Abstract
Purpose
To investigate potential application of poly(amidoamine) (PAMAM) dendrimers for improving the delivery of SN-38.
Methods
Complexes of SN-38 with generation 4 amine terminated PAMAM dendrimers were synthesized with varying amounts of drug. Stability of the complexes as well as influence of complexation on permeability across and cellular uptake by Caco-2 cells was evaluated.
Results
The complexes were stable at pH 7.4 and drug was released at pH 5. A tenfold increase in permeability and more than hundredfold increase in cellular uptake of the complexes with respect to free SN-38 was observed.
Conclusions
Studies suggest that complexation with PAMAM dendrimers has the potential to improve the oral bioavailability of SN-38.
Keywords: camptothecin, oral drug delivery, PAMAM dendrimer, SN-38
INTRODUCTION
7-Ethyl-10-hydroxy-camptothecin (SN-38) is a potent topoisomerase I poison (1) and a biologically active metabolite of irinotecan hydrochloride (CPT-11). SN-38 has potent antitumor activity, approximately 1,000-fold more active than CPT-11, but suffers from limited direct clinical applications because of poor aqueous solubility. Conversely, SN-38’s water-soluble bipiperidine prodrug CPT-11 is a promising antitumor agent, displaying a broad spectrum of activity against various neoplasms and pleiotropically drug-resistant tumors (2,3). However, its oral bioavailability is merely 8% (4) and it displays gastrointestinal toxicity resulting in diarrhea, which is widely recognized as its dose-limiting toxicity (5). Low systemic availability upon oral administration may be partly attributed to limited drug absorption caused by the concerted action of the drug-efflux pump, P-gp, and metabolizing enzyme CYP3A4, both highly expressed in enterocytes (6,7). Finally, the fraction of the CPT-11 dose that reaches the liver intact is converted into SN-38 by hepatic carboxylesterases. The combined dose-limiting toxicity of CPT-11 and the poor aqueous solubility of SN-38 suggest that there is a clear need for efficient delivery of SN-38, preferably through the oral route of administration. The aim of the present study was to investigate the potential application of poly(amidoamine) (PAMAM) dendrimers for improving the oral bioavailability of SN-38.
PAMAM dendrimers are well defined macromolecules with controlled structure, globular shape and a high density of tunable surface functional groups in their periphery. Studies from our laboratory (8–10) and others (11–13) point to the potential of PAMAM dendrimers in oral drug delivery. A diverse array of in-vitro studies have demonstrated the influence of generation, surface charge and hydrophobicity on the transepithelial transport of PAMAM dendrimers (10,14–16). PAMAM dendrimers with positive surface charge at physiological pH were found to permeate across the intestinal barrier to a higher extent compared to their neutral and negatively charged counterparts. Recently, we demonstrated that surface acetylation minimized cytotoxicity while maximizing transepithelial permeability of fourth generation, positively charged, amine terminated PAMAM dendrimers (16). In the current research we report the complexation of SN-38 with fourth generation PAMAM dendrimers (G4; Table I; Fig. 1). G4 dendrimers were used due to their appreciable permeability and higher number (64) of amine groups present on their surface for obtaining polymer complexes with differential ratios of SN-38 molecules per dendrimer. Stability of these water soluble SN-38-PAMAM dendrimer complexes at physiological (7.4) and acidic pH (5.0) simulating endocytic environment, as well as, the influence of this complexation on permeability and cellular uptake is reported. Increased oral bioavailability associated with the observed increased solubility as well as permeability of SN-38-PAMAM dendrimer complexes will help to reduce dose related toxicity of SN-38. Most of the formulations, currently under development for camptothecin analogs are for intravenous administration. Use of PAMAM dendrimers in this study stages a platform for development of an oral formulation for SN-38 with higher therapeutic index.
Table I.
Characteristics of PAMAM Dendrimers Studied
| PAMAM dendrimer | Feed molar ratio | # of SN-38 molecules complexeda | % wt/wt of SN-38 | MW calculated | Solubility (mg/ml) |
|---|---|---|---|---|---|
| G4S5 | 8 | 5 | 12.14 | 16,179 | >10 |
| G4S11 | 16 | 11 | 23.32 | 18,537 | >10 |
| G4S16 | 32 | 16 | 28.48 | 22,074 | <5 |
Determined from NMR data.
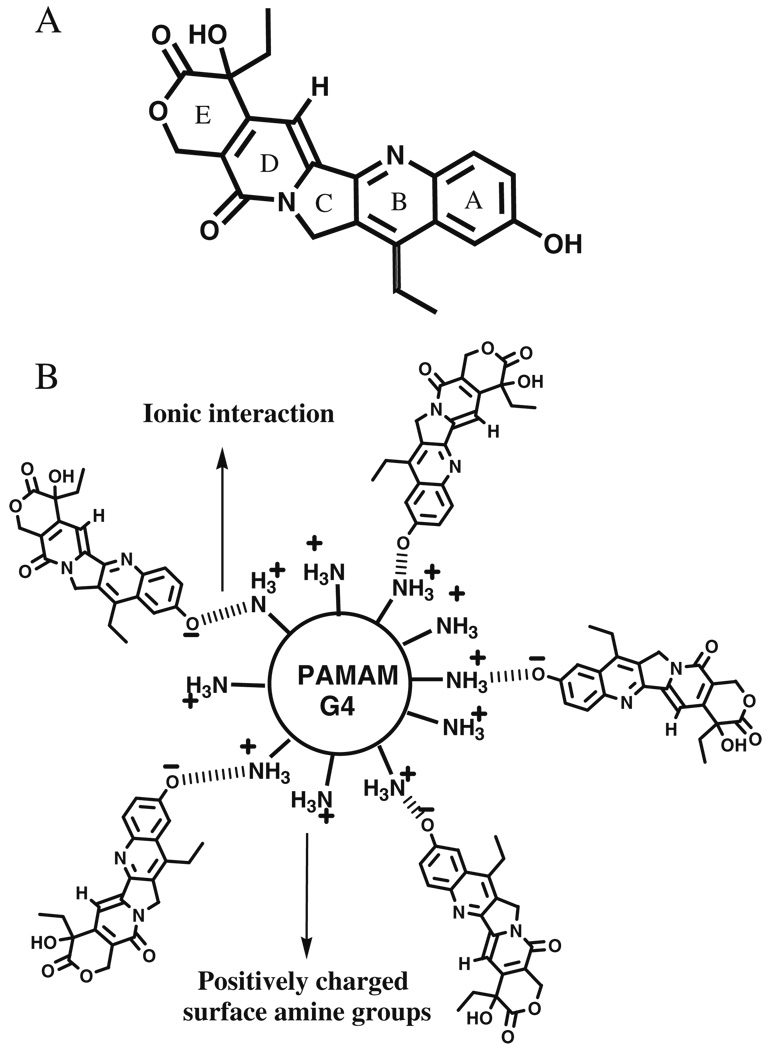
Fig. 1.
A Structure of SN-38, B Schematic representation of G4S5.
MATERIALS AND METHODS
Materials
SN-38 was supplied (Abatra technologies Co. Ltd., Xian, China) as a yellow powder (molecular formula C22H20O5N2) where identification of SN-38 was performed with ultraviolet (UV), infrared as well as nuclear magnetic resonance (NMR) with purity checked by high-performance liquid chromatography (98.2%) and melting point (specification 228–235°C, analysis result 229–233°C). PAMAM-G4NH2 (molecular weight=14,215, 64 amine end groups), acetic anhydride, [14C]-mannitol (specific activity 50 mCi/mmol), D2O and Triton X-100 were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). [3H]-Acetic anhydride was purchased from American Radiolabeled Chemicals. Superose 12 HR 16/50 column was purchased from Amersham Pharmacia Biotech (Piscataway, NJ, USA). PD-10 columns (disposable desalting column) were purchased from GE Healthcare Biosciences Corp (Piscataway, NJ, USA). Caco-2 cells were purchased from American Type Cell Culture (ATCC, Rockville, MD, USA). WST-1 cell proliferation reagent was purchased from Roche Applied Science (Indianapolis, IN, USA). 1H NMR and 13C NMR spectra were obtained using Varian 500 MHz FT NMR. The NMR solvent used was D2O unless otherwise indicated. All other chemicals used were analytical grade. Circular dichroism (CD) spectra were recorded using Jasco J-810 spectropolarimeter (cuvette path length=1 mm).
Complexation of SN-38 with PAMAM Dendrimers
Complexes are designated as GxSy where x represents the PAMAM dendrimer generation and y represents the number of SN-38 molecules complexed. A typical procedure for complexation, as described for G4S5 was as follows: SN-38 (0.005 g, 14.1 µmol) was added into the solution of G4-PAMAM dendrimer (0.025 g, 1.76 µmol) in dimethyl sulfoxide (DMSO; 10 mL) and the solution was stirred for 48 h at room temperature. DMSO was evaporated in vacuo to obtain crude G4S5 complex. The crude complex was re-dissolved in water and purified by extensive dialysis against distilled water using dialysis membrane of 1000 MWCO (Spectrum Laboratories, Inc., Rancho Dominguez, CA, USA). The product was further purified by size exclusion chromatography using PD-10 column. Purified PAMAM dendrimer was then freeze dried and solid product was stored at 4°C. G4S11 and G4S26 were prepared following a similar procedure with 16 and 32 M equivalents of SN-38 respectively.
Synthesis of Radiolabeled PAMAM Dendrimers
Radiolabeled PAMAM dendrimer complexes (G4S5, G4S11, G4S16) were prepared using [3H]-labeled acetic anhydride, following a procedure describe previously (16). The crude product was dialyzed against distilled water for 48 h using dialysis membranes of 1000 MWCO (Spectrum Laboratories, Inc., Rancho Dominguez, CA, USA). The polymers were further purified by size exclusion chromatography using a PD-10 column. The specific activity for each radiolabeled polymer was in the range of 2–3 mCi/mmol.
Drug Release
To determine in-vitro release characteristics, complexes (G4S5 and G4S11, 2 mg/mL) were dissolved in neutral (pH 7.4) and acidic (pH 5.0) buffers and stirred continuously at 37°C. At various time points 10 µL of the solution was used to separate free drug from the polymer complex. Separation was achieved using PD-10 column and the fractions collected were used to quantify, by fluorimetry (Ex/Em 375/550 nm) the amount of drug retained in the complex and the amount of drug released.
Cell Culture
Caco-2 cells (passages 30–60) were grown at 37°C in an atmosphere of 5% CO2 and 95% relative humidity. Cells were maintained in T-75 flasks using Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, 1% non-essential amino acids, 10,000 units per milliliter penicillin, 10,000 µg/mL streptomycin and 25 µg/mL amphotericin. Medium was changed every other day and cells were passaged at 70–90% confluency using 0.25% trypsin/ethylenediamine tetraacetic acid solution. Incubation buffer consisted of Hank’s balanced salt solution (HBSS) supplemented with 10 mM N-(2-hydroxyethyl)piperazine-N’-(2-ethanesulfonic acid) hemisodium salt buffer (pH 7.4).
Cytotoxicity Assay
Caco-2 cells were seeded (50,000 cells/well) in 96-well cell culture plates (Corning, Inc., Corning, NY, USA) and maintained for 48 h. The cells were washed twice with HBSS and incubated with 100 µl of SN-38 or PAMAM dendrimer-SN-38 complex solutions in HBSS. After 2 h, the cells were washed with HBSS to remove the dendrimers and replaced with 100 µl HBSS. Thereafter, 10 µl of cell proliferation reagent WST-1 was added to each well, followed by an incubation period of 4 h. Absorbance was measured at 440 nm using a SpectraMax 384 Plus plate reader (Molecular Devices, Sunnyvale, CA, USA). HBSS was used as a negative control.
Cellular Uptake of PAMAM Dendrimers
To determine cellular uptake, Caco-2 cells were seeded with a cell density of 80,000 cells per well on cell culture-treated 12-well plates (Becton Dickinson). After 48 h, cells were washed with HBSS and incubated with 1 and 10 µM radiolabeled complexes or 5 and 10 µM SN-38 for 5, 15 and 30 min respectively. At the end of the incubation period the cells were washed twice with ice-cold HBSS buffer to stop the uptake process. The cells were then lysed with 1 N NaOH solution which was then neutralized with HCl. The cell-associated radioactivity was determined using liquid scintillation counting (Beckman Coulter, Fullerton, CA, USA). The uptake data was normalized to total protein content determined using bicinchoninic acid protein assay kit (Pierce, Evanston, IL, USA).
Transepithelial Transport
Caco-2 cell monolayers were seeded (80,000 cells/well) on 12-well Transwell® cell culture inserts (3 µM pore size). After the cells reached confluency (21–28 days in culture) the transport of complexes and SN-38 from apical-to-basolateral direction was investigated at a donor concentration of 1 and 10 µM for G4S5 and 5 and 50 µM for SN-38. Prior to experiments, the cells were washed with HBSS buffer and then 500 µL of the [3H]-labeled polymer/SN-38 was added to the apical side of each insert while 1,500 µL of the HBSS buffer was added on the basolateral side. Samples were collected at 0, 30, 60, 90 and 120 min from the basolateral compartment and analyzed for accumulated radiolabel using a liquid scintillation counter (Beckman Coulter, Fullerton, CA, USA). Concentration of SN-38 in the receiver compartment was determined by fluorimetry (Ex/Em 375/550 nm). The apparent permeability (Papp) coefficients were calculated as follows:
| (1) |
where dM represents the change in dendrimer accumulation on the basolateral side, C0 the concentration on the apical side of the membrane, S the surface area of the insert (1.12 cm2) and dt the change in time. Monolayers were considered to be confluent when Papp for mannitol was less than 2×10−6 cm/s.
Cell monolayer integrity was monitored during transport studies by using an epithelial voltohmmeter (EVOM™; World Precision Instruments, Inc., Sarasota, FL, USA) to measure the transepithelial electrical resistance (TEER). TEER values (ohm per square centimeter) were corrected for background (obtained from inserts without cells) and normalized to untreated inserts (percent control). After 120 min the dosing solution was removed and cells were washed with HBSS. Subsequently, cells were incubated in DMEM and placed at 37°C overnight. After 24 h TEER was measured to assess reversibility of tight junctional perturbance.
Statistical Analysis
Statistical analysis was carried out using Student’s t-test with a probability value p<0.05 considered statistically significant.
RESULTS AND DISCUSSION
There is a growing interest in the use of polymers for delivery of therapeutic compounds lacking suitable pharmacokinetic parameters (17,18) and PAMAM dendrimers are emerging as promising candidates for oral drug delivery (9,11,13). These polymers are transported across epithelial cell monolayers via trans- and para-cellular permeation pathways (19). There is a desire to use higher generation PAMAM dendrimers since with each increase in generation the number of functional groups available for complexation or conjugation with drugs, increases. Positively charged dendrimers are transported to a higher extent compared to their neutral and negatively charged counterparts. Recent studies in our laboratory suggest that there is a linear correlation between the number of free amine groups and cellular toxicity; thus cytotoxicity of higher generation (G4) amine terminated PAMAM dendrimers can be reduced by conjugation to drug molecules while maintaining their transepithelial permeability (16). In the present work we hypothesize that complexation of SN-38 with fourth generation amine terminated PAMAM dendrimers will increase its solubility and thereby, may improve its pharmacokinetic parameters.
Synthesis and Characterization of Dendrimer-SN-38 Complexes
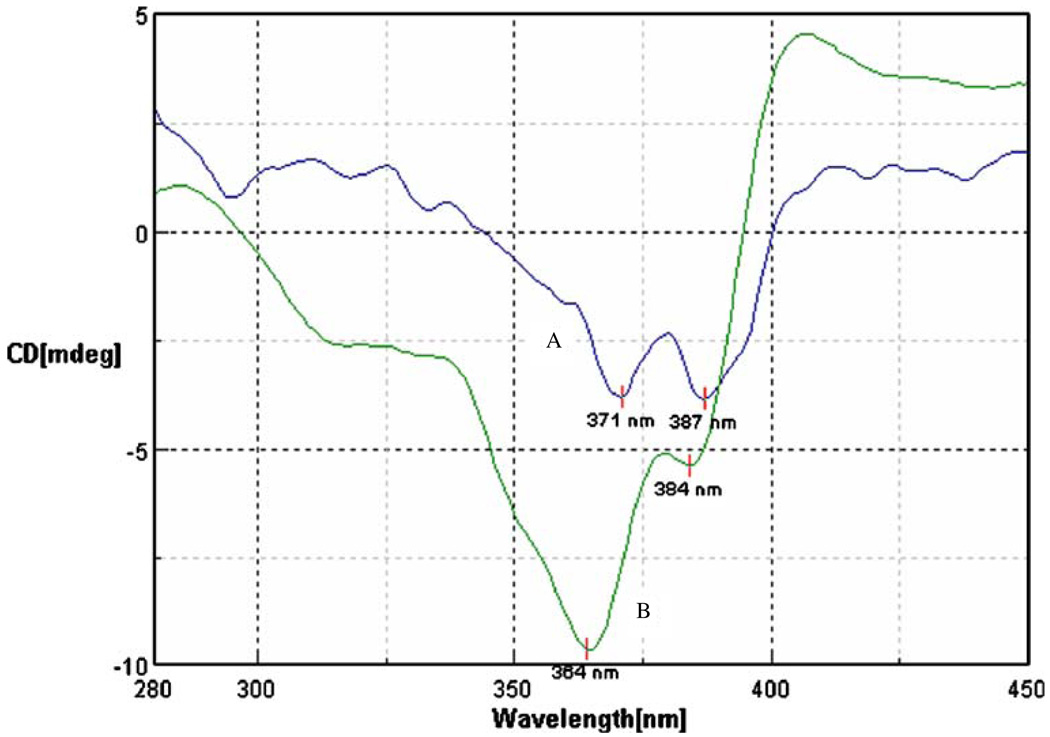
Complexes containing 5, 11 and 16 molecules of SN-38 for each dendrimer were prepared. 1H NMR revealed the formation of complexes rather than a physical mixture. Aromatic signals originating from SN-38 in the complex were broad indicating lower T2 relaxation times. Comparison of T2 relaxation time for various proton signals (Table II) in complex and free SN-38, confirmed the formation of complex as opposed to physical mixture. T2 relaxation times for aromatic protons were reduced to 46–56 ms compared to 520 ms in free SN-38. T2 relaxation times for aliphatic signals originating from methyl protons in SN-38 were reduced to 22–29 ms compared to >250 ms in free SN-38. These values were similar to T2 relaxation times for protons in G4 PAMAM dendrimer. Number of SN-38 molecules attached to the PAMAM dendrimer was determined by calculating the ratios of integral values of the signal for upfield CH3 protons originating from SN-38 and integral values of the signal for – CH2– protons from PAMAM dendrimer. The closed lactone (E) ring is widely regarded as being an essential pharmacophore for activity against cancer cells, and the ring opening is thought to result in a loss of drug potency; however, the lactone (closed) and carboxylate (open) forms of the E ring may be at equilibrium, depending on microenvironmental pH (5). In previous investigations with SN-38 and other camptothecin analogs, a reversal of the sign for CD bands was observed after the hydrolysis of the closed lactone (E) ring to carboxylate (20). In current studies, no changes in the sign of the CD bands were observed for the complexes when compared to SN-38 (Fig. 2), confirming presence of lactone ring in the complexes. The nature of drug complexation with dendrimer may be simple physical entrapment, or involve ionic interactions. A bathochromic shift in the UV absorption spectra (data not shown) of the complex which is characteristic of the deprotonation of the A ring hydroxyl group was observed indicating the involvement of phenolic OH in drug complexation. It might be speculated that an ionic interaction between phenolic OH and the surface amine groups on the PAMAM dendrimer can lead to formation of a complex leading to increased water solubility. All the complexes showed increased water solubility compared to free drug. An increase in the hydrophobicity associated with an increase in drug loading reduced the solubility of complexes from 10 mg/mL (G4S5, G4S11) to less than 5 mg/mL (G4S16; Table I). Cytotoxicity, permeability and cellular uptake studies were evaluated using only G4S5 and G4S11 due to the higher solubility of these complexes.
Table II.
T2 Relaxation Time of Protons for G4S5, G4 and SN-38
| ppm signal | T2 relaxation time (s) |
|||
|---|---|---|---|---|
| SN-38 | G4 | Complex | ||
| Aromatic signals | 7.56, 7.34, 7.03, 6.74 | – | – | 0.046–0.056 |
| 8.03, 7.41, 7.24 | >0.520 | – | – | |
| Aliphatic protons | 1.30, 0.88 | >0.230 | – | – |
| 1.13 | – | – | 0.022–0.049 | |
| 3.31 | – | 0.075 | – | |
| 3.37 | – | – | 0.211 | |
| 3.25 | – | 0.225 | – | |
| 3.24 | – | – | 0.072 | |
| 3.14 | – | – | 0.185 | |
| 2.3–3.0 | – | 0.100 | 0.100 | |
Fig. 2.
Circular dichroism spectra of A SN-38 and B G4S5. Spectra were recorded from 0.01 mM solution of equivalent concentrations of SN-38.
Drug Release
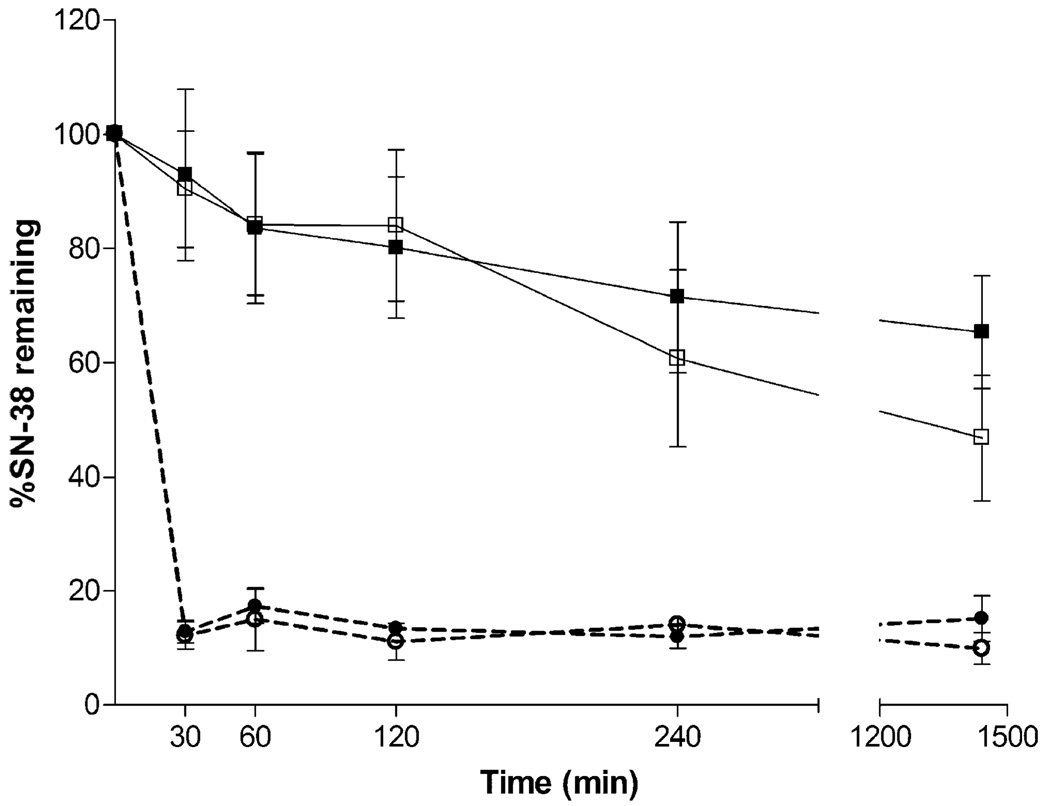
Due to the various physiological environments encountered by the drug–dendrimer complexes during their transport from the gastrointestinal tract to the systemic circulation, we determined the stability of these complexes at a range of pH values. Initial studies suggested that G4S5 is relatively stable at pH 7.4 thus retaining >80% of the drug in the first 2 h at pH 7.4 (Fig. 3). The amount of drug retained by G4S5 reduced to 60% after 4 h and 46% after 24 h. G4S11 showed similar trend with little improvement in stability. For G4S11, 65% of the drug was still retained on the polymer after 24 h. Initial stability studies in acidic environment (pH 1–2) suggested that the drug is released rapidly from the complex (data not shown). Further studies at milder acidic conditions were conducted to observe the release of the drug at simulated endocytic environment (pH 5.0). Both the complexes retained <15% and <10% of the drug after 30 min and 24 h respectively at these milder acidic conditions. It is important to realize that equilibrium exists between neutral phenolic OH and deprotonated phenolic OH in SN-38 (21). It can be postulated that at acidic pH the equilibrium will shift towards neutral form of the phenolic OH thus eliminating the ionic interactions between deprotonated phenolic OH and dendrimer, thereby releasing the free drug. Similar observations were reported previously where binding of polarity responsive probe 5-(di-methylamino)-1-napthalene sulfonic acid (DNS) with amine terminated PAMAM dendrimer was studied at several pH levels (22). Optimal binding was observed when both DNS and PAMAM dendrimers were in ionic forms. At lower pH when DNS was present in the protonated form, no binding occurred.
Fig. 3.
Stability of polymer-SN-38 complexes ( ) G4S5 and (
) G4S5 and ( ) G4S11 at pH 7.4, and (
) G4S11 at pH 7.4, and ( ) G4S5 and (
) G4S5 and ( ) G4S11 at pH 5.
) G4S11 at pH 5.
Various other investigators have studied release of free drug after incubation of dendrimers containing drug in buffered solutions. Patri et al. (23) have reported more than 70% release of free methotrexate within first 2.5 h from non covalent methotrexate-dendrimer inclusion complex. Complete release of efavirenz within first 24 h from drug containing polypropylenimine (PPI) dendrimers while significantly slower release from t-Boc glycine conjugated and mannose conjugated PPI dendrimers was reported by Dutta et al. (24). Drug release was also found to be controlled by molecular architecture (25) of dendrimer and introducing poly(ethylene oxide) chains on the periphery of the dendrimer (26,27). These observations suggest that further studies need to be conducted to avoid the premature release as well as control the release of SN-38 from the complexes. Encapsulation of these complexes can also be considered as an alternative to avoid premature release of SN-38 from the complexes in harsh GI environment.
Cytotoxicity and Cellular Uptake Studies
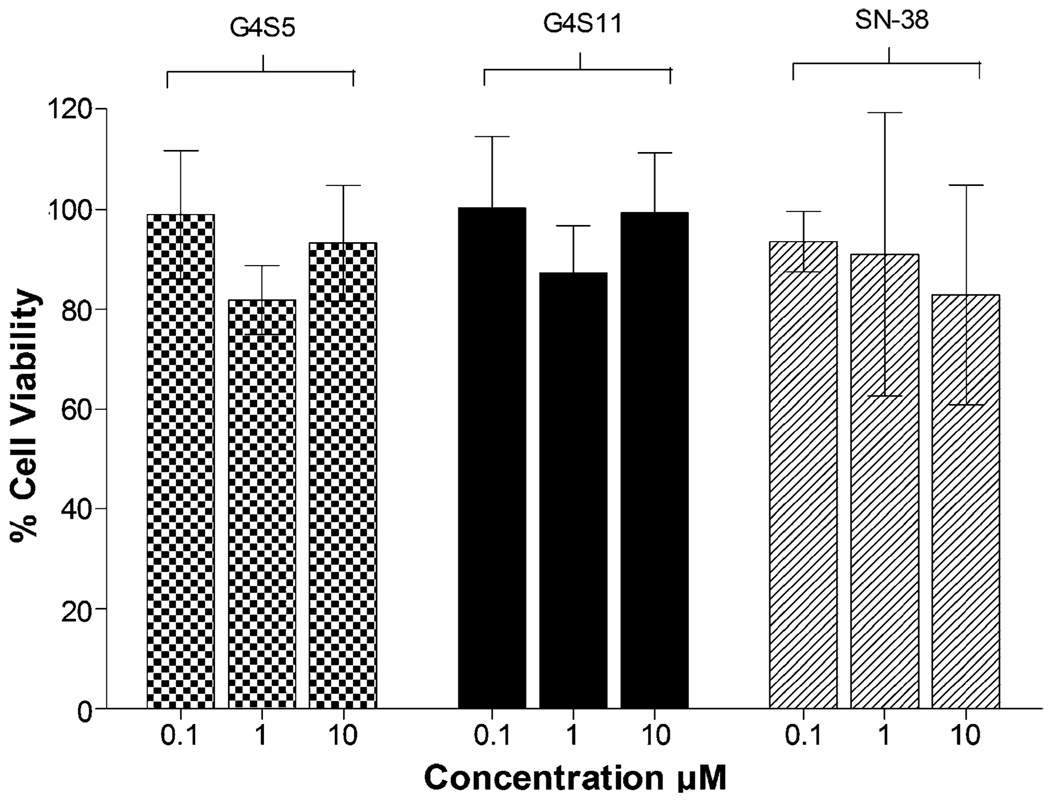
Previously we showed that G4 PAMAM dendrimers are toxic at concentrations higher than 10 µM (16). Based on these results lower concentrations (1 and 10 µM) were used for the current permeability studies. To further rule out the possibility of higher permeability associated with cellular toxicity, cytotoxicity was evaluated for all the complexes. No appreciable toxicity (cell viability>90%) was observed for the complexes after incubation for 2 h with Caco-2 cells at 0.1 µM (Fig. 4). Further tenfold (1 µM) and 100-fold (10 µM) increase in concentration did not decrease cell viability. SN-38 was nontoxic at these concentrations and time points.
Fig. 4.
Viability of Caco-2 cells after incubation for 2 h with ( ) G4S5, (
) G4S5, ( ) G4S11 and (
) G4S11 and ( ) SN-38 at 0.1,1 and 10 µM. Results are expressed as Mean ± SEM (n=6).
) SN-38 at 0.1,1 and 10 µM. Results are expressed as Mean ± SEM (n=6).
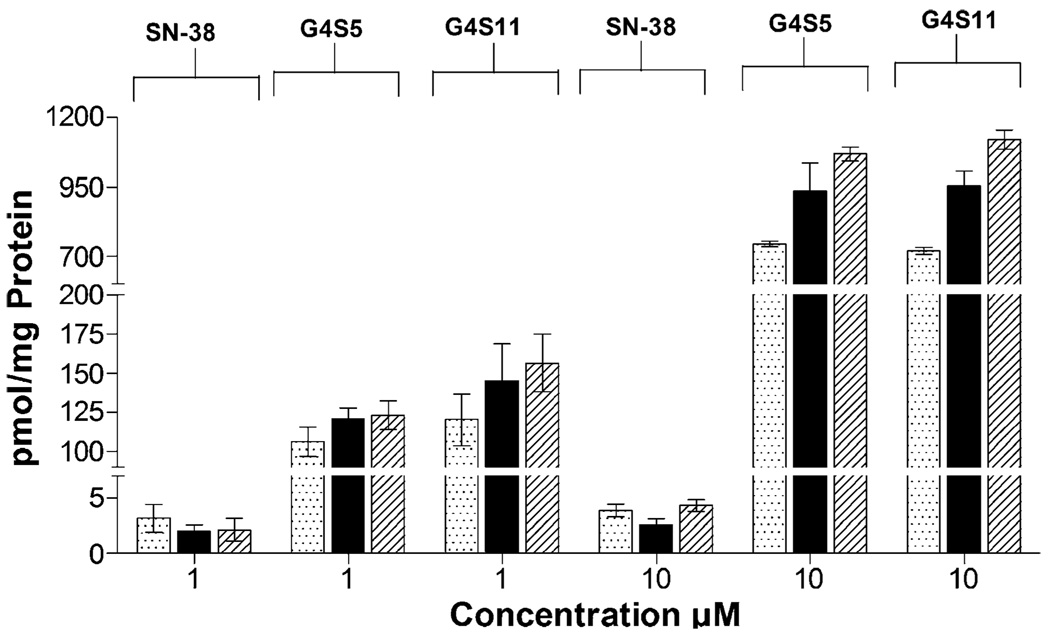
Investigation of the cellular entry of polymer complexes in Caco-2 cells revealed a linear increase in uptake with respect to time for G4S5 and G4S11 assuming steady state conditions after 5 min of incubation at 1 µM (Fig. 5). A further eight and sevenfold increase in uptake was observed for G4S5 and G4 S11 respectively at 10 µM. The uptake values for complexes were significantly higher (p<0.05) than those for free SN-38 and three to fourfold higher compared to values reported for unmodified dendrimers (16).
Fig. 5.
Cellular uptake (picomole of dendrimer per milligram of protein) of 1 and 10 µM of G4S5, G4S11 and SN-38 at ( ) 5 min, (
) 5 min, ( ) 15 min and (
) 15 min and ( ) 30 min. Results are expressed as Mean ± SEM (n=6).
) 30 min. Results are expressed as Mean ± SEM (n=6).
Molecular weight and geometry of the polymer influence cellular uptake kinetics. Recently, Seib and co-workers (28) have shown that PAMAM dendrimers enter cells at relatively higher levels compared to other linear and hyperbranched polymers. When effect of generation was examined, fourth generation dendrimers showed a tenfold higher uptake than third generation dendrimer. Further, we postulate that increased hydrophobicity associated with higher SN-38 content in the complex leads to higher cellular uptake of these complexes when compared to unmodified dendrimers. Previous studies in our laboratory have demonstrated that endocytosis contributes to the internalization and intracellular trafficking of cationic and anionic PAMAM dendrimers across Caco-2 cells (8,19). It is likely that PAMAM dendrimer-SN-38 complexes may follow similar pathways for cellular entry and transport, possibly delivering SN-38 to acidic vesicles such as lysosomes. Although additional studies are necessary to determine the overall contribution of endocytosis to the cellular uptake mechanism of SN-38-dendrimer complexes, release studies in this report suggest that drug will be released rapidly once exposed to an acidic environment. In tumor cells, this intracellular ‘active’ release mechanism would be an essential component before SN-38 can act on its target, nuclear topoisomerase I. However, for oral delivery, premature release in the stomach may result in decreased translocation of the complexes across intestinal epithelial barrier. As a consequence, for successful oral delivery of PAMAM–SN-38 complexes, protection from the acidic environment of the stomach will be necessary.
Permeability Across Caco-2 Cell Monolayers
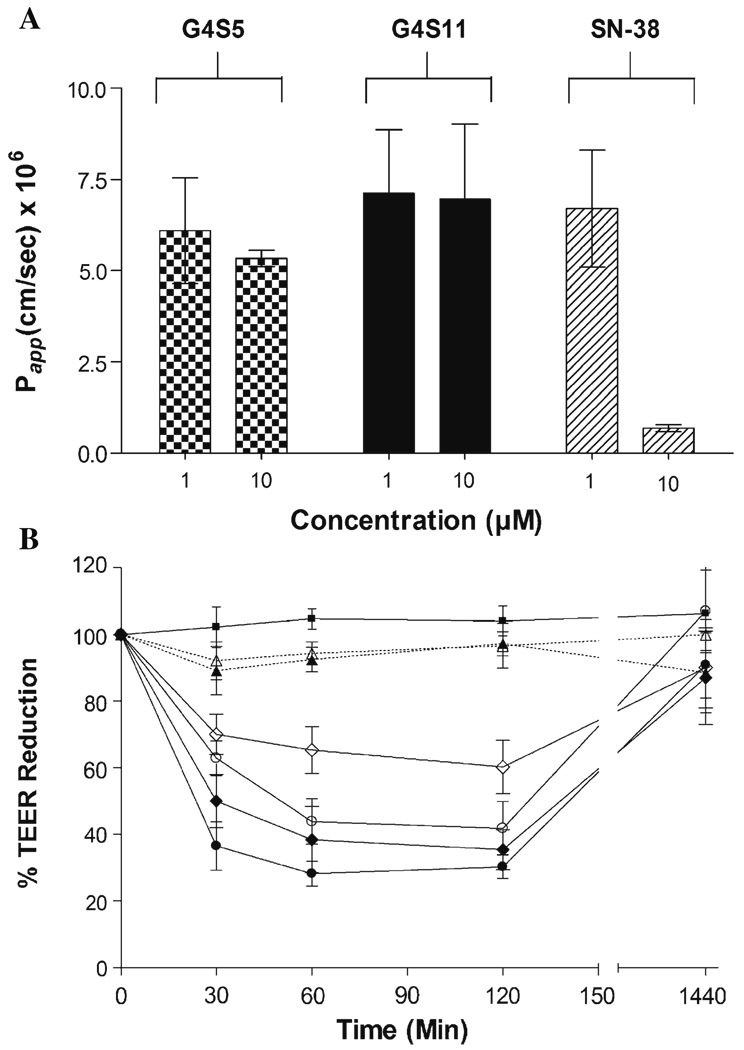
The relative stability of complexes after 2 h at pH 7.4 led us to evaluate the influence of complexation on the permeability of SN-38 across Caco-2 cell monolayers. At the lowest concentration studied (1 µM), apparent permeability coefficients (Papp’s) of G4S5, G4S11 and SN-38 were 6.1×10−6, 7.1×10−6 and 6.7×10−6 cm/s, respectively (Fig. 6A). The permeability values of the complexes were three to fourfold higher than the permeability of unmodified dendrimer (Papp= 1.7×10−6) reported previously (16). This might be due to an increase in the hydrophobicity associated with the complexation of SN-38 to PAMAM dendrimer. Previous studies from our laboratory have demonstrated the influence of hydrophobicity on permeability of PAMAM dendrimers (15). It is important to note that although the permeability values for complexes are similar to that of SN-38, the flux for SN-38 corresponds to the amount of drug present in the complexes. Thus total amount of SN-38 transported is in the order of G4S11 > G4S5 > SN-38. A ten fold increase in concentration (10 µM) increased the flux of polymer complexes across epithelial cells thus maintaining Papp. However, no such increase in flux with increase in concentration was observed for SN-38, consequently lowering Papp for SN-38. Similar findings have been reported by Yamamoto and colleagues (7) who observed a decrease in the transport rate of CPT-11 and SN-38 with increasing concentration. It was postulated that SN-38 was actively transported by P-glycoprotein due to the higher basolateral to apical transport rates observed for SN-38 (7). Observed higher permeability coefficients of G4S5 and G4S11 compared to free SN-38 might be due to the decrease in the efflux of SN-38 when complexed with PAMAM dendrimer. In accordance with our previous findings a concentration and time dependant decline in TEER was observed with G4S5 as well as G4S11 (Fig. 6B). A sudden drop in TEER was observed after the first 30 min and TEER values remained the same until 2 h, while reverted back in the next 24 h after removal of the complexes. As evident from Fig. 6B, SN-38 did not affect TEER values significantly.
Fig. 6.
A Permeability of ( ) G4S5, (
) G4S5, ( ) G4S11 and (
) G4S11 and ( ) SN-38 across Caco-2 cell monolayers after 120 min. B Effect of G4S5, G4S11 and SN-38 on Transepithelial Electrical Resistance (TEER): (
) SN-38 across Caco-2 cell monolayers after 120 min. B Effect of G4S5, G4S11 and SN-38 on Transepithelial Electrical Resistance (TEER): ( ) Control, (
) Control, ( ) SN-38 (1 µM), (
) SN-38 (1 µM), ( )SN-38 (10 µM), (
)SN-38 (10 µM), ( )G4S5 (1 µM), (
)G4S5 (1 µM), ( )G4S5 (10 µM), (
)G4S5 (10 µM), ( )G4S11 (1 µM), (
)G4S11 (1 µM), ( ) G4S11 (10 µM). Results expressed as Mean ± SEM (n=6).
) G4S11 (10 µM). Results expressed as Mean ± SEM (n=6).
In conclusion, these studies provide evidence that water soluble SN-38-PAMAM dendrimer complexes can be used for improving the transepithelial transport of SN-38. Further efforts are required to prepare SN-38 containing PAMAM dendrimers which will be stable in the harsh gastric environment. These studies point to the pH stimuli responsive release of SN-38 from the complex which can be useful for intracellular delivery of SN-38 in target cancer cells.
ACKNOWLEDGMENTS
The authors appreciate the efforts of Dr. Kelly Hom for assisting with NMR studies. Financial support was provided by the Department of Defense multidisciplinary postdoctoral fellowship award (W81XWH-06-1-0698) to Rohit Kolhatkar, a University System of Maryland Integrated Nanobio seed grant through the Maryland Department of Business and Economic Development and NIH R01EB07470.
REFERENCES
- 1.Ulukan H, Swaan PW. Camptothecins: a review of their chemotherapeutic potential. Drugs. 2002;62:2039–2057. doi: 10.2165/00003495-200262140-00004. [DOI] [PubMed] [Google Scholar]
- 2.Shimada Y, Rothenberg M, Hilsenbeck SG, Burris HA, III, Degen D, Von Hoff DD. Activity of CPT-11 (irinotecan hydrochloride), a topoisomerase I inhibitor, against human tumor colony-forming units. Anticancer Drugs. 1994;5:202–206. doi: 10.1097/00001813-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Houghton PJ, Cheshire PJ, Hallman JC, Bissery MC, Mathieu-Boue A, Houghton JA. Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-(4-[1-piperidino]-1-piperidino)-carbonyloxy-camptothecin against human tumor xenografts: lack of cross-resistance in vivo in tumors with acquired resistance to the topoisomerase I inhibitor 9-dimethylaminomethyl-10-hydroxycamptothecin. Cancer Res. 1993;53:2823–2829. [PubMed] [Google Scholar]
- 4.Calabresi P, Chabner BA, Hardman JG, Limbird LE. Chemotherapy of Neoplastic Diseases, Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. New York: 10McGraw-Hill (Medical Publishing Division); 2001. p. 1424. [Google Scholar]
- 5.Ulukan H, Muller MT, Swaan PW. Downregulation of topoisomerase I in differentiating human intestinal epithelial cells. Int. J. Cancer. 2001;94:200–207. doi: 10.1002/ijc.1463. [DOI] [PubMed] [Google Scholar]
- 6.Haaz MC, Rivory L, Riche C, Vernillet L, Robert J. Metabolism of irinotecan (CPT-11) by human hepatic microsomes: participation of cytochrome P-450 3A and drug interactions. Cancer Res. 1998;58:468–472. [PubMed] [Google Scholar]
- 7.Yamamoto W, Verweij J, de Bruijn P, de Jonge MJ, Takano H, Nishiyama M, Kurihara M, Sparreboom A. Active transepithelial transport of irinotecan (CPT-11) and its metabolites by human intestinal Caco-2 cells. Anticancer Drugs. 2001;12:419–432. doi: 10.1097/00001813-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kitchens KM, Foraker AB, Kolhatkar RB, Swaan PW, Ghandehari H. Endocytosis and interaction of poly (amidoamine) dendrimers with Caco-2 cells. Pharm. Res. 2007;24:2138–2145. doi: 10.1007/s11095-007-9415-0. [DOI] [PubMed] [Google Scholar]
- 9.Kitchens KM, El-Sayed ME, Ghandehari H. Transepithelial and endothelial transport of poly (amidoamine) dendrimers. Adv. Drug Deliv. Rev. 2005;57:2163–2176. doi: 10.1016/j.addr.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 10.El-Sayed M, Ginski M, Rhodes C, Ghandehari H. Transepithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. J. Control. Rel. 2002;81:355–365. doi: 10.1016/s0168-3659(02)00087-1. [DOI] [PubMed] [Google Scholar]
- 11.Najlah M, Freeman S, Attwood D, D'Emanuele A. In vitro evaluation of dendrimer prodrugs for oral drug delivery. Int. J. Pharm. 2007;336:183–190. doi: 10.1016/j.ijpharm.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 12.D'Emanuele A, Jevprasesphant R, Penny J, Attwood D. The use of a dendrimer-propranolol prodrug to bypass efflux transporters and enhance oral bioavailability. J. Control. Rel. 2004;95:447–453. doi: 10.1016/j.jconrel.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Wiwattanapatapee R, Carreno-Gomez B, Malik N, Duncan R. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: a potential oral delivery system? Pharm. Res. 2000;17:991–998. doi: 10.1023/a:1007587523543. [DOI] [PubMed] [Google Scholar]
- 14.El-Sayed M, Ginski M, Rhodes C, Ghandehari H. Influence of surface chemistry of poly(amidoamine) dendrimers on Caco-2 cell monolayers. J. Bioact. Comp. Polym. 2003;18:7–22. [Google Scholar]
- 15.Kitchens KM, Kolhatkar RB, Swaan PW, Eddington ND, Ghandehari H. Transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers: influence of size, charge and fluorescent labeling. Pharm. Res. 2006;23:2818–2826. doi: 10.1007/s11095-006-9122-2. [DOI] [PubMed] [Google Scholar]
- 16.Kolhatkar RB, Kitchens KM, Swaan PW, Ghandehari H. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconj. Chem. 2007;18:2054–2060. doi: 10.1021/bc0603889. [DOI] [PubMed] [Google Scholar]
- 17.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 18.Heath F, Haria P, Alexander C. Varying polymer architecture to deliver drugs. AAPS J. 2007;9:E235–E240. doi: 10.1208/aapsj0902026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Sayed M, Rhodes CA, Ginski M, Ghandehari H. Transport mechanism(s) of poly (amidoamine) dendrimers across Caco-2 cell monolayers. Int. J. Pharm. 2003;265:151–157. doi: 10.1016/s0378-5173(03)00391-0. [DOI] [PubMed] [Google Scholar]
- 20.Nabiev I, Fleury F, Kudelina I, Pommier Y, Charton F, Riou JF, Alix AJ, Manfait M. Spectroscopic and biochemical characterisation of self-aggregates formed by antitumor drugs of the camptothecin family: their possible role in the unique mode of drug action. Biochem. Pharmacol. 1998;55:1163–1174. doi: 10.1016/s0006-2952(97)00508-x. [DOI] [PubMed] [Google Scholar]
- 21.Solntsev KM, Sullivan EN, Tolbert LM, Ashkenazi S, Leiderman P, Huppert D. Excited-state proton transfer reactions of 10-hydroxycamptothecin. J. Am. Chem. Soc. 2004;126:12701–12708. doi: 10.1021/ja047821e. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Tomalia DA, Thomas J. Unusual pH-dependant polarity changes in PAMAM dendrimers: evidence for pH-responsive conformational changes. Macromolecules. 2000;33:9169–9172. [Google Scholar]
- 23.Patri AK, Kukowska-Latallo JF, Baker JR., Jr Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Del. Rev. 2005;57:2203–2214. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Dutta T, Agashe HB, Garg M, Balakrishnan P, Kabra M, Jain NK. Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro. J. Drug Target. 2007;15:89–98. doi: 10.1080/10611860600965914. [DOI] [PubMed] [Google Scholar]
- 25.Dhanikula RS, Hildgen P. Influence of molecular architecture of polyether-co-polyester dendrimers on the encapsulation and release of methotrexate. Biomaterials. 2007;28:3140–3152. doi: 10.1016/j.biomaterials.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Kojima C, Kono K, Maruyama K, Takagishi T. Synthesis of polyamidoamine dendrimers having poly(ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconjug. Chem. 2000;11:910–917. doi: 10.1021/bc0000583. [DOI] [PubMed] [Google Scholar]
- 27.Bhadra D, Bhadra S, Jain S, Jain NK. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int. J. Pharm. 2003;257:111–124. doi: 10.1016/s0378-5173(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 28.Seib FP, Jones AT, Duncan R. Comparison of the endocytic properties of linear and branched PEIs, and cationic PAMAM dendrimers in B16f10 melanoma cells. J. Control. Rel. 2007;117:291–300. doi: 10.1016/j.jconrel.2006.10.020. [DOI] [PubMed] [Google Scholar]