Abstract
Scurfy (Sf) mice lack CD4+Foxp3+ regulatory T cells (Treg) and develop fatal multi-organ inflammation (MOI) mediated by CD4+ T cells. Introducing Il2-/- gene into Sf mice (Sf.Il2-/-) inhibited inflammation in skin and lung. As a major integrin receptor for the organs, we compared CD103 expression on the CD4+ T cells of B6, Il2-/-, Sf and Sf.Il2-/- mice. CD103+CD4+ T cells but not CD8+ T cells or CD11c+ dendritic cells were significantly up-regulated only in Sf mice, indicating Il2-/- dominantly and specifically inhibited CD103 up-regulation in Sf CD4+ T cells. In addition, Treg CD103 expression was not reduced in Il2-/- mice. Introducing CD103-/- into Sf mice inhibited inflammation in skin and lung as compared with age-matched Sf mice but they died around 7 wks old with inflammation developed in skin, lungs and colon, demonstrating fatal MOI induced by CD103-independent mechanism. Transfer of Sf CD103+CD4+ T cells induced MOI more rapidly than CD103-CD4+ T cells, indicating the presence of CD103-dependent mechanism for inflammation. In vitro stimulation with anti-CD3+anti-CD28 beads confirmed that CD103 induction in the CD4+Foxp3- T cells in Il2-/- and Sf.Il2-/- is defective and cannot be restored by rIL-2 or rIL-15. The data indicate that IL-2 is required for optimal CD103 induction on CD4+ T cells in Sf mice and this effect contributes to inflammation in an organ-specific manner. IL-2 also has additional roles because the protection of skin and lung inflammation in Sf.Il2-/- but not Sf.CD103-/- mice is life-long and Sf.Il2-/- mice have longer lifespan than Sf.CD103-/- mice.
Keywords: Regulatory T cells, Integrin, Organ Inflammation, Autoimmunity
Introduction
Thymocyte differentiation and selection impart distinct functions with different surface marker phenotypes into different T cell populations. In the CD4+ T cell compartment, two subsets are generated: a CD25- set that responds to Ag in the host and a CD25+ set that suppresses these Ag-specific responses (reviewed in Refs. 1-3). Many studies suggest that autoreactive T cells with moderate affinities against self-Ag could escape negative selection and exit to the periphery (4-7). These autoreactive T cells in the periphery are suppressed by the CD4+CD25+ regulatory T cells (Treg)3 (1-3). In the absence of IL-2 such as the case of Il2-/- mice, Treg in the thymus are significantly reduced but Treg in the periphery are compensated by IL-15/IL-15R signal (8, 9). The CD4+CD25+ Treg also express the transcription factor Foxp3, and Foxp3 expression in CD4+ T cells offers a more stringent definition for the “naturally occurring” Treg (10-12). Thymic generation of Treg is critically dependent on the Foxp3 gene which is linked to chromosome X (13, 14). Scurfy (Sf) mice, which bear a defective Foxp3 gene and totally lack Treg, invariably develop fatal MOI diseases within 4 wks after birth. In newborn boys, Foxp3 mutation also resulted in fatal IPEX (immune-dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome (15).
Sf mice begin to develop MOI at 9-d old and soon afterward severe inflammation in skin, lung, and tail and moderate inflammation in the liver follow. In contrast, Il2-/- mice have a significantly longer lifespan of 6-25 wks and the most often reported organ inflammation is colitis which usually begins after weaning and progresses thereafter (17). These differences could be explained by the relatively dispensable nature of IL-2, but not Foxp3, in the generation and maintenance of Treg. However, IL-2 is also critically important for the expansion of CD4+Foxp3- T cells and their effector functions that are involved in inflammation and tissue damage (18-21). Given the complexity of immune system regulation, novel roles of IL-2 remain to be uncovered. Thus, lacking these mechanisms in Il2-/- mice could also explain why they displayed significantly reduced inflammation responses as well.
To determine the roles of Treg and IL-2 in the control of MOI, we introduced the Il2-/- mutation into Sf mice. These mice, despite totally lacking Treg, still lived significantly longer than Sf mice. Introducing the Il2-/- gene into Sf mice did not reduce lymphoproliferation nor FasL (CD178) expression (22). Rather, it controlled the MOI in an organ-specific manner such that the pattern resembled that of Il2-/- mice. Most notably, it prevented the inflammation in the skin and tail and greatly reduced inflammation in the lung but the liver inflammation and colitis associated with Il2-/- remained (22). However, how this “organ-specific” regulation of inflammation beyond the Treg checkpoint took place remains unanswered (22).
The αE(CD103)β7 integrin of lymphocytes is a cell surface receptor that interacts with E-cadherin on epithelial cells (23-25), resulting in signal transduction and long stay of CD103+ T cells in non-lymphoid tissues such as skin, lung and gastrointestinal organs that express E-cadherin (26-28). In normal mice, CD103 is highly expressed on CD8+ T cells and intestinal epithelial lymphocytes (IEL) are highly enriched for CD103+CD8+ T cells (29-31). Other lymphoid cells that express CD103 include Treg, dendritic cells, and γδ T cells (32-34). In contrast to CD8+ T cells, approximately 45% of CD4+ intestinal T cells and a small fraction (4-7%) of the CD4+ T cells in the LN are CD103+ (30, 35). Relatively little is known regarding the regulation of CD103 expression. TGF-β1 alone appears sufficient to up-regulate CD103 expression by increasing αε mRNA levels (35-37). Runx3 transcription factor has been shown to increase CD103 expression and to suppress CD4 expression during CD4+CD8+ thymocyte transition into CD8+ T cells (38).
Because CD4+ T cells from Sf mice transferred MOI to Rag1-/- recipients (39), we hypothesize that the uncontrolled T cell activation in Sf mice up-regulates CD103 expression on CD4+ T cells and enables them to travel to E-cadherin+ target organs to induce inflammation. We further propose that IL-2 is a critical component in the up-regulation of CD103 on these inflammation-inducing T cells, thus explaining the inhibition of skin, lung and tail inflammation in Il2-/- and Sf.Il2-/- mice. Our hypotheses predict an up-regulation of CD103+CD4+ T cells in Sf and the long-lived Sf.Faslpr/lpr but not Il2-/- and Sf.Il2-/- mice. In addition, introducing CD103-/- mutation into Sf mice should inhibit inflammation in those organs that use E-cadherin to attract the inflammation-inducing CD103+CD4+ T cells. These hypotheses, predictions, and some novel findings are presented in this study.
Materials and Methods
Mice
C57BL/6 (B6), B6.Faslpr/lpr, B6.Il2+/-, B6.129S2(C)-Itgαεtm1Cmp/J (CD103-/-), B6.Cg-Foxp3sf/x/J, and B6.129S7-Rag1tm/Mom/J (Rag1-/-) mice were obtained from the Jackson Laboratories, Bar Harbor, ME. B6.Il2-/- mice were obtained by breeding B6.Il2+/- mice as previously described (20). B6.Cg-Foxp3sf/x/J mice were bred with male B6 mice to produce Sf mice (Foxp3sf/Y). Mice (Sf.Il2-/-) carrying both Il2-/- and Foxp3sf/Y genes and mice (Sf.Faslpr/lpr) carrying both Faslpr/lpr and Foxp3sf/Y genes were generated as previously described (22). Female Foxp3sf/x/J mice were crossed with male CD103-/- mice to generate Foxp3sf/xCD103+/- female. These mice were then backcrossed with CD103-/- male to generate Foxp3sf/yCD103-/- (Sf.CD103-/-) mice and Foxp3sf/xCD103-/- female for further breeding. Presence of the Il2-/-, Faslpr/lpr, integrin αε and Foxp3sf mutation was confirmed by PCR as detailed in the Jackson Laboratory's website. Mice were examined twice weekly for clinical signs of diseases including manifestation of skin inflammation, prolapse, body weight loss, and wasting etc. All animal experiments were approved by the Animal Care and Use Committee of the University of Virginia.
Flow cytometry
Axillary, brachial, inguinal, cervical and facial LN from sex- and age-matched B6, Il2-/-, Sf, and Sf.Il2-/- mice were isolated, pooled, and single cell suspensions were prepared in PBS (21). Leukocytes in the lungs and liver were prepared by digestion with collagenase type-IA (Sigma-Aldrich), followed by filtration through nylon mesh and Ficoll-Hypaque gradient centrifugation. Similar procedure was used to isolate leukocytes in the skin by digesting the skin that covers the areas of head and neck with collagenase type-1A (2 mg/ml). Lymphocytes from the small intestines, intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) were isolated as follows. The intestines were flushed with sterile PBS and Peyer's patches were removed. The intestines were cut in to 0.5 cm pieces and digested with collagenase type-IA (2 mg/ml) and DNase I (10μg/ml) at 37°C with shaking at 150 rpm for 30 m in Hank's Balanced Salt Solution (Mediatech) supplemented with 2% FCS, 2 mM DTT and 2 mM EDTA. The cell suspension was filtered through a 100 mesh Nylon filter (BD Bioscience) and the lymphocytes were harvested by centrifugation through a 45–70% discontinuous Percoll gradient. Cells were suspended in 100 μl of PBS solution (containing 4 mg of bovine serum albumin and 1 μg of anti-FcR mAb 2.4G2) and incubated with 0.2 μg of various fluorescent antibodies for 30 minutes at 4°C. FITC, PE, or PE-Cy5 conjugated anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-CD11c (N418), anti-MHC class II (M5/114.15.2), anti-CD25 (PC61), anti-CD103 (2E7), CD49d (R1-2), CD29 (HMb1-1), CCR6 (140706), CCR9 (CW1-2), CCR10 (2G12; Biolegend), CXCR3 (CXCR3-173) and Integrin α4β7 (DATK-32) mAb were obtained from BD Biosciences or eBioscience. Staining for CD4+FoxP3+ T cells was carried out using the commercial kit from eBioscience. At least 104 stained cells were analyzed using a FACScan equipped with CellQuest (BD Biosciences). Post acquisition analyses were carried out using FlowJo™ software (Tree Star, Inc, OR).
Cell Purification and in vitro stimulation
CD4+ T cells were purified using the CD4+ T cell negative purification kit (Miltenyi Biotec). The CD4+CD103+ T cells were purified by using PE-labeled anti-CD103 mAb and anti-PE beads (Miltenyi Biotec) according to the manufacturer's protocols. Purified CD4+ T cells (106) or un-fractionated LN cells (2×106) from age and sex-matched B6, Il2-/-, Sf and Sf.Il2-/- were cultured with anti-CD3/anti-CD28 beads (Invitrogen) at a cell/bead ratio of 1 in a 48-w culture plate for 72 h. IL-2 (30 IU/ml), IL-15 (100 ng/ml) and TGF-β1 (2.5 ng/ml), obtained from Peprotech, were added either alone or in combination during activation.
Adoptive transfer
LN cells (15×106) from Sf.Il2-/-, Sf.Faslpr/lpr or Sf.CD103-/- mice were injected I.V. into adult Rag1-/- male mice. Histological examination was conducted at 4-7 wks after transfer on H&E-stained sections of various organs.
Histology
Tissues/Organs from age-matched males of various strains were fixed with 10% neutral buffered formalin (Fisher Scientific) and sections of paraffin-embedded tissue were stained with H&E. Tissues/Organs examined included skin, ear, lung, colon, tail and liver.
Results
Sf but not Il2-/- and Sf.Il2-/- mice display severe inflammation in skin, tail and lung
We have recently reported a detailed comparison of inflammation among various organs of Sf, Il2-/- and Sf.Il2-/- mice (22). The major organs that displayed severe inflammation in Sf but not Il2-/- and Sf.Il2-/- mice were skin, tail and lung whereas all mice were found to have a moderate level of inflammation in the liver. On the other hand, colitis was observed in Il2-/- and Sf.Il2-/- that lived beyond weaning but not in Sf mice that rarely lived beyond weaning. Although these observations indicate that the phenotype associated with Il2-/- mutation is dominant over Sf, the mechanism(s) responsible was not addressed in that study.
CD103+CD4+ T cells are increased in Sf but not Il2-/- and Sf.Il2-/- mice
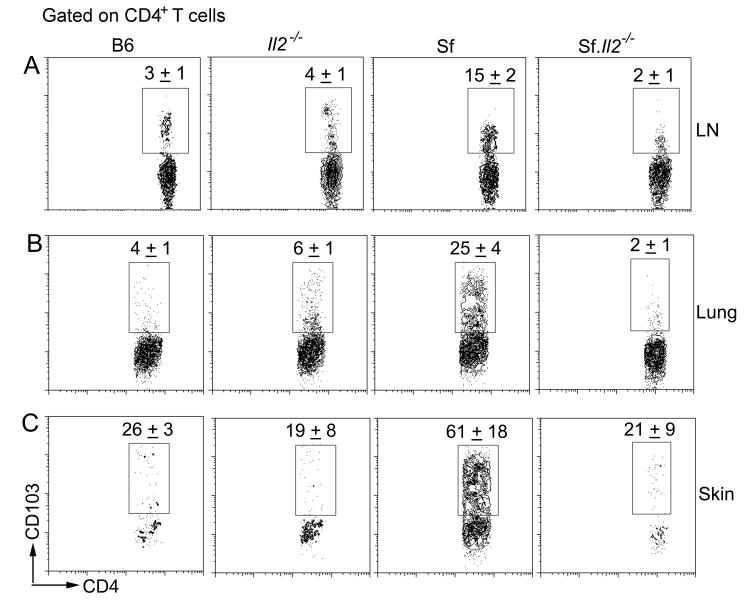
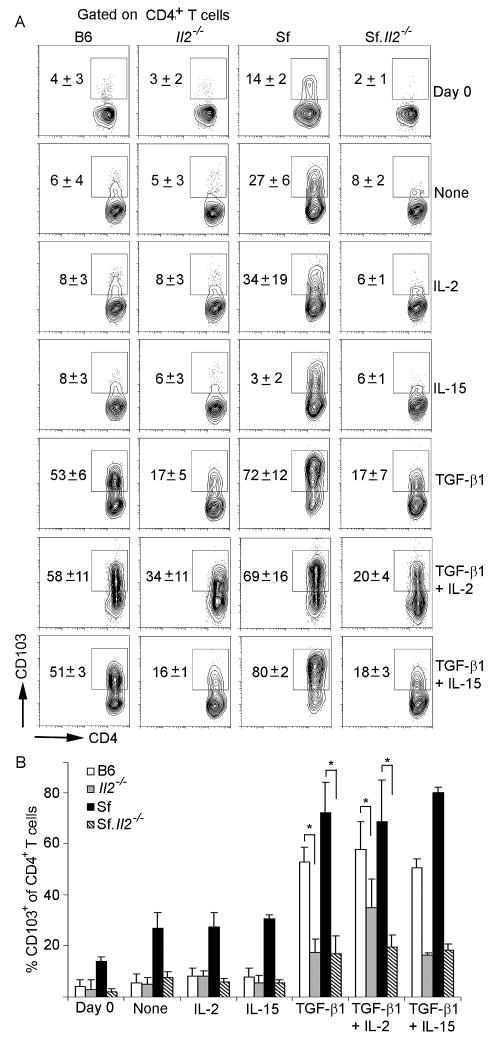
Because the inflammation-inducing cells in Sf mice are CD4+ T lymphocytes and CD103 plays a critical role in the homing of cells to cutaneous and mucosal organs, we determined the expression of CD103+CD4+ T cells among B6, Sf, Il2-/-, and Sf.Il2-/- mice (Fig. 1). As shown in Fig. 1A, the fraction of CD4+ T cells that expressed CD103 was low, ranged between 3±1% in the LN of normal B6 mice. In contrast, the fraction of CD103+CD4+ T cells in Sf mice was 15±2%, presumably due to the spontaneous activation resulted from the loss of Treg. Interestingly, CD103+ expression on the CD4+ T cells of Il2-/- mice was not increased and remained in the range of 4±1%. Importantly, the lack of increase in CD103 expression on CD4+ T cells was also observed in Sf.Il2-/- mice (2±1%), indicating that this is not due to the presence in Il2-/- mice the residual Treg that suppress T cell activation and CD103 expression but rather a real dominant effect of Il2-/- operated at a stage after the Treg checkpoint (Fig. 1A). It should be emphasized that the IL-2 requirement for the up-regulation of CD103+CD4+ T cells was not observed by comparing Il2-/- with B6 mice. Only by comparing Sf with Sf.Il2-/- mice was this dominant phenotype observed, indicating that IL-2 requirement for CD103 expression is associated with spontaneous CD4+ T cell activation in Sf mice. Although inflammation signal has been implicated in the down-regulation of CD103 expression on Treg (40), inflammation did not down-regulate CD103 expression in our system because high CD103 expression on CD4+ T cells was observed in the highly inflamed tissues of Sf mice.
Fig. 1.
Sf mice display IL-2-dependent high expression of CD103+CD4+ T cells. Lymphocytes from LN (A), lungs (B) and skin (C) of B6, Il2-/-, Sf, and Sf.Il2-/- mice were counted and stained for CD103 and CD4 as described in “Materials and Methods”. Total CD103+CD4+ were calculated. Three to five mice per group were used.
Skin and lungs in Il2-/- and Sf.Il2-/- but not Sf mice are deficient in CD103+CD4+ T cells
Because CD103 expression is not increased in the LN of Il2-/- and Sf.Il2-/- mice, we determined the expression of CD103+CD4+ T cells in the skin and lungs. Like LN cells, the % of CD103+CD4+ T cells were increased in the skin and lungs of Sf mice. In contrast, the % of CD103+CD4+ T cells were low in Il2-/- and Sf.Il2-/- mice and were not significantly different from that observed in the skin and lungs of B6 mice (Fig. 1B and 1C). Table 1 summarized the total number of T cells, CD4+ T cells, and CD103+CD4+ T cells in the LN, lungs, and skin. As a result of lymphoproliferation, LN cell numbers in Il2-/- and Sf mice were high but significantly higher numbers of total T cells were observed in the LN of Sf.Il2-/- mice. Similar findings were observed for CD4+ T cells. In contrast to LN, high numbers of total T cells and total CD4+ T cells were observed in the skin and lungs of Sf mice but not Il2-/- and Sf.Il2-/-, reflecting the lack of skin and lung inflammation in these mice. The total CD103+CD4+ T cells in the LN of Sf mice are very high but are moderate in the LN of Il2-/- and Sf.Il2-/- mice even though the latter contained twice as many CD4+ T cells (Table 1). Thus, only Sf mice contained high CD103+CD4+ T cells in their LN, skin, and lungs and this increase correlated with organ-inflammation. The data suggest that CD103+CD4+ T cells are the critical inflammation-inducing T cells in Sf mice and the lack of these cells in Il2-/- and Sf.Il2-/- mice is one reason that these mice failed to develop inflammation in the skin and lungs.
Table 1.
Il2-/- inhibits the preferential expression of CD103+CD4+ T cells in the LN, skin and lungs of Sf mice.a
| Mice | Total cells | CD4+ T cells | CD103+CD4+ T cells |
|---|---|---|---|
| LN (number of cells × 106) | |||
| B6 | 17 ± 2 | 6 ± 1 | 0.2 ± 0.02 |
| Il2-/- | 62 ± 17 | 24 ± 7 | 0.9 ± 0.02 |
| Sf | 71 + 14 | 25 ± 4 | 4.2 ± 0.5 |
| Sf.Il2-/- | 131 ± 35 | 43 ± 14 | 0.9 ± 0.2 |
| Lung (number of cells × 105) | |||
| B6 | 21 ± 2 | 4 ± 1 | 0.2 ± 0.01 |
| Il2-/- | 22 ± 2 | 5 ± 2 | 0.25 ± 0.02 |
| Sf | 56 ± 9 | 19 ± 4 | 5 ± 0.7 |
| Sf.Il2-/- | 26 ± 4 | 8 ± 3 | 0.2 ± 0.04 |
| Skin (number of cells × 104) | |||
| B6 | 26 ± 4 | 0.9 ± 0.3 | 0.25 ± 0.08 |
| Il2-/- | 18 ± 3 | 0.8 ± 0.2 | 0.2 ± 0.05 |
| Sf | 190 ± 60 | 82 ± 49 | 35 ± 10 |
| Sf.Il2-/- | 13 ± 9 | 1.6 ± 1.2 | 0.3 ± 0.08 |
Lymphocytes were obtained from LN, lungs and skin of indicated mice and counted. Cells were stained for CD4 and CD103 and the total number of CD4+ and CD103+CD4+ T cells were calculated. The data were obtained from 4-5 mice for each group.
IL-2-dependent regulation of CD103 is specific to CD4+Foxp3- T cells
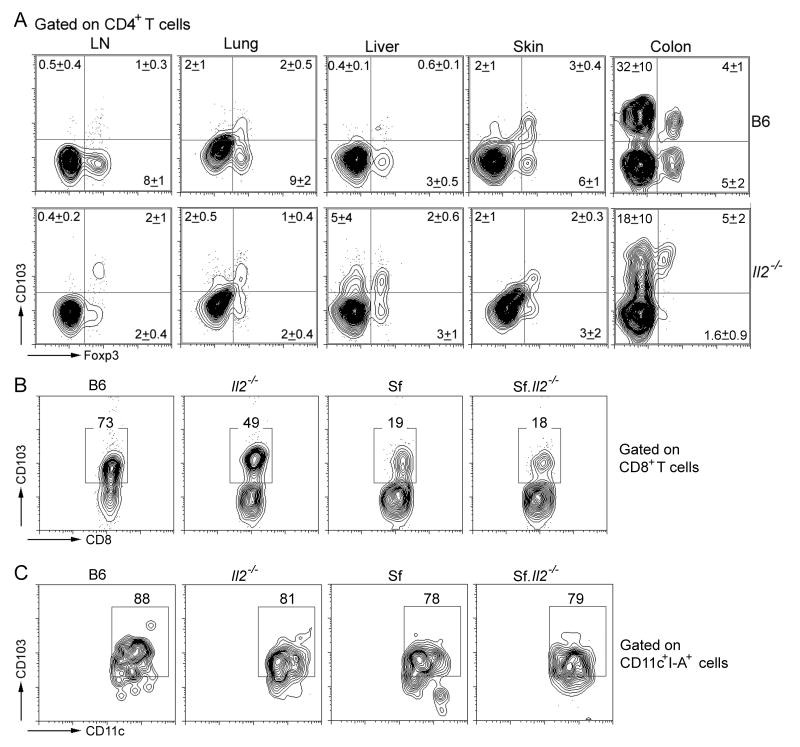
Because a significant portion of CD4+Foxp3+ Treg express CD103 and because Il2-/- mice have a significant level of CD4+Foxp3+ Treg in the periphery, it becomes important to determine whether the Treg in Il2-/- mice express low CD103 like their CD4+Foxp3- T cell counterparts. We determined the total number of Treg and the CD103 expression on Treg isolated from LN, lung, liver, skin, and colon (Fig. 2A). In agreement with a previous report, the % of CD4+Foxp3+ Treg in the LN CD4+ T cells in Il2-/- mice (4%) is about 33% that present in B6 counterpart (9%). However, the total CD4+ T cells in the LN of Il2-/- mice are 2-3 times more than B6 (22, this study). Thus, the total number of Treg was only slightly reduced in the Il2-/- mice. Moreover, the ratio of CD103+Treg to CD103-Treg was higher in Il2-/- mice than B6 control. Similar findings and trends were observed in the other organs examined (Fig. 2A). The CD103+Treg in Il2-/- colon appear to have lower Foxp3 expression than B6 control but somewhat higher CD103 expression. In addition, CD103-Treg was almost absent, suggesting a strong local environmental influence on CD103 expression on Treg (40). The presence of CD103+Treg in Il2-/- mice should provide additional force to protect the skin and lungs from inflammation amid the reduced levels of CD103+CD4+ inflammation-inducing T cells.
Fig. 2.
IL-2-dependent regulation of CD103 is specific to CD4+Foxp3- T cells. (A) CD103 expression is not inhibited in the Treg of Il2-/- mice. Lymphocytes were obtained from LN, lungs, liver, skin and colon from B6 and Il2-/- mice (N=4) and stained for CD103, Foxp3 and CD4. The numbers represent mean±S.D. from gated CD4+ T cells. The intensity of staining may be reduced slightly by protease treatment for lymphocyte preparations. Please note the CD103 expression was high on Treg and non-Treg isolated from colon. (B) CD103 expression is down-regulated in Il2-/-, Sf, and Sf.Il2-/- mice as compared with B6 control. LN cells from these mice were stained for CD8 and CD103 as described in Materials and Methods. (C) No significant changes in CD103 expression on CD11c+ DC from Il2-/-, Sf. and Sf.Il2-/- as compared with B6 control. LN cells from these mice were stained for CD11c and CD103 as described in Materials and Methods. Representative data from at least 3 experiments are presented for B and C.
The preferential regulation of CD103 expression by IL-2 on CD4+ T cells can only be observed by comparing CD103+CD4+ T cell expression levels among B6, Sf, Il2-/- and Sf.Il2-/- mice. This activation-dependent expression of CD103 is in contrast to the “constitutive” expression of CD103 on CD8+ T cells observed in normal mice. The % of CD103+CD8+ T cells in the LN of B6, Sf, Il2-/-, and Sf.Il2-/- mice are 73%, 49%, 19%, and 18%, respectively (Fig. 2B). Thus, unlike CD4+ T cells, CD103 expression on CD8+ T cells is down-regulated in Sf, Il2-/- mice and Sf.Il2-/- mice.
Because CD103 is also expressed on CD11c+ DC cells, we determined if they were reduced in Il2-/- and Sf.Il2-/- mice as compared with B6 and Sf mice, respectively. No significant change in the expression of CD103 on the CD11c+ DC cells was observed in all cases (Fig. 2C). Taken together, these observations indicate that the IL-2-dependent up-regulation of CD103 expression is specific to CD4+ T cells in Sf mice.
CD103 is a major target of IL-2-regulated receptor for Sf T cell trafficking
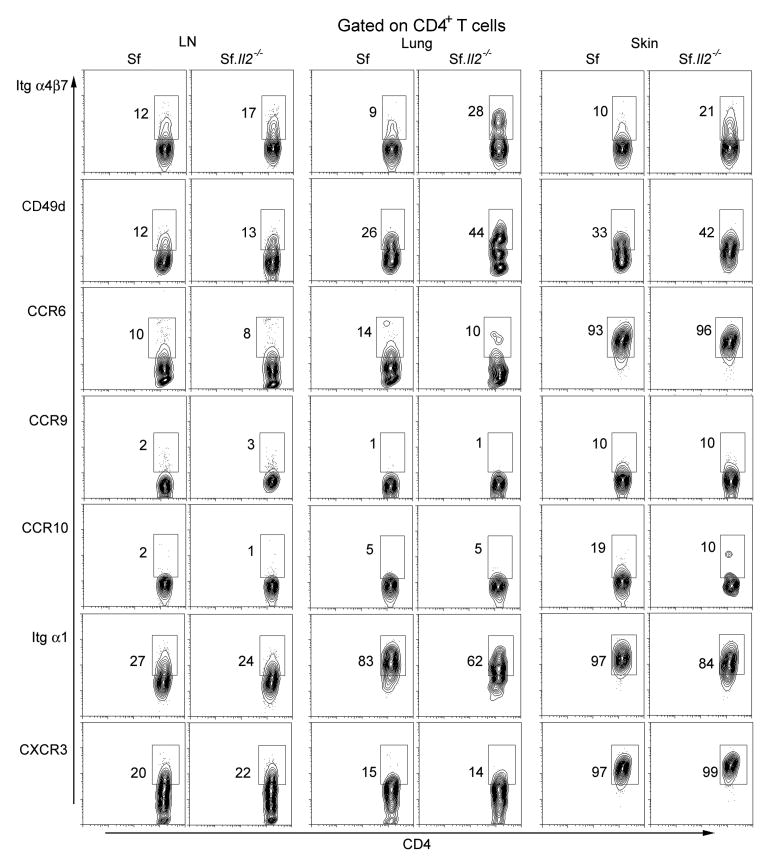
We examined several cell surface receptors involved in T cell trafficking using available mAb to determine if CD103 expression on Sf CD4+ T cells is a major target of IL-2-depedent regulation (Fig. 3). None of the receptors examined were significantly different in the LN CD4+ T cells between Sf and Sf.Il2-/- mice. We also examined skin CD4+ T cells of Sf mice since they contain the highest expression of CD103+CD4+ T cells. The result shows that additional receptors for trafficking/retention are regulated although the extent is not as remarkable as CD103. Interestingly, CD49d and α4β7 expression was increased in Sf.Il2-/- mice as compared with Sf counterpart. These observations suggest that IL-2 regulates multiple cell surface receptors involved in trafficking and that CD103 is a major but not necessarily the only target of such regulation under the limited number of target receptors examined.
Fig. 3.
Expression of various chemokine receptors and integrins on the CD4+ T cells from the LN, lung, and skin of 3 wks old Sf and Sf.Il2-/- mice. Cells were isolated as described in the “Materials and Methods” and viable (7AAD-negative) CD4 gated cells were analyzed by flow cytometry. The data presented is a representative of three independent experiments.
Colon but not other gastrointestinal organs remain inflamed in Il2-/- and Sf.Il2-/- mice
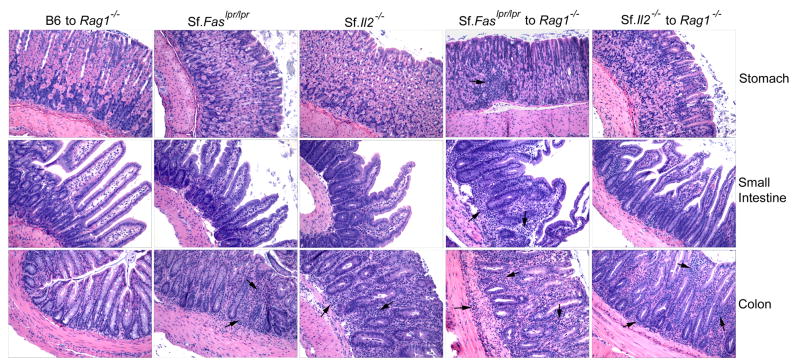
The question why colon remained inflamed in Il2-/- mice is addressed further in Sf.Il2-/- and Sf.Faslpr/lpr mice. Although Sf mice do not display colitis and inflammation in other gastrointestinal organs, they contain CD4+ T cells capable of inducing inflammation in these organs in Rag1-/- recipients upon adoptive transfer (39). The absence of inflammation in the gastrointestinal organs could be due to pre-weaning condition, short lifespan of 4 wk, and lacking sufficient time for the inflammation to occur. Because Sf.Il2-/- and Sf.Faslpr/lpr mice lived beyond weaning, we compared their gastrointestinal organs for inflammation. Prolongation of lifespan to 10 wk beyond weaning did not allow inflammation to develop in the stomach and small intestine in Sf.Faslpr/lpr mice. Like Sf.Il2-/- mice, inflammation was observed in the colon. We also transferred LN cells from Sf.Il2-/- and Sf.Faslpr/lpr mice to Rag1-/- recipients. In contrast to Sf.Faslpr/lpr LN cells that transferred inflammation to stomach, small intestine, and colon, LN cells from Sf.Il2-/- transferred colitis but not inflammation in the stomach and intestine (Fig. 4). These observations suggest that colon is unique in the IL-2-controlled inflammation process and additional components other than CD103 expression on CD4+ T cells might play a role in the development of colitis induced by Il2-/- defect.
Fig. 4.
Sf.Il2-/- mice transfer inflammation in the colon but not in stomach and small intestine like Sf.Faslpr/lpr mice do. LN cells from 8-12 wks old Sf.Faslpr/lpr and Sf.Il2-/- mice were transferred I.V. into adult Rag1-/- recipients and tissue inflammation was determined 8 wks after. Stomach, small intestine, and colon from the donors and the recipients were processed, H&E stained, and examined under microscope. Sf.Il2-/- mice but not Sf.Faslpr/lpr also failed to transfer inflammation in the skin and lung (not shown).
Effect of CD103-/- on MOI in Sf mice
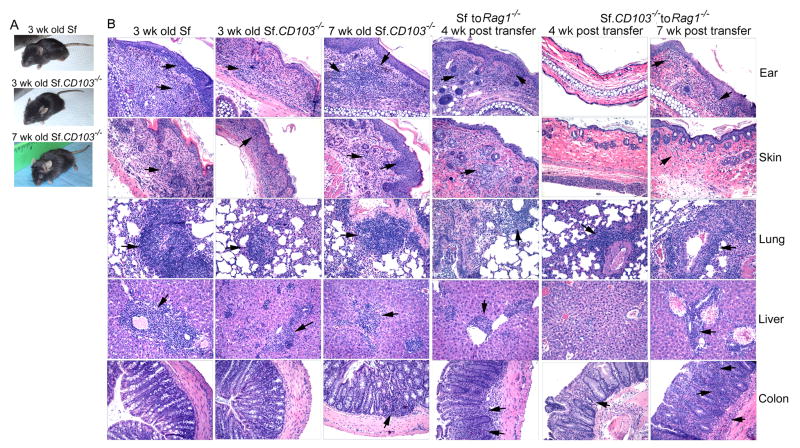
If CD103+CD4+ T cells are required for inflammation in the skin and lungs of Sf mice, targeted knockout of CD103 should inhibit such inflammation. This interpretation is tested by breeding the CD103-/- mutation into Sf mice (Fig. 5). The result showed a considerable inhibition of inflammation in the ears, skin, tail, and lungs as compared with age-matched Sf control (Fig. 5A and 5B). Moreover, inflammation in the liver remained in the Sf.CD103-/- mice (Fig. 5B). Despite initial inhibition of MOI, significant inflammation in the liver, skin and lungs and moderate colitis and inflammation in tail were observed in Sf.CD103-/- mice that lived beyond weaning. As a result, the lifespan of Sf.CD103-/- mice was only slightly prolonged to about 7-8 wks that is significantly shorter than Sf.Il2-/- mice, suggesting that IL-2 plays additional roles in the inflammation response other than regulating CD103 expression on CD4+ T cells. Interestingly, leukocyte infiltration was weak around epithelial cell areas, most notably in the ears as compared with that in the Sf mice (Fig. 5B, top panels). Both skin and lung showed mild inflammation in 3 wks old Sf.CD103-/- mice but severe inflammation developed in the 7 wks old Sf.CD103-/- mice. Leukocyte infiltration was observed in the sinusoids of the liver of Sf.CD103-/- mice in addition to perivascular infiltration that was mainly observed in Sf mice (Fig. 5B, 4th row panels). The moderate colitis in Sf.CD103-/- mice suggests that CD103-CD4+ T cells could induce colitis but the short lifespan prevented a full-blown inflammation in the colon (Fig. 5B, bottom panels). It is important to note that the total peripheral lymphocytes of B6.CD103-/- mice are inherently less than B6 by about 30%. The total peripheral lymphocytes in Sf.CD103-/- mice is also significantly less than Sf mice by about 40-50% and this could have impacted the extent of organ-specific inflammation as well. On the other hand, this reduction of cell number underscores the power of CD103-independent mechanisms for MOI. To address this issue, we transferred equal numbers of LN cells from Sf and Sf.CD103-/- mice into adult Rag1-/- males and examined various organs for inflammation 4 wks and 7 wks later (Fig. 5B, columns 4, 5 and 6). At 4 wks post transfer, all the organs examined for the Rag1-/- mice that received LN cells from Sf mice showed leukocyte infiltration and destruction of organ architecture (Fig 5B, column 4). On the other hand, at the 4 wks time point, the inflammation in the ear and skin was undetectable for the mice that received LN cells from Sf.CD103-/- mice, although mild to moderate inflammation could be seen in the liver, lung and colon. The data demonstrate that CD103+CD4+ T cells are more effective in inducing skin and lung inflammation through CD103-dependent mechanism. However, at 7 wks post transfer, both groups displayed external signs of inflammation on the ears and skin. Upon histological examination, inflammation was observed in ear, skin, lungs, liver and colitis with various degrees of intensity (Fig. 5B, column 6). Taken together, these observations demonstrate that MOI mediated totally by CD103-independent mechanisms exist as demonstrated in Sf.CD103-/- mice. Thus, additional regulatory mechanisms must be used in Sf.Il2-/- mice to account for their life-long protection of skin and lung inflammation and their longer lifespan than Sf.CD103-/- mice.
Fig. 5.
Effect of CD103-/- on tissue inflammation in Sf mice. Sf (3 wks old), Sf.CD103-/- (3 wks old), and Sf.CD103-/- (7 wks old) mice were photographed (A) and various tissues were processed for histological analysis (B). LN cells from 3 wks old Sf and Sf.CD103-/- mice (15×106 cells) were transferred to adult Rag1-/- male and various tissues were processed for histological analysis 4 and 7 wks later (B, right panels). The data is representative of two independent experiments each contained 2 mice in each group.
In vitro regulation of CD103 expression on CD4+ T cells
To better understand the role of IL-2 in the regulation of CD103 expression on CD4+ T cells, we conducted in vitro stimulation assays. LN CD4+ T cells were purified from B6, Il2-/-, Sf and Sf.Il2-/- mice and activated with anti-CD3/anti-CD28 beads in the presence or absence of IL-2, IL-15 and TGF-β1. As shown in Fig 6, TCR stimulation alone resulted in a robust net increase in CD103 expression on Sf CD4+ T cells (27±6%), whereas a weak net increase was observed for B6 (6±4%), Il2-/- (5±3%) and Sf.Il2-/- (8±2%) samples. Addition of IL-2 and IL-15 did not further increase CD103 expression (row 3 and row 4), suggesting the culture system is limited by TGF-β1. Indeed, addition of TGF-β1 dramatically increased CD103+CD4+ T cells to 53±6% and 72±12% for B6 and Sf mice but only 17±5% and 17±7% for Il2-/- and Sf.Il2-/- samples, respectively. Addition of IL-2 together with TGF-β1 also failed to increase CD103 expression on Sf.Il2-/- CD4+ T cells (20±4%). Similar treatment of age- and sex-matched Il2-/- mice also failed to completely restore CD103 expression to the same level as B6 control and this failure is statistically significant (Fig. 6B). Furthermore, addition of IL-15, either alone or together with TGF-β1, did not influence the expression of CD103 on these CD4+ T cells (last row). Taken together, these observations indicate that the CD103 expression defect in Sf.Il2-/- mice can be recapitulated under the in vitro stimulation condition but the defect cannot be restored by the exogenous IL-2 and IL-15.
Fig. 6.
Role of IL-2 in CD103 expression on CD4+ T cells examined under in vitro activation. (A) Pooled LN CD4+ T cells from B6, Sf, Il2-/- and Sf.Il2-/- mice were purified by negative selection and stimulated for 3 d with anti-CD3+anti-CD28 beads in the presence or absence of rIL-2 (30 IU/ml), rIL-15 (100 ng/ml), or TGF-β1 (2.5 ng/ml) as described in “Materials and Methods”. Viable (7-AAD negative) cells gated on CD4+ T cells were analyzed for CD103 expression by flow cytometry. In separate experiments, both rIL-2 and rIL-15 potently stimulated the proliferation of memory CD8+ T cells, indicating they are biologically active. The data is representative of four independent experiments. The number in each panel is the M±S.D. of all experiments. (B) Statistical analysis of the data indicates that the different expression of CD103+CD4+ T cells between B6 and Il2-/- mice and between Sf and Sf.Il2-/- mice are significant as shown by students t-test (* indicates p value <0.01)
Discussion
A major point of the study is the observation that CD103 expression on CD4+ T cells was significantly up-regulated in Sf mice and this up-regulation was not observed in Il2-/- mice and was inhibited in Sf.Il2-/- mice. The in vivo regulation of CD103 expression on CD4+ T cells and its dependence on IL-2 is novel because CD103 expression on CD4+ T cells has not been appreciated even though they are the critical cells directly responsible for the induction of many autoimmune and inflammation responses. In addition, other than TGF-β1, few activators for CD103 expression are known and our identification that IL-2 is critically required for CD103 up-regulation in CD4+ T cells in Sf mice represents an important breakthrough and opens up new avenues in this area of research. It is significant that not only have we demonstrated IL-2-dependent regulation of CD103 expression in CD4+ T cells in Sf mice but also we have correlated this regulation with the development of inflammation in skin and lungs. Because CD4+ T cells of Sf mice transferred MOI, the link between IL-2-dependent CD103 expression on CD4+ T cells and inhibition of inflammation in Sf.Il2-/- mice in an “organ-specific” manner suggests that the interaction between CD103+CD4+ T cells and E-cadherin+ target organs plays a role in the inflammation process.
An exception is the observation that colons are strongly inflamed in Il2-/- mice. Annacker et al, have shown that CD4+CD45RBhigh T cells from CD103-/- mice could transfer colitis to Balb/c.scid/scid recipients (41). In another study, transfer of CD4+CD45RBhigh T cells into Balb.scid/scid recipients induced both colitis and psoriasis but only skin-infiltrating T cells predominantly expressed the P-selectin ligand and E-selectin ligand (Cutaneous Lymphocyte Ag) cells, correlating organ-specific inflammation with specific T cell subsets (42). It is interesting that colitis is preferentially or selectively induced in many animal models in which a common defect affecting general immune regulation appears to be the culprit. Also, colon may be more sensitive to inflammation-inducing T cells because it anatomically and environmentally subjects to constant immune stimulation from different immunogens and microbiota (43, 44). Given the high sensitivity of colon to inflammation, the residual CD103+CD4+ T cells in Il2-/- and Sf.Il2-/- may be sufficient to induce inflammation in the colon. This appears not to be the case because the colonic CD4+ T cells from Sf.Il2-/- mice (5-wk old) were low in CD103 expression whereas extremely high CD103+CD4+ T cells were observed in the colon of Sf mice that survived a few days after weaning (data not shown). Thus, mechanism for colitis independent from CD103+CD4+ T cells must exist as indicated by our study of Sf.CD103-/- mice and the study by Annacker et al (41).
The molecular mechanism by which IL-2 induces CD103 up-regulation in CD4+ T cells is under investigation. The first question to be addressed is whether IL-2 regulates CD103 expression through the induction of TGF-β1 and/or its receptors. TGF-β1-specific ELISA kit with a sensitivity of detecting ∼ 100 pg/ml (Promega) failed to detect active TGF-β1 in the sera of B6, Il2-/-, Sf, and Sf.Il2-/- mice. In addition, there were no differences in the expression of total TGF-β1 in these samples after acid treatment for latent TGF-β1 (data not shown). Thus, IL-2 may be required for the induction of TGF-β receptors as suggested by TGF-βR1 mRNA analysis that showed a 4-fold increase in Sf CD4+ T cells as compared with Sf.Il2-/- T cells (unpublished observation). We also activated T cells with anti-CD3/anti-CD28 beads and observed increased CD103 expression on CD4+ T cells from Sf mice but not other mice tested. Addition of excess active TGF-β1 (2.5 ng/ml versus <120 pg/ml latent form in culture supernatants) strongly increased CD103 expression on the CD4+ T cells Sf and B6 mice, but not much in Il2-/- and Sf.Il2-/- samples. Importantly, IL-2 plus TGF-β1 also failed to completely restore the CD103 expression on Il2-/- and Sf.Il2-/- CD4+ T cells. It is likely that the inflammation has driven these cells beyond the point in such a way that response to IL-2 and TGF-β1 cannot be totally restored under the in vitro system.
Introducing CD103-/- into Sf mice inhibited the inflammation in skin, tail, and lungs initially, but the CD103-independent inflammation eventually developed around 3 wk after weaning at which time the mice became moribund. In contrast, Il2-/- mutation-mediated inhibition of skin and lung are long-lasting throughout the lifespan of the mice. In addition, CD103-/- mutation prolongs the lifespan of Sf mice significantly shorter than Il2-/- mutation. Thus, Il2-/- must play other roles in addition to regulating CD103 expression on CD4+ T cells in the inflammatory process. The results obtained with Sf.CD103-/- mice suggest that other integrins and chemokine receptors for cell-trafficking and retaining are involved in the MOI. Selective and preferential use of specific chemokines/receptors systems have been described for skin, lungs, and gut (42, 45, 46). We compared the expression of some of these receptors on target organs between Sf and Sf.Il2-/- mice using limited number of available mAb (Fig. 3). The data suggest that IL-2 regulates several receptors but CD103 remains to be one of the major targets of regulation. Given the limited target receptors examined and the fact that CD103-independent mechanisms exist for both skin and lung inflammation, we compared the mRNA levels of various chemokine receptors between the CD4+ T cells of Sf and Sf.Il2-/- mice. Our preliminary data identified super-expression of cysteinyl leukotriene receptor 1, leukotriene B4 receptor 1, integrin αE, CCR1, CXCL2, CCR6, and CCR8 in Sf CD4+ T cells as compared with Sf.Il2-/- CD4+ T cells. Thus, IL-2 appears to be a master regulator for several trafficking/retention receptors that can account for the CD103-independent mechanisms observed in Sf.CD103-/- mice.
As a receptor for E-cadherin and signal transduction, CD103 controls the trafficking and retaining of lymphocytes in target organs. Thus, CD103/E-cadherin interaction is the final stage of cell trafficking and the beginning of immune reaction in the target organs. Many studies have suggested the importance of CD103 expression in the mucosal and cutaneous inflammation process. The present study demonstrates that IL-2, by regulating such a critical molecule involved in target organ inflammation, facilitates organ-specific inflammation in a manner opposing its role as a positive regulator of Treg that inhibit inflammation. Most importantly, our studies using Il2-/- mice consider its effects on not only Treg but also on IL-2-dependent mechanisms beyond Treg check point and beyond CD103-dependent mechanism. In this regard, it is of great significance that we have established that CD103-independent inflammation-inducing mechanism is capable of fully inducing the fatal MOI in the absence of Treg, implying that CD103-targeted therapy will be unlikely to produce long-lasting protection against inflammation of E-cadherin+ organs. Our study further accentuates the role of IL-2 in regulating multiple mechanisms of T cells such that lifelong protection from spontaneous skin and lung inflammation from inflammation-inducing CD4+ T cells can be succeeded in Sf.Il2-/- mice.
Footnotes
Supported by NIH grants DE-017579 and AR-051203 (to STJ), AR-047988 and AR-049449 (to SMF), AI-079906 (to SJS) and a grand-in-aid from the Beirne B. Carter Center of Immunology (to RS).
Abbreviations used in this paper: Intestinal Epithelial Lymphocytes (IEL), Multi-Organ Inflammation (MOI), Scurfy (Sf), CD4+Foxp3+ regulatory T cells (Treg).
Disclosures The authors have no conflict of interest.
References
- 1.Wing K, Fehervari Z, Sakaguchi S. Emerging possibilities in the development and function of regulatory T cells. Int Immunol. 2006;18:991–1000. doi: 10.1093/intimm/dxl044. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–5972. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 5.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: A large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 6.Yan J, Mamula MJ. Autoreactive T cells revealed in the normal repertoire: escape from negative selection and peripheral tolerance. J Immunol. 2002;168:3188–3194. doi: 10.4049/jimmunol.168.7.3188. [DOI] [PubMed] [Google Scholar]
- 7.Anderson AC, Kuchroo VK. Expression of self-antigen in the thymus: A little goes a long way. J Exp Med. 2003;198:1627–1629. doi: 10.1084/jem.20031803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3+CD25+CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antony PA, Paulos CM, Ahmadzadeh M, Akpinarli A, Palmer DC, Sato N, Kaiser A, Hinrichs CS, Klebanoff CA, Tagaya Y, Restifo NP. Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J Immunol. 2006;176:5255–5266. doi: 10.4049/jimmunol.176.9.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 11.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Gavis MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 13.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 14.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 15.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. J Med Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Benoist C, Mathis D. How defects in central tolerance impinge on a deficiency in regulatory T cells. Proc Nat Acad Sci USA. 2005;102:14735–14740. doi: 10.1073/pnas.0507014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 18.Refaeli Y, van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2–regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 19.Kung JT, Beller D, Ju ST. Lymphokine regulation of activation-induced apoptosis in T cells of IL-2 and IL-2R beta knockout mice. Cell Immunol. 1998;185:158–163. doi: 10.1006/cimm.1998.1282. [DOI] [PubMed] [Google Scholar]
- 20.Xiao S, Sung SSJ, Fu SM, Ju ST. Combining Fas mutation with interleukin-2 deficiency prevents colitis and lupus. J Biol Chem. 2003;278:52730–52738. doi: 10.1074/jbc.M308707200. [DOI] [PubMed] [Google Scholar]
- 21.Sharma R, Bagavant H, Jarjour WN, Sung SSJ, Ju ST. The role of Fas in the immune system biology of IL-2Rα knockout mice: Interplay among regulatory T cells, inflammation, hemopoiesis, and apoptosis. J Immunol. 2005;175:1965–1973. doi: 10.4049/jimmunol.175.3.1965. [DOI] [PubMed] [Google Scholar]
- 22.Zheng L, Sharma R, Gaskin F, Fu SM, Ju ST. A novel role of IL-2 in organ-specific autoimmune inflammation beyond regulatory T cell checkpoint: Both IL-2 knockout and Fas mutation prolong lifespan of Scurfy mice but by different mechanisms. J Immunol. 2007;179:8035–8041. doi: 10.4049/jimmunol.179.12.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the α(E)β(7) integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 24.Dogan A, Wang ZD, Spencer J. E-cadherin expression in intestinal epithelium. J Clin Pathol. 1995;48:143–146. doi: 10.1136/jcp.48.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Karecla PI, Bowden SJ, Green SJ, Kilshaw PJ. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin alpha M290 beta 7 (alpha E beta 7) Eur J Immunol. 1995;25:852–856. doi: 10.1002/eji.1830250333. [DOI] [PubMed] [Google Scholar]
- 27.Lefrancois L, Parker CM, Olson S, Muller W, Wagner N, Schon MP, Puddington L. The role of β-7 integrins in CD8 T cell trafficking during an antiviral immune response. J Exp Med. 1999;189:1631–1638. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JMG, Mandelbrot DA, Shaw SK, Russell GJ, Murphy EA, Chen YT, Nelson WJ, Parker CM, Brenner MB. Direct and regulated interaction of integrin αEβ7 with E-cadherin. J Cell Biol. 1998;140:197–210. doi: 10.1083/jcb.140.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell G, Parker C, Cepek K, Mandelbrot D, Sood A, Mizoguchi E, Ebert E, Brenner M, Bhan A. Distinct structural and functional epitopes of the αEβ7 integrin. Eur J Immunol. 1994;24:2832–2841. doi: 10.1002/eji.1830241138. [DOI] [PubMed] [Google Scholar]
- 30.Cerf-Bensussan N, Jarry A, Brousse N, Lisowska-Grospierre B, Guy-Grand D, Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987;17:1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- 31.Kilshaw PJ, Baker KC. A unique surface antigen on intraepithelial lymphocytes in the mouse. Immunol Lett. 1988;18:149–154. doi: 10.1016/0165-2478(88)90056-9. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A. Expression of the integrin αEβ7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc Nat Acad Sci USA. 2002;99:13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lefrancois L, Barrett TA, Havran WL, Puddington L. Developmental expression of the α(IEL)β(7) integrin on T cell receptor γδ and T cell receptor αβ T cells. Eur J Immunol. 1994;24:635–640. doi: 10.1002/eji.1830240322. [DOI] [PubMed] [Google Scholar]
- 35.Kilshaw PJ, Murant SJ. A new surface antigen on intraepithelial lymphocytes in the intestine. Eur J Immunol. 1990;20:2201–2007. doi: 10.1002/eji.1830201008. [DOI] [PubMed] [Google Scholar]
- 36.Parker CM, Cepek K, Russell GJ, Shaw SK, Posnett DN, Schwarting R, Brenner MB. A family of beta 7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci USA. 1992;89:1924–1928. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson PW, Green SJ, Carter C, Coadwell J, Kilshaw PJ. Studies on transcriptional regulation of the mucosal T-cell integrin αEβ7 (CD103) Immunology. 2001;103:146–154. doi: 10.1046/j.1365-2567.2001.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grueter B, Petter M, Egawa T, Laule-Kilian K, Aldrina CJ, Wuerch A, Ludwig Y, Fukuyama H, Wasremann H, Waldschuetz R, Moroy J, Taniuchi I, Steimle V, Littman DR, Ehlers M. Runx3 regulates integrin αE/CD103 and CD4 expression during development of CD4-/CD8+ T cells. J Immunol. 2005;175:1694–1705. doi: 10.4049/jimmunol.175.3.1694. [DOI] [PubMed] [Google Scholar]
- 39.Sharma R, Jarjour WN, Zheng L, Gaskin F, Fu SM, Ju ST. Large functional repertoire of regulatory T-cell suppressible autoimmune T cells in scurfy mice. J Autoimmunity. 2007;29:10–19. doi: 10.1016/j.jaut.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suffia I, Reckling SK, Salay* G, Belkaid Y. A Role for CD103 in the Retention of CD4+CD25+ Treg and Control of Leishmania major Infection. J Immunol. 2005;174:5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 41.Annacker O, Coombes JL, Malmstrom v, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie f. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu A, Hong K, Berg EL, Ehrhardt RO. Tissue specificity of E- and P-selectin ligands in Th1-mediated chronic inflammation. J Immunol. 1999;163:5086–5093. [PubMed] [Google Scholar]
- 43.Pamer EG. Immune responses to commensal and environmental microbes. Nat Immunol. 2007;8:1173–1177. doi: 10.1038/ni1526. [DOI] [PubMed] [Google Scholar]
- 44.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor CCR4 and the CCR10 ligand cutaneous T cell–attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Vries IJM, Langeveld-Wildschut EG, van Reijsen FC, Bihari IC, Bruijnzeel-Koomen CAFM, Thepen T. Nonspecific T-cell homing during inflammation in atopic dermatitis: expression of cutaneous lymphocyte-associated antigen and integrin αEβ7 on skin-infiltrating T cells. J Allergy Clin Immunol. 1997;100:694–701. doi: 10.1016/s0091-6749(97)70175-1. [DOI] [PubMed] [Google Scholar]