Abstract
Background
In a recent cohort study, prolongation of the corrected QT interval (QTc) was associated with independent, increased risk of sudden cardiac death (SCD). We evaluated determinants of prolonged QTc as well as the relationship of prolonged QTc to SCD risk among patients with coronary artery disease (CAD) in the general population.
Methods and Results
A case-control design was utilized. Cases were SCDs with coronary artery disease (CAD) among a metropolitan area of 1,000,000 residents (2002-06) and controls were area residents with CAD, but no history of SCD. All cases were required to have an ECG suitable for QTc analysis prior and unrelated to the occurrence of SCD. A total of 373 cases and 309 control subjects met criteria for analysis. Mean QTc was significantly longer in cases vs. controls (450±45 vs. 433±37 ms, p<0.0001). In a multivariate model, gender, diabetes mellitus and QTc prolonging drugs were significant determinants of QTc prolongation in control subjects. In a logistic regression model predicting SCD, diabetes [OR 1.97 (1.32 – 2.96)] and use of QTc prolonging drugs [OR 2.90 (1.92 – 4.37)] were significant predictors of SCD among subjects with normal or borderline QTc. However, abnormally prolonged QTc in the absence of diabetes and QT-prolonging medications was the strongest predictor of SCD [OR 5.53, (3.20-9.57)].
Conclusions
Diabetes and QTc-affecting drugs determined QTc prolongation and were predictors of SCD in CAD. However, idiopathic abnormal QTc prolongation was associated with five-fold increased odds of SCD. A continued search for novel determinants of QTc prolongation, such as genomic factors, is likely to enhance risk stratification for SCD in CAD.
Keywords: Death, sudden, epidemiology, population, ventricular, repolarization, risk stratification
INTRODUCTION
A mechanistic link between the prolonged corrected QT interval (QTc) and increased risk of fatal arrhythmogenesis is well established by the detailed investigation of the relatively rare monogenic, long QT syndromes (1, 2). However, QTc prolongation has also been associated with increased risk of SCD in a non-long QT syndrome community-based cohort of unrelated individuals (3, 4). The potential of prolonged cardiac repolarization as a stratifier of risk among unrelated individuals in the general population merits further evaluation.
Coronary artery disease (CAD) is the condition most commonly associated with sudden cardiac death (SCD). At present, severe left ventricular (LV) systolic dysfunction is an established predictor of SCD but less than one-third of all SCD cases will have severe LV dysfunction (5, 6). Enhancement of risk stratification of risk stratification among CAD patients is likely to have significant impact on SCD prevention. QTc prolongation has emerged as a possible candidate but determinants of QTc prolongation among individuals with CAD remain unclear. In general, QTc prolongation is more common among diabetics (7) with a good correlation reported between prolonged QTc and overall cardiac mortality in diabetes (8, 9). QT-prolonging non-cardiac medications have also been implicated as contributors to SCD risk (10).
The Oregon Sudden Unexpected Death Study (Ore-SUDS) is an ongoing investigation of SCD among all residents of a large US community (11). Based on the hypothesis that diabetes and QT-prolonging medications can cause QTc prolongation, analyses were conducted to evaluate the determinants of QTc among patients with CAD. We also evaluated whether prolonged QTc was a predictor of SCD risk in this population.
METHODS
Ascertainment of Subjects
Detailed methods have been published earlier (6, 11). Briefly, the ongoing Oregon Sudden Unexpected Death Study (Ore-SUDS) prospectively identified all cases of SCD that occurred among residents of the Portland, OR metropolitan area (pop. approx. 1,000,000) during Feb 2002-Jan 2005 from the emergency medical response system, the Medical Examiner’s office, and local hospitals. During Feb 2005-Jan 2006, identification was limited to the majority subset identified by first responders or investigated by the medical examiner. SCD was defined as a sudden unexpected pulseless condition of likely cardiac etiology. If un-witnessed, SCDs were those in which patients were found dead within 24 hours of having last been seen alive and in normal state of health. Subjects with likely SCD were assigned a diagnosis of SCD after a review of available medical records and the circumstances of arrest; survivors of SCD were included. Subjects with chronic terminal illnesses (e.g. cancer), known non-cardiac causes of sudden death (e.g. pulmonary embolism, CVA), traumatic deaths and overdoses were excluded. Cases were also required to have documented significant CAD or if aged ≥50 years were assumed to have CAD (based on 95% likelihood of CAD in SCD cases aged≥50 years) (12, 13). CAD was defined as ≥50% stenosis of a major coronary artery or history of myocardial infarction, coronary artery bypass grafting or percutaneous coronary intervention.
During the same time period a control group of subjects from the same geographic region were identified who had CAD, but no history of SCD. They had either been transported by the Emergency Medical Response system for complaints suggestive of ongoing coronary ischemia, recruited from clinics of participating health systems, or received a coronary angiogram revealing significant CAD. After consent was obtained, medical records for each potential control subject were reviewed; those with documented CAD (as defined above) were enrolled.
Measurement of QTc interval from the 12-lead ECG
All cases and controls were required to have a 12-lead ECG in sinus rhythm. For SCD cases, the most recent ECG available in the medical records, prior to and unrelated to the cardiac arrest, was used. For control subjects, ECGs were obtained from the post-enrollment visit to the study site in one-third of the subjects, from the day of ascertainment for 40%, and prior to ascertainment for the remaining 27%. All subjects with ECG findings of QRS ≥120 ms were excluded from analysis. A standard 12-lead ECG tracing at 25 mm/s paper speed and 10 mm/mV amplitude was used, with measurements conducted manually using calipers. The QT interval was measured from the beginning of the earliest onset of the QRS complex to the end of the T wave. The end of the T wave was defined as the return of the descending limb to the TP baseline when not followed by a U wave or if distinct from the following U wave. If a second low-amplitude repolarization wave interrupted the terminal portion of the T wave, the T-wave offset was measured as the nadir between the T and U wave. After measuring in all precordial and limb leads, the longest QT interval was recorded. Two trained personnel performed separate, blinded measurements on a subset of ECGs. The QT interval was corrected (QTc) using Bazett’s formula (14). Data were analyzed using gender-specific QTc categories as defined in the Rotterdam study [males ≤430 (normal), 431-450 (borderline), >450 (abnormal); females ≤450 (normal), 451-470 (borderline), >470 (abnormal)] (4).
LV ejection fraction was used to assess the association of LV function and QTc interval for the subset of patients with ejection fraction measured prior to arrest (cases) or ascertainment (controls) by echocardiogram, angiogram, or MUGA. To identify drugs that prolong the QT interval and/or induce Torsades de Pointes ventricular arrhythmia, we used the exhaustive set of lists from the Arizona Center for Education and Research on Therapeutics (http://www.arizonacert.org/medical-pros/drug-lists/drug-lists.htm).
Statistical analysis
Independent samples t-tests and Pearson’s chi-square tests were used for bivariate case-control comparisons of continuous and categorical variables, respectively. A general linear model was used for analysis of covariance to evaluate significant independent predictors of QTc interval length in control study subjects, as well as potential interaction among covariates. Potential predictors of the QTc interval were age, gender, history of MI, diabetes, obesity, and use of QT-prolonging medications. All two-way interactions between significant main effects terms were evaluated. A model was also run for the subgroup of control patients with LV function assessment available.
Finally, univariate logistic models were used to estimate the association between SCD and QTc, diabetes, and QT-prolonging medications, and a multiple logistic regression model was used to estimate odds ratios for SCD associated with abnormal QTc (vs. normal or borderline QTc) adjusted for age, gender, history of MI, diabetes, obesity, and use of QT-prolonging medications. Abnormal QTc was compared to the normal and borderline QTc groups combined based on initial results showing that borderline QTc had no elevation in risk compared to normal QTc. Two-way interactions were also evaluated between all covariates and abnormal QTc, and between diabetes and QT-prolonging medications. Main effects terms were retained in the logistic model for any factors with significant interaction. When significant interactions were identified, we used the full model to estimate odds ratios for SCD for relevant patient subgroups.
For all analyses, main effects terms were considered significant if p<0.05. For identification of interaction, p<0.20 was considered potentially significant; p-values for each interaction term were reported.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
In the four year period Feb. 1, 2002 to Jan. 31, 2006, 1678 adult cases of SCD (age ≥ 18) were identified in the Portland, Oregon metropolitan area. Of these, 682 (41%) had ECGs available. Of the 682 cases, 403 (59%) had ECGs prior to arrest with sinus rhythm and QRS < 120 ms. Reasons for exclusion of case ECGs were timing (46%), abnormal rhythms (29%), and QRS ≥ 120 ms (25%). In the same time period, 400 control subjects were identified; 379 (86%) had an ECG performed, and 309 (82%) had an ECG with sinus rhythm and QRS < 120 ms. Control ECGs were excluded due to abnormal rhythms (37%) and QRS interval ≥ 120 ms (25%). For both cases (51%) and controls (87%), the majority of ECGs were done within a year of the SCD occurrence or control ascertainment, respectively.
Among the 403 case subjects with ECGs meeting criteria, 373 also had documented CAD or assumed CAD based on age. All control subjects had documented CAD (n=309). Basic demographics and clinical characteristics of cases and controls are summarized in Table 1. Cases were older, had a higher frequency of diabetes, medications that prolong the QT interval and severe LV dysfunction (Table 1).
Table 1.
Demographics and SCD risk factors in case and control subjects
| Cases (n = 373) | Controls (n = 309) | p-value* | |
|---|---|---|---|
| Age, y | 70 ± 13 | 64 ± 12 | <0.0001 |
| Male, % | 60 | 64 | 0.24 |
| History of MI | 33% | 28% | 0.23 |
| History of diabetes | 40% | 33% | 0.06 |
| Obese† | 35% | 38% | 0.37 |
| Severe LV Dysfunction (EF ≤ 35%)‡ | 22% | 9% | 0.004 |
| Using medication with risk of causing Torsades de Pointes (qtdrugs.org List 1) | 7.5% | 3.9% | 0.05 |
| Using medication that may prolong QT interval (qtdrugs.org Lists 2 or 3) | 38% | 26% | 0.0005 |
| Any medication from Lists 1-3 | 43% | 27% | <0.0001 |
p-value from t-tests for continuous variables and chi-square tests for categorical variables.
BMI ≥30; BMI available for 309 cases and 291 controls
EF from echo, angiogram, or MUGA for 188 cases and 116 controls
Reliability of QT measurement
ECGs for a subset of patients in this analysis (197 cases and 276 controls) were randomly selected to be read by a second reader. The intra-class correlation coefficient comparing the first and second reader for the QT interval was 0.92 (95% CI 0.90 – 0.93), and for the RR interval was 0.94 (95% CI 0.93 – 0.95), indicating excellent reliability in the method of reading ECGs.
Greater prolongation of QTc interval in cases of sudden cardiac death
Cases were significantly more likely than controls to have an abnormally prolonged QTc interval (Table 2; p<0.0001). Mean QTc was 17 ms longer in cases (p<0.0001; Table 2). Figure 1 shows the distribution of QTc in case and control subjects. Prolongation of the QTc interval was consistently associated with SCD status across demographic and clinical characteristics, except in subjects using QT-prolonging drugs, subjects with diabetes, and subjects with severe LV dysfunction (Table 2).
Table 2.
QTc categories and mean QTc interval in case and control subjects, stratified by demographics and clinical characteristics
| Cases (n = 373) | Controls (n = 309) | Difference in ms | p-value* | |
|---|---|---|---|---|
| QTc category† (n, %) | ||||
| Normal | 164 (44%) | 179 (58%) | <0.0001 | |
| Borderline | 63 (17%) | 66 (21%) | ||
| Abnormal | 146 (39%) | 64 (21%) | ||
| QTc ≥ 500 ms (n, %) | 47 (13%) | 10 (3%) | <0.0001 | |
| Mean QTc, all subjects | 450 ± 45 | 433 ± 37 | 17 | <0.0001 |
| Gender | ||||
| Males | 446 ± 47 (n=224) | 429 ± 38 (n=199) | 17 | <0.0001 |
| Females | 457 ± 40 (n=149) | 442 ± 32 (n=110) | 15 | 0.001 |
| Age | ||||
| 65 and over | 452 ± 46 (n=224) | 434 ± 37 (n=137) | 18 | <0.0001 |
| Under 65 | 448 ± 44 (n=149) | 433 ± 36 (n=172) | 15 | 0.009 |
| History of MI | ||||
| Yes | 453 ± 43 (n=122) | 432 ± 32 (n=88) | 21 | <0.0001 |
| No | 449 ± 46 (n=251) | 434 ± 38 (n=221) | 15 | 0.0001 |
| Diabetes | ||||
| Yes | 452 ± 43 (n=148) | 443 ± 40 (n=101) | 9 | 0.11 |
| No | 449 ± 46 (n=225) | 429 ± 34 (n=208) | 20 | 0.0001 |
| Obese‡ | ||||
| Yes | 454 ± 48 (n=107) | 435 ± 33 (n=111) | 19 | 0.0007 |
| No | 449 ± 44 (n=202) | 433 ± 39 (n=180) | 16 | 0.0001 |
| Severe LV Dysfunction§ | ||||
| Yes | 457 ± 47 (n=42) | 446 ± 27 (n=11) | 11 | 0.45 |
| No | 459 ± 44 (n=146) | 434 ± 33 (n=105) | 25 | <0.0001 |
| Using any medication known to prolong QT or increase risk of TdP | ||||
| Yes | 449 ± 46 (n=161) | 446 ± 42 (n=84) | 3 | 0.64 |
| No | 451 ± 44 (n=212) | 429 ± 33 (n=225) | 22 | <0.0001 |
p-value from Pearson chi-square test for categorical variables and independent samples t-test for continuous variables;
QTc categories: Males ≤430 ms (normal), 431-450 ms (borderline), >450 ms (abnormal); Females ≤450 ms (normal), 451-470 ms (borderline), >470 ms (abnormal).
BMI ≥30, BMI available for 309 cases and 291 controls;
EF from echo, angiogram, or MUGA for 188 cases and 116 controls.
Figure 1.
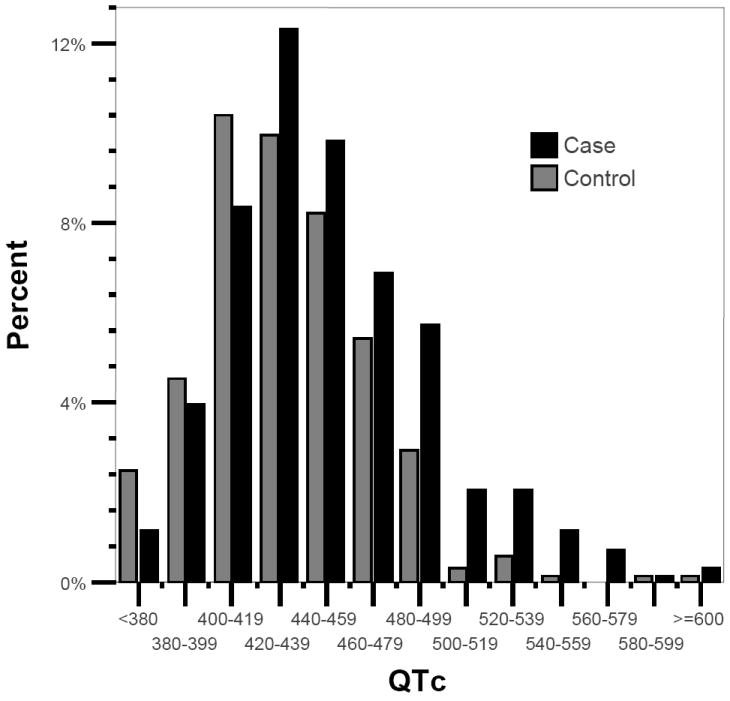
Distribution of the heart-rate corrected QT interval (QTc) in case and control subjects
Determinants of QTc interval prolongation
In the analysis of covariance model, age, history of MI, and obesity did not significantly predict QTc interval when controlling for the other variables (p≥0.43). In a final multivariate model only gender (p=0.01), diabetes (p=0.0007), use of QT prolonging medications (p=0.0003), and the interaction between diabetes and medications were significant (p=0.08). The adjusted (least squares) mean QTc interval was longer in females than males (534 vs. 446 ms) (Table 3). Use of QT-prolonging medications and diabetes were significantly associated with the QTc interval, but their effects depended on each other (the test for interaction indicated marginal significance; p= 0.08). Diabetics using QT-prolonging medications had a significantly longer adjusted mean QTc interval compared to other patients (p≤0.01) (Table 3). Because age has been associated with QTc in previous studies, we repeated analyses retaining age in the ANCOVA model, and obtained very similar results (data not shown). In the subset of individuals with measurement of LV ejection fraction available (188 cases and 116 controls), severe LV dysfunction was non-significantly associated with a longer QTc (452 vs 439, p=0.21).
Table 3.
Predictors of QTc duration among control subjects in multivariate analysis of covariance model*
| Adjusted mean | p-value† | |
|---|---|---|
| Gender | 0.01 | |
| Male | 435 | |
| Female | 446 | |
| Diabetes* QT-prolonging medications interaction | 0.08 | |
| No diabetes and no QTmeds | 428 | |
| With diabetes and no QTmeds | 437 | |
| No diabetes and use of QTmeds | 436 | |
| With diabetes and use of QTmeds | 462‡ |
Abbreviations: QTmeds = QT-prolonging medications.
Model predicting QTc in control subjects (with coronary artery disease but no SCD) included age, gender, history of myocardial infarction (MI), diabetes, obesity, and use of QT medications;
p value from analysis of covariance F statistic;
Mean differs from three other groups (p≤0.01) in post-hoc test using Tukey-Kramer adjustment for multiple comparisons.
Use of QTc-interval prolonging medications
Case subjects were significantly more likely to be taking QT-prolonging medications (p<0.0001, Table 4). The vast majority of QTc drugs prescribed in either group were non-cardiac. For both groups, the majority of patients were taking a single QT-prolonging medication (Table 4). We were able to establish that for 160 (43%) of the 373 case subjects in this analysis, the date the medications were noted was within 45 days of the ECG. Similarly, 144 (39%) of the 373 case subjects were on the listed medications within the 45 days prior to arrest.
Table 4.
Use of QT-prolonging medications among cases and controls
| Cases (n = 373) | Controls (n=309) | p-value | |
|---|---|---|---|
| Any QT-prolonging medication* | 161 (43%) | 84 (27%) | <0.0001 |
| Type of QT-prolonging medications | |||
| Cardiac† | 16 (10%) | 7 (8%) | 0.68 |
| Non-cardiac | 145 (90%) | 77 (92%) | |
| Number of QT-prolonging medications | |||
| None | 212 (57%) | 225 (73%) | 0.0002 |
| 1 | 133 (36%) | 73 (24%) | |
| 2 | 25 (6%) | 11 (3%) | |
| 3 or 4 | 3 (1%) | 1 (<1%) |
QT-prolonging medications included antiarrhythmics, antibiotics, antidepressants, antihypertensives, antipsychotics, anti-epileptic medications, bronchodilators, diuretics, lithium, and methadone.
Cardiac QT-prolonging medications = antiarrhythmic agents that prolong QT interval; all others defined as non-cardiac.
QTc remained a significant predictor of SCD among subjects not taking QT-prolonging medications
In the univariate logistic model, abnormal QTc and borderline QTc were compared to normal QTc. Abnormal QTc was significantly associated with SCD (odds ratio (OR) 2.5, 95% confidence interval (CI) 1.7-3.6), while borderline QTc was not (OR 1.0, 95% CI 0.7-1.6). In univariate models, diabetes (OR 1.4, 95% CI 1.0-1.9) and QT-prolonging medications (OR 2.0, 95% CI 1.5-2.8) were also associated with SCD.
In the initial multiple logistic regression models, borderline QTc had odds ratios close to 1.0, and thus the normal and borderline QTc categories were combined as a reference category. History of MI and obesity were not associated with SCD, and were removed from the model, as was the interaction term for diabetes and QT-prolonging medications (p=0.36). The final model included age (p<0.0001); gender (p=0.42) (retained based on an a priori decision to adjust for gender); abnormally-prolonged QTc (p<0.0001); QT-prolonging medications (p<0.0001); and diabetes (p=0.001) as main effects terms, and interaction terms for diabetes and abnormal QTc (p=0.007) and QT-prolonging medications and abnormal QTc (p=0.0009).
Table 5 presents odds ratios for SCD from the multivariable model. Among subjects without diabetes and not using QT-prolonging medications (idiopathic QTc prolongation), an abnormally long QTc quintupled odds of SCD (OR 5.53, 95% CI 3.20 – 9.57). In subjects with diabetes who were not using QT-prolonging medications, an abnormally prolonged QTc remained associated with SCD, but the magnitude of the effect was attenuated (OR 2.04, 95% CI 1.08 – 3.85). In subjects using QT-prolonging medications, there was no significant association between abnormal QTc and SCD, whether diabetes was present or absent (p>0.14).
Table 5.
Factors independently associated with sudden cardiac death (SCD) from the full multiple logistic regression model: QTc prolongation*, diabetes, and use of QT-prolonging medications.
| Odds Ratio (95% Confidence Interval)† | |
|---|---|
| Patient subgroup from full model | Abnormal QTc vs. Normal/Borderline QTc (reference) |
| No diabetes and no QTmeds | 5.53 (3.20 – 9.57) |
| With diabetes and no QTmeds | 2.04 (1.08 – 3.85) |
| No diabetes and use of QTmeds | 1.60 (0.83 – 3.05) |
| With diabetes and use of QTmeds | 0.59 (0.29 – 1.19) |
| Patient subgroup from full model | Diabetes vs. No Diabetes (reference) |
| Normal/borderline QTc | 1.97 (1.32 – 2.96) |
| Abnormal QTc | 0.73 (0.39 – 1.34) |
| Patient subgroup from full model | Use of QT-Prolonging Meds vs. No Use (reference) |
| Normal/borderline QTc | 2.90 (1.92 – 4.37) |
| Abnormal QTc | 0.84 (0.45 – 1.54) |
Abbreviations: QTmeds = QT-prolonging medications.
QTc categories: Males ≤430 ms (normal), 431-450 ms (borderline), >450 ms (abnormal); Females ≤450 ms (normal), 451-470 ms (borderline), >470 ms (abnormal).
From full multiple logistic regression model adjusting for age (p<0.0001), gender (p=0.42), abnormal QT interval vs normal or borderline (p<0.0001), use of QT medications (yes/no) (p<0.0001), diabetes (yes/no) (p=0.001), and interaction terms for QT interval and diabetes (p=0.007), the QT interval and use of QT medications (p=0.0009).
From the same multivariable models we also estimated the adjusted effects of diabetes and QT-prolonging drugs on odds of SCD. Due to the interaction between both of these factors and the QTc interval, we present estimates for their effects by QTc interval category (normal/borderline QTc and abnormal QTc, Table 5). Both diabetes and use of QT-prolonging medications doubled or nearly tripled odds of SCD, but only in subjects with normal or borderline QTc. Neither diabetes nor QT-prolonging medications were related to SCD among subjects with an abnormally-prolonged QTc (p>0.30).
Finally, the full logistic regression model in the subset of patients with data on ejection fraction (188 cases and 116 controls) showed that severe LV dysfunction was significantly associated with SCD (p=0.002), and there was significant interaction between LV dysfunction and the QTc interval (p=0.03). Among patients with normal or borderline QTc, severe LV dysfunction was strongly associated with SCD (OR 5.11, 95% CI 1.83-14.22); this association was not observed in subjects with abnormal QTc (p=0.86).
DISCUSSION
Summary of main findings
In this population-based sample of subjects with coronary disease, QTc interval duration was significantly greater in cases (those who suffered SCD) vs. controls (those who did not have a history of SCD). Gender, diabetes, and use of QT-prolonging medications were significant predictors of QT interval prolongation. In multiple logistic regression analyses, abnormally prolonged QTc in the absence of diabetes and without use of QT-prolonging medications was an important determinant of SCD risk (OR 5.53, 95% CI 3.20 – 9.57).
The vast majority (at least 80%) of all cases of SCD are associated with significant CAD (12, 15). At present, the only available predictor of SCD risk employed in clinical practice is severe LV systolic dysfunction. However, recent population-based studies have reported that only a minority of patients (20-30%) are found to have LV systolic dysfunction prior to SCD (5, 6, 16). In fact, the majority have either normal or mild-moderately decreased LV systolic dysfunction (6). As a consequence, there is an urgent need to identify additional novel and clinically relevant predictors of SCD in patients with coronary disease. In early small case-control studies, QTc prolongation was identified as a predictor of SCD in patients who suffered myocardial infarction (17). Most recently, the Rotterdam Heart Study provided strong evidence of a doubling of SCD risk with abnormal QTc prolongation among middle-aged as well as older members of this large cohort (3, 4, 18). Therefore, abnormal QTc prolongation has significant potential for use in enhancing risk stratification as well as future prevention of SCD. However, before this potential can be exploited, it would be important to understand the etiology of QTc prolongation among patients with CAD. In the current study, DM and QT-prolonging drugs were independent determinants of QTc prolongation in the control population. In addition, patients with both of these factors had a significantly longer QT interval than patients with neither or only one of these factors.
Both DM and QT-prolonging drugs have been previously associated with increased SCD risk. The association of DM with SCD risk was first identified in the Paris Prospective Study from analysis performed among >6,000 middle-aged, healthy male Parisian civil servants who were enrolled and followed for over 23 years (19, 20). The US Nurses Study and the Physicians Health Study (21, 22), as well as a retrospective clinical database analysis from a health cooperative in Seattle (23), have reported similar findings. While these observations implicate DM as an important factor in pathogenesis of SCD, our findings indicate that this relationship may be complex. Diabetes appears to confer risk of SCD only in subjects with normal or borderline QTc interval. The association between non-cardiac QT-prolonging drugs and SCD was evaluated from a large longitudinal observational database in the Netherlands and use of a non-cardiac QT-prolonging drug was associated with a significantly increased risk of SCD (adjusted OR: 2.7; 95% CI: 1.6–4.7), nearly identical to the findings reported in the present study. However, the present study evaluated all QTc prolonging drugs, cardiac and non-cardiac. The fact that QT-prolonging drugs were associated with SCD occurrence in the absence of QTc-prolongation has potential implications for their clinical utilization and follow-up of patients. A potential mechanistic explanation has recently been reported by Hinterseer et al (24). This report noted that in some patients with drug-induced torsades, beat to beat or short-term variability of the QT interval may be more useful as a predictor of arrhythmic risk, than absolute value of the QTc.
The most interesting and somewhat unexpected finding of the present study is that abnormally prolonged QTc of unknown etiology was a powerful independent predictor of SCD. When comparing two groups, both of which have coronary disease and similar co-morbidities, a five-fold increase in odds is a significant finding. If not DM or drugs, what then are the potential etiologies of prolonged QTc in coronary disease? It has been postulated that heart failure could be a form of acquired, abnormally prolonged ventricular repolarization (25). In the present study however, while there was a strong association (5-fold increased risk) between severe LV dysfunction and SCD in patients with normal QTc, this was not observed for patients with borderline/abnormally prolonged QTc. While this appears contrary to some observations published in the literature, these findings likely relate to the nature of the population. Both cases and controls have significant coronary disease, high prevalence of diabetes as well as other conditions that indicate high risk. Individuals with QT-prolongation who also have diabetes and are using QT-prolonging medications may have additional risk for SCD conferred by co-morbidities. In the present study, two other co-morbidities chronic renal insufficiency and chronic obstructive pulmonary disease, were significantly more common in subjects with prolonged QTc, diabetes, and QT-prolonging medications than in subjects with idiopathic prolonged QTc (data not shown). With many competing risks, the SCD risk attributable to QTc-prolongation may become attenuated.
Our findings of SCD cases with prolonged QTc in the absence of DM, QT-prolonging drugs or severe LV dysfunction, suggests there may be genetic factors contributing to QTc prolongation. There is a growing body of literature regarding the contribution of common genetic variants to cardiac repolarization. Bezzina and colleagues demonstrated that a common polymorphism in the KCNH2 gene influenced QT interval length in healthy individuals in the MONICA study (26). Further evidence of the influence of common gene variants on the QT interval came from a linkage disequilibrium based SNP association study of the KORA population in Germany (27). More recently, Arking, Pfeuffer and co-workers performed a genome-wide association study on subjects from the KORA cohort (28) and identified a common genetic variant in NOS1AP (CAPON), a regulator of neuronal nitric oxide synthase, as a new target that modulates cardiac repolarization. Several other studies have since been published that have confirmed the role of this common genetic variant as a determinant of the QT interval (29-31). Newton-Cheh and colleagues, using a candidate gene variation approach in the Framingham Heart Study population, found that two common genetic variants at the KCNH2 locus were associated with continuous QT interval duration and were able to replicate these observations in an independent sample of the same population. Albert and co-workers recently evaluated the role of repolarization-prolonging SCN5A gene variants in 113 SCD cases from 2 large prospective cohorts of women (Nurses’ Health Study) and men (Health Professional Follow-Up Study) (32). Found in a significantly higher frequency in cases vs. controls (10% vs. 1.6%), functionally significant mutations and rare variants in SCN5A are likely to have contributed to SCD risk among women, but not among men. Taken together, published studies and the present study would suggest that abnormally prolonged ventricular repolarization may be a causative factor in the pathophysiology of SCD in patients with CAD, and not just a marker of increased morbidity. Therefore, the continued identification of gene variants that determine QT interval duration has become an important scientific priority in the field (33).
This study has some potential limitations. Approximately half of case subjects (47%) had medically-documented CAD but the remainder were assumed to have CAD based on prior studies showing >95% of SCDs at age ≥ 50 have significant CAD at autopsy (12, 13). However, sensitivity analyses showed that the results were very similar when analysis was restricted to cases with documented CAD. Though all ECGs with QRS≥120 ms were excluded from analysis, we also considered possible confounding of the QTc-SCD association by QRS duration and did not find evidence of any. Finally, in a population-based study of this nature, the exact timing of medications can be difficult to determine. However, we were able to determine that for 160 (43%) of the 373 case subjects in this analysis, the date the medications were noted was within 45 days of the ECG. In addition, the large proportion (up to 50%) of patients with sudden death as a first cardiac event may have infrequent visits to their health care provider. For some of these patients, medications noted more remotely from the ECG may represent the medications taken when the ECG was performed. Similarly, we were able to establish that 144 (39%) of the 373 case subjects were on the listed medications within the 45 days prior to arrest, indicating that a substantial number of patients were likely to be on the listed medications during the time of cardiac arrest.
Conclusions
These findings underscore the importance of prolonged QTc as an independent predictor of SCD risk in the general population, even in the presence of CAD and diabetes. Idiopathic QTc prolongation was a strong, significant predictor, resulting in a five-fold increased risk of SCD. Since common as well as rare genetic variants are likely to be important contributors to cardiac ventricular repolarization, continued identification of these factors is likely to enhance risk stratification and prevention of SCD in patients with coronary disease.
Acknowledgments
The authors would like to acknowledge the significant contribution of American Medical Response, Portland/Gresham fire departments and the Oregon State Medical Examiner’s office. The authors thank Eric Stecker for his critical review of the manuscript.
Funding sources: Supported by NIH National Heart Lung and Blood Institute R01 HL088416 and a Hopkins-Reynolds Clinical Cardiovascular Center grant to Sumeet S. Chugh.
Footnotes
Conflict of Interest Disclosures: SS Chugh: None; K Reinier: None; T Singh: None; A Uy-Evanado: None; C Socoteanu: None; D Peters: None; R Mariani: None; K Gunson: None; J Jui: None.
References
- 1.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A., Jr The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–44. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 3.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–94. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 4.Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 5.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–9. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 6.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–6. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 7.Veglio M, Borra M, Stevens LK, Fuller JH, Perin PC. The relation between QTc interval prolongation and diabetic complications. The EURODIAB IDDM Complication Study Group. Diabetologia. 1999;42:68–75. doi: 10.1007/s001250051115. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso CR, Salles GF, Deccache W. QTc interval prolongation is a predictor of future strokes in patients with type 2 diabetes mellitus. Stroke. 2003;34:2187–94. doi: 10.1161/01.STR.0000085084.15144.66. [DOI] [PubMed] [Google Scholar]
- 9.Rana BS, Lim PO, Naas AA, Ogston SA, Newton RW, Jung RT, Morris AD, Struthers AD. QT interval abnormalities are often present at diagnosis in diabetes and are better predictors of cardiac death than ankle brachial pressure index and autonomic function tests. Heart. 2005;91:44–50. doi: 10.1136/hrt.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straus SM, Sturkenboom MC, Bleumink GS, Dieleman JP, van der Lei J, de Graeff PA, Kingma JH, Stricker BH. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;26:2007–12. doi: 10.1093/eurheartj/ehi312. [DOI] [PubMed] [Google Scholar]
- 11.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–75. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Kannel WB, Gagnon DR, Cupples LA. Epidemiology of sudden coronary death: population at risk. Can J Cardiol. 1990;6:439–44. [PubMed] [Google Scholar]
- 13.Kannel WB, Schatzkin A. Sudden death: lessons from subsets in population studies. J Am Coll Cardiol. 1985;5:141B–149B. doi: 10.1016/s0735-1097(85)80545-3. [DOI] [PubMed] [Google Scholar]
- 14.Bazett H. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 15.Myerburg RJ, Interian A, Jr, Mitrani RM, Kessler KM, Castellanos A. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol. 1997;80:10F–19F. doi: 10.1016/s0002-9149(97)00477-3. [DOI] [PubMed] [Google Scholar]
- 16.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, Wellens HJ. Out-of-hospital cardiac arrest in the 1990’s: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–5. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation. 1978;57:1074–7. doi: 10.1161/01.cir.57.6.1074. [DOI] [PubMed] [Google Scholar]
- 18.Moss AJ. QTc prolongation and sudden cardiac death: the association is in the detail. J Am Coll Cardiol. 2006;47:368–9. doi: 10.1016/j.jacc.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Balkau B, Jouven X, Ducimetiere P, Eschwege E. Diabetes as a risk factor for sudden death. Lancet. 1999;354:1968–9. doi: 10.1016/S0140-6736(99)04383-4. [DOI] [PubMed] [Google Scholar]
- 20.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99:1978–83. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 21.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 22.Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343:1355–61. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 23.Jouven X, Lemaitre RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26:2142–7. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 24.Hinterseer M, Thomsen MB, Beckmann BM, Pfeufer A, Schimpf R, Wichmann HE, Steinbeck G, Vos MA, Kaab S. Beat-to-beat variability of QT intervals is increased in patients with drug-induced long-QT syndrome: a case control pilot study. Eur Heart J. 2008;29:185–90. doi: 10.1093/eurheartj/ehm586. [DOI] [PubMed] [Google Scholar]
- 25.Marban E. Heart failure: the electrophysiologic connection. J Cardiovasc Electrophysiol. 1999;10:1425–8. doi: 10.1111/j.1540-8167.1999.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 26.Bezzina CR, Verkerk AO, Busjahn A, Jeron A, Erdmann J, Koopmann TT, Bhuiyan ZA, Wilders R, Mannens MM, Tan HL, Luft FC, Schunkert H, Wilde AA. A common polymorphism in KCNH2 (HERG) hastens cardiac repolarization. Cardiovasc Res. 2003;59:27–36. doi: 10.1016/s0008-6363(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 27.Pfeufer A, Jalilzadeh S, Perz S, Mueller JC, Hinterseer M, Illig T, Akyol M, Huth C, Schopfer-Wendels A, Kuch B, Steinbeck G, Holle R, Nabauer M, Wichmann HE, Meitinger T, Kaab S. Common variants in myocardial ion channel genes modify the QT interval in the general population: results from the KORA study. Circ Res. 2005;96:693–701. doi: 10.1161/01.RES.0000161077.53751.e6. [DOI] [PubMed] [Google Scholar]
- 28.Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M, West K, Kashuk C, Akyol M, Perz S, Jalilzadeh S, Illig T, Gieger C, Guo CY, Larson MG, Wichmann HE, Marban E, O’Donnell CJ, Hirschhorn JN, Kaab S, Spooner PM, Meitinger T, Chakravarti A. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–51. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 29.Aarnoudse AJ, Newton-Cheh C, de Bakker PI, Straus SM, Kors JA, Hofman A, Uitterlinden AG, Witteman JC, Stricker BH. Common NOS1AP variants are associated with a prolonged QTc interval in the Rotterdam Study. Circulation. 2007;116:10–6. doi: 10.1161/CIRCULATIONAHA.106.676783. [DOI] [PubMed] [Google Scholar]
- 30.Lehtinen AB, Newton-Cheh C, Ziegler JT, Langefeld CD, Freedman BI, Daniel KR, Herrington DM, Bowden DW. Association of NOS1AP genetic variants with QT interval duration in families from the Diabetes Heart Study. Diabetes. 2008;57:1108–1114. doi: 10.2337/db07-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Post W, Shen H, Damcott C, Arking DE, Kao WH, Sack PA, Ryan KA, Chakravarti A, Mitchell BD, Shuldiner AR. Associations between genetic variants in the NOS1AP (CAPON) gene and cardiac repolarization in the old order Amish. Hum Hered. 2007;64:214–9. doi: 10.1159/000103630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albert CM, Nam EG, Rimm EB, Jin HW, Hajjar RJ, Hunter DJ, MacRae CA, Ellinor PT. Cardiac sodium channel gene variants and sudden cardiac death in women. Circulation. 2008;117:16–23. doi: 10.1161/CIRCULATIONAHA.107.736330. [DOI] [PubMed] [Google Scholar]
- 33.Newton-Cheh C, Shah R. Genetic determinants of QT interval variation and sudden cardiac death. Curr Opin Genet Dev. 2007;17:213–21. doi: 10.1016/j.gde.2007.04.010. [DOI] [PubMed] [Google Scholar]


