Abstract
Background
Little is known about the pattern of genetic and environmental influences on symptoms of anxiety and depression (SxAnxDep) from childhood to early adulthood.
Method
Parental- and self-reported levels of SxAnxDep were assessed at ages 8–9, 13–14, 16–17 and 19–20 years in 2508 twins from the Swedish Twin Study of Child and Adolescent Development (TCHAD). Analysis conducted using the Mx program included SxAnxDep by parental and self-report.
Results
The best-fit model revealed one genetic risk factor for SxAnxDep acting at ages 8–9, 13–14, 16–17 and 19–20, and new sets of genetic risk factors ‘coming on line’ in early adolescence, late adolescence and early adulthood. Together, these genetic factors were very strong influences on the levels of SxAnxDep reported in common by parents and twins with heritability estimates, correcting for rater- and time-specific effects, ranging from 72% to 89%. The first genetic factor, which accounted for 72% of the variance in SxAnxDep at ages 8–9, attenuated sharply in influence, accounting for only 12% of the variance by ages 19–20. No evidence was found for shared environmental influences. Although not statistically significant, the correlation between genetic risk factors for SxAnxDep in males and females declined with advancing age.
Conclusions
Genetic effects on SxAnxDep are developmentally dynamic from middle childhood to young adulthood, demonstrating both genetic innovation and genetic attenuation. The attenuation might explain the low levels of continuity observed for anxiety and depressive disorders from childhood to adulthood. Differences in genetic risk factors for SxAnxDep in males and females may increase during development.
Keywords: Anxiety, depression, development, genetic effects, twins
Introduction
Studies in children (Edelbrock et al. 1995; Topolski et al. 1997; Eley & Stevenson, 1999; Rice et al. 2002; Boomsma et al. 2005), adolescents (Thapar & McGuffin, 1994; Topolski et al. 1997; Rice et al. 2002) and adults (Kendler et al. 1986; Mackinnon et al. 1990) suggest that genetic risk factors substantially influence individual differences in symptoms of anxiety and depression (SxAnxDep). However, as most of these studies are cross-sectional, we know little about the impact of genetic risk factors over time, particularly during childhood and adolescence – a time when levels of SxAnxDep and prevalence of anxiety and mood disorders change substantially (Twenge & Nolen-Hoeksema, 2002; Costello et al. 2005; Angold & Costello, 2006). Changing genetic risk factors for SxAnxDep during development would have implications both for research (e.g. molecular genetic and follow-up studies) and for treatment and prevention.
In this study, we examined the development of genetic and environmental risk factors for SxAnxDep in a population-based cohort of Swedish twins assessed four times from age 8 to 20 years. Our period of observation included puberty, a developmental transition of special interest because gonadal hormones impact on brain mechanisms influencing SxAnxDep (Killgore et al. 2001; Steiner et al. 2003; Toufexis et al. 2006; Walf & Frye, 2006; Koshibu & Levitt, 2008).
Our primary goal was to discriminate between two patterns for the development of genetic risk factors for SxAnxDep. The first, or ‘developmentally stable’ pattern, predicts a single set of genetic risk factors that impacts on SxAnxDep throughout development. The second, or ‘developmentally dynamic’ pattern, predicts that genetic effects on SxAnxDep vary over time through genetic innovation, in which new genes that were previously without effect on SxAnxDep become active, and/or genetic attenuation, in which genes that impact at one developmental age decline in their influence during subsequent periods.
We had two secondary goals:
To determine the nature of shared and unique environmental influences on SxAnxDep from childhood to young adulthood.
To explore the impact of sex on genetic risk factors for SxAnxDep. In particular, does the similarity of genetic influences on SxAnxDep in males and females decline from childhood to young adulthood?
Method
Sample
This study, the Swedish Twin Study of Child and Adolescent Development (TCHAD), began with all twin pairs born in Sweden between May 1985 and December 1986, where both twins were alive and residing in Sweden in 1994 (Lichtenstein et al. 2007). They have been assessed four times for SxAnxDep by a mailed questionnaire: ages 8–9 to parents (n=1109 or 75% response of those eligible), ages 13–14 to parents (n =1063, 73%) and children (n =2263, 78%), ages 16–17 to parents (n =1067, 74%) and children (n =2369 children, 82%), and ages 19–20 to parents (n =619, 78%) and children (n =1705, 59%). Each questionnaire was approved by the Ethics Committee of the Karolinska Institute, Stockholm, Sweden. No informed consent was required because, according to Swedish rules, response to the questionnaire constitutes consent. Zygosity was based on well-validated questions to twins and parents chosen from by a discriminant analysis of 106 pairs with zygosity determined by DNA markers (Lichtenstein et al. 2007).
Measures
Parental-reported SxAnxDep was obtained by using the items from the Anxious/Depressed subscale from the Child Behavior Checklist (CBCL; Achenbach, 1991a) when the twins were aged 8 to 17 years, and from the Adult Behavior Checklist at ages 19–20 (Achenbach & Rescorla, 2003). Self-reported SxAnxDep items came from the same scale from the Youth Self-Report CBL (Achenbach, 1991b) when the twins were ages 13–17, and from the Adult Self-Report form at ages 19–20 (Achenbach & Rescorla, 2003). For this study, we used the 12 items from the Anxious/ Depressed subscale that was in common across these forms. All items were scored on a three-point scale: not true, somewhat or sometimes true, and very true or often true.
Because item performance could vary by age, sex or rater, we calculated, separately for each of our 14 combinations, factor loadings for each item in the program Mplus (Muthen & Muthen, 2004), using a robust weighted least squares estimator. Given the polychotomous nature of the item responses, these loadings were used to generate estimated factors scores by probit regression coefficients.
Data analysis
For these analyses of SxAnxDep, we began with 2718 twin individuals of whom 210 had no available data and/or unknown zygosity. The remaining 2508 individuals came from 1261 complete pairs and 14 single twins. Of these pairs, 246 were female monozygotic (MZ), 185 female dizygotic (DZ), 247 male MZ, 180 male DZ and 403 opposite-sex DZ pairs.
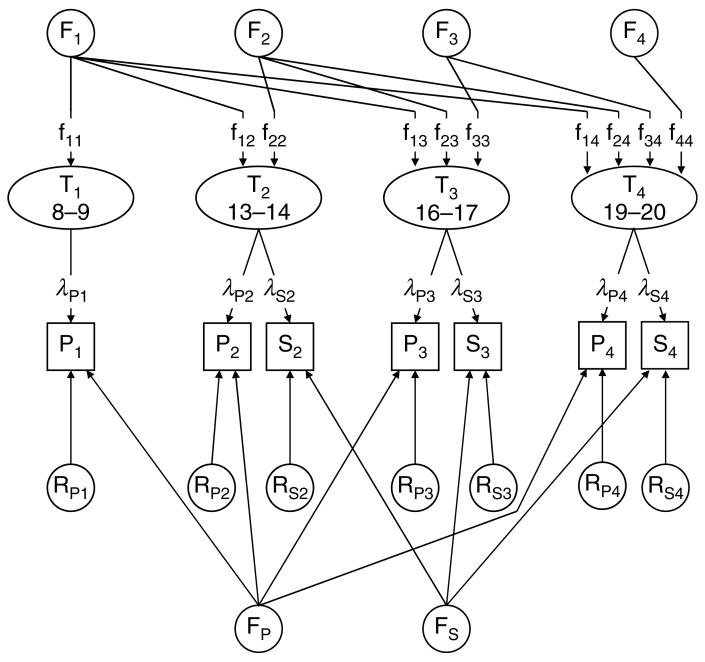
The model used in these analyses is illustrated in Fig. 1 for additive genetic effects. The model has four major features. First, it contains four latent SxAnxDep scores (T1–T4) that reflect the ‘true’ level of SxAnxDep at ages 8–9, 13–14, 16–17 and 19–20, here called times 1, 2, 3 and 4. Second, these latent variables are indexed by ratings of SxAnxDep by parental report (P) or self-report (S). Both reporters are available for times 2, 3 and 4, whereas only parental report is available for time 1. The paths λP and λS reflect the degree to which the parent- and self-reported SxAnxDep ratings index the latent level of SxAnxDep. Third, the genetic and environmental influences on the latent levels of SxAnxDep at times 1, 2, 3 and 4 are modeled as a Cholesky decomposition. This developmentally informative approach divides genetic risk into four factors (F1–F4). The first (F1) begins in childhood (ages 8–9) and is continually active over the entire developmental period. The strength of its effect at each age is reflected in the path coefficients f11, f12, f13 and f14. The second factor begins in early adolescence (ages 13–14) and impacts on times 2, 3 and 4 via paths f22, f23 and f24. The third factor starts in late adolescence (ages 16–17) and impacts on times 3 and 4 via paths f33 and f34. The fourth and final factor acts only at time 4 (young adulthood; ages 19–20), via path f44. The ‘developmentally stable’ hypothesis predicts that genetic liability to SxAnxDep originates solely in the first factor with no later genetic innovation. The ‘developmentally dynamic’ hypothesis predicts both genetic innovation (new genetic variation impacting on SxAnxDep emerging later in development) and genetic attenuation (declining impact over time of the genetic factors acting early in development).
Fig. 1.
The model used in these analyses presented for one source of liability such as additive genetic effects. The model contains four latent scores for symptoms of anxiety and depression (SxAnxDep) (T1–T4) reflecting the true level of SxAnxDep at time 1 (age 8–9), time 2 (age 13–14), time 3 (age 16–17) and time 4 (age 19–20). These latent variables are indexed by ratings of SxAnxDep by parental report (P) (available for times 1–3) and by self-report (S) (available for times 2–4). The degree to which the parent- and self-reported ratings of SxAnxDep index the true latent level of symptoms is reflected by the paths λP and λS. The genetic and environmental influences on the latent SxAnxDep scores are modeled as a Cholesky decomposition. See text article for further details.
Fourth, the model contains two reporter-specific common factors, one each for parent (Fp) and self (Fs), as well as rater- and time-specific residuals. The reporter-specific common factors allow the model to estimate genetic and environmental influences on ratings that are unique to the parents or to the children.
Our analyses focused on the latent measures of SxAnxDep reported by both parents and children (T1–T4) because these measures are likely to be most valid, reflecting both the subjective and objective manifestations of anxiety and depression. Reporter-specific factors (Fp, Fs) are present in the model but are not focused upon here.
Estimates of heritability and shared environmental effects obtained in this model are not comparable to those obtained from standard twin models because in standard twin models, errors of measurement contribute to individual specific environment, thereby reducing estimates of heritability and shared environment. Our use of multiple raters permits us to distinguish true individual specific environment, which impacts on the latent SxAnxDep scores (T1–T4), from the errors of measurement, which contribute to the rater-specific effects (P1–P4 and S2–S4).
We examined qualitative and quantitative sex effects on SxAnxDep. Qualitative sex effects, which arise when genetic factors influencing a trait are not identical in males and females, are measured by the genetic correlation, rg, which can vary from zero (i.e. entirely distinct sets of genes in the two sexes) to unity (i.e. identical genetic factors impacting on males and females). We also assessed qualitative sex effects by the ratio of the observed correlation in DZ opposite-sex pairs to the geometric mean of that observed in male–male and female–female DZ pairs. If the same familial effects are influencing males and females, this ratio should approximate unity. Quantitative sex effects arise when the same genetic factors impact in males and females but to different degrees. This was implemented by allowing all path coefficients to be estimated separately by sex. This is not identical to the scalar sex limitation model described by (Neale et al. 2006) as it does not constrain the genetic correlation matrices to be the same in males and females.
Analyses were performed using the Mx software package (Neale et al. 2003). Factor scores for SxAnxDep were relatively normally distributed and were treated as a continuous trait. To evaluate the fit of our entire model, we used the Bayesian Information Criterion (BIC; Schwarz, 1978), which performs well with complex models (Markon & Krueger, 2004). The lower the BIC value, the better the balance of explanatory power and parsimony.
Results
Table A1 (available in online Appendix) shows the mean (±standard error) of the raw score for the SxAnxDep scale as a function of sex, age and reporter. Higher rates of symptoms were seen for females versus males and for self-report versus parental report. Symptoms increased with age in females whereas for males they declined through adolescence and then increased in young adulthood. Table 1 shows the correlations between self-reports and parental reports of SxAnxDep within and across time. At ages 13–14, 17–18 and 19–20, the correlations in self- and parental ratings of SxAnxDep varied across a narrow range from +0.33 to +0.35. In adjacent waves, the within-rater correlations for self- and parental ratings were similar, ranging from +0.47 to +0.57 and decline monotonically with increasing passage of time. The cross-time cross-rater correlations were lower and, for adjacent assessment periods, ranged from +0.18 to +0.30.
Table 1.
Correlations for symptoms of anxiety and depression as a function of age and reporter (self versus parent)
| Age
|
|||||||
|---|---|---|---|---|---|---|---|
| Age 13–14
|
Age 16–17
|
Age 19–20
|
|||||
| Age | Reporter | Parent | Self | Parent | Self | Parent | Self |
| 8–9 | Parent | 0.48 | 0.18 | 0.42 | 0.19 | 0.35 | 0.15 |
| 13–14 | Parent | – | 0.33 | 0.57 | 0.30 | 0.46 | 0.24 |
| 13–14 | Self | – | – | 0.25 | 0.50 | 0.23 | 0.40 |
| 16–17 | Parent | – | – | – | 0.34 | 0.52 | 0.27 |
| 16–17 | Self | – | – | – | – | 0.29 | 0.52 |
| 19–20 | Parent | – | – | – | – | – | 0.35 |
Our model fitting began with a full model including both quantitative and qualitative sex effects (model I in Table 2). We first attempted to simplify this model by dropping the quantitative sex effects (model II), which markedly improved the model fit as indexed by the BIC. Of note, in this model, the genetic correlations between males and females in model II were estimated at +1.00, 0.72, 0.71 and 0.55 at ages 8–9, 13–14, 16–7 and 19–20 respectively. This intriguing pattern, sugestive of declining similarity of genetic risk factors for SxAnxDep in males and females, is explored in more detail below. Next, we rigorously tested for these qualitative sex effects in model III. Despite the suggestive pattern of estimates, the model fit improved when all genetic correlations were constrained to 1.00.
Table 2.
Model fitting results: a multi-rater developmental model for symptoms of anxiety and depression
| Model | Compared to model | Description | Δχ2 | Δdf | ΔBIC |
|---|---|---|---|---|---|
| I | Fulla | – | – | – | |
| II | I | Drop quantitative sex effects | +113.9 | +54 | −135.8 |
| III | I | Model II+drop qualitative sex effects | +116.1 | +58 | −149.1 |
| IV | Model III+constraining measurement portion of modelb | – | – | – | |
| V | IV | Model IV+no time 4 C factor | 0.0 | +1 | −9.4 |
| VI | IV | Model V+no time 3 C factor | 0.0 | +3 | −28.3 |
| VII | IV | Model VI+no time 2 C factor | 0.0 | +6 | −56.6 |
| VIII | IV | Model VII+no time 1 C factor | +1.3 | +10 | −93.1 |
| IX | IV | Model VIII+no time 4 E factor | +1.3 | +11 | −102.5 |
| Xc | IV | Model IX+no time 3 E factor | +3.0 | +13 | −119.7 |
| XI | IV | Model X+no time 2 E factor | +51.4 | +16 | −99.6 |
| XII | IV | Model X+no time 4 A factor | +27.6 | +14 | −104.5 |
df, Degrees of freedom; BIC, Bayesian Information Criterion.
−2 log-likelihood 31774.2, df=12 486, BIC=32935.5.
−log-likelihood 31890.6, df=12 544, BIC=32409.8.
Best-fit model.
Our goal was then to determine which of the paths in the model III were required to explain the pattern of correlations between and within twin pairs within and across time. To facilitate this effort, in model IV, we constrained the measurement portion of our model to the estimates obtained in model III (Table A2, online). All subsequent models modify only the genetic and environmental influences on the latent measures of SxAnxDep (T1 to T4). We compare the resultant fit to that obtained by model IV.
In models V, VI, VII and VIII, we constrained to zero the shared environmental or C effects at times 4, 3, 2 and 1. The model fit, as reflected by the BIC, improved with each step. Next, we constrained to zero the unique environmental or E effects at times 4, 3 and 2 in models IX, X and XI respectively. The fit further improved in models IX and X (Table 2), but setting to zero the E effects at time 2 results in a substantial deterioration in the BIC. Finally, in model XII, we constrained to zero the genetic effects at time 4. This also caused the model fit to deteriorate, thereby indicating that model X was our best-fit model.
Parameter estimates for this model, along with 95% confidence intervals, are shown in Table 3 and results for the genetic factors illustrated in Fig. 2. Seven results are noteworthy. First, shared environmental factors did not contribute to the rater-independent latent SxAnxDep scores. Second, genetic factors played a strong role in influencing symptoms of SxAnxDep as indexed by self- and parent ratings. Heritability was estimated at 72, 89, 84 and 79% for times 1, 2, 3 and 4 respectively.
Table 3.
Parameter estimates and 95% confidence intervals for the best-fit model (model X) for symptoms of anxiety and depression (T1 to T4)a
| λ path
|
Residual
|
Genetic factors
|
Unique environmental factor
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor (age) | P | S | P | S | Total a2 (%) | 1 | 2 | 3 | 4 | Total e2 (%) | 1 | 2 |
| 1 | 0.77 | – | 0.33 | – | 72 | 0.85 | – | – | – | 28 | 0.53 | – |
| (8–9) | 0.42–0.94 | 0.00–0.58 | 0.79–0.90 | 0.44–0.61 | ||||||||
| 2 | 0.73 | 0.45 | 0.56 | 0.65 | 89 | 0.46 | 0.83 | – | – | 11 | 0.22 | 0.25 |
| (13–14) | 0.62–0.83 | 0.38–0.54 | 0.50–0.61 | 0.55–0.75 | 0.36–0.55 | 0.77–0.87 | 0.11–0.33 | 0.13–0.36 | ||||
| 3 | 0.65 | 0.49 | 0.49 | 0.58 | 84 | 0.48 | 0.58 | 0.53 | – | 16 | 0.13 | 0.38 |
| (16–17) | 0.55–0.75 | 0.41–0.58 | 0.00–0.56 | 0.00–0.66 | 0.37–0.58 | 0.49–0.66 | 0.43–0.60 | 0.00–0.26 | 0.26–0.48 | |||
| 4 | 0.75 | 0.45 | 0.57 | 0.69 | 79 | 0.34 | 0.42 | 0.37 | 0.60 | 21 | 0.04 | 0.46 |
| (19–20) | 0.65–0.86 | 0.38–0.53 | 0.43–0.65 | 0.58–0.76 | 0.20–0.46 | 0.32–0.52 | 0.23–0.50 | 0.51–0.68 | −0.13 to 0.21 | 0.33–0.58 | ||
a2, Heritability, or the proportion of variance in fear scores resulting from genetic factors; e2, the proportion of variance in fear scores resulting from unique environmental factors.
The λ path, depicted in Fig. 1, connects the latent liability to fears to the report level of fears from parent-report (P) and self-report (S).
Results for the ‘upper part’ of the model only (see Fig. 1), which includes symptoms of anxiety and depression (SxAnxDep) shared by parent- and self-reporters. Parameter estimates constrained to equality across the sexes and the value of rg set to 1.0. Parameter estimates for the lower part of the model are available in Table A2 (online).
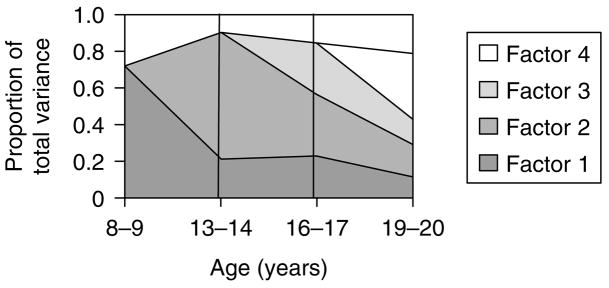
Fig. 2.
The proportion of total variance in symptoms of anxiety and depression (SxAnxDep) accounted for by genetic factors through development. The y axis represents the total phenotypic variance so the sum of all the factors equals the total heritability. The first genetic factor, which starts at ages 8–9, is represented in dark grey. An intermediate grey represents the second genetic factor starting at ages 13–14. Light grey represents the third genetic factor starting at ages 16–17 and white represents the fourth genetic factor acting only at ages 19–20.
Third, consistent with the predictions of the ‘developmentally dynamic’ hypothesis, we found evidence for genetic innovation in genetic factors 2, 3 and 4. That is, in addition to the temporally stable genetic influences that begin at ages 8–9, as illustrated in Fig. 2, the model demonstrated substantial new genetic influences on SxAnxDep emerging at each of the three subsequent ages: 13–14, 16–17 and 19–20. Fourth, we also saw strong evidence for genetic attenuation. The first genetic factor accounted for 72% of the variance in our latent measures of SxAnxDep at ages 8–9 but declines steeply in influence, and by ages 19–20 accounted for only 12% of that variance. A less dramatic but similar decline is seen for factor 2. The attenuation of these genetic factors is not likely to result from chance factors as the parameter estimates in the youngest and oldest age periods have non-overlapping confidence intervals.
Fifth, unique environmental factors accounted for 28, 11, 16 and 21% of the variance in SxAnxDep as reported by both parent and child. The effects of the first unique environmental factor attenuated sharply over time. By contrast, the impact of the second unique environmental factor grew over time, suggesting that some environmental experiences not shared with twins between the ages of 8–9 and 13–14 have an enduring impact on SxAnxDep. Sixth, at each of the three time-points when we had reports from both parent and child, the λP path was higher than the λS path, suggesting that parental ratings were contributing more strongly to the latent index of anxiety and depression than was self-report. Seventh, parameter estimates for the reporter-specific parent- and self-report factors for SxAnxDep are shown in Table A2 (online). Examining rater-specific effects unique to parents, estimates of shared environment substantially exceeded that for genetic effects whereas for the rater-specific factors unique to the twins, the reverse pattern was seen.
Although our global analyses did not support the presence of qualitative sex effects, model II showed intriguing evidence suggestive of declining genetic correlations between males and females with development. Given our prior interest in this question, and the low power for detecting qualitative sex effects even with samples of our size (Prescott & Gottesman, 1993), we pursued this issue further in an exploratory manner. Our best-fit model X produced a –2 log likelihood value of 31893.6 with 12 557 degrees of freedom. This model constrained the genetic correlation (ra) in males and females to equal unity across all four ages. If we added to this model a single parameter, a linear decline in ra across age, the –2 log likelihood improved by 1.1 units and estimated ra was equal to 1.00, +0.90, +0.80 and +0.70 at ages 8–9, 13–14, 16–17 and 19–20 respectively. The fit by BIC was moderately worse than that seen for the best-fit model (Δ 8.4 units) and the fit by Akaike’s Information Criterion (AIC; Akaike, 1987), another widely used and validated fit index for structural modeling (Williams & Holahan, 1994), was slightly worse (Δ 0.9 units).
To determine whether this effect was seen in both parental and twin reports, we examined the ratio of correlations in SxAnxDep scores in opposite- to same-sex DZ twins. With parental ratings, this ratio was 1.50, 1.14, 0.88 and 0.64 respectively across the four waves from ages 8–9 to 19–20. Using self-ratings, this ratio, from ages 13–14 to 19–20 was 0.85, 0.62 and 0.62 respectively.
Discussion
Developmentally dynamic genetic effects
This study sought to discriminate ‘developmentally stable’ versus ‘developmentally dynamic’ patterns of genetic risk factors for SxAnxDep from childhood to young adulthood. Our results support the developmentally dynamic hypothesis. Our best-fit model showed evidence for both genetic innovation and attenuation. Genetic innovation was demonstrated by strong new genetic effects on SxAnxDep ‘coming on line’ at ages 13–14, 16–17 and 19–20. Attenuation was evident as the first genetic risk factors accounted for far more of the variance in SxAnxDep at ages 8–9 than 19–20. The importance of genetic factors influencing SxAnxDep at ages 13–14 declined less dramatically over time.
Four studies are particularly relevant to the current report. First, Scourfield et al. (2003) examined parental reports of depressive symptoms in Welsh twins at the mean ages of 11 and 14 and, in accord with our findings, demonstrated both genetic innovation and attenuation. Second, by contrast, O’Connor et al. (1998) examined twin and sibling pairs, using a composite measure of parental and self-report depression, at the mean ages of 13 and 15 and found no evidence for genetic innovation and slight genetic attenuation. Third, Silberg et al. (2001) demonstrated a different kind of dynamic genetic effect on SxAnxDep in childhood and adolescence. Genetic influences on depressive symptoms in female twins aged 14–17 reflected the liability to symptoms of overanxious disorder and phobias assessed at ages 8–13. Finally, a prior study of situational, animal and blood injury fears in the TCHAD study (Kendler et al. 2008) also showed robust genetic attenuation and innovation.
Other results
Our study had two subsidiary goals, the first of which was to clarify the nature of environmental influences on SxAnxDep from childhood to young adulthood. We found no statistical evidence for a shared environmental contribution to SxAnxDep. However, the unique environment was important and developmentally dynamic. The first unique environmental factor significantly influenced SxAnxDep at the younger ages but rapidly attenuated in impact with age. However, the second unique environmental factor increased in importance through development. Environmental experiences not shared with the co-twin occurring soon after puberty can have an effect on levels of SxAnxDep that endures into early adulthood.
The second subsidiary goal of this report was to explore sex effects on the genetic risk factors for SxAnxDep. We were particularly interested in the possibility of puberty-associated changes in the relationship between genetic influences on SxAnxDep in males and females. Our data suggested that genetic influences on symptoms of SxAnxDep in males and females were very similar in childhood. We have intriguing but only suggestive evidence that these genetic influences become more distinct from ages 13 to 20. Although these results were considerably short of being statistically significant, they were noteworthy because they are what would be expected if genetic influences on SxAnxDep are partly moderated by gonadal hormonal exposures. This exposure becomes increasingly divergent in males and females after puberty. These tentative results are also consistent with a range of prior research including (i) evidence that the prevalence difference in major depression in the sexes appears at puberty and is linked more directly to changes in gonadal steroids than in morphological changes (Angold et al. 1998, 1999), (ii) findings in adult samples that genetic risk factors for major depression are only partly correlated in males and females (Kendler et al. 2001, 2006) and (iii) multiple findings in animals and human that gonadal hormones influence multiple neurobiological systems that potentially impact on levels of mood and anxiety (Steiner et al. 2003; Toufexis et al. 2006; Walf & Frye, 2006; Koshibu & Levitt, 2008). Clearly, replication is needed, in large samples, that would ideally incorporate into the analyses measurements of pubertal stages. The plausibility of these findings is increased by a seeing, in both parental- and self-report ratings, a declining resemblance of levels of SxAnxDep over time in opposite-sex versus same-sex DZ twins (although the decline in parents begins with greater similarity in opposite-sex versus same-sex DZ twins, a pattern not expected under any theory of which we are aware).
Implications
If replicated, our results have two important implications. First, molecular genetic studies of anxiety and depression during development will need to move beyond static models to capture the true complexity of gene action. This point is well illustrated by a recent molecular genetic study that showed, across multiple large samples, a gene×age interaction for a single nucleotide polymorphism (SNP) (rs1455832) in the gene ROBO1 (Lasky-Su et al. 2008). The CC genotype of this SNP was strongly related to obesity early in life but the magnitude of this association declined substantially with age, exactly the pattern expected given genetic attenuation.
Second, the low levels of continuity of anxiety and depressive disorders from childhood to adulthood (Last et al. 1996; Weissman et al. 1999; Ost & Treffers, 2001) would be an explicable result of genetic attenuation and innovation.
Limitations
These results should be considered in the context of five potentially important methodological limitations. First, these results are from a single birth cohort in Sweden and may not extrapolate to other ethnic groups. Second, it would have been desirable to examine separately symptoms of anxiety and depression. We attempted to obtain stable and distinct anxiety and depression subscales from the CBCL Anxious/Depressed subscale in a number of ways and failed consistently. Our results therefore apply only to the scale that we used and might have differed if we had had other more detailed measures of anxious and depressive symptoms.
Third, some of our findings might have arisen artifactually because we had only parental reports at ages 8–9. To examine this question, we developed an alternative model that did not combine parent and twin ratings but approached as closely as possible our best-fit model. We fitted this directly to our seven measures of SxAnxDep: parental report at ages 8–9, 13–14, 16–17 and 19–20 and self-report at ages 13–14, 16–17 and 19–20. Reassuringly, this less elegant model showed all the major trends noted above, including evidence for genetic attenuation in both the parental-and self-report data. For example, the first genetic factor loaded +0.53 on the wave I parental report but loaded only 0.38, 0.18 and 0.19 on the wave 2, 3 and 4 parental reports.
Fourth, we were surprised that our model results indicated that parental ratings were better indices of the latent levels of SxAnxDep than self-ratings. These findings may have resulted from the model attempting to explain the consistently higher levels of twin resemblance seen in the parent- versus child-reported SxAnxDep scores and thus may not reflect a general feature of parent versus self-ratings.
Fifth, there was insufficient information to examine jointly developmental changes in levels of SxAnxDep reported by both parent and child and those specific to parental or child report. Our analyses focused on the former because of their greater validity. As seen in Table A2 (online), substantial familial effects unique to individual reporters were evident. Of note, although the familial influences on parent-specific ratings were largely shared-environmental in origin, for the child-specific ratings familial effects were almost entirely genetic.
Acknowledgments
This study was supported in part by NIH grants MH068643 and MH-65322, the Swedish Council for Working Life and Social Research (project 2004-0383) and the Swedish Research Council (2004-1415).
Footnotes
Note
Supplementary material accompanies this paper on the Journal’s website (http://journals.cambridge.org).
Declaration of Interest
None.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, VT: 1991a. [Google Scholar]
- Achenbach TM. Manual for the Youth Self-Report and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, VT: 1991b. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms and Profiles. University of Vermont, Research Center for Children, Youth and Families; Burlington, VT: 2003. [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Angold A, Costello EJ. Puberty and depression. Child and Adolescent Psychiatric Clinics of North America. 2006;15:919–937. doi: 10.1016/j.chc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychological Medicine. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, van Beijsterveldt CE, Hudziak JJ. Genetic and environmental influences on Anxious/ Depression during childhood: a study from the Netherlands Twin Register. Genes, Brain and Behavior. 2005;4:466–481. doi: 10.1111/j.1601-183X.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child and Adolescent Psychiatric Clinics of North America. 2005;14:631–648. doi: 10.1016/j.chc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Edelbrock C, Rende R, Plomin R, Thompson LA. A twin study of competence and problem behavior in childhood and early adolescence. Journal of Child Psychology and Psychiatry. 1995;36:775–785. doi: 10.1111/j.1469-7610.1995.tb01328.x. [DOI] [PubMed] [Google Scholar]
- Eley TC, Stevenson J. Exploring the covariation between anxiety and depression symptoms: a genetic analysis of the effects of age and sex. Journal of Child Psychology and Psychiatry. 1999;40:1273–1282. [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Annas P, Neale MC, Eaves LJ, Lichtenstein P. A longitudinal twin study of fears from middle childhood to early adulthood: evidence for a developmentally dynamic genome. Archives of General Psychiatry. 2008;65:421–429. doi: 10.1001/archpsyc.65.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychological Medicine. 2001;31:605–616. doi: 10.1017/s0033291701003907. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner C, Pedersen N. A Swedish national twin study of lifetime major depression. American Journal of Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath A, Martin NG, Eaves LJ. Symptoms of anxiety and depression in a volunteer twin population. The etiologic role of genetic and environmental factors. Archives of General Psychiatry. 1986;43:213–221. doi: 10.1001/archpsyc.1986.01800030023002. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12:427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Koshibu K, Levitt P. Gene×environment effects: stress and memory dysfunctions caused by stress and gonadal factor irregularities during puberty in control and TGF-alpha hypomorphic mice. Neuropsychopharmacology. 2008;33:557–565. doi: 10.1038/sj.npp.1301436. [DOI] [PubMed] [Google Scholar]
- Lasky-Su J, Lyon HN, Emilsson V, Heid IM, Molony C, Raby BA, Lazarus R, Klanderman B, Soto-Quiros ME, Avila L, Silverman EK, Thorleifsson G, Thorsteinsdottir U, Kronenberg F, Vollmert C, Illig T, Fox CS, Levy D, Laird N, Ding X, McQueen MB, Butler J, Ardlie K, Papoutsakis C, Dedoussis G, O’Donnell CJ, Wichmann HE, Celedon JC, Schadt E, Hirschhorn J, Weiss ST, Stefansson K, Lange C. On the replication of genetic associations: timing can be everything! American Journal of Human Genetics. 2008;82:849–858. doi: 10.1016/j.ajhg.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last CG, Perrin S, Hersen M, Kazdin AE. A prospective study of childhood anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1502–1510. doi: 10.1097/00004583-199611000-00019. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Tuvblad C, Larsson H, Carlstrom E. The Swedish Twin study of CHild and Adolescent Development: the TCHAD study. Twin Research and Human Genetics. 2007;10:67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- Mackinnon AJ, Henderson AS, Andrews G. Genetic and environmental determinants of the lability of trait neuroticism and the symptoms of anxiety and depression. Psychological Medicine. 1990;20:581–590. doi: 10.1017/s0033291700017086. [DOI] [PubMed] [Google Scholar]
- Markon KE, Krueger RF. An empirical comparison of information-theoretic selection criteria for multivariate behavior genetic models. Behavioral Genetics. 2004;34:593–610. doi: 10.1007/s10519-004-5587-0. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide. Muthen & Muthen; Los Angeles, CA: 2004. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. Department of Psychiatry, Virginia Commonwealth University Medical School; Box 980126, Richmond, VA 23298: 2003. [Google Scholar]
- Neale MC, Roysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and G×E interaction. Twin Research and Human Genetics. 2006;9:481–489. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Neiderhiser JM, Reiss D, Hetherington EM, Plomin R. Genetic contributions to continuity, change, and co-occurrence of antisocial and depressive symptoms in adolescence. Journal of Child Psychology and Psychiatry. 1998;39:323–336. [PubMed] [Google Scholar]
- Ost LG, Treffers PDA. Onset, course, and outcome for anxiety disorders in children. In: Silverman WK, Treffers PDA, editors. Anxiety Disorders in Children and Adolescents : Research, Assessment and Intervention. Cambridge University Press; NY, New York: 2001. pp. 293–312. [Google Scholar]
- Prescott CA, Gottesman I. Power limitations in detecting heterogeneity of genetic effects: the case of sex differences in alcoholism. Paper presented at the Annual Meeting of the Society for Research on Psychopathology; Chicago. October 1993.1993. [Google Scholar]
- Rice F, Harold GT, Thapar A. Assessing the effects of age, sex and shared environment on the genetic aetiology of depression in childhood and adolescence. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2002;43:1039–1051. doi: 10.1111/1469-7610.00231. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Annual Statistics. 1978;6:461–464. [Google Scholar]
- Scourfield J, Rice F, Thapar A, Harold GT, Martin N, McGuffin P. Depressive symptoms in children and adolescents: changing aetiological influences with development. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44:968–976. doi: 10.1111/1469-7610.00181. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Rutter M, Eaves L. Genetic and environmental influences on the temporal association between earlier anxiety and later depression in girls. Biological Psychiatry. 2001;49:1040–1049. doi: 10.1016/s0006-3223(01)01161-1. [DOI] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. Journal of Affective Disorders. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Thapar A, McGuffin P. A twin study of depressive symptoms in childhood. British Journal of Psychiatry. 1994;165:259–265. doi: 10.1192/bjp.165.2.259. [DOI] [PubMed] [Google Scholar]
- Topolski TD, Hewitt JK, Eaves LJ, Silberg JL, Meyer JM, Rutter M, Pickles A, Simonoff E. Genetic and environmental influences on child reports of manifest anxiety and symptoms of separation anxiety and overanxious disorders: a community-based twin study. Behavioral Genetics. 1997;27:15–28. doi: 10.1023/a:1025607107566. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Hormones and Behavior. 2006;50:539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Nolen-Hoeksema S. Age, gender, race, socioeconomic status, and birth cohort differences on the children’s depression inventory: a meta-analysis. Journal of Abnormal Psychology. 2002;111:578–588. doi: 10.1037//0021-843x.111.4.578. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Wolk S, Wickramaratne P, Goldstein RB, Adams P, Greenwald S, Ryan ND, Dahl RE, Steinberg D. Children with prepubertal-onset major depressive disorder and anxiety grown up. Archives of General Psychiatry. 1999;56:794–801. doi: 10.1001/archpsyc.56.9.794. [DOI] [PubMed] [Google Scholar]
- Williams L, Holahan P. Parsimony-based fit indices for multiple-indicator models: do they work? Structural Equation Modeling. 1994;1:161–189. [Google Scholar]