Abstract
The ability of Toll-like Receptor (TLR) agonists to promote adaptive immune responses is attributed to their ability to robustly activate innate immunity. However, it has been observed that, for adjuvants in actual use in research and vaccination, TLR signaling is dispensable for generating humoral immunity. Here, we examined the role of the TLR5 and MyD88 in promoting innate and humoral immunity to flagellin using a prime/boost immunization regimen. We observed that eliminating TLR5 greatly reduced flagellin-induced cytokine production, except for IL-18, and ablated DC maturation but did not significantly impact flagellin’s ability to promote humoral immunity. Elimination MyD88, which will ablate signaling through TLR and IL-1β/IL-18 generated by nod-like receptors (NLR), reduced, but did not eliminate flagellin’s promotion of humoral immunity. In contrast, loss of the innate immune receptor for profilin-like protein (PLP), TLR11, greatly reduced the ability of PLP to elicit humoral immunity. Together, these results indicate that, firstly, the degree of innate immune activation induced by TLR agonists may be in great excess of that needed to promote humoral immunity and, secondly, there is considerable redundancy in mechanisms that promote the humoral immune response upon innate immune recognition of flagellin. Thus, it should be possible to design innate immune activators that are highly effective vaccine adjuvants yet avoid the adverse events associated with systemic TLR activation.
Keywords: TLR11, profilin-like protein, adjuvant, Ipaf, TLR4, cytokines, dendritic cells
INTRODUCTION
Toll-like receptor (TLR)-mediated recognition of structural components of microbial pathogens plays a key role in the initiation of host defense. Specifically, these germline-encoded pattern recognition receptors (PRR) recognize and initiate immune responses to a wide variety of microbial patterns, including those of bacteria, viruses, parasites, nucleic acids, carbohydrates, and lipids. [1–4]. TLR agonists are classified into three broad categories: nucleic acids (TLRs 3, 7, 8, 9), lipids/lipopeptides (TLRs 4, 1/2, 2/6), and protein (TLRs 5, 11). Of particular importance to this study, TLR5 recognizes the bacterial protein flagellin [5], in its soluble/monomeric form, while TLR11 recognizes profilin-like protein (PLP), made by T. gondii [6]. Activation of most TLRs, including TLR5 and TLR11, by their cognate ligands results in rapid nuclear translocation of the transcription factor NF-κB and, consequently, synthesis and secretion of a panel of proinflammatory cytokines. Another class of PRR thought to play an important role in innate immunity is the Nod-like receptors (NLR), which are expressed in the cytosol. Of particular relevance to this study, 2 NLR proteins, Ipaf and Naip5, have been reported to signal in response to flagellin that attains an intracellular location [7–10]. In contrast to TLRs, the primary consequence of Ipaf signaling is not to induce transcription or protein synthesis but rather to activate caspace-1, which results in inflammasome-mediated processing/secretion of pro IL-1β and IL-18 to their mature bioactive forms [11]. All TLRs, except TLR3, signal, at least in part, via the myeloid differentiation primary-response protein 88 (MyD88). Consequently, MyD88-deficient mice have been a very useful tool in investigating the roles of TLR signaling in numerous processes. However, MyD88 is also required for signaling by the IL-1 and IL-18 receptors. As these cytokines important components of NLR signaling, MyD88-deficient mice have deficiencies in both NLR and TLR plays a key role in function.
PRR signaling is thought to play a key role in both the primary immune clearance of pathogens and in promoting development of protective responses to prevent against future encounters of similar pathogens [12]. Such ability of TLR-mediated signaling to promote adaptive immunity has led to development of approaches utilizing TLR agonists as vaccine adjuvants. TLR agonists currently being developed for use as vaccine adjuvants include monophosporyl lipid A (MPL), CpG ODN, and single stranded RNA/imidazoquinolins, which are ligands of TLR4, TLR9, and TLR7/8, respectively [13–17]. There has recently been particular interest in the TLR5 agonist, bacterial flagellin, in part, because being a protein, it can be readily formulated as a fusion-protein with a variety of antigens and, furthermore, is amenable to being used as a DNA-based adjuvant. Flagellin expression with bacterial or viral antigens leads to innate immune functions, potent humoral immunity, and protection against challenge with viruses including influenza A and West Nile virus, bacterial infection such as Y. pestis [18–23]. In addition to promoting adaptive immunity to other antigens, flagellin is also a major target of adaptive immunity. Specifically, upon infection with Salmonella species, flagellin is a dominant antigen for CD4+ and CD8+ T cell activation and humoral immunity [24–27]. Flagellin is also a major target of adaptive immunity in Crohn’s disease [28]. Purified flagellin has been reported to induce Th1 and Th2 responses, and IgG and IgM to itself and to other antigens indicative of the wide range of adaptive immune responses it promotes [29–31]. While early studies on flagellin’s elicitation of Ig indicated it was a thymus-independent antigen, particularly when in a polymerized state (i.e. flagella), recent studies performed in T-cell deficient mice indicate that generation of Ig to flagellin monomers or polymerized flagella are absolutely T-cell dependent [32].
A substantial amount of research has been devoted in recent years to studying the means by which TLR signaling can promote adaptive immunity. Such studies have led to the view that function of adjuvants in general and TLR-based adjuvants in particular results from their ability to induce cytokine production and DC maturation. However, this notion has been challenged recently in that Nemazee and colleagues reported that a classical adjuvant, namely complete Freund’s adjuvant, and an adjuvant that includes a synthetic TLR4 agonist, namely Ribi/MPL, both appeared to function efficiently in mice deficient in the two major TLR signaling adaptor proteins MyD88 and TRIF [33].
The goal of this study was to examine the role of innate immunity in the promotion of humoral immunity using purified flagellin and mice lacking the molecules that mediate TLR- and/or NLR- mediated recognition of this molecule. We demonstrate a critical role for both TLR5 and MyD88 in mediating innate immunity to flagellin, particularly acute systemic cytokine production and dendritic cell activation, but, surprisingly, loss of TLR5 did not have a substantial impact on the humoral response to purified flagellin or co-administered ovalbumin. MyD88-deficiency reduced, but did not eliminate flagellin’s promotion of humoral immunity. These results suggest that it may be possible to develop vaccine adjuvants that effectively promote humoral immunity without inducing robust activation of innate immunity.
RESULTS
Generation of flagellin-specific antibodies in mice lacking TLR5 or MyD88
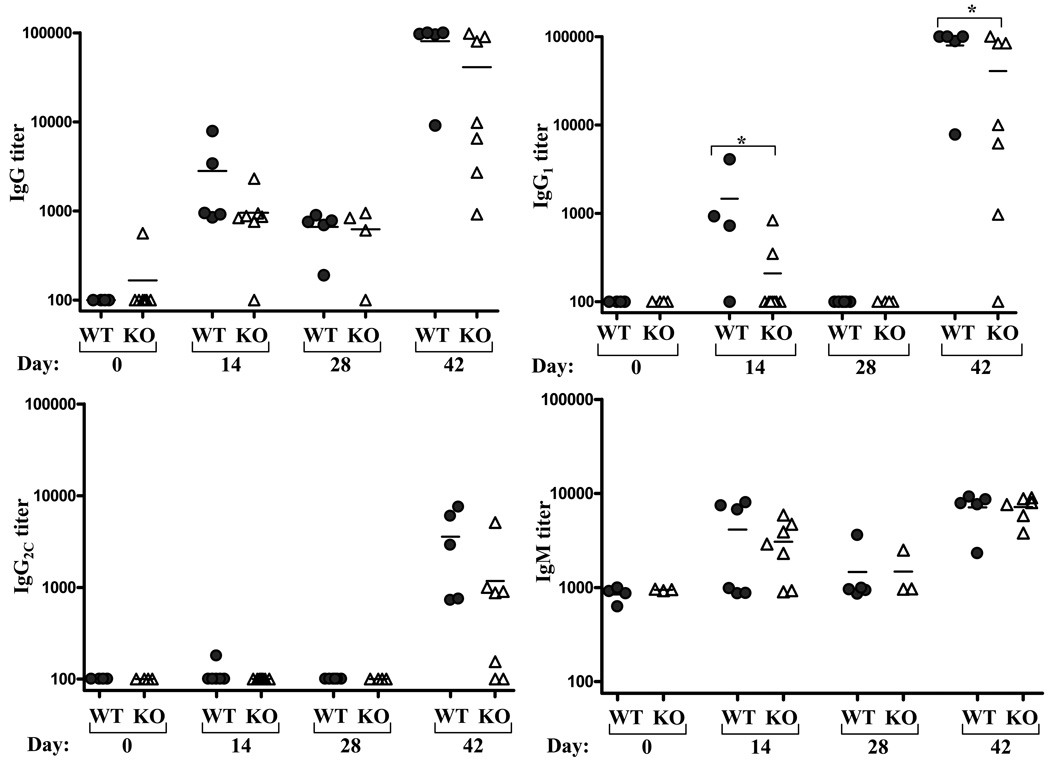
A substantial body of research supports the notion that TLRs are important mediators of innate immunity and, consequently, key initiators of adaptive immunity. However, as flagellin is one of only two known well-defined protein TLR ligands, and minimal work has been done with their receptor knockout mice to date, little is known regarding the nature of the adaptive immune response to a T cell-dependent protein TLR ligand. Given that, at least when measured in a primary response, MyD88−/− mice do not make antibody responses to purified flagellin, and failed to make antibodies in vaccine models in which flagellin serves as an adjuvant [34], we expected that TLR5−/− mice would lack antibody responsiveness to flagellin. To test this notion TLR5−/− mice and WT littermates were injected with 50 µg of purified flagellin on day 0 and day 28, and serum isolated on days 0, 14, 28, and 42. Serum was assayed for levels of flagellin-specific total IgG, IgG1, IgM, IgG2c, and IgG3. In WT mice, flagellin elicited a strong IgG1 response while IgG2c was only detected following the boost injection. Analysis of sera on days 31 and 35 revealed the antibody response increased with greater rapidity following the boost injection (data not shown) suggesting the response following the second inoculation is that of a classic memory-type response. Relative increases in IgM were only moderate. Surprisingly, relative to WT mice, TLR5−/− mice exhibited only modest reductions of flagellin-specific Ig titers, which only achieved statistical significance in the case of IgG1 (figure 1). Flagellin-specific IgG3 was undetectable in all mouse groups (data not shown).
Figure 1. Induction of flagellin-specific humoral immunity in TLR5-deficient mice.
Mice lacking TLR5 and WT littermates were immunized with 50µg flagellin on days 0 and 28. Flagellin-specific Ig was assayed on indicated days by ELISA. Each point represents the serum antibody titer to flagellin for an individual mouse. Grey circles, wild type controls (WT); open triangles TLR5−/− (KO). *-indicates statistically significant difference between WT and KO (p<0.05).
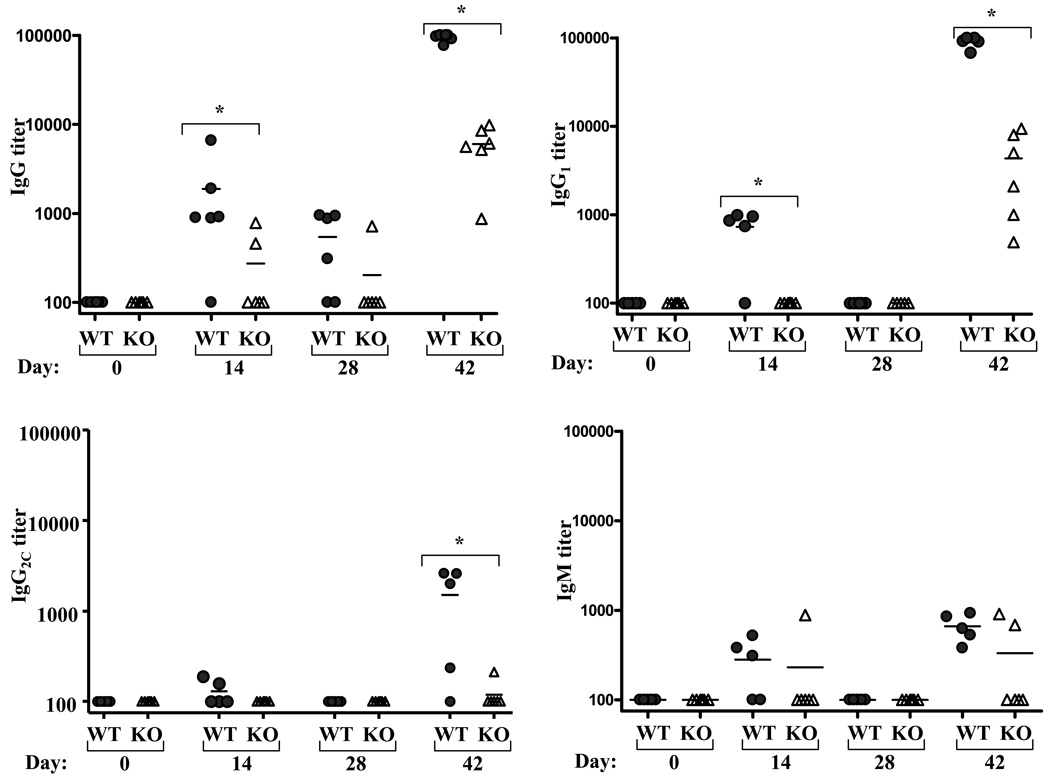
In light of previous reports from our lab and others that demonstrated a requirement for MyD88 in flagellin-induced antibody responses [32, 34], we next examined the role of MyD88 in the prime/boost immunization regimen described above. In accordance with previous findings, flagellin-specific Ig was markedly reduced in MyD88−/− relative to similarly-treated WT mice with most MyD88 failing to exhibit a detectable titer in response to primary injection (figure 2). Total IgG and IgG1 were readily detectable following flagellin in MyD88−/− mice given a second injection of flagellin, yet, unlike TLR5−/− mice, flagellin-specific IgG and IgG1 titers of MyD88−/− mice were markedly less than those of wild type mice. In further contrast to TLR5−/−, MyD88 were unable to produce significant levels of anti-flagellin IgG2c. In addition, while flagellin-specific IgM was elevated by day 7 following boost, their titers dropped by day 14 while those of WT mice remained elevated. These results suggest that there exists a MyD88-dependent, TLR5-independent means by which flagellin promotes the humoral immune response to itself. However, these results indicate that neither TLR5 nor MyD88 are absolutely critical for an adaptive immune response to flagellin.
Figure 2. Induction of flagellin-specific humoral immunity in MyD88-deficient mice.
Mice lacking MyD88 and WT control mice were immunized with 50µg flagellin on days 0 and 28. Flagellin-specific Ig was assayed on indicated days by ELISA. Each point represents the serum antibody titer to flagellin for an individual mouse. Grey circles, wild type controls (WT); open triangles MyD88−/− (KO). *-indicates statistically significant difference between WT and KO (p<0.05).
Flagellin’s elicitation of Ig in TLR5-deficient mice not due to activation of other TLRs
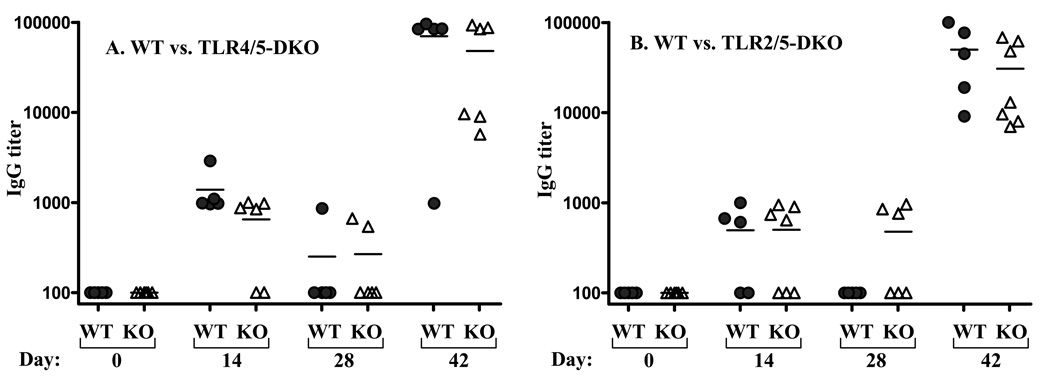
One potential explanation of why flagellin elicited antibodies efficiently in mice lacking TLR5 was that, despite it being HPLC-purified and undergoing rigorous in vitro and in vivo purity tests [30, 35], our flagellin contained a contaminant that was activating another TLR. We first considered the possibility that our flagellin contained LPS, which is known to contaminate many purified proteins. While our previous demonstration that flagellin’s ability to elicit antibodies was not diminished in C3H/HeJ mice, which lack functional TLR4, suggest LPS-induced signaling was not involved in this process [32], it seemed possible that our flagellin may still contain a minute amount of LPS that serves as a “back-up” innate immune activator in TLR5−/− mice. To address this possibility, we generated TLR5−/−/TLR4−/− mice and evaluated their ability to generate antibodies in response to the prime/boost inoculation regimen described above. We observed that TLR5−/−/TLR4−/− mice generated antibodies to flagellin as robustly as identically-treated WT mice (figure 3). Thus, TLR4-mediated LPS-induced signaling has no role in the ability of our flagellin preparation to elicit antibodies. Furthermore, these results rule out the possibility that flagellin itself is recognized by TLR4 - a possibility suggested by the report that TLR5/TLR4 heteromeric complexes mediated flagellin-induced induce nitric oxide production by macrophages [36].
Figure 3. Induction of flagellin-specific humoral immunity in lacking TLRs 4and 5 or 2 and 5.
Mice lacking indicated TLRs and WT littermates were immunized with 50µg flagellin on days 0 and 28. Flagellin-specific Ig was assayed on indicated days by ELISA. Each point represents the serum antibody titer to flagellin for an individual mouse. A. Grey circles, wild type littermates (WT); open triangles TLR4−/−,TLR5−/− (DKO). B. Grey circles, wild type littermates (WT); open triangles TLR2−/−,TLR5−/− (DKO).*-indicates statistically significant difference between WT and KO (p<0.05).
We next generated mice lacking both TLR2 and TLR5 to address the possibilities that either flagellin can signal through TLR2 and/or that our flagellin preparation might contain TLR2 agonists such as lipopeptide, which is present in all gram negative bacteria. Furthermore, as TLR2 can dimerize with TLR1 and TLR6, and is necessary for their function, studying responses of TLR5−/−/TLR2−/− mice also addresses the possibility that our flagellin might have ligands for these TLRs. Analogous to the case for TLR4, flagellin elicited self-specific antibodies equally well in WT mice vs. mice lacking both TLR5 and TLR2 (figure 3B). Lastly because TLR11, the receptor for the T. gondii protein “profilin-like protein” [6] is the TLR most related to TLR5 and has been suggested as a possible flagellin receptor [37], we examined the role of this TLR in generation of flagellin-specific Ig. We observed that, in response to flagellin treatment, TLR11−/− mice generated antibodies to a similar extent as WT mice (data not shown) arguing against this possibility.
Flagellin’s adjuvanticity is maintained in the absence of TLR5
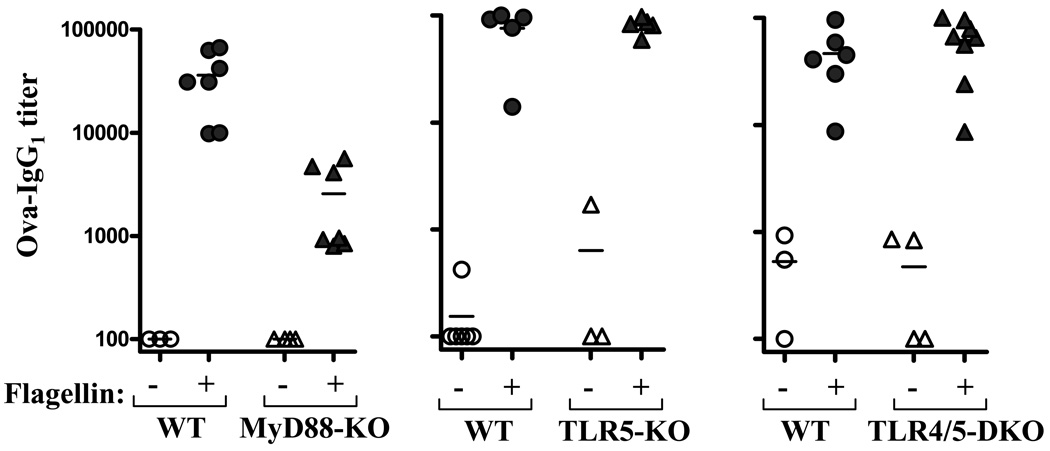
Next, we examined the role of TLRs in flagellin’s adjuvant function. Mice were injected with 50 µg of ovalbumin alone, or ovalbumin mixed with 50 µg of flagellin, on day 0 and 28. This regimen is similar to that which Didier-Laurent et. al. observed results in a strong MyD88-dependent IgG1 response to ovalbumin following the second injection of ovalbumin/flagellin [34]. In accordance with this study, we observed that MyD88−/− mice given ovalbumin/flagellin exhibited anti-ovalbumin antibody titers that were significantly less than similarly-treated WT mice (figure 4). However, anti-ovalbumin titers in MyD88−/− mice given ovalbumin/flagellin were still substantially higher than MyD88−/− mice given ovalbumin alone. Thus, absence of MyD88 significantly reduced but did not eliminate flagellin’s ability to promote humoral immunity. In contrast to MyD88−/− mice, flagellin promoted antibodies to ovalbumin as efficiently in TLR5−/− mice as in WT littermates indicating that TLR5 is not required for flagellin to promote the humoral immune response to a bystander antigen. We repeated this experiment using only 10 µg of flagellin. This smaller dose of flagellin also robustly promoted generation of anti-ovalbumin antibodies in both WT and TLR5−/− mice (antibody titers to mice given ovalbumin only and ovalbumin/flagellin were, respectively, WT: 513 ± 38 and 26,654 ± 3842 vs. TLR5−/−: 546 ± 146 and 34,676 ± 8632, NS). Flagellin also efficiently promoted antibody responses to ovalbumin in mice lacking both TLR5 and TLR4 arguing against the notion that a contaminating TLR4 ligand plays an important role in the responses we observed in TLR5-deficient mice.
Figure 4. Ovalbumin-specific humoral immunity in TLR-deficient mice inoculated with ovalbumin and flagellin.
Mice (WT and indicated TLR-deficient mice) were immunized with ovalbumin alone or co-injected with 50µg ovalbumin and 50µg flagellin on days 0 and 28. Ovalbumin-specific IgG1 was assayed on day 42 by ELISA. Each point represents the serum IgG1 titer to ovalbumin for an individual mouse. Circles, wild type controls (WT) triangles, indicated TLR-deficient mice. A “+” sign indicates mice were given flagellin in addition to ovalbumin. *-indicates statistically significant difference between WT and KO (p<0.05).
We further addressed the possibility of contaminants via biochemical approaches. First, our preparation of flagellin was exposed to trypsin immobilized on agarose beads. Such treatment will cleave proteins such as flagellin into peptides that lack ability to activate TLR5, but does not alter the bioactivity of numerous other TLR agonists (e.g. LPS, CpG DNA, PGN). The ability of such trypsin-treated flagellin to serve as an adjuvant was assessed by measuring anti-ovalbumin antibodies in mice injected with ovalbumin alone or ovalbumin + trypsin-treated flagellin. We observed that trypsin treatment eliminated all of flagellin’s ability to promote antibodies to ovalbumin (mice getting ovalbumin only or ovalbumin/trypsinsized flagellin had ant-ova titers below 100-limit of detection). Thus, our preparation of flagellin does not contain any non-proteinaceous materials with adjuvant function. Lastly, we examined whether a mock preparation of flagellin had any adjuvant function. Specifically, we performed all the steps we utilize to generate purified flagellin but did so using supernatant from the isogenic flagellin-deficient Salmonella mutant Sl3201 (fliC−/fljB−), and isolated the same HPLC fractions that would have contained flagellin if WT Salmonella had been utilized. Mice were inoculated with ovalbumin or ovalbumin + mock flagellin (amount equivalent to above experiments using actual flagellin) and ovalbumin-specific antibodies measured. Such mock flagellin had no ability to promote antibodies to ovalbumin (data not shown), further indicating that the adjuvant activity observed herein is indeed that of flagellin rather than a contaminant.
Flagellin-induced cytokine production is mainly TLR5-dependent
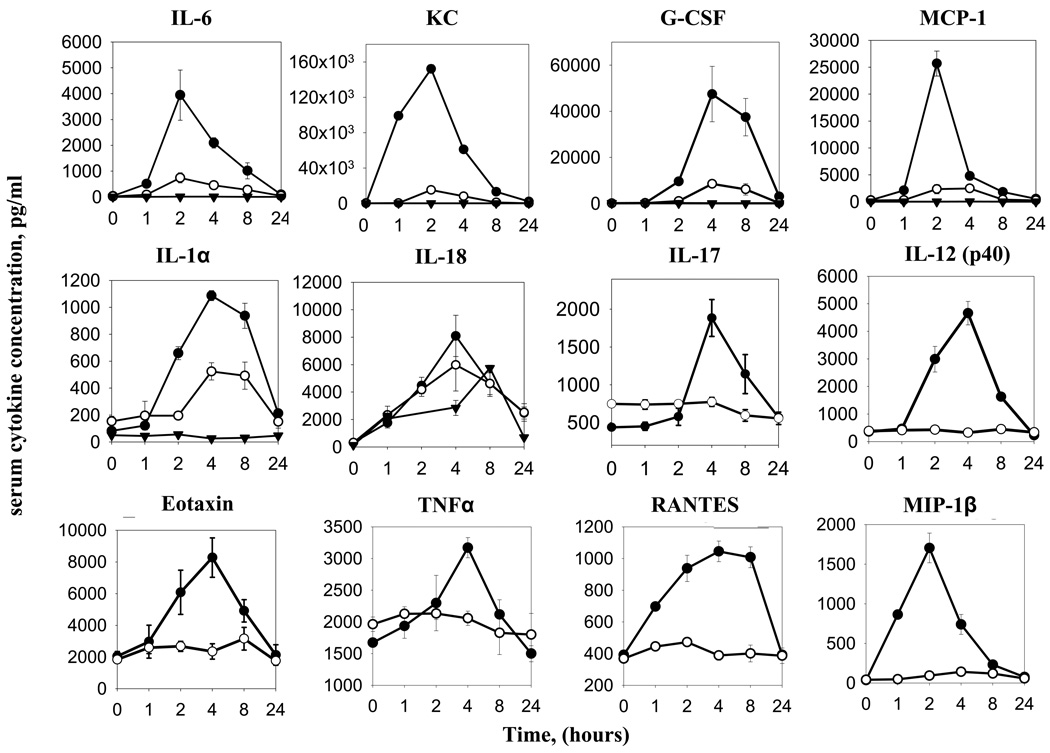
To understand how flagellin might maintain full adjuvant function in the absence of TLR5 and partial adjuvant function in absence of MyD88, we examined early events in the innate immune response that are thought to shape adaptive immunity. In general, TLR signaling is thought to promote adaptive immunity by mediating secretion of cytokines and cell surface expression of co-stimulatory molecules on APC [38]. Thus, we examined how loss of TLR5 and MyD88 affected these events in response to flagellin. First, we examined the role TLR5 plays in systemic innate immune responses to purified flagellin. Though previous studies have reported flagellin-induced cytokine production is dependent on TLR5 and MyD88 in vivo [5, 39–43], we broadly investigated if the absence of either of these two molecules leads to a complete loss of cytokines, a delay in cytokine production, or merely a weakened response over a 24 hour period. Flagellin-injected TLR5−/−, MyD88−/−, and WT control mice were bled at 0, 1, 2, 4, 8 or 24 h and their serum were analyzed using a multiplex cytokine assay. Loss of TLR5 resulted in a complete absence of IL-12 (p40), TNFα, IL-17, RANTES, MIP1β, and Eotaxin, cytokines normally produced in wild type mice (figure 5). Flagellin did induce statistically significant levels of IL-6, MCP-1, and G-CSF in TLR5−/− mice with kinetics similar to that seen in WT littermates, although at greatly reduced levels relative to these WT mice. In contrast, loss of TLR5 only moderately reduced serum elevations in IL-1α and had no significant impact upon induction of IL-18. Mice lacking MyD88 exhibited a complete loss of elevation in all measured serum cytokines in response to flagellin except for IL-18, which was significantly induced albeit at a moderately reduced level relative to WT mice. Flagellin did not induce detectable elevations in serum IL-1β, in WT nor KO mice, consistent with our previous finding that flagellin’s elicitation of specific antibodies procedes normally in IL-1R-deficient mice [44].
Figure 5. Serum cytokine levels in TLR5−/− and MyD88−/− given flagellin.
TLR5−/−, TLR5+/+, and MyD88−/− mice were bled before any treatment (0 hour), then immunized I.P. once with 50µg flagellin and bled 1, 2, 4, 8, or 24 hours later. Closed circles, TLR5+/+ mice; open circles, TLR5−/− mice, open triangles, MyD88−/− mice. Note MyD88−/− mice sera were analyzed only for IL-6, KC, G-CSF, MCP-1, IL-1α, and IL-18. Dqata are means ± SEM, n=4–5 mice.
Cytosolic flagellin receptors are not required for humoral immune responses to flagellin
Recent findings demonstrate that Ipaf and Birc1e/Naip5, members of the cytosolic nucleotide-binding oligomerization domain (NOD)-leucine-rich repeat (LRR) family of proteins, are required for cytosolic flagellin to activate caspase-1 and induce IL-1β secretion in Salmonella-infected macrophages and Legionella [7, 8, 45]. That flagellin induced IL-18 production in both TLR5−/− and MyD88−/− mice is in accordance with these findings although this pathway is not thought to be capable of recognizing “free”, or extracellular, flagellin [7, 8]. Moreover, this observation suggested that IL-18 might drive adaptive immunity in these TLR-deficient mice. To assess the role IL-18 may play in flagellin’s promotion of adaptive immunity, IL-18−/− mice were injected with flagellin and flagellin-specific Ig measured 14 d later. No significant difference in flagellin-specific IgG titer was noted in IL-18−/− mice compared to wild-type controls, indicating that IL-18 is not required promote flagellin-specific humoral immunity (data not shown).
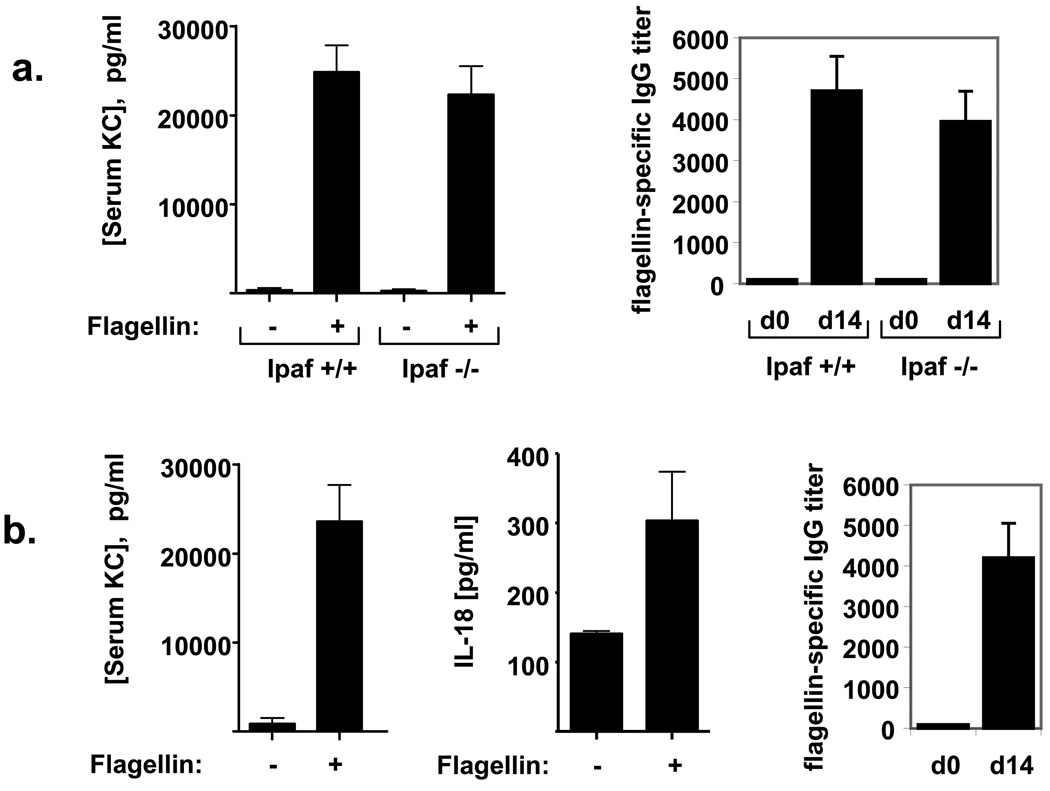
We next examined the contribution of Ipaf to flagellin-induced immune responses in vivo. While our approach utilized purified flagellin and thus lacks the active cytosolic entry provided by the Salmonella Pathogenicity Island 1 Type III secretion system, it was conceivable that flagellin may enter a cell’s cytosol via an unknown mechanism and induce an immune response [46]. Thus, we examined how loss of Ipaf impacted flagellin-induced cytokine production and elicitation of antibodies. KC was measured to serve as a general indicator of a TLR5-mediated cytokine while IL-18 was measured as a potential indicator of activation of NLR-mediated detection of flagellin. We observed that Ipaf−/− mice exhibited WT-levels of acute serum KC and flagellin-specific-IgG, suggesting that this TLR5-independent pathway is not required for these flagellin-induced immune responses, at least in TLR5-competent mice (figure 6A). Our efforts to discern whether the flagellin-induced IL-18 production exhibited by TLR5−/− and MyD88−/− mice was mediated by Ipaf did not prove informative in that we were unable to detect any increase in serum IL-18 in both IPaf−/− and their the wild type littermate controls mice (data not shown), possibly due to a difference in mouse background strain or housing conditions of these mice. Interestingly, Ipaf−/− mice did exhibit lower basal levels of serum IL-18 in comparison to their wild type littermate controls, suggesting some degree of Ipaf-dependence for IL-18 production.
Figure 6. Innate and humoral immune responses to flagellin in Ipaf−/− and A/J mice.
Mice were immunized once with 50µg flagellin/100µl PBS/mouse. (a) Ipaf−/− and wild type control mice. Serum KC levels at 4hr; flagellin-specific IgG titers on day 0 and day 14. (b) A/J (non-functional Naip5 allele) mice. Serum KC and IL-18 levels at 2hr; flagellin-specific IgG titers on day 0 and day 14. Data are means +/− SEM, n=5–6 mice.
Naip5 (Birc1e), another NLR, has been found to restrict intracellular replication of Legionella pneumophila in mouse macrophages [47]. An inability to detect intracellular flagellin in macrophages and mice containing a non-functional Naip5 allele (A/J mice) confers susceptibility to Legionella infection [10, 48]. We thus investigated if the intracellular flagellin signaling molecule Naip5 may have an effect on immune responses to flagellin. Although there is not an isogenic control strain, carrying functional Naip5, to which we could directly compare A/J mice, we observed that, relative to WT mice on the C57BL/6 and Balb/c backgrounds, A/J mice exhibited robust levels of acute serum KC acute and flagellin-specific IgG, suggesting that this cytosolic flagellin detector also does not impact humoral immunity to flagellin (figure 6B). Interestingly, A/J mice also exhibited elevations in serum IL-18 in response to flagellin. This result is in accordance with recent general findings that some aspects of Ipaf function, in particular caspase-1 activation, are independent of Naip5 [49, 50].
TLR5 and MyD88 are needed for in vivo APC activation
DC activation is considered a critical step in the initiation of adaptive immunity [51] and, indeed, flagellin can induce strong upregulation of co-stimulatory molecules on DC when administered in vivo [34, 37]. Such DC activation is commonly measured in vivo by administering a potential stimulus, isolating splenocytes, and measuring surface expression levels of CD80, CD86, or CD40 on CD11chi cells by flow cytometry. Thus, to understand how flagellin promotes adaptive immunity, we utilized this approach to examine how loss of TLR5 or MyD88 impacted upon the ability of flagellin to induce DC activation. In agreement with previous reports, these splenic DC from TLR5−/− and MyD88−/− mice did not upregulate /activation six hours after primary or secondary flagellin treatment (figure 7 and data not shown) [34, 40]. To address the possibility that DC activation might be merely delayed, we performed similar analysis 24h following flagellin treatment but again observed that CD11chi cells from TLR5−/− mice failed to upregulate these cell surface markers in response to flagellin (data not shown). Potent LPS-induced CD40, CD80 (B7.1), and CD86 (B7.2) upregulation demonstrated that our TLR5−/− mice retained the ability to respond normally to another TLR ligand (data not shown). Thus, flagellin’s promotion of humoral immunity in TLR5−/− and MyD88−/− mice occurred without detectable activation of splenic DC.
Figure 7. Effect of loss of TLR5 and MyD88 on flagellin induced activation of splenic DC.
Wild type, TLR5−/−, and MyD88−/− mice were injected once I.P. with 50µg flagellin/100µl PBS/mouse or 100µl PBS/mouse. After 6 hours splenocytes were collected, stained, and analyzed by flow cytometry. Histograms show expression levels of CD80, CD86, and CD40 on CD11chigh gated live cells. Filled gray area, PBS-treated mice; heavy line, flagellin-treated mice. These data are from an individual experiment utilizing spleens pooled from 3 mice per condition. The results are representative of 3 separate experiments that showed an identical patterns of results.
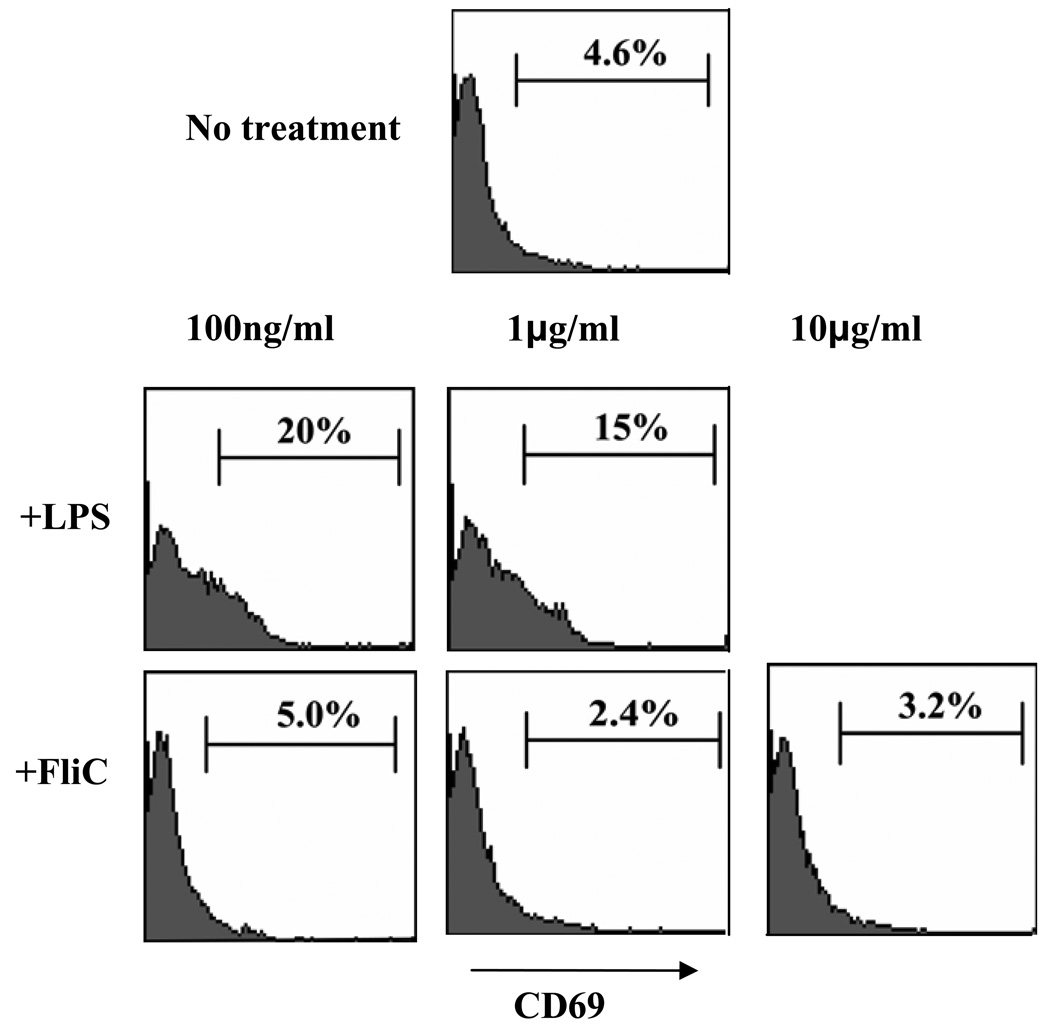
It has been reported that direct activation of MyD88 in B-cells is required to elicit robust humoral immunity in response to TLR agonists and that B-cells express TLR5, at least at the mRNA level [52]. Thus, we sought to determine if purified flagellin directly activates resting B cells ex vivo. Purified splenic B cells were incubated with flagellin (100ng/ml – 10µg/ml) or a positive control stimulus, LPS, which is known to activate these cells. B-cell activation was assessed by measuring surface expression of CD69. Neither low (1µg/ml) nor high (100µg/ml) concentrations of flagellin upregulated CD69 expression, while various doses of LPS were able to induce impressive upregulation of this molecule (figure 8). These results suggest that flagellin does not induce direct activation of B-cells and thus, direct activation of B-cells by flagellin is unlikely to play a role in flagellin’s promotion of humoral immunity.
Figure 8. Effect of flagellin on CD69 expression of splenic B cells ex vivo.
Purified resting splenic B cells were stimulated in culture at 1×106 cells/well with media or indicated concentrations of LPS or flagellin. After 24hr, cells were collected and analyzed by flow cytometry. Histograms show expression levels of CD69 on CD19+ live cells. The results are from a single experiments and representative of 3 parallel experiments that showed an identical patterns of results.
TLR11−/− mice do not generate profilin-like protein specific antibodies
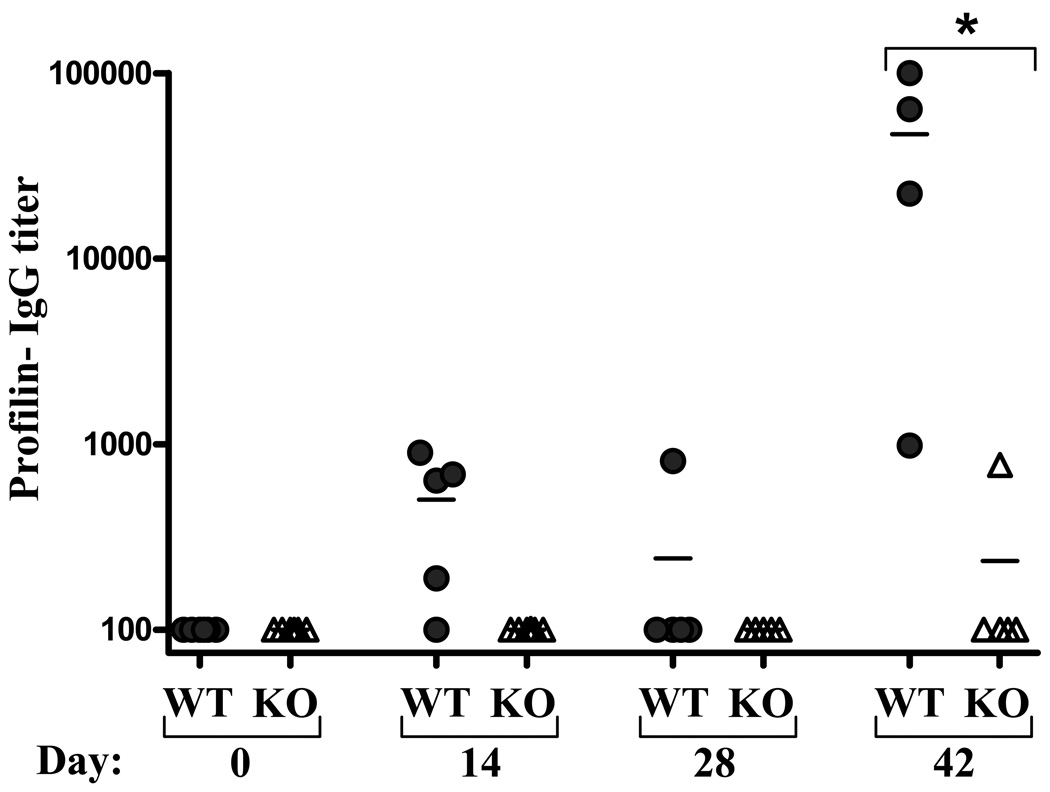
Profilin-like protein (PLP) is an immunodominant antigen of Toxoplasma gondii, which has been shown to be recognized by TLR11 [6, 53]. As PLP and TLR11 is the only other established protein ligand-TLR cognate pair, we investigated whether the relative unimportance of a TLR for generating specific antibodies to its purified protein ligand was a general phenomenon or was specific for flagellin/TLR5. Mice lacking TLR11 and WT control mice were administered a prime/boost regimen of PLP analogous to that utilized for flagellin. We observed that, in contrast to the TLR5/flagellin interaction, generation of PLP-specific antibodies was greatly diminished in TLR11−/− mice when assayed in response to both primary injection and 14 days after boosting (figure 9). Thus, PLP, appears to elicit humoral immunity via activation of a single non-redundant pathway of innate immunity. In contrast, flagellin may utilize multiple pathways of activating innate immunity so that even markedly reducing its ability to activate innate immunity will not severely attenuate its ability to promote humoral immune responses.
Figure 9. Induction of PLP-specific humoral immunity in TLR11-deficient mice.
Mice lacking TLR11 and WT littermates were immunized with 10µg T. gondii profiling-like protein (PLP) on days 0 and 28. PLP-specific Ig was assayed on indicated days by ELISA. Each point represents the serum antibody titer to PLP for an individual mouse. Grey circles, wild type controls (WT); open triangles TLR11−/− (KO). *-indicates statistically significant difference between WT and KO (p<0.05).
DISCUSSION
Nearly two decades ago, C.A. Janeway Jr. proposed a two-signal model for activation of adaptive immunity to non-self antigens. “Signal one” was defined as the interaction between a specific ligand and its antigen receptor, while “signal two” constituted host cell activation via a microbially-induced, antigen recognition-independent event [54]. This model provided a potential explanation for the well-established ability of bacterial products to promote adaptive immune responses. The discovery of toll-like receptors and the deciphering of how they signal in response to various ligands provided this model a molecular mechanism by which innate immunity controls adaptive immunity, namely by activating signaling events that induced expression of immunostimulatory/pro-inflammatory cytokines and surface co-stimulatory molecules on APC [55–57]. This model held that flagellin’s ability to promote adaptive immunity to itself and by-stander antigens was underlaid by its ability to activate TLR5 rather the long-held notion that flagellin was highly immunogenic due to its ability to polymerize and thus cross-link antigen receptors [30, 32]. However, herein we observed that loss of TLR5 did not have a substantial impact upon the ability of flagellin to promote the T-cell dependent antibody response to itself or a bystander antigen. This observation parallels the recent report by Nemazee and colleagues, that both a classic adjuvant based on mycobacterial extract and one based on a synthetic TLR4 agonist functioned independently of all known TLR signaling pathways [33]. Thus, at least in the case of humoral immunity, which is the basis of protection of most currently used vaccines, TLR signaling may not be as important as previously suggested. However, our conclusions go beyond simply supporting some aspects of their work. Rather, by measuring the extent to which loss of TLR5 and MyD88 impacted upon various aspects of the flagellin-induced innate response, our study allows for mechanistic assessment of how flagellin may be promoting adaptive immunity in the absence of TLR5.
The ability of flagellin to function as an adjuvant in TLR5-deficient mice occurred despite a substantial, but not complete, loss of cytokine induction and without detectable maturation of splenic DC. We utilized a variety of approaches to investigate whether flagellin’s bioactivity in TLR5-deficient mice was the result of a potential contaminant in our flagellin preparation but did not find evidence to support this possibility. Interestingly, mice lacking TLR5 and TLR4, which was our prime suspect to recognize any potential contaminant, appeared to respond slightly better to flagellin than mice lacking only TLR5. The reason for this difference may result from TLR5-deficient mice having basal alterations in gene expression that can be envisaged to influence subsequent immune responses to exogenous ligands [58]. Such basal alterations are not present in mice lacking both TLR4 and TLR5 thus potentially accounting for modestly greater antibody responses elicited by flagellin in TLR5−/−/TLR4−/− mice. While the experiments we have performed herein may not allow us to be certain that our flagellin does not contain some substance other than flagellin that can promote immune responses, regardless, we can conclude that the level of cytokine production and DC maturation induced by flagellin in WT mice is clearly in great excess of what is required to promote a humoral immune response. Yet, although differences in antibody responses between WT and TLR5−/− mice differed only modestly, that responses were modestly higher and more uniform in WT mice suggests that the robust cytokine/DC response of WT mice may increase the likelihood that individual mice will make a robust antibody response. This notion is consistent with clinical studies in which synthetic analogs of CpG DNA added to a hepatitis B vaccine markedly increased the rate of sero-conversion following primary inoculation while only moderately increasing titers following multiple inoculations [59].
Flagellin also functioned as an adjuvant in mice lacking MyD88 although to a significantly lesser extent than in WT mice. A potential explanation for this difference is that MyD88−/− mice are not only deficient in signaling by TLR5 but, because MyD88 is required for signaling in response to both IL-1β and IL-18, are effectively deficient in being able to ultimately respond to signals initiated via nod-like receptors (NLR) such as Ipaf, which function in large part by generating mature IL-1β and IL-18 via post-translational processing [7]. Thus, in the absence of TLR5 signaling, perhaps flagellin’s adjuvanticity is mediated by NLR-generated IL-18. Intriguingly, recent reports indicate NLR-mediated IL-18 generation is central the mechanism by which aluminum-based adjuvants function [60, 61]. Should the ability of flagellin to promote humoral immunity in TLR-deficient mice rely on IL-18 via a mechanism analogous to that used by Alum, it may explain why flagellin’s adjuvant function is reduced, but not entirely eliminated, in MyD88-null mice in that a portion of Alum’s adjuvant function is MyD88-independent but IL-18 dependent [62].
While the extent of the innate immune response induced by flagellin may be far in excess of what is needed to promote a humoral immune response, the above-described difference between TLR5 and MyD88 suggest that residual innate response observed in flagellin-treated TLR5−/− mice may indeed be limiting to this aspect of the adaptive immune response. This notion would predict that simultaneous elimination of TLR5, Ipaf, Naip5, and perhaps other yet to be defined flagellin receptors would completely ablate flagellin’s ability to function as an adjuvant. By this reasoning, it seems reasonable to speculate that the profoundly reduced ability of TLR11-null mice to generate antibodies to PLP reflects that mice lack additional significant means of innately recognizing this molecule. Such lack of redundancy could reflect that PLP is unique to select parasites while flagellin, in mediating motility, plays a central role in the function of many bacteria. Indeed, the evolutionary loss of functional TLR11 from humans argues that immune recognition of PLP may not be especially important.
Thus, in conclusion, we do not question Dr. Janeway’s proposal that innate immune recognition of conserved microbial patterns by germ-line encoded receptors is necessary for adaptive immune responses to microbes. Nor do we question the notion that TLRs are the front line class of such receptors. Rather, we propose a model with redundancy in which molecules with critical microbial function can be recognized by a series of germ-line encoded receptors. Some of these receptors may be viewed as “alternate recognition molecules” in that they promote low-level signals that may be hard to detect in vivo, yet such signals are immunologically highly significant in that they can promote adaptive immunity particularly in the case of recall responses. The robust signals generated by a product’s “primary” innate immune receptor may be more important for acute responses to microbes but may also assure rapid generation of primary adaptive responses, which could be important in clearance of a primary infection. These findings have important implications for vaccine design in that they show robust adaptive immune responses are achievable without potent TLR-mediated innate immune responses, which are often associated with adverse events. Consequently, perhaps targeting of non-TLR immune sensors may ultimately provide the safest means of generating robust adaptive immune responses and thus should be considered in vaccine development.
MATERIAL AND METHODS
Mice
TLR5−/− and MyD88−/− mice were generated as previously described [63, 64]. TLR5−/− mice used here were backcrossed eight times onto a C57BL/6J background, and wild type littermates used as controls. MyD88−/− mice on a C57BL/6J background were a gift of Melanie Sherman (Emory University, Atlanta, GA). These mice were bred at Emory University. C57BL/6J, B6.129-Tlr2tm1Kir/J (Tlr2−/−), B6.129P2-Il18tm1Aki/J (Il18−/−), and A/J (non-functional Naip5) were obtained from The Jackson Laboratory (Bar Harbor, ME). Except for A/J mice, these mice are all on the C57BL/6 background. TLR11−/− mice are on a mixed background. These mice were generated S. Ghosh (Yale University, New Haven, CT) [65] and provided to us by Doug Golenbock (UMass Med. ctr. Worcester, MA). Ipaf−/− mice were generated as previously described [7]. TLR5−/−TLR4−/− mice and controls were generated by first crossing TLR5−/− N7 males and C57BL/10ScNJ (Tlr4 gene deletion) females (The Jackson Laboratory), then crossing the heterozygous F1 generation. Confirmation of double deficient and wild type littermates was done by genotyping the F2 generation for Tlr5 and for Tlr4. TLR5−/−TLR2−/− mice and wild type controls were generated by first crossing TLR5−/− N7 females with TLR2−/− males (Jackson Laboratory), then crossing the heterozygous F1 generation. Genotyping of the F2 generation for Tlr5 and Tlr2 identified double-deficient mice and wild type littermates. All mice with the exception of Ipaf−/− and Ipaf+/+ were housed at Emory University. Animal studies were approved by the Institutional Animal Care and Use Committee of Emory University.
Injections
Native flagellin was isolated from Salmonella typhimurium and its purity verified as previously described [30, 35]. Flagellin injections were given intraperitoneally (I.P.) at 50µg/100µl PBS/mouse. Ovalbumin (grade IV, Sigma, St. Louis, MO) was given I.P. at 50µg/100µl PBS/mouse. LPS from Salmonella typhimurium (Sigma) was given at 25µg/100µl PBS/mouse. Profilin-like protein was isolated from Toxoplasma gondii as previously described [6]. Profilin-like protein was administered I.P. at 10µg/100µl PBS//mouse. Mouse serum was isolated from blood obtained from puncture of the submandibular pouch. Sera were stored at −20°C or −80°C until use.
Dendritic cell isolation and flow cytometry
Splenic CD11c-hi cells, herein referred to as splenic DC, were isolated from 3 pooled spleens of mice injected I.P. 6 hr or 24hr previously with 50µg flagellin/100µl PBS/mouse, 25µg LPS/100µl PBS/mouse or 100µl PBS/mouse. Spleens were digested with 1mg/ml collagenase type IV (Worthington Biochemical Corp., Lakewood, NJ) in complete DMEM + 2% FBS for 30 minutes at 37°C. Single cell suspensions were treated with ammonium chloride to lyse red blood cells, washed twice in PBS supplemented with 2mM EDTA and 1%FBS and mesh filtered before staining for flow cytometry. Flow cytometric stains were performed for 30 minutes at 4°C in 2.4G2 hybridoma culture supernatant (anti-I-Ak/FcγRIII/II) to block nonspecific binding. Splenocytes were stained with anti-mouse APC-CD11c, FITC-CD80 (B7-1), FITC-CD86 (B7-2), and FITC-CD40 (eBioscience). Data were collected on a FACSCalibur cytometer (Becton Dickinson, Franklin Lakes, NJ) and analyzed using CellQuest software (BD Biosciences, San Jose, CA).
Cytokine analysis
TLR5−/− and MyD88−/− mouse sera were evaluated for cytokines using a custom multiplex cytokine assay kit according to the manufacturer’s specifications (Bio-Rad, Hercules, CA). Analysis was performed on a Luminex 100 machine running Bioplex Manager version 4.0 (Bio-Rad). Additional cytokine analyses were obtained using mouse IL-6, mouse KC, and mouse TNFα cytokine ELISA kits (R&D Systems, Minneapolis, MN).
Antibody analysis
Antibody ELISA plates (MP Biomedicals, Solon, OH) were coated with 2µg OVA or 100ng Salmonella typhimurium FliC/well in 0.1M NaHCO3 buffer (pH 9.6) overnight at 4°C. Plates were washed in ELISA wash buffer (HBSS, 0.5% goat serum, 0.1% Tween-20) and serum applied in various dilutions for one hour at 37°C. After three additional washes, HRP-conjugated sheep anti-mouse IgG (GE Healthcare, Piscataway, NJ), HRP-conjugated goat anti-mouse IgG1 (Caltag Laboratories, Burlingame, CA), HRP-conjugated goat anti-mouse IgG2c (Southern Biotech, Birmingham, AL), or donkey biotinylated-anti-mouse IgM (Jackson Immunoresearch, West Grove, PA) were added 1:1000, 1:2000, 1:2000, and 1:1000, respectively, in wash buffer for one hour at 37°C. Anti-IgM plates were further incubated for 30 minutes with 100µg/ml streptavidin-HRP (Jackson Immunoresearch) at 37°C. Plates were developed using tetramethylbenzidine (TMB) substrate (Kierkegaard and Perry Laboratories—KPL, Gaithersburg, MD), stopped using H2SO4, and read at 450nm on a SpectraMax Plus plate reader (Molecular Devices, Sunnyvale, CA). Antibody titers were defined by the reciprocal of the serum dilution equivalent to three times ELISA plate background (coated, no sample).
Statistical analysis
Significance was determined using both Student’s t-test and Mann-Whitney test (GraphPad Prism software, San Diego, CA), which agreed in every instance. Differences were noted as significant when p<0.05.
ACKNOWLEDGEMENTS
We thank Ifor Williams, Joshy Jacob, Brian Evavold, Melanie Sherman, Alan Sher (NIH), and the late Dr. Kirk Ziegler for helpful advice on these studies. We also thank Kristy Szretter and Matam Vijay-Kumar for technical assistance. This work was supported by an NIH (DK061417) to A.T. Gewirtz. This research utilized NIH Digestive Disease Research and Development Center (DDRDC) that is supported by NIH grant DK064399.
Abbreviations
- TLR
Toll-like receptor
- LPS
Lipopolysacharride
- DC
Dendritic cell
References
- 1.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 2.Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 4.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 6.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 7.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 8.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 9.Coers J, Vance RE, Fontana MF, Dietrich WF. Restriction of Legionella pneumophila growth in macrophages requires the concerted action of cytokine and Naip5/Ipaf signalling pathways. Cell Microbiol. 2007;9:2344–2357. doi: 10.1111/j.1462-5822.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 10.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Nunez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. doi: 10.1111/j.1462-5822.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 13.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher S, Steffy K, Averett D. Masked oral prodrugs of toll-like receptor 7 agonists: a new approach for the treatment of infectious disease. Curr Opin Investig Drugs. 2006;7:702–708. [PubMed] [Google Scholar]
- 15.Hammerbeck DM, Burleson GR, Schuller CJ, Vasilakos JP, Tomai M, Egging E, Cochran FR, Woulfe S, Miller RL. Administration of a dual toll-like receptor 7 and toll-like receptor 8 agonist protects against influenza in rats. Antiviral Res. 2007;73:1–11. doi: 10.1016/j.antiviral.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Kline JN, Krieg AM, Waldschmidt TJ, Ballas ZK, Jain V, Businga TR. CpG oligodeoxynucleotides do not require TH1 cytokines to prevent eosinophilic airway inflammation in a murine model of asthma. J Allergy Clin Immunol. 1999;104:1258–1264. doi: 10.1016/s0091-6749(99)70022-9. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz K, Storni T, Manolova V, Didierlaurent A, Sirard JC, Rothlisberger P, Bachmann MF. Role of Toll-like receptors in costimulating cytotoxic T cell responses. Eur J Immunol. 2003;33:1465–1470. doi: 10.1002/eji.200323919. [DOI] [PubMed] [Google Scholar]
- 18.Honko AN, Sriranganathan N, Lees CJ, Mizel SB. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect Immun. 2006;74:1113–1120. doi: 10.1128/IAI.74.2.1113-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS, Lee JJ, Song HC, Kim JM, Choy HE, Chung SS, Kweon MN, Rhee JH. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun. 2006;74:694–702. doi: 10.1128/IAI.74.1.694-702.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Applequist SE, Rollman E, Wareing MD, Liden M, Rozell B, Hinkula J, Ljunggren HG. Activation of innate immunity, inflammation, and potentiation of DNA vaccination through mammalian expression of the TLR5 agonist flagellin. J Immunol. 2005;175:3882–3891. doi: 10.4049/jimmunol.175.6.3882. [DOI] [PubMed] [Google Scholar]
- 22.Huleatt JW, Nakaar V, Desai P, Huang Y, Hewit D, Jacobs A, Tang J, McDonald W, Song L, Evans RK, Umlauf S, Tussey L, Powell TJ. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine. 2008;26:201–214. doi: 10.1016/j.vaccine.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 23.McDonald WF, Huleatt JW, Foellmer HG, Hewitt D, Tang J, Desai P, Price A, Jacobs A, Takahashi VN, Huang Y, Nakaar V, Alexopoulou L, Fikrig E, Powell TJ. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J Infect Dis. 2007;195:1607–1617. doi: 10.1086/517613. [DOI] [PubMed] [Google Scholar]
- 24.Bergman MA, Cummings LA, Alaniz RC, Mayeda L, Fellnerova I, Cookson BT. CD4+-T-cell responses generated during murine Salmonella enterica serovar Typhimurium infection are directed towards multiple epitopes within the natural antigen FliC. Infect Immun. 2005;73:7226–7235. doi: 10.1128/IAI.73.11.7226-7235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McSorley SJ, Cookson BT, Jenkins MK. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol. 2000;164:986–993. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 26.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infect Immun. 2000;68:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salerno-Goncalves R, Wyant TL, Pasetti MF, Fernandez-Vina M, Tacket CO, Levine MM, Sztein MB. Concomitant induction of CD4+ and CD8+ T cell responses in volunteers immunized with Salmonella enterica serovar typhi strain CVD 908-htrA. J Immunol. 2003;170:2734–2741. doi: 10.4049/jimmunol.170.5.2734. [DOI] [PubMed] [Google Scholar]
- 28.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunningham AF, Khan M, Ball J, Toellner KM, Serre K, Mohr E, MacLennan IC. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur J Immunol. 2004;34:2986–2995. doi: 10.1002/eji.200425403. [DOI] [PubMed] [Google Scholar]
- 30.McSorley SJ, Ehst BD, Yu Y, Gewirtz AT. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J Immunol. 2002;169:3914–3919. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]
- 31.Strindelius L, Filler M, Sjoholm I. Mucosal immunization with purified flagellin from Salmonella induces systemic and mucosal immune responses in C3H/HeJ mice. Vaccine. 2004;22:3797–3808. doi: 10.1016/j.vaccine.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Sanders CJ, Moore DA, 3rd, Williams IR, Gewirtz AT. Humoral immune response to flagellin requires T cells and activation of innate immunity. J Immunol. 2006;177:2810–2818. doi: 10.4049/jimmunol.177.5.2810. [DOI] [PubMed] [Google Scholar]
- 33.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Didierlaurent A, Ferrero I, Otten LA, Dubois B, Reinhardt M, Carlsen H, Blomhoff R, Akira S, Kraehenbuhl JP, Sirard JC. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J Immunol. 2004;172:6922–6930. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- 35.Gewirtz AT, Simon PO, Jr, Schmitt CK, Taylor LJ, Hagedorn CH, O'Brien AD, Neish AS, Madara JL. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest. 2001;107:99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizel SB, Honko AN, Moors MA, Smith PS, West AP. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J Immunol. 2003;170:6217–6223. doi: 10.4049/jimmunol.170.12.6217. [DOI] [PubMed] [Google Scholar]
- 37.Tsujimoto H, Uchida T, Efron PA, Scumpia PO, Verma A, Matsumoto T, Tschoeke SK, Ungaro RF, Ono S, Seki S, Clare-Salzler MJ, Baker HV, Mochizuki H, Ramphal R, Moldawer LL. Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J Leukoc Biol. 2005;78:888–897. doi: 10.1189/jlb.0105051. [DOI] [PubMed] [Google Scholar]
- 38.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol. 2001;1:209–219. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 39.Moors MA, Li L, Mizel SB. Activation of interleukin-1 receptor-associated kinase by gram-negative flagellin. Infect Immun. 2001;69:4424–4429. doi: 10.1128/IAI.69.7.4424-4429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galan JE, Flavell RA, Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen-Nissen E, Hawn TR, Smith KD, Nachman A, Lampano AE, Uematsu S, Akira S, Aderem A. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J Immunol. 2007;178:4717–4720. doi: 10.4049/jimmunol.178.8.4717. [DOI] [PubMed] [Google Scholar]
- 42.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 43.Weinmann AS, Mitchell DM, Sanjabi S, Bradley MN, Hoffmann A, Liou HC, Smale ST. Nucleosome remodeling at the IL-12 p40 promoter is a TLR-dependent, Rel-independent event. Nat Immunol. 2001;2:51–57. doi: 10.1038/83168. [DOI] [PubMed] [Google Scholar]
- 44.Sanders CJ, Moore DA, 3rd, Williams IR, Gewirtz AT. Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. J Immunol. 2008;180:7184–7192. doi: 10.4049/jimmunol.180.11.7184. [DOI] [PubMed] [Google Scholar]
- 45.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 46.Sun YH, Rolan HG, Tsolis RM. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J Biol Chem. 2007;282:33897–33901. doi: 10.1074/jbc.C700181200. [DOI] [PubMed] [Google Scholar]
- 47.Diez E, Lee SH, Gauthier S, Yaraghi Z, Tremblay M, Vidal S, Gros P. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat Genet. 2003;33:55–60. doi: 10.1038/ng1065. [DOI] [PubMed] [Google Scholar]
- 48.Wright EK, Goodart SA, Growney JD, Hadinoto V, Endrizzi MG, Long EM, Sadigh K, Abney AL, Bernstein-Hanley I, Dietrich WF. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr Biol. 2003;13:27–36. doi: 10.1016/s0960-9822(02)01359-3. [DOI] [PubMed] [Google Scholar]
- 49.Lamkanfi M, Amer A, Kanneganti TD, Munoz-Planillo R, Chen G, Vandenabeele P, Fortier A, Gros P, Nunez G. The Nod-like receptor family member Naip5/Birc1e restricts Legionella pneumophila growth independently of caspase-1 activation. J Immunol. 2007;178:8022–8027. doi: 10.4049/jimmunol.178.12.8022. [DOI] [PubMed] [Google Scholar]
- 50.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, Monack DM, Tsolis RM, Vance RE. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 52.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 53.Yarovinsky F, Kanzler H, Hieny S, Coffman RL, Sher A. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+ T cell response. Immunity. 2006;25:655–664. doi: 10.1016/j.immuni.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Medzhitov R, Janeway CA., Jr On the semantics of immune recognition. Res Immunol. 1996;147:208–214. doi: 10.1016/0923-2494(96)87222-1. [DOI] [PubMed] [Google Scholar]
- 56.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 57.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 58.Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, Cameron DW, Heathcote J. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24:693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 60.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 63.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, Hemmi H, Coban C, Kawai T, Ishii KJ, Takeuchi O, Miyasaka M, Takeda K, Akira S. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 64.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 65.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]