SUMMARY
Breathing automaticity and CO2 regulation are inseparable neural processes. The retrotrapezoid nucleus (RTN), a group of glutamatergic neurons that express the transcription factor Phox2b, may be a crucial nodal point through which breathing automaticity is regulated to maintain CO2 constant. This review updates the analysis presented in prior publications. Additional evidence that RTN neurons have central respiratory chemoreceptor properties is presented but this is only one of many factors that determine their activity. The RTN is also regulated by powerful inputs from the carotid bodies and, at least in the adult, by many other synaptic inputs. We also analyze how RTN neurons may control the activity of the downstream central respiratory pattern generator. Specifically, we review the evidence which suggests that RTN neurons a) innervate the entire ventral respiratory column, and b) control both inspiration and expiration. Finally, we argue that the RTN neurons are the adult form of the parafacial respiratory group in neonate rats.
Keywords: Central chemoreceptors, breathing, Phox2b, retrotrapezoid nucleus, parafacial respiratory group
1. Introduction
This review is about the regulation of breathing automaticity and the putative role of the retrotrapezoid nucleus (RTN) in this process. Neuronal chemosensitivity in general (pH-sensitivity) and the central chemoreflex in particular are briefly discussed but the main purpose of the exercise is to point out that both topics are in many ways separate from the fundamental question of CO2 homeostasis and the regulation of breathing automaticity.
A complex network of lower brainstem neurons, called the respiratory central pattern generator (CPG), generates the respiratory rhythm, defines the onset and offset of the various respiratory motor outflows and regulates the frequency and intensity of these periodic outflows (Duffin, 2004; Feldman and Del Negro, 2006; Smith et al., 2007). The output of the CPG is automatically adjusted to maintain arterial PCO2 within narrow limits (Feldman et al., 2003). This regulation matches the excretion of CO2 to its metabolic production and prevents the deleterious effects of pH changes on protein function. Although the tight regulation of arterial PCO2 through breathing has been thoroughly described since the very beginning of the 20th century (Haldane and Priestley, 1905), the molecular and cellular mechanisms that underlie this critical aspect of homeostasis are not elucidated.
2. Hypothesis and overview
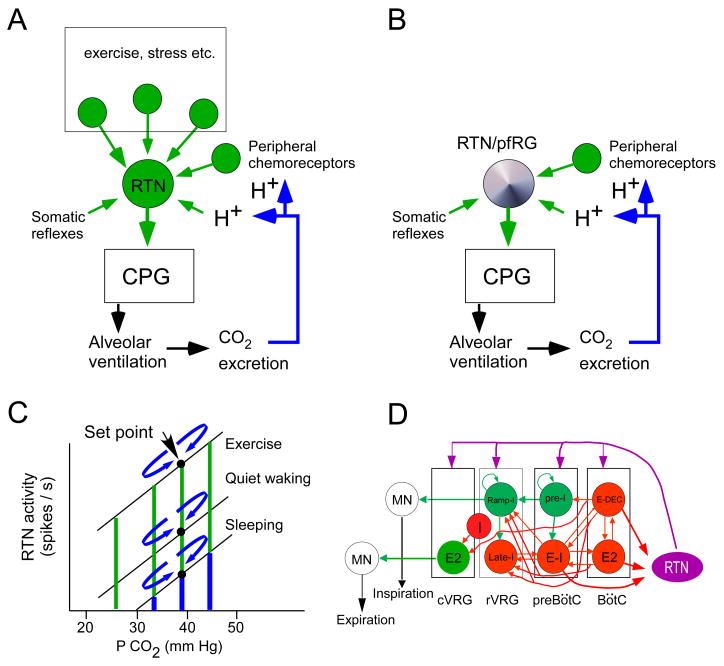
Our working hypothesis for how breathing automaticity is regulated to maintain PCO2 constant is in many ways an elaboration of the Loeschcke model (Loeschcke, 1982) (Fig. 1A). We propose that the RTN is a major source of tonic excitatory drive to both rhythm generating and pattern generating components of the CPG. Many other tonic drives to the respiratory centers exist but, according to our hypothesis, the excitatory drive that originates from the RTN is particularly, perhaps uniquely, important for CO2 stability because the discharge of these neurons is exquisitely sensitive to changes in blood gases (CO2 and oxygen). This sensitivity to small changes in blood gases is due to the combination of two factors: the intrinsic pH sensitivity of the RTN neurons and the powerful input that these cells receive from the carotid bodies (Fig. 1A). The potential importance of the carotid body input cannot be stressed enough given their potent contribution to breathing automaticity under normocapnic conditions in unanesthetized mammals (Smith et al., 2006). Finally, in this review, we also discuss the possibility that the resting, i.e. CO2-independent, activity of RTN neurons derives from intrinsic pacemaker properties at birth and are later replaced by conventional synaptic drives (Fig. 1B). We have developed several of these ideas previously (Guyenet et al., 2008; Guyenet, 2008). The present review is an update.
Figure 1. Role of RTN in breathing: a working hypothesis.
A: In the adult, the RTN no longer possesses intrinsic pacemaker properties (Mulkey et al., 2004). We postulate that the discharge of RTN neurons is regulated by their “chemical drive” (intrinsic pH-sensitivity and inputs from peripheral chemoreceptors) and by synaptic inputs that develop along with various behaviors that require large changes in respiration (emotions, stress and particularly physical exercise). B: In the neonate the RTN, a subset of neurons previously identified as the pfRG, is a cluster of pH-sensitive excitatory neurons endowed with intrinsic bursting activity (Onimaru et al., 2008). We hypothesize that the intrinsic bursting properties of these neurons provide a background activity that enables these cells to be up and down regulated by the local pH and, presumably, also by inputs from peripheral chemoreceptors. C: graphic representation of how RTN neurons might regulate arterial PCO2 in the adult. The chemical drive of the RTN neurons is represented in blue and the other drives (synaptic drives from within the brain) in green. Any small deviation of PCO2 from the set-point translates into an increase or decrease in the chemical drive of the RTN cells; the effect is to increase or decrease ventilation and bring PCO2 back to the set-point (mechanism represented by the curved blue arrows). When metabolic requirements are low, the chemical drive may be the predominant factor that maintains the activity of the RTN neurons. D: theoretical concept of how RTN might differentially control the respiratory rhythm, the inspiratory motor outflow and the activity of expiratory muscles. The core components of the rhythm and pattern generating circuit are redrawn after Rybak et al. ( 2007). Excitatory neurons that presumably function via fast ionotropic transmission are in green. The pre-I glutamatergic neurons are the primary oscillator of the preBötC (Feldman and Del Negro, 2006). Inhibitory neurons that use ionotropic GABAergic or glycinergic transmission are in red (Rybak et al., 2007). RTN neurons are depicted in magenta to convey the notion that their post-synaptic effect may be mediated by a slow signaling pathway (“tonic drive”, Rybak et al. ( 2007)). Expiratory pre-motor neurons (E2, i.e. E-AUG) are located in the cVRG. These neurons derive their expiratory-augmenting membrane trajectory from inhibitory volleys (GABA or glycine) during the I and the post-I (E-DEC) phases of the respiratory cycle (Iscoe, 1998). To account for evidence that the RTN region regulates expiration (Janczewski and Feldman, 2006b), we hypothesize that a subset of RTN neurons provides a form of tonic excitatory drive to the expiratory pre-motor neurons of the cVRG (E2, green). RTN neurons collectively innervate the cVRG, rVRG, preBötC and BötC regions but the exact neuronal targets within these regions are unknown and it is also unknown whether the cVRG, rVRG, preBötC and BötC are differentially innervated by subsets of RTN neurons. Some of the ramp-I premotor neurons located in the dorsal respiratory group (not represented) may also be targeted by RTN neurons which innervate this region of the NTS. Finally, RTN neurons have a respiratory modulation with an intensity roughly proportional to that of the central respiratory drive (Guyenet et al., 2005a). This modulation could be a form of feedback that originates from inhibitory interneurons with early-I, E-DEC and E-2 activity (Guyenet et al., 2005a). The location of these inhibitory neurons in the ventral respiratory group where indicated is only a guess.
Our theory presumes that a limited number of CNS neurons mediate the central and peripheral effects of CO2 on the CPG under normal physiological circumstances, i.e. under normocapnia as opposed to during asphyxia or during exposure to abnormally high levels of inspired CO2. This is a working hypothesis, not a demonstrated fact. This hypothesis is rooted in the belief that what defines a central respiratory chemoreceptor neuron is the specificity of its projections, its intrinsic properties and the nature of its postsynaptic effects rather than a molecular pH-sensor that is expressed exclusively by this class of cell.
Many experimental observations appear to contradict our interpretation but others, especially the most recent genetic evidence, strongly support the concept (Gozal, 1998; Amiel et al., 2003; Dubreuil et al., 2008). The first two sections of this review represent an effort to analyze the possible origin of some of the contradictions.
3. Central respiratory chemoreception
The respiratory rhythm presumably originates from a nest of synaptically coupled excitatory neurons located in the region of the pre-Bötzinger complex (Butera et al., 1999) whereas the timing of the various motor outflows seems to be defined by sets of mutually inhibitory interneurons that discharge during specific phases of the respiratory cycle, e.g. early-inspiratory, post-inspiratory, late-inspiratory, inspiratory, etc.(Duffin, 2004; Feldman and Del Negro, 2006; Smith et al., 2007). All existing models of the CPG postulate that both rhythm generating and pattern generating neurons receive “tonic excitatory drives” without which the system could not oscillate. The most powerful of these drives is usually thought to derive from entities called central respiratory chemoreceptors. Originally, central chemoreceptors were conceived of as specialized pH sensitive neurons that were extrinsic to the CPG (Loeschcke, 1982). However, specialized neurons are not required a priori to account for the CO2–dependent excitatory drive to the CPG. Central respiratory chemosensitivity could conceivably be an emergent property of the respiratory network caused by the intrinsic pH-sensitivity of a large proportion of its participating neurons. In fact, acid-sensitivity is quite commonly observed in the lower brainstem in vitro, in both neurons and glia (Putnam et al., 2004; Nichols et al., 2008; Chernov et al., 2008) and the activity of many channels and membrane receptors is altered by pH within a physiologically pertinent range (7.0 to 7.5) in vitro (Wellner-Kienitz et al., 1998; Jiang et al., 2001; Scheid et al., 2001; Jiang et al., 2005). Any combination of these widely expressed membrane proteins could, in theory, underlie central respiratory chemosensitivity. This working hypothesis (the distributed chemosensitivity hypothesis (Guyenet et al., 2008)) justifiably continues to exert a major influence in the field of respiration. Unfortunately, no conclusive evidence has been produced in its favor since it has not been possible to demonstrate that the deletion of a specific channel or combination thereof eliminates or even significantly attenuates central chemosensitivity. If it is correct that chemosensitivity relies on a large number of widely expressed channels, this particular experimental goal may never be achieved.
Despite evidence that pH-sensitivity is very widespread in vitro, a majority of investigators believe that central respiratory chemosensitivity relies on a limited number of neuronal groups. The raphe, the RTN, the nucleus of the solitary tract and the locus coeruleus are most often mentioned (Dean et al., 1989; Richerson, 2004; Nattie and Li, 2008) but the list also includes the orexinergic neurons and the fastigial nucleus (Martino et al., 2006; Deng et al., 2007; Williams et al., 2007). Many authors exclude from this list the neurons that are believed to be intrinsic to the CPG. This exclusion appears somewhat arbitrary because the evidence supporting the idea that specific neuronal groups are responsible for central chemosensitivity (respiratory stimulation caused by artificial brain acidification in vivo, effect of neuronal lesions on the chemoreflex in vivo and effect of pH on neurons in vitro) also supports the candidacy of the CPG and the respiratory motor neurons. For instance, in selected in vitro preparations, a large majority of CPG neurons are either hyperpolarized or depolarized by acidification, even in the presence of tetrodotoxin (Kawai et al., 1996). Acidification of the premier rhythmogenic locus, the preBötzinger complex, increases the breathing frequency markedly in vivo (Solomon et al., 2000) and acidification of the rVRG, the site of phrenic pre-motor neurons, increases PND amplitude, also in vivo (Nattie and Li, 1996). Finally motor neurons are acid-sensitive in vitro, owing to the presence of TASK channels (Bayliss et al., 2001).
So, the question remains as to how widely distributed central respiratory chemosensitivity is (Nattie and Li, 2008). Is it virtually ubiquitous in the CPG and all its tonic drives as suggested by the above quoted work, widespread in a large fraction of the above neurons, or limited to a few or a single cluster of neurons? A definitive answer cannot be provided at this time but important clues were recently derived from the study of the congenital central hypoventilation syndrome (CCHS) and its mouse model (Mellins et al., 1970; Gozal, 1998; Amiel et al., 2003; Dubreuil et al., 2008). This human developmental disease, known since 2003 to be due to various mutations of PHOX2B, is characterized by a virtually complete loss of central respiratory chemosensitivity and a failure of breathing automaticity that occurs only while sleeping in all but the most severely affected patients (Gozal, 1998; Amiel et al., 2003; Dauger et al., 2003). Despite a lack of responsiveness to CO2, CCHS patients breathe relatively normally while awake (Gozal, 1998) and must, therefore, have a largely intact CPG. If one accepts this interpretation, two conclusions follow inevitably. The first is that central respiratory chemosensitivity cannot be explained by the intrinsic pH-sensitivity of the CPG or the downstream motor neurons. This conclusion contradicts the interpretations of several of the previously mentioned animal experiments and should be an invitation to revisit the potential limitations of the experimental evidence used to arrive at such divergent interpretations. The second conclusion is that central chemosensitivity must rely on one or a few groups of neurons whose prenatal development or survival is directly or indirectly Phox2b-dependent (Dauger et al., 2003). The list includes several brainstem nuclei (catecholaminergic neurons, the nucleus of the solitary tract and the RTN) already suspected to play a role in central chemoreception based on other evidence. However, the mouse model of the disease suggests that the RTN could be the most critical structure, at least at birth, because this nucleus fails to develop whereas all other Phox2b-dependent brain areas appear on first approximation to be normal (Dubreuil et al., 2008).
4. Potential limitations of the various methods used to identify central respiratory chemoreceptors
4.1 Interpreting neuronal pH-sensitivity in vitro and in vivo
The ability of neurons to respond to acidification in vitro is a criteria commonly used to identify potential central chemoreceptors. As mentioned above, acid-sensitivity in vitro is in fact very widespread in the brainstem and elsewhere (Wang and Richerson, 2000). Differences in pH-responsiveness between regions (the “chemosensory index”) are anything but dramatic (Dean et al., 1990; Putnam et al., 2004; Nichols et al., 2008; Chernov et al., 2008) although, at temperatures approaching the physiological level (30° C), RTN neurons have a dynamic range of response to pH that is larger than that reported for other brain regions (up to 0–12 Hz between pH 7.5 and 6.9) (Guyenet et al., 2005b).
In such in vitro experiments, acid sensitivity is most often tested on spontaneously active neurons and on the resting membrane potential of silent cells (Dean et al., 1990; Putnam et al., 2004; Mulkey et al., 2007b; Nichols et al., 2008; Chernov et al., 2008). This sampling method biases the investigator against identifying potential effects of acidification on voltage-activated conductances, which can only be examined in active neurons. Accordingly, the prevalence of acid sensitivity in brain neurons in vitro could conceivably be even greater than what has been reported (15–60% depending on the region).
Neurons recorded in slices at room temperature, and particularly in cell culture, have essentially no active synaptic inputs and are therefore in a very low conductance state (Destexhe et al., 2003). Accordingly, small membrane currents that may have next to no effect in vivo because of the high conductance state that typically prevails under such conditions could change the activity of many neurons dramatically in vitro but only under those conditions. Such differences could conceivably explain why motor neurons are acid-sensitive in vitro (Bayliss et al., 2001) and yet may not contribute to central respiratory chemosensitivity. The issue of low and high conductance state may also account for the difficulty in showing that serotonergic neurons respond to CO2 in vivo, despite the fact that such neurons are clearly acid-sensitive both in slices and, even more so, in culture (Veasey et al., 1995; Severson et al., 2003; Mulkey et al., 2004). In short, the fact that neurons respond to acidification in slices or in culture is evidence that such neurons might be affected by changes in PCO2 in vivo but this evidence is not sufficient to prove that the these neurons would respond similarly in vivo and its predictive value cannot be assessed a priori.
The notion of high and low neuronal conductance also concerns experimentation performed in vivo since anesthesia can change the membrane conductance and synaptic inputs of neurons in ways that are cell-specific, with some neurons being silenced and others extremely activated depending on their connectivity and the anesthetic used. For example, it is conceivable that the lack of responsiveness of serotonergic neurons to CO2 under halothane anesthesia could be due to an unusually high conductance state of the serotonergic neurons induced by the anesthetic (Mulkey et al., 2004). This explanation evidently does not account for the fact that only 25% of presumed serotonergic neurons respond to CO2 in the unanesthetized cat (Veasey et al., 1995). However, if one assumes that during sleep these cells are subjected to strong inhibitory inputs, it could account for the fact that the minority of serotonergic neurons that do respond to CO2 during waking no longer respond during sleep (Veasey et al., 1995),.
Evidence that neurons respond consistently and similarly to CO2 in vitro and in vivo would therefore be highly desirable to assess whether such cells could be central respiratory chemoreceptors. Unfortunately, such evidence is difficult to obtain because, in the respiratory system especially, the response to hypercapnia in vivo is likely to be synaptically mediated in most cell types. Only in rare cases such as the RTN, has it been possible to show that neurons can still respond to CO2 in vivo under conditions of at least partial synaptic blockade (Mulkey et al., 2004).
4.2 CO2 homeostasis and the “central chemoreflex” are two different things
To assess the role of specific groups of neurons in central respiratory chemoreception, a common paradigm consists of examining whether the elimination of such neurons alters the central chemoreflex, i.e. reduces the stimulation of breathing caused by hypercapnic hyperoxia (5% or more CO2 is typically used) in the absence of anesthesia. Interpreting the resulting data requires consideration of several factors. First, rodents are capable of consciously detecting levels of atmospheric CO2 that are as low as 2–3 times the normal ambient concentration (0.08%) and this detection involves olfactory cells (Hu et al., 2007). Secondly, high levels of CO2 such as those used to elicit the chemoreflex in awake rats or mice, elicit a strong aversive response in humans (Moosavi et al., 2003) and most likely do so in all mammals. Therefore, exposing unanesthetized mammals, especially rodents, to high levels of CO2 presumably activates breathing via several mechanisms. Lower brainstem mechanisms that are related to those that are studied under anesthesia are undoubtedly involved but this reflex probably also engages mechanisms related to behavioral arousal and perhaps outright stress. The latter responses could be indirect consequences of the activation of lower brainstem chemoreceptors but they could also be due to effects of pH on unrelated neurons, hypothetically the locus coeruleus, raphe, orexinergic neurons etc.. This perspective, which considers the effects of high CO2 levels as a form of visceronociceptive reflex (Saper, 2002), may help understand why elimination of a key vigilance-promoting pathway such as the orexin system attenuates the “chemoreflex” in unanesthetized rodents in a state-dependent manner, i.e. during waking but not during sleep (Deng et al., 2007). Orexinergic neurons respond to acidification in slices (Williams et al., 2007) and could conceivably be directly CO2-responsive in vivo but an alternative explanation could simply be that these neurons stimulate the breathing network as part of a much broader role in maintaining the waking state. A similar case could probably be made with respect to the serotonergic neurons which have broad brain projections and a well known wake-promoting role (Lu et al., 2006). The arousal caused by central chemoreceptor stimulation (Phillipson et al., 1977; Berthon-Jones and Sullivan, 1984) is undoubtedly crucial to survival in the context of airway obstruction or central apneas (Narkiewicz and Somers, 1997; Gozal, 1998) but, under normal circumstances, CO2 homeostasis only involves minuscule changes in arterial PCO2 that produce no conscious sensation.
4.3 What does topical acidification of a brain region in vivo mimic?
Topical acidification using dialysis probes or microinjection of an inhibitor of carbonic anhydrase is used to identify regions that may contain central chemoreceptors. This very sensible paradigm is nonetheless based on several unproven assumptions. The first one is that the region of interest would be comparably acidified when arterial PCO2 increases. This is very difficult to prove and there is evidence that extracellular acidification in response to hypercapnia varies considerably depending on the brain region, perhaps because of regional differences in the blood brain barrier (reviewed by Nattie ( 2007)). The second assumption, fairly benign though also difficult to prove, is that the mechanical disruption of the local tissue does not change the buffering properties of the neuronal environment. The third assumption is that nerve terminals from distantly located chemoreceptors (hypothetically RTN neurons, subsets of serotonergic neurons, etc.) do not contribute to the pH sensitivity of the area that is being acidified. This possibility could theoretically explain why regions such as the pre-Bötzinger complex, the rVRG or even the NTS respond to local acidification (Nattie and Li, 1996; Solomon et al., 2000) since these regions are innervated by central chemoreceptor candidates such as the RTN.
5. Definition and anatomy of the RTN
Smith and colleagues ( 1989) gave the name RTN to a sparse collection of neurons located under the facial motor nucleus which could be retrogradely labeled from both the dorsal and ventral respiratory groups. The RTN is among various brainstem structures that are retrogradely labeled following injections of pseudorabies virus into the diaphragm (Dobbins and Feldman, 1994), an anatomical finding that is consistent with physiological evidence that this region regulates the inspiratory motor outflow. Indeed, stimulation, inhibition or acidification of the RTN region in the adult rat and cat in vivo has produced results consistent with the notion that it contains neurons that provide a CO2-regulated excitatory drive to the respiratory network at large (Feldman et al., 2003). Since the mid-70s, the ventral medullary surface, including but not limited to the region that is currently defined as the RTN, has been shown to contain neurons that are responsive to PCO2 via pH and the same broad region is also known to express c-Fos in awake intact rats following exposure to high levels of CO2 (Fukuda and Honda, 1975; Sato et al., 1992; Putnam et al., 2004). The RTN was more precisely outlined by Nattie and colleagues on functional and anatomical terms in the adult rat (Cream et al., 2002). In 2006, this region was shown to contain a very distinctive cluster of small VGLUT2-positive, i.e. glutamatergic, neurons that express high levels of the transcription factor Phox2b (Stornetta et al., 2006). Unlike other Phox2b-positive neurons located in the vicinity, these cells are neither cholinergic nor catecholaminergic (Stornetta et al., 2006; Takakura et al., 2008). This cell cluster does not extend further caudal than the level of the Bötzinger complex and is therefore no longer present at the level of the hypoglossal rootlets where many CO2-sensitive neurons have also been detected in the past by electrophysiology or c-Fos expression.
Each side of the brain contains about 1000 such Phox2b/VGLUT2-positive non catecholaminergic neurons in the rat (Takakura et al., 2008). They form two uneven clusters located at opposite ends of the facial motor nucleus which are joined by a sparse superficial strip of similar-looking cells (Takakura et al., 2008). Collectively, these neurons innervate the lower brainstem regions that contain the CPG, namely the entire ventral respiratory column (Bötzinger, pre-Bötzinger, rVRG and cVRG), the ventrolateral nucleus of the solitary tract and the dorsolateral pons (Rosin et al., 2006; Alheid and McCrimmon, 2008). These regions also contain many neurons that regulate functions other than breathing (e.g. cardiovascular and other autonomic functions) and some of these “non-respiratory” neurons could also be RTN targets. Most RTN neurons can be antidromically activated from both the ventral respiratory column and the dorsal pons indicating that they innervate both structures (Mulkey et al., 2004). RTN neurons project neither to the spinal cord nor to the hypoglossal nucleus (Mulkey et al., 2004; Rosin et al., 2006), therefore their presumptive targets within the respiratory network include rhythm/pattern generating neurons and or pre-motor neurons but not motor neurons. This projection pattern demarcates RTN neurons from other putative central chemoreceptors such as the serotonergic, orexinergic or noradrenergic neurons which innervate much broader regions of the brain including all forms of motor neurons. Whether subsets of serotonergic, orexinergic or noradrenergic neurons target the respiratory network selectively is unknown.
In the remainder of this review, the term RTN will be used to refer specifically to the above-defined bilateral cluster of superficial neurons that express Phox2b and VGLUT2 but contain neither cholinergic nor catecholaminergic biosynthetic enzymes. This choice is justified by the fact that, at this time, these characteristics seem to define a functionally and anatomically homogeneous cell group. Further subdivisions will have to be introduced in the future if it can be shown that the same region of the brain contains other types of neurons involved in breathing or if subsets of Phox2b/VGLUT2 neurons can be defined on functional grounds. For example, we recently found that about half of the RTN neurons express galanin, but this observation remains without a functional correlate (Stornetta et al., 2008). Finally, although the term RTN suggests the existence of a “nucleus”, it should be clear that the Phox2b/VGLUT2 positive cells do not form an architectonically distinct entity, i.e., a cell grouping that can be identified using a non-specific neuronal label (NeuN, cresyl violet). The RTN is part of the medullary reticular formation and, as such, the cell bodies and, most dramatically, the dendrites of the Phox2b/VGLUT2 positive cells are thoroughly intermingled with those of many other types of neurons which could also potentially influence breathing, most notably the C1 neurons. This intermingling is especially pronounced at the caudal end of the RTN.
6. RTN neurons function as central respiratory chemoreceptors in the adult
By definition, central respiratory chemoreceptors should possess the three following properties (Richerson et al., 2005; Guyenet et al., 2008). They should respond to small increments in arterial PCO2 in vivo by a vigorous increase in their rate of discharge. This response should be caused primarily by a direct effect of pH on these cells, although this criteria may need some adjustment pending definitive proof of the contribution of satellite cells (glial cells, vasculature etc.) to the CO2 sensitivity of central chemoreceptor neurons (Spyer et al., 2004; Gourine et al., 2005). Finally, an increase of the activity of central respiratory chemoreceptors should cause a measurable, preferably large, stimulation of breathing. This last property also implies that central respiratory chemoreceptors should have mono- or pauci-synaptic connections with the CPG.
RTN neurons fulfill the three criteria that define central chemoreceptors and they seem to conform to the simplest of many conceivable models, i.e. a group of acid-activated excitatory neurons that drive the CPG. These cells are vigorously activated by increasing PaCO2 in vivo (Mulkey et al., 2004; Guyenet, 2008). This characteristic is independent of the anesthetic (halothane, isoflurane, urethane, urethane-chloralose and pentobarbital; unpublished results of P. Guyenet, S. Abbott, M. Fortuna, T. Moreira and A. Takakura.) and it persists after silencing the CPG pharmacologically (by using, for example, ionotropic glutamate receptor blockade, or morphine)(Mulkey et al., 2004). The latter evidence means that the stimulation of RTN neurons by CO2 in vivo is not secondary to the activation of the pattern generator. It does not rule out the possibility that the activation of RTN neurons by CO2 in the absence of glutamate blocker could be partly due to excitatory synaptic inputs from other putative chemoreceptors (e.g. serotonergic, aminergic neurons or solitary tract nucleus (NTS) neurons).
A large fraction of the CO2 response of RTN neurons in vivo is presumably due to their intrinsic pH-sensitivity because these neurons are also strongly activated by mild extracellular acidification in vitro. This property has been demonstrated in coronal slices of mouse and rat brain (post-natal age 6–13 days) where the pH-sensitive neurons have been positively identified as VGLUT2- and Phox2b-expressing by single cell PCR (Mulkey et al., 2004; 2007b). As mentioned above, the pH-sensitivity of RTN neurons in slices is far greater at 30 degrees Celsius than at room temperature and approaches that observed in vivo under conditions of partial synaptic blockade (Guyenet et al., 2005b). The uniform pH-sensitivity of RTN neurons has been confirmed in a Phox2b-EGFP transgenic mouse in which RTN neurons were randomly selected for recording based on their fluorescence (unpublished results of R. Lazarenko, P. Guyenet and D. Bayliss). The pH-sensitivity of RTN neurons maintained in slices at room temperature is unchanged after blocking purinergic receptors, ionotropic glutamate, glycine and GABA receptors (Mulkey et al., 2004). The same study showed that alkalization enhances a background potassium current in the presence of tetrodotoxin. This evidence strongly suggests that these cells are intrinsically responsive to acidification. The responsible channel is not identified but does not appear to be TASK-1 or TASK-3 (Mulkey et al., 2007b). Also, there is no evidence that the pH-sensitive background potassium current present in RTN neurons accounts entirely for their acid-sensitivity.
Finally and importantly, the selective activation of RTN neurons stimulates breathing. The earliest supportive evidence has been that excitatory transmitters such as glutamate or TRH stimulate breathing when they are microinjected into the region of the RTN in vivo (Nattie and Li, 1995; Cream et al., 1999). The Phox2b/VGLUT2 neurons are most likely responsible for these effects because these cells are activated by iontophoretic application of the above mentioned excitatory transmitters in vivo and by bath applications of these substances in slices (Mulkey et al., 2007a). More importantly, a vigorous increase in breathing is produced by activating these Phox2b-expressing neurons selectively with pulsed laser light following their transfection with the light-activated cationic channel channelrhodopsin-2 (unpublished results of S. Abbott, R. Stornetta and P. Guyenet).
In summary, RTN neurons fulfill the three criteria that define central chemoreceptors. They are activated by acidification in vitro. They are strongly activated by hypercapnia in vivo, they innervate selectively the regions of the brain that contain the respiratory pattern generator and their selective activation produces a vigorous stimulation of breathing. Although RTN neurons are probably intrinsically sensitive to pH, some type of satellite cells (e.g. glial cells, vascular cells, or pericytes) may also mediate a portion of their activation by CO2 (Spyer et al., 2004; Gourine et al., 2005).
The relative contribution of RTN vs. other neurons to central respiratory chemoreception is unclear at this time. What has been examined so far is primarily the contribution of various neuronal groups to the chemoreflex as opposed to the regulation of breathing automaticity by CO2 which, as mentioned, generally occurs without significant change in PCO2. The chemoreflex is attenuated by selective elimination of numerous cell groups besides the RTN (orexin, serotonin, locus coeruleus) and many of these cells are pH-sensitive in vitro. Whether the attenuation of the chemoreflex caused by these lesions is due to the fact that these neurons have the ability to detect changes in arterial PCO2 in vivo is unknown (orexin neurons) or still controversial (serotonergic neurons)(Veasey et al., 1995; Mulkey et al., 2004). Even if one assumes that they do detect pH changes caused by severe hypercapnia, the fact that these neurons contribute to CO2 stabilization via effects on breathing under normocapnic conditions remains to be established. Some of these neurons (serotonergic in particular) innervate the RTN and may enhance the response of the latter neurons to hypercapnia (Mulkey et al., 2007a; Dias et al., 2008).
Chronic lesions of RTN neurons increase the apneic threshold of anesthetized adult rats. The change is marginal when less than half of the RTN cells are eliminated. It is considerable (from 5 to 8% CO2) in animals with around 70% cell loss but these animals remain viable (Takakura et al., 2008). This evidence could mean that many other types of CNS neurons make an equal or larger contribution than the RTN to central chemosensitivity but this interpretation must await the results of more complete destructions of the RTN. The possibility that RTN neurons could be the principal central respiratory chemoreceptors at birth is suggested by the Phox2b27Ala/+ mouse model of CCHS (Dubreuil et al., 2008). Indeed, this mouse lacks a response to CO2 and the only anatomical defect that was reported is the quasi absence of the RTN neurons as defined in this review. An alternative explanation of the respiratory deficit exhibited by the Phox2b27Ala/+ mouse is that the RTN neurons are the main convergence point for all other forms of central chemoreceptors, an interpretation that only reinforces the view that these neurons are critical for CO2 stabilization. A third, albeit highly speculative explanation, is that RTN neurons must be present to drive specific downstream elements of the CPG that are the “real chemoreceptors”.
7. Synaptic inputs to RTN neurons in the adult
Adult RTN neurons tagged with EGFP in a Phox2b-EGFP mouse receive numerous conventional excitatory inputs (asymmetric synapses, presumed to be glutamatergic) and inhibitory inputs (symmetric synapses, presumed to be GABAergic or glycinergic) (unpublished EM results by T. Milner, R. Stornetta, D. Bayliss and P. Guyenet). This observation reinforces the view that the level of activity of RTN cells also depends on conventional synaptic drives.
RTN neurons receive potent polysynaptic excitatory inputs from the carotid body (Takakura et al., 2006; Guyenet, 2008). These inputs, along with the presumably intrinsic response of the RTN neurons to brain PCO2, constitute what can be described collectively as their “chemical drive”. RTN neurons are therefore a chemosensory integrating center and the excitatory drive that these cells contribute to the pattern generator is a function both of brain PCO2 and of arterial gases as detected by peripheral chemoreceptors. Under normal physiological conditions, one would expect that the chemical drive of RTN neurons would be fairly constant since arterial PCO2 changes very little (Fig. 1C).
Based on the retrograde transport of neuronal tracers, the RTN region is a convergence point for inputs from the ventrolateral medulla, the nucleus of the solitary tract, the hypothalamus, the periaqueductal gray matter, the spinal cord, the dorsolateral pons and the raphe nuclei (Cream et al., 2002; Rosin et al., 2006). However, the RTN region contains the dendrites of many cell types (the C1 neurons in particular). Few of the inputs to this region have been definitively shown to control the activity of the RTN chemosensitive neurons and none have been shown to do so via direct synaptic contacts. RTN neurons are excited by TRH, serotonin and substance P and are therefore up-regulated by subsets of raphe neurons (Mulkey et al., 2007a). The activation of RTN neurons by peripheral chemoreceptors is probably mediated by a direct glutamatergic input from carotid body-responsive NTS neurons located in the caudal part of the NTS (Takakura et al., 2006). Inhibitory inputs to RTN neurons originate from the NTS and/or the ventrolateral medulla (Guyenet et al., 2005a; Moreira et al., 2007; Takakura et al., 2007). Some of these inputs relay sensory information from lung stretch receptors and J-receptors whereas others appear to mediate some form of feedback from components of the CPG such as early-inspiratory, post-inspiratory and late expiratory neurons (Guyenet et al., 2005a; Moreira et al., 2007; Takakura et al., 2007) (Fig. 1D). The functional significance of the CPG feedback to adult RTN neurons needs further evaluation. Formally, these inputs are somewhat reminiscent of the inhibitory input that the pfRG receives from the CPG at birth although this inhibition does not occur always or selectively during the inspiratory phase in adult RTN neurons in vivo (Onimaru and Homma, 2003; Guyenet et al., 2005a). Functionally, this feedback could account for the fact that, in vagotomized animals, the total phrenic outflow (product of rate and amplitude) and the activity of RTN neurons are both a saturable function of arterial PCO2 (Eldridge et al., 1981). This property is probably not due to the fact that the RTN or respiratory motor neurons reach their maximum discharge rate at saturating levels of CO2 because both neurons can reach higher levels of activity during hypothalamic stimulation (unpublished observations by M. Fortuna and P. Guyenet).
Given the plethora of respiratory modulated inputs that converge on RTN neurons, one would expect the respiratory modulation of RTN neurons to be both highly dependent on the preparation, the anesthetic and the PCO2 level. Indeed, although weak at low levels of CO2, the respiratory modulation of RTN neurons is greatly accentuated as the central respiratory drive increases and the respiratory pattern varies among RTN neurons and may even be species-specific (Guyenet et al., 2005a). This is the case of the respiratory modulation of the nearby C1 neurons (Janig and Habler, 2003). If this view is correct, the assumption that the discharge patterns of RTN neurons observed under anesthesia or in the Suzue preparation are similar in intact animals of the same developmental age (Wittmeier et al., 2008) seems hazardous.
8. Is the RTN a source of tonic drive to the CPG?
The word “tonic” drive conveys the notion of a postsynaptic effect that is roughly uniform throughout the respiratory cycle. In the older literature on breathing, the word probably referred to neurons whose discharge was hypothesized to be uniform throughout the respiratory cycle. In theory, such neurons could indeed exert a tonic effect by continually releasing a short-acting transmitter. However, a “tonic” drive could also be produced by neurons with phasic discharges, including neurons with respiratory phase-selective discharges, provided that these neurons signal via a low-pass filtered postsynaptic mechanism rather than via fast-acting, e.g. ionotropic, transmission. The available evidence, though still circumstantial, is compatible with the possibility that the RTN signals via such a slow mechanism. The first argument is the most indirect. As indicated above, the respiratory modulation of RTN neurons is highly variable from preparation to preparation, depends greatly on the degree of activation of the respiratory network and may even be species-specific (Nattie et al., 1993; Guyenet et al., 2005a). Yet, despite the differences in respiratory patterns, inhibiting RTN neurons always produces the same effect in cats or rats regardless of the respiratory drive, namely a reduction in phrenic rate and amplitude, whereas activating RTN cells always produces the opposite results. Therefore the mean discharge rate of RTN neurons probably matters far more than their specific respiratory pattern. The second argument is somewhat more direct. Light stimulation of RTN neurons transfected with channelrhodopsin-2 produces an activation of the phrenic nerve discharge that persists for tens of seconds after the end of the stimulus period (unpublished results of S. Abbott, R. Stornetta and P. Guyenet). This phenomenon has kinetic properties that are very reminiscent of the respiratory after-discharge elicited in cats by stimulating a carotid sinus nerve or skelettal muscle afferents except for the shorter time constant in rats vs. cats (t1/2 = 12 vs. 48 s)(Eldridge et al., 1981). There are many possible interpretations of the after-discharge but a simple one would be that the transmitter(s) released by RTN neurons elicit(s) a postsynaptic response that has a long half-life (about 12 seconds in rats). If this interpretation is correct, RTN neurons would not have the ability to dictate the time of onset of an inspiratory cycle that has a period of 1 s or less in rats. However such a long-lasting postsynaptic effect would be appropriate to underlie the “tonic” excitatory drive that rhythm/pattern generating neurons are thought to receive from central chemoreceptors. By contrast, judging from the many studies that have used spike-triggered averaging as an experimental approach, the ON-OFF respiratory neurons that are known to generate the respiratory pattern exert short-lived post-synaptic effects that are mediated by classic ionotropic transmission (glutamate, GABA, glycine)(Feldman and McCrimmon, 1999). The postsynaptic effects of RTN neurons are not known. Given that these cells are glutamatergic, some of their actions could be mediated by group I glutamatergic metabotropic receptors (mGluR1 and mGluR5) which are present on presumed rhythmogenic neurons (Pace et al., 2007).
In summary, in the adult at least, the possibility that RTN neurons contribute a tonic excitatory drive to various components of the CPG is compatible with the available evidence and deserves further scrutiny.
9. Relationship between the RTN and the perinatal parafacial respiratory group, pfRG
The parafacial respiratory group, pfRG, refers to a collection of twice-bursting respiratory neurons that have originally been identified in the vicinity of the facial motor nucleus in the Suzue preparation, a brainstem and spinal cord preparation obtained from the rat immediately after birth (Suzue, 1984; Onimaru et al., 2006). These neurons discharge both before and after the phrenic nerve and their inspiratory pause is due to an inhibitory input (reviewed by Ballanyi et al.( 1999)). Their given name (pre-inspiratory, pre-I, neurons) deliberately emphasizes the pre-inspiratory portion of their discharge which is thought by some investigators to initiate the activity of the preBötzinger oscillator on a cycle by cycle basis (Onimaru et al., 2006). The pfRG is therefore viewed as having “rhythmogenic” properties. Some of these neurons appear to have intrinsic bursting properties at birth and possibly since embryonic day 19 in rats (Onimaru et al., 1995; Onimaru and Homma, 2002).
An enduring issue with the pfRG concept has been that the pre-I pattern has not been identified within the medulla oblongata in older or more intact preparations. Under very specific pharmacological conditions however, the abdominal muscles of juvenile (7–13 day-old) rats do have a pfRG-like activity pattern but the presence of this pattern within the medulla oblongata and, a fortiori, in the RTN has not been reported (Janczewski and Feldman, 2006a). A second issue is that the pre-I pattern seems to be shared by many cell types in the Suzue preparation, most notably by neurons located in regions that, in more intact or older preparations, contain expiratory-augmenting neurons (Bötzinger region, cVRG, lumbar motor neurons)(Taccola et al., 2007). A very recent report by Onimaru and colleagues has clarified this issue considerably (Onimaru et al., 2008). The most rostral and superficial group of pre-I neurons, i.e. those that are located very close to the ventral medullary surface under the facial motor nucleus, have precisely the same phenotype as the RTN neurons of the adult. These pre-I neurons are not catecholaminergic, they express Phox2b and possibly VGLUT2, they have a dendritic structure that is highly reminiscent of that of the RTN neurons of the adult and they are acid-sensitive similar to adult RTN neurons. This evidence leaves little doubt that the very superficial pre-I neurons of the Suzue preparation that lie under the facial motor nucleus are the neonatal precursors of the RTN neurons as defined in this review or, at the very least, are the precursors of a subset of these cells. However, the same report (Onimaru et al., 2008) also describes another group of pre-I cells located in the Bötzinger region which lack Phox2b, are acid-insensitive and have a very different dendritic structure. This evidence demonstrates that the Suzue preparation contains multiple types of “pre-I” neurons, even in close proximity to the facial motor nucleus. Some of the pre-I neurons that are outside the RTN may be late-E neurons that develop a post-inspiratory rebound for lack of post-inspiratory inhibition. In support of this hypothesis, the glycinergic late-expiratory neurons of the Bötzinger region develop such a post-I rebound discharge in adult rats exposed to hypoxia and hypercapnia (Fortuna et al., 2008), classic post-I, e.g. E-DEC, neurons are apparently not or very rarely found in the Suzue preparation (Ballanyi et al., 1999) and neurons that receive chloride-mediated inhibitory volleys during both the pre- and post-I period have been identified in this preparation (Arata et al., 1998). Whether the post-inspiratory discharge of the RTN neurons in the Suzue preparation is a post-inspiratory rebound (Wittmeier et al., 2008) also needs to be demonstrated.
At postnatal age P7 and later, RTN neurons seem to have lost their intrinsic bursting properties judging from recordings made in coronal slices and in vivo (Mulkey et al., 2004; 2007a; 2007b). From this point on, the level of activity of RTN neurons in vivo presumably relies on a combination of synaptic drives and intrinsic pH-sensitivity as indicated above. The biological significance of the intrinsic pacemaker properties of neonatal RTN neurons is not fully understood at present. These properties may be vestigial, i.e. the remains of an oscillator used at earlier evolutionary times for other purposes (branchial movements), or they could be maintained because they are essential for breathing automaticity at the time of birth, i.e. before RTN neurons receive their mature innervation. The latter interpretation is consistent with the severe hypoventilation observed at this stage of life in two genetic models in which this nucleus is absent but more caudal regions of the CPG, in particular the preBötzinger complex, seem to be present and functional (Dubreuil et al., 2008; Pagliardini et al., 2008).
Understanding whether the neonatal pfRG is “rhythmogenic” or has a “tonic” modulatory role on the downstream CPG will require knowing the neuronal targets of the RTN neurons and the types of postsynaptic effects that these cells elicit, at birth and later in life. Evidence does suggest that some form of pre-I neuron exerts conventional short-lasting excitatory or inhibitory synaptic effects in the Suzue preparation (Onimaru et al., 1992). However, as noted above, the pre-I population is very heterogeneous in this preparation. Evidence that these short time-scale interactions originate from the Phox2b expressing RTN neurons as opposed to other types of pre-I neurons is needed.
In conclusion, there is little doubt that RTN neurons drive the CPG both during the perinatal period and later on in life. These excitatory neurons may change from rhythmogenic to simply modulatory as they mature but in all cases the cells seem to be subject to regulation by pH.
10. Does the RTN regulate inspiratory activity, expiratory activity or both?
The pre-inspiratory nature of the discharge of RTN neurons in the Suzue preparation is the principal argument supporting the view that these neurons might be part of a master inspiratory oscillator that triggers bursting within the pre-Bötzinger complex (Onimaru and Homma, 2003). The recent realization that lumbar expiratory motor neurons also have a twice-bursting “pre-I” pattern, not only in the Suzue preparation (Taccola et al., 2007) but in intact neonate rats treated with fentanyl and an NMDA receptor blocker (Janczewski et al., 2002), has spurred the opposite concept, namely that the pfRG is an “oscillator” that regulates expiratory activity (Feldman and Del Negro, 2006; Janczewski and Feldman, 2006b). A theoretical model, the “pacemaker handshake synchronization mechanism” endeavors to explain how the pfRG might both entrain the preBötC and drive a downstream expiratory oscillator (Wittmeier et al., 2008). The unanswered questions seem to be fourfold: 1) Are the RTN neurons, as defined in this review, the hypothesized pfRG expiratory oscillator? 2) Is a different population of RTN neurons involved? 3) Is the hypothesized expiratory oscillator a subset of the RTN neurons presently defined? 4) Is the postulated expiratory oscillator downstream from a subset of RTN neurons, the latter providing a chemosensory drive to the former? The answers to these questions will evidently require the cellular identification of the neurons suspected to form the pfRG expiratory oscillator. What is clear at this time is that stimulation of RTN neurons facilitates inspiratory motor activity and this finding is consistent in preparations ranging from the Suzue preparation to the unanesthetized adult rat.
The neuronal targets of RTN neurons are presently unknown but a degree of functional heterogeneity among RTN neurons is highly plausible with distinct subsets of these neurons potentially targeting distinct components of the CPG (e.g. rhythm generating neurons, selected pools of pre-motor neurons, etc.).
11. Conclusions
In the adult as in the neonate, RTN neurons function as central respiratory chemoreceptors (Dubreuil et al., 2008; Guyenet, 2008; Onimaru et al., 2008). In the adult, the activity of RTN neurons is also controlled by the peripheral chemoreceptors and by many synaptic inputs that require further characterization. RTN neurons are probably the adult form of a subset of the cells originally classified as pfRG neurons at birth in the Suzue preparation (Onimaru et al., 2008). Neonatal RTN neurons have intrinsic bursting activity but there is no evidence that this property persists for more than a few days after birth. At later times, synaptic inputs probably provide the background activity necessary to enable small changes in CO2 to up- or down-regulate the downstream CPG, thereby contributing to stabilizing arterial PCO2. Many investigators view the neonatal RTN as having a rhythmogenic function with three proposed variations on this theme: the first that this nucleus is a master inspiratory oscillator (Onimaru and Homma, 2003), the second that it is a master expiratory oscillator (Janczewski and Feldman, 2006b) and the third that it is both (Wittmeier et al., 2008). These discrepant theories will no doubt be resolved when the targets of individual RTN neurons and their postsynaptic actions are better known.
The prenatal differentiation or survival of RTN neurons in mice is eliminated by the PHOX2B mutation most commonly found in CCHS patients (Amiel et al., 2003; Dubreuil et al., 2008). The mice described by Dubreuil et al.(2008) and another mouse strain in which the RTN does not develop (Pagliardini et al., 2008) breathe poorly at birth suggesting that, at this age, RTN neurons are required for breathing automaticity as well as for central chemoreception. Although the RTN clearly participates in both functions in the adult (Nattie and Li, 2006; Takakura et al., 2008), there is no proof that this nucleus is as critical to respiration in adulthood as it seems to be at birth. However, this possibility has not been adequately tested because only partial lesions of this nucleus have been produced in the adult (Takakura et al., 2008) whereas the genetic approach in mice could conceivably have destroyed all of the relevant cells (Dubreuil et al., 2008).
The way in which RTN neurons regulate the CPG needs clarification. RTN is composed of around 2000 neurons in rat, a non-trivial number which suggests that some degree of functional heterogeneity among these neurons is very likely to exist. This heterogeneity is supported by three lines of evidence: 1) the RTN region controls both inspiration and expiration, 2) collectively, RTN neurons innervate the entire ventral respiratory column and, 3) the respiratory pattern of RTN neurons displays considerable within-animal heterogeneity. Efficient control of automatic breathing, hence proper CO2 stabilization, almost certainly requires that rhythm generating neurons and selected pools of pre-motor neurons be differentially controlled. A reasonable working hypothesis is that subclasses of RTN neurons have the ability to differentially regulate several breathing parameters (inspiration vs. expiration, breathing rate, etc.) to suit various physiological conditions.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health to PGG: HL74011 and HL28785.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol. 2008 doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de PL, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Arata A, Onimaru H, Homma I. Possible synaptic connections of expiratory neurons in the medulla of newborn rat in vitro. NeuroReport. 1998;9:743–746. doi: 10.1097/00001756-199803090-00033. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Talley EM, Sirois JE, Lei QB. TASK-1 is a highly modulated pH-sensitive ‘leak’ K+ channel expressed in brainstem respiratory neurons. Resp Physiol. 2001;129:159–174. doi: 10.1016/s0034-5687(01)00288-2. [DOI] [PubMed] [Google Scholar]
- Berthon-Jones M, Sullivan CE. Ventilation and arousal responses to hypercapnia in normal sleeping humans. J Appl Physiol. 1984;57:59–67. doi: 10.1152/jappl.1984.57.1.59. [DOI] [PubMed] [Google Scholar]
- Butera RJ, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. I. Bursting pacemaker neurons. J Neurophysiol. 1999;82:382–397. doi: 10.1152/jn.1999.82.1.382. [DOI] [PubMed] [Google Scholar]
- Chernov M, Putnam RW, Leiter JC. A computer model of mammalian central CO2 chemoreception. Adv Exp Med Biol. 2008;605:301–305. doi: 10.1007/978-0-387-73693-8_52. [DOI] [PubMed] [Google Scholar]
- Cream C, Li A, Nattie E. The retrotrapezoid nucleus (RTN): local cytoarchitecture and afferent connections. Respir Physiol Neurobiol. 2002;130:121–137. doi: 10.1016/s0034-5687(01)00338-3. [DOI] [PubMed] [Google Scholar]
- Cream C, Nattie E, Li A. TRH microdialysis into the RTN of the conscious rat increases breathing, metabolism, and temperature. J Appl Physiol. 1999;87:673–682. doi: 10.1152/jappl.1999.87.2.673. [DOI] [PubMed] [Google Scholar]
- Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neurosci. 1990;36:207–216. doi: 10.1016/0306-4522(90)90363-9. [DOI] [PubMed] [Google Scholar]
- Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Focal CO2 dialysis in raphe obscurus (ROb) does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus (RTN) J Appl Physiol. 2008 doi: 10.1152/japplphysiol.00120.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnoea and specific loss of parafacial neurons. Proc Natl Acad Sci USA. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J. Functional organization of respiratory neurones: a brief review of current questions and speculations. Exp Physiol. 2004;89:517–529. doi: 10.1113/expphysiol.2004.028027. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Gill-Kumar P, Millhorn DE. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, McCrimmon DR. Neural control of breathing. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. Academic Press; San Diego: 1999. pp. 1063–1090. [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, Plasticity, Chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna MG, West GH, Stornetta RL, Guyenet PG. Botzinger expiratory-augmenting neurons and the parafacial respiratory group. J Neurosci. 2008;28:2506–2515. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y. pH-sensitive cells at ventro--lateral surface of rat medulla oblongata. Nature. 1975;256:317–318. doi: 10.1038/256317a0. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gozal D. Congenital central hypoventilation syndrome: an update. Pediatr Pulmonol. 1998;26:273–282. doi: 10.1002/(sici)1099-0496(199810)26:4<273::aid-ppul7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005a;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005b;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Haldane JS, Priestley JG. The regulation of the lung-ventilation. J Physiol. 1905;32:225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56:433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006a;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006b;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janig W, Habler HJ. Neurophysiological analysis of target-related sympathetic pathways--from animal to human: similarities and differences. Acta Physiol Scand. 2003;177:255–274. doi: 10.1046/j.1365-201X.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol. 2005;145:115–126. doi: 10.1016/j.resp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Jiang C, Xu HX, Cui NR, Wu JP. An alternative approach to the identification of respiratory central chemoreceptors in the brainstem. Resp Physiol. 2001;129:141–157. doi: 10.1016/s0034-5687(01)00301-2. [DOI] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Muckenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem--spinal cord preparation of the neonatal rat. J Physiol. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Martino PF, Hodges MR, Davis S, Opansky C, Pan LG, Krause K, Qian B, Forster HV. CO2/H+ chemoreceptors in the cerebellar fastigial nucleus do not uniformly affect breathing of awake goats. J Appl Physiol. 2006;101:241–248. doi: 10.1152/japplphysiol.00968.2005. [DOI] [PubMed] [Google Scholar]
- Mellins RB, Balfour HH, Jr, Turino GM, Winters RW. Failure of automatic control of ventilation (Ondine’s curse). Report of an infant born with this syndrome and review of the literature. Medicine (Baltimore) 1970;49:487–504. [PubMed] [Google Scholar]
- Moosavi SH, Golestanian E, Binks AP, Lansing RW, Brown R, Banzett RB. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J Appl Physiol. 2003;94:141–154. doi: 10.1152/japplphysiol.00594.2002. [DOI] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, West GH, Guyenet PG. Inhibitory input from slowly adapting lung stretch receptors to retrotrapezoid nucleus chemoreceptors. J Physiol. 2007;580:285–300. doi: 10.1113/jphysiol.2006.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007a;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007b;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens. 1997;15:1613–1619. doi: 10.1097/00004872-199715120-00062. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol. 2006;101:1596–1606. doi: 10.1152/japplphysiol.00347.2006. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Chemoreceptors, breathing, and pH. Seldin and Giebisch’s The Kidney. In: Alpern RJ, Hebert SC, editors. Physiology & Pathophysiology. 4. 1–2. Elsevier; 2007. pp. 1587–1600. [Google Scholar]
- Nattie EE, Gdovin M, Li A. Retrotrapezoid nucleus glutamate receptors: control of CO2-sensitive phrenic and sympathetic output. J Appl Physiol. 1993;74:2958–2968. doi: 10.1152/jappl.1993.74.6.2958. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Rat retrotrapezoid nucleus iono- and metabotropic glutamate receptors and the control of breathing. J Appl Physiol. 1995;78:153–163. doi: 10.1152/jappl.1995.78.1.153. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group in the rat. J Appl Physiol. 1996;81:1987–1995. doi: 10.1152/jappl.1996.81.5.1987. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception is a complex system function that involves multiple brainstem sites. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Med Biol. 2008;605:348–352. doi: 10.1007/978-0-387-73693-8_61. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Intrinsic burst generation of preinspiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Exp Brain Res. 1995;106:57–68. doi: 10.1007/BF00241356. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I, Iwatsuki K. Excitation of inspiratory neurons by preinspiratory neurons in rat medulla in vitro. Brain Res Bull. 1992;29:879–882. doi: 10.1016/0361-9230(92)90159-u. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma T. Development of the rat respiratory neuron network during the late fetal period. Neurosci Res. 2002;42:209–218. doi: 10.1016/s0168-0102(01)00322-4. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. CO2-Sensitive Preinspiratory Neurons of the Parafacial Respiratory Group Express Phox2b in the Neonatal Rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBotzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Ren J, Gray PA, Vandunk C, Gross M, Goulding M, Greer JJ. Central respiratory rhythmogenesis is abnormal in lbx1- deficient mice. J Neurosci. 2008;28:11030–11041. doi: 10.1523/JNEUROSCI.1648-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson EA, Kozar LF, Rebuck AS, Murphy E. Ventilatory and waking responses to CO2 in sleeping dogs. Am Rev Respir Dis. 1977;115:251–259. doi: 10.1164/arrd.1977.115.2.251. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO(2) and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain ph homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res. 2007;165:201–220. doi: 10.1016/S0079-6123(06)65013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. J Appl Physiol. 1992;73:96–100. doi: 10.1152/jappl.1992.73.1.96. [DOI] [PubMed] [Google Scholar]
- Scheid P, Putnam RW, Dean JB, Ballantyne D. Special issue: central chemosensitivity. Respir Physiol Neurobiol. 2001;129:1–278. doi: 10.1016/s0034-5687(01)00297-3. [DOI] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100:13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, O’Neill MH. CO2/H+ chemoreception in the cat pre-Botzinger complex in vivo. J Appl Physiol. 2000;88:1996–2007. doi: 10.1152/jappl.2000.88.6.1996. [DOI] [PubMed] [Google Scholar]
- Spyer KM, Dale N, Gourine AV. ATP is a key mediator of central and peripheral chemosensory transduction. Exp Physiol. 2004;89:53–59. doi: 10.1113/expphysiol.2003.002659. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Spirovski D, Moreira TS, Takakura AC, West GH, Gwilt JM, Pilowsky PM, Guyenet PG. Galanin is a selective marker of the retrotrapezoid nucleus in rats. J Comp Neurol. 2008 doi: 10.1002/cne.21897. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccola G, Secchia L, Ballanyi K. Anoxic persistence of lumbar respiratory bursts and block of lumbar locomotion in newborn rat brainstem spinal cords. J Physiol. 2007;585:507–524. doi: 10.1113/jphysiol.2007.143594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, West GH, Gwilt JM, Colombari E, Stornetta RL, Guyenet PG. GABAergic pump cells of solitary tract nucleus innervate retrotrapezoid nucleus chemoreceptors. J Neurophysiol. 2007;98:374–381. doi: 10.1152/jn.00322.2007. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WA, Richerson GB. Chemosensitivity of non-respiratory rat CNS neurons in tissue culture. Brain Res. 2000;860:119–129. doi: 10.1016/s0006-8993(00)02033-3. [DOI] [PubMed] [Google Scholar]
- Wellner-Kienitz MC, Shams H, Scheid P. Contribution of Ca2+-activated K+ channels to central chemosensitivity in cultivated neurons of fetal rat medulla. J Neurophysiol. 1998;79:2885–2894. doi: 10.1152/jn.1998.79.6.2885. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmeier S, Song G, Duffin J, Poon CS. Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc Natl Acad Sci USA. 2008;105:18000–18005. doi: 10.1073/pnas.0809377105. [DOI] [PMC free article] [PubMed] [Google Scholar]