Abstract
Introduction
Where virologic monitoring is not routinely available, immunologic criteria are commonly used to determine treatment failure while on antiretroviral therapy (ART). However, few have studied CD4+ response and its relationship to subsequent clinical outcomes in a programmatic setting.
Methods
We analyzed cohort data from Zambia to investigate whether 6- and 12-month CD4+ response following ART initiation was associated with later mortality. We used Cox proportional hazard models that accounted for different strata of baseline CD4+ counts and adjusted for age, gender, clinical stage, tuberculosis co-infection, baseline hemoglobin, initial ART regimen, and adherence behavior.
Results
We analyzed data from two cohorts, from 6 months onward (n=24,366; median follow-up=467 days, IQR 222, 791) and from 12 months onward (n= 17,920; median follow-up = 423 days, IQR 191, 689). In the post-6 month analysis, hazard for death was significantly higher when absolute CD4+ response was <100 cells/μL (adjusted hazard ratio [AHR]=2.25, 95%CI=1.91–2.64), relative response was <10% above baseline (AHR=2.60, 95%CI=2.12–3.19), and absolute CD4+ count was <100 cells/μL (AHR=2.79, 95%CI=2.26–3.45). In the post-12 month analysis, mortality was associated with rise in absolute CD4+ cell count <200 cells/μL (AHR=2.41, 95%CI=1.83–3.17), relative rise in CD4+ cell count of <10% above baseline (AHR=3.41, 95%CI=2.51–4.64), and absolute CD4+ count at 12 months was <100 cells/μL (AHR=4.11, 95%CI=2.96–5.68).
Conclusion
Commonly used definitions for immunologic treatment failure are associated with elevated mortality risk among patients on ART.
Keywords: HIV, immunologic response, CD4+ cell count, mortality, treatment failure, sub-Saharan Africa, Zambia
Introduction
In resource-constrained settings, where access to viral load testing is typically unavailable, long-term assessment of antiretroviral therapy (ART) response presents operational challenges. Without the means to detect viremia, many programs must rely on immunologic (i.e. CD4+) response to diagnose treatment failure,1–4 despite the lack of clear scientific evidence validating such criteria. The World Health Organization (WHO), for example, established “reasonable working definitions” in 2006,5 based on expert opinion rather than clinical or epidemiologic studies. In this study, we investigate the relationship between immunologic response following ART initiation and risk for subsequent death. Field validations such as this are urgently needed, so that appropriate long-term assessment of ART can be made and utilization of second-line therapy can be improved among patients in need.6
Methods
We conducted a cohort analysis of patients accessing HIV care and treatment in Lusaka, Zambia. This program has been previously described.1, 7 Briefly, HIV-infected patients are enrolled into one of 18 primary care centers within the Lusaka district. They undergo history and physical exam (including WHO clinical staging) and blood is collected for CD4+ cell count. Those with < 200 CD4+ cells/μL; WHO stage 4; or WHO stage 3 and < 350 CD4+ cells/μl are eligible for ART. First-line ART regimens comprise a non-nucleoside reverse transcriptase inhibitor (NNRTI; nevirapine or efavirenz) with a nucleoside reverse transcriptase inhibitor “backbone” of lamivudine and either zidovudine or stavudine. Beginning July 2007, the Zambian Ministry of Health introduced tenofovir and emtricitabine as alternative components for first-line therapy. Over the first 6 months, patients initiating ART undergo an intensive visit schedule to assess adherence and regimen toxicities. Clinical visits occur every 3 months; repeat CD4+ screening occurs every 6 months.
In our setting, viral load testing is available but limited. To assess for treatment failure, clinicians review the patient’s immunologic and clinical response to therapy. Immunologic failure is defined locally as: (1) CD4+ cell count rise of <50 cells/μL during the first 6 months of therapy; (2) persistent CD4+ cell count <100 cells/μL after 12 months of ART; (3) decline in CD4+ cell count to value below that of pre-treatment baseline; or (4) CD4+ cell count decline of >30% from on-treatment peak. Clinical treatment failure is defined as progression of HIV disease, signaled by new or recurrent stage 3 or 4 condition, when ART has been given for at least 6 months. When both immunologic and clinical treatment failure are diagnosed, providers are advised to switch the patient to a second-line regimen. When clinical and immunologic criteria are discordant (i.e., one suggests failure and the other does not), viral load testing is performed to further guide care.8
We developed two separate cohorts for this analysis. The first cohort comprised individuals >15 years of age who: (1) initiated and continued ART for greater than 6 months in the Lusaka program, and (2) had available baseline and 6-month CD4+ cell counts. Those who were switched to second-line therapy before 6 months were excluded. The second cohort comprised adults who initiated and continued ART for greater than 12 months and used analogous inclusion and exclusion criteria. For each analysis cohort, we categorized patients according to several CD4+ response criteria, including: absolute CD4+ cell change, absolute CD4 cell count, and drop below baseline value. In addition, for the 12-month analysis, we evaluated the relative drop from peak post-therapy CD4+ as a criterion. Individuals meeting criteria were included in both cohorts for this analysis.
We performed post-6 month and post-12 month survival analyses using Cox proportional hazards regression to understand hazard of death among patients meeting various criteria for CD4+ cell response. We examined each criterion separately; we did not consider combinations of criterion for CD4+ cell response. Multivariable models adjusted for potential confounders: age, gender, WHO stage at ART initiation, tuberculosis co-infection status, baseline hemoglobin, initial ART regimen, and adherence behavior. Adherence was measured over the pre-observation period – either the first 6 months or the first 12 months – using a variation of the medication possession ratio (MPR). We divided the number of days late for pharmacy refills by total days on therapy, and then subtracted that percentage from 100%. Because patients in the Lusaka district are provided a buffer stock of three days with each drug dispensation; we began calculating lateness on the fourth day after a missed pharmacy appointment.9, 10
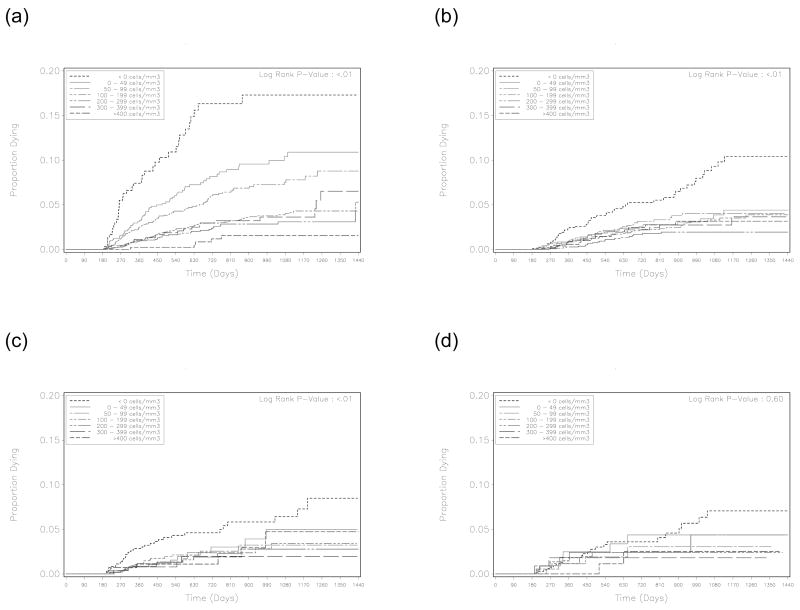
Individuals who were switched to second-line therapy in the observation window (i.e. after 6 or 12 months) were censored on the date of regimen change. As shown in other reports,11 initial CD4+ cell count was demonstrated as an effect modifier in this cohort (Figure 1). For this reason, we calculated hazard ratios for each CD4+ response criterion across five different baseline CD4+ categories: ≤ 100 cells/μL, 101–200 cells/μL, 201–300 cells/μL, 301–400 cells/μL, > 400 cells/μL. From these, we calculated a weighted summary measure based on the population’s relative distribution of CD4+ cell count at enrollment. All analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC). This analysis was approved by the Institutional Review Boards of the University of Zambia (Lusaka, Zambia) and the University of Alabama at Birmingham (Birmingham, Alabama, USA).
Figure 1.
Kaplan-Meier survival curves by absolute CD4+ response at 6 months, stratified according to baseline CD4+ result: (A) < 100 cells/μL, (B) 100–199 cells/μL, (C) 200–299 cells/μL, and (D) ≥ 300 cells/μL. Because of notable differences in mortality trends across strata, we used a weighted summary measure to calculate overall hazard for death across all measures of CD4+ response.
Results
From May 1, 2004 to June 2, 2008, 38,150 patients enrolled ≥ 6 months prior to the data freeze date and had a baseline CD4+ result. Of these, 4,535 (11%) were more than 30 days late for a scheduled appointment or had withdrawn from the program, 2,996 (7%) had died, and 168 (<1%) had switched to second-line therapy prior to 6 months. Of the remaining 30,451 patients, 24,366 (80%) had a documented 6-month CD4+ test result. Those included in the analysis were more likely to have ≥ 95% adherence to ART when compared to those without 6-month CD4+ test result (73% vs. 64%; p< 0.01). Otherwise, differences between the two groups may have reached statistical significance with respect to age, body mass index, baseline CD4+ count distribution, baseline hemoglobin, entry WHO stage, and ART regimen (all p≤ 0.01), but none were believed to be clinically meaningful (Table 1). From 6 months onward, mortality rate in this cohort was 2.0 per 100 patient-years (95%CI = 1.9, 2.2); rate for follow-up losses was 10.4 per 100 patient-years (95%CI = 10.1, 10.8); and rate for switching to second-line therapy was 1.4 per 100 patient-years (95%CI = 1.2, 1.5). Median observation time after 6 months was 467 days (IQR 222, 791).
Table 1.
Comparison of patients included and excluded in the two analysis cohorts based on availability of 6-month or 12-month CD4+ cell result
| 6 month cohort |
12 month cohort |
|||||
|---|---|---|---|---|---|---|
| Included (N = 24,366) | Excluded (N = 6,085) | P | Included (N = 17,920) | Excluded (N = 5,420) | P | |
| Age, median (Q1, Q3) | 35 (30, 41) | 35 (30, 41) | <0.01+ | 35 (30, 42) | 35 (30, 41) | <0.01+ |
| Female, n (%) | 15,086 (61.9%) | 3,702 (60.8%) | 0.12* | 11,212 (62.6%) | 3,328 (61.4%) | 0.12* |
| Body Mass Index | ||||||
| Female, median kg/m2 (Q1, Q3) | 19.5 (17.8, 21.4) | 19.3 (17.6, 21.2) | 0.01+ | 19.5 (17.8, 21.5) | 19.3 (17.6, 21.1) | <0.01+ |
| Male, median kg/m2 (Q1, Q3) | 20.4 (18.3, 22.9) | 20.2 (18.1, 22.8) | <0.01+ | 20.3 (18.3, 22.9) | 20.2 (18.0, 22.7) | <0.01+ |
| CD4+ count at baseline, median cells/μL (Q1, Q3) | 132 (70, 198) | 132 (67, 201) | 0.69+ | 130 (69, 195) | 129 (67, 198) | 0.78+ |
| ≥ 400 cells/μL, n (%) | 511 (2.1%) | 169 (2.8%) | <0.01* | 341 (1.9%) | 133 (2.5%) | <0.01* |
| 300–399 cells/μL, n (%) | 1,456 (6.0%) | 373 (6.1%) | 1,000 (5.6%) | 343 (6.3%) | ||
| 200–299 cells/μL, n (%) | 3,987 (16.4%) | 1,019 (16.7%) | 2,865 (16.0%) | 860 (15.9%) | ||
| 100–199 cells/μL, n (%) | 9,441 (38.7%) | 2,232 (36.7%) | 6,984 (39.0%) | 1,988 (36.7%) | ||
| < 100 cells/μL | 8,971 (36.8%) | 2,292 (37.7%) | 6,730 (37.6%) | 2,096 (38.7%) | ||
| Hemoglobin, median g/dL (Q1, Q3) | 10.9 (9.6, 12.3) | 10.9 (9.4, 12.3) | <0.01+ | 10.9 (9.6, 12.3) | 10.8 (9.4, 12.2) | <0.01+ |
| >10.0 g/dL, n (%) | 15,249 (69.3%) | 3,580 (66.6%) | <0.01* | 11,025 (69.6%) | 3,207 (66.2%) | <0.01* |
| 8.0 – 10.0 g/dL, n (%) | 5,076 (23.1%) | 1,321 (24.6%) | 3,666 (23.2%) | 1,200 (24.8%) | ||
| < 8.0 g/dL, n (%) | 1,686 (7.7%) | 478 (8.9%) | 1,139 (7.2%) | 435 (9.0%) | ||
| Tuberculosis at enrollment, n (%) | 3,268 (13.4%) | 866 (14.2%) | 0.09* | 2,360 (13.2%) | 718 (13.2%) | 0.88* |
| WHO Stage | ||||||
| I/II, n (%) | 8,102 (33.4%) | 1,743 (28.9%) | <0.01* | 5,939 (33.3%) | 1,530 (28.4%) | <0.01* |
| III, n (%) | 14,116 (58.2%) | 3,683 (61.0%) | 10,422 (58.4%) | 3,300 (61.2%) | ||
| IV, n (%) | 2,053 (8.5%) | 612 (10.1%) | 1,489 (8.3%) | 558 (10.4%) | ||
| Regimen | ||||||
| ZDV + 3TC + NVP, n (%) | 11,458 (47.2%) | 2,665 (43.9%) | <0.01* | 9,088 (50.9%) | 2,617 (48.4%) | 0.02* |
| ZDV + 3TC + EFV, n (%) | 942 (3.9%) | 232 (3.8%) | 676 (3.8%) | 200 (3.7%) | ||
| D4T + 3TC + NVP, n (%) | 9,302 (38.3%) | 2,443 (40.3%) | 7,125 (39.9%) | 2,284 (42.2%) | ||
| D4T + 3TC + EFV, n (%) | 1,367 (5.6%) | 386 (6.4%) | 972 (5.4%) | 307 (5.7%) | ||
| TDF + FTC + NVP, n (%) | 446 (1.8%) | 123 (2.0%) | 0 (0.0%) | 1 (0.0%) | ||
| TDF + FTC + EFV, n (%) | 784 (3.2%) | 215 (3.5%) | 10 (0.1%) | 3 (0.1%) | ||
| Adherence according to medication possession ratio | ||||||
| ≥ 95%, n (%) | 17,716 (72.7%) | 3,918 (64.4%) | <0.01* | 11,594 (64.7%) | 2,994 (55.2%) | <0.01* |
| 80% – 94%, n (%) | 4,609 (18.9%) | 1,281 (21.1%) | 4,957 (27.7%) | 1,743 (32.2%) | ||
| < 80%, n (%) | 2,041 (8.4%) | 886 (14.6%) | 1,369 (7.6%) | 683 (12.6%) | ||
Chi-square test, + Wilcoxon rank sum test
ZDV = zidovudine, D4T = stavudine, 3TC = lamivudine, NVP = nevirapine, EFV = efavirenz, TDF = tenofovir, FTC = emtricitabine
Overall, 32,121 patients with baseline CD4+ result enrolled ≥12 months prior to the data freeze date. Of these, 5,380 (17%) were more than 30 days late for a scheduled appointment or had withdrawn from the program, 3,210 (10%) had died, and 191 (1%) had started second-line ART prior to 12 months. Of the remaining 23,340 patients, 17,920 (77%) had a recorded 12-month CD4+ value. Those included and excluded from the analysis differed across multiple medical and demographic characteristics; with the exception of adherence, however, none were believed to be clinically meaningful (Table 1). From 12 months onward, mortality rate in this cohort was 1.5 per 100 patient-years (95%CI = 1.4, 1.7); rate for follow-up losses was 9.4 per 100 patient-years (95%CI = 9.0, 9.8); and rate for switching to second-line therapy was 1.8 per 100 patient-years (95%CI = 1.6, 1.9). Median observation time after 12 months was 423 days (IQR 191, 689).
When we applied our local criteria for immunologic treatment failure at 6 months, 2681 (11%) had a decline of CD4+ cell count to pre-therapy level or below and 5488 (23%) demonstrated a CD4+ cell rise of < 50 cells/μL. By definition, individuals who meet the first criteria also met the second. For those in the post-12 month observational cohort, 1452 (8%) had a decline of CD4+ cell count to or below pre-therapy levels; 761 (4%) had a CD4+ cell count less than 100 cells/μL; and 2844 (19%) had a CD4+ cell count decline of >30% from on-treatment peak. 1171 (8%) met two criteria and 320 (2%) met all three criteria.
We investigated discrete cut-offs for mortality associated with different CD4+ response thresholds at 6 months. In adjusted models that accounted for baseline CD4+ strata, the hazard for death was significantly higher when the absolute CD4+ response was < 100 cells/μL (adjusted hazard ratio [AHR] = 2.25, 95% CI = 1.91, 2.64), relative response was < 10% above baseline (AHR = 2.60, 95% CI = 2.12, 3.19), and absolute CD4+ count under 100 cells/μL (AHR = 2.79, 95% CI = 2.26, 3.45). Similar trends were noted at 12 months. Mortality following 12 months of ART was associated with rise in absolute CD4+ cell count of less than 200 cells/μL (AHR = 2.41, 95% CI = 1.83, 3.17), relative rise in CD4+ cell count of < 10% above baseline (AHR = 3.41, 95% CI = 2.51, 4.64), and CD4+ cell count at 12 months under 100 cells/μL (AHR = 4.11, 95% CI = 2.96, 5.68). In the subset of patients in the 12-month cohort with ≥ 3 documented CD4+ tests, those with a drop of ≥ 50% from the peak CD4+ value had a greater than three-fold hazard for death when compared to those without such a decline. We provide further stratification of CD4+ response criteria at 6 and 12 months in Tables 2 and 3 respectively.
Table 2.
Immunologic response at 6 months and subsequent risk of mortality in Lusaka, Zambia
| Crude HR (95% CI) | Adjusted HR (95% CI)* | |||
|---|---|---|---|---|
| Absolute change in CD4+ cell count from baseline | N = 24,366 | N = 21,865 | ||
| Below baseline | n = 2,681 | 3.69 (2.43 – 5.60) | n = 2,389 | 3.94 (2.53 – 6.14) |
| 0 – 49 cells/μL | n = 2,807 | 2.58 (1.68 – 3.96) | n = 2,548 | 2.28 (1.45 – 3.57) |
| 50 – 99 cells/μL | n = 3,969 | 2.26 (1.49 – 3.44) | n = 3,599 | 1.86 (1.20 – 2.89) |
| 100 – 199 cells/μL | n = 7,536 | 1.43 (0.95 – 2.16) | n = 6,793 | 1.12 (0.73 – 1.73) |
| 200 – 299 cells/μL | n = 4,094 | 1.07 (0.68 – 1.67) | n = 3,659 | 1.01 (0.63 – 1.60) |
| 300 – 399 cells/μL | n =1,815 | 1.37 (0.84 – 2.23) | n = 1,588 | 1.08 (0.64 – 1.83) |
| ≥ 400 cells/μL | n = 1,464 | 1.0 | n = 1,289 | 1.0 |
| CD4+ cell count in relation to baseline | N = 24,366 | N = 21,865 | ||
| > 20% below baseline | n = 1,503 | 2.45 (1.95 – 3.08) | n = 1,335 | 3.16 (2.45 – 4.09) |
| > 10% to ≤ 20% below baseline | n = 565 | 1.77 (1.17 – 2.69) | n = 498 | 2.38 (1.52 – 3.73) |
| ≤ 10% below to < 10% above baseline | n = 1,414 | 1.82 (1.38 – 2.39) | n = 1,285 | 2.19 (1.61 – 2.99) |
| ≥ 10% to < 20% above baseline | n = 818 | 1.10 (0.71 – 1.70) | n = 731 | 1.40 (0.87 – 2.27) |
| ≥ 20% above baseline | n = 20,066 | 1.0 | n = 18,015 | 1.0 |
| Absolute CD4+ cell count at 6 months | N = 24,366 | N = 21,865 | ||
| < 100 cells/μL | n = 1,738 | 3.92 (3.21 – 4.79) | n = 1,552 | 3.77 (2.99 – 4.76) |
| 100 – 149 cells/μL | n = 2,480 | 2.27 (1.83 – 2.81) | n = 2,190 | 2.39 (1.89 – 3.03) |
| 150 – 199 cells/μL | n = 3,280 | 1.61 (1.30 – 2.00) | n = 2,963 | 1.69 (1.34 – 2.15) |
| ≥ 200 cells/μL | n = 16,868 | 1.0 | n = 15,160 | 1.0 |
Model adjusted for age, gender, WHO stage at ART initiation, tuberculosis co-infection status, baseline hemoglobin, adherence behavior, and initial ART regimen. Each criterion for immunologic response was modeled separately.
Table 3.
Immunologic response at 12 months and subsequent risk of mortality in Lusaka, Zambia
| Crude HR (95% CI) | Adjusted HR (95% CI)* | |||
|---|---|---|---|---|
| Absolute change in CD4+ cell count from baseline | N = 17,920 | N = 15,727 | ||
| Below baseline | n = 1,452 | 4.56 (2.68 – 7.78) | n = 1,303 | 6.55 (3.63 – 11.82) |
| 0 – 49 cells/μL | n = 1,513 | 3.65 (2.11 – 6.29) | n = 1,335 | 3.53 (1.93 – 6.47) |
| 50 – 99 cells/μL | n = 2,355 | 2.67 (1.56 – 4.54) | n = 2,108 | 2.44 (1.36 – 4.38) |
| 100 – 199 cells/μL | n = 5,379 | 2.02 (1.22 – 3.34) | n = 4,732 | 1.74 (1.00 – 3.03) |
| 200 – 299 cells/μL | n = 3,651 | 1.15 (0.66 – 2.01) | n = 3,188 | 0.95 (0.51 – 1.76) |
| 300 – 399 cells/μL | n = 1,904 | 1.39 (0.76 – 2.52) | n = 1,638 | 1.25 (0.65 – 2.42) |
| ≥ 400 cells/μL | n = 1,666 | 1.0 | n = 1,423 | 1.0 |
| CD4+ cell count in relation to baseline | N = 17,920 | N = 15,727 | ||
| > 20% below baseline | n = 813 | 2.97 (2.10 – 4.21) | n = 733 | 4.75 (3.23 – 6.97) |
| > 10% to ≤ 20% below baseline | n = 290 | 2.13 (1.10 – 4.14) | n = 269 | 3.20 (1.55 – 6.60) |
| ≤ 10% below to < 10% above baseline | n = 845 | 1.63 (1.05 – 2.54) | n = 747 | 2.50 (1.56 – 4.01) |
| ≥ 10% to < 20% above baseline | n = 472 | 1.14 (0.57 – 2.31) | n = 416 | 1.73 (0.80 – 3.71) |
| ≥ 20% above baseline | n = 15,500 | 1.0 | n = 13,562 | 1.0 |
| Absolute CD4+ cell count at 6 months | N = 17,920 | N = 15,727 | ||
| < 100 cells/μL | n =761 | 5.53 (4.09 – 7.48) | n = 672 | 5.35 (3.79 – 7.55) |
| 100 – 149 cells/μL | n = 1,365 | 2.82 (2.07 – 3.85) | n = 1,209 | 2.85 (2.01 – 4.04) |
| 150 – 199 cells/μL | n = 2,073 | 1.62 (1.17 – 2.24) | n = 1,836 | 1.61 (1.12 – 2.31) |
| ≥ 200 cells/μL | n = 13,721 | 1.0 | n = 12,010 | 1.0 |
| Drop from peak CD4+ cell count (analysis limited to patients with ≥ 3 values) | N = 15,092 | N = 13,269 | ||
| ≥ 50% drop from peak | n = 947 | 3.14 (2.23 – 4.42) | n = 836 | 3.23 (2.23 – 4.67) |
| 30% – 49% drop from peak | n = 1,624 | 1.31 (0.89 – 1.94) | n = 1,432 | 1.33 (0.87 – 2.05) |
| < 30% drop from peak | n = 4,141 | 1.02 (0.75 – 1.38) | n = 3,678 | 1.19 (0.87 – 1.65) |
| No drop from peak | n = 8,380 | 1.0 | n = 7,323 | 1.0 |
Model adjusted for age, gender, WHO stage at ART initiation, tuberculosis co-infection status, baseline hemoglobin, adherence behavior, and initial ART regimen. Each criterion for immunologic response was modeled separately.
Discussion
In this programmatic analysis, a blunted CD4+ response at either 6 or 12 months post-ART initiation was associated with higher subsequent mortality. While poor immunologic response was consistently associated with adverse patient outcomes across various criteria, underlying causes of death were not tracked. Patients identified by poor immunologic response would require further clinical and/or laboratory evaluation to differentiate treatment failure from other medical conditions (e.g. untreated opportunistic infections).
Immunologic response has been well-correlated with clinical outcomes among HIV-infected patients on ART. However, definitions for adequate CD4+ recovery have varied greatly across studies. Moore and colleagues demonstrated that two criteria at 6 months post-initiation – increase of <25 cells/μL from baseline and absolute cell count of <200 cells/μL – were associated with elevated risk or death and/or AIDS-related event among patients with documented virologic suppression.12 Modeling from the Aquitaine cohort suggested that incremental increases of 50 cell/μL resulted in 60% reductions in opportunistic infections.13 Data from 13 developed world cohorts demonstrated a protective effect from death and AIDS as CD4+ response rose categorically.14 Other criteria have included CD4+ change of greater than 100 cells/μL and CD4+ recovery to over 200 cells/μL.15, 16 For the purposes of this analysis, we adapted commonly referenced criteria and prognostic thresholds from international treatment guidelines. These results support use of the three criteria recommended by the World Health Organization for determining poor immunologic response: (1) fall of CD4+ cell count to pre-therapy value or below, (2) 50% fall from peak on-treatment value, and (3) persistent CD4+ levels below 100 cells/μL.5 Our findings also suggest that another criterion may be useful in the identification of patients at high risk for mortality: minimum rise in absolute CD4+ cell count over time.
Because baseline CD4+ cell count is a demonstrated effect modifier for CD4+ response,11 we did not include it as a covariate in our Cox proportional hazards models. Instead, we performed a stratified analysis according to baseline CD4+ category and then calculated a summary hazard ratio by weighing each category according to its relative frequency in the study population. The primary advantage of such an approach is that is provides an overall summary statistic at the population level. With the complexity of our planned analysis and the multiple models used to generate our findings, this was an important consideration in our design. External validity of study findings could be negatively impacted by such an approach, since it is dependent on the distribution of baseline CD4+ cell counts within the target group. Given our large cohort size, however, we believe our findings are representative of our population and others like it in the region.
In our multivariable analyses, we adjusted for patient adherence over the pre-observation period (i.e. first 6 months or first 12 months). Adherence was measured according to MPR, a crude metric based on pharmacy refills. In our previous work, MPR of less than 80% over the first 12 months of ART was associated with nearly two-fold hazard for death (adjusted HR = 1.7; 95%CI = 1.4, 2.3).9 We recognize that bias could be introduced by including adherence behavior in our Cox proportional hazard models. Because adherence may lie on the causal pathway from blunted CD4+ cell response and mortality, by controlling for it we could be underestimating the hazard for death associated with different measures of CD4+ cell response. Despite this potential bias, we demonstrated significantly elevated risk for mortality in patients with poor CD4+ cell response, independent of previous adherence behaviors.
A significant proportion of individuals met immunologic criteria for treatment failure at 6 and 12 months (i.e., the start of observation), yet switch to second-line therapy appeared infrequent in both cohorts (< 2.0 per 100 patient-years). Two factors may have contributed to these findings. Like others,17–21 we have recognized the poor performance of CD4+ criteria in predicting virologic failure, particularly among asymptomatic patients. For this reason, individuals with discordant clinical and immunologic responses to ART undergo virologic screening to confirm treatment failure. In a study of these patients, Goldman et al found that up to 74% had undetectable viremia and thus did not require switch to second-line therapy.10 Another contributing factor to the low switch rate may be reluctance among health providers to prescribe second-line regimens when there are currently no third-line alternatives available. We have observed this locally and the phenomenon has been reported in other regional programs.6, 22
We recognize several limitations to this analysis. First, a notable proportion of eligible patients (11% at 6 months and 17% at 12 months) was lost to follow-up and thus excluded. Although greater ascertainment of this population would have been preferable, we believe our analysis cohorts were representative of those seeking care at these time points in large African ART programs. Similar retention rates have been observed in other programmatic cohorts in the region.23 Secondly, each of the proposed immunologic criteria was evaluated in a separate multivariable model; we did not consider the predictive value of combined thresholds due to reasons of complexity. Whether such multi-level models would be of use clinically is currently unknown. Our use of all-cause mortality made it difficult to delineate the relationships between immunologic response and AIDS-related events. Because viral load testing is very limited in our setting,8 we were also unable to correlate CD4+ response and virologic failure, a potentially more meaningful outcome in the context of clinical decision-making. Finally, because of the nature of our data collection methods and the limited diagnostic capacity for key opportunistic infections, we did not include clinical disease progression in our predictive models. Inclusion of such data would have added substantively and should be considered for future analyses of ART outcomes in resource-constrained settings.
In summary, our findings emphasize the importance of immunologic response as measured by serial CD4+ measurements in identifying patients at elevated risk for mortality. To improve clinical outcomes, this marker should prompt diagnostic work-up for opportunistic infections, comprehensive assessment of adherence, and/or early diagnosis of treatment failure using virologic testing where available. Given the poor correlation between CD4+ response and virologic failure,18–21 switching to second-line therapy without such evaluations may be premature and could limit future treatment options.
Acknowledgments
The authors acknowledge the Zambian Ministry of Health for consistent and high-level support of operations research in the context of HIV program expansion. The work reported herein was supported in part by the President’s Emergency Plan for AIDS Relief through a multi-country grant to the Elizabeth Glaser Pediatric AIDS Foundation from the U.S. Department of Health and Human Services and Centers for Disease Control and Prevention Global AIDS Program (cooperative agreement U62/CCU12354), and a grant for Operations Research for AIDS Care and Treatment in Africa from the Doris Duke Charitable Foundation (2005047). Additional investigator salary or trainee support is provided by the National Institutes of Health (K23-AI01411; K01-TW06670; P30-AI027767) and a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (2007061). The findings and conclusions included herein are solely the responsibility of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006 Aug 16;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 2.Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006 Apr 22;367(9519):1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 3.Wools-Kaloustian K, Kimaiyo S, Diero L, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS. 2006 Jan 2;20(1):41–48. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]
- 4.Akileswaran C, Lurie MN, Flanigan TP, Mayer KH. Lessons learned from use of highly active antiretroviral therapy in Africa. Clin Infect Dis. 2005 Aug 1;41(3):376–385. doi: 10.1086/431482. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: towards universal access. Geneva: 2006. [Google Scholar]
- 6.Pujades-Rodriguez M, O’Brien D, Humblet P, Calmy A. Second-line antiretroviral therapy in resource-limited settings: the experience of Medecins Sans Frontieres. AIDS. 2008 Jul 11;22(11):1305–1312. doi: 10.1097/QAD.0b013e3282fa75b9. [DOI] [PubMed] [Google Scholar]
- 7.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007 Oct 24;298(16):1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 8.Goldman JD, Cantrell RA, Mulenga LB, et al. Simple adherence assessments to predict virologic suppression among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS Res Hum Retroviruses. doi: 10.1089/aid.2008.0035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi BH, Cantrell RA, Zulu I, et al. Adherence to first-line antiretroviral therapy affects non-virologic outcomes among patients on treatment for greater than 12 months in Lusaka, Zambia. doi: 10.1093/ije/dyp004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman JD, Cantrell RA, Mulenga LB, et al. Simple adherence assessments to predict virologic failure among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS Res Hum Retroviruses. 2008 Aug;24(8):1031–1035. doi: 10.1089/aid.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nash D, Katyal M, Brinkhof MW, et al. Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS. 2008 Nov 12;22(17):2291–2302. doi: 10.1097/QAD.0b013e3283121ca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore DM, Hogg RS, Chan K, Tyndall M, Yip B, Montaner JS. Disease progression in patients with virological suppression in response to HAART is associated with the degree of immunological response. AIDS. 2006 Feb 14;20(3):371–377. doi: 10.1097/01.aids.0000196180.11293.9a. [DOI] [PubMed] [Google Scholar]
- 13.Binquet C, Chene G, Jacqmin-Gadda H, et al. Modeling changes in CD4-positive T-lymphocyte counts after the start of highly active antiretroviral therapy and the relation with risk of opportunistic infections: the Aquitaine Cohort, 1996–1997. Am J Epidemiol. 2001 Feb 15;153(4):386–393. doi: 10.1093/aje/153.4.386. [DOI] [PubMed] [Google Scholar]
- 14.Chene G, Sterne JA, May M, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003 Aug 30;362(9385):679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 15.Piketty C, Weiss L, Thomas F, Mohamed AS, Belec L, Kazatchkine MD. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic responses to a protease inhibitor-containing regimen. J Infect Dis. 2001 May 1;183(9):1328–1335. doi: 10.1086/319861. [DOI] [PubMed] [Google Scholar]
- 16.Anastos K, Barron Y, Cohen MH, et al. The prognostic importance of changes in CD4+ cell count and HIV-1 RNA level in women after initiating highly active antiretroviral therapy. Ann Intern Med. 2004 Feb 17;140(4):256–264. doi: 10.7326/0003-4819-140-4-200402170-00007. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds SJ, Nakigozi G, Newell K, et al. Failure of immunologic criteria to appropriately identify antiretroviral treatment failure in Uganda. AIDS. 2009 Feb 7; doi: 10.1097/QAD.0b013e3283262a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisson GP, Gross R, Strom JB, et al. Diagnostic accuracy of CD4 cell count increase for virologic response after initiating highly active antiretroviral therapy. AIDS. 2006 Aug 1;20(12):1613–1619. doi: 10.1097/01.aids.0000238407.00874.dc. [DOI] [PubMed] [Google Scholar]
- 19.Mee P, Fielding KL, Charalambous S, Churchyard GJ, Grant AD. Evaluation of the WHO criteria for antiretroviral treatment failure among adults in South Africa. AIDS. 2008 Oct 1;22(15):1971–1977. doi: 10.1097/QAD.0b013e32830e4cd8. [DOI] [PubMed] [Google Scholar]
- 20.Badri M, Lawn SD, Wood R. Utility of CD4 cell counts for early prediction of virological failure during antiretroviral therapy in a resource-limited setting. BMC Infect Dis. 2008;8:89. doi: 10.1186/1471-2334-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore DM, Awor A, Downing R, et al. CD4+ T-Cell Count Monitoring Does Not Accurately Identify HIV-Infected Adults With Virologic Failure Receiving Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2008 Nov 4; doi: 10.1097/QAI.0b013e318186eb18. [DOI] [PubMed] [Google Scholar]
- 22.Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther. 2007;12(1):83–88. [PubMed] [Google Scholar]
- 23.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007 Oct 16;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]