Abstract
Recent neuroimaging studies have demonstrated that both remembering the past and simulating the future activate a core neural network including the medial temporal lobes. Regions of this network, in particular the medial temporal lobes, are prime sites for amyloid deposition and are structurally and functionally compromised in Alzheimer's disease (AD). While we know some functions of this core network, specifically episodic autobiographical memory, are impaired in AD, no study has examined whether future episodic simulation is similarly impaired. We tested the ability of sixteen AD patients and sixteen age-matched controls to generate past and future autobiographical events using an adapted version of the Autobiographical Interview. Participants also generated five remote autobiographical memories from across the lifespan. Event transcriptions were segmented into distinct details, classified as either internal (episodic) or external (non-episodic). AD patients exhibited deficits in both remembering past events and simulating future events, generating fewer internal and external episodic details than healthy older controls. The internal and external detail scores were strongly correlated across past and future events, providing further evidence of the close linkages between the mental representations of past and future.
Keywords: Autobiographical, medial temporal lobes, prospection, specificity, fluency
Alzheimer's disease (AD) is characterized by the presence of amyloid plaques and neurofibrillary tangles (National Institute on Aging, 1997; Khachaturian, 1985). Recent neuropathology and neuroimaging work has determined that such pathology is initially focused in a number of brain regions including the medial prefrontal and parietal cortices, as well as lateral parietal and temporal regions (McKee et al., 2006). Amyloid deposition is correlated with disrupted metabolic function, particularly in posterior temporoparietal regions (Buckner, et al., 2005; McKee et al., 2006). Atrophy is also correlated with this pathology, and has been found to exist in medial prefrontal, medial and lateral parietal, and medial and lateral temporal regions (Buckner et al., 2005). Notably, these regions comprise a neural network that supports functions such as the default mode and episodic memory (Buckner et al., 2005). It is not surprising then that episodic memory, including autobiographical memory for past experiences, is disrupted early in AD (e.g., Addis & Tippett, 2004; Budson et al., 2007; Dorrego et al., 1999; Greene & Hodges, 1996; Greene, Hodges, & Baddeley, 1995; Kopelman, 1989; Leyhe, Muller, Milian, Eschweiler, & Saur, in press; Piolino et al., 2003; Sagar, Cohen, Sullivan, Corkin, & Growdon, 1988).
Recent neuroimaging work has extended the cognitive functions supported by this network, demonstrating that these regions are not only robustly engaged when remembering past events but also when imagining novel scenarios that might happen in future (e.g., Addis, Pan, Vu, Laiser, & Schacter, in press; Addis, Wong, & Schacter, 2007; Botzung, Dankova, & Manning, 2008; Hassabis, Kumaran, & Maguire, 2007; Okuda et al., 2003; Szpunar, Watson, & McDermott, 2007, for review, see Schacter, Addis, & Buckner, 2007, 2008). Based on these and related findings, Schacter & Addis (2007a, 2007b) put forward the constructive episodic simulation hypothesis, which holds that in order to imagine a future event, one must extract details from episodic memory and flexibly recombine them into a coherent simulation. As such, representations of past and future events contain contextual, perceptual and other event details stored in episodic memory, and rely on similar cognitive processes during construction (e.g., self-referential processing and imagery). It is these commonalities which are thought to result in the striking overlap in neural activity when remembering and imagining (Schacter & Addis, 2007a, 2007b).
The engagement of this common core network during both remembering and imagining in healthy young adults leads to the prediction that the functional and structural compromise of the network would be associated not only with deficits in remembering but also imagining. Consistent with this prediction, patients with amnesia as a result of damage to regions in this network exhibit not only difficulties with memory for past experiences but also imagining novel experiences. One study found that four of five amnesic patients with damage to a critical region in this network - the hippocampus - had difficulties imagining new experiences (Hassabis, Kumaran, Vann, & Maguire, 2007). In particular, the descriptions of experiences of these patients lacked the richness of detail and coherence that was evident in age-matched control subjects. Moreover, patient K.C., who developed severe amnesia after a head injury that damaged the medial temporal and frontal lobes, is also unable to envisage future events (Rosenbaum et al., 2005; Tulving, 1985). Another amnesic patient, D.B., is also documented as having deficits in both episodic memory and future thinking (Klein, Loftus, & Kihlstrom, 2002), however, the locus of brain damage in this case is not clear.
We have recently examined episodic memory and future simulation abilities in older adults (Addis, Wong, & Schacter, 2008). The connectivity of the core network is disrupted with increasing age, likely as a result of declines in white matter integrity (Andrews-Hanna et al., 2007), and many regions comprising this network, such as the hippocampus, are atrophied in healthy older adults. The degree of atrophy correlates with the decline in cognitive abilities supported by the core network, such as episodic memory (Kramer et al., 2007). We asked young and older adults to generate past and future events in response to cue words (nouns) and describe the event in as much detail as possible for 3 minutes (Addis et al., 2008). Transcripts of events were parsed into internal episodic details (i.e., episodic details internal to the central event being described, e.g., visuospatial details, context, emotions and thoughts from the experience) and external details (i.e., non-episodic information including semantic details). Older adults generated fewer internal episodic details relative to younger adults. In contrast, there was no evidence of an age-related deficit in the generation of external details; in fact, older adults produced significantly more external details than younger adults. Importantly, this pattern was evident for both past and future events, and the internal and external detail scores were tightly correlated across past and future events. Interestingly, the deficit in internal details correlated with the integrity of relational memory (as assessed by recall of verbal paired associates), supporting the idea that hippocampal function and the ability to integrate various details into a coherent event is an important component of future simulation.
As noted earlier, the core network exhibits further structural and functional compromises in patients with AD; in addition to age-related structural changes, brain regions comprising this network are characteristic sites of amyloid deposition (Buckner et al., 2005). Moreover, patients with AD typically exhibit deficits, relative to healthy older adults, in functions supported by this network, such as the episodic and semantic aspects of autobiographical memory. For instance, Gilboa et al. (2005) found that in AD patients, episodic autobiographical memory function was correlated with the volume of bilateral medial temporal regions and anterior lateral temporal cortex. In contrast, semantic autobiographical memory was correlated with volume of bilateral anterior and posterior lateral temporal cortex and right prefrontal cortex. Numerous studies have examined the integrity of the episodic and semantic components of autobiographical memory in AD, and most studies document some level of impairment in one or both types of memory (e.g., Addis & Tippett, 2004; Budson et al., 2007; Dorrego et al., 1999; Greene & Hodges, 1996; Greene et al., 1995; Kopelman, 1989; Leyhe et al., in press; Piolino et al., 2003; Sagar et al., 1988).
The majority of these studies have used the Autobiographical Memory Interview (AMI, Kopelman, Wilson, & Baddeley, 1990) or autobiographical fluency (Dritschel, Williams, Baddeley, & Nimmo-Smith, 1992). These tests probe episodic and semantic memory separately, despite the fact that in natural discourse, personal semantic knowledge forms an integral part of a description of an episodic event (Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002; Murphy, Troyer, Levine, & Moscovitch, 2008). Both measures assess personal semantic memory by recall of names (the AMI also probes recall of addresses) from various lifetime periods, while episodic memory is assessed by the recall of specific past events from across the lifespan. It has been argued that these tests are unmatched in terms of difficulty, sensitivity, content, and psychometric characteristics (Ivanoiu, Cooper, Shanks, & Venneri, 2006; Murphy et al., 2008). In an attempt to rectify this problem, one recent study of AD and semantic dementia used a modified version of the AMI that had first been matched for difficulty in control participants and included both cued and free recall measures of episodic and semantic memory (Ivanoiu et al., 2006). The authors found that while AD patients exhibited deficits for both episodic and semantic aspects of autobiographical memory relative to controls, they were significantly less impaired on the semantic component relative to patients with mild and moderate semantic dementia. Conversely, the episodic autobiographical memory impairment was more severe for those with mild to moderate AD than those with mild semantic dementia. Interestingly, though, patients with the most severe level of semantic dementia also exhibited deficits on the episodic component of autobiographical memory, suggesting that severe semantic impairments can affect the ability to recall episodic events. This deficit likely reflects the fact that when describing past events, one typically provides both episodic and semantic forms of information, woven together into a narrative. Thus, these standard measures of the episodic component of autobiographical memory are likely contaminated by semantic memory (Murphy et al., 2008).
The Autobiographical Interview (AI) was designed to overcome such limitations and allow assessment of the episodic and semantic aspects of a narrative describing a specific past event. Although this task has not yet been used to assess memory for past events in AD, it has been used with patients characterized by amnestic mild cognitive impairment (MCI) (Murphy et al., 2008). MCI refers to patients who likely have underlying neuropathology, are not cognitive normal, but do not meet criteria for dementia. MCI is thought to represent the transition stage between normal aging and dementia. Indeed, studies suggest that approximately 70% of patients given a diagnosis of MCI will go on to develop dementia (Petersen & Negash, 2008). Patients with the amnestic type of MCI are those individuals who show an isolated memory impairment, are otherwise functioning well, and do not meet criteria for AD or other dementia (Petersen et al., 2001). Most patients with amnestic MCI go on to develop AD (Petersen & Negash, 2008). Results revealed that while amnestic MCI patients exhibited a significant reduction in the number of internal episodic details comprising autobiographical memories of past events, they also exhibited a significant increase in the number of external semantic details relative to the control group for past events from across the lifespan (note, however, that Leyhe et al. (in press) report declines in both episodic and semantic details of recent autobiographical memories in MCI patients using the AMI). Interestingly, this pattern of decreased internal and increased external details parallels findings with healthy older adults relative to younger adults for both past (Addis et al., 2008; Levine et al., 2002) and future (Addis et al., 2008) events.
These findings suggest that when faced with deficits in the ability to generate internal details, participants may, when describing episodic events, exhibit an increased reliance on external semantic information if such information is available. Whether a similar pattern is also evident even when pathological changes are more severe, as in AD, is an open question. On the one hand, with the progression of neuropathology in AD beyond the medial temporal regions to encompass lateral temporal regions, and the associated decline of semantic memory, there could also be a decline in the production of both internal and external details when describing past and future events. On the other hand, given the overproduction of external information in the face of declining episodic memory in healthy aging and MCI, if a reduction in external details is evident in AD patients, it likely will not be as severe as the decline in internal details. We attempted to distinguish between these two possibilities in the present study, which as far as we know is the first to examine future episodic simulation in AD. Using an adapted version of the AI, we probed the ability of AD patients and healthy older controls to imagine future events, as well as remember recent past events and remote past events from across the lifespan. In line with the constructive episodic simulation hypothesis, and based on the tight correspondence of past and future events previously documented in healthy older adults (Addis et al., 2008), we expect similar patterns of decline across past and future events in AD patients. Moreover, the amount of internal and external detail generated for past, future and remote events should be strongly correlated.
Methods
Participants
Sixteen patients with a diagnosis of mild AD as determined by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria (NINCDS-ADRDA, McKhann et al., 1984) and sixteen age-matched controls with no significant history of other neurological or psychiatric impairment completed this study (demographic information is presented in Table 1). Participants were excluded if they were characterized by having clinically significant depression, alcohol or drug use, cerebrovascular disease, traumatic brain damage, or if English was not their primary language. Patients with AD were excluded if their Mini Mental State Examination (Folstein, Folstein, & McHugh, 1975) score fell below 20. Healthy older adults were excluded if they had a first-degree relative with a history of AD, another neurodegenerative disorder, or dementia. The groups did not differ significantly in age (p = .49), or education (p = .10). The apparent difference in gender-ratio between the groups (Control group, 6 male/10 female; AD group, 11 male/5 female) was not significant, χ2 (1, N=32) = 3.14 (p = 0.08). Consistent with a diagnosis of mild AD, MMSE scores were significantly lower in the AD group (p < .001). Informed written consent was obtained in a manner approved by the Bedford VA Hospital Institutional Review Board. Participants were given the option of completing testing either in the laboratory or in their own home. Twenty-five participants (15 AD, 10 Controls) opted to complete testing at home, while 7 participants (1 AD, 6 Controls) came into the laboratory. One potential concern is that participants tested at home might perform at a higher level than those tested in the lab because the home environment provides access to personal cues that could aid episodic retrieval or simulation. Thus, the ANOVA analyses conducted on the AI internal and external detail scores were also ran using “testing location” as the Between-Groups variable to ensure that location was not responsible for any group effects.
Table 1.
Demographic and neuropsychological characteristics of participants
| Group means (SD): |
||
|---|---|---|
| Demographic/neuropsychological characteristic | Control | AD |
| Demographics | ||
| Gender | 6M/10F | 11M/5F |
| Age (years) | 78.75 (5.17) | 77.06 (8.10) |
| Education (years) | 16.38 (2.68) | 14.69 (2.73) |
| Standard Neuropsychological Tests | ||
| Boston Naming Test - Short Form (max 15) | 14.31 (1.14) | 12.13 (3.56) |
| Category (semantic) fluency (total score; no max)* | 47.63 (9.82) | 26.75 (10.90) |
| CERAD Recall (max 30)* | 24.5 (3.48) | 10.31 (3.38) |
| CERAD Delayed Recall (max 10)* | 8.31 (1.78) | 1.06 (1.65) |
| CERAD Delayed Recognition (max 10)* | 10.0 (0.00) | 6.19 (2.48) |
| Mini-Mental State Examination (max 30)* | 29.63 (.719) | 24.38 (3.18) |
| Phonemic (FAS) fluency (total score; no max) | 49.13 (12.67) | 35.13 (17.17) |
| Trail Making Test Part A (seconds; no max)* | 29.69 (7.14) | 75.88 (16.31) |
| Trail Making Test Part B (seconds; no max)* | 56.50 (17.09) | 206.69 (77.89) |
| Verbal Paired Associates I (Recall Total Score; max 32)* | 21.56 (6.50) | 1.44 (3.29) |
| Verbal Paired Associates II (Recall Total Score; max 8)* | 6.56 (1.46) | 0.25 (0.77) |
| Cambridge Behaviour Prospective Memory Test | ||
| Time score (max 4)* | 3.44 (1.26) | 0.19 (0.75) |
| Event score (max 4)* | 2.25 (1.61) | 0.00 (0.00) |
| Object score (max 5)* | 4.13 (1.78) | 0.06 (0.25) |
| Place score (max 5)* | 4.06 (1.81) | 0.06 (0.25) |
Note: Standard deviations given in parentheses. AD = Alzheimer's disease group; CERAD = Consortium to Establish a Registry for Alzheimer's Disease; F = female; M = male; max = maximum score attainable
p < .003 (Bonferroni corrected threshold)
Neuropsychological Testing Session
During an initial one-hour session, a battery of standard neuropsychological tests was administered to assess various aspects of cognitive function including prospective memory, immediate and delayed recall of relational memory, executive functioning and aphasic impairments.
The Cambridge Behaviour Prospective Memory Test (Groot, Wilson, Evans, & Watson, 2002)
Participants were given a series of tasks consisting of 4 event-related tasks (i.e. a certain task was to be carried out following a specified event) and 4 time-based tasks (i.e. a certain task was to be carried out at a specified time) to be completed throughout the testing session. Prior to instructions of the task, subjects were told that they could use anything to help them remember to carry out the tasks (a sheet of paper and a pen were visible), but that the instructions would not be repeated. The time-based tasks used in the present study were: (1) closing an open notebook 3 minutes into the testing session; (2) reminding the experimenter not to forget his keys after 15 minutes; (3) asking for a copy of the newspaper after 20 minutes; (4) writing the date on a pad of paper after 20 minutes. The event-based tasks used were: (1) placing a computer case on the floor when an alarm set for 5 minutes rings; (2) changing pens after completing the fourth test; (3) giving an envelope with a message written on it to the experimenter when he says “there are 10 minutes left”; note this instruction was given after the alarm rang; (4) reminding the experimenter of the five hidden objects when he tells the subject that the testing session was over. One point was given for each task successfully carried out at the correct time (time score) or after the correct event occurred (event score).
In addition, five objects were shown to the subject and hidden (1) phone cord placed inside a computer case; (2) white out placed inside experimenter's pocket; (3) paper clip placed under experimenter's chair; (4) breath mints placed inside experimenter's back-pack; and (5) envelope moistener placed underneath testing table. At the end of testing, the subject was required to recall the identity of the objects (object score - one point for each remembered object) and where it was hidden (object score - one point was given for each remembered location).
Standard Neuropsychological Tests
A number of standard tests were administered in the following order: (1) Verbal Paired Associates I (VPA-I); (2) Verbal Paired Associates II (VPA-II); Trail Making Test - Part A (TMT-A); (4) Trail Making Test - Part B (TMT-B); (5) Phonemic (FAS) Fluency; (6) Boston Naming Test - Short Form (BNT-15); (7) the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) word list memory tasks (Recall, Delayed-Recall and Delayed-Recognition); (8) MMSE. A filler task was used in between VPA-I and VPA-II in order to provide the required time delay. To better characterize the control and AD groups, semantic (category) fluency data (total score for animals, fruits and vegetables) are also reported. However, it should be noted these data were not collected in the same session as the other neuropsychological data, but were obtained from another neuropsychological assessment conducted an average of 252 days (SD = 178 days) before or after the AI session.
Famous Names
Three-alternative forced choice recognition task (Westmacott & Moscovitch, 2002). During the delay between VPA-I and VPA-II, participants completed a famous name recognition test to assess semantic memory. The famous names were the same as those used by Westmacott and colleagues, which included entertainers, athletes, politicians and other newsworthy people whose fame was of limited duration. In the present study, famous Canadian names were removed as the current participants were American. Thus 20 famous name recognition trials for each of 6 decades (1940s through 1990s) were administered. Each trial comprised one famous name and two foil names matched for gender and ethnicity, presented simultaneously on a computer screen. Trials from different decades were presented randomly. Participants were asked to identify which of the three names belonged to a famous person and the experimenter recorded the response using the keyboard. A recognition accuracy score was calculated for each decade.
Adapted Autobiographical Interview (AI) Session
Approximately one week following neuropsychological testing, participants completed an adapted AI session (Levine et al., 2002).
(1) Past-Future AI task
The first AI task probed both past and future events (Addis et al., 2008). Participants generated past and future events in response to cue words (Crovitz & Schiffman, 1974). We provided a guideline for the temporal distance of events to be within the past or next few months in order to encourage similar temporal distances for past and future events, especially given that for past events, older adults could sample from an entire lifetime but could not do the same for future events. Five past and five future event trials were completed. All trials for one temporal direction (past or future) were completed before beginning the trials for the other temporal direction. Conditions were blocked in this manner to reduce load and facilitate older adults' understanding of the instructions for each condition. The order of presentation of condition was counterbalanced, resulting in two versions of the task (past then future; future then past). Two lists of five cue words (see Appendix A1) cycled through past and future conditions, resulting in four counterbalanced versions of the past-future AI task. Participants were randomly assigned to one of these versions. Although the order of presentation of condition was counterbalanced across subjects, and the word list cycled through conditions, the presentation of cue words within a condition was random.
A1.
Lists of cue words used in the Past-Future AI Task
| List 1 | List 2 |
|---|---|
| CAR | SHOES |
| STAIN | TOWER |
| OVEN | ENGINE |
| ARM | BABY |
| APPLE | LEMON |
(2) Remote Memory AI Task
Participants then completed a remote memory AI task; this condition always followed the past-future task due to a concern that this remote memory AI task might affect the results of the past-future AI task. For the remote memory AI task, participants were asked to generate an event from each of five lifetime periods (early childhood, up to 10 years old; teenage years, 11 to 18 years old; early adulthood, 19 to 35 years old; middle adulthood, 36 to 55 years old; recent adulthood, 55 to 65 years old). As with the past-future AI task, a cue word was provided on every trial to facilitate memory retrieval. Two versions of this AI task were created: one where the order of lifetime periods ascended from childhood to recent adulthood; and one where the order of lifetime periods descended from recent adulthood to childhood. Two lists of five cue words cycled through these two versions, resulting in four partially-counterbalanced versions of the remote memory AI task (see Appendix A2). Participants were randomly assigned to one of these versions.
A2.
Lists of cue words used in the Remote Memory AI Task
| List 1 | List 2 |
|---|---|
| PHOTOGRAPH | TOY |
| PIANO | WINE |
| HORSE | SHIP |
| INSECT | LETTER |
| TREE | TRUCK |
Stimuli
Cues for the past-future and remote memory AI tasks comprised twenty nouns high in concreteness (M = 6.87, SD =0.19), imageability (M = 5.86, SD =.31) and Thorndike-Lorge frequency (M = 1.72, SD =0.24) taken from the Clark and Paivio (2004) extended norms. Cues were divided into four lists of five nouns matched for frequency, imageability and concreteness. Analysis of variance (ANOVA) confirmed that the lists used in these tasks did not differ significantly in concreteness [F(3,16) = .25, p = .86], imageability [F(3,16) = .22, p = .88] or Thorndike-Lorge frequency [F(3,16) = .45, p = .72]. Two of these cue-word lists were used for the past-future AI task, and two for the remote memory AI tasks (see Appendix A).
Interview
Participants were instructed to recall or imagine an event and to generate as much detail as possible within a three minute time-limit. The event generated in response to a cue word did not have to strictly involve the named object; participants were encouraged to freely associate so that they were successful in generating an event. Each event was required, however, to be temporally and contextually specific (i.e., episodic), occurring over minutes or hours, but not more than one day. Future events had to be plausible given the participant's plans, and novel, that is, not previously experienced by the participant. Participants were asked to, when possible, experience events from a field (i.e., seeing the event from the perspective of being there) rather than an observer perspective (i.e., observing the self from an external vantage point).
For the duration of each trial, the relevant cue word was displayed on a computer screen along with the task instruction (“recall past event” or “imagine future event”) and time-period (“past few months”, “next few months”, or for the remote AI task, the relevant lifetime period). When necessary, general probes were given to clarify instructions and encourage further description of details. After three minutes, a bell sounded to indicate the end of the trial. Participants then dated the event and rated it on a five-point scale for level of detail (1 = vague with no/few details, 5 = vivid), emotionality (i.e., intensity of emotion experienced upon recalling/imagining the event; 1=detachment, 5=highly emotional) and personal significance (i.e., how life-changing the event is; 1=insignificant, 5=life-changing). Participants then estimated or predicted the temporal distance of the event from the present. The interview took approximately 1-2 hours. Participants were tested individually, and responses were recorded using a digital audio-recorder for later transcription.
Scoring
The standardized AI scoring procedure (Levine et al., 2002) was used. The events were scored by a trained rater (L.M.) blind to group membership and the hypothesis of this study. This rater (L.M.) scored events in a manner that was highly reliable with another two raters in our laboratory (R.M. and L.P.). Reliability was established using a training set of 20 past and future events taken from our original study (Addis et al., 2008) that employed the same adapted AI protocol as the current study. An intraclass correlation analysis indicated that the three raters scored this set of transcripts in a highly reliable manner (two-way mixed model; standardized Cronbach's alpha: internal detail score, .964; external detail score, .905).
Each event was scored in the following manner. The central event was first identified; if more than one event was mentioned, the event discussed in most detail that occurred over a brief timeframe was selected as the central event. The average proportion of trials in which more than one event was mentioned was .11 (SD = .10). The transcription was then segmented into distinct details (i.e., chunks of information, e.g., a unique occurrence or thought), and these details were categorized as internal (episodic information relating to the central event) or external (nonepisodic information including semantic details, extended events and repetitions). For each event, the number of internal and external details was tallied, and each total was then averaged across the five events in each condition to create an internal and external AI score for each condition in each participant.
Results
Neuropsychological Tests
In order to characterize the neuropsychological deficits in the AD group, we ran a series of independent samples t-tests probing group differences on various neuropsychological tests (Table 1). Using a threshold corrected for multiple comparisons (Bonferroni p value = .003), there was evidence of significant impairment in the AD group relative to the control group on the following tests: category fluency, all scores of the Cambridge Behaviour Prospective Memory Test (i.e., time, event, object and place scores), the CERAD recall, delayed recall and delayed recognition tests, MMSE (consistent with a diagnosis of AD in the AD group), Trail Making Test A and B, Verbal Paired Associates I and II. Notably, the AD group performed on floor for all of the prospective memory scores. Although numerical differences were evident for performance on the Boston Naming Test and Phonemic (FAS) Fluency, these differences did not survive the Bonferroni-corrected threshold, possibly due to increased variance in the AD group. However, given the large numerical differences between the group means, we do not wish to assert that these functions are preserved in the patient group.
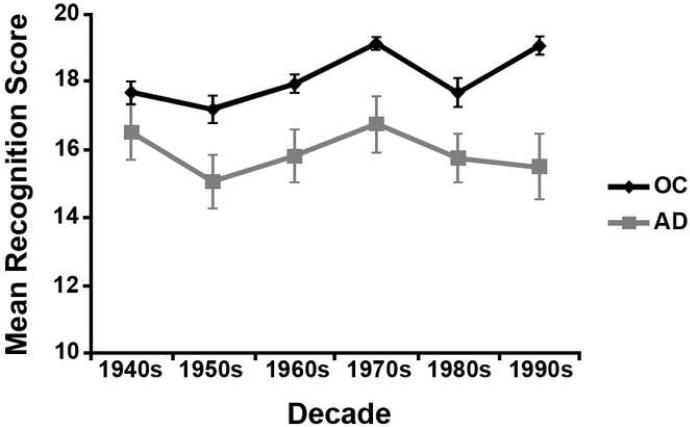
Performance on the Famous Names three-alternative forced choice recognition task (see Figure 1) was analyzed using a repeated-measures ANOVA with one within-subjects factor (decade) and one between-subjects factor (group). This test revealed a main effect of group, F(1,30) = 8.20, p = .01, with AD patients exhibiting significantly poorer recognition performance (M = 15.90) than control participants (M = 18.12). There was also a significant main effect of decade, F(1,115.66) = 6.41, p < .001, and a decade by group interaction, F(1,115.66) = 2.62, p = .04. Post-hoc bonferroni t-tests revealed that recognition accuracy is higher for names from the 70s relative to names from the 50s, 60s and 80s, and for names from the 90s relative to the 50s. The group by decade interaction likely reflects the fact that while older controls exhibited highest accuracy for names from the 70s and 90s, AD patients exhibited their best performance for names from the 40s and 70s.
Figure 1.
Mean recognition accuracy score for each decade (1940s to 1990s) for AD patients and healthy older controls on the three-alternative forced choice famous names recognition task.
Phenomenology of past, future and remote events
The phenomenological qualities of past, future and remote autobiographical events are presented in Table 2. To ensure that temporal distance of events did not differ significantly between groups or temporal directions (past and future), dates of events (converted to weeks from the present) were analyzed using repeated-measures ANOVA. Temporal distance did not differ by temporal direction, F(1,30) = .84, p = .37. Importantly, temporal distance did not differ between groups, F(1,30) = 1.54, p = .22, nor was there an interaction of temporal direction and group, F(1,30) = .57, p = .47, meaning that any group differences on the AI cannot be accounted for by temporal distance. For remote events, an independent samples t-test confirmed that the temporal distance of remote events also did not differ between groups, t = -1.49, p = .15.
Table 2.
Phenomenological qualities of past, future and remote autobiographical events
| Group | Past events | Future events | Remote events |
|---|---|---|---|
| Mean temporal distance of event from the present (sd) | |||
| Control Group | 9.85 weeks (8.88) | 9.56 weeks (4.30) | 47.68 years (5.37) |
| AD Group | 8.37 weeks (12.60) | 5.40 weeks (3.16) | 51.37 years (8.34) |
| Mean rating of subjective detail (sd)* | |||
| Control Group | 4.40 (0.53) | 3.88 (0.76) | 3.99 (0.62) |
| AD Group | 4.14 (0.61) | 3.77 (0.68) | 4.27 (0.70) |
| Mean rating of emotional intensity of event (sd) | |||
| Control Group | 3.59 (0.94) | 3.50 (0.92) | 3.59 (0.87) |
| AD Group | 3.58 (0.98) | 3.36 (1.01) | 3.68 (1.16) |
| Mean rating of personal significance of event (sd) | |||
| Control Group | 3.58 (1.05) | 3.73 (0.94) | 3.69 (0.97) |
| AD Group | 3.45 (0.84) | 3.45 (0.98) | 3.94 (0.78) |
Note. Standard deviations given in parentheses. Past and future events did not differ significantly in terms of temporal distance, emotional intensity or personal significance. There were no group differences on any of these dimensions for past, future or remote events.
Past events were more detailed than future events, p < .001.
We also examined whether the phenomenological qualities of events differed across group and temporal direction, given that such differences could change interpretations of other effects. Wilcoxon Sign tests indicated that there were no differences between past and future events in terms of emotional intensity (p = .18) or personal significance (p = .58). There was, however, evidence that past events were rated as more detailed than future events (p < .001), in line with previous findings that experienced events are more detailed than imagined events (M. K. Johnson, Foley, Suengas, & Raye, 1988). Importantly, Mann-Whitney U tests showed that there were no group differences in detail, emotional intensity or personal significance for past, future or remote events (all p values were above 0.18).
Verification of past events
We corroborated a sample of the five past events generated by 13 of the AD patients with their spouses (3 patients/spouses could not be contacted). This verification process occurred approximately 1.5 years after the data were collected, and thus it was only feasible to enquire about those events that the spouse would be likely to remember years later (i.e., we did not attempt to verify seemingly trivial events). For 11 patients, we enquired about 2 events and the spouse verified both events for all patients. For another 2 patients, we enquired about 1 event, and the event from one of these patients was verified. Thus, we were unable to verify the past event in only 1 of 13 patients; even in the single instance of non-confirmation, the spouse was unsure of whether the event had occurred or not rather than characterizing the event as a likely confabulation. These observations suggest that the rate of confabulation in these mild AD patients is very low, consistent with evidence suggesting that confabulation is typically evident only in patients with frontal (particularly ventromedial) pathology (Baddeley & Wilson, 1986; Kopelman, 2002; Kopelman, Wilson, & Baddeley, 1989)
Adapted Autobiographical Interview - Past-Future AI Task
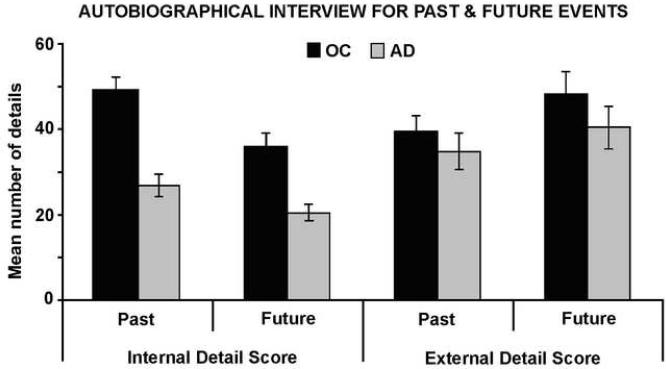
We conducted a 2 (Temporal Direction: Past, Future) x 2 (Detail: Internal, External) x 2 (Group: Control, AD) mixed factorial ANOVA with repeated factors of Temporal Direction and Detail and between factor of Group. Of most interest here, we found a significant main effect of Group, with AD patients producing fewer details when describing past and future events than did healthy control participants, F(1,30) = 17.26, p < .001 (see Figure 2). Although the mean difference between the control and AD group was more pronounced for internal (mean difference = 18.95) than external (mean difference = 6.23), the interaction of Detail and Group failed to reach significance, F(1,30)=3.08, p = .09. There was a significant main effect of Detail, F(1,30) = 4.43, p = .04, reflecting increased generation of external versus internal details across groups and temporal direction. There was also a significant interaction of Detail and Temporal Direction, F(1,30) = 15.73, p < .001. Post-hoc bonferroni tests revealed that this interaction reflected higher amounts of internal detail for past (M= 37.97) than future (M=28.20) events (p<.001), while external detail was higher for future (M=44.25) than past (M=37.18) events (p=.02). No other interactions were significant (all p values > .26).
Figure 2.
Mean number of internal and external details generated for past and future events by patients with AD and healthy older controls. Error bars represent standard errors of the means.
In order to assess whether the testing location influenced the results, we re-computed a 2 (Temporal Direction: Past, Future) x 2 (Detail: Internal, External) x 2 (Testing Location: Home, Laboratory) mixed factorial ANOVA with repeated factors of Temporal Direction and Detail and between factor of Testing Location. Importantly, there was no main effect of Testing Location, F(1,30)=2.90, p=.10. Although the 6 control subjects and 1 AD subject tested in the laboratory generated, on average, more details (M=42.74) than the 15 AD and 10 control subjects tested at home (M=35.26), this pattern suggests that being tested at home in an environment rich with cues does not result in higher levels of detail. In fact, this trend may reflect the fact that only higher functioning older adults and AD patients might choose to travel into the laboratory for testing. Indeed the mean MMSE for those participants tested in the lab (28.14) was numerically higher than those tested at home (26.68), though it is noted this difference was not significant, t(30)=.98, p=.34. Testing Location did not interact significantly with Detail, F(1,30)=0.31, p=.58, or Temporal Direction, F(1,30)=0.14, p=.72.
The ability to generate internal and external detail could be greatly influenced by deficits in fluency abilities, as measured by phonemic (FAS) and semantic (category) fluency. As reported above, the AD patients were significantly impaired on category fluency, and showed a non-significant reduction in performance on phonemic fluency. Given that these deficits could confound the results on the AI, we re-ran the analysis including the fluency measures as covariates. Moreover, the number of females was higher in the control group, and because females tend to have longer and more detailed memories of past experiences than males (Fivush & Buckner, 2003), we also included gender as a covariate. Thus, we computed a 2 (Temporal Direction: Past, Future) x 2 (Detail: Internal, External) x 2 (Group: Control, AD) mixed factorial ANCOVA with repeated factors of Temporal Direction and Detail, between factor of Group, and three covariates (FAS, Category Fluency and Gender). Although none of these covariates reached significance, it seems the FAS covariate explained more variance, F(1,27)=3.55, p=.07, than Category Fluency, F(1,27)=.25, p=.63, or Gender, F(1,27)=.40, p=.53. Certainly, including these covariates resulted in some changes in the results. For instance, including the covariates eliminated the main effect of Detail, F(1,27)=0.05, p=.82; in the previous analysis, this effect had reflected more significantly more external than internal details generated overall. Additionally, the interaction of Detail and Temporal Direction, which in the previous analysis had reflected the higher number of internal details for past vs. future events contrasting with the higher number of external details for future vs. past events, was no longer significant, F(1,27) = 0.27, p = .61. The elimination of these two effects with the inclusion of gender and fluency covariates, and the trend towards significance for the FAS covariate, suggests that the over-generation of external details, particularly for future events, is related to phonemic fluency abilities. Although the overall Group effect remained significant, F(1,27)=5.96, p=.02), there is evidence to suggest that this difference was only apparent for internal details. Specifically, inclusion of the fluency covariates resulted in a slight strengthening of the interaction of Detail and Group, F(1,27)=3.53, p = .07. Although this interaction is still only a trend, including the fluency covariates reduced the estimated mean group difference for external details from 6.23 to 0.59, while it increased the estimated group difference for internal details from 18.95 to 20.90. Indeed, bonferroni post-hoc tests indicates this interaction reflects a significant group difference for internal (p < .001) but not external (p=.95) detail. This pattern suggests that including gender and fluency covariates controls for the higher production of external details in healthy controls relative to AD patients, making this group difference less significant. In contrast, the significant group difference for internal details is not affected by the covariates. Overall, these results indicate that there is a significant deficit in generating internal, but not external, details for both past and future events even when group differences in phonemic fluency are accounted for.
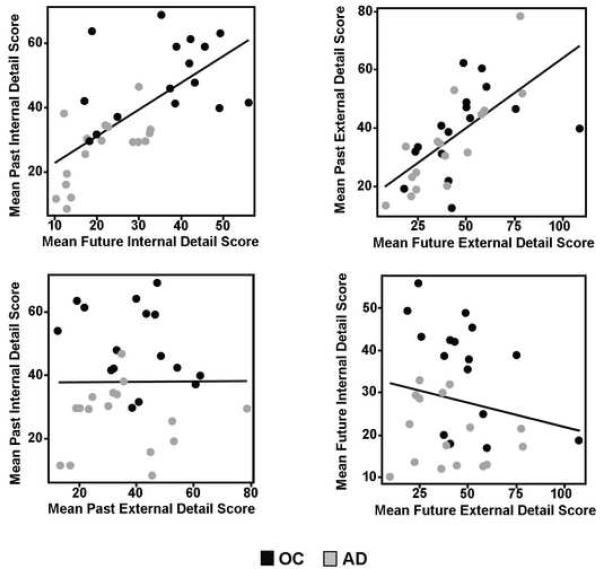
Correlations between past and future AI scores were computed across all subjects. To control for overall level of cognitive decline, performance on the MMSE was partialled from all correlations. We replicated previous findings of strong correlations between past and future internal (r = .60, p < .001) and external (r = .64, p < .001) scores (Figure 3). In contrast, past internal and external scores were uncorrelated (r = -.13, p = .25) as were future internal and external scores (r = -.24, p = .10). Partial correlations, controlling not only for MMSE but also phonemic (FAS) fluency, semantic (category) fluency and gender, were computed. Overall, the pattern of correlations did not change. Past and future internal (r = .62, p < .001) and external (r = .61, p < .001) scores were again strongly correlated. Past internal and external scores remained uncorrelated (r = -.20, p = .15), though the correlation between future internal and external scores approached significance (r = -.30, p = .06).
Figure 3.
Scatter plots and regression lines showing the correlations between the numbers of internal details in generated past and future events (top left, r = .60, p < .001), the numbers of external details in generated past and future events (top right, r = .64, p < .001), the numbers of internal and external details in generated past events (bottom left, r = -.13, p = .25), and the numbers of internal and external details in generated future events (bottom right; r = -.24, p = .10). Partialling out phonemic and semantic fluency abilities and gender did not change the overall pattern of correlations, except that the correction of future internal and external details approached significance (p = .06).
A possible concern, at least in the AD group, is that confabulation of past events underlies the strong correspondence between past and future events: if patients were actually fabricating `memories' of past events, then they would have been imagining events in both the past and future conditions. However, as noted earlier, the verification of past events generated by AD patients indicates that confabulation is almost non-existent in this group of mild AD patients. Moreover, it is notable that the correlations between past and future events are evident not only in AD patients but also healthy control participants, who are very unlikely to confabulate.
Adapted Autobiographical Interview - Remote Events
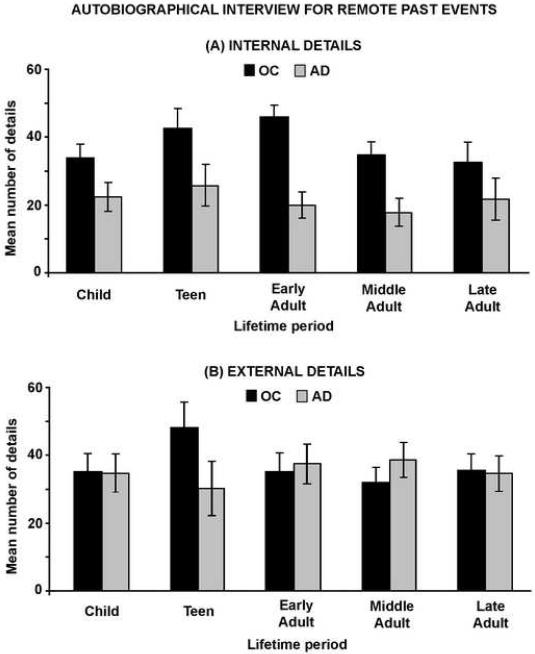
In order to analyze remote memory across the lifespan, we conducted a 5 (Lifetime Period: Childhood, Teenage Years, Early Adulthood, Middle Adulthood, Recent Adulthood) x 2 (Detail: Internal, External) x 2 (Group: Control, AD) mixed factorial ANOVA with repeated factors of Lifetime Period and Detail and between factor of Group. The only significant effect was that of Group, F(1,30)=7.90, p < .01 (see Figure 4). Although subjects generated more external (M=35.62) than internal (M = 29.64) details overall, this difference did not reach significance, F(1,30)=2.99, p=.09. Similarly, although the mean difference between the control and AD group was more pronounced for internal (mean difference = 16.00) than external (mean difference = 3.36), the interaction of Detail and Group failed to reach significance, F(1,30)=3.34, p = .08. The effect of life-time period approached significance, F(2.93,87.10)=2.30, p = .08, and although it appeared that only the control group exhibited a `reminiscence bump' - enhanced recall of events from teenager and early adult years -the interaction of Group and Lifetime Period failed to reach significance, F(2.93,87.10)=2.38, p = .08.
Figure 4.
Mean number of internal (top panel) and external (bottom panel) details generated for remote past events by patients with AD and healthy older controls. Five lifetime periods were assessed: early childhood, up to 10 years old; teenage years, 11 to 18 years old; early adulthood, 19 to 35 years old; middle adulthood, 36 to 55 years old; recent adulthood, 55 to 65 years old. Error bars represent standard errors of the means.
We also conducted a 5 (Lifetime Period: Childhood, Teenage Years, Early Adulthood, Middle Adulthood, Recent Adulthood) x 2 (Detail: Internal, External) x 2 (Group: Control, AD) mixed factorial ANCOVA with repeated factors of Lifetime Period and Detail, between factor of Group, and three covariates (FAS, Category Fluency and Gender). Controlling for fluency ability and gender resulted in the elimination of the main effect of Group, F(1,27)=1.18, p=.29. Indeed, including the covariates brought the estimated marginal group means closer together, by slightly reducing the control group (from 37.47 to 35.29) and slightly increasing the AD group mean (from 27.79 to 29.97). Moreover, including covariates also reduced the significance of any effects related to Detail or Lifetime Period. Thus, the trends evident for the main effect of Detail, Lifetime Period, the interaction of Detail and Group and the interaction of Lifetime Period and Group were no longer evident (p values > .33). As was evident for the Past-Future AI analysis, it was the phonemic (FAS) fluency covariate that was the most influential covariate, F(1,27)=4.18, p=.05; semantic (Category) fluency, F(1,27)=0.001, p=.97, and gender, F(1,27)=0.53, p=.47, explained little variance. These results suggest that for remote memory, the group difference in the ability to generate internal and external details is, at least in part, related to differences in phonemic fluency.
Correlations between remote AI scores and past-future AI scores were computed across all subjects, controlling for MMSE. This analysis revealed strong correlations between remote and past scores for internal (r = .61, p < .001) and external (r = .52, p = .004) details. A similar correlation was evident for remote and future external details (r = .69, p < .001) but it did not reach significance for internal details (r = .35, p = .07). We also conducted partial correlations, controlling not only for MMSE but also phonemic (FAS) fluency, semantic (category) fluency and gender. Overall, the pattern of correlations did not change. The magnitude of the correlations between remote and past scores for internal (r = .54, p = .001) and external (r = .45, p = .01) details were slightly reduced but remained significant. The correlation between remote and future external details remained significant (r = .67, p < .001), and the trend for a significant correlation of remote and future internal details further approached significance when the effect of fluency measures was partialled out (r = .30, p = .06).
Discussion
Autobiographical Interview of Past and Future Events
This study is the first to investigate whether AD patients exhibit impairments in the ability to simulate future events. Given that in AD, the regions of the core network found to support remembering and imagining in young adults are structurally and functionally compromised, we hypothesized that AD patients should exhibit deficits not only in remembering but also future simulation. The results of the current study support this hypothesis. Relative to healthy older adults, the patients generated significantly fewer internal episodic details when describing both past and future events.
It is worth noting that this deficit in generating internal details persisted even when phonemic and semantic fluency abilities and gender were included as covariates. Moreover, this group difference cannot be accounted for by differences in the phenomenology of events generated by AD and control participants; the mean temporal distance of events from the present, the emotional intensity, and personal significance did not differ by group. Further, the group difference cannot be explained by testing location. The majority of AD patients were tested at home, an environment rich with potential retrieval cues. Even so, these patients exhibited a deficit in the ability to generate internal episodic details for past and future events. Moreover, the ANOVA examining the effect of testing location revealed no difference in the ability to generate past and future details. The number of details generated was numerically higher in those subjects who were tested in the laboratory. However, this trend may reflect only that more higher-functioning participants choose to travel into the laboratory for testing.
The finding that AD patients showed a deficit in generating internal details for imagined future episodes is consistent with a growing number of findings that populations with deficits in episodic memory, including older adults (Addis et al., 2008), amnesic patients (Hassabis, Kumaran, Vann et al., 2007) and depressed individuals (Williams et al., 1996), also experience difficulties in imagining specific future episodes. As we observed in the current study, even if some description of a past or future event is produced, it appears that the event representation lacks the rich level of specific episodic details typically generated by healthy controls (Addis et al., 2008; Hassabis, Kumaran, Vann et al., 2007). Moreover, Hassabis and colleagues (2007) found that amnesic patients with hippocampal damage exhibited not only a deficit in the number of details generated but also the integration of the details into a coherent event representation. These findings also converge with recent neuroimaging findings suggesting that the hippocampus is not only engaged by remembering and imagining (e.g., Addis et al., 2007; Botzung et al., 2008; Hassabis, Kumaran, & Maguire, 2007; Okuda et al., 2003, see Schacter and Addis, 2009, for a review of related evidence) but that hippocampal activity is specifically correlated with the amount of detail comprising past and future events (Addis & Schacter, 2008). The constructive episodic simulation hypothesis (Schacter & Addis, 2007a, 2007b) proposes that the hippocampus is crucial to future simulation because it enables not only the extraction of relevant details, but the recombination and integration of these various details into coherent future events. Others have proposed that the role of medial temporal regions in remembering and imagining reflects the process of constructing a vivid and spatially coherent scene (i,e., scene construction; Hassabis & Maguire, 2007). Indeed, patients with hippocampal damage exhibit a lack of spatial coherence when imagining scenes (Hassabis, Kumaran, Vann et al., 2007). However, an impaired ability to construct a scene likely reflects difficulty in retrieving details from episodic memory. Thus, given (1) the role of the hippocampus in retrieving details from episodic memory and the process of constructing a scenario, and (2) that the hippocampus is a prime site of neuropathology in AD, it is not surprising the patients in the present study generated significantly fewer episodic details than healthy controls.
However, the hippocampus is not the only region that is commonly activated by past and future events, and thus other regions in the core network may be critical to the deficits evident here. There is evidence of reduced glucose metabolism, atrophy and amyloid deposition in medial and lateral parietal regions. In particular, medial parietal regions such as the posterior cingulate and precuneus may be involved early in the course of AD - along with the medial temporal lobes (Buckner et al., 2005). Medial parietal regions are strongly engaged by both remembering past events (Cabeza & St Jacques, 2007; Svoboda, McKinnon, & Levine, 2006; Wagner, Shannon, Kahn, & Buckner, 2005) and imagining future events (Schacter, Addis, & Buckner, 2007; Spreng, Mar, & Kim, 2009); damage to this region can result in memory deficits (e.g., Gainotti, Almonti, Di Betta, & Silveri, 1998; Heilman et al., 1990; Rudge & Warrington, 1991; Valenstein et al., 1987). In a recent study, Summerfield, Hassabis and Maguire (2009) examined the contributions of medial parietal cortex to the representation of real and imaginary events that featured the self or others. They found the precuneus to be active across various types of event representations. Given that all the event types required some degree of visual imagery, this finding is consistent with a suggested link between the precuneus and episodic imagery processes (Fletcher et al., 1995). Importantly, this study was able to distinguish between the contributions of subregions of the precuneus, retrosplenial cortex and posterior cingulate. Specifically, subregions of the precuneus and the posterior cingulate were modulated by the `realness' of the event. Although the paradigm in this study differed from the current one in that representations of imagined events were recalled from a prior construction and not constructed for the first time, the finding that the posterior cingulate responds to the `realness' of an event still suggests that dysfunction of this region might result in a differential deficit in the ability to remember real experiences. However, this was not evident in the present study, where there was no effect of temporal direction, and both past and future events were affected. Summerfield et al. (2009) and others (S. C. Johnson et al., 2002; Maddock, 1999; Northoff & Bermpohl, 2004; Northoff et al., 2006) have linked the activity of posterior cingulate and retrosplenial cortex to the `selfness' and emotionality of event representations. However, the AD group did not differ from controls in terms of the subjective ratings of emotionality and personal significance (i.e., the importance of the event to the self) of the past and future events generated. This observation suggests that if there is dysfunction in medial parietal regions, it did not affect the emotional and/or self components of the events generated. More relevant here, others have suggested that retrosplenial cortex, along with the precuneus, is specifically related to the recollection of details (Wagner et al., 2005). Thus, retrosplenial dysfunction could underlie the impaired generation of internal details evident in the AD group. It is not possible in the current study, however, to tease apart whether the reduction in internal details is related to dysfunction of the medial temporal lobes, the retrosplenial cortex, or both regions.
There is evidence of dysfunction in lateral parietal cortex in Alzheimer's disease (Buckner et al., 2005). Recent neuroimaging (Svoboda et al., 2006; Wagner et al., 2005) studies have also implicated the lateral parietal region in episodic memory retrieval; moreover, this region is also engaged during the simulation of future events (Schacter et al., 2007; Spreng et al., 2009). These observations have led to debate regarding the nature of the lateral parietal contribution to episodic memory. Some have argued it is the perception that the information being retrieved is “old” (Wheeler & Buckner, 2004), while others have proposed this region may support attention to internal memory representations (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008; Wagner et al., 2005). Others have suggested this region, along with medial parietal regions, is responsive to the retrieval of contextual details (Wagner et al., 2005). Davidson et al. (2008) recently examined memory function in six patients with lateral parietal damage. They found evidence that recollection but not familiarity was disrupted on an anterograde episodic memory task. However, with respect to a retrograde autobiographical memory, neither recollection nor familiarity was impaired. Using the Autobiographical Interview, they found that on average, patients with lateral parietal lesions tended to generate more external and fewer internal details than control subjects. However, only in one patient was the overproduction of external details significantly different from controls, and the reduction of internal details was not significant in any of the patients. These findings would suggest, then, that dysfunction in the lateral parietal cortex is not as detrimental to the ability to access episodic details as hippocampal dysfunction appears to be (Addis et al., 2008; Hassabis, Kumaran, Vann et al., 2007).
Not all of the neural regions reported as active during the imagining of future events are also engaged by remembering past events. For instance, neuroimaging studies indicated that the right frontal pole is involved in the construction of future events (Addis et al., 2007; Okuda et al., 2003). Moreover, activity in the frontopolar region correlates with the amount of future-oriented or intentional detail comprising the future event (Addis & Schacter, 2008; Okuda et al., 2003). Lesion and neuroimaging studies have also implicated this region in prospective memory (Burgess, Quayle, & Frith, 2001; Burgess, Veitch, de Lacy Costello, & Shallice, 2000; Okuda et al., 1998). Interestingly, in the present study, the AD group was very impaired on a measure of prospective memory, in line with previous work (e.g., Duchek, Balota, & Cortese, 2006; Huppert & Beardsall, 1993; Martins & Damasceno, 2008). Whether or not this severe prospective memory deficit reflects early dysfunction in the frontopolar region, it still may contribute to the reduction of details generated, at least for future events.
Another aim of the current study was to ascertain whether patients with AD would exhibit deficits in the non-episodic content of past and future events. Other studies using the AI have reported that while healthy older adults generate fewer internal details than younger adults for both past (Addis et al., 2008; Levine et al., 2002) and future (Addis et al., 2008) events, they also generate more external details than young adults. This pattern of decreased internal and increased external details for past events has also been reported in MCI patients relative to healthy older adults (Murphy et al., 2008). While this general pattern is suggestive of an increased reliance on external semantic details when unable to generate internal episodic details, we were not certain whether AD patients would also exhibit this pattern. Although most patients (approximately 70%) with amnestic MCI have underlying AD pathology, the assumption is that in this early pre-clinical stage of the disease the pathology predominantly affects medial temporal lobe function, producing deficits in episodic memory in the relative absence of other cognitive deficits (Petersen & Negash, 2008). Because in AD the neuropathological changes extend beyond the medial temporal regions to other cortical areas including the lateral temporal regions that support semantic memory, we speculated that external details might be impaired in our AD patients as well. Consistent with this possibility, Leyhe et al. (in press) documented declines in both episodic and semantic aspects of autobiographical memory for recent events in a population of MCI patients who they characterized as more impaired than the MCI patients studied by Murphy et al. (2008) that exhibited selective deficits in remembering episodic details. Moreover, it was possible that if there is an impairment in external details, it may be tempered by the tendency to overproduce external details and thus might not be as severe as the deficit in internal details. Indeed, this was what we found: there was some decline in the production of external details in AD patients relative to healthy controls, but unlike the impairment in internal details, the decline in external details was not statistically significant when fluency abilities were accounted for.
Even though the lack of a significant deficit in semantic autobiographical memory could be related to the typical overproduction of external details on the AI, this finding contrasts with many studies that do report impairment of personal semantics in AD (e.g., Addis & Tippett, 2004; Graham & Hodges, 1997; Greene & Hodges, 1996; Greene et al., 1995; Hou, Miller, & Kramer, 2005; Kopelman, 1989). It is important to consider the fact that this study examined semantic autobiographical memory when it is retrieved as an integral part of an event description. Other studies reporting personal semantic deficits in AD have used the Autobiographical Memory Interview (Kopelman et al., 1990) or autobiographical fluency (Dritschel et al., 1992); both methods assess semantic autobiographical knowledge by probing memory for personal facts (e.g., names of childhood friends, addresses, etc.). However, personal semantic memory on these measures have been found to load onto working memory measures, such as divided attention (Greene et al., 1995). Thus, it is possible that retrieving personal semantic information in the context of an episodic event description provides some form of executive support for retrieving semantic information, and as such, a significant deficit is not evident on the AI.
Neuropsychological testing revealed that the AD patients do exhibit some level of impairment of general semantic information. For instance, performance on the recognition of famous names was impaired for names encountered across the lifespan. Moreover, the AD patients were significantly impaired on semantic (Category) fluency. However, although performance was impaired relative to healthy controls on other aspects of semantic memory, such as confrontation naming of objects (e.g., on the Boston Naming Test), this group difference was not significant. This finding suggests that in this sample of AD patients there was some sparing of semantic knowledge, possibly enough to still enable the generation of external details when describing past and future events.
Overall, the present results, in conjunction with the findings of Murphy et al. (2008), are consistent with the idea that memory function in amnestic MCI is at an intermediary point between healthy aging and clinical AD (Petersen & Negash, 2008). While amnestic MCI patients exhibit significant declines in memory for internal episodic details, as do AD patients, it appears they can still rely on strategies also used by older adults -- such as overproduction of external details -- when describing past and future events. This pattern of performance likely reflects the extent of neuropathological changes within the core network in patients with MCI versus AD. Most patients with amnestic MCI show AD pathology mainly in the medial temporal lobes and anterior cingulate (e.g., Chetelat et al., 2002). Other regions of the core network engaged by remembering and imagining, such as lateral temporal regions, are largely spared in amnestic MCI. This pattern is in contrast to the pattern of AD neuropathology in patients with clinical AD, in which prefrontal, parietal, and lateral temporal cortices are also sites of amyloid deposition (Buckner et al., 2005). It is thought lateral temporal regions in particular support the retrieval of conceptual autobiographical information which may be incorporated into descriptions of past and future events (Schacter et al., 2007; Schacter, Addis, & Buckner, 2008). Thus it is not surprising that AD patients exhibit some semantic memory deficits, including being less able to generate external details. These findings do raise an interesting direction for future neuroimaging studies, to determine whether the medial aspects of the core network support the episodic component of future simulation while more lateral regions support the semantic component.
Correspondence between Past and Future Events
There was a striking similarity between performance on the past and future events tasks. Not only was the pattern of results (significant decline for internal details; non-significant decline for external details) evident across both the past and future tasks, there was strong correspondence across performance on the tasks. Specifically, there were positive correlations between the past and future internal (.60) and external (.64) AI scores, and these strong correlations were evident even when controlling for general cognitive decline (MMSE), phonemic (FAS) and semantic (Category) fluency. These results replicate the pattern of correlations between AI scores we previously found with young and older adults (Addis et al., 2008). Moreover, the correspondence across past and future is consistent with neuroimaging evidence indicating that remembering past events and simulating future events relies on the same core network (e.g., Addis et al., in press; Addis et al., 2007; Botzung et al., 2008; Hassabis, Kumaran, & Maguire, 2007; Okuda et al., 2003; Szpunar et al., 2007, for review, see Schacter, Addis, & Buckner, 2007, 2008).
Also consistent with previous findings, only the correlations between past and future scores were significant; correlations between past internal and external scores and between future internal and external scores were not significant (Addis et al., 2008). However, it is noted that the negative correlation between future internal and external scores approached significance when the covariates were included (r = -.299, p = .06). This finding suggests that when controlling for cognitive decline and fluency abilities, those subjects who generate more external details generate fewer internal details.
This is not to say that there are no differences across past and future events. Our initial ANOVA revealed a significant interaction of temporal direction and detail. This interaction reflected more internal details for past than future events, consistent with previous findings that representations of previously experienced events are more detailed than imagined events (Addis et al., in press; Addis et al., 2008; D'Argembeau & van der Linden, 2004; M. K. Johnson et al., 1988). This interaction also resulted from future events containing more external details than past events, perhaps indicating the individuals rely more heavily on access to semantic information in generating an event simulation because episodic details are less available. That this interaction was no longer significant once phonemic fluency abilities were accounted for suggests that phonemic fluency may play an important role in the production of external details for future events in particular.
Remote and Prospective Memory
In order to fully characterize the autobiographical memory deficits of the present sample of AD patients, we also had subjects complete the AI for remote events spanning five lifetime periods. While the AD group showed a significant deficit for generating internal and external details relative to older controls, this group difference was eliminated once gender, semantic (Category) fluency and phonemic (FAS) fluency abilities were accounted for. Moreover, the phonemic fluency covariate in this analysis was significant. This pattern of results suggests that, although phonemic fluency is related to the generation of external details on the past-future AI task, with respect for remote events, phonemic fluency is important for generating both external and internal details. This observation highlights the importance of accounting for fluency abilities when examining deficits in remote autobiographical memory in AD, particularly when using measures such as the AI, and likely autobiographical fluency (Dritschel et al., 1992). However, controlling for fluency abilities and gender did not alter the overall pattern of significant correlations between the internal and external detail scores for remote memory and the past-future AI task.
As mentioned briefly above, we assessed non-personal semantic memory function across the lifespan using the Famous Names three-alternative forced choice recognition task (Westmacott & Moscovitch, 2002). This test examines memory for names of famous individuals who entered popular culture in the US during different decades of the 20th century. Using this test, Westmacott and colleagues (Westmacott, Freedman, Black, Stokes, & Moscovitch, 2004) found that patients with AD exhibited a temporal gradient, with better recognition of names from more remote time periods. In the present study, we did find a significant group by time-period interaction. While both groups show enhanced recall of names from the 70s, the pattern across the other decades is broadly consistent with AD patients showing preservation of more remote semantic memory (e.g., names from the 40s) and controls showing better recognition of recent names (e.g., names from the 90s).
We also assessed the integrity of prospective memory in AD, using the Cambridge Behaviour Prospective Memory Test (Groot et al., 2002). This task revealed that in mild AD, prospective memory function is very much impaired irrespective of the cue used (e.g., to remember at a specified time or after a specified event occurs) or the time of information to be remembered (e.g., object or place information). This finding is consistent with other reports that prospective memory is impaired early in AD (e.g., Duchek et al., 2006; Huppert & Beardsall, 1993; Martins & Damasceno, 2008), and also in preclinical AD / MCI (Jones, Livner, & Backman, 2006; Troyer & Murphy, 2007).
In summary, the major finding of this study is that patients with AD exhibit deficits not only in remembering past events but also simulating future events. This impairment predominantly affects the ability to generate the episodic details comprising past and future events. Although there was some evidence of a reduction in the number of non-episodic details produced by AD patients, this was not significant. There was a tight correspondence between past and future events, with the number of internal details and the number of external details showing strong correlations between past and future events. These findings converge with previous neuropsychological and neuroimaging work, and further supports the idea advanced in the constructive episodic simulation hypothesis that being able to remember one's past is crucial for imagining one's future.
Acknowledgements
We thank Lucia Lee for transcribing interviews and Lara Markstein, Regina Musicaro and Ling Pan for scoring interviews. We also thank anonymous reviewers for their helpful comments. This research was supported by National Institute on Aging (NIA) grant AG08441, awarded to DLS and R01 AG025815 and P30 AG13846 to AEB. This material is also the result of work supported with resources and the use of facilities at the Bedford VA Hospital in Bedford, MA.
Appendix A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Pan L, Vu M-A, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2008.10.026. in press. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. Effects of detail and temporal distance of past and future events on the engagement of a common neural network. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- Addis DR, Tippett LJ. Memory of myself: autobiographical memory and identity in Alzheimer's disease. Memory. 2004;12:56–74. doi: 10.1080/09658210244000423. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychological Science. 2008;19:33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Wilson B. Amnesia, autobiographical memory and confabulation. In: Rubin DC, editor. Autobiographical Memory. Cambridge University Press; New York, N.Y.: 1986. pp. 225–252. [Google Scholar]
- Botzung A, Dankova E, Manning L. Experiencing past and future personal events: Functional neuroimaging evidence on the neural bases of mental time travel. Brain and Cognition. 2008;66:202–212. doi: 10.1016/j.bandc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer's Disease: evidence for a relationship between default activity, amyloid, and memory. The Journal of Neuroscience. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson AE, Simons JS, Waring JD, Sullivan AL, Hussion T, Schacter DL. Memory for the September 11, 2001, terrorist attacks one year later in patients with Alzheimer's disease, patients with mild cognitive impairment, and healthy older adults. Cortex. 2007;43:875–888. doi: 10.1016/s0010-9452(08)70687-7. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature Reviews Neuroscience. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Jacques P., St Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13:1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- Clark JM, Paivio A. Extensions of the Paivio, Yuille, and Madigan (1968) norms. Behavior Research Methods, Instruments and Computers. 2004;36:371–383. doi: 10.3758/bf03195584. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Schiffman H. Frequency of episodic memories as a function of their age. Bulletin of the Psychonomic Society. 1974;4:517–518. [Google Scholar]
- D'Argembeau A, van der Linden M. Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: Influence of valence and temporal distance. Consciousness & Cognition. 2004;13:844–858. doi: 10.1016/j.concog.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Anaki D, Ciaramelli E, Cohn M, Kim AS, Murphy KJ, et al. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–1755. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrego MF, Sabe L, Cuerva AG, Kuzis G, Tiberti C, Boller F, et al. Remote memory in Alzheimer's disease. Journal of Neuropsychiatry & Clinical Neurosciences. 1999;11:490–497. doi: 10.1176/jnp.11.4.490. [DOI] [PubMed] [Google Scholar]
- Dritschel BH, Williams JM, Baddeley AD, Nimmo-Smith I. Autobiographical fluency: a method for the study of personal memory. Memory and Cognition. 1992;20:133–140. doi: 10.3758/bf03197162. [DOI] [PubMed] [Google Scholar]
- Duchek J, Balota DA, Cortese M. Prospective memory and apoliprotein E in healthy aging and early stage Alzheimer's disease. Neuropsychology. 2006;20:633–644. doi: 10.1037/0894-4105.20.6.633. [DOI] [PubMed] [Google Scholar]
- Fivush R, Buckner JP. Constructing gender and identity through autobiographical narratives. In: Fivush R, Haden C, editors. Autobiographical Memory and the Construction of a Narrative Self: Developmental and Cultural Perspectives. Erlbaum; Hillsdale, NJ: 2003. pp. 149–168. [Google Scholar]
- Fletcher P, Frith C, Baker SC, Shallice T, Frackowiak RS, Dolan R. The mind's eye - precuneus activation in memory-related imagery. Neuroimage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SL, McHugh PR. `Mini-Mental State', practical methods for grading the cognitive state of patients for clinicians. Journal of Psychiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Almonti S, Di Betta AM, Silveri MC. Retrograde amnesia in a patient with retrosplenial tumour. Neurocase. 1998;4:519–526. [Google Scholar]
- Gilboa A, Ramirez J, Köhler S, Westmacott R, Black SE, Moscovitch M. Retrieval of autobiographical memory in Alzheimer's Disease: Relation to volumes of medial temporal lobe and other structures. Hippocampus. 2005;15:535–550. doi: 10.1002/hipo.20090. [DOI] [PubMed] [Google Scholar]
- Graham KS, Hodges JR. Differentiating the roles of the hippocampal complex and the neocortex in long-term memory storage: evidence from the study of semantic dementia and Alzheimer's disease. Neuropsychology. 1997;11:77–89. doi: 10.1037//0894-4105.11.1.77. [DOI] [PubMed] [Google Scholar]
- Greene JDW, Hodges JR. The fractionation of remote memory: Evidence from a longitudinal study of dementia of Alzheimer type. Brain. 1996;119:129–142. doi: 10.1093/brain/119.1.129. [DOI] [PubMed] [Google Scholar]
- Greene JDW, Hodges JR, Baddeley AD. Autobiographical memory and executive function in early dementia of Alzheimer type. Neuropsychologia. 1995;33:1647–1670. doi: 10.1016/0028-3932(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Groot YCT, Wilson BA, Evans J, Watson P. Prospective memory functioning in people with and without brain injury. Journal of the International Neuropsychological Society. 2002;8:645–654. doi: 10.1017/s1355617702801321. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. The Journal of Neuroscience. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends in Cognitive Sciences. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Watson RT, Day A, Valenstein E, Hammond E, et al. Frontal hypermetabolism and thalamic hypometabolism in a patient with abnormal orienting and retrosplenial amnesia. Neuropsychologia. 1990;28:161–169. doi: 10.1016/0028-3932(90)90098-9. [DOI] [PubMed] [Google Scholar]
- Hou CE, Miller BL, Kramer JH. Patterns of autobiographical memory loss in dementia. International Journal of Geriatric Psychiatry. 2005;20:809–815. doi: 10.1002/gps.1361. [DOI] [PubMed] [Google Scholar]
- Huppert FA, Beardsall L. Prospective memory impairment as an early indicator of dementia. Journal of Clinical & Experimental Neuropsychology. 1993;15:805–821. doi: 10.1080/01688639308402597. [DOI] [PubMed] [Google Scholar]
- Ivanoiu A, Cooper JM, Shanks MF, Venneri A. Patterns of impairment in autobiographical memory in the degenerative dementias constrain models of memory. Neuropsychologia. 2006;44:1936–1955. doi: 10.1016/j.neuropsychologia.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Foley MA, Suengas AG, Raye CL. Phenomenal characteristics of memories for perceived and imagined autobiographical events. Journal of Experimental Psychology: General. 1988;117:371–376. [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Jones S, Livner A, Backman L. Patterns of prospective and retrospective memory impairment in preclinical Alzheimer's disease. Neuropsychology. 2006;20:144–152. doi: 10.1037/0894-4105.20.2.144. [DOI] [PubMed] [Google Scholar]
- Khachaturian ZS. Diagnosis of Alzheimer's disease. Archives of Neurology. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Memory and temporal experience: The effects of episodic memory loss on an amnesic patient's ability to remember the past and imagine the future. Social Cognition. 2002;20:353–379. [Google Scholar]
- Kopelman MD. Disorders of memory. Brain. 2002;125:2152–2190. doi: 10.1093/brain/awf229. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Remote and autobiographical memory, temporal context memory and frontal atrophy in Korsakoff and Alzheimer patients. Neuropsychologia. 1989;27:437–460. doi: 10.1016/0028-3932(89)90050-x. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Wilson B, Baddeley A. The Autobiographical Memory Interview. Thames Valley Test Company; Suffolk, U.K.: 1990. [Google Scholar]
- Kopelman MD, Wilson BA, Baddeley AD. The Autobiographical Memory Interview: a new assessment of autobiographical and personal semantic memory in amnesic patients. Journal of Clinical & Experimental Neuropsychology. 1989;11:724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;23:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyhe T, Muller S, Milian M, Eschweiler GW, Saur R. Impairment of episodic and semantic autobiographical memory in patients with mild cognitive impairment and early Alzheimer's disease. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2009.04.018. in press. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Maddock RJ. Retrosplenial cortex and emotion: New insights from functional imaging studies of the human brain. Trends in Neurosciences. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Martins SP, Damasceno BP. Prospective and retrospective memory in mild Alzheimer's disease. Arquivos de Neuro-Psiquiatria. 2008;66:318–322. doi: 10.1590/s0004-282x2008000300006. [DOI] [PubMed] [Google Scholar]
- McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, et al. Visual association pathology in preclinical Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2006;65:621–630. doi: 10.1097/00005072-200606000-00010. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of AD: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on AD. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Murphy KJ, Troyer AK, Levine B, Moscovitch M. Episodic, but not semantic, autobiographical memory is reduced in amnestic mild cognitive impairment. Neuropsychologia. 2008;46:3116–3123. doi: 10.1016/j.neuropsychologia.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain - A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- National Institute on Aging Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiology of Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, et al. Thinking of the future and the past: The roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Yamadori A, Kawashima R, Tsukiura T, Fukatsu R, et al. Participation of the prefrontal cortices in prospective memory: evidence from a PET study in humans. Neuroscience Letters. 1998;253:127–130. doi: 10.1016/s0304-3940(98)00628-4. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectrums. 2008;13:45–53. doi: 10.1017/s1092852900016151. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Belliard S, Matuszewski V, Lalevee C, De la Sayette V, et al. Autobiographical memory and autonoetic consciousness: triple dissociation in neurodegenerative diseases. Brain. 2003;126:2203–2219. doi: 10.1093/brain/awg222. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Köhler S, Schacter DL, Moscovitch M, Westmacott R, Black SE, et al. The case of K.C.: contributions of a memory-impaired person to memory theory. Neuropsychologia. 2005;43:989–1021. doi: 10.1016/j.neuropsychologia.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rudge P, Warrington EK. Selective impairment of memory and visual perception in splenial tumours. Brain. 1991;114:349–360. doi: 10.1093/brain/114.1.349. [DOI] [PubMed] [Google Scholar]
- Sagar HJ, Cohen NJ, Sullivan EV, Corkin S, Growdon JH. Remote memory function in Alzheimer's disease and Parkinson's disease. Brain. 1988;111:185–206. doi: 10.1093/brain/111.1.185. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2007a;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The ghosts of past and future. Nature. 2007b;445:27. doi: 10.1038/445027a. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2009;364:1245–1253. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. The prospective brain: Remembering the past to imagine the future. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. Annals of the New York Academy of Sciences. 2008 doi: 10.1196/annals.1440.001. in press. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Cortical midline involvement in autobiographical memory. Neuroimage. 2009;44:1188–1200. doi: 10.1016/j.neuroimage.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer AK, Murphy KJ. Memory for intentions in amnestic mild cognitive impairment: Time- and event-based prospective memory. Journal of the International Neuropsychological Society. 2007;13:365–369. doi: 10.1017/S1355617707070452. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychologist. 1985;25:1–12. [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110:1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Westmacott R, Freedman M, Black SE, Stokes KA, Moscovitch M. Temporally graded semantic memory loss in Alzheimer's disease: Cross-sectional and longitudinal studies. Cognitive Neuropsychology. 2004;21:353–378. doi: 10.1080/02643290342000375. [DOI] [PubMed] [Google Scholar]
- Westmacott R, Moscovitch M. Temporally graded semantic memory loss in amnesia and semantic dementia: Further evidence for opposite gradients. Cognitive Neuropsychology. 2002;19:135–163. doi: 10.1080/02643290143000123. [DOI] [PubMed] [Google Scholar]
- Wheeler M, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ellis NC, Tyers C, Healy H, Rose G, MacLeod AK. The specificity of autobiographical memory and imageability of the future. Memory and Cognition. 1996;24:116–125. doi: 10.3758/bf03197278. [DOI] [PubMed] [Google Scholar]