Abstract
In the present study we examined brain and liver derived proteasome complexes to elucidate if there is a differential susceptibility in proteasome complexes from these tissues to undergo inactivation following exposure to oxidative stressors. We then examined the influence of aging and dietary restriction (DR) on the observed proteasome inactivation. Studies used a filtration based methodology that allows for enrichment of proteasome complexes with less tissue than is required for traditional chromatography procedures. Our results indicate that the brain has much lower levels of overall proteasome activity, and exhibits increased sensitivity to hydrogen peroxide mediated inactivation as compared to proteasome complexes derived from the liver. Interestingly, the brain proteasome complexes did not appear to have increased susceptibility to 4-hydroxynonenal (HNE)-induced inactivation. Surprisingly, aging and DR induced minimal effects on oxidative stress mediated proteasome inhibition. These results indicate that the brain not only has lower levels of proteasome activity as compared to the liver, but is also more susceptible to inactivation following exposure to some (but certainly not all) oxidative stressors. Our data also suggest that aging and DR may not significantly modulate the resistance of the proteasome to inactivation in some experimental settings.
Keywords: 4-hydroxynonenal, Aging, Brain, Liver, Oxidative stress, Proteasome
INTRODUCTION
The proteasome proteolytic system is responsible for degradation of oxidized and ubiquitinated proteins in both the nucleus and cytoplasm (1–3). Structurally the proteasome is a multiprotein complex that consists of a catalytic core, the 20S proteasome, with additional cap-like structures capable of binding to the 20S to form a 26S proteasome complex. The 19S (PA700) and 11S cap-like structures are the best characterized of the 26S proteasome cap structures (4–8). The beta subunits of eukaryotic proteasome exhibit multiple endopeptidase activities including chymotrypsin-like (ChT-L), trypsin-like (T-L) and peptidylglutamyl-peptide hydrolase (PGPH) activities. These peptidase activities mediate the hydrolysis of proteins at hydrophobic, basic, and acidic residues, respectively (1, 9,10).
The proteasome is involved in a variety of cellular processes including regulating the level of misfolded and damaged proteins (11,12), regulating cell cycle progression (13,14), transcriptional regulation (15,16), modulating apoptosis (17), and contributing to the regulation of both immune and stress responses (18–20). Inhibition of the proteasome complexes has been shown to occur in aging tissues and age-related neurodegerative diseases (21–26). The observed age-related decreases in proteasome activity, in both in vitro and in vivo settings, are believed to be mediated by several individual and/or coordinated effects of aging. For example, studies have demonstrated a potential role for proteasome inhibition occurring as the result of increased levels of oxidized and/or aggregated proteins (27), increased oxidative modifications to the proteasome complexes (23,28–33), and deleterious changes in proteasome composition (25,29). Inhibition of proteasome in both aging and age related diseases are believed to contribute to cellular dysfunction and further cellular deterioration (26, 34, 35). This is based largely on the fact that proteasome inhibition is sufficient to recapitulate many aspects of aging and age-related disease (36–38), and the observation that increases in proteasome levels is sufficient to extend cellular lifespan and increase resistance to a variety of stressors (39–40).
Dietary restriction (DR) has been shown to increase life span and retard the age-related decline of several physiological functions and age related pathologies (41). DR was found to reduce the levels of oxidatively damaged proteins in liver (42, 43) and increase the resistance of neurons to age-related stressors (44). DR has been linked to the regulation of proteasome function, most notably increasing the basal levels of overall proteasome activity in the aging liver (45) and muscle (46). At present, it is unclear whether DR modulates stress-induced inactivation of proteasome complexes, which could potentially serve as a mechanism by which DR promotes beneficial effects on proteasome function. Cumulatively our data indicate that the brain has much lower levels of proteasome activity as compared to the liver, and is much more vulnerable to inactivation following exposure to pro-oxidative stress environment, which together may have important implications to understanding to the relative vulnerabilities of the brain and liver to toxicity following exposure to stressors. Because aging and DR had minimal effects on each of these analyses our data raise interesting questions with regards to how aging and DR may promote changes in proteasome-mediated protein degradation in the brain and liver.
MATERIALS
The substrates Boc-Leu-Arg-Arg-AMC (PGPH activity) and Suc-Leu-Leu-Val-Tyr-AMC (ChT-L activity) were purchased from Bachem America’s Inc., (Torrance, CA). The substrate Boc-Leu-Ser-Thr-Arg-7-Amido-4-methylcoumarin (T-L activity) was purchased from Sigma Aldrich Company (St Louis, MO). The YM-100 centricon filters were purchased from Millipore Corporation (Bedford, MA). 4-Hydroxy-2-nonenal (HNE) was purchased from Cayman Chemical Company (Ann Arbor, MI). All other items were purchased from Sigma Aldrich Inc. (St. Louis, MO).
Animal tissues
Male Helicobacter-free F344/Brown Norway (F344 × BN F1) rats were obtained from the NIA Dietary Restriction (DR) colony. The rats in this study consisted of 6 three month-old ad libitum (AL), 6 twenty five month-old AL, and 6 twenty five month-old DR rodents. All animals were handled and euthanatized in accordance with IACUC approved protocols at the Pennington Biomedical Research Center.
Filtration of tissue lysates to remove low molecular weight proteases
Liver and brain tissues were homogenized in lysis buffer containing 20 mM Tris-HCl pH-7.4, 5 mM Magnesium chloride, 2 mM ATP, 100 mM NaCl and 0.5 mM DTT as described previously (47). Lysates were centrifuged at 25,000 X g for 45 minutes. The supernatant obtained was diluted with 1 volume of lysis buffer and filtered through the YM-100 centricon filters to remove low molecular weight proteases. The proteins remaining on the filter were collected (here forth referred to as retentate), subjected to protein assays, and then utilized for further proteasome activity assays.
In-Gel proteasome activity assay
The activity of the proteasome in the retentate was analyzed using 4% Native-PAGE, essentially as described by Glickman et al. (48). The protein samples were run on Native PAGE at 120 V for approximately 3 hrs at 4°C. The proteasome activity was visualized by incubating the gels in activity buffer containing 20mM Tris-Cl pH-7.8, 2mM ATP, 5 mM KCl, 5mM Magnesium chloride, 0.5mM DTT and 100 μM of Suc-Leu-Leu-Val-Tyr-AMC, (substrate for ChT-L enzyme activity). Following 30 minute incubation the 26S proteasome bands were visualized under a UV light source at a wavelength of 360 nm. To visualize the 20S proteasome activity, the same gel was incubated in the activity buffer (without ATP) containing 0.02% SDS and visualized under a UV light source.
Assays for proteasome activity
Assays for proteasome activity were performed as described previously by our laboratory (47) using the fluorogenic substrates for PGPH, ChT-L, and T-L activities. The reaction was conducted in 250 μl of activity assay buffer within a 96 well black assay plate with clear bottom containing 50 μg/ml of liver or brain retentate, 20mM Tris HCl, pH 7.8, 1 mM EDTA, 0.5 mM DTT and 5mM MgCl2, 2mM ATP and 50 μM of the corresponding substrate. Samples were then administered the indicated concentrations of H202 or HNE. The reaction mixture was incubated for 1 hour at 37°C and the fluorescence of the released AMC product was measured in Molecular Devices plate reader at an emission wavelength of 355 nm and an excitation wavelength of 460 nm. The background fluorescence values obtained by incubating the lysates with MG132 were subtracted from activity values as described previously by our laboratory (47), (37). The proteasome activity per mg of the brain or liver protein per hour was calculated from the fluorescence values and all subsequent data expressed as percent control values.
RESULTS
Analysis of 26S and 20S proteasome activity present in 100 kDa cutoff filtrate
Because of the limited availability of tissues to utilize for aging and DR studies, we sought to establish a method that allowed for the analysis of proteasome activity in small amounts of tissue, which currently is not possible with chromatography procedures that are commonly utilized by our laboratory and others. Additionally, we wanted to develop an assay that allowed for the monitoring of proteasome complexes without the presence of free-proteasome subunits, which are capable of generating background peptidase activity in commonly utilized peptide based assays of proteasome activity. To this end we conducted studies in which liver lysates were generated and filtered using filter sets that had a 100 kDa cutoff, and thus allowed for free proteasome subunits (< 40 kDa) to be separated from proteasome complexes. When we subjected the crude lysate, 100 kDa retentate, or filtrate to in-gel analysis of proteasome activity and observed the presence of significant 26S (ATP stimulated) and 20S (SDS stimulated) proteasome activity in the crude lysate and retentate (Figure 1), that was not observed in the filtrate. Analysis of retentates from liver and brain revealed that the majority peptidase activity was inhibited by the proteasome inhibitor MG-132 (Figure 2), consistent with the majority of peptidase activity being mediated by proteasome complexes in the retentate. Interestingly, the liver was observed to have nearly twice the amount of proteasome activity as compared to the brain (Figure 2).
Figure 1. In-gel assay for peptidase activity of the proteasome.
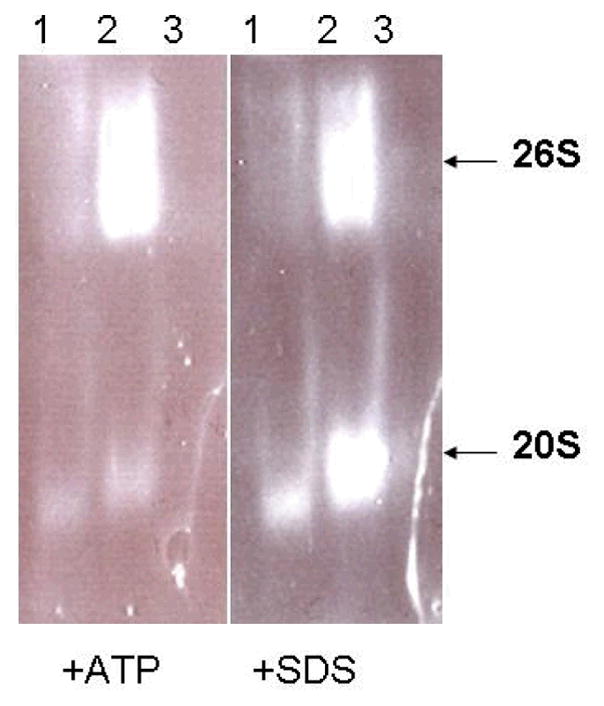
Liver lysates from 3 month-old rats were analyzed for proteasome activity presence in the crude homogenate (1), >100 kDa retentate (2) and filtrate (3). Equal concentrations of the samples were separated on a 4% native PAGE gel and assayed for the presence of 26S (ATP stimulated) and 20S (SDS stimulated) proteasome complexes via visualization of ChT-L activity of the proteasome. These data show an enrichment of proteasome complexes in the retentate relative to the homogenate or filtrate.
Figure 2. Analysis of brain and liver proteasome activity in the retentate fractions.
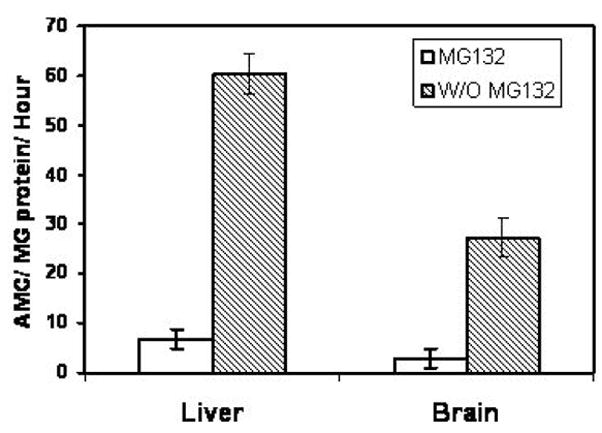
The liver and brain retentates from 3 month-old rats were analyzed for the rates of ChT-L activity in the presence and absence of the proteasome inhibitor MG132. These data demonstrate that the liver possesses significantly higher levels of proteasome activity relative to the brain and that MG132 suppresses the majority of observed peptidase activity consistent with the involvement of the proteasome in observed peptidase activity.
Effects of hydrogen peroxide on liver and brain proteasome activity
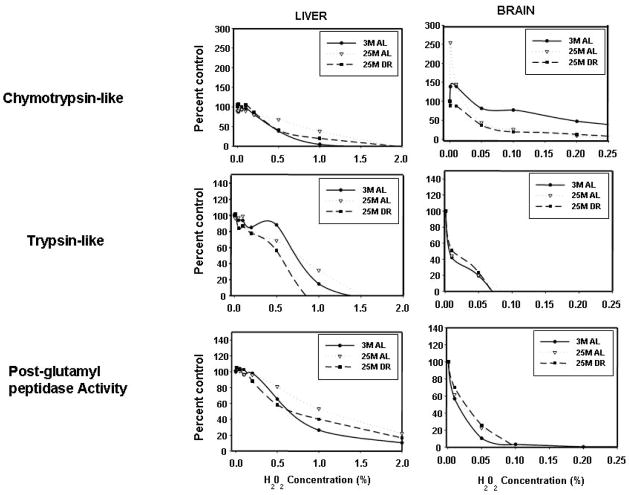
Incubation of both liver and brain proteasome complexes with hydrogen peroxide induced a dose-dependent inhibition of ChT-L, T-L, and PGPH activities of the proteasome (Figure 3). Brain proteasome complexes exhibited dramatic sensitivities to hydrogen peroxide mediated inactivation of T-L, ChT-L, and PGPH activities (Figure 3), as compared to the liver. In the presence of hydrogen peroxide liver proteasomes showed relative IC50 values of 0.729, 0.429, and 0.644 for T-L, ChT-L, and PGPH respectively. In the presence of hydrogen peroxide brain proteasomes showed relative IC50 values of 0.006, 0.184 and 0.015 for T-L, ChT-L, and PGPH activities respectively. Interestingly, extremely low levels of hydrogen peroxide were observed to stimulate chymotrypsin-like activity selectively in proteasome complexes from the brains of 3- and 25-month old rodents (Figure 3). In our overall analyses aging and DR did not exhibit significant effects on the amount of hydrogen peroxide-induced inactivation of the proteasome peptidase activities.
Figure 3. Effect of H202 on ChT-L, T-L, and PGPH activities of the proteasome.
The liver and brain retentates from 3 month-old AL, 25 month-old AL, and 25 month-old DR rats were analyzed for proteasome inactivation following treatment with increasing concentrations of H202. The effect of H202 on ChT-L, T-L, and PGPH proteasome activities were analyzed.
Effects of HNE on liver and brain proteasome Activity
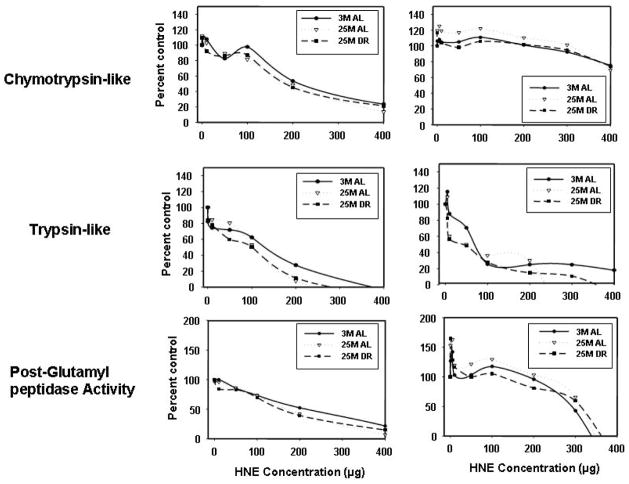
Incubation with the lipid peroxidation product HNE inhibited each of the peptidase activities of both liver and brain proteasome complexes (Figure 4), and did so in a dose dependent manner. Interestingly, brain ChT-L activity was observed to exhibit increased resistance to HNE-induced inactivation (Figure 4) as compared to the liver. Brain and liver proteasome complexes exhibited similar levels of HNE induced inactivation of T-L-like and PGPH activities (Figure 4). In the presence of HNE, Liver proteasomes showed relative IC50 values of 129, 215, 217 for T-L, ChT-L and PGPH activities respectively. Brain proteasomes showed an approximate relative IC50 values of 72, 280, and 290 for T-L, ChT-L and PGPH activities respectively. Extremely low levels of HNE were observed to selectively stimulate PGPH activity in proteasome complexes from the brain (Figure 4). In our overall analyses aging and DR did not exhibit significant effects on the overall effects of HNE on proteasome peptidase activities.
Figure 4. Effect of HNEon ChT-L, T-L, and PGPH activities of the proteasome.
The liver and brain retentates from 3 month-old AL, 25 month-old AL, and 25 month-old DR rats were analyzed for proteasome inactivation following treatment with increasing concentrations of HNE. The effect of HNEon ChT-L, T-L, and PGPH proteasome activities were analyzed.
DISCUSSION
The proteasome contributes to multiple cellular processes ranging from proliferation, differentiation, and apoptosis (1, 3, 18, 49). Accumulation of abnormal (oxidized, unfolded, cross-linked, glycated) proteins is known to occur in mammalian cells during both in vitro and in vivo aging due a toxic combination of elevated levels of reactive oxygen species (ROS), decreased removal of and repair of damaged molecules, and decreased replacement of essential molecules (30, 35,50,51). Cumulatively, these events promote the development oxidative stress. Oxidatively damaged proteins may cross-link each other, form aggregates which can disrupt cellular homeostasis, and accumulate in cells and tissues resulting in impaired cellular functioning (41). The brain contains high levels of polyunsaturated fatty acids, which upon oxidation can form neurotoxic lipid peroxidation products including HNE (52–54). Post mitotic neurons injured by oxidative stress cannot be readily replaced, potentially compounding the effects of oxidative injury over a lifetime, and potentially leading to a number of neurological disorders. Chronic alcohol use induces the oxidative stress in liver via the inflammatory response of Kupffer cells and other types (macrophages, neutrophils, lymphocytes) in response to elevated gut-derived endotoxin plasma levels (55). This leads to amplified formation of ROS and cell-toxic or profibrogenic cytokines including TNF and TGF which promote fibrogenesis, hepatocellular carcinoma and liver injury (56, 57). However it is clear that for the liver to undergo such toxicity there must be both high levels of ROS and a chronic exposure to ROS. It appears from current studies that the brain is much less capable of withstanding such prolonged periods of oxidative stress. As such, while oxidative stress is linked to aging and toxicity in the liver and brain, it appears that the liver is much more resistant to the toxicity of oxidative stress as compared to the brain. This may be due to the high mitotic capacity of the liver relative to the brain, which allows it to regenerate damaged cells, but may also be due to other factors. Our data suggest that elevated levels of proteasome activity in the liver (relative to the brain) may potentially play a role in promoting the ability of the liver to withstand periods of exposure to oxidative stress relative to the brain. By having increased levels of proteasome activity the liver could potentially be able to remove deleterious proteins which are generated as the result of exposure to oxidative stressors. In addition to having increased levels of proteasome activity, our studies indicate that liver proteasomes are able to sustain their proteolytic activity in the presence of elevated levels of hydrogen peroxide, consistent with a model whereby enhanced preservation of proteasome activity contributes to viability of liver following exposure to stressors.
Our data may have important implications for understanding why the brain is so succeptible to cell loss and dysfunction in response to oxidative stress injury and age-related diseases of the nervous system. Having a predisposition to have proteasome activity lowered to a toxic level, via reduced basal levels of activity and increased sensitivity to hydrogen peroxide, the brain is extremely vulnerable to the toxic effects of some endogenous proteasome inhibitors. For example, hydrogen peroxide rapidly inactivates brain proteasomes relative to the proteasomes found in liver. Such inhibition of the proteasome could thereby promote further disturbances in cellular and tissue homeostasis and contribute to the aging and cell dysfunction. However, the fact that brain proteasomes are not more vulnerable to HNE-induced inactivation suggests that brain proteasomes may be able to perform at a higher level in the face of some selective forms of ROS or lipid peroxidation products. It is interesting to note that previous studies from our laboratory demonstrated that neural cells were able to maintain viability and proteasome function following low level exposure to chronic ROS (58), which was associated with increased expression of selective proteasome subunits. These data suggest that the cells of the brain have both compositional alterations in the proteasome, and potential translational alterations in proteasome subunit expression, which can modulate the ability of brain proteasomes (and presumably proteasomes from other tissues) to successfully respond to the presence of some oxidative stressors.
Declines in proteasome activitiy are observed with age (22, 25, 36, 59) with some studies reporting the ability of DR to rescue the proteasome activity in aging animals (42, 43, 45, 61, 65). The levels of proteasome were found to be similar during aging in these previous studies, with the observed the decline in proteasome activity mainly attributed to the changes in the proteasome subunit composition and/or the presence of deleterious posttranslational modifications (including oxidative modifications). The inhibition of the proteasome has also been linked through a variety of experiments to play a role in aging (25, 36,42). In previous studies DR was found to increase the levels of heat shock proteins and increase the 20S proteasome activity in Fisher 344 rat muscle (61). The amount of the proteasome present in brain tissues was found to be lower than that observed in the muscle, and to differ in composition as compared to the muscle (60,62). Interestingly, 2D PAGE analysis and immunoblotting analysis indicated that brain and liver samples from young rodents have similar composition of proteasome subunits as well as same substrate specificity (35, 41, 60, 62). However, interferon induced immunoproteasome subunits like LMP7, LMP2 and MECL were observed to be elevated in the liver and lung, as compared to the brain (35,63). Genetic depletion of the LMP2 and LMP7 subunits in the brain and liver exacerbate age-related decreases in proteasome activities, and promoted age-related increases in oxidative stress (64). The differences in the susceptibility of the brain and liver proteasomes to oxidative stress could be due to the presence of different proteasome subtypes (constitutive versus immunoproteasome), or due in part to different levels of 26S versus 20S proteasomes in these different tissues (65). Together, these studies highlight the importance of understanding how different subtypes of proteasomes are affected by aging and DR in the brain and other tissues.
While chromatography techniques have been invaluable in establishing the biochemistry of the 20S and 26S proteasomes, the fact that they require the use of large amount of tissue has made it difficult to study proteasome biology in settings where the amount of tissue available is extremely limited. In the present study we demonstrated that a filtration based assay allows for rapid enrichment of proteasome complexes from both liver and brain lysates. This methodology may be extremely important to opening up the exploration of proteasome biology in experimental conditions where the amount of tissue available is very limited (as in current study). Continuing to expand our methodologies for studying the biology and biochemistry of the proteasome are essential to accurately understanding the role this important enzyme plays in both biological and pathological conditions.
Acknowledgments
This work was supported by grants from the National Institute of Aging (AG0257701, AG029885).
Abbreviations
- AL
ad libitum
- DR
dietary restriction
- NIA
National Institute of Aging
- ROS
reactive oxygen species
References
- 1.Coux O, Tanka K, Goldberg AL. Structure and functions of the 20S and 26S proteosomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 2.Dahlmann B, Kuehn L, Reinauer H. Studies on the activation by ATP of the 26S proteasome complex from rat skeletal muscle. Biochem J. 1995;309:195–202. doi: 10.1042/bj3090195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilt W, Wolf DH. Proteasomes: destruction as a programme. Trends Biochem Sci. 1996;21:96–102. [PubMed] [Google Scholar]
- 4.Dubiel W, Rechsteiner M. The 19S regulatory complex of the 26S proteasome. Adv Mol Cell Biol. 1998;27:129–163. [Google Scholar]
- 5.Tanahashi N, Tsurumi C, Tamura T, Tanaka K. Molecular structure of 20S and 26S proteasomes. Enzyme Protein. 1993;47:241–251. doi: 10.1159/000468683. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka K, Tsurumi C. The 26S proteasome: subunits and functions. Mol Biol Rep. 1997;24:3–11. doi: 10.1023/a:1006876904158. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K. Proteasomes: structure and biology. J Biochem. 1998;123:195–204. doi: 10.1093/oxfordjournals.jbchem.a021922. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura T, Kameyama K, Takagi T, Ikai A, Tokunaga F, Koide T, Tanahashi N, Tamura T, Cejka Z, Baumeister W, Tanaka K, Ichihara A. Molecular characterization of the “26S” proteasome complex from Rat liver. J Struct Biol. 1993;111:200–211. doi: 10.1006/jsbi.1993.1050. [DOI] [PubMed] [Google Scholar]
- 9.Dick TP, Nussbaum AK, Deeg M, Heinemeyer W, Groll M, Schirle M, Keilholz W, Stevanovic S, Wolf DH, Huber R, Rammensee HG, Schild H. Contribution of proteasomal β-subunits to the cleavage of peptide substrates analysed with yeast mutants. J Biol Chem. 1998;273:25637–26646. doi: 10.1074/jbc.273.40.25637. [DOI] [PubMed] [Google Scholar]
- 10.Groll M, Ditzel A, Lowe J, Stock D, Bochtler M, Bartunik HD, Huber R. Structure of the 20S proteosome from yeast at 2.4A resolution. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 11.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. The FASEB Journal. 1997;11:526–534. [PubMed] [Google Scholar]
- 12.Poppek D, Grune T. Proteasomal defense of oxidative protein modifications. Antioxid Redox Signal. 2006;8:173–184. doi: 10.1089/ars.2006.8.173. [DOI] [PubMed] [Google Scholar]
- 13.Bowerman B, Kurz T. Degrade to create: developmental requirements for ubiquitin-mediated proteolysis during early C. elegans embryogenesis. Development. 2006;133:773–784. doi: 10.1242/dev.02276. [DOI] [PubMed] [Google Scholar]
- 14.Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem. 2003;278:35857–35860. doi: 10.1074/jbc.R300018200. [DOI] [PubMed] [Google Scholar]
- 15.Collins GA, Tansey WP. The proteasome: A utility tool for transcription? Curr Opin Genet Dev. 2006;16:197–202. doi: 10.1016/j.gde.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Lipford JR, Deshaies RJ. Diverse roles for ubiquitin-dependent proteolysis in transcriptional activation. Nat Cell Biol. 2003;5:845–850. doi: 10.1038/ncb1003-845. [DOI] [PubMed] [Google Scholar]
- 17.Naujokat C, Hoffmann S. Role and function of the 26S proteasome in proliferation and apoptosis. Lab Invest. 2002;82:965–980. doi: 10.1097/01.lab.0000022226.23741.37. [DOI] [PubMed] [Google Scholar]
- 18.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Maldonado MA. The Ubiquitin-Proteasome System and Its role in Inflammatory and Autoimmune Diseases. Cell Mol Immunol. 2006;3:255–261. [PubMed] [Google Scholar]
- 20.Xiao B-G, Link H. Immune regulation with in the central nerous system. J Neurol sci. 2006;157:1–12. doi: 10.1016/s0022-510x(98)00049-5. [DOI] [PubMed] [Google Scholar]
- 21.Cecarini V, Bonfili L, Amici M, Angeletti M, Keller JN, Eleuteri AM. Amyloid peptides in different assembly states and related effects on isolated and cellular proteasomes. Brain Res. 2008;1209:8–18. doi: 10.1016/j.brainres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Keller JN, Gee J, Ding Q. The proteasome in brain aging. Ageing Res Rev. 2002;1:279–293. doi: 10.1016/s1568-1637(01)00006-x. [DOI] [PubMed] [Google Scholar]
- 23.Leon TI, Lim BO, Yu BP, Lim Y, Jeon EJ, Park DK. Effect of dietary restriction on age-related increase of liver susceptibility to peroxidation in rats. Lipids. 2001;36:589–593. doi: 10.1007/s11745-001-0761-1. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan PG, Dragicevic NB, Deng JH, Bai Y, Dimayuga E, Ding Q, Chen Q, Bruce-Keller AJ, Keller JN. Proteasome inhibition alters neural mitochondrial homeostasis and mitochondria turnover. J Biol Chem. 2004;279:20699–20707. doi: 10.1074/jbc.M313579200. [DOI] [PubMed] [Google Scholar]
- 25.Vigouroux S, Briand M, Briand Y. Linkage between the proteasome pathway and Neurodegerative diseases and aging. Mol Neurobiol. 2004;30:201–221. doi: 10.1385/MN:30:2:201. [DOI] [PubMed] [Google Scholar]
- 26.Widmer R, Ziaja I, Grune T. Protein oxidation and degradation during aging: role in skin aging and neurodegeneration. Free Radic Res. 2006;40:1259–1268. doi: 10.1080/10715760600911154. [DOI] [PubMed] [Google Scholar]
- 27.Friguet B, Stadtman ER, Szweda LI. J Biol Chem. Vol. 269. 1994. Modification of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal. Formation of cross-linked protein that inhibits the multicatalytic protease; pp. 21639–21643. [PubMed] [Google Scholar]
- 28.Amici M, Lupidi G, Angeletti M, Fioretti E, Eleuteri AM. Peroxynitrite-induced oxidation and its effects on isolated proteasomal systems. Free Radic Biol Med. 2003;34:987–996. doi: 10.1016/s0891-5849(02)01369-2. [DOI] [PubMed] [Google Scholar]
- 29.Carrard G, Dieu M, Raes M, Toussaint O, Friguet B. Impact of ageing on proteasome structure and function in human lymphocytes. Int J Biochem Cell Biol. 2003;35:728–732. doi: 10.1016/s1357-2725(02)00356-4. [DOI] [PubMed] [Google Scholar]
- 30.Farout L, Friguet B. Proteasome function in aging and oxidative stress: implications in protein maintenance failure. Antioxid Redox Signal. 2006;8:205–216. doi: 10.1089/ars.2006.8.205. [DOI] [PubMed] [Google Scholar]
- 31.Friquet B, Swezda LI. Inhibition of the multicatalytic proteinase by 4-hydroxy-2-nonenal cross linked protein. FEBS lett. 1997;405:21–25. doi: 10.1016/s0014-5793(97)00148-8. [DOI] [PubMed] [Google Scholar]
- 32.Keller JN. Age-related neuropathology, cognitive decline, and Alzheimer’s disease. Ageing Res Rev. 2006;5:1–13. doi: 10.1016/j.arr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 34.Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem. 2008;389:203–209. doi: 10.1515/BC.2008.029. [DOI] [PubMed] [Google Scholar]
- 35.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10:S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 36.Kapphahn RJ, Bigelow EJ, Ferrington DA. Age-dependent inhibition of proteasome chymotrypsin-like activity in the retina. Exp Eye Res. 2007;84:646–54. doi: 10.1016/j.exer.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNaught KS, Olanow CW. Proteasome inhibitor-induced model of Parkinson’s disease. Ann Neurol. 2006;60:243–247. doi: 10.1002/ana.20936. [DOI] [PubMed] [Google Scholar]
- 38.Torres C, Lewis L, Cristofalo VJ. Proteasome inhibitors shorten replicative life span and induce a senescent-like phenotype of human fibroblasts. J Cell Physiol. 2003;207:845–853. doi: 10.1002/jcp.20630. [DOI] [PubMed] [Google Scholar]
- 39.Chen Q, Thorpe J, Dohmen JR, Li F, Keller JN. Ump1 extends yeast lifespan and enhances viability during oxidative stress: central role for the proteasome? Free Radic Biol Med. 2006;40:120–126. doi: 10.1016/j.freeradbiomed.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 40.Chondrogianni N, Tzavelas C, Pemberton AJ, Nezis IP, Rivett AJ, Gonos ES. Overexpression of proteasome beta 5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates. J Biol Chem. 2005;280:11840–11850. doi: 10.1074/jbc.M413007200. [DOI] [PubMed] [Google Scholar]
- 41.Aksensova MV, Aksensova MY, Carney JM, Butterfield DA. Protein oxidation and enzyme activity decline in old brown Norway rats are reduced by dietary restriction. Mech Ageing Dev. 1998;100:157–168. doi: 10.1016/s0047-6374(97)00133-4. [DOI] [PubMed] [Google Scholar]
- 42.Wachsman JT. The beneficial effects of dietary restriction: reduced oxidative damage and enhanced apoptosis. Mutat Res. 1996;350:25–34. doi: 10.1016/0027-5107(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 43.Yu BP. Aging and oxidative stress: modulation by dietary restriction. Free Radic Biol Med. 1996;21:651–668. doi: 10.1016/0891-5849(96)00162-1. [DOI] [PubMed] [Google Scholar]
- 44.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- 45.Goto S, Takahashi R, Radak Z, Sharma R. Beneficial biochemical outcomes of late-onset dietary restriction in rodents. Ann N Y Acad Sci. 2007;1100:431–441. doi: 10.1196/annals.1395.048. [DOI] [PubMed] [Google Scholar]
- 46.Radak Z, Takahashi R, Kumiyama A, Nakamoto H, Ohno H, Ookawara T, Goto S. Effect of aging and late onset dietary restriction on antioxidant enzymes and proteasome activities, and protein carbonylation of rat skeletal muscle and tendon. Exp Gerontol. 2002;37:1423–1430. doi: 10.1016/s0531-5565(02)00116-x. [DOI] [PubMed] [Google Scholar]
- 47.Li F, Zhang L, Craddock J, Bruce-Keller AJ, Dasuri K, Nguyen A, Keller JN. Aging and dietary restriction effects on ubiquitination, sumoylation, and the proteasome in the heart. Mech Ageing Dev. 2008;129:515–521. doi: 10.1016/j.mad.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glickman MH, Rubin DM, Fried VA, Finley D. The regulatory particle of the Saccharomyces cerevisiae proteasome. Mol Cell Biol. 1998;18:3149–3162. doi: 10.1128/mcb.18.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres CA, Perez VI. Proteasome modulates mitochondrial function during cellular senescence. Free Radic Biol Med. 2008;44:403–414. doi: 10.1016/j.freeradbiomed.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rattan SI. Increased molecular damage and heterogeneity as the basis of aging. Biol Chem. 2008;389:267–272. doi: 10.1515/BC.2008.030. [DOI] [PubMed] [Google Scholar]
- 51.Rattan SI. Theories of biological aging: Genes, proteins, and free radicals. Free Radical Research. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- 52.Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN. Protein oxidation and cellular homeostasis: Emphasis on metabolism. Biochim Biophys Acta. 2007;773:93–104. doi: 10.1016/j.bbamcr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 53.Grune T, Davies KJ. The proteasomal system and HNE-modified proteins. Mol Aspects Med. 2003;24:195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 54.Keller JN, Mark RJ, Bruce AJ, Blanc E, Rothstein JD, Uchida K, Waeg G, Mattson MP. 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience. 1997;80:685–696. doi: 10.1016/s0306-4522(97)00065-1. [DOI] [PubMed] [Google Scholar]
- 55.Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol. 1993;142:367–373. [PMC free article] [PubMed] [Google Scholar]
- 56.Maher JJ. Interactions between hepatic stellate cells and the immune system. Semin Liver Dis. 2001;21:417–426. doi: 10.1055/s-2001-17555. [DOI] [PubMed] [Google Scholar]
- 57.Tsukamoto H. Cytokine regulation of hepatic stellate cells in liver fibrosis. Alcohol Clin Exp Res. 1999;23:911–916. [PubMed] [Google Scholar]
- 58.Ding Q, Reinacker K, Dimayuga E, Nukala V, Drake J, Butterfield DA, Dunn JC, Martin S, Bruce-Keller AJ, Keller JN. Role of the proteasome in protein oxidation and neural viability following low-level oxidative stress. FEBS Lett. 2003;546:228–232. doi: 10.1016/s0014-5793(03)00582-9. [DOI] [PubMed] [Google Scholar]
- 59.Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer’s disease. J Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- 60.Akaishi T, Sawada H, Yokosawa H. Properties of 26S proteasome purified from rat skeletal muscles: Comparison with those of 26S proteasome from the rat brain. Biochem Mol Biol Int. 1996;39:1017–1021. doi: 10.1080/15216549600201172. [DOI] [PubMed] [Google Scholar]
- 61.Selsby JT, Judge AR, Yimlamai T, Leeuwenburgh C, Dodd SL. Life long calorie restriction increases heat shock proteins and proteasome activity in soleus muscles of Fisher 344 rats. Experimental Gerontology. 2005;40:37–42. doi: 10.1016/j.exger.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 62.Akaishi T, Shiomi T, Sawada H, Yokosawa H. Purification and properties of the 26S proteasome from the rat brain: evidence for its degradation of myelin basic protein in a ubiquitin-dependent manner. Brain Res. 1996;722:139–44. doi: 10.1016/0006-8993(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 63.Noda C, Tanahashi N, Shimbara N, Hendil KB, Tanaka K. Tissue distribution of constitutive proteasomes, immunoproteasomes, and PA28 in rats. Biochem Biophys Res Commun. 2000;277:348–354. doi: 10.1006/bbrc.2000.3676. [DOI] [PubMed] [Google Scholar]
- 64.Ding Q, Martin S, Dimayuga E, Bruce-Keller AJ, Keller JN. LMP2 knock-out mice have reduced proteasome activities and increased levels of oxidatively damaged proteins. Antioxid Redox Signal. 2006;8:130–135. doi: 10.1089/ars.2006.8.130. [DOI] [PubMed] [Google Scholar]
- 65.Dahlmann B, Ruppert T, Kuehn L, Merforth S, Kloetzel PM. Different proteasome subtypes in a single tissue exhibit different enzymatic properties. J Mol Biol. 2000;303:643–53. doi: 10.1006/jmbi.2000.4185. [DOI] [PubMed] [Google Scholar]