Abstract
Structural modification of the frontline antitubercular isonicotinic acid hydrazide (INH) provides lipophilic adaptations (3-46) of the drug in which the hydrazine moiety of the parent compound has been chemically blocked from the deactivating process of N2-acetylation by N-arylaminoacetyl transferases. As a class, these compounds show high levels of activity against Mycobacterium tuberculosis in vitro and in tuberculosis-infected macrophages. They provide strong protection in tuberculosis-infected mice and have low toxicity. With some representatives of this class achieving early peak plasma concentrations approximately three orders of magnitude above minimum inhibitory concentration, they may serve as tools for improving our understanding of INH-based treatment modalities, particularly for those patients chronically underdosed in conventional INH therapy.
Keywords: Tuberculosis, Acetylation, Isoniazid, Schiff Base
1. Introduction
Having been used as the cornerstone of antituberculosis therapy for more than half a century, much has been learned about the biochemistry and multifaceted modes of action of the frontline drug isoniazid (INH, 1, Fig. 1). Indeed, with the enormous global burden of tuberculosis and the alarming rise in the number of clinical isolates displaying drug resistance or increased virulence [1-4], INH has become the single most researched antitubercular agent [5-9]. The problem of resistance has necessitated combination regimens for tuberculosis since the earliest days of chemotherapy. Researchers have recently begun to better understand the complex interactions among the pathogen, host and antimicrobial agent that relate to resistance and thus require combination therapy [10-13]. It is desirable to maintain drug concentrations within the mammalian host at levels sufficiently high to restrict amplification of drug-resistant mutant subpopulations. As a practical matter, drugs for combination therapy are most efficacious when their achievable serum concentration levels permit this restriction of amplification, given concomitant low toxicity. Advancement of these important drug characteristics may make it possible to shorten the current lengthy therapeutic regimens and to address the physiologically-controlled underdosing of certain patient groups, both of which have been identified as pivotal factors in the rise of acquired and primary drug resistance in the clinic [14, 15].
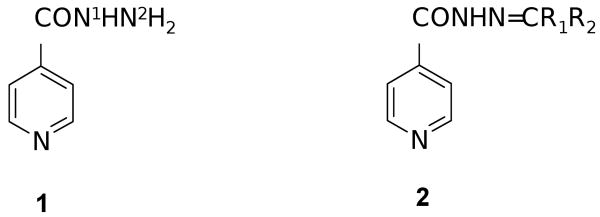
Figure 1.
The antitubercular isonicotinic acid hydrazide (1) and its Schiff base congeners (2). Position N2 of 1 is subject to deactivating acetylation by the class of enzymes known as N-arylaminoacetyl transferases (NATs). The deactivation phenomenon is associated with the rise of resistance. Compounds 2 are blocked towards this enzymatic process.
There is an increasing body of evidence to the effect that many of the same molecular characteristics determined to enhance bioavailability of antitubercular drugs also have the potential to suppress the rise of resistance [16-18]. It has been argued that, where significant increases can be made in serum concentration levels, drug efficacy can be enhanced, even against moderately drug-resistant organisms [19-21]. Persuasive data strongly suggest that a significant impediment to the implementation of long-sought intermittent treatment regimens, such as rifapentine and isoniazid given once per week, is the comparatively low level of isoniazid that is achievable within the host [22]. With a companion drug that is more pharmacokinetically suitable, intermittent rifapentine-based therapy might become a reality [23]. Several recent experiments indicate that the incorporation of hydrophobic moieties into the framework of INH can enhance penetration of the drug into the tissues of the mammalian host and into the waxy cell wall of the bacterium. This strategy for drug design has been proposed as a vehicle for controlled study of the growth cycle of the pathogen, as well as a means of augmenting fundamental drug activity [24-27]. Previous work on several members of this class, particularly the aromatic derivatives, had been carried out some years ago and had demonstrated good activities by the methods then available [28-37]. These compounds have continued to attract the attention of medicinal chemists up to the present day [38-43]. For example, a series of (E)-N′-(monsubstituted-benzylidene)isonicotinoyl-hydrazides was recently evaluated to good effect, with several of these aromatic compounds showing potent activities in vitro [44], although structure-activity relationships have not yet become clear. There is evidence that such structurally-modified drugs gain efficacy as the result of suppression of xenobiotic transformation [28-32], and that they obtain noteworthy concentration in several organs of tuberculosis-infected experimental animals, perhaps most significantly in caseation bodies [33]. These compounds also possess a vital characteristic of antimycobacterials, namely, that toxicity is low [32, 34-44].
Structurally, the modifications engendering these desirable drug properties are optimally made at N2 of the INH framework. Such modifications block the resulting molecule against the action of N-arylaminoacetyl transferases (NATs). These enzymes are found in both mycobacteria and their mammalian hosts, and they deactivate INH by means of reaction at N2. Consistent with a survival stratagem in which pathogenic bacteria control their environment through acetylation [45, 46], the NATs have been implicated in the development of resistance, particularly among those patients known as the “fast acetylators,” for whom there is an inherent problem of chronic underdosing of INH under genetic control [47]. Evaluations of pharmacokinetic diversity among patients treated with INH indicate that its sources are complex and that the problem of underdosing is more widespread than once believed, underscoring the need for clinicians to know the acetylator status of their patients [48]. Underdosing typically places fast acetylators in jeopardy for selective amplification of drug resistant organisms within a mutant selection range of serum drug concentrations. Improving serum drug concentrations should narrow this range [49-51], and structurally blocking INH toward the actions of NATs at N2 may thus combat the rise of resistance. We previously reported results on one of these lipophilic compounds modified at N2 (46) that combined low toxicity with serum concentration levels as much as three orders of magnitude above minimum inhibitory concentration (MIC) [32]. We now report findings from our further experiments in vitro and in vivo on Schiff base derivatives of INH (2, Fig. 1), including results from a new short-course therapy model in mice. In line with the above discussion, our rationale has been to prepare Schiff bases with enhanced lipophilicity and to examine their efficacy against Mycobacterium tuberculosis with up-to-date methods. Our objective is the development of these compounds as tools for probing interactions among pathogen, host and drugs in INH-based treatment modalities.
2. Results and Discussion
2.1. Chemistry
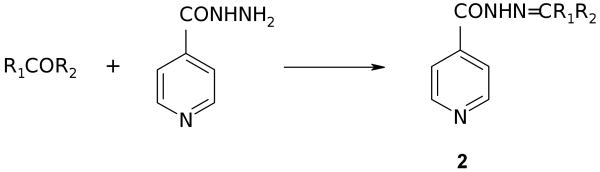
Compounds 3-46 are readily prepared in good yields and purity by functionalization of INH at N2. Treatment of INH with the appropriate ketone or aldehyde for 1-2 hours in boiling alcohol typically leads without complication to the condensation product, the Schiff base, which is generally isolated as a crystalline solid in analytical purity (Fig. 2). Carbonyl precursors included substituted aromatic aldehydes, long chain aliphatic aldehydes, palindromic dialdehydes, α,β-unsaturated aldehydes, cinnamaldehydes, aliphatic ketones, aryl alkyl ketones and keto esters. In a representative example, INH reacted at reflux for 1.25 hours with a molar equivalent of 2-benzyloxybenzaldehyde in ethanol to produce compound 2 (R1 = H, R2 = 2-(OCH2C6H5)C6H4), 3) as a white crystalline solid, 90%, m.p.: 185°C. FT-IR: ν (cm-1) 3227 (hydrazone NH), 1653 (acylhydrazone carbonyl). 1H NMR: δ (ppm) 8.85 (1H, singlet, azomethine), 8.75 (2H, doublet, pyridine alpha-protons), 7.98-7.01 (9H, multiplets, aromatics and pyridine beta-protons), 5.20 (2H, singlet, benzyl protons). 13C NMR: δ (ppm) 161.89 and 157.36 (azomethine and carbonyl). HRMS (FAB MH+): (C20H18N3O2) Calc 332.1399. Found: 332.1414. Anal. (C20H17N3O2) Calc C 72.49, H 5.17. Found: C 72.59, H 5.35. The calculated value of the logarithm of the octanol/water partition coefficient (C log P) for this compound (4.57) is greater than that of INH (0.49), consistent with the improved solubility observed for this compound in a number of organic solvents and indicative of enhanced lipophilicity [52].
Figure 2.
Preparation of Schiff bases from carbonyl precursors.
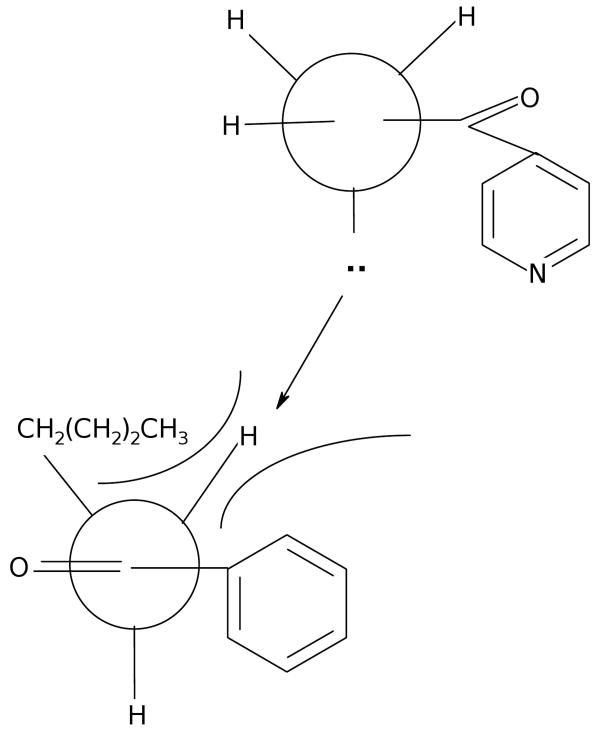
Our results on the preparation of the Schiff bases included in this study are summarized in Table 1. The Schiff bases may be conveniently divided into aromatic compounds (3-22) and aliphatic derivatives (23-46). Using the methods described, recrystallization was generally not required. In some cases, it was necessary to concentrate the reaction mixture to half volume and to precipitate the product with ether. As alternative solvents, both water and toluene produced inferior results in synthesis. The compounds were stable for prolonged periods on the shelf when not stored in direct light. All the Schiff bases had C log P values considerably greater (more lipophilic) than did INH itself (Table 1). In laboratory practice, experimental estimates of partitioning between aqueous and organic phases for a number of these compounds (see Experimental protocols) also demonstrated their organic solubilities to be several orders of magnitude greater than that of INH. During our investigations, we found that there were some structural limits on the procedure. Thus we observed that aryl alkyl ketones, such as 4 with alkyl groups larger than methyl, required strenuous conditions for reaction, namely, refluxing in dimethyl sulfoxide (DMSO). We attribute this lower reactivity to a sterically congested approach (Fig. 3) for nucleophilic attack in which the lone pair on N2 must stand orthogonal to the conjugated π system of the remainder of the INH molecule. With alkyl groups larger than methyl in the ketone component of the transition state, the conformational preference for the lone pair on N2 renders this nitrogen poorly poised for nucleophilic attack. Such stereoelectronic effects have been observed previously in the reactions of acylhydrazines as nucleophiles [53].
Table 1. Preparation of Schiff Bases 2.
| Entry | Compound | R1 | R2 | % Yield | mp, °C | C log Pa | MIC, μg/mLb |
|---|---|---|---|---|---|---|---|
| 1. | 3 | H | 2-(OCH2C6H5)C6H4 | 90 | 185 | 4.57 | 0.06c |
| 2. | 4 | (CH2)4CH3 | C6H5 | 64 | 116-117 | 4.49 | 0.39 |
| 3. | 5 | H | 4-BrC6H4 | 91 | 218-219 | 3.84 | 3.13 |
| 4. | 6 | H | 2-ClC6H4 | 66 | 218-221 | 3.56 | 0.78 |
| 5. | 7 | H | 3,4-F2C6H3 | 86 | 195-196 | 3.32 | 1.56 |
| 6. | 8 | H | 4-IC6H4 | 92 | 243 | 4.30 | 0.20 |
| 7. | 9 | H | 3-I-4,5-(OCH3)2C6H2 | 90 | 234 | 3.80 | 0.20 |
| 8. | 10 | H | 3-I-4-OH-5-OCH3C6H2 | 87 | 221 | 3.76 | 0.39 |
| 9. | 11 | H | 4-ClC6H5 | 94 | 215-216 | 3.56 | 0.20 |
| 10. | 12 | H | 3,4-Cl2C6H3 | 88 | 241-244 | 4.08 | 0.20 |
| 11. | 13 | H | 2,6-F2C6H3 | 100 | 239 | 3.32 | 0.10 |
| 12. | 14 | H | 2,3-Cl2C6H3 | 98 | 227 | 4.08 | 0.78 |
| 13. | 15 | H | 2,6-Cl2C6H3 | 97 | semisolid | 4.08 | <6.25 |
| 14. | 16 | H | 2-NO2C6H4 | 97 | 230 | 1.06 | 0.39 |
| 15. | 17 | H | 4-Cl-3-NO2C6H3 | 100 | 231 | 1.58 | 0.39 |
| 16. | 18 | CH3 | C6H5 | 92 | 169-170 | 2.68 | <6.25 |
| 17. | 19 | H | 4-CH3(CH2)5OC6H4 | 87 | 135-138 | 5.49 | 0.39 |
| 18. | 20 | H | 3-NO2C6H4 | 85 | 225 | 1.06 | 0.125c |
| 19. | 21 | H | C6H5 | 98 | 197 | 3.04 | 0.05 |
| 20. | 22 | H | 4-CH3(CH2)3OC6H4 | 91 | 148-149 | 4.00 | 0.10 |
| 21. | 23 | H | t-CH=CHCH2CH3 | 54 | 161-164 | 2.24 | 0.05 |
| 22. | 24 | H | t-CH=CHCH3 | 73 | 198-201 | 1.85 | <6.25 |
| 23. | 25 | H | t-CH=CHCH2CH2CH3 | 93 | 152-154 | 2.64 | <6.25 |
| 24. | 26 | H | t-CH=CH(CH2)3CH3 | 84 | 152-154 | 3.03 | <6.25 |
| 25. | 27 | H | C=CCH3CH2CH2CH=CH2 | 59 | 124-125 | 2.97 | 0.10 |
| 26. | 28 | H | CH2CHCH3CH2CH2CH=C(CH3)2 | 29 | 78-80 | 3.58 | 0.20 |
| 27. | 29 | CH3 | CH3 | 48 | 161-162 | 1.70 | 0.10 |
| 28. | 30 | CH2Ph | CH2CO2CH2CH3 | 90 | 104 | 3.78 | 0.10 |
| 29. | 31 | R1R2 = CCH2CH(CH3)CH2C(CH3)2CH2 | 76 | 149-151 | 3.94 | <0.025 | |
| 30. | 32 | H | CH=C(C6H5)2 | 86 | 223 | 4.43 | 0.10 |
| 31. | 33 | H | t-CH=CH-4-OCH3C6H4 | 89 | 218 | 2.73 | 0.20 |
| 32. | 34 | H | t-CH=CH-2-NO2C6H4 | 94 | 204 | 1.08 | 0.25c |
| 33. | 35 | H | C(n-C3H7)=CH(CH2)3CH3 | 84 | 121 | 4.10 | 0.50 |
| 34. | 36 | H | C(i-C3H7)=CHCH2CH(CH3)2 | 99 | 129 | 3.97 | 0.06c |
| 35. | 37 | H | t-CH=CH-2-OCH3C6H4 | 92 | 206 | 2.81 | 0.10 |
| 36. | 38 | H | t-CCH3=CHC6H5 | 90 | 200 | 3.34 | 0.50 |
| 37. | 39 | H | CH=NNHCOC5H4N | 90 | >300 (d)d | 1.58 | 0.10 |
| 38. | 40 | CH2CH2CH3 | CH2CO2CH2CH3 | 98 | 89-90 | 2.96 | 0.39 |
| 39. | 41 | CH3 | CH2CO2CH2CH3 | 85 | 99 | 1.93 | 0.20 |
| 40. | 42 | H | 4-(CH=NNHCOC5H4N)C6H4 | 90 | >300 (d)d | 4.04 | 0.05 |
| 41. | 43 | CH2CH2C6H5 | CO2CH2CH3 | 90 | 104 | 4.24 | 0.10 |
| 42. | 44 | H | CH3(CH2)11CH2 | 73 | 89-91 | 5.73 | 0.20 |
| 43. | 45 | H | CH3(CH2)10CH2 | 73 | 92-93 | 5.33 | 0.20 |
| 44. | 46 | R1R2 = C(CH2CH2)2CH2 | 81 | 167-168 | 2.85 | 0.03 | |
Calculated using HyperChem Pro, Version 7.5. For INH, C log P is 0.49.
MIC90 against M. tuberculosis strain H37Rv, unless otherwise noted. MIC INH control 0.03-0.06 μg/mL.
MIC90 against M. tuberculosis strain Erdman. MIC INH control 0.03-0.06 μg/mL. MIC PAS control 0.03-0.06 μg/mL.
Decomposed without melting.
Figure 3.
With alkyl groups larger than methyl in the ketone component, nucleophilic attack by the hydrazide moiety is hindered.
2.2. Biological Assays
In a standard primary screen against Mycobacterium tuberculosis strain H37Rv, all the compounds in this study (3-46) were active and displayed minimum inhibitory concentrations necessary to inhibit growth of the organism by 90% of less than 6.25 μg/mL [54]. Activities in vitro were also determined against M. tuberculosis strain Erdman using a broth dilution method, referred to known antituberculars as standards [32]. Individual MIC values are reported in Table 1. The mean of the MIC values for the forty-four compounds tested was 1 μg/mL, with most having individual values considerably less than this. Compounds displaying MICs of 0.06 μg/mL or less were derived from cyclohexanones (31, 46), benzaldehydes (3, 21) and enals (23, 24), having an average C log P value of 3.1. Compound 3 provides a representative example of the biological activities of the Schiff bases, demonstrating good potency against the pathogen (strain Erdman) with an MIC of 0.06 μg/mL (INH control 0.06 μg/mL, para-aminosalicylic acid (PAS) control 0.06 μg/mL).
Thirty-one of the more active compounds were evaluated for cytotoxicity in VERO cells at concentrations ten times the MIC (Table 2) [54]. After 72 hours exposure, viability was assessed using a standard cell proliferation assay, in which the selection criterion was a selectivity index (SI, IC50/MIC) greater than 10. Nearly all of the Schiff bases met this criterion, giving an acceptable range between their active and cytotoxic concentrations. For the eleven compounds with outstanding SI values exceeding 1000, the mean C log P was 3.2.
Table 2. Selectivity Indices of Schiff Bases 2a.
| Entry | Compound | SI (IC50/MIC) |
|---|---|---|
| 1. | 4 | >25 |
| 2. | 5 | 12 |
| 3. | 6 | >128 |
| 4. | 8 | >50 |
| 5. | 9 | 297 |
| 6. | 10 | >160 |
| 7. | 11 | >50 |
| 8. | 12 | >50 |
| 9. | 13 | >1250 |
| 10. | 14 | >32 |
| 11. | 17 | >64 |
| 12. | 19 | 1.5 |
| 13. | 20 | 8 |
| 14. | 21 | 4000 |
| 15. | 22 | >2000 |
| 16. | 23 | 712 |
| 17. | 28 | >1000 |
| 18. | 29 | >10000 |
| 19. | 30 | >1250 |
| 20. | 31 | >2000 |
| 21. | 32 | 68 |
| 22. | 33 | >50 |
| 23. | 35 | 1.60 |
| 24. | 36 | >10 |
| 25. | 38 | >250 |
| 26. | 40 | >2631 |
| 27. | 41 | >5000 |
| 28. | 42 | >126 |
| 29. | 43 | >1250 |
| 30. | 45 | 374 |
| 31. | 46 | >40000 |
Data from TAACF.54 Evaluation for cytotoxicity in VERO cells at concentrations 10 times the MIC for M. tuberculosis H37Rv. SI INH control >40000. The activity criterion for this assay is an SI >10.
For eleven of the compounds, efficacy in vitro was further explored in a tuberculosis-infected macrophage model [54]. This assay measures killing of M. tuberculosis strain Erdman in monolayers of mouse bone marrow macrophages. In Table 3, EC90 and EC99 show the lowest concentrations giving 90% and 99% reduction in colony-forming units at 7 days (compared to drug-free controls) at four-fold concentrations equivalent to 0.25, 1, 4 and 16 times the MIC. In this model, EC90 represents bacteriostatic activity and EC99 indicates bactericidal activity. For INH, EC90 is 0.03 mg/mL and EC99 is 0.42 mg/mL. Most of the Schiff bases gave EC90 and EC99 values equal to or less than those of INH. The ratio EC90/MIC (Table 3) gives a measure of bioavailability and metabolism of the compound within the living host cell, comparing the in vitro activity against the bacillus to the activity against the bacillus while it lives within the host. For INH, the ratio is unity. Whenever the ratio is less than 16, it is concluded that there has been an effective reduction in residual mycobacterial growth. This condition is fulfilled by all eleven of the compounds assessed. The data thus indicate that the compounds are active against both extracellular and intracellular organisms. For the eleven compounds displaying excellent efficacies within the tuberculosis-infected macrophage model, the mean value of C log P was 3.5.
Table 3. Efficacies within Tuberculosis-Infected Macrophagesa.
| Entry | Compound | EC90, μg/mL | EC99, μg/mL | EC90/MIC |
|---|---|---|---|---|
| 1. | 4 | 0.050 | 0.25 | 0.13 |
| 2. | 11 | 0.04 | 0.14 | 0.20 |
| 3. | 12 | 0.106 | 0.596 | 0.53 |
| 4. | 13 | 0.037 | 0.15 | 0.37 |
| 5. | 14 | 0.071 | 0.30 | 0.09 |
| 6. | 17 | 0.078 | 0.30 | 0.20 |
| 7. | 23 | 0.028 | 0.529 | 0.56 |
| 8. | 30 | 0.010 | 0.211 | 0.10 |
| 9. | 31 | 0.063 | 0.15 | 2.52 |
| 10. | 32 | 0.028 | 0.121 | 0.28 |
| 11. | 46 | 0.08 | 0.42 | 2.7 |
Data from TAACF.54 EC90 INH control 0.03 μg/mL. EC99 INH control 0.42 μg/mL. EC90/MIC INH control 1. The ratio provides a measure of bioavailability and metabolism of the active agent within the living host cell; whenever the ratio is less than 16, it is said to indicate effective reduction in residual mycobacterial growth.
For our studies in vivo, we used a short-course treatment method that has recently been described [55]. This method provides a demanding test of the activities of candidate drugs and has the advantage of cutting in half the time required to produce results in animal studies, compared to the traditional four-week treatment model. In brief, C57BL/6 mice are infected intranasally with approximately one million viable M. tuberculosis organisms. Treatment begins one day post-infection and is administered for two days. Mice are sacrificed three days post-infection, their right lungs are removed aseptically, and cell counts are determined. Mice are only able to present a strong immune response to control the growth of M. tuberculosis about ten to fourteen days after initial infection, so the reduction in mycobacteria using the two-day treatment regimen is entirely drug related. Three aspects of the model have been established: (1) If a test compound shows activity after two days of treatment, then the compound must have reached the lung after oral delivery, it being known that there is no detectable circulation of the pathogen within the first four days of infection after intranasal deposition. Bacterial reduction observed in the lung after treatment is thus due to mycobacterial killing within the lung itself. (2) If a test drug has activity in the model, it has entered the macrophage in which the bacteria reside. (3) Activity indicates the drug is killing the bacteria within the phagosome of the macrophage. The short-course murine model thus provides a rigorous and robust indication of the efficacy of an antimycobacterial agent. In the short-course therapy model, representative compound 3 and INH were dosed at 25 mg/kg of body weight. No ill effects or overt toxicity were noted in the animals, in keeping with our observations on Schiff base 46, for which the maximally tolerated dose in mice was determined to be 1000 mg/kg [32]. Compared to early untreated controls, compound 3 displayed strong activity, giving 1.01 log10 reduction in colony-forming units (CFU). This was somewhat more protection than provided by INH, which gave 0.92 log10 CFU reduction. Results in vivo for selected Schiff bases are provided in Table 2. These compounds generally appeared to have strong activities in murine models. For the Schiff bases examined in vivo, the mean C log P value was 3.7.
3. Conclusions
The Schiff bases of INH were readily prepared for evaluation against M. tuberculosis in good yield and purity using inexpensive commonly-available reagents. The structural modification of the INH framework described here provides a lipophilic adaptation of isoniazid in which the hydrazine unit has been chemically blocked from the deactivating process of N2-acetylation by NATs. Across the class, these compounds show strong levels of activity in vitro and in experimental animals. In each of the biological assays, high potency was observed for compounds with values of C log P considerably greater than that for INH itself, and this appears to be the most significant structure-activity relationship. The exploration of more subtle structure-activity relationships is the subject of ongoing work in our laboratory. With some representatives of this class achieving early peak plasma concentrations approximately three orders of magnitude above MIC [32], the Schiff bases may serve as discovery tools in probing INH-based treatment modalities, particularly for those patients chronically underdosed in conventional INH therapy.
4. Experimental protocols
4.1. Chemistry
4.1.1. General
Elemental analyses were carried out by Galbraith Laboratories, Knoxville, Tennessee, USA. Melting points (m.p., °C) were taken in open capillary tubes using a Mel-Temp apparatus (Laboratory Devices, Cambridge, MA, USA), and are corrected. Infrared (FT-IR) spectra were recorded on a Perkin-Elmer Spectrum One Fourier transform spectrophotometer fitted with a universal attenuated total reflectance sampling accessory, reported in wavenumbers (ν, cm−1). Most reactants, reagents and solvents were obtained from Aldrich Chemical Company (Milwaukee, Wisconsin, USA) and Lancaster Synthesis Incorporated (Windham, New Hampshire, USA) and were used as received. All preparations were carried out in a manner similar to that for compound 3. In some cases, it was necessary to concentrate the reaction mixture to half volume and add ether to precipitate the product. A number of the Schiff bases were further characterized by their exchange reactions with 2,4-dinitrophenylhydrazine to produce the corresponding highly insoluble 2,4-dinitrophenylhydrazones, readily identified by their melting points. A representative procedure for this method is provided for compound 29. Our data for activities in vitro and in vivo for compound 46 (2, R1-R2 = (CH2)5) have been previously reported [32]. Nuclear magnetic resonance (NMR) spectra were taken on a Bruker 300 Fourier transform instrument in dimethyl sulfoxide-d6, recorded at 300 megahertz (1H NMR) or 75 megahertz (13C NMR) and are reported in parts per million delta (δ) downfield from internal tetramethylsilane as reference, with coupling constants given in cycles per second (cps). In some proton spectra, only signals in the region 0-10 ppm are reported. High resolution mass spectra (HRMS, fast atom bombardment method) and low resolution mass spectra were determined at the NIH Mass Spectrometry Facility at Michigan State University, East Lansing, Michigan, USA, unless otherwise noted. Values for the calculated logarithm of the octanol-water partitioning coefficient (C log P) were obtained using the QSAR properties function of HyperChem Pro, Version 7.5.
Safety Notes
Gloves were worn during the chemical synthesis, and the reactions were carried out in the hood. In general, any scale-up of preparations of compounds with relatively high proportions of nitrogen and oxygen was done with due caution. No specific safety problems were encountered with the methods given below. No attempt was made to optimize yields.
4.1.2. Representative Example of Schiff Base Formation. N2-(2-Benzyloxy)benzylidenyl Isonicotinic Acid Hydrazide (3)
INH (1.37 g, 10.0 mmol) was mixed with absolute ethanol (15 mL) and the mixture brought to the boil, producing a slurry. Barely sufficient additional ethanol was then added to give a homogeneous solution at reflux. 2-Benzyloxybenzaldehyde (2.12 g, 10.0 mmol) was added dropwise over 5 minutes and washed with 3 mL of ethanol. The reaction mixture was refluxed for 1.25 hour, then allowed to cool slowly and to stand over night, producing a white crystalline solid, which was filtered off and dried. Yield: 2.88 g, (90%), m.p.: 185°C. FT-IR: ν (cm-1) 3227, 3077, 1653, 1598, 1549, 1371, 1249. 1H NMR: δ (ppm) 8.85 (1H, s), 8.75 (2H, d, J = 7 cps), 7.98-7.01 (9H, m, from which emerged at 7.88 (2H, d, J = 7 cps), 5.20 (2H, s). 13C NMR: δ (ppm) 161.89, 157.36, 150.61, 144.51, 140.85, 137.02, 132.15, 128.90, 128.38, 127.88, 126.14, 123.46, 122.69, 121.91, 121.36, 113.48, 70.07. HRMS (FAB MH+): (C20H18N3O2) Calc 332.1399. Found: 332.1414. Anal. (C20H17N3O2) Calc C 72.49, H 5.17. Found: C 72.59, H 5.35.
4.1.3 Isonicotinoylhydrazone of n-Hexanophenone (4)
To a homogeneous solution of INH (0.45 g, 3.30 mmol) in boiling DMSO (11 mL) was added n-hexanophenone (1.0 mL, 5.40 mmol). The mixture was refluxed for an hour, the solution allowed to cool for 20 minutes, and water (9 mL) added. After several minutes a solid began to form. The solid was filtered off and washed with ether (3 × 10 mL) to give the title compound as a white solid. Yield: 0.62 g (64%), m.p.: 116-117°C. FT-IR: ν (cm-1) 3166, 1649, 1602, 1542, 1347, 1325, 1284, 1145, 1090, 1068, 834. 1H NMR: δ (ppm) 11.30 (1H, br s), 8.78 (2H, d, J = 7 cps), 7.91 (2H, d, J = 7 cps), 7.65-7.21 (5H, m), 2.89 (m, 2H), 1.53-1.20 (m, 6H), 0.81 (m, 3H). Anal. (C18H21N3O) Calc C 73.20, H 7.10. Found: C 73.23, H 7.16.
4.1.4 N2-4-Bromobenzylidenyl Isonicotinic Acid Hydrazide (5)
Yield: 1.65 g (91%), m.p.: 218-219°C. FT-IR: ν (cm-1) 3252, 3079, 1657, 1603, 1589, 1551, 1290, 1067, 822. 1H NMR: δ (ppm) 12.18 (1H, br s), 8.79 (2H, d, J = 7 cps), 8.49 (1H, s), 7.86 (2H, d, J = 7 cps), 7.70 (4H, aromatic pseudoquartet). Anal. (C13H10N3OBr) Calc C 51.34, H 3.31. Found: C 51.32, H 3.30.
4.1.5 N2-2-Chlorobenzylidenyl Isonicotinic Acid Hydrazide (6)
Yield: 1.08 g (66%), m.p.: 218-221°C. FT-IR: ν (cm-1) 3060, 1673, 1600, 1552, 1412, 1341, 1281, 1216, 1156, 1047, 1027, 1001, 842. 1H NMR: δ (ppm) 8.95 (1H, s), 8.80 (2H, d, J = 7 cps), 8.05 (1H, m), 7.88 (2H, d, J = 7 cps), 7.48 (4H, m). Anal. (C13H10N3OCl) Calc C 60.13, H 3.88. Found: C 60.11, H 3.86.
4.1.6 N2-3,4-Difluorobenzylidenyl Isonicotinic Acid Hydrazide (7)
Yield: 1.46 g (86%), m.p.: 195-196°C. FT-IR: ν (cm-1) 3159, 1679, 1567, 1512, 1376, 1280, 1218, 1160, 1134, 1071, 850, 814, 757. 1H NMR: δ (ppm) 12.20 (1H, br s), 8.80 (2H, d, J = 7 cps), 8.44 (1H, s), 8.00-7.36 (5H, m, from which emerged at 7.82 a doublet, J = 7 cps). Anal. (C13H9N3OF2) Calc C 59.77, H 3.47. Found: C 59.44, H 3.56.
4.1.7 N2-4-Iodobenzylidenyl Isonicotinic Acid Hydrazide (8)
Yield: 1.39 g (92%), m.p.: 243°C. FT-IR: ν (cm-1) 3229, 3063, 1654, 1606, 1583, 1543, 1478, 1404, 1393, 1359, 1290, 1218, 1148, 1066, 1057, 996, 959, 936, 922, 881, 846, 828, 809. 1H NMR: δ (ppm) 8.80 (2H, d, J = 7 cps), 8.40 (1H, s), 7.82 (4H, m, aromatic pseudoquartet), 7.54 (2H, d, J = 7 cps). 13C NMR: δ (ppm) 162.08, 150.67, 148.39, 140.72, 138.08, 133.91, 129.40, 121.89, 97.64. HRMS (FAB MH+): (C13H11N3OI) Calc 351.9947. Found: 351.9946. Anal. (C13H10N3OI) Calc C 44.47, H 2.87. Found: C 44.49, H 3.03.
4.1.8 N2-3-Iodo-4,5-dimethoxybenzylidenyl Isonicotinic Acid Hydrazide (9)
Yield: 3.15 g (90%), m.p.: 234°C. FT-IR: ν (cm-1) 3200, 2980, 2929, 1673, 1599, 1585, 1538, 1494, 1474, 1455, 1408, 1358, 1273, 1235, 1216, 1190, 1173, 1150, 1063, 1046, 994, 960, 898, 860, 843. 1H NMR: δ (ppm) 8.58 (2H, d, J = 7 cps), 8.13 (1H, s), 7.61 (2H, d, J = 7 cps), 7.51 (1H, s), 7.22 (1H, s), 3.69 (3H, s), 3.54 (3H, s). 13C NMR: δ (ppm) 162.08, 152.88, 150.67, 150.29, 147.43, 140.72, 132.56, 129.68, 121.90, 111.18, 93.37, 60.35, 56.36. HRMS (FAB MH+): (C15H15N3O3I) Calc 412.0158. Found: 412.0158. Anal. (C15H14N3O3I) Calc C 43.81, H 3.43. Found: C 43.87, H 3.53.
4.1.9 N2-3-Iodo-4-hydroxy-5-methoxybenzylidenyl Isonicotinic Acid Hydrazide (10)
Yield: 3.46 g (87%), m.p.: 221°C. FT-IR: ν (cm-1) 3192, 3035, 2866, 1648, 1585, 1572, 1550, 1493, 1464, 1413, 1379, 1343, 1324, 1305, 1290, 1197, 1182, 1168, 1146, 1063, 1050, 1007, 986, 956, 905, 856, 833, 796, 762, 730. 1H NMR: δ (ppm) 10.20 (1H, br), 8.75 (2H, d, J = 7 cps), 8.28 (1H, s), 7.82 (2H, d, J = 7 cps), 7.62 (1H, s), 7.36 (1H, s), 3.92 (3H, s). 13C NMR: δ (ppm) 161.91, 150.63, 148.94, 148.23, 147.63, 140.86, 130.82, 127.59, 121.88, 109.38, 84.71, 56.45. HRMS (FAB MH+): (C14H13N3O3I) Calc 398.0002. Found: 398.0007. Anal. (C14H12N3O3I) Calc C 42.34, H 3.05. Found: C 42.21, H 3.22.
4.1.10 N2-4-Chlorobenzylidenyl Isonicotinic Acid Hydrazide (11)
Yield: 94%, m.p.: 215-216°C. FT-IR: ν (cm-1) 3167, 1660, 1611, 1597, 1412, 1219, 1158, 1118, 1087, 1012, 952, 1000, 879, 838, 818, 751, 722. Anal. (C13H10N3OCl) C, 60.13; H, 3.88. Found: C, 60.05; H, 3.89.
4.1.11 N2-3,4-Dichlorobenzylidenyl Isonicotinic Acid Hydrazide (12)
Yield: 88%, m.p.: 241-244°C. FT-IR: ν (cm-1) 3178, 1682, 1590, 1552, 1413, 1352, 1278, 1215, 1147, 1121, 1078, 1062, 1028, 999, 952, 937, 886, 846, 813, 747, 722. Anal. (C13H9N3OCl2) C 53.08, H 3.08. Found: C 52.85, H 3.20.
4.1.12 N2-2,6-Difluorobenzylidenyl Isonicotinic Acid Hydrazide (13)
Yield: 100%, m.p.: 239°C (uncorr). FT-IR: ν (cm-1) 3161, 1654, 1624, 1608, 1569, 1550, 1412, 1305, 1238, 1154, 1076, 1066, 1000, 960, 926, 840, 782, 758, 728. Anal. (C13H9N3OF2) C 59.77, H 3.47. Found: C 59.92, H 3.70.
4.1.13 N2-2,3-Dichlorobenzylidenyl Isonicotinic Acid Hydrazide (14)
Yield: 98%, m.p.: 227°C (uncorr). FT-IR: ν (cm-1) 3188, 1687, 1602, 1585, 1547, 1497, 1411, 1350, 1278, 1248, 1214, 1189, 1155, 1141, 1096, 1060, 1042, 997, 972, 940, 848, 783, 742, 708. Anal. (C13H9N3OCl2) C 53.08, H 3.08. Found: C 53.16, H 3.20.
4.1.14 N2-2,6-Dichlorobenzylidenyl Isonicotinic Acid Hydrazide (15)
Yield: 97%, semisolid. FT-IR: ν (cm-1) 3150, 1681, 1605, 1592, 1555, 1416, 1354, 1274, 1222, 1192, 1148, 1000, 925, 842, 791, 778, 752, 720. Anal. (C13H9N3OCl2) C 53.08, H 3.08. Found: C 53.28, H 3.21.
4.1.15 N2-2-Nitrobenzylidenyl Isonicotinic Acid Hydrazide (16)
Yield: 97%, m.p.: 230°C (uncorr). FT-IR: ν (cm-1) 3188, 1678, 1602, 1556, 1515, 1413, 1315, 1287, 1273, 1214, 1149, 1139, 1062, 998, 964, 932, 920, 880, 856, 848, 836, 788, 744. Anal. (C13H10N4O3) C 57.78, H 3.73. Found: C 57.51, H 3.65.
4.1.16 N2-4-Chloro-3-nitrobenzylidenyl Isonicotinic Acid Hydrazide (17)
Yield: 100%, m.p.: 231°C (uncorr). FT-IR: ν (cm-1) 3189, 1685, 1599, 1558, 1530, 1412, 1351, 1277, 1252, 1214, 1154, 1125, 1074, 1061, 1049, 998, 962, 942, 896, 846, 823, 748, 721. Anal. (C13H9N4O3Cl) C 51.25, H 2.98. Found: C 51.20, H 2.90.
4.1.17 Isonicotinoylhydrazone of Acetophenone (18)
Yield: 92%, m.p.: 169-170°C. FT-IR: ν (cm-1) 3173, 1652, 1599, 1540, 1288, 1150, 1103, 975, 835, 757, 722. Anal. (C14H13N3O) C 70.27, H 5.48. Found: C 70.18, H 5.51.
4.1.18 N2-4-Hexyloxybenzylidenyl Isonicotinic Acid Hydrazide (19)
Yield: 87%, m.p.: 135-138°C. FT-IR: ν (cm-1) 3234, 3062, 1650, 1608, 1572, 1549, 1513, 1415, 1398, 1297, 1240, 1179, 1154, 1127, 1112, 1070, 1032, 993, 976, 961, 941, 922, 863, 846, 832, 810, 752, 725. Anal. (C19H23N3O2) C, 70.06; H, 7.07. Found: C, 70.07; H, 7.07.
4.1.19 N2-3-Nitrobenzylidenyl Isonicotinic Acid Hydrazide (20)
Yield: 85%, m.p.: 225°C (uncorr). FT-IR: ν (cm-1) 3232, 1691, 1609, 1600, 1548, 1524, 1412, 1352, 1314, 1271, 1141, 1101, 1062, 996, 961, 948, 887, 843, 827, 815, 750, 739, 710. Anal. (C13H10N4O3) C 57.78, H 3.73. Found: C 57.69, H 3.77.
4.1.20 N2-Benzylidenyl Isonicotinic Acid Hydrazide (21)
Yield: 98%, m.p.: 197°C, lit m.p. [59] 194-195 °C. FT-IR: ν (cm-1) 3192, 1691, 1598, 1565, 1412, 1354, 1284, 1150, 1081, 1058, 998, 952, 920, 845, 767, 724.
4.1.21 N2-4-Butoxylbenzylidenyl Isonicotinic Acid Hydrazide (22)
Yield: 91%, m.p.: 148-149°C. FT-IR: ν (cm-1) 3272, 1650, 1260. Anal. (C17H19N3O2) C 68.68, H 6.44. Found: C 68.84, H 6.61.
4.1.22 N2-2-trans-Pentenylidenyl Isonicotinic Acid Hydrazide (23)
The reaction of trans-2-pentenal (0.84 g, 10.0 mmol) with INH (0.822 g, 6 mmol) in ethanol (20 mL) for 2 hours was followed by concentration to half volume and the addition of ether (25 mL). After standing over night, the title compound was obtained by filtration from the reaction mixture and drying. Yield: 0.71 g (54%), m.p.: 161-164°C. FT-IR: ν (cm-1) 3237, 2725, 1654, 1638, 1546, 1293, 1001. 1H NMR: δ (ppm) 11.75 (1H, br s), 8.78 (2H, br d, J = 7 cps), 8.07 (1H, d, J = 9 cps), 7.88 (2H, br d, J = 7 cps), 6.35 (2H, m), 2.25 (2H, m), 1.05 (3H, t, J = 6 cps). 13C NMR: δ (ppm) 161.68, 151.55, 150.62, 146.12, 140.85, 126,70, 121.81, 25.69, 13.03. HRMS (FAB MH+): (C11H14N3O) Calc 204.1137. Found: 204.1139. Anal. (C11H13N3O) Calc C 65.01, H 6.45. Found: C 65.18, H 6.42.
4.1.23 N2-2-trans-Butenylidenyl Isonicotinic Acid Hydrazide (24)
Yield: 0.83 g (73%), m.p.: 198-201°C. FT-IR: ν (cm-1) 3178, 2725, 1664, 1637, 1578, 1547, 1299, 983. 1H NMR: δ (ppm) 11.78 (1H, br s), 8.75 (2H, br d, J = 7 cps), 8.05 (1H, m), 7.78 (2H, d, J = 7 cps), 6.28 (2H, m), 1.88 (m, 3H). 13C NMR: δ (ppm) 161.71, 151.49, 150.61, 140.85, 139.83, 128.95, 121.80, 18.73. HRMS (FAB MH+): (C10H12N3O) Calc 190.0980. Found: 190.0983. Anal. (C10H11N3O) Calc C 63.48, H 5.86. Found: C 63.79, H 5.94.
4.1.24 N2-2-trans-Hexenylidenyl Isonicotinic Acid Hydrazide (25)
Yield: 1.21 g (in two crops, 93%), m.p.: 152-154°C. FT-IR: ν (cm-1) 3223, 2724, 1654, 1638, 1545, 1299, 994. 1H NMR: δ (ppm) 11.78 (1H, br s), 8.76 (2H, d, J = 7 cps), 8.06 (1H, m), 7.78 (2H, d, J = 7 cps), 6.26 (2H, m), 2.22 (2H, m), 1.45 (2H, sextet, J = 6 cps), 0.90 (3H, t, J = 6 cps). 13C NMR: δ (ppm) 161.71, 151.50, 150.62, 144.49, 140.85, 127.82, 121.81, 34.62, 21.66, 13.86. HRMS (FAB MH+): (C12H16N3O) Calc 218.1284. Found: 218.1284. Anal. (C12H15N3O) Calc C 66.34, H 6.96. Found: C 66.33, H 7.13.
4.1.25 N2-2-trans-Heptenylidenyl Isonicotinic Acid Hydrazide (26)
Yield: 1.16 g (in two crops, 84%), m.p.: 152-154°C. FT-IR: ν (cm-1) 3245, 2726, 1656, 1633, 1545, 1296, 1006, 962. 1H NMR: δ (ppm) 11.78 (1H, br s), 8.76 (2H, d, J = 7 cps), 8.08 (1H, m), 7.78 (2H, d, J = 7 cps), 6.27 (2H, m), 2.20 (2H, m), 1.37 (4H, m), 0.85 (3H, t, J = 6 cps). 13C NMR: δ (ppm) 161.69, 151.50, 150.62, 144.73, 140.84, 127.66, 121.81, 32.25, 30.54, 21.99, 14.07. HRMS (FAB MH+): (C13H18N3O) Calc 232.1450. Found: 232.1443. Anal. (C13H17N3O) Calc C 67.51, H 7.41. Found: C 67.38, H 7.53.
4.1.26 N2-3,7-Dimethyl-2,6-octadienylidenyl Isonicotinic Acid Hydrazide (27)
Compound 27 was prepared from INH (0.83 g, 6.06 mmol) and citral (2 mL). The citral employed was the commercial mixture of geranial and neral (D = 0.888 g/mL). Yield: 0.97 g (59%), m.p.: 124-125°C. FT-IR: ν (cm-1) 3178, 3028, 1638, 1548, 1297. Anal. (C16H21N3O) Calc C 70.82, H 7.80. Found: C 70.78, H 7.99.
4.1.27 N2-3,7-Dimethyl-6-octenylidenyl Isonicotinic Acid Hydrazide (28)
Schiff base 28 was formed from INH (0.85 g, 6.20 mmol) and citronellal (2 mL). Yield: 0.49 g (29%), m.p.: 78-80°C. FT-IR: ν (cm-1) 3226, 3060, 1651, 1619, 1544, 1410, 1296, 1215, 1135, 1067, 1032, 975, 906, 844, 757, 722. Anal. (C16H23N3O) Calc C 70.30, H 8.48. Found: C 70.35, H 8.78.
4.1.28 N2-Isopropylidenyl Isonicotinic Acid Hydrazide (29)
The Schiff base was produced from the reaction of INH (0.82 g, 6.00 mmol) with acetone (2 mL). It was necessary to concentrate the reaction mixture to half volume and then to precipitate the product with ether. Yield: 0.51 g (48%), m.p.: 161-162°C. FT-IR: ν (cm-1) 3181, 3060, 1654, 1632, 1534, 1298. 1H NMR: δ (ppm) 10.74 (1H, br s), 8.72 (2H, d, J = 7 cps), 7.78 (2H, d, J = 7 cps), 2.01 (3H, s), 1.91 (3H, s). Anal. (C9H11N3O) Calc C 61.00, H 6.25. Found: C 60.95, H 6.21. This material was further characterized by its exchange reaction with 2,4-dinitrophenylhydrazine to produce the 2,4-dinitrophenylhydrazone of acetone, in a procedure used for a number of compounds described in this work but only exemplified here. Thus compound 29 (0.135 g) was dissolved in the minimum volume (5 mL) of absolute ethanol. To this solution was then added standard 2,4-dinitrophenylhydrazine reagent (10 mL) [55], at room temperature. Formation of a yellow precipitate was instantaneous. The mixture was warmed to just below the boiling point and swirled continuously. The mixture was then cooled, and the resulting yellow-orange 2,4-dinitrophenylhydrazone of acetone (72%) was allowed to stand for a few hours, then filtered off by gravity, m.p. 127-128°C (from ethanol); mixed m.p. with an authentic specimen (prepared directly from acetone and 2,4-dinitrophenylhydrazine by the same method) 127-128°C; lit m.p. 128°C [56-58].
4.1.29 N2-1-Ethoxycarbonyl-3-phenylpropylidenyl Isonicotinic Acid Hydrazide (30)
Yield: 1.72 g (90% in two crops), m.p.: 104°C. FT-IR: ν (cm-1) 3250, 1702, 1664, 1592, 1553, 1510, 1399, 1245, 1143, 1074, 1022, 941, 843. Anal. (C18H19N3O3) Calc C 66.45, H 5.89. Found: C 66.10, H 5.96
4.1.30 N2-3,5,5-Trimethylcyclohexylidenyl Isonicotinic Acid Hydrazide (31)
Yield: 1.55 g (76%), m.p.: 149-151°C. FT-IR: ν (cm-1) 3174, 1649, 1627, 1552, 1533, 1338, 1292. 1031. 1H NMR: δ (ppm) 10.91 and 10.08 (1H, br singlets), 8.69 (2H, d, J = 7 cps), 7.71 (2H, d, J = 7 cps), 2.94-0.78 (16H, m). Anal. (C15H21N3O) Calc C 69.47, H 8.16. Found: C 69.42, H 8.17.
4.1.31 N2-β-Phenylcinnamylidenyl Isonicotinic Acid Hydrazide (32)
Yield: 0.83 g (86%), m.p.: 223°C. FT-IR: ν (cm-1) 3186, 1648, 1603, 1564, 1546, 1522, 1298, 1284, 1155, 1132, 1062, 1016, 842, 770. Anal. (C21H17N3O) Calc C 77.04, H 5.23. Found: C 76.78, H 5.36.
4.1.32 N2-4-Methoxycinnamylidenyl Isonicotinic Acid Hydrazide (33)
Yield: 2.51 g (89%), m.p.: 218°C. FT-IR: ν (cm-1) 3221, 3044, 2840, 1649, 1622, 1603, 1576, 1537, 1509, 1456, 1442, 1415, 1362, 1318, 1305, 1291, 1259, 1242, 1213, 1173, 1143, 1110, 1086, 1069, 1048, 1026, 982, 949, 934, 909, 841, 824, 812, 754. 1H NMR: δ (ppm) 8.54 (2H, d, J = 7 cps), 8.20 (1H, d, J = 9 cps), 7.80 (2H, d, J = 7 cps), 7.57 (2H, d, J = 9 cps), 6.98 (4H, m), 3.78 (3H, s). 13C NMR: δ (ppm) 161.70, 160.35, 151.75, 150.62, 140.84, 140.17, 129.13, 128.80, 123.32, 121.85, 114.65, 55.55. HRMS (FAB M+): (C16H16N3O2) Calc 282.1243. Found: 282.1241. Anal. (C16H16N3O2) Calc C 68.31, H 5.37. Found: C 68.43, H 5.38.
4.1.33 N2-2-Nitrocinnamylidenyl Isonicotinic Acid Hydrazide (34)
Yield: 2.77 (94%), m.p.: 204°C. FT-IR: ν (cm-1) 3243, 3044, 1655, 1624, 1606, 1595, 1569, 1552, 1536, 1510, 1472, 1444, 1408, 1363, 1344, 1290, 1248, 1216, 1180, 1169, 1134, 1070, 1056, 975, 942, 910, 859, 840, 828, 780, 755, 717. 1H NMR: δ (ppm) 8.84 (2H, d, J = 7 cps), 8.28 (1H, d, J = 9 cps), 8.02 (2H, m), 7.85 (2H, d, J = 7 cps), 7.75 (1H, t, J = 9 cps), 7.60 (1H, t, J = 9 cps), 7.38 (1H, d, J = 12 cps), 7.15 (1H, dd, J = 12, 9 cps). 13C NMR: δ (ppm) 161.98, 150.68, 150.55, 148.17, 140.59, 134.09, 133.94, 133.36, 130.68, 130.23, 130.05, 128.73, 121.87. Anal. (C15H12N4O3) Calc C 60.80, H 4.08. Found: C 60.68, H 4.12.
4.1.34 N2-2-Propyl-2-heptenylidenyl Isonicotinic Acid Hydrazide (35)
The aldehyde used was the commercial mixture of cis and trans isomers. Yield: 2.29 g (84%), m.p.: 121°C. FT-IR: ν (cm-1) 3210, 3075, 2956, 2927, 2870, 1651, 1629, 1566, 1551, 1455, 1401, 1367, 1304, 1218, 1160, 1061, 1048, 976, 872, 838, 756, 723. 1H NMR: δ (ppm) 11.68 (1H, br s), 8.78 (2H, d, J = 7 cps), 7.99 (1H, s), 7.78 (2H, d, J = 7 cps), 5.89 (1H, t, J = 7 cps), 2.39-1.99 (4H, m), 1.53-1.28 (6H, m), 0.90 (6H, t, J = 7 cps). 13C NMR: δ (ppm) 161.65, 154.00, 150.57, 141.91, 141.09, 137.53, 121.79, 31.25, 28.01, 27.33, 22.22, 21.89, 14.30, 14.13. Anal. (C16H23N3O) Calc C 70.30, H 8.48. Found: C 70.32, H 8.62.
4.1.35 N2-2-Isopropyl-5-methyl-2-hexenylidenyl Isonicotinic Acid Hydrazide (36)
The aldehyde used was the commercial mixture of cis and trans isomers. Yield: 2.71 g (99%), m.p.: 129°C. FT-IR: ν (cm-1) 3250, 3060, 2955, 2869, 1650, 1549, 1462, 1408, 1362, 1301, 1216, 1149, 1066, 993, 965, 892, 843, 754. 1H NMR: δ (ppm) 11.75-11.50 (1H, br m), 8.78 (2H, m), 8.51 and 7.90 (1H, m), 7.78 (2H, m), 5.91-5.62 (1H, m), 3.05 (1H, septet, J = 6 cps), 2.12 (2H, m), 1.65 (1H, m), 1.19-0.78 (12H, m). 13C NMR: δ (ppm) 168.43, 161.66, 150.57, 149.44, 147.74, 142.72, 142.60, 142.23, 141.07, 140.94, 139.42, 139.21, 123.25, 121.79, 37.28, 35.94, 28.75, 28.55, 27.85, 26.76, 22.63, 22.56, 22.41, 20.89. Anal. (C16H23N3O) Calc C 70.30, H 8.48. Found: C 70.23, H 8.48.
4.1.36 N2-2-Methoxycinnamylidenyl Isonicotinic Acid Hydrazide (37)
Yield: 2.59 g (92%), m.p.: 206°C. FT-IR: ν (cm-1) 3246, 3023, 2994, 2839, 1646, 1615, 1595, 1581, 1540, 1487, 1465, 1436, 1408, 1368, 1314, 1291, 1245, 1216, 1186, 1172, 1141, 1107, 1067, 1044, 1025, 1001, 956, 909, 838, 748. 1H NMR: δ (ppm) 11.95 (1H, br s), 8.82 (2H, d, J = 7 cps), 8.23 (1H, d, J = 9 cps), 7.80 (2H, d, J = 7 cps), 7.72-6.98 (6H, m), 3.82 (3H, s). 13C NMR: δ (ppm) 161.83, 157.30, 152.00, 150.64, 140.82, 135.18, 130.84, 128.07, 126.29, 123.30, 121.86, 111.92, 55.90. Anal. (C16H15N3O2) Calc C 68.31, H 5.37. Found: C 67.92, H 5.39.
4.1.37 N2-2-trans-2-Methylcinnamylidenyl Isonicotinic Acid Hydrazide (38)
Yield: 2.38 g (90%), m.p.: 200°C. FT-IR: ν (cm-1) 3198, 3015, 2863, 1650, 1620, 1598, 1572, 1550, 1493, 1439, 1402, 1362, 1325, 1305, 1212, 1159, 1074, 1015, 989, 966, 931, 877, 862, 839, 748, 724. 1H NMR: δ (ppm) 8.78 (2H, d, J = 7 cps), 8.24 (1H, s), 7.92 (2H, d, J = 7 cps), 7.58-7.28 (5H, m), 6.89 (1H, s), 2.14 (3H, s). 13C NMR: δ (ppm) 160.02, 152.68, 148.79, 139.11, 136.17, 134.65, 132.75, 127.90, 126.96, 126.38, 120.03, 11.31. Anal. (C16H15N3O) Calc C 72.43, H 5.70. Found: C 72.19, H 5.67.
4.1.38 Glyoxal Di-isonicotinoylhydrazone (39)
This compound was prepared from glyoxal and isonicotinic acid hydrazide (2 equivalents). Yield: 90%, m.p.: >300°C (dec). FT-IR: ν (cm-1) 3198, 3052, 1666, 1579, 1535, 1407, 1310, 1288, 1213, 1156, 1063, 963, 915, 842, 763, 730. Anal. (C14H12N6O2) Calc C 56.75, H 4.08. Found: C 56.85, H 4.10.
4.1.39 Ethyl Butyryl Acetate Isonicotinoylhydrazone (40)
Yield: 98%, m.p.: 89-90°C. FT-IR: ν (cm-1) 3163, 1733, 1675, 1655, 1627, 1599, 1558, 1548, 1426, 1406, 1336, 1298, 1261, 1196, 1162, 1095, 1044, 1030, 992, 974, 918, 886, 842, 762, 722. Anal. (C14H19N3O3) Calc C 60.64, H 6.91. Found: C 60.26, H 7.02.
4.1.40 Ethyl Acetoacetate Isonicotinoylhydrazone (41)
Yield: 85%, m.p.: 99°C. FT-IR: ν (cm-1) 3200, 1733, 1690, 1655, 1635, 1598, 1554, 1535, 1406, 1336, 1301, 1268, 1182, 1142, 1067, 1037, 840, 756, 721, 672. Anal. (C12H15N3O3) Calc C 57.82, H 6.07. Found: C 57.88, H 6.26.
4.1.41 Terephthalaldehyde Di-isonicotinoylhydrazone (42)
This compound was prepared from terephthalaldehyde and isonicotinic acid hydrazide (2 equivalents). Yield: 90%, m.p.: >300°C (dec). FT-IR: ν (cm-1) 3246, 3067, 1653, 1600, 1543, 1507, 1408, 1293, 1215, 1154, 1107, 1068, 969, 923, 839, 812, 755, 719. Anal. (C20H16N6O2) Calc C 64.51, H 4.33. Found: C 64.59, H 4.44.
4.1.42 Isonicotinoylhydrazone of Ethyl 2-Oxo-4-phenylbutyrate (43)
Yield: 90%, m.p.: 104°C. FT-IR: ν (cm-1) 3251, 1702, 1685, 1592, 1554, 1511, 1417, 1403, 1301, 1246, 1213, 1143, 1112, 1074, 1022, 994, 942, 875, 843, 798, 764, 754, 721, 706. Anal. (C18H19N3O3) C 66.45, H 5.89. Found: C 66.10, H 5.96.
4.1.43 N2-Tetradecanylidenyl Isonicotinic Acid Hydrazide (44)
Yield: 73%, m.p.: 89-91°C. FT-IR: ν (cm-1) 3258, 1654, 1624, 1553, 1410, 1295, 1221, 1158, 1120, 1099, 1065, 1042, 962, 846, 753, 718. Anal. (C20H33N3O) C 72.46, H 10.04. Found: C 72.20, H 10.33.
4.1.44 N2-4-Tridecanylidenyl Isonicotinic Acid Hydrazide (45)
Yield: 73%, m.p.: 92-93°C. FT-IR: ν (cm-1) 3260, 3066, 1654, 1624, 1597, 1548, 1412, 1294, 1041, 846, 754, 727. Anal. (C19H31N3O) C 71.88, H 9.84. Found: C 71.68, H 10.14.
4.1.45 Experimental Estimates of Relative Lipophilicities of Schiff Bases
To a weighed amount of the Schiff base of interest (0.75 mmol) in a Florence flask containing a stirring bar was delivered by pipet distilled water (5 mL) and also by pipet chloroform (5 mL). The flask was sealed with a cork and paraffin, and the contents were magnetically stirred for 30 minutes. The cork, paraffin and stirring bar were carefully removed, and the contents of the flask were transferred to a separatory funnel and separated. The individual layers were allowed to evaporate to dryness over night on a pre-weighed watchglass. The ratio of amounts of dry compound remaining after evaporation of the layers was obtained, then compared to the same ratio for INH as standard, expressing the comparison as follows:
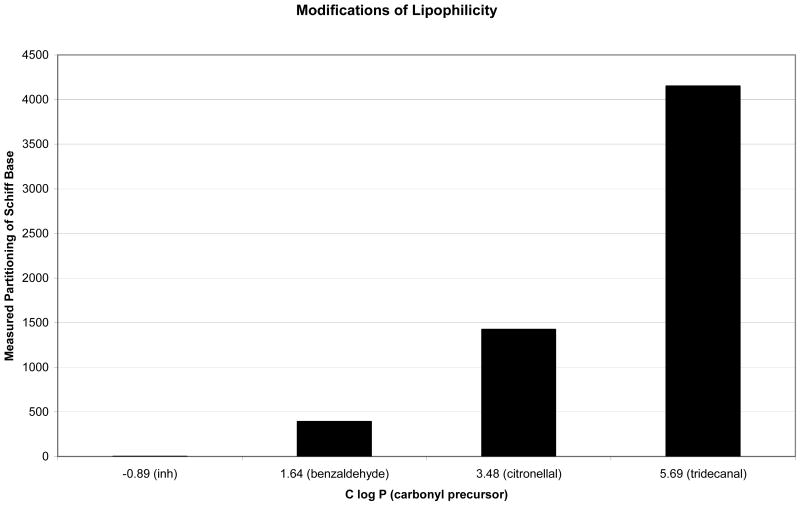
where Acmpd(solvent) refers to the amount of compound in chloroform or water, as appropriate, and AINH(solvent) refers to the amount of INH in chloroform or water, as appropriate. Representative examples of these estimates are shown in Figure 4, in which results are plotted against C log P [51] of the carbonyl precursor to the Schiff base. In this way, we observed that functionalization at N2 led to lipophilicities as much as several orders of magnitude greater than that of INH itself.
Figure 4.
Bar graph depicting the relative lipophilicities of INH and its Schiff bases of benzaldehyde, citronellal and tridecanal.
3.2. Biological methods
M. tuberculosis ATCC 35801 (strain Erdman) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). INH was purchased from Sigma Chemical Company (St. Louis, MO, USA). For testing, a given Schiff base was dissolved in dimethyl sulfoxide and subsequently diluted in distilled water. INH was dissolved in distilled water. Stock solutions were filter-sterilized by passage through a 0.22 um-pore-size membrane filter and stored at -20°C until use. The drugs were prepared each morning, before experimentation. With respect to testing against this isolate, the MICs of all antimicrobial agents were determined in modified 7H10 broth (7H10 agar formulation with agar and malachite green omitted; pH 6.6) supplemented with 10% Middlebrook oleic acid-albumin-dextrose-catalase (OADC) enrichment (Difco Laboratories, Detroit, MI, USA) and 0.05% Tween 80 [60]. The MICs of the antimicrobial agents were determined by a broth dilution method [61]. The organism was grown in the modified 7H10 broth with 10% OADC enrichment and 0.05% Tween 80 on a rotary shaker at 37°C for 5 days. The culture suspension was diluted in modified 7H10 broth to yield 100 Klett units/mL (Photoelectric Colorimeter, Manostat Corporation, New York, NY, USA), or approximately 5 × 107 cfu/mL. The size of the inoculum was determined by titration and counting from triplicate 7H10 agar plates (BBL Microbiology Systems, Cockeysville, MD, USA) supplemented with 10% OADC enrichment. The plates were incubated at 37°C in ambient air for 4 weeks before counting of the colonies. Results in vitro (strain H37Rv) were also determined according to the fully-documented protocols of the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF), of the US National Institutes of Health [54]. In the latter case, primary screening had indicated that all compounds had MIC values less than 6.25 μg/mL.
For the short-course therapy studies, four-week-old female C57BL/6 mice (Charles River, Wilmington, MA, USA) were infected intranasally with approximately one million viable M. tuberculosis organisms. Treatment began one day post-infection and was administered for two days. The agents were administered by gavage: the INH control and a given Schiff base were dosed at 25 mg/kg of body weight. Mice were sacrificed by carbon dioxide inhalation three days post-infection. Their right lungs were removed aseptically and were ground in a tissue homogenizer (IdeaWorks! Laboratory Devices, Syracuse, NY, USA). The number of viable organisms was determined by titration on 7H10 agar plates. The plates were incubated at 37°C in ambient air for 4 weeks before counting of the colonies. Control groups of infected but untreated mice were sacrificed at the initiation of therapy (early controls) or at the end of the treatment period (late controls). The complete procedure for the short-course therapy model has been reported [55]. For conventional four-week infection studies, the methods used have been noted [32]. In brief, four-week-old female outbred CD-1 mice (Charles River, Wilmington, MA, USA) were infected intravenously through a caudal vein. Each mouse received approximately 107 viable organisms suspended in 0.2 mL of modified 7H10 broth. There were eight mice per group. Treatment began 1 week after infection. Therapy was given 5 days per week for 4 weeks. Otherwise, procedures were identical to those for the short-course model. The use of animals complied with institutional policies and national guidelines.
Table 4. In Vivo Activities of Schiff Bases.
| Compound | Group | Log CFU/Lung |
|---|---|---|
| 3a | Early Controls | 6.50 ± 0.14 |
| Late Controls | 6.37 ± 0.24 | |
| Isoniazid | 5.58 ± 0.28 | |
| Schiff Base 3 | 5.49 ± 0.63 | |
| 36b | Early Controls | 6.38 ± 0.14 |
| Late Controls | 6.51 ± 0.13 | |
| Isoniazid | 4.96 ± 0.13 | |
| Schiff Base 36 | 5.49 ± 0.26 | |
| 38b | Early Controls | 6.38 ± 0.14 |
| Late Controls | 6.51 ± 0.13 | |
| Isoniazid | 4.96 ± 0.13 | |
| Schiff Base 38 | 5.22 ± 0.36 | |
| 46c | Early Controls | 6.74 ± 0.98 |
| Late Controls | 8.80 ± 0.18 | |
| Isoniazid | 4.71 ± 0.89 | |
| Schiff Base 46 | 4.65 ± 0.27 |
Groups of six mice, short course therapy model.
Groups of five mice, short course therapy model.
Groups of eight mice, traditional four week therapy model.
Acknowledgments
The authors thank the staff of the Tuberculosis Antimicrobial Acquisition and Coordinating Facility, co-ordinated by the Southern Research Institute, Birmingham, Alabama, USA, under a research and development contract with the National Institute of Allergy and Infectious Diseases (NIAID) of the US National Institutes of Health (NIH). Mass spectra were determined at the NIH Mass Spectrometry Facility at Michigan State University, East Lansing, Michigan, USA. This work was supported by the Global Alliance for Tuberculosis Drug Development and by grant 1 R15 AI48397-01 from the Division of Acquired Immunodeficiency Syndrome, NIAID, NIH. MJH thanks Wellesley College for the grant of a sabbatical.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davies J. In: Genetics and Tuberculosis, Novartis Foundation Symposia. Chadwick DJ, editor. Vol. 217. John Wiley and Sons, Limited; Chichester, England: 1998. pp. 195–208. [Google Scholar]
- 2.Miesel L, Rozwarski D, Sacchettini J, Jacobs W. In: Genetics and Tuberculosis, Novartis Foundation Symposia. Chadwick DJ, editor. Vol. 217. John Wiley and Sons, Limited; Chichester, England: 1998. pp. 209–227. [DOI] [PubMed] [Google Scholar]
- 3.Barry CE. Mycobacteria and TB. Issues Infect Dis. 2003;2:137–150. [Google Scholar]
- 4.Takiff HE. In: Multidrug-resistant tuberculosis. Bastian I, Portaels F, editors. Kluwer Academic Publishers; Boston, Massachusetts: 2000. pp. 79–84. [Google Scholar]
- 5.Ventura C, Martins F. Journal of Medicinal Chemistry. 2008;51:612–624. doi: 10.1021/jm701048s. [DOI] [PubMed] [Google Scholar]
- 6.Sriram D, Yogeeswari P, Madhu K. Bioorganic and Medicinal Chemistry Letters. 2005;15:4502–4505. doi: 10.1016/j.bmcl.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Sinha N, Jain S, Tilekar A, Upadhayaya R, Kishore N, Jana G, Arora SK. Bioorganic and Medicinal Chemistry Letters. 2005;15:1573–1576. doi: 10.1016/j.bmcl.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 8.Parikh SL, Xiao G, Tonge P. Biochemistry. 2000;39:7645–7650. doi: 10.1021/bi0008940. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan T, Truglio J, Boyne M, Novichenok P, Zhang X, Stratton C, Li HJ, Kaur T, Amin A, Johnson F, Slayden R, Kisker C, Tonge P. Chemical Biology. 2006;1:43–53. doi: 10.1021/cb0500042. [DOI] [PubMed] [Google Scholar]
- 10.Drlica K, Zhao X. Clinical Infectious Diseases. 2007;44:681–688. doi: 10.1086/511642. [DOI] [PubMed] [Google Scholar]
- 11.Drlica K. J Antimicrob Chemother. 2003;52:11–17. doi: 10.1093/jac/dkg269. [DOI] [PubMed] [Google Scholar]
- 12.Diacon A, Patientia R, Venter A, Helden Pv, Smith P, McIlleron H, Maritz J, Donald P. Antimicrobial Agents and Chemotherapy. 2007;51:2994–2996. doi: 10.1128/AAC.01474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu T, Zhao X, Li X, Hansen G, Blondeau J, Drlica K. J Antimicrob Chemother. 2003;52:61–64. doi: 10.1093/jac/dkg268. [DOI] [PubMed] [Google Scholar]
- 14.Augustynowicz-Kopec E, Zwolska Z. Acta Poloniae Pharmaceutica. 2002;59:443–447. [PubMed] [Google Scholar]
- 15.Keus K, Houston S, Melaku Y, Burling S. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97:614–618. doi: 10.1016/s0035-9203(03)80048-2. [DOI] [PubMed] [Google Scholar]
- 16.Broussy S, Bernardes-Genisson V, Quemard A, Meunier B, Bernardou J. Jounal of Organic Chemistry. 2005;70:10502–10510. doi: 10.1021/jo051901z. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan TJ, Truglio JJ, Boyne ME, Novichenok P, Zhang X, Stratton CF, Li HJ, Kaur T, Amin A, Johnson F, Slayden RA, Kisker C, Tonge PJ. ACS Chemical Biology. 2006;1:43–53. doi: 10.1021/cb0500042. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y. Ann Rev Pharmacol Toxicol. 2005;45:529–564. doi: 10.1146/annurev.pharmtox.45.120403.100120. [DOI] [PubMed] [Google Scholar]
- 19.Cynamon MH, Zhang Y, Harpster T, Cheng S, DeStefano MS. Antimicrobial Agents and Chemotherapy. 1999;43:2922–2924. doi: 10.1128/aac.43.12.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagannath C, Emanuele M, Hunter R. International Journal of Antimicrobial Agents. 2000;15:55–63. doi: 10.1016/s0924-8579(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 21.Jagannath C, Wells A, Mshvildadze M, Olsen M, Sepulveda E, Emanuele M, Hunter R, Dasgupta A. Life Sciences. 1999;64:1733–1738. doi: 10.1016/s0024-3205(99)00111-3. [DOI] [PubMed] [Google Scholar]
- 22.The Tuberculosis Trials Consortium. The Lancet. 2002;360:528–534. [Google Scholar]
- 23.Laurenzi M, Ginsberg A, Spigelman M. Infectious Disorders - Drug Targets. 2007;7:105–119. doi: 10.2174/187152607781001817. [DOI] [PubMed] [Google Scholar]
- 24.Mohamad S, Ibrahim P, Sadikun A. Tuberculosis. 2004;84:56–62. doi: 10.1016/j.tube.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Rastogi N, Goh K. Antimicrobial Agents and Chemotherapy. 1990;34:2061–2064. doi: 10.1128/aac.34.11.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rastogi N, Moreau B, Capmau M, Goh K, David H. Zentralbl Bakteriol Mikrobiol Hyg [A] 1988;268:456–462. doi: 10.1016/s0176-6724(88)80123-8. [DOI] [PubMed] [Google Scholar]
- 27.Vigorita M, Ottana R, Maccari R, Monforte F, Bisignano G, Pizzimenti F. Boll Chim Farmaceutico. 1998;137:267–276. [PubMed] [Google Scholar]
- 28.DeMoen P, Janssen P, Keere Bvd. Arch Internat Pharmacodyn et de Therap. 1954;98:427. [PubMed] [Google Scholar]
- 29.Iwainsky H. In: Antituberculosis Drugs. Bartmann K, editor. Springer-Verlag; New York: 1988. pp. 489–490. [Google Scholar]
- 30.Orlowski VEH, Rosenfeld M, Wolter H, Schunk R. Arzneimittel Forschung. 1976;26:409–416. [PubMed] [Google Scholar]
- 31.Hearn M, Cynamon M. Drug Design and Discovery. 2003;18:103–108. [PubMed] [Google Scholar]
- 32.Hearn M, Cynamon M. J Antimicrob Chemother. 2004;53:185–191. doi: 10.1093/jac/dkh041. [DOI] [PubMed] [Google Scholar]
- 33.Krueger-Thiemer E. In: Tuberkulose-Forschungsinstitut Borstel Jahresbericht 1956-1957. Freerksen E, editor. Berlin: 1957. p. 331. [Google Scholar]
- 34.Antony S, Ynares C, Dummer J. Clin Transplantation. 1997;11:34–37. [PubMed] [Google Scholar]
- 35.Brost B, Newman R. Obstetrics and Gynecology Clinics of North America. 1997;24:659–673. doi: 10.1016/s0889-8545(05)70329-6. [DOI] [PubMed] [Google Scholar]
- 36.Crema A. Giorn ital chemterap. 1955:45–54. [PubMed] [Google Scholar]
- 37.Crema A, Baroli F, Ferrero E. Boll soc ital biol sper. 1955;31:244–246. [PubMed] [Google Scholar]
- 38.Elliott A, Mitchison D. In: Tuberculosis: Back to the Future. Porter J, McAdam K, editors. John Wiley and Sons, Limited; Chichester, England: 1994. pp. 247–249. [Google Scholar]
- 39.Sarich T, Youssefi M, Zhou T, Adams S, Wall R, Wright J. Arch Toxicology. 1996;70:835–840. doi: 10.1007/s002040050347. [DOI] [PubMed] [Google Scholar]
- 40.Starke J. Primary Care. 1996;23:861–881. doi: 10.1016/s0095-4543(05)70367-5. [DOI] [PubMed] [Google Scholar]
- 41.Timbrell J, Wright J. Human Toxicology. 1984;3:485–495. doi: 10.1177/096032718400300603. [DOI] [PubMed] [Google Scholar]
- 42.Timbrell J, Wright J, Baillie T. Clinical Pharmacology and Therapeutics. 1977;22:602–608. doi: 10.1002/cpt1977225part1602. [DOI] [PubMed] [Google Scholar]
- 43.Georgieva N, Gadjeva V. Biochemistry (Moscow) 2002;67:588–591. doi: 10.1023/a:1015558514432. [DOI] [PubMed] [Google Scholar]
- 44.da Silva Lourenço M, de Lima Ferreira M, Nora de Souza M, Amado Peralta M, Alves Vasconcelos T, das Graças M, Henriques MO. Eur J Med Chem. 2008;43:1344–1347. doi: 10.1016/j.ejmech.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee S, Keitany G, Li Y, Wang Y, Ball H, Goldsmith E, Orth K. Science. 2006;312:1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
- 46.Worby CA, Dixon JE. Science. 2006;312:1150–1151. doi: 10.1126/science.1128785. [DOI] [PubMed] [Google Scholar]
- 47.Augustynowicz-Kopec E, Zwolska Z. Acta Poloniae Pharmaceutica. 2002;59:452–457. [PubMed] [Google Scholar]
- 48.McIlleron H, Wash P, Burger A, Norman J, Folb P, Smith P. Antimicrobial Agents and Chemotherapy. 2006;50:1170–1177. doi: 10.1128/AAC.50.4.1170-1177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nuermberger E, Grosset J. Eur J Clin Microbiol Infect Dis. 2004;23:243–255. doi: 10.1007/s10096-004-1109-5. [DOI] [PubMed] [Google Scholar]
- 50.Kinzig-Schippers M, Tomalik-Scharte D, Jetter A, Scheidel B, Jakob V, Rodamer M, Cascorbi I, Doroshyenko O, Sorgel F, Fuhr U. Antimicrobial Agents and Chemotherapy. 2005;49:1733–1738. doi: 10.1128/AAC.49.5.1733-1738.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balcells M, Thomas S, Godfrey-Faussett P, Grant A. Emerging Infectious Diseases. 2006;12:744–751. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Online at SciFinder Scholar. American Chemical Society 2002 [Google Scholar]
- 53.Hearn MJ, Prisch SB. Organic Preparations and Procedures International. 1981;13:421–424. [Google Scholar]
- 54.Secrist J, Anathan S, Kwong C, Maddry J, Reynolds R, Poffenberger A, Michael M, Miller L, Krahenbuhl J, Adams L, Biswas A, Franzblau S, Rouse D, Winfield D, Brooks J, Orme I. Antimicrobial Agents and Chemotherapy. 2001;45:1943–1946. [Google Scholar]
- 55.Shoen C, DeStefano M, Sklaney M, Monica B, Slee A, Cynamon M. J Antimicrob Chemother. 2004;53:641–645. doi: 10.1093/jac/dkh124. [DOI] [PubMed] [Google Scholar]
- 56.Vogel AI. A Text-book of Practical Organic Chemistry. Third. John Wiley and Sons, Incorporated; New York; New York: 1956. p. 422. [Google Scholar]
- 57.Behforouz M, Bolan J, Flynt M. J Org Chem. 1985;50:1186. [Google Scholar]
- 58.Hearn M, Sy K. Bulletin des Societes Chimiques Belges. 1989;98:339–342. [Google Scholar]
- 59.Windholz Martha., editor. The Merck index: an encyclopedia of chemicals and drugs. Ninth. Merck; Rahway, New Jersey: 1976. p. 587. [Google Scholar]
- 60.Vestal AL. Public Health Service publication no 1995. Laboratory Division, National Communicable Disease Center; Atlanta, Georgia: 1969. pp. 113–115. [Google Scholar]
- 61.Wong CS, Palmer GS, Cynamon MH. J Antimicrob Chemother. 1988;22:863–866. doi: 10.1093/jac/22.6.863. [DOI] [PubMed] [Google Scholar]