phases per respiratory cycle. This included: inspiration, post-inspiration (stage I) and active expiration (stage II; see also Bianchi & Gestreau, 2009 – this Special Issue). Fig 1 depicts these 3 phases by comparing phrenic and recurrent laryngeal motor outflows. This pattern seems to hold true in mouse and rat including newborn rats (Abdala et al. 2009; Dutschmann et al. 2000; Paton, 1996b; 1997; 2006). Stage II expiration (termed here as late-E) is best observed when recording from abdominal motor nerves under high respiratory drive states such as hypercapnia (Fig 2). Note that hypoxia, hypercapnia and ischemia will bring into play late-E activity, but late-E is absent under normoxia and normocapnia. This activity accentuates expiratory pumping via abdominal muscle contraction and thereby reduces expiratory time to allow respiratory frequency to increase. Note also, that during the expression of late-E activity, the onset of hypoglossal motor activity occurs coincidently and earlier relative to phrenic than when late-E is absent. Thus, hypoglossal pre-inspiratory discharge is both longer in duration and advanced in onset during forced expiration; this is important for reducing upper airway resistance during abdominal expiratory pumping (Fig 2; Abdala et al. 2009).
Figure 2. Eupneic pattern of respiratory motor activity in situ.
In control conditions (normocapnia) phrenic (PN) and hypoglossal (HN) have an inspiratory ramp shaped activity envelope whereas abdominal nerve (AbN; lumbar segment 1) exhibits small amplitude post-inspiratory activity. Using 10% carbon dioxide (i.e. 5% above normocapnic conditions), central respiratory drive was raised. This resulted in generation of augmenting expiratory activity in the AbN outflow (Late-E; arrowed) and advanced the onset of pre-inspiratory HN activity relative to PN; the latter indicating reduced airway resistance during both the forced expiration and inspiration. The pattern of PN now showed an abrupt onset in discharge (see Abdala et al. 2009).
3. Putting differences in experimental preparations aside
The above description is based on that from the arterially perfused in situ preparation of decerebrate rat (Paton, 1996a; 1996b). This preparation provides a phrenic motor pattern that is similar to that reported from an in vivo decerebrate rat (e.g. Shen et al. 2003). The slower frequency reflects the absence of phasic pulmonary stretch receptor feedback and cooler operating temperature (31°C). However, it is distinct to that reported from in vitro preparations of neonatal rat and mouse either transverse slice (thick or thin), en bloc brainstem or brainstem spinal cord. Why are there these differences? A proposal is, that the physical compartmentalization and/or change in metabolic state that accompany the in vitro preparation enforce a reorganization of the neuronal mechanisms generating respiratory motor pattern. The following section describes an attempt to unify the central respiratory control field by providing an explanation as to why different preparations (in vitro versus in situ/in vivo) produce different respiratory motor patterns. But, importantly, it also reveals novel insights into the hierarchical organization of the ponto-medullary network and multiple mechanisms for rhythm generation that operate in a state-dependent manner.
4. The 3-2-1 hypothesis of respiratory rhythm/pattern generation
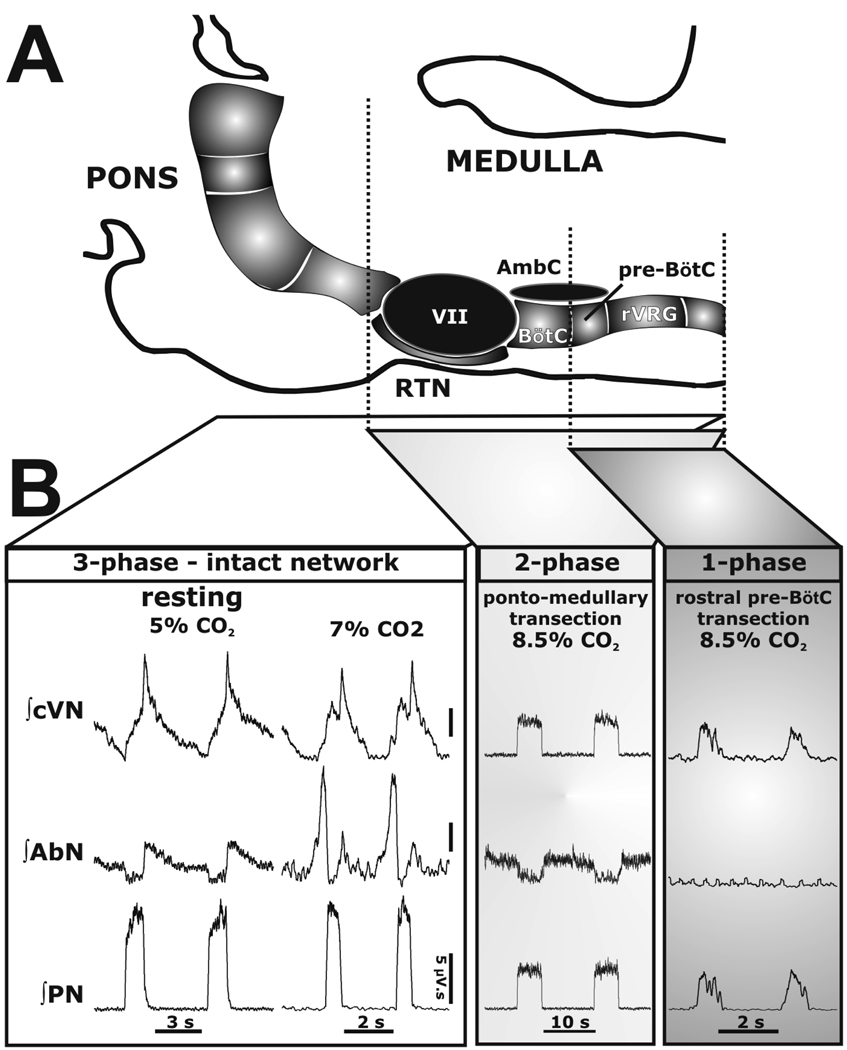
The hypothesis is that there is both spatial and functional compartmentalization of the respiratory network. If this is the case, then logically it should be possible to divide it up. Sequential rostral to caudal transections through the ponto-medullary respiratory network within the in situ perfused rat brainstem-spinal cord preparation allowed us to show that network dynamics reorganized rapidly (within a respiratory cycle or two) and new rhythmogenic mechanisms emerge (Rybak et al. 2007; Smith et al. 2007). Thus, the three-phase respiratory rhythm (eupneic pattern) transformed to a two-phase and then to a one-phase rhythm as the network was physically reduced using precision micro-vibratome slicing. Based on histological reconstruction of boundaries from sliced brainstems of multiple preparations, expression of the three-phase rhythm required the presence of the pons, whereas generation of the two-phase rhythm depended on the integrity of Bötzinger (BötC) and pre-Bötzinger complexes (pre-BötC; see Ruangkittisakul & Ballanyi, 2009 – this Special Issue; Ruangkittisakul et al. 2009 – this Special Issue) and interactions between them. The one phase-pattern was generated within the pre-Bötzinger complex and disappeared once this region was transected caudally (Fig 3).
Figure 3. 3-2-1 phase hypothesis of respiration.
A: Schematic drawing depicting the spatial arrangement of the ventral respiratory column viewed sagittally. Microtransections of the brainstem are indicated by the vertical dashed lines. Abbreviations: AmbC: compact nucleus ambiguus; BötC: Bötzinger Complex; LRt: lateral reticular nucleus; Pn: pontine nucleus; pre-BötC: pre- Bötzinger Complex; RTN/vlPF: retrotrapezoid nucleus and ventrolateral parafacial regions; rVRG: rostral ventral respiratory group; V: trigeminal motor nucleus; VII: facial motor nucleus. B: Activity patterns of phrenic (PN), abdominal (AbN), and central vagus (cVN) nerves from an intact preparation (3-phase pattern) during eucapnia (5% CO2) and hypercapnia (7% CO2), and after a ponto-medullary transection. The latter resulted in a 2-phase pattern in which late-E abdominal bursts are abolished during hypercapnia (8.5% CO2). After a transection at the rostral boundary of the pre-BötC, a 1-phase inspiratory pattern was evoked and all expiratory motor activity was abolished.
Clearly this approach is extreme, so could one obtain these three- to two-or two- to one- phase transitions without physically cutting through the ponto-medullary neuraxis? This was approached by reducing chloride-mediated synaptic inhibition (low extracellular Cl∓ concentration in ponto-medullary intact preparations; Smith et al. 2007), or lowering CO2 (Smith et al. 2007) or blocking glycine receptors with strychnine (Busselberg et al. 2001; Dutschmann & Paton, 2002). All these manipulations transformed the three-phase to a two-phase respiratory pattern a response similar to that reported after inactivation of the Kolliker Fuse (Dutschmann & Herbert, 2006). Moreover, a one-phase pattern (analogous to gasping) could be produced during ischemia (Paton et al. 2006). In all cases, the blockade of persistent sodium current (INaP) abolished the one-phase rhythm in stark contrast to the three-phase and two-phase rhythms which are not dependent on this current (Smith et al. 2007). These results suggest that the three-phase and two-phase rhythms depend on inhibitory synaptic interactions between networks of neurons located in two core adjacent compartments – the BötC and pre-BötC, with essential tonic drive arising from different rostral regions. The one-phase rhythm, however, depends on INaP and local synaptic excitation confined to the pre-BötC only. Thus, these data suggest that the ponto-medullary respiratory network has numerous rhythmogenic capabilities that are distinct and operate at multiple levels of network organization. This provides both robustness and back-up, and undoubtedly allows expression of motor patterns specific for various physiological and/or pathophysiological respiratory behaviors. Finally, the two- and one- phase respiratory motor patterns may map onto those that are observed in vitro. Therefore, it becomes essential to know what regions of the network are being isolated in slice preparations (BötC and pre-BötC or pre-BötC only) as both use distinct neuronal mechanisms to generate rhythmic motor outputs. In en bloc brainstem or brainstem-spinal cord preparations extreme metabolic conditions such as hypercapnia and hypoxia in the deeper brainstem tissue may impair network connectivity and synaptic functions, resulting in either a two or one-phase pattern. Such metabolic disturbances were mimicked by depressing glycinergic synaptic inhibition and raising levels of extracellular potassium (see St-John et al. 2001). This resulted in switching from a 3-phase eupneic pattern to a one phase pattern (see St-John et al. 2001).
5. The pre-BötC: a kernel with essential connections
The results from Smith et al. (1991; 2007) indicate that the pre-BötC when isolated physically can generate respiratory rhythmicity but in the intact ponto-medullary brainstem is functionally embedded into a broader network. Clearly it can operate in multiple modes of rhythm generation, such as intrinsic bursting, which does not require phasic inhibition, or a tonic activity mode which needs phasic inhibition to exhibit rhythmic bursting activity (Smith et al. 2000). It is proposed that the mechanism by which the pre-BötC generates rhythmic activity are based on synaptic inputs (phasic and tonic) from many levels of the central neuraxis including both the BötC (providing phasic inhibition) and from more rostral structures including the retrotrapezoid nucleus (RTN; see Guyenet et al 2009 – this Special Issue; Onimaru et al. 2009 – this Special Issue) and the pons (controlling the state of the pre-BötC). These inputs define the mode of operation of the pre-BötC and hence the rhythmogenic mechanism expressed by the entire brainstem respiratory network.
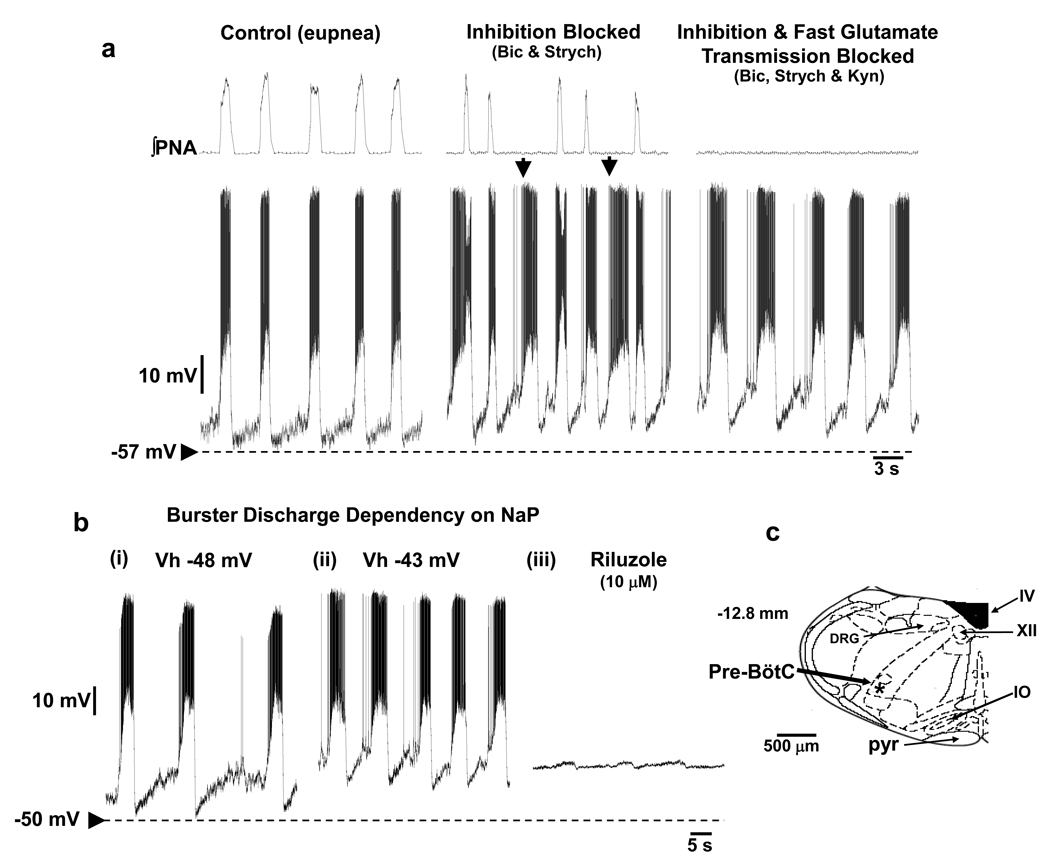
Until recently, intrinsic burster neurons of the pre-BötC had only been described in neonatal tissue in vitro, with no functioning pons. Evidence now exists demonstrating the first description of pre-BötC bursters in mature mice (Paton 1997) and rats (Fig 4; Paton et al. 2006; St-John et al. 2009) with an intact pontomedullary brainstem. In the mature rat, these neurons have an intrinsic ability to generate bursts in the absence of ionotropic mediated synaptic inputs. They are located in both the pre-BötC and extend caudally into the rostral ventral respiratory column, they have axons that cross the midline and some, but not all, are glutamatergic (St-John et al. 2009). In the intact network, when the intrinsic bursting property is suppressed (see below), these bursters are inspiratory neurons such that their firing is either coincident with phrenic motor outflow or just precedes it. Further, in the intact ponto-medullary system intrinsic bursting is suppressed by chloride mediated synaptic inhibition. The blockade of GABAA and glycine receptors depolarize these neurons and they demonstrate ectopic bursting (Fig 4). As with in vitro bursters of neonatal rats, it is clear that the frequency of burst generation is voltage-dependent. However, it was found that even at the upper limit of their intrinsic bursting frequency was slower than the eupneic rhythm (St-John et al. 2009) suggesting another mechanism for eupneic breathing. Finally, intrinsic bursters are sensitive to metabolic hypoxia (induced by cyanide), which is consistent with their role in gasp generation (St-John et al. 2009). Indeed, this intrinsic property makes them ideal for operating in low oxygen environments when synaptic inhibition is likely to fail (Schmidt et al. 1995). In sum, based on recent evidence (St-John et al. 2009), it seems unlikely that the intrinsic bursting property of these burster neurons is expressed during eupnea. During eupnea, the intrinsic bursting property of pacemakers or groups of pacemakers discussed earlier (see Feldman & Del Negro, 2006) might have no crucial role in driving normal breathing. However, the ionic conductances of these neurons may play critical roles in shaping the pattern of discharge of these neurons during eupnea.
Figure 4. Respiratory burster neurons recorded from a ponto-medullary intact in situ rat preparation.
Prior to their synaptic isolation, burster neurons were characterized as inspiratory and held relatively hyperpolarized during eupnea (a). Bursters depolarized subsequent to blockade of glycine and GABAA receptors (1µM strychnine, 20 µM bicuculline) when ectopic bursts that did not correspond to a phrenic burst were evident (arrowed). Blockade of ionotropic glutamate receptors arrested phrenic discharge and resulted in intrinsic bursting which was voltage-dependent (bi,ii) and sensitive to blockade of persistent sodium current. Some bursters were found in the pre-BötC (c) while others were in the rostral ventral respiratory group region (see St-John et al. 2009 for details).
6. Forced breathing: recruitment of an expiratory oscillator
The abdominal motor outflow targets accessory muscles of breathing. During quiet breathing these muscles provide postural tone and a foundation on which the thoracic pump muscle can operate but do not show respiratory rhythmic contraction. However, during increased respiratory drive as during exercise or hypercapnia, abdominal muscle pumping is recruited to provide a mechanism for active (or forced) expiratory pumping. It remains unclear where the oscillator for this active expiratory activity resides but the parafacial respiratory group (pFRG) has been suggested (Feldman & Del Negro, 2006).
The location of the pFRG partially overlaps with the RTN (Fortuna et al., 2008). The RTN plays a critical role in controlling the respiratory rhythm and has appropriate connectivity to/from the pons and ventral respiratory column as well as input from peripheral chemoreceptors (see Guyenet 2008; Guyenet et al. 2008; Mulkey et al. 2004; Nattie 2001). The RTN senses changes in arterial tension of CO2 (central chemosensitivity) in both anaesthetised and conscious rats. In addition, inactivation of the RTN in anaesthetised rats causes apnea that cannot be reversed by concurrent peripheral chemoreceptor stimulation (Mulkey et al. 2004) whereas activation of the RTN stimulates breathing (Abbott et al. 2009). Recordings from this region indicate that chemosensitive neurons fire tonically and are only weakly respiratory modulated even with high levels of hypercapnia in adult anaesthetised rats (Guyenet 2008). Yet others describe phasic expiratory neurons that fire in late and early expiration - so-called “pre-inspiratory” (or biphasic expiratory) in in vitro en bloc brainstem/spinal cord preparations of neonatal rats. Onimaru et al. (1987; 1988; 2006) proposed that this “pre-inspiratory” activity originates from a primary (pre-inspiratory) oscillator located in the pFRG that entrains a secondary (inspiratory) oscillator in the pre-BöC. Others, using both in vitro en bloc and in vivo preparations of newborn rats, reported that the same biphasic-E activity originating in pFRG drives abdominal muscles activity (Janczewski et al., 2002), although they did not substantiate this claim by providing central unitary recordings. Notwithstanding, Janczewski and Feldman (2006) proposed that the same biphasic-E activity is generated by an “expiratory oscillator” located in RTN/pFRG that interacts reciprocally with the pre-BötC inspiratory oscillator, and that the coupling of these two oscillators represents a fundamental mechanism for respiratory rhythm generation (Feldman & Del Negro, 2006; Janczewski & Feldman, 2006). This concept opposes that suggested by Onimaru et al. (1987, 1988 & 2006) who believe that the RTN/pFRG provides inspiratory promoting oscillations. How might these opposing ideas be reconciled?
Recent data from Abdala et al. (2009) using the unanesthetised decerebrate in situ rat indicate that suppression of the RTN does not cause apnoea. Rather, it depresses post-inspiratory activity during normocapnia and totally abolishes late-E abdominal activity that is evoked during hypercapnia (Fig 5; Abdala et al. 2009). Further, in conditions of hypercapnia a sub-population of RTN neurons discharged during late expiration and they were comparable to those of Onimaru et al. (1987, 1988 & 2006). These neurons were quiescent during normocapnia, but, their phenotype, projection patterns and whether they are originators or followers remain unknown.
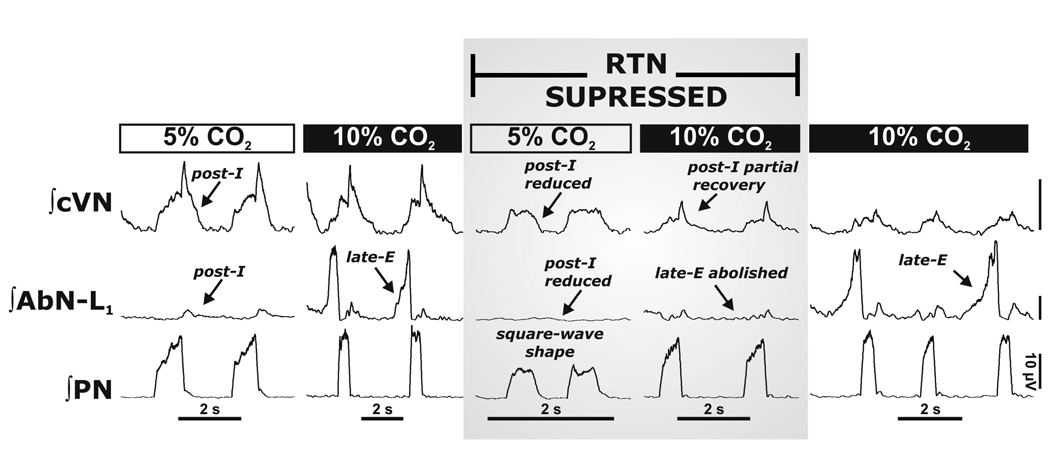
Figure 5. Reversible inactivation of the RTN abolished the 3-phase respiratory pattern and hypercapnia evoked Late-E abdominal nerve activity.
The RTN/vlPF was inactivated by bilateral microinjections of isoguvacine hydrochloride (GABAA receptor agonist). This resulted in a depression of post-inspiratory motor output on both AbN and central vagus (cVN) nerves, and the phrenic nerve pattern (PN) was transformed to a “square-wave” shape. RTN/vlPF suppression also abolished late-E AbN bursts during hypercapnia (10% CO2). Note that hypercapnia partially reinstated post-inspiratory activity during RTN/vlPF inactivation. Late-E activity recovered after isoguvacine washed out (∼1 hr; right panel). All traces show integrated nerve activities.
Thus, there is accumulating evidence indicating that in the mature rat the RTN region is essential for expression of late-E activity and for abdominal pumping during hypercapnia. It appears that oscillatory activity within the RTN of mature rats is absent in normocapnia but emerges with hypercapnia. Data from Abdala et al. (2009) contest the importance of the RTN/pFRG as a fundamental rhythmogenic mechanism during normal breathing (see Feldman & Del Negro, 2006; Janczewski & Feldman, 2006). The origin of the late-E discharges in RTN is unknown and may be within this nucleus or from other regions such as the pons; if the latter, then the RTN may act as a relay to retroambigual abdominal motoneurons. As demonstrated below it is clear that abdominal motor activity is highly plastic and can be up-regulated under chronic pathological conditions of intermittent hypoxia and become expressed during normocapnia.
7. Plasticity of respiratory modulation of sympathetic activity: novel insights into pathological states
Physiologically it is essential to match cardiac output with respiratory minute volume to ensure efficient delivery of oxygen to the tissues not only in resting conditions but also during physiological challenges. Figure 1 shows that the central networks regulating breathing and the cardiovascular system are coupled including respiratory modulation of both heart rate and thoracic sympathetic nerve activity; the latter typically occurs during late inspiration and early post-inspiratory phases. Since the work of Anrep (1936), it was known that respiratory sinus arrhythmia was, in part, generated by central cross-talk and independent from afferent feedback. It is pertinent to mention that pathologies relating to central respiratory arrhythmias may well have cardiovascular abnormalities which may, in part, precipitate the respiratory disorder as well as vice versa. Below are two examples describing the plasticity of central respiratory-sympathetic coupling and how this relates to common pathologies.
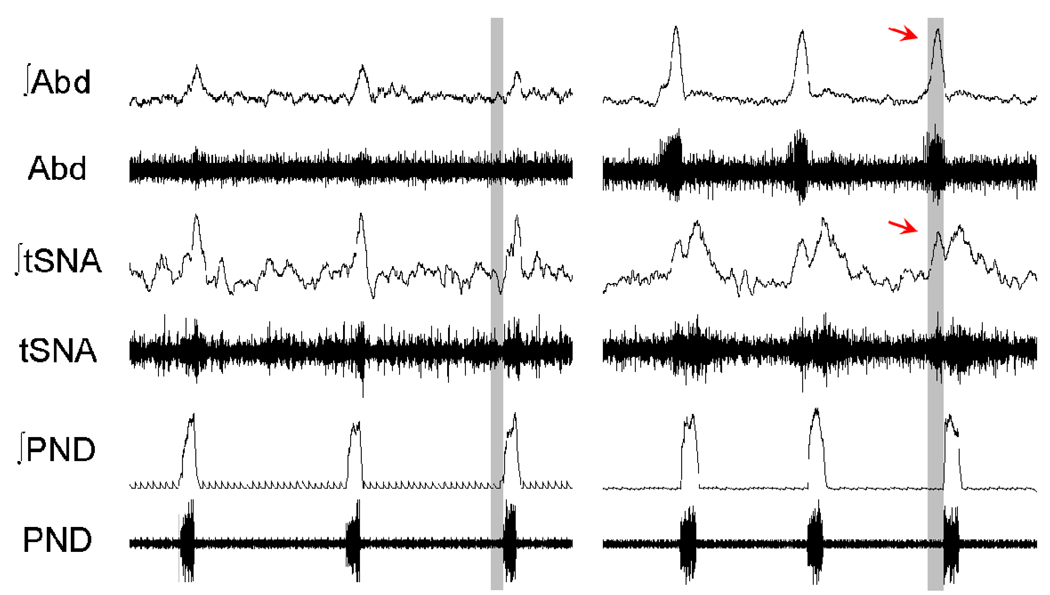
The point was made above (Fig 5) that late-E abdominal motor activity (active or forced expiration) is recruited under conditions of high respiratory drive such as hypercapnia. However, recent data indicate that abdominal expiratory activity can be expressed in normocapnia in rats after treatment with chronic intermittent hypoxia (CIH, Fig 6; Zoccal et al. 2008). Following 10 days of CIH, rats exhibited late-E abdominal activity that persisted beyond the treatment paradigm. The animals exhibited a significant increase in mean arterial pressure of more than 10 mmHg, which characterizes hypertension. Interestingly, the respiratory modulation of sympathetic nerve activity in CIH treated rats (but not controls) included an additional late-E related burst (Zoccal et al. 2008), which could provide an explanation for the increased arterial pressure. Thus, abdominal late-E activity is highly plastic, recruited following repeated peripheral chemoreceptor stimulation (by CIH) and may provide clues to the sympathoexcitability and hypertension found in patients with repeated sleep apnoeas (obstructive or central).
Figure 6. Chronic intermittent hypoxia causes long term plasticity in respiratory modulation of sympathetic activity.
Cardiovascular and respiratory control systems can only work efficiently when coupled. In control conditions, rats show inspiratory/post-inspiratory modulation of thoracic sympathetic nerve activity (tSNA) relative to phrenic discharge (PND). This modulation is chronically altered in juvenile rats exposed to chronic intermittent hypoxia (CIH) for 10 days. In CIH treated rats an additional burst of sympathetic activity emerges, which correlated with the development of a Late-E burst in the abdominal motor outflow (Abd). The latter may well contribute to the hypertension generated in the CIH model of sleep apnoea.
A second example of plasticity within central respiratory-cardiovascular circuits is the observation that the normal inspiratory-post-inspiratory modulation of sympathetic activity is augmented in the spontaneously hypertensive (SH) rat (Simms et al. 2009), an established model of human hypertension. Moreover, this enhanced modulation translated into Traube-Hering waves in arterial pressure that were significantly larger than those in normotensive control rats. The functional relevance of this amplified modulation in SH rats was demonstrated by reversibly arresting the respiratory rhythm generator with hypocapnia. On re-starting the central rhythm generator (by returning to normocapnia), there was an increase in arterial pressure of ∼20 mmHg, which reflected that produced following reinstatement of respiratory-sympathetic coupling. This evidence strengthened the causality argument that aberrant cardiorespiratory coupling can contribute to pathological states.
8. Conclusions
As described herein, in addition to generating the eupneic three-phase rhythmic pattern of breathing, the respiratory rhythm generator can, under certain conditions, generate other breathing patterns, such as apneusis, gasping and breathing with forced expiratory activity. These patterns reflect the dynamics of the ponto-medullary circuit and the multiple state-dependent mechanisms that can be adopted for rhythm generation. These different respiratory motor patterns have been equated with the notion of hierarchically organized compartments spanning the ponto-medullary respiratory regions each with its distinct neuronal mechanisms for operation. The review also alludes to the plasticity within the respiratory network and how chronic manipulation of breathing (e.g. with CIH) can cause pathology, such as hypertension. It remains to be elucidated how the fundamental ponto-medullary architecture changes to cause other pathological states such as Cheyne-Stokes, Kussmaul’s and Biot’s breathing. All said, revealing a mechanistic understanding of any breathing pathology relies on a complete understanding of respiratory and cardiovascular activities paralleled with iterative exchanges with multiscale computational models. This combined approach has every chance of providing novel guidance in the design of new therapeutic treatments of common cardiorespiratory diseases.
ACKNOWLEDGEMENTS
The supported of the National Institutes of Health (NIH) and British Heart Foundation is acknowledged. JFRP is the recipient of a Royal Society Wolfson Research Merit Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b–expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala APL, Rybak IA, Smith JC, Paton JFR. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins, and implications for respiratory rhythm generation. J. Physiol. 2009 doi: 10.1113/jphysiol.2008.167502. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anrep GV, Pascual W, Rossler R. Respiratory variations of the heart rate. II. The central mechanism of the sinus arrhythmia and the inter-relationships between central and reflex mechanism. Proc. Royal Soc. Lond. 1936;119:218–230. (Series B) [Google Scholar]
- Bianchi AL, Gestreau C. The respiratory network: An overview of a half century research. Respir. Physiol. Neurobiol. 2009 doi: 10.1016/j.resp.2009.04.019. ?, ? - ? (This issue) [DOI] [PubMed] [Google Scholar]
- Büsselberg D, Bischoff AM, Paton JFR, Richter DW. Loss of glycinergic inhibition reveals two modes of respiratory rhythm generation. Pflügers Arch. 2001;441:444–449. doi: 10.1007/s004240000453. [DOI] [PubMed] [Google Scholar]
- Dick TE, Baekey DM, Paton JFR, Lindsey BG, Morris KF. Cardio-respiratory coupling depends on the pons. Respir. Physiol. Neurobiol. 2009 doi: 10.1016/j.resp.2009.07.009. ?, ? - ? (This issue) [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Paton JFR. Glycinergic inhibition is essential for coordinating cranial and spinal respiratory motor outputs in the neonatal rat. J. Physiol. 2002;543:643–653. doi: 10.1113/jphysiol.2001.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Wilson RJ, Paton JFR. Respiratory activity in neonatal rats. Auton Neurosci. 2000;84:19–29. doi: 10.1016/S1566-0702(00)00177-6. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat. Rev. Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna MG, West GH, Stornetta RL, Guyenet PG. Bötzinger expiratory-augmenting neurons and the parafacial respiratory group. J Neurosci. 2008;28:2506–2515. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J. Appl. Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SB, Depuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Respir. Physiol. Neurobiol. 2009 doi: 10.1016/j.resp.2009.02.001. ?, ? - ? (This issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J. Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J. Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J. Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir. Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Localization of respiratory rhythm-generating neurons in the medulla of brainstem-spinal cord preparations from newborn rats. Neurosci. Lett. 1987;78:151–155. doi: 10.1016/0304-3940(87)90624-0. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Primary respiratory rhythm generator in the medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1988;445:314–324. doi: 10.1016/0006-8993(88)91194-8. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. Phox2b, RTN/pFRG neurons and rhythmogenesis. Respir. Physiol. Neurobiol. 2009 doi: 10.1016/j.resp.2009.03.007. ?, ? - ? (This issue) [DOI] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J. Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Paton JFR. A working heart-brainstem preparation of the mouse. J. Neurosci. Meth. 1996a;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JFR. The respiratory network in the ventrolateral medulla of the mature mouse studied in a working heart-brainstem preparation. J.Physiol. 1996b;493:819–831. doi: 10.1113/jphysiol.1996.sp021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR. Rhythmic bursting of pre- and post- inspiratory neurones during central apnoea in mature mice. J. Physiol. 1997;502:623–639. doi: 10.1111/j.1469-7793.1997.623bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR, Abdala APL, Koizumi H, Smith JC, St.-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat. Neurosci. 2006;9:311–313. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Nolan PJ. Similarities in reflex control of laryngeal and cardiac vagal motor neurones. Resp. Physiol. 2000;119:101–111. doi: 10.1016/s0034-5687(99)00105-x. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Ballanyi K. Structure-function analysis of inspiratory pre-Bötzinger complex networks in “calibrated” newborn rat brainstem slices. Respir. Physiol. Neurobiol. 2009 doi: 10.1016/j.resp.2009.04.020. ?, ? - ? (This issue) [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Okada A, Oku Y, Koshiya N, Ballanyi K. Fluorescence imaging of active respiratory networks. Respir. Physiol. Neurobiol. 2009 doi: 10.1016/j.resp.2009.02.012. ?, ? - ? (This issue) [DOI] [PubMed] [Google Scholar]
- Richter DW. Generation and maintenance of the respiratory rhythm. J Exp. Biol. 1982;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog. Brain. Res. 2007;165:201–220. doi: 10.1016/S0079-6123(06)65013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Bellingham MC, Richter DW. Adenosinergic modulation of respiratory neurones and hypoxic responses in the anaesthetized cat. J. Physiol. 1995;483:769–781. doi: 10.1113/jphysiol.1995.sp020621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Li YM, Duffin J. Inhibitory connections among rostral medullary expiratory neurones detected with cross-correlation in the decerebrate rat. Pflugers Arch. 2003;446:365–372. doi: 10.1007/s00424-003-1024-0. [DOI] [PubMed] [Google Scholar]
- Simms AE, Paton JFR, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in neonatal and juvenile spontaneously hypertensive rats: does it contribute to hypertension? J. Physiol. 2009;587:597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Butera RJ, Koshiya N, Del Negro C, Wilson CG, Johnson SM. Respiratory rhythm generation in neonatal and adult mammals: The hybrid pacemaker-network model. Respir. Physiol. Neurobiol. 2000;122:131–147. doi: 10.1016/s0034-5687(00)00155-9. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AA, Koizumi H, Rybak IA, Paton JFR. Spatial and Functional Architecture of the Mammalian Brainstem Respiratory Network: A Hierarchy of Three Oscillatory Mechanisms. J. Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM, Paton JFR. Role of pontile mechanisms in the neurogenesis of eupnea. Respir. Physiol. Neurobiol. 2004;143:321–332. doi: 10.1016/j.resp.2004.05.010. [DOI] [PubMed] [Google Scholar]
- St.-John WM, Rybak IA, Paton JFR. Potential switch from eupnea to fictive gasping following blockade of glycine transmission and potassium channels. Am. J. Physiol. 2002;283:R721–R731. doi: 10.1152/ajpregu.00004.2002. [DOI] [PubMed] [Google Scholar]
- St.-John WM, Stornetta RL, Guyenet PG, Paton JFR. Location and properties of respiratory neurones with putative intrinsic bursting properties in the rat in situ. J. Physiol. 2009 doi: 10.1113/jphysiol.2009.170308. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LH, Braga VA, Pickering AE, Paton JFR, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J. Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]