Abstract
Lysostaphin sensitivity was evaluated as a rapid screening test to differentiate Staphylococcus aureus from other species of staphylococci and micrococci. A total of 168 strains of staphylococci, 108 of which were S. aureus, were cultured overnight in brain infusion broth. Gram stains were peformed before and after a 1:10 dilution of the culture was exposed to 2 micrograms of lysostaphin per ml at 37 degrees C for 30 min. A reduction of 90% or greater in the number of organisms seen on comparison of the pre- and posttreatment Gram stains was considered a "positive" test result and was found in 106 of 108 S. aureus strains; 60 of 60 non-S. aureus staphylococci had a negative test result, showing no difference between the pre- and posttreatment Gram stains. Identical results were obtained using commerical blood culture media in place of brain heart infusion broth. Also studied prospectively were 100 blood or broth cultures which the clinical microbiology laboratory identified as containing gram-positive cocci suggestive of staphylococci. All 33 cultures later found to contain S. aureus gave positive test results; 67 of 67 non-S. aureus staphylococci, micrococci, and steptococci were negative.
Full text
PDF

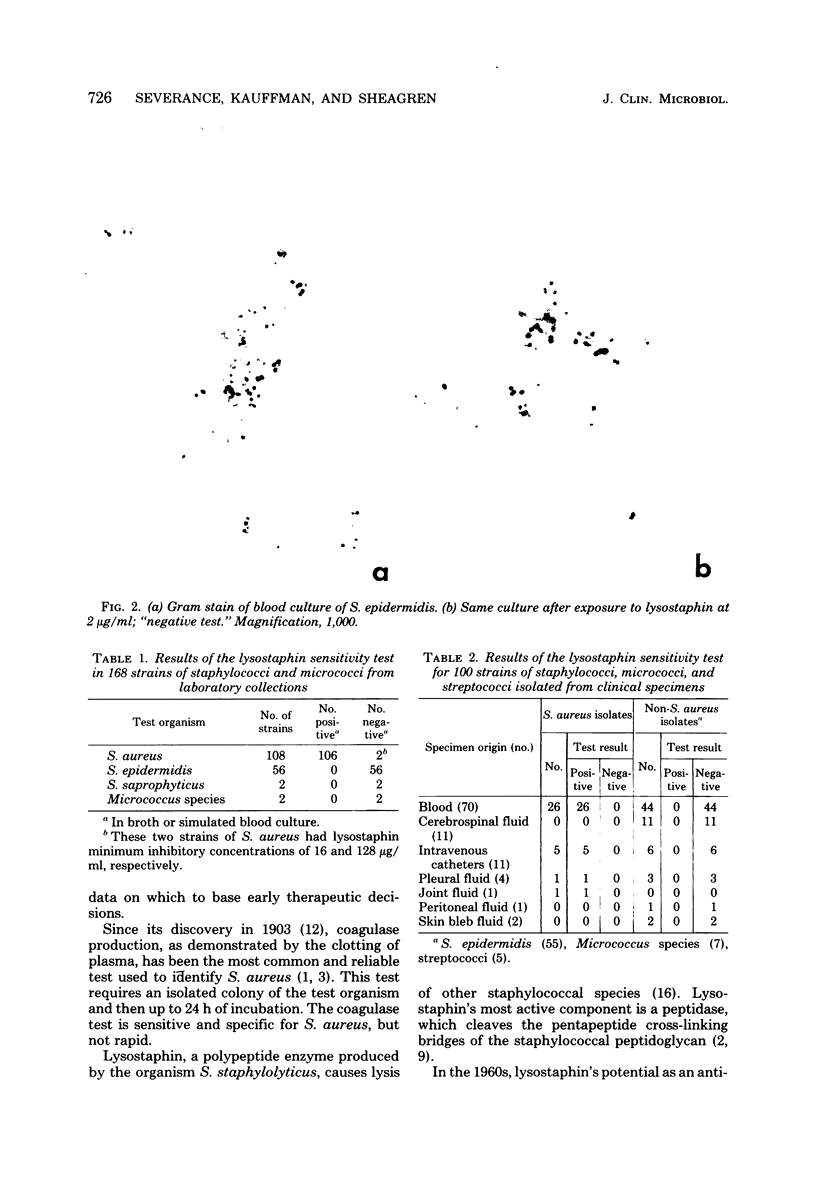

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWDER H. P., ZYGMUNT W. A., YOUNG J. R., TAVORMINA P. A. LYSOSTAPHIN: ENZYMATIC MODE OF ACTION. Biochem Biophys Res Commun. 1965 Apr 23;19:383–389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- HARRISON E. F., CROPP C. B. COMPARATIVE IN VITRO ACTIVITIES OF LYSOSTAPHIN AND OTHER ANTISTAPHYLOCOCCAL ANTIBIOTICS ON CLINICAL ISOLATES OF STAPHYLOCOCCUS AUREUS. Appl Microbiol. 1965 Mar;13:212–215. doi: 10.1128/am.13.2.212-215.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddaeus H., Heczko P. B., Pulverer G. Evaluation of the lysostaphin-susceptibility test for the classification of staphylococci. J Med Microbiol. 1979 Feb;12(1):9–15. doi: 10.1099/00222615-12-1-9. [DOI] [PubMed] [Google Scholar]
- Iversen O. J., Grov A. Studies on lysostaphin. Separation and characterization of three enzymes. Eur J Biochem. 1973 Oct 5;38(2):293–300. doi: 10.1111/j.1432-1033.1973.tb03061.x. [DOI] [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. R., White A. The selective activity of lysostaphin in vivo. J Lab Clin Med. 1967 Jul;70(1):1–8. [PubMed] [Google Scholar]
- Pulverer G., Jeliaszewicz J. Susceptibility of Staphylococcus epidermidis to lysostaphin and major antimicrobial agents. Chemotherapy. 1973;19(2):109–114. doi: 10.1159/000221445. [DOI] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. LYSOSTAPHIN: A NEW BACTERIOLYTIC AGENT FOR THE STAPHYLOCOCCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUHARDT V. T., SCHINDLER C. A. LYSOSTAPHIN THERAPY IN MICE INFECTED WITH STAPHYLOCOCCUS AUREUS. J Bacteriol. 1964 Sep;88:815–816. doi: 10.1128/jb.88.3.815-816.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Melly M. A., Koenig M. G. Lysostaphin: an enzymatic approach to staphylococcal disease. II. In vivo studies. Yale J Biol Med. 1967 Feb;39(4):230–244. [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kloos W. E. A simple test system for the separation of staphylococci from micrococci. J Clin Microbiol. 1975 Mar;1(3):337–338. doi: 10.1128/jcm.1.3.337-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J. Structures of the cell wall peptidoglycans of Staphylococcus epidermidis Texas 26 and Staphylococcus aureus Copenhagen. II. Structure of neutral and basic peptides from hydrolysis with the Myxobacter al-1 peptidase. Biochemistry. 1969 May;8(5):2192–2202. doi: 10.1021/bi00833a061. [DOI] [PubMed] [Google Scholar]
- Zygmunt W. A., Browder H. P., Tavormina P. A. Lytic action of lysostaphin on susceptible and resistant strains of Staphylococcus aureus. Can J Microbiol. 1967 Jul;13(7):845–853. doi: 10.1139/m67-111. [DOI] [PubMed] [Google Scholar]
- Zygmunt W. A., Browder H. P., Tavormina P. A. Susceptibility of coagulase-negative staphylococci to lysostaphin and other antibiotics. Appl Microbiol. 1968 Aug;16(8):1168–1173. doi: 10.1128/am.16.8.1168-1173.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt W. A., Tavormina P. A. Lysostaphin: model for a specific enzymatic approach to infectious disease. Prog Drug Res. 1972;16:309–333. doi: 10.1007/978-3-0348-7081-8_7. [DOI] [PubMed] [Google Scholar]




