Abstract
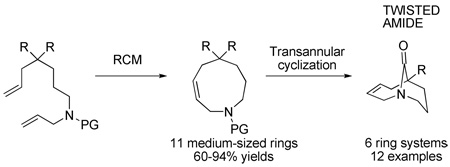
The sequential RCM to construct a challenging medium-sized ring followed by a transannular cyclization across a medium-sized ring delivers previously unattainable twisted amides from simple acyclic precursors.
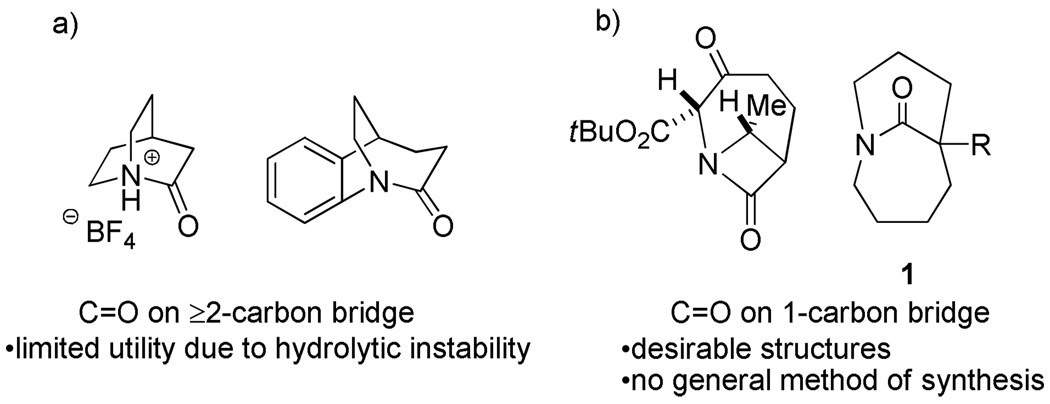
Many lactams incorporating nitrogen at a bridgehead position contain non-planar or “twisted” amides. 1 Due to limited overlap of the nitrogen lone pair of electrons with the π-system of the carbonyl group, such compounds are extremely sensitive to hydrolysis and display other kinds of reactivity that are sharply divergent from that of standard lactams.2 Moreover, since a fully twisted amide represents the transition state of the cis–trans amide bond isomerization essential in protein folding,3 it has been suggested that compounds that contain nonplanar amides could be useful as inhibitors of proline isomerases.4 However, due to inherent strain and the enhanced lability to water, twisted amides have not fulfilled their promise as biological tools.
The vast majority of bridged amides place the carbonyl group on a bridge containing two or more carbons (Figure 1a). Although less common, we have recently shown that one-carbon bridged twisted amides 1 (Figure 1b) are substantially more persistent in aqueous solutions.5
Figure 1.
Some twisted amides (a) with the C=O bond placed on a 2- or 3-carbon7 or (b) on a 1-carbon bridge.8
This arises from the relatively relaxed ring sizes present in 1 and the fact that the ring-opened amino acid corresponding to this structure is destabilized by transannular interactions. However, the amide bond in 1 is substantially distorted from planarity and the lactam displays reactivity that belie this nature.6
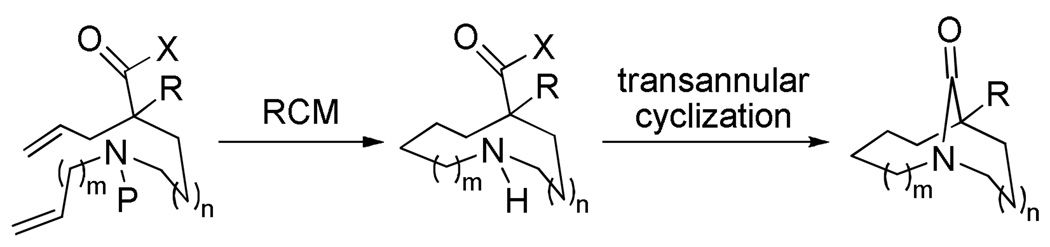
In general, existing synthetic approaches to one-carbon bridged twisted amides are limited to particular structural types9 and do not allow for synthesis of larger number of diverse analogues.10 There is no general method of synthesis of one-carbon bridged twisted amides. The observation that lactams 1 can reform in water once hydrolyzed, plus the rich history of transannular cyclizations in synthesis,11 (including limited precedent from the twisted amide chemistry),12 suggested that such ring systems might be accessible using a direct cyclization approach. Although only limited precedent supported the synthesis of medium-ring nitrogen containing heterocycles with appropriately placed amine and carboxylic acid derivative functionalities,13 we believed that, if successful, RCM would allow for rapid construction of diverse precursors to the key cyclization.14 Herein, we report the realization of these ideas to provide a highly general solution to the problem of one-carbon bridged twisted amide synthesis (Scheme 1).
Scheme 1.
RCM/cyclization strategy.
Our initial investigations focused on the preparation of the [4.3.1] bicyclic ring system previously studied in this laboratory.5,6,8b Thus, malonate 2a was prepared and subjected to range of RCM conditions. After extensive experimentation it was found that Hoveyda–Grubbs 2 catalyst15 most effectively led to the 9-membered heterocycle 3a (Table 1). Use of these conditions allowed synthesis of a series of analogues containing various amine substitutions, including readily removable carbamate groups (Table 1, entries 12 and 13).16
Table 1.
Optimization of RCM.
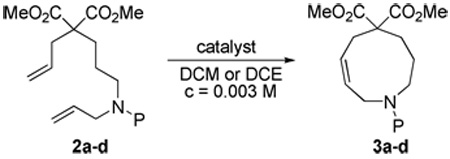 | ||||||
|---|---|---|---|---|---|---|
| entry | diene [P] |
catalyst | mol [%] |
T [°C] |
t [h] |
yield [%]a |
| 1 | Ts (2a) | G1 | 100 | 40 | 26 | 87 |
| 2 | Ts (2a) | Fürstner | 50 | 24 | 25 | 54 |
| 3 | Ts (2a) | Fürstner | 20 | 40 | 22 | 41 |
| 4 | Ts (2a) | G2 | 50 | 40 | 21 | 43 |
| 5 | Ts (2a) | G2 | 20 | 80 | 21 | 76 |
| 6 | Ts (2a) | G2 | 5 | 80 | 21 | 72 |
| 7 | Ts (2a) | G2c | 5 | 80 | 21 | 69 |
| 8 | Ts (2a) | HG2 | 5 | 80 | 21 | 81 |
| 9 | Ts (2a) | HG2d | 5 | 80 | 8 | 87b |
| 10 | Ts (2a) | HG2e | 5 | 80 | 8 | 95b |
| 11 | Ns (2b) | HG2d | 5 | 80 | 16 | 93b |
| 12 | Boc (2c) | HG2d | 5 | 80 | 17 | 85b |
| 13 | Cbz (2d) | HG2c | 5 | 80 | 8 | 89b |
Determined by 1H NMR.
Isolated yields.
With Ti(OiPr)4.
Argon bubbled through the reaction.
Open to air. G1 = Grubbs catalyst 1, G2 = Grubbs catalyst 2, Fürstner = Fürstner catalyst, HG2 = Hoveyda-Grubbs catalyst 2. RCM = ring closing metathesis. Ns = 2-Nitrobenzenesulfonyl. Cbz = Carbobenzyloxy.
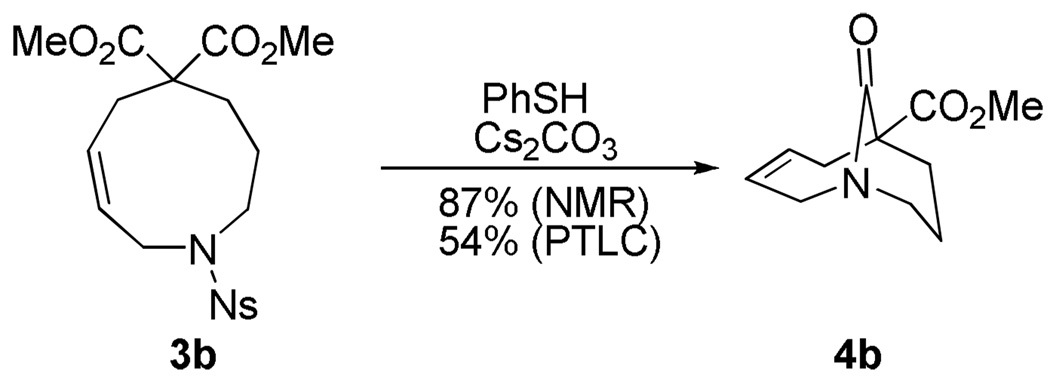
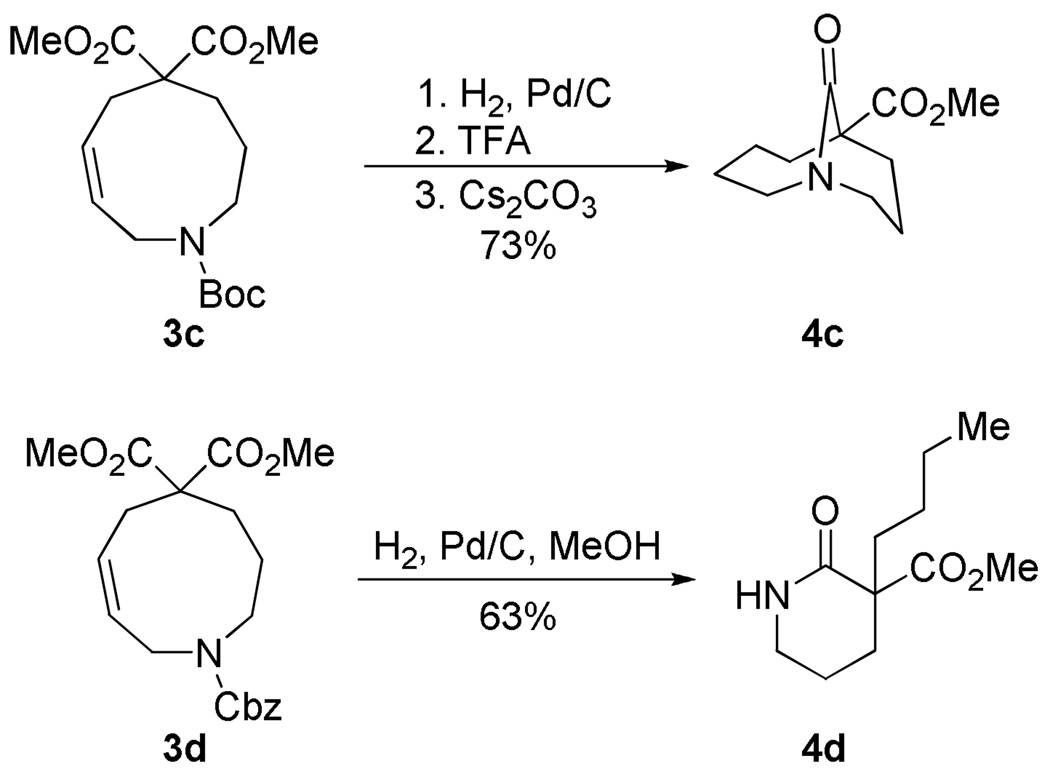
We now wished to determine whether the desired lactams could be obtained via direct cyclization of the substrates. Previously, we had determined that some bicyclic amino acids analogous to 3 were in equilibrium with their closed forms (even in water), but that the hydrolysis reactions were irreversible if the medium-sized ring adopted a conformation with the carboxylic acid in an exo position. In the present cases, we controlled for this through the use of gem-diester substitution. In the event, deprotection and cyclization of the Ns precursor could be carried out in a single operation to deliver 4b under very mild conditions (Scheme 2). Although this material showed modest sensitivity to flash chromatography, it could be isolated in ca. 50% yield after PTLC.
Scheme 2.
Synthesis of [4.3.1] lactam.
We have also determined that the Boc precursor 3c could be utilized for preparation of twisted amides (Scheme 3, top). In contrast, the use of Cbz derivatives could be problematic. Deprotection and cyclization of 3d (Scheme 3, bottom) proceeded smoothly, but the twisted amide proved to be unstable to the hydrogenation conditions, giving piperidone 4d by C-N ring cleavage.6
Scheme 3.
Synthesis from orthogonally protected systems.
The sequential RCM/transannular cyclization strategy was extended to a series of dienes, thus providing a systematic series of twisted lactam ring systems (Table 2). In general, the RCM reactions proceeded in very good yields. All of the medium-sized rings save one (entry 3) were obtained as exclusive cis double bond isomers. This study provides very rare examples of the successful use of catalytic RCM in the formation of 9- and 10-membered nitrogen containing ring systems with minimal conformational constraints.17 Furthermore, the cyclization of the medium ring amino diesters to bridged lactams proved gratifyingly general. Although the cyclization of compound 3g proved sluggish under our initially identified conditions, the [5.3.1] twisted amide could be generated by treatment with DBU after deprotection (entry 3). While malonate could not be applied for preparation of the [4.4.1] system (entry 5) due to competing decarboxylation, use of the phenyl acetate (entry 6) allowed for preparation of the desired compound, albeit in conservative yield. The experiment in entry 7 was performed to explore the effect of leaving group on cyclization reaction. Replacing methoxide with phenoxide dramatically improved the yield of the transannular cyclization, delivering lactam 4j in 86% yield.
Table 2.
Synthesis of bridged lactams.
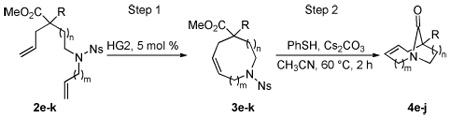 | |||||
|---|---|---|---|---|---|
| entry | series (n, m) |
R | ring system |
yields, step 1/2 [%] |
notes |
| 1 | e (1, 1) | CO2Me | [4.2.1] | 90/75 | – |
| 2 | f (1, 2) | CO2Me | [5.2.1] | 94/85 | a |
| 3 | g (2, 2) | CO2Me | [5.3.1] | 90/64 | b, c |
| 4 | h (1, 3) | CO2Me | [6.2.1] | 92/41 | d |
| 5 | i (3, 1) | CO2Me | [4.4.1] | 79/0 | – |
| 6 | j (3, 1) | Ph | [4.4.1] | 76/33 | e |
| 7 | j (3, 1) | Phf | [4.4.1] | 60/86 | g |
Step 2 run for 13 h.
Compound 5c obtained as 5:1 mixture of Z/E isomers.
Step 2: (i) PhSH, Cs2CO3; (ii) DBU, PhMe, 200 °C, 3 h.
Step 2: (i) PhSH, Cs2CO3; (ii) DBU, PhMe, 180 °C, 12 h.
Step 2: (i) PhSH, Cs2CO3; (ii) DBU, PhMe, 220 °C, 10 h.
PhO2C instead of MeO2C.
Step 2 run at 110 °C for 16 h.
Several methods to prepare saturated lactams were also investigated (Table 3). This normally straightforward process was complicated by the tendency of some twisted amides to undergo unusual C-N cleavage reaction under mild hydrogenolysis conditions.6 Thus, when twisted amides prepared in the current study were treated with standard hydrogenolysis conditions, [4.3.1] and [4.4.1] scaffolds showed the highest reactivity, participating in C-N cleavage to the corresponding monocyclic amides (Table 3; entries 1 and 2), while [4.2.1], [5.2.1], [5.3.1] and [6.2.1] twisted amides were less reactive, undergoing only traditional reduction to the saturated analogues (entry 3; only products shown). As expected, allylic olefins are more susceptible to hydrogenolysis than isolated bonds. Interestingly, when hydrogenation of [4.4.1] scaffold was carried out in the presence of Willkinson’s catalyst, the amide bond remained intact and the saturated amide was obtained in high yield (entry 4).
Table 3.
Hydrogenation/Hydrogenolysis of bicyclic lactams.
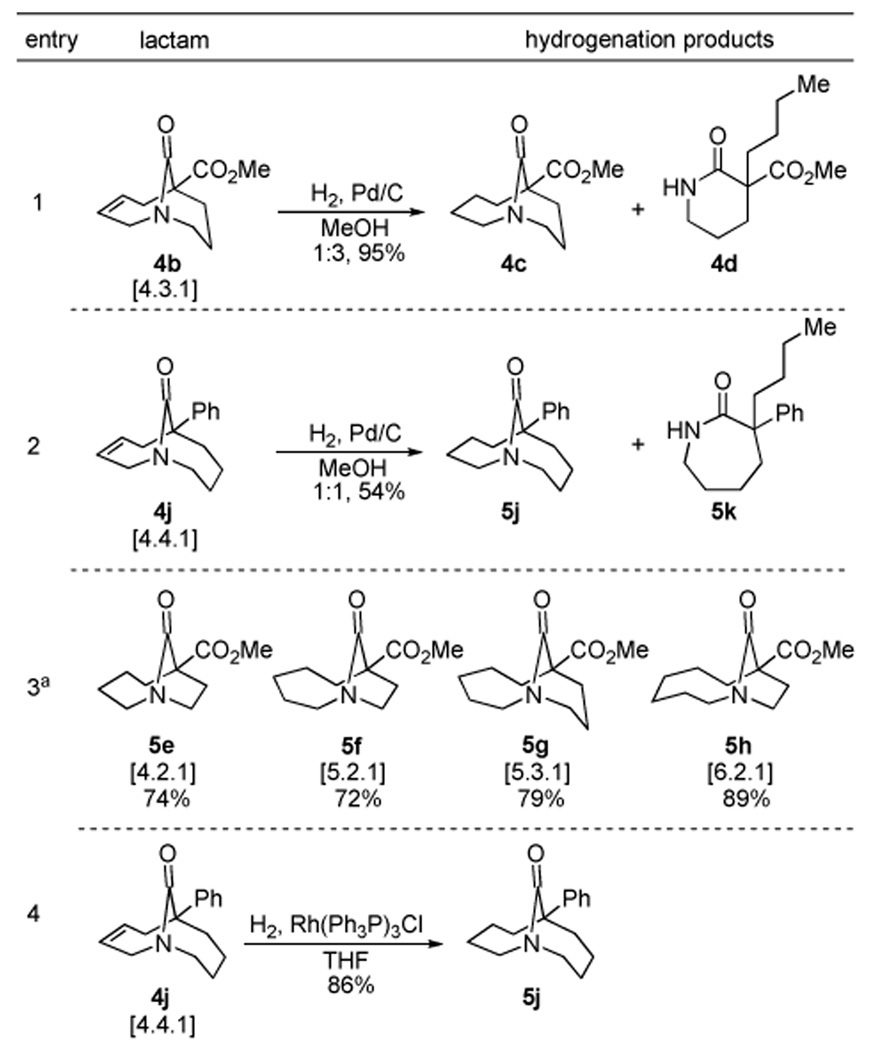 |
Only products of hydrogenation of corresponding unsaturated lactams 4e–h shown.
One goal of the present project is to obtain a series of varied ring systems to allow systematic exploration of the effect of amide twist on chemical and spectroscopic properties. For example, non-planar amides often display spectral features consistent with less “amide-like” and greater “ketone-like” nature of the carbonyl group.18 Although the medium-bridged lactams prepared herein do not contain perfectly orthogonal Nlone pair–carbonyl groupings, most of the ring systems prepared here show substantially higher 13C chemical shifts for the carbonyl carbon and higher carbonyl stretches in the infrared spectra, consistent with a substantial degree of twist (Table 4). As expected, several of the ring systems containing larger rings are able to relax into values closer to those of analogous fused lactams (entries 3 and 5). We note that the infrared stretching frequences cover a range that begins at that for a “normal” amide (entry 6) and go all the way to a value that would be expected for a saturated, “normal” ketone (entry 1). This bodes well for the use of this suite of compounds for the systematic evaluation of the effect of amide bond geometry on reactivity. Interestingly, there is not a perfect correlation between IR stretching frequencies and 13C NMR carbonyl chemical shifts (also noted by Yamada18), suggesting that subtle electronic effects beyond bond angle likely effect these parameters.
Table 4.
Spectroscopic properties of saturated lactams.
| entry | lactam | ring system |
lactam C=O 13C [ppm] |
lactam IR νC=O [cm−1] |
|---|---|---|---|---|
| 1 | 5e | [4.2.1] | 183.4 | 1716 |
| 2 | 5f | [5.2.1] | 180.1 | 1693 |
| 3 | 5h | [6.2.1] | 173.4 | 1685 |
| 4 | 4c | [4.3.1] | 181.0 | 1679 |
| 5 | 5g | [5.3.1] | 176.6 | 1647 |
| 6 | 5j | [4.4.1] | 186.3 | 1643 |
In summary, a sequential RCM/transannular cyclization strategy has been developed that provides access to an expanded family of one-carbon bridged lactams. This route highlights the rapid assembly of previously inaccessible, functionalized ring systems from readily available starting materials. The mild conditions for the transannular cyclization allow for isolation of strained amides by a route similar to classical amide bond formation but facilitated by close proximity of the reactive functionalities. Work is currently underway on further development of this methodology and its application to the synthesis of a whole gamut of twisted amides. As already exemplified by hydrogenolysis reactions, this in turn will allow for the systematic study of strain influence on chemical and biological properties of amide bonds.
Supplementary Material
Acknowledgment
This work was supported by the National Institute of General Medical Sciences (GM-49093).
Footnotes
Supporting Information Available: Experimental procedures and spectral data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For reviews see: Hall HK, Elshekeil A. Chem. Rev. 1983;83:549. Greenberg A. In: The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science. Greenberg A, Breneman CM, Liebman JF, editors. Wiley-Interscience; 2000. p. 47. Clayden J, Moran WJ. Angew. Chem., Int. Ed. 2006;45:7118. doi: 10.1002/anie.200603016.
- 2.For leading references see: Greenberg A, Moore DT, DuBois TD. J. Am. Chem. Soc. 1996;118:8658. Kirby AJ, Komarov IV, Feeder N. J. Chem. Soc., Perkin Trans. 2. 2001;4:522. Brown R. In: The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science. Greenberg A, Breneman CM, Liebman JF, editors. Wiley-Interscience; 2000. p. 85. Mujika JI, Mercero JM, Lopez X. J. Am. Chem. Soc. 2005;127:4445. doi: 10.1021/ja044873v.
- 3.(a) Shao H, Jiang X, Gantzel P, Goodman M. Chem. Biol. 1994;1:231. doi: 10.1016/1074-5521(94)90015-9. [DOI] [PubMed] [Google Scholar]; (b) Fischer G, Schmid FX. In: Molecular Chaperones and Folding Catalysts: Regulation, Cellular Functions and Mechanisms. Bakau B, editor. CRC; 1999. p. 461. [Google Scholar]; (c) Schmid FX. Protein Folding in the Cell. Vol. 59. 2002. p. 243. [Google Scholar]
- 4.Greenberg A. In: The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science. Greenberg A, Breneman CM, Liebman JF, editors. Wiley-Interscience; 2000. p. 78. [Google Scholar]
- 5.Szostak M, Yao L, Aube J. J. Org. Chem. 2009;74:1869. doi: 10.1021/jo802192v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei Y, Wrobleski AD, Golden JE, Powell DR, Aube J. J. Am. Chem. Soc. 2005;127:4552. doi: 10.1021/ja050214m. [DOI] [PubMed] [Google Scholar]
- 7.(a) Tani K, Stoltz BM. Nature. 2006;441:731. doi: 10.1038/nature04842. [DOI] [PubMed] [Google Scholar]; (b) Somayaji V, Brown RS. J. Org. Chem. 1986;51:2676. [Google Scholar]
- 8.(a) Williams RM, Lee BH, Miller MM, Anderson OP. J. Am. Chem. Soc. 1989;111:1073. [Google Scholar]; (b) Yao L, Aube J. J. Am. Chem. Soc. 2007;129:2766. doi: 10.1021/ja068919r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.For the only examples of one-carbon medium bridged amides, see: (a) two examples from Ref. 8. Arata Y, Kobayash T. Chem. Pharm. Bull. 1972;20:325. Schill G, Priester CU, Windhovel UF, Fritz H. Tetrahedron. 1987;43:3747.
- 10.For failed attempts to form one-carbon bridged amides, see: Amide coupling reactions: (a) Ref. 9c. Intramolecular SN2 displacement: Smissman EE, Wirth PJ, Abernethy D, Ayres JW. J. Org. Chem. 1972;37:3486. doi: 10.1021/jo00795a020. Smissman EE, Ayres JW. J. Org. Chem. 1971;36:2407. doi: 10.1021/jo00816a005. Smissman EE, Robinson RA, Matuszak AJ. J. Org. Chem. 1970;35:3823. doi: 10.1021/jo00836a054. Brouillette WJ, Friedrich JD, Muccio DD. J. Org. Chem. 1984;49:3227. Selenium-mediated electrophilic cyclization: Toshimitsu A, Terao K, Uemura S. Tetrahedron Lett. 1984;25:5917. Toshimitsu A, Terao K, Uemura S. J. Org. Chem. 1987;52:2018. Claisen condensation: Smissman EE, Wirth PJ, Glynn DR. J. Org. Chem. 1975;40:281. doi: 10.1021/jo00899a012. Direct RCM: Doodeman R. University of Amsterdam; 2002. Preston AJ, Gallucci JC, Paquette LA. J. Org. Chem. 2006;71:6573. doi: 10.1021/jo0611162. Lei Y. University of Kansas; 2006. (bond isomerization was observed rather than formation of strained twisted amides). [2+2] cycloaddition: Booker-Milburn KI, Anson CE, Clissold C, Costin NJ, Dainty RF, Murray M, Patel D, Sharpe A. Eur. J. Org. Chem. 2001;8:1473. Oxidative coupling: Szostak M, Aubé J. Unpublished results.
- 11.For a pioneering work, see: Leonard NJ, Fox RC, Oki M, Chiavarelli S. J. Am. Chem. Soc. 1954;76:630. For selected examples from the recent literature, see: Balskus EP, Jacobsen EN. Science. 2007;317:1736. doi: 10.1126/science.1146939. Chandler CL, List B. J. Am. Chem. Soc. 2008;130:6737. doi: 10.1021/ja8024164. Vital P, Hosseini M, Shanmugham MS, Gotfredsen CH, Harris P, Tanner D. Chem. Commun. 2009;14:1888. doi: 10.1039/b822955d.
- 12.(a) Ref. 9c. Bashore CG, Samardjiev IJ, Bordner J, Coe JW. J. Am. Chem. Soc. 2003;125:3268. doi: 10.1021/ja028152c.
- 13.For reviews, see: Maier ME. Angew. Chem., Int. Ed. 2000;39:2073. doi: 10.1002/1521-3773(20000616)39:12<2073::aid-anie2073>3.0.co;2-0. Nubbemeyer U. Stereoselective Heterocyclic Synthesis Iii. Vol. 216. 2001. p. 125. Michaut A, Rodriguez J. Angew. Chem., Int. Ed. 2006;45:5740. doi: 10.1002/anie.200600787. For selected approaches from the recent literature, see: Kan T, Fujiwara A, Kobayashi H, Fukuyama T. Tetrahedron. 2002;58:6267. David O, Meester WJN, Bieraugel H, Schoemaker HE, Hiemstra H, van Maarseveen JH. Angew. Chem., Int. Ed. 2003;42:4373. doi: 10.1002/anie.200351930. Klapars A, Parris S, Anderson KW, Buchwald SL. J. Am. Chem. Soc. 2004;126:3529. doi: 10.1021/ja038565t.
- 14.For selected reviews, see: Phillips AJ, Abell AD. Aldrichimica Acta. 1999;32:75. Fürstner A. Angew. Chem., Int. Ed. 2000;39:3013. Deiters A, Martin SF. Chem. Rev. 2004;104:2199. doi: 10.1021/cr0200872. Chattopadhyay SK, Karmakar S, Biswas T, Majumdar KC, Rahaman H, Roy B. Tetrahedron. 2007;63:3919. Compain P. Adv. Synth. Catal. 2007;349:1829.
- 15.For a review, see: Hoveyda AH, Gillingham DG, Van Veldhuizen JJ, Kataoka O, Garber SB, Kingsbury JS, Harrity JPA. Org. Biomol. Chem. 2004;2:8. doi: 10.1039/b311496c. For recent examples of the use of Hoveyda-Grubbs catalysts in demanding RCM reactions, see: Brown MK, Hoveyda AH. J. Am. Chem. Soc. 2008;130:12904. doi: 10.1021/ja8058414. Farina V, Shu C, Zeng X, Wei X, Han Z, Yee NK, Senanayake CH. Org. Process Res. Dev. 2009;13:250.
- 16.For selected examples of RCM reactions influenced by the presence of carbamate or ester functionalities, see: Campagne JM, Ghosez L. Tetrahedron Lett. 1998;39:6175. Brouwer AJ, Liskamp RMJ. J. Org. Chem. 2004;69:3662. doi: 10.1021/jo0358325. Fürstner A, Langemann K. J. Am. Chem. Soc. 1997;119:9130.
- 17.For selected examples of RCM reactions in formation of 9- and 10-membered nitrogen containing ring systems, see: Bamford SJ, Goubitz K, van Lingen HL, Luker T, Schenk H, Hiemstra H. J. Chem. Soc., Perkin Trans. 1. 2000;3:345. Fürstner A, Guth O, Duffels A, Seidel G, Liebl M, Gabor B, Mynott R. Chem.--Eur. J. 2001;7:4811. doi: 10.1002/1521-3765(20011119)7:22<4811::aid-chem4811>3.0.co;2-p. Enders D, Lenzen A, Backes M, Janeck C, Catlin K, Lannou MI, Runsink J, Raabe G. J. Org. Chem. 2005;70:10538. doi: 10.1021/jo0518093.
- 18.Yamada S. Rev. Heteroat. Chem. 1999;19:203. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.