We identified meniscal damage, meniscal extrusion, and any high-grade MR feature as baseline risk factors for fast cartilage loss over a 30-month period in a population with no or early osteoarthritis.
Abstract
Purpose:
To assess baseline factors that may predict fast tibiofemoral cartilage loss over a 30-month period.
Materials and Methods:
The Multicenter Osteoarthritis (MOST) study is a longitudinal study of individuals who have or who are at high risk for knee osteoarthritis. The HIPAA-compliant protocol was approved by the institutional review boards of all participating centers, and written informed consent was obtained from all participants. Magnetic resonance (MR) images were read according to the Whole-Organ Magnetic Resonance Imaging Score (WORMS) system. Only knees with minimal baseline cartilage damage (WORMS ≤ 2.5) were included. Fast cartilage loss was defined as a WORMS of at least 5 (large full-thickness loss, less than 75% of the subregion) in any subregion at 30-month follow-up. The relationships of age, sex, body mass index (BMI), ethnicity, knee alignment, and several MR features (eg, bone marrow lesions, meniscal damage and extrusion, and synovitis or effusion) to the risk of fast cartilage loss were assessed by using a multivariable logistic regression model.
Results:
Of 347 knees, 90 (25.9%) exhibited cartilage loss, and only 20 (5.8%) showed fast cartilage loss. Strong predictors of fast cartilage loss were high BMI (adjusted odds ratio [OR], 1.11; 95% confidence interval [CI]: 1.01, 1.23), the presence of meniscal tears (adjusted OR, 3.19; 95% CI: 1.13, 9.03), meniscal extrusion (adjusted OR, 3.62; 95% CI: 1.34, 9.82), synovitis or effusion (adjusted OR, 3.36; 95% CI: 0.91, 12.4), and any high-grade MR-depicted feature (adjusted OR, 8.99; 95% CI: 3.23, 25.1).
Conclusion:
In participants with minimal baseline cartilage damage, the presence of high BMI, meniscal damage, synovitis or effusion, or any severe baseline MR-depicted lesions was strongly associated with an increased risk of fast cartilage loss. Patients with these risk factors may be ideal subjects for preventative or treatment trials.
Magnetic resonance (MR) imaging directly depicts articular cartilage and has sensitivity superior to that of radiography for detection of progressive cartilage damage (1,2). Longitudinal studies (3–15) of osteoarthritic (OA) knees have shown that MR-depicted tibiofemoral (TF) cartilage loss is associated with older age, female sex, higher body mass index (BMI), African American ethnicity, smoking, varus malalignment, a high degree of synovitis, large bone marrow lesions, anterior cruciate ligament tears, meniscal tears, and meniscal extrusion. Risk factors for patellofemoral cartilage loss overlap partially with those for TF cartilage loss, but also seem to be distinct (15–17).
Owing to the slowly progressive course of the disease, epidemiologic OA studies require large cohorts that need to be followed over relatively long periods. It would be useful to identify a subgroup of patients with no or early disease who are at high risk for fast cartilage loss. Such patients would be ideal for testing new treatments and should have the greatest need for preventative maneuvers or treatments.
By using a semiquantitative approach and focusing only on MR features in a small cohort, Biswal et al (18) found that baseline anterior cruciate ligament and meniscal tears and cartilage lesions in the central weight-bearing regions were risk factors for more rapid cartilage loss, which was not defined. By using volumetric cartilage morphometry as the outcome, Raynauld et al (6) differentiated a population of patients with symptomatic OA into three subgroups on the basis of the rate of global cartilage volume loss after 24 months. Baseline predictors of faster cartilage volume loss were severe meniscal extrusion, severe medial meniscal tears, bone marrow lesions, high BMI, and age. Despite the potential utility, there are little additional data on radiologic or clinical predictors of fast cartilage loss that would allow investigators to identify high-risk populations.
The aim of our study was to identify predictors of fast TF cartilage loss, as semiquantitatively defined on MR images, over a period of 30 months in a cohort of subjects who have no structural OA but are at risk of developing OA or who have early radiographic evidence of OA. We examined a set of putative risk factors for fast cartilage loss, including age, sex, BMI, ethnicity, varus and valgus mechanical knee alignment, and several MR features.
Materials and Methods
A.G. is the president of and F.W.R. is the vice president of Boston Imaging Core Lab (Boston, Mass), a company that provides radiologic image assessment services. A.G. is a shareholder in Synarc (San Francisco, Calif).
Study Subjects
Subjects were participants in the Multicenter Osteoarthritis (MOST) study, a prospective epidemiologic study aimed at identifying risk factors for incident and progressive knee OA in 3026 persons aged 50–79 years who either had radiographic knee OA or were at high risk for developing the disease. They were recruited from two U.S. communities, Birmingham, Ala, and Iowa City, Iowa, through mass mailing of letters and study brochures, which were supplemented by media and community outreach campaigns. The Health Insurance Portability and Accountability Act–compliant study protocol was approved by the Institutional Review Boards at the University of Iowa; University of Alabama, Birmingham; University of California, San Francisco; and Boston University Medical Campus. We obtained written informed consent from all participants.
Subjects were excluded from the MOST study if they had had or planned to have bilateral knee replacement surgery; were unable to walk without assistance; were planning to move out of the area in the next 3 years; or had rheumatoid arthritis (19), ankylosing spondylitis, psoriatic arthritis, chronic reactive arthritis, renal insufficiency requiring hemodialysis or peritoneal dialysis, or a history of cancer (except nonmelanoma skin cancer).
Radiographs
At the baseline clinic visit, all subjects underwent weight-bearing posteroanterior fixed flexion knee radiographs with the protocol developed by Peterfy et al (20) and an acrylic plastic (Plexiglas) positioning frame (SynaFlexer; Synarc). Long-limb radiographs were acquired with a 14 × 51-inch cassette. Mechanical alignment was measured as the angle formed by the intersection of the femoral and the tibial mechanical axes. The femoral mechanical axis was considered to be the line from the center of the femoral head through the center of the knee, and the tibial mechanical axis was considered to be the line drawn from the center of the ankle to the center of the knee. Neutral alignment was defined as an angle between 179° and 181°; varus malalignment, as an angle less than or equal to 178°; and valgus malalignment, as an angle greater than or equal to 182°.
A musculoskeletal radiologist who was not an author and a rheumatologist (D.T.F.), both with over 10 years experience reading study radiographs and over 30 years clinical experience, who were blinded to clinical data, graded all baseline posteroanterior radiographs according to the Kellgren-Lawrence scale (21). TF OA was considered to be present at radiography if the Kellgren-Lawrence score was greater than or equal to 2. If the readers disagreed on the presence of OA on radiographs, readings were adjudicated by a panel of three readers (two nonauthors and D.T.F.).
MR Acquisition
MR images of both knees were obtained for all participants with a 1.0-T dedicated MR system (OrthOne; ONI Medical Systems, Wilmington, Mass) with a circumferential extremity coil by using fat-suppressed fast spin-echo intermediate-weighted sequences in the sagittal (repetition time msec/echo time msec, 4800/35; section thickness, 3 mm; intersection gap, 0 mm; sections, 32; matrix, 288 × 192; signals acquired, two; field of view, 140 mm2; echo train length, eight) and axial (4680/13; section thickness, 3 mm; intersection gap, 0 mm; sections, 20; matrix, 288 × 192; signals acquired, two; field of view, 140 mm2; echo train length, eight) planes and a short inversion time inversion-recovery sequence in the coronal plane (6650/15; inversion time, 100 msec; section thickness, 3 mm; intersection gap, 0 mm; sections, 28; matrix, 256 × 192; signals acquired, two; field of view, 140 mm2; echo train length, eight). MR images were obtained at the baseline and 30-month follow-up visits.
MR Interpretation
MR examinations were selected for semiquantitative assessment in one or more of three substudies of the MOST study: (i) a cohort study of risk factors for radiologic progression of OA consisting of randomly selected knees with either patellofemoral or TF OA, (ii) a case-control study of risk factors for incident radiographically depicted OA, and (iii) a case-control study of risk factors for new onset of consistent frequent knee pain (22). Altogether, 1096 knees were chosen for MR assessment.
Two musculoskeletal radiologists (F.W.R. and A.G., with 6 and 8 years experience, respectively, with standardized semiquantitative MR assessment of knee OA), who were blinded to radiographic OA grade and clinical data, evaluated the MR images by using the Whole-Organ Magnetic Resonance Imaging Score (WORMS) (23). The WORMS system is a validated research tool for semiquantitative assessment of knee OA. WORMS was first introduced by Peterfy et al in 1999 (24) and was published in 2004 (23). Reliable standardized scoring systems allow expert semiquantitative readings of MR images of the knees to be used as outcome measures for epidemiologic trials and have furthered the understanding of the natural history of OA (9–11, 15,25,26). An overview of the WORMS system is presented in Table 1.
Table 1.
Overview of WORMS System
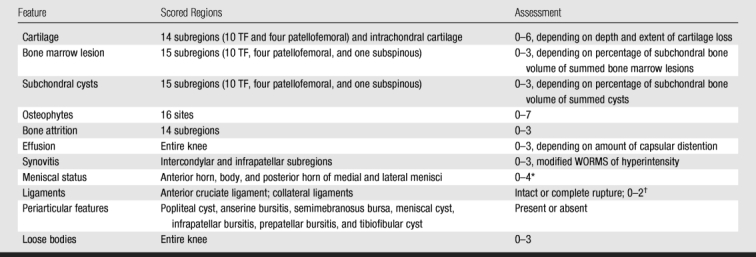
Source.—Reference 23.
* 1 = minor radial or parrot-beak tear, 2 = nondisplaced tear or prior surgical repair, 3 = displaced tear or partial resection, 4 = complete maceration or destruction or complete resection.
† 0 = normal, 1 = thickened, 2 = torn.
Baseline and follow-up MR images were read in pairs, with the chronological order known to the readers. The following joint structures were assessed in our study: cartilage morphology and signal intensity, subchondral bone marrow lesions (poorly delineated hyperintense areas directly adjacent to the subchondral plate on MR images), meniscal status, synovitis, effusion, and anterior cruciate ligament status. Cartilage signal intensity and morphology were scored according to the WORMS system from 0 to 6 in five subregions each in the medial and lateral TF compartments, for a total of 10 TF subregions. Bone marrow lesions were scored from 0 to 3 on the basis of the extent of regional involvement. Meniscal status was graded from 0 to 4 in the anterior horn, the body segment, and the posterior horn of the medial and lateral menisci. In addition to the WORMS, meniscal extrusion of the medial and lateral meniscal body was scored on the coronal image according to the method in previous publications (11,12). The anterior cruciate ligament was scored either as intact or torn. Although not part of the WORMS system, signal intensity alterations in the infrapatellar and intercondylar regions of the Hoffa fat pad were scored from 0 to 3 as a surrogate for synovial thickening, in accordance with the literature (8,27,28). Joint effusion was graded from 0 to 3 in terms of the estimated maximum distention of the synovial cavity (23). Joint effusion and synovitis were analyzed as a combined feature and were defined as any score equal to or greater than 1, either for effusion or for infrapatellar and intercondylar signal intensity alterations in the Hoffa fat pad.
Inclusion Criteria and Definition of Fast Cartilage Loss
Eligible knees included in our analysis (347 of 1096) were those that did not have a cartilage morphology score of greater than 2.5 in any of the 10 TF subregions at baseline imaging. These knees had no defects, small focal superficial defects, or fissure-like full-thickness defects that were less than 1 cm wide. We defined a knee as having fast cartilage loss if its cartilage score was 5 or 6 in at least one subregion at the follow-up visit (ie, if at least one subregion had multiple areas of focal full-thickness lesions or a single full-thickness lesion wider than 1 cm but including less than 75% of the subregion [grade 5] or if there was full-thickness loss in at least 75% of the subregion [grade 6]). Cartilage loss was considered to be slow if the maximum score was less than 5 in all subregions, but the cartilage score worsened in at least one subregion at follow-up.
Statistical Analysis
We examined the relation of each baseline characteristic to the risk of fast or slow cartilage loss by using the logistic regression model. Slow and fast cartilage loss were analyzed as separate outcome variables. Risk factors analyzed were age, sex, BMI, ethnicity, mechanical knee alignment, and baseline MR features (eg, bone marrow lesions, meniscal tears, maceration and resection, meniscal extrusion, synovitis or effusion, and anterior cruciate ligament tears).
All MR features were divided into two categories: present (score ≥ 1) and absent (score = 0). In addition, knees were dichotomized on the basis of MR features into a reference group (knees with all MR feature scores ≤ 1) and a group with high-grade MR abnormalities (knees with a score ≥ 2 for any baseline MR feature). We used the logistic regression model to assess the risk of fast or slow cartilage loss for the group with high-grade abnormalities after adjusting for all demographic risk factors. All characteristics were included in the multivariable regression model. We used the generalized estimating equation model to account for the correlation between two knees from the same subject. All statistical calculations were performed by using software (SAS, version 9.1 for Windows; SAS Institute, Cary, NC). We considered a two-tailed P value of less than .05 to indicate a significant difference.
Results
A total of 347 knees in 336 subjects (mean age, 61.1 years ± 7.8 [standard deviation]) were included. On average, the subjects were overweight (mean BMI, 29.5 kg/m2 ± 4.7), and there were more women (65.2%, 219 of 336) than men (34.8%, 117 of 336). The majority (85.6%, 297 of 347) of knees did not have established TF OA (Kellgren-Lawrence score of 0 or 1) at baseline. Over the 30-month observational period, 74.1% (257 of 347) of knees did not show any cartilage loss, 20.2% (70 of 347) exhibited slow cartilage loss, and 5.8% (20 of 347) showed fast cartilage loss.
The interreader reliability (weighted κ) for the different features was 0.62 for bone marrow lesions, 0.65 for synovitis or joint effusion, 0.65 for meniscal extrusion, 0.78 for cartilage morphology, and 0.80 for meniscal status.
The association between demographic characteristics and the risk of slow or fast cartilage loss is presented in Table 2. The crude ORs represent the association between a certain baseline characteristic and the risk of cartilage loss. In addition, we constructed a multivariable regression model to assess whether such an association was confounded by other characteristics. The results are listed in Tables 2 and 3. High BMI at baseline was significantly associated (P = .04) with an increased risk of fast cartilage loss. For a unit increase in BMI, the odds of fast cartilage loss increased by 11% (OR, 1.11; 95% CI: 1.01, 1.23). There was a trend for varus knees to have a higher risk of fast cartilage loss (OR, 1.99; 95% CI: 0.66, 6.00); however, the effect was not significant. There were no significant associations of age, sex, or ethnicity with the risk of fast cartilage loss.
Table 2.
Associations between Cartilage Loss and Baseline Demographic Characteristics
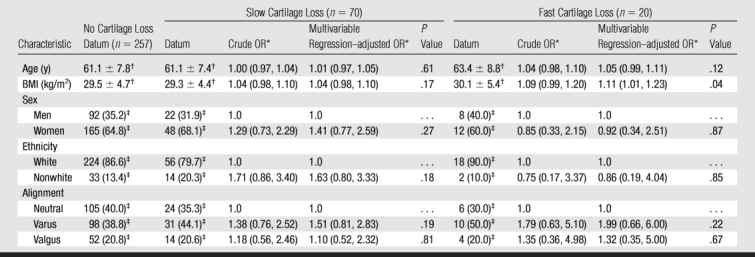
Note.—OR = odds ratio.
* Data are ORs, with 95% confidence intervals (CIs) in parentheses. Characteristics with an OR of 1.0 were used as reference.
† Data are mean ± standard deviation.
‡ Data are number of knees, with percentage in parentheses.
Table 3.
Associations between Cartilage Loss and Baseline MR Imaging Features
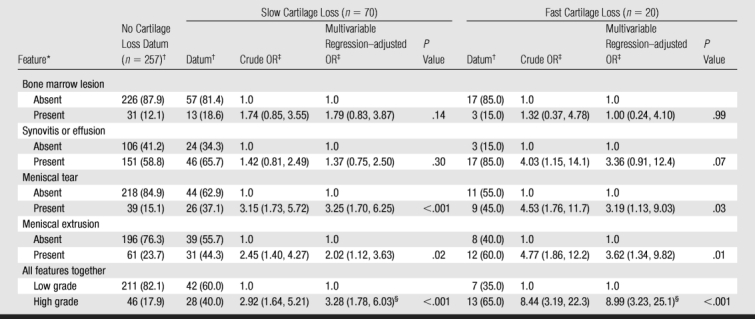
* Absent = score of 0, high grade = any score of 2 or higher, low grade = all scores of 1 or lower, present = score of 1 or higher.
† Data are number of knees, with percentage in parentheses.
‡ Data are ORs, with 95% CIs in parentheses. Characteristics with an OR of 1.0 were used as reference.
§ Adjusted for age, sex, race, BMI, and alignment.
Table 4 shows the frequencies of the different baseline MR features and the single maximum baseline score per knee, illustrating that only a minority of knees exhibited high-grade features at baseline.
Table 4.
Distribution of Maximum Scores for MR Features in 347 Knees
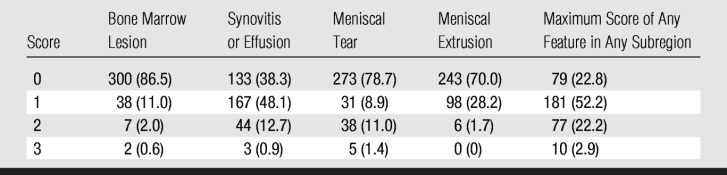
Note.—Data are numbers of knees, with percentages in parentheses.
Table 3 depicts the relation of each MR feature to the risk of both slow and fast cartilage loss. No anterior cruciate ligament tears were observed in the study sample. Meniscal tears and meniscal extrusion at baseline were both associated with an increased risk of fast (P = .03 and .01, respectively) and slow (P < .001 and P = .02, respectively) cartilage loss (Fig 1). There was a suggestion (P = .07) that synovitis and effusion at baseline increased the risk of fast cartilage loss (OR, 3.36; 95% CI: 0.91, 12.40) (Fig 2). There was a strong association between any high-grade MR imaging abnormalities and the risk of fast (OR, 8.99; 95% CI: 3.23, 25.1) and slow (OR, 3.28; 95% CI: 1.78, 6.03) cartilage loss by using the multivariable regression–adjusted OR.
Figure 1a:
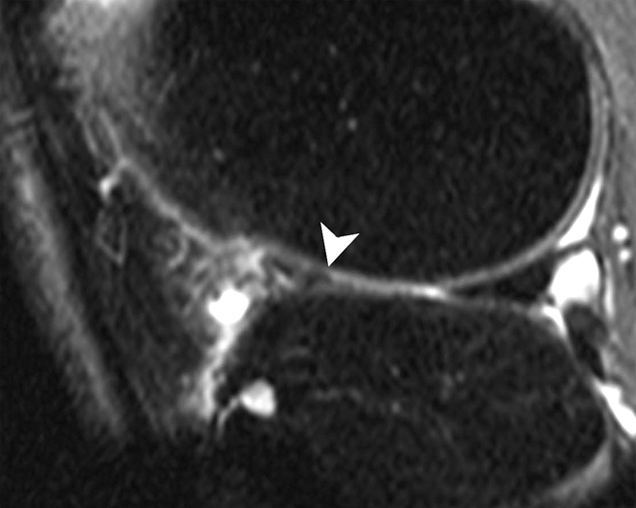
Sagittal intermediate-weighted fat-suppressed MR images (4800/35) show fast cartilage loss in lateral compartment between (a) baseline and (b) follow-up. (a) Maceration (arrowhead) of anterior horn of lateral meniscus. (b) Diffuse cartilage loss in central and posterior parts of lateral femur and in central region of lateral tibia (arrowheads).
Figure 1b:
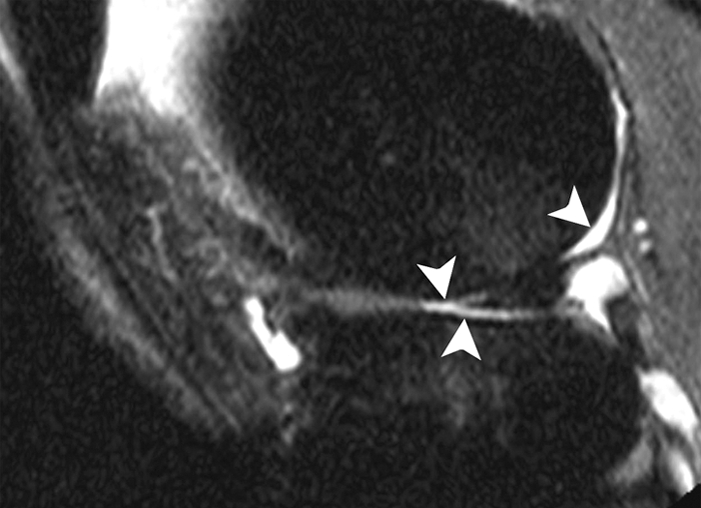
Sagittal intermediate-weighted fat-suppressed MR images (4800/35) show fast cartilage loss in lateral compartment between (a) baseline and (b) follow-up. (a) Maceration (arrowhead) of anterior horn of lateral meniscus. (b) Diffuse cartilage loss in central and posterior parts of lateral femur and in central region of lateral tibia (arrowheads).
Figure 2a:
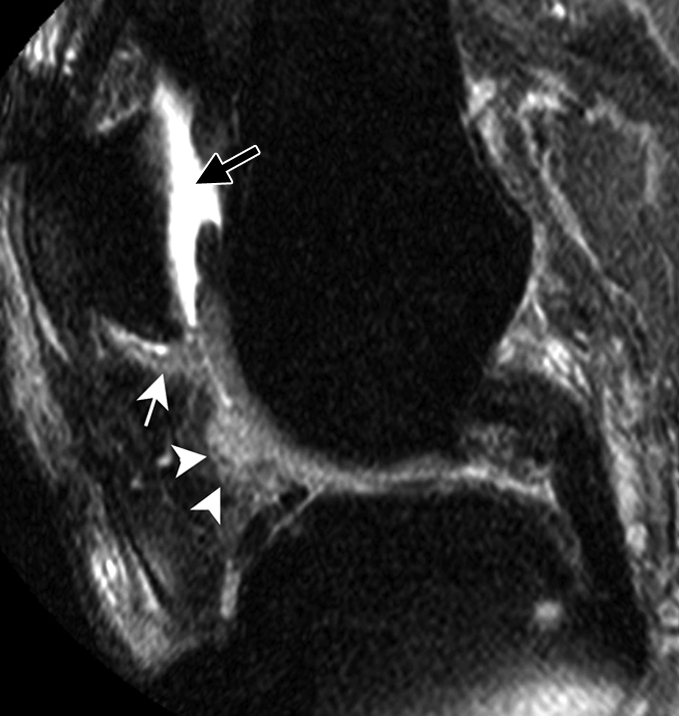
Sagittal intermediate-weighted fat-suppressed MR images (4800/35) show fast cartilage loss in medial compartment between (a,b) baseline and (c) follow-up. (a) Effusion (black arrow) and marked synovitic infiltration of Hoffa fat pad in intercondylar (arrowheads) and infrapatellar (white arrow) regions. (b) Small superficial cartilage defect (arrow) in central region of medial femoral condyle. (c) Massive cartilage loss and denudation of bone (arrowheads) in central region of medial femoral condyle. Note also diffuse cartilage damage in central part of tibial plateau.
Figure 2b:
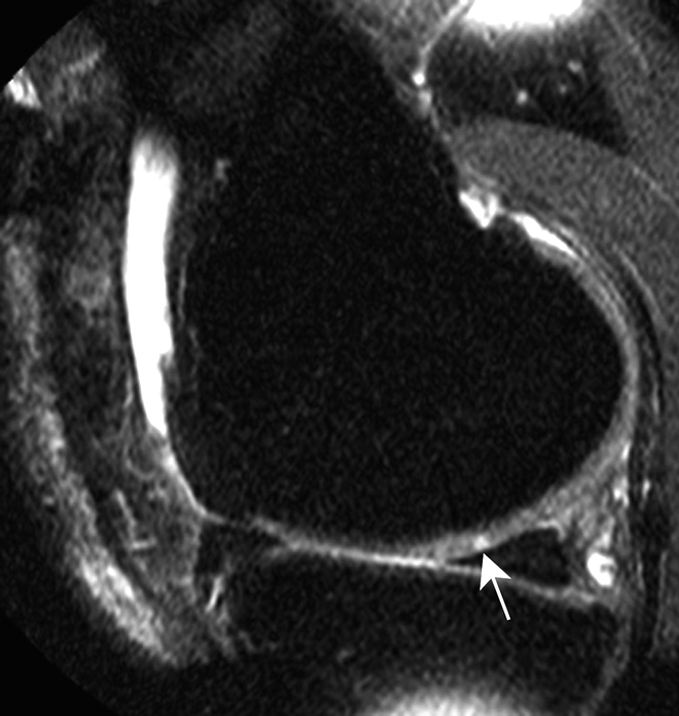
Sagittal intermediate-weighted fat-suppressed MR images (4800/35) show fast cartilage loss in medial compartment between (a,b) baseline and (c) follow-up. (a) Effusion (black arrow) and marked synovitic infiltration of Hoffa fat pad in intercondylar (arrowheads) and infrapatellar (white arrow) regions. (b) Small superficial cartilage defect (arrow) in central region of medial femoral condyle. (c) Massive cartilage loss and denudation of bone (arrowheads) in central region of medial femoral condyle. Note also diffuse cartilage damage in central part of tibial plateau.
Figure 2c:
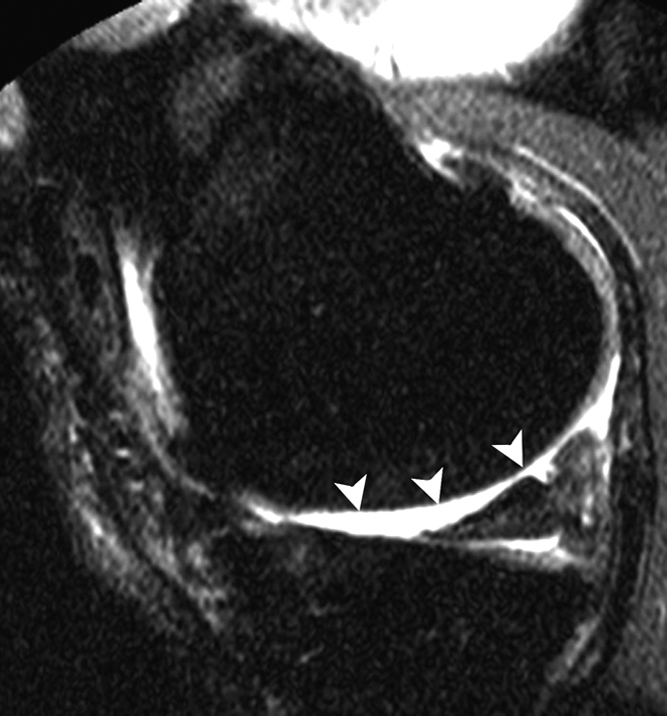
Sagittal intermediate-weighted fat-suppressed MR images (4800/35) show fast cartilage loss in medial compartment between (a,b) baseline and (c) follow-up. (a) Effusion (black arrow) and marked synovitic infiltration of Hoffa fat pad in intercondylar (arrowheads) and infrapatellar (white arrow) regions. (b) Small superficial cartilage defect (arrow) in central region of medial femoral condyle. (c) Massive cartilage loss and denudation of bone (arrowheads) in central region of medial femoral condyle. Note also diffuse cartilage damage in central part of tibial plateau.
Discussion
In knees with early structural OA and those at risk of developing OA, we identified meniscal damage or extrusion and any high-grade MR abnormalities as predictors of fast TF cartilage loss over a 30-month period. We identified baseline BMI as the only demographic risk factor for fast cartilage loss. However, we were not able to unequivocally distinguish knees that would have slow cartilage loss from those that would have fast cartilage loss because some of the baseline features predicted both slow and fast cartilage loss.
Researchers in most available studies that investigate progression or incidence of OA have applied joint space width measurements from radiographs as a surrogate outcome for cartilage loss (29,30). However, as these measures are indirect and reflect not only cartilage status but also pathologic changes of other intrinsic joint structures, MR imaging has become the method of choice for cartilage assessment (2,6,11, 31–34). Since there is no existing definition of fast cartilage loss as evaluated with a semiquantitative scoring method, we needed to create one to appropriately analyze potential associated risk factors. The majority of knees will show either no or subtle cartilage loss during the observational period (34–36). An optimal definition of fast cartilage loss would show a large amount of cartilage loss in a given period of time. A less stringent definition of cartilage loss would increase the number of subjects included but would blur the distinction between fast and slow loss. We used a stringent definition, with knees progressing from no or only minor focal defects to large full-thickness cartilage damage. Alternative definitions are possible, and ongoing large epidemiologic studies (eg, the Osteoarthritis Initiative) will help to determine which definitions are the most appropriate for a given research question.
Among the demographic characteristics we studied, we found that only baseline BMI was a predictor of fast cartilage loss. As obesity is one of the few established risk factors for incident radiographic OA, it is not surprising that obesity may also precede and be a predictor for fast cartilage loss, especially in knees without definite OA on radiographs (30,37). The other demographic features were not predictors of fast cartilage loss with our inclusion criteria.
Investigators in few studies have assessed MR-defined predictors for longitudinal cartilage loss, and those in most of the studies (8,9,13,38) have investigated knees with OA established on radiographs. Raynauld et al (38) reported correlations between subchondral bone marrow lesion change and cartilage volume loss in the same compartment. Our findings did not show such an association, possibly because we noted only the presence of bone marrow lesions at baseline and did not differentiate between small and large bone marrow lesions. Also, we used a knee-based approach rather than a subregional approach that would take into account associations between bone marrow lesions and directly adjacent cartilage status. A strong association of bone marrow lesions and cartilage loss has been shown longitudinally for subregional semiquantitative approaches (9,10). An increased risk of subsequent cartilage loss for knees with established OA and baseline complete anterior cruciate ligament tears has been reported previously (13,18). However, as no baseline anterior cruciate ligament tears were observed in our sample, we could not analyze any association between anterior cruciate ligament status and subsequent cartilage loss in patients with little or no OA on radiographs at baseline.
The strong association of baseline medial meniscal damage and malposition with consequent cartilage loss in the same compartment in subjects with OA is well established (11,15). Subjects without OA but with baseline meniscal tears have shown an increased risk of progression of cartilage lesions when compared with a control group without meniscal tears (18). Several large studies (39,40) have shown a highly increased risk for radiographically visible OA in patients with baseline partial meniscectomy or meniscal damage. Our results confirmed the highly increased risk of cartilage loss for subjects with baseline meniscal damage and extrusion.
A study limitation that needs to be acknowledged is the limited assessment of meniscal extrusion on non–weight bearing MR images. The images used to evaluate the degree of extrusion were acquired in a static position with the subject supine. During flexion and extension, the menisci move along an anterior-posterior axis. Boxheimer et al (41) demonstrated that extrusion of the medial meniscus depends on knee position, rotation, and load in a small cohort of asymptomatic subjects. The number patients exhibiting extrusion increased markedly in the loaded weight-bearing position. Whether meniscal extrusion always represents a true pathologic condition that may negatively affect the articular cartilage surface is under debate, as meniscal extrusion seems to also be prevalent in asymptomatic knees without OA (42–44). However, one may expect that, under weight-bearing conditions, the amount of extrusion and its prevalence in the study population would be even higher than the measurements we obtained in the non–weight bearing supine position.
To assess the degree of synovial activation of the joint, we used the surrogates of signal intensity alterations in the Hoffa fat pad and joint effusion and then applied the maximum score of both MR features as an estimate of severity (8,23,27). Hill et al (8) reported an association of moderate and severe baseline synovitis with an increased risk of cartilage loss in the TF compartment at follow-up. Our findings support this finding and showed a trend toward an increased risk of fast cartilage loss for any degree of baseline synovial activation, although the association was not statistically significant after adjusting for other risk factors.
Although we initially analyzed each MR risk factor just for presence, we also performed an analysis of high-grade baseline lesions and dichotomized the knees into those with scores of only 0 or 1 in each category and those with a score of 2 or higher for any baseline feature. By using this approach, we found that knees with prevalent high-grade lesions were at a highly increased risk for fast TF cartilage loss. Several studies (8–10) have found a direct association between baseline lesion grade and risk of subsequent cartilage loss.
We are not aware of any study that has tried to define the time period during which fast cartilage loss is to be expected and may be detectable at MR imaging. We decided to analyze knees that were imaged at baseline and at 30 months. Whether this is the ideal interval remains to be shown. Recent articles (34,35) from the Osteoarthritis Initiative report only small changes during a 12-month interval by using morphometric measurements.
We were not able to analyze the full array of possible baseline predictors of fast cartilage loss as they were not available to us at the time of analysis. Genetic predisposition is certainly one of the most important factors to be considered (45,46). Serum biomarkers may also play an important role in predicting progression (47,48). Occupational and nutritional risk factors (49–51) were not included but are worth mentioning.
In addition, we analyzed each risk factor separately. In a much larger study, a highly increased risk for cartilage loss might be proved for subjects with several concomitant MR and demographic risk factors. For the MR features, we used a knee-based approach and examined only the presence or absence of baseline features. A compartmental or subregional approach might show stronger associations because several baseline risk factors for local cartilage loss have been identified on MR images (9–11, 14,52). However, for some of the analyzed predictors (eg, anterior cruciate ligament tears, effusion, synovitis, and possibly meniscal damage), a subregional association likely is incorrect (8,13,53,54). We focused on the TF joint only; an analysis of predictors of patellofemoral cartilage loss might yield other results (15–17).
In summary, we identified meniscal damage, meniscal extrusion, and any high-grade MR feature as baseline risk factors for fast cartilage loss over a 30-month period in a population with no or early structural OA. Baseline synovitis and effusion increased the risk of fast cartilage loss, but not significantly. Among demographic characteristics, only higher baseline BMI predicted fast cartilage loss. We did not succeed in defining baseline predictors that can unequivocally differentiate slow from fast cartilage loss. However, by including knees with meniscal damage and extrusion or high-grade MR features at baseline, a subpopulation at high risk of progressive cartilage loss was identified. Our findings are a step in characterizing subjects at risk for progressive cartilage loss, which may be of relevance for eligibility in clinical trials and epidemiologic studies.
Advances in Knowledge.
Subjects who have early structural osteoarthritis (OA) or who are at risk for OA may be at higher risk for fast progressive cartilage loss if they have a high body mass index or show signs of meniscal damage, meniscal extrusion, or any high-grade lesion at baseline MR imaging.
Unequivocal differentiation of risk factors for fast versus slow cartilage loss was not possible.
Implications for Patient Care.
Preventative measures to avoid cartilage loss should include avoidance of obesity.
To decrease study intervals and sample size, cohorts may be screened for certain baseline demographic and MR imaging risk factors for longitudinal cartilage loss.
Acknowledgments
We thank the participants and staff of the MOST study at the clinical sites in Birmingham, Ala, and Iowa City, Iowa, and at the Coordinating Center at University of California, San Francisco. We acknowledge the valuable contributions of Burton Sack, MD, of Boston, Mass, who was one of the reviewers of the knee radiographs.
Received December 18, 2008; revision requested February 9, 2009; revision received March 24; accepted March 31; final version accepted April 6.
Funding: This work was supported by National Institute on Aging (grants U01-AG-18947, U01-AG-18832, U01-AG-19069, and U01-AG-18820).
See Materials and Methods for pertinent disclosures.
Abbreviations:
- BMI
- body mass index
- CI
- confidence interval
- MOST
- Multicenter Osteoarthritis
- OA
- osteoarthritis
- OR
- odds ratio
- TF
- tibiofemoral
- WORMS
- Whole-Organ Magnetic Resonance Imaging Score
References
- 1.Amin S, LaValley MP, Guermazi A, et al. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum 2005; 52: 3152– 3159 [DOI] [PubMed] [Google Scholar]
- 2.Bruyere O, Genant H, Kothari M, et al. Longitudinal study of magnetic resonance imaging and standard x-rays to assess disease progression in osteoarthritis. Osteoarthritis Cartilage 2007; 15: 98– 103 [DOI] [PubMed] [Google Scholar]
- 3.Davies-Tuck ML, Wluka AE, Wang Y, et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage 2008; 16: 337– 342 [DOI] [PubMed] [Google Scholar]
- 4.Ding C, Cicuttini F, Scott F, Cooley H, Boon C, Jones G. Natural history of knee cartilage defects and factors affecting change. Arch Intern Med 2006; 166: 651– 658 [DOI] [PubMed] [Google Scholar]
- 5.Amin S, Niu J, Guermazi A, et al. Cigarette smoking and the risk for cartilage loss and knee pain in men with knee osteoarthritis. Ann Rheum Dis 2007; 66: 18– 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther 2006; 8: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cicuttini F, Wluka A, Hankin J, Wang Y. Longitudinal study of the relationship between knee angle and tibiofemoral cartilage volume in subjects with knee osteoarthritis. Rheumatology (Oxford) 2004; 43: 321– 324 [DOI] [PubMed] [Google Scholar]
- 8.Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis 2007; 66: 1599– 1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter DJ, Zhang Y, Niu J, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum 2006; 54: 1529– 1535 [DOI] [PubMed] [Google Scholar]
- 10.Roemer FW, Guermazi A, Javaid MK, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST study—a longitudinal multicenter study of knee osteoarthritis. Ann Rheum Dis doi:10.1136/ard.2008.096834. Published online October 1, 2008 Accessed December 23, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum 2006; 54: 795– 801 [DOI] [PubMed] [Google Scholar]
- 12.Hunter DJ, Zhang YQ, Tu X, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum 2006; 54: 2488– 2495 [DOI] [PubMed] [Google Scholar]
- 13.Amin S, Guermazi A, Lavalley MP, et al. Complete anterior cruciate ligament tear and the risk for cartilage loss and progression of symptoms in men and women with knee osteoarthritis. Osteoarthritis Cartilage 2008; 16: 897– 902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapoor D, Eckstein F, Dunlop DD, et al. Do meniscal tears, laxity, and malalignment predict subsequent cartilage loss in osteoarthritic knees? [abstr]. Arthritis Rheum 2007; 56( suppl): S129. [DOI] [PubMed] [Google Scholar]
- 15.Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum 2008; 58: 1716– 1726 [DOI] [PubMed] [Google Scholar]
- 16.Kalichman L, Zhu Y, Zhang Y, et al. The association between patella alignment and knee pain and function: an MRI study in persons with symptomatic knee osteoarthritis. Osteoarthritis Cartilage 2007; 15: 1235– 1240 [DOI] [PubMed] [Google Scholar]
- 17.McAlindon T, Zhang Y, Hannan M, et al. Are risk factors for patellofemoral and tibiofemoral knee osteoarthritis different? J Rheumatol 1996; 23: 332– 337 [PubMed] [Google Scholar]
- 18.Biswal S, Hastie T, Andriacchi TP, Bergman GA, Dillingham MF, Lang P. Risk factors for progressive cartilage loss in the knee: a longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum 2002; 46: 2884– 2892 [DOI] [PubMed] [Google Scholar]
- 19.Karlson EW, Sanchez-Guerrero J, Wright EA, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol 1995; 5: 297– 302 [DOI] [PubMed] [Google Scholar]
- 20.Peterfy CG, Lynch JA, Miaux Y, et al. Non-fluoroscopic method for flexed radiography of the knee that allows reproducible joint-space width measurement [abstr]. Arthritis Rheum 1998; 41( suppl): S361 [Google Scholar]
- 21.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957; 16: 494– 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felson DT, Niu J, Guermazi A, et al. The development of knee pain correlates with enlarging bone marrow lesions on MRI. Arthritis Rheum 2007; 56: 2986– 2992 [DOI] [PubMed] [Google Scholar]
- 23.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004; 12: 177– 190 [DOI] [PubMed] [Google Scholar]
- 24.Peterfy CG, White D, Tirman P, et al. Whole-organ evaluation of the knee in osteoarthritis using MRI [abstr]. Ann Rheum Dis 1999; 38( suppl): S342 [Google Scholar]
- 25.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med 2003; 139: 330– 336 [DOI] [PubMed] [Google Scholar]
- 26.Reichenbach S, Yang M, Eckstein F, et al. Do cartilage volume or thickness distinguish knees with and without mild radiographic osteoarthritis? the Framingham study. Ann Rheum Dis doi:10.1136/ard.2008.099200. Published online February 4, 2009 Accessed March 15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill CL, Gale DG, Chaisson CE, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol 2001; 28: 1330– 1337 [PubMed] [Google Scholar]
- 28.Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, An T, Negendank WG. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging 1995; 13: 177– 183 [DOI] [PubMed] [Google Scholar]
- 29.Felson DT, Zhang Y, Hannan MT, et al. The incidence and natural history of knee osteoarthritis in the elderly: the Framingham Osteoarthritis Study. Arthritis Rheum 1995; 38: 1500– 1505 [DOI] [PubMed] [Google Scholar]
- 30.Spector TD, Hart DJ, Doyle DV. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general population: the effect of obesity. Ann Rheum Dis 1994; 53: 565– 568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cicuttini F, Hankin J, Jones G, Wluka A. Comparison of conventional standing knee radiographs and magnetic resonance imaging in assessing progression of tibiofemoral joint osteoarthritis. Osteoarthritis Cartilage 2005; 13: 722– 727 [DOI] [PubMed] [Google Scholar]
- 32.Cicuttini FM, Wluka AE, Wang Y, Stuckey SL. Longitudinal study of changes in tibial and femoral cartilage in knee osteoarthritis. Arthritis Rheum 2004; 50: 94– 97 [DOI] [PubMed] [Google Scholar]
- 33.Eckstein F, Maschek S, Wirth W, et al. Change in femorotibial cartilage volume and subregional cartilage thickness over 1 year: data from the Osteoarthritis Initiative progression subcohort [abstr]. Arthritis Rheum 2007; 56( suppl): S283 [Google Scholar]
- 34.Eckstein F, Maschek S, Wirth W, et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis 2009; 68: 674– 679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter DJ, Niu J, Zhang Y, et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis 2009; 68: 349– 356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelletier JP, Raynauld JP, Berthiaume MJ, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther 2007; 9: R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham study. Arthritis Rheum 1997; 40: 728– 733 [DOI] [PubMed] [Google Scholar]
- 38.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann Rheum Dis 2008; 67: 683– 688 [DOI] [PubMed] [Google Scholar]
- 39.Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: the Multicenter Osteoarthritis study. Arthritis Rheum 2009; 60: 831– 839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum 2004; 50: 2811– 2819 [DOI] [PubMed] [Google Scholar]
- 41.Boxheimer L, Lutz AM, Zanetti M, et al. Characteristics of displaceable and nondisplaceable meniscal tears at kinematic MR imaging of the knee. Radiology 2006; 238: 221– 231 [DOI] [PubMed] [Google Scholar]
- 42.Rennie WJ, Finlay DB. Meniscal extrusion in young athletes: associated knee joint abnormalities. AJR Am J Roentgenol 2006; 186: 791– 794 [DOI] [PubMed] [Google Scholar]
- 43.Puig L, Monllau JC, Corrales M, Pelfort X, Melendo E, Caceres E. Factors affecting meniscal extrusion: correlation with MRI, clinical, and arthroscopic findings. Knee Surg Sports Traumatol Arthrosc 2006; 14: 394– 398 [DOI] [PubMed] [Google Scholar]
- 44.Ding C, Martel-Pelletier J, Pelletier JP, et al. Knee meniscal extrusion in a largely non-osteoarthritic cohort: association with greater loss of cartilage volume. Arthritis Res Ther 2007; 9: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valdes AM, Loughlin J, Oene MV, et al. Sex and ethnic differences in the association of ASPN, CALM1, COL2A1, COMP, and FRZB with genetic susceptibility to osteoarthritis of the knee. Arthritis Rheum 2007; 56: 137– 146 [DOI] [PubMed] [Google Scholar]
- 46.Kraus VB, Jordan JM, Doherty M, et al. The Genetics of Generalized Osteoarthritis (GOGO) study: study design and evaluation of osteoarthritis phenotypes. Osteoarthritis Cartilage 2007; 15: 120– 127 [DOI] [PubMed] [Google Scholar]
- 47.Garnero P, Peterfy C, Zaim S, Schoenharting M. Bone marrow abnormalities on magnetic resonance imaging are associated with type II collagen degradation in knee osteoarthritis: a 3-month longitudinal study. Arthritis Rheum 2005; 52: 2822– 2829 [DOI] [PubMed] [Google Scholar]
- 48.Otterness IG, Weiner E, Swindell AC, Zimmerer RO, Ionescu M, Poole AR. An analysis of 14 molecular markers for monitoring osteoarthritis: relationship of the markers to clinical end-points. Osteoarthritis Cartilage 2001; 9: 224– 231 [DOI] [PubMed] [Google Scholar]
- 49.McAlindon TE, Felson DT, Zhang Y, et al. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham study. Ann Intern Med 1996; 125: 353– 359 [DOI] [PubMed] [Google Scholar]
- 50.Felson DT, Hannan MT, Naimark A, et al. Occupational physical demands, knee bending, and knee osteoarthritis: results from the Framingham study. J Rheumatol 1991; 18: 1587– 1592 [PubMed] [Google Scholar]
- 51.Felson DT, Niu J, Clancy M, et al. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum 2007; 56: 129– 136 [DOI] [PubMed] [Google Scholar]
- 52.Hernandez-Molina G, Guermazi A, Niu J, et al. Central bone marrow lesions in symptomatic knee osteoarthritis and their relationship to anterior cruciate ligament tears and cartilage loss. Arthritis Rheum 2008; 58: 130– 136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roemer FW, Guermazi A, Hunter DJ, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis Cartilage 2009; 17( 6): 748– 753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lynch JA, Javaid MK, Roemer FW, et al. Associations of medial meniscal tear and extrusion with the sites of cartilage loss in the knee: results from the MOST study. Arthritis Rheum 2008; 58( suppl): S235– S236 [Google Scholar]


