Abstract
It has long been known that lymphopoiesis is transiently suppressed during pregnancy, which can be experimentally simulated by estrogen treatment. We now confirm with Rag1/GFP reporter mice that early lymphoid progenitors in the lineage marker- c-kitHi Sca1+, hematopoietic stem cells (HSC)-enriched fraction of bone marrow are particularly depressed in these circumstances. Hematopoietic and environmental cells are both potential hormone targets and, because of this complexity, very little is known regarding mechanisms. We have now identified soluble Frizzled-related protein 1 (sFRP1) as an estrogen inducible gene in stromal cells, whose expression corresponded to inability to support lymphopoiesis. Bone-lining stromal cells express sFRP1, and the transcripts were elevated by pregnancy or estrogen injection. Estrogen receptor-α was essential for both lymphoid suppression and induction of the sFRP family. SFRP1 has been mainly described as an antagonist for complex Wnt signals. However, we found that sFRP1, like Wnt3a, stabilized β-catenin and blocked early lymphoid progression. Myelo-erythroid progenitors were less affected by sFRP1 in culture, which was similar to estrogen with respect to lineage specificity. HSC expressed various Frizzled receptors, which markedly declined as they differentiated to lymphoid lineage. Thus, hormonal control of early lymphopoiesis in adults might partly relate to sFRP1 levels.
Keywords: B Cells, Stem Cells, Stromal Cells, Hematopoiesis, Reproductive Immunology
Introduction
Lymphocytes are produced throughout life from self-renewing hematopoietic stem cells (HSC) and are indispensable elements of the immune system. Much has been learned about molecular mechanisms that regulate lymphocyte differentiation (1, 2). For example, it is now clear that lymphoid gene expression in progenitor cells is determined by levels and combinations of key transcription factors cooperating or cross competing with each other in a hierarchy (3). HSC give rise to multipotent progenitors (MPP) that lack extensive self-renewal ability and eventually segregate into the various blood cell lineages. Lymphoid-primed MPP in the mouse represent a rare lineage marker negative (Lin-) Sca1+ c-kitHi CD150- CD27+ Flk-2+ subset of bone marrow (BM) cells, and some fraction of them replenish all of the lymphocyte populations, including B cells (4). Knowledge about extracellular cues that direct early steps in lymphocyte formation is incomplete, but cytokines such as stem cell factor and Flt-3 ligand are certainly important. Cell adhesion molecules allow stem and progenitor cells to be in proximity to stromal cells that produce those factors, as well as ligands in the extremely complex Notch and Wnt families that control many differentiation events (5).
There is evidence that sex steroids play important regulatory roles in lymphocyte production. All cells in the lymphoid differentiation series decline dramatically in the BM of pregnant mice, while they are abnormally elevated in castrated male, ovariectomized female, hypogonadal and androgen receptor deficient animals (6). Treatment with estrogen does not precisely simulate all of the pregnancy related changes, but it restores B lymphopoiesis in hypogonadal mice to the normal range and selectively depletes lymphoid progenitors in normal animals (7, 8). In fact, sensitivity to estrogen made it possible to identify primitive cells in the MPP fraction that can generate T and B lymphocytes (9). These early lymphoid progenitors (ELP) are not homogenous, but express different combinations of lymphoid genes such as terminal deoxynucleotidyl transferase (TdT) and Rag1 (10).
BM contains multiple cell types that could be hormone targets, accounting in part for the modest progress made in determining exactly how sex steroids regulate lymphopoiesis. Lymphoid progenitors express estrogen and androgen receptors in a developmental age dependent manner (11). Furthermore, they are directly sensitive to estrogen in stromal cell-free, serum-free cultures (12). Transplantation studies with estrogen receptor (ER) deficient mice also implicated hematopoietic cells in this process (13). However, other findings suggest that stromal cells can make suppressive factors for lymphopoiesis when exposed to hormone (14). We have now identified one such substance as soluble Frizzled-related protein 1 (sFRP1).
Soluble Frizzled-related proteins (SFRPs) are among several types of extracellular regulators for Wnt signaling (15). The 5 members of this family contain a characteristic cysteine-rich domain (CRD) that shares 30-50% homology with Frizzled for Wnt. Several studies have shown that sFRPs can antagonize Wnt signaling by interrupting the binding of Wnt to Frizzled (16, 17). However, it is not clear whether all sFRPs down-regulate Wnt signaling or how specific these interactions are. There are at least 19 Wnt proteins that bind to 9 Frizzled or other receptors and exhibit their activities depending on receptor context (18). Furthermore, recent studies demonstrated that sFRPs can even function through Wnt-independent mechanisms (19, 20). These complex observations make it extremely difficult to define the physiological role of sFRPs. They are known to be expressed in BM (21), and may have a role in maintenance of bone density, but little is known about their importance for normal blood cell formation.
At least one Wnt ligand, Wnt3a, can slow the differentiation of HSC, and purified HSC from Bcl-2 transgenic mice were propagated in culture with this as the primary stimulus (22). The best studied of several Wnt signaling pathways involves stabilization of intracellular β-catenin that moves to the nucleus and activates genes through association with TCF/LEF transcription factors (23). Experimental introduction of constitutively active β-catenin preserves the multipotential status of HSC and restores primitive properties to committed myeloid and lymphoid progenitors (24, 25). Wnt5a appears to limit late stages of B lymphopoiesis and may function as a tumor suppressor (26). On the other hand, stromal cell production of Wnt5a promotes the formation of primitive lymphoid cells (27). It is interesting that Wnt5a can inhibit Wnt3a function in a β-catenin signaling pathway independent fashion (28). Alternatively, and depending on which Frizzled receptor is expressed, the same ligand can stimulate canonical β-catenin pathway (29). These examples account for the interest in Wnt family molecules as potential regulators of lympho-hematopoiesis. However, they also illustrate the complexity that accounts for the difficulty in ascribing roles under normal steady-state conditions.
An initial goal of this study was to compare how pregnancy and estrogen treatment affect ELP identified in Rag1/GFP reporter mice. The aim was to better characterize the most proximal event in lymphocyte formation that might be hormone regulated. Two strategies were then used to identify stromal cell genes that were both estrogen regulated and associated with inability to support B lymphopoiesis. We found that sFRP1 was not only up-regulated in estrogen treated mice but capable of selectively suppressing lymphocyte formation. Moreover, the protein stabilized β-catenin in hematopoietic progenitors without exogenous Wnt. The findings suggest a functional relationship between sex steroids and Wnt signaling in the regulation of initial stages of lymphopoiesis.
Materials and Methods
Animals, cell and compound sources
Rag1/GFP knock-in mice have been described (10, 30, 31). BALB/c mice were obtained at 5 weeks of age from Japan Clea. Human mesenchymal cells derived from fetal BM (fetal MC) were established from anonymous tissues obtained from the Anatomic Gift Foundation. Adult human mesenchymal stem cells (adult MSC) were obtained from BioWhittaker. ERα and ERβ knock-out mice have been previously described (32, 33). BMS2 and OP42, murine BM stromal lines, were maintained in 10% FCS-containing DMEM (high glucose) or α-MEM, respectively. Propyl Pyrazole Triol (34) and ERB-041 (35) were obtained from the Wyeth. Recombinant sFRP1-4 and Wnt3a were obtained from R&D systems.
Antibodies
FITC-anti-Mac1 (M1/70), Gr-1 (Ly-6G; RB6-8C5), erythroid (TER-119), CD8a (53-6.7), CD19 (1D3), CD45R/B220 (RA3/6B2), and CD34 (RAM34) Abs; PE-anti-Mac1, CD45R/B220, CD19, c-kit (2B8), Sca1 (Ly6A/E; D7), and IL-7Rα (SB/199.1) Abs; biotin-anti-Mac1, Gr-1, CD45R/B220, erythroid, and CD3 (145-2C11) Abs were obtained from BD Bioscience. APC-anti-CD45R/B220 and c-kit Abs were purchased from eBioscience. Purified anti-Mac1, Gr-1, erythroid, CD3 (145-2C11), CD45RA (14.8) and CD19 Abs from BD Bioscience were used for depleting Lin+ cells, followed by incubation with goat anti-rat IgG-coated magnetic beads (Miltenyi Biotechnology). A rabbit polyclonal Ab to sFRP1 (ab4193) and an anti-β-catenin Ab (14) were from abcam and BD Bioscience, respectively.
Flow cytometry and cell sorting
Cells were stained with Abs indicated in each figure, and analyzed with FACScalibur or FACSaria. Sorting of Lin- c-kitHi or Lin- c-kitLo cells from BALB/c mice was performed as previously described (12). In some experiments, cells from Rag1/GFP heterozygotes were used to isolate Lin- IL-7Rα- c-kitHi Sca1+Rag1/GFP- (HSC enriched) or Lin- IL-7Rα- c-kitHi Sca1+ Rag1/GFP+ (ELP) cells. CLP (Lin- IL-7Rα+ c-kitLo Sca1Lo/-) and CMP (Lin- IL-7Rα- c-kitHi Sca1- CD34+ FcγRII/IIILo) were sorted from C57BL/6 mice as described (24, 36, 37).
Differential display PCR
Total RNA from fetal MC and adult MSC was first digested with 10 U of DNase I. The Differential Display PCR was performed using the RNAimage Kit (GeneHunter). Three different reverse transcription (RT) reactions were carried out on 0.2 μg of DNA-free RNA using a 1 b anchor oligo-dT primer, H-T11A, H-T11G, H-T11C (where H = AAGC) to generate different fractions of cDNAs. PCR reactions were then performed on 1:10 aliquots of the RT mixtures using the same anchor oligo-dT primer (3-primer) and a 13-mer primer that contained a 7 base arbitrary sequence (5-primer). The sequences of differentially expressed cDNAs were determined using an ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems).
Gene arrays
BMS2 and ERα-deficient stromal cells were treated with 10-7 M estrogen for 24h and mRNA was extracted. Gene Discovery Array Mouse version 1.1 (Genome Systems) blots were probed according to manufacturer’s instructions. Briefly, mRNA was bound and eluted from oligo dT cellulose, and 2.5 μg of each mRNA was labeled during reverse transcription using [alpha 33P] dCTP (2000-4000 Ci/mmol). Following hybridization to probes, arrays were exposed and imaged with a Molecular Dynamics STORM phosphorimager (Sunnyvale). The gel files were sent to Genome Systems for analysis.
Stromal cell-free lymphocyte culture
Sorted cells were cultured in 24-well culture plates (3000-5000 cells/well) with SF-03 medium (Sanko-junyaku) containing 1% FCS, 50 μM 2-mercaptoethanol, 2 mM L-glutamine, 1 ng/mL rm IL-7, 100 ng/mL rm FL and 20 ng/mL rm SCF, and fed every 4 days. At the end of culture, cells were counted and analyzed by flow cytometry.
Immunohistochemical staining
Tissues were fixed with 10% buffered formalin and embedded in paraffin. SFRP1 immunohistochemical staining was performed using a 3-step immuno—alkaline phosphatase method, and alkaline phosphatase—labeled streptavidin (DAKO). Rabbit Ig (DAKO) was used as negative control. The staining was performed in PBS containing 1% of BSA in the presence or absence of 10 μg/ml sFRP1.
Cell cycle and apoptosis
Sorted Lin- c-kitHi cells were cultured in stromal-free lymphocyte culture conditions for 24-36 h. At the end of culture, the cells were labeled with 10 μM BrdU for 45 min using the BD Biosciences BrdU labeling system. The cells were fixed for 10 min at room temperature in 4% paraformaldehyde/PBS, permeabilized, and then stained with FITC-conjugated anti-BrdU and 7AAD. Apoptotic events were identified as cells with subdiploid DNA content.
RT-PCR and quantitative real-time PCR
Total RNA was prepared from sorted cells and subjected to the cDNA synthesis using ThermoScriptTM RT-PCR System (Invitrogen). PCR used a combination of ampli-Taq DNA polymerase (Takara) and TaqStart Ab (Clontech) at 40 cycles for ERα and β, or 35 cycles for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Real-time PCR analyses were performed using SYBR Green Mater Mix (Takara). The thermal cycling conditions for the real-time PCR were 5 min at 95 °C to active SYBR Ex Taq, followed by 40 cycles of denaturation, annealing and extension. The mean number of cycles to the threshold of fluorescence detection was calculated for each sample, and β-actin expression was quantified to normalize the amount of cDNA in each sample. Semi-quantitative RT-PCR was performed for Frizzled gene expression as described (9). Primers for all of these reactions are available on request.
Statistical analyses
P values were calculated by using the unpaired Student’s t-test.
Results
Pregnancy and estrogen inhibit very early steps of lymphopoiesis
Previous studies have demonstrated that lymphocyte differentiation is strongly suppressed during pregnancy (38, 39). However, high resolution analysis of the earliest events in lymphopoiesis had not been performed. Therefore, we used Rag1/GFP heterozygous females at 14.5-19.5 day of gestation and compared their BM to non-pregnant females of the same age. Profiles of CD43 and CD45R/B220 expression showed that the CD43Hi CD45R/B220+ (proB-enriched) and CD43Lo CD45R/B220+ (pre B-enriched) fractions were preferentially depleted during pregnancy (Fig. 1A). Progenitors in the CD43Hi CD45R/B220+ fraction with clear expression of Rag1/GFP were particularly suppressed (Fig. 1A). This reporter system has been useful for identifying primitive Rag1 expressing ELP among the Lin- Sca1+ c-kitHi (LSK) fraction (10, 30). ELP were nearly absent from the BM of pregnant mice (Fig. 1B).
Figure 1.
Early stages of B lymphopoiesis are blocked by pregnancy and estrogen treatment.
BM cells of control mice or pregnant Rag1/GFP heterozygotes were stained with the indicated Ab and analyzed in flow cytometry. Whole BM cells were stained with CD45R/B220 and CD43 (A, upper), and Lin- cells were gated and resolved with c-kit and Sca1 staining (B, upper). The CD45R/B220+ CD43Hi fraction and the LSK fraction were further resolved with Rag-1/GFP intensity (A, B, lower). The numbers in each panel are percentages of the gated population. The figures represent data obtained on day 19.5 of gestation. Rag1/GFP heterozygous males were intraperitoneally given a single injection of estrogen (1 mg/mouse) and analyzed at the indicated time after injection. BM Lin- cells were resolved with c-kit and Sca1 (C, upper), and then the LSK fraction was further resolved according to Rag1/GFP intensity (C, lower). Cell numbers in the indicated populations were calculated (D). The data show mean ± SD values from 4 mice in each group, and the figures are representative of 2 independent trials. Significant differences from control (0h) values are indicated by an asterisk (p<0.05) or two asterisks (p<0.01). (E) BM LSK cells of estrogen-injected (at 96h after injection) or control mice were subdivided according to the expression of Flk2/Flt3 and Rag1/GFP. Splenic LSK cells of the same mice were also evaluated (F).
We previously demonstrated that estrogen, one of the pregnancy-related steroids, selectively suppressed Flk2+ CD27+ progenitors capable of generating B and/or T lineage cells among the LSK fraction (9). To allow comparison to the above results with pregnant mice, these experiments were repeated using Rag1/GFP reporter mice. Heterozygous males received single intraperitoneal injections of estrogen, and changes in lymphopoiesis were monitored at intervals over the following 96 h. Decreases in Rag1/GFP expressing cells in the LSK fraction became evident as early as 24h after injection, and total numbers of Rag1+ ELP were reduced 10 fold by 96 h, while there were less severe depletions in total Rag1/GFP-/dim LSK cells (Fig. 1C and 1D). Thus, pregnancy and estrogen treatment are similar in preferentially depleting the earliest known stages of lymphopoiesis in BM. To more precisely analyze proportional change in the LSK fraction, we combined anti-Flk2/Flt3 Ab with Rag1/GFP expression because Flk2+ LSK cells were known as lymphoid-primed MPP (4). Indeed, the Flk2+ LSK contained Rag1+ ELP as a subpopulation. We found that Flk2+ Rag1- as well as Flk2+ Rag1+ cells were sensitive to estrogen, while the Flk2- Rag1- LSK fraction was sustained (Fig. 1E). Of note, no increase of Rag1/GFP+ LSK cells was observed in spleen, peripheral blood or thymi (Fig. 1F and data not shown). In addition, numbers of CFU-IL-7, corresponding to IL-7 responding pro/preB cells also decreased with slower kinetics (data not shown). These results suggested that mobilization or rapid differentiation were unlikely explanations for the depletion of ELP.
Human lymphopoiesis is hormone regulated
Molecular requirements for human B cell formation are not well understood and the process is less efficient in culture than with murine lymphoid progenitors (40). However, B cells slowly emerge when human cells are co-cultured with murine stromal cells (41), and we obtained better results with adult MSC (Fig. 2A, 2B). That is, substantial numbers of CD19+ CD13- B lineage cells were produced from umbilical cord blood CD34+ CD38- cells within 4 weeks of culture without any exogenous cytokines. In contrast, fetal MC did not support B lymphopoiesis.
Figure 2.
Establishment of two types of stromal-cocultures which differ in lymphopoiesis supporting capability.
CD34+ CD38- human hematopoietic progenitors were cultured on fetal MC or adult MSC, and the output of CD19+ cells were evaluated (A and B). The figures represent 3 independent experiments. Influence of estrogen on the cell growth from human CD34+ CD38- progenitors was tested in the co-culture using adult MSC (C). RT-PCR was used to amplify transcripts for ERα and β in adult MSC (D). MCF7, a human breast cancer line, was used as a positive control.
Importantly, the output of CD19+ cells on adult MSC decreased substantially when estrogen was added to the cultures (Fig. 2C). Culture studies done with murine stromal cells and lymphoid progenitors suggest that both are potential targets for hormone regulation (12, 14). Transcripts for ERα and ERβ were detected by RT-PCR in the MSC (Fig. 2D). Human cord blood progenitors do not generate lymphocytes in stromal cell-free cultures, but do not themselves express estrogen receptors (11). We conclude that at least one hormone sensitive human mesenchymal cells can support formation of human B lineage lymphocytes.
SFRPs are candidate inhibitors of lymphopoiesis
The focus of our next experiments was on identifying molecular mechanisms involved in estrogen action on lymphopoiesis. Our previous studies demonstrated that estrogen can directly suppress B lineage progenitors (12). It inhibited production of CD45R/B220+ CD19+ B lineage lymphocytes from early progenitors in stromal cell-free, serum-free culture, but accumulation of CD45R/B220+ CD19- cells was observed (12). The results shown in Fig. 1 demonstrate that estrogen treatment and normal pregnancy affect lymphopoiesis prior to acquisition of CD45R/B220. In addition, as shown in Fig. 2, estrogen suppressed human B cell growth in MSC co-cultures from cord blood progenitors that do not express estrogen receptors (11). Thus, the focus of the present experiments was on identifying mechanisms involving bone marrow stromal cells.
Stromal cells might fail to support human B lymphopoiesis because of their inability to produce requisite molecules and a differential display PCR method suggested that a number are preferentially made by adult MSC (Figure 3A, 3B). However, there was no evidence to implicate any in lymphopoiesis and we considered the alternative possibility. That is, fetal marrow stromal cells might elaborate a suppressive substance as was previously found to be the case for one murine stromal cell sub-clone (42). SFRP1 had those characteristics, and interest in this molecule increased further when RT-PCR with its specific primers confirmed that the transcripts were induced by treatment of adult MSC with estrogen (Fig. 3C). Immunohistochemical analysis showed that bone-lining osteoblast-like stromal cells and osteoclasts expressed sFRP1 protein (Fig. 3D, large orange arrow). While most hematopoietic cells appeared negative, endothelial cells (small black arrow), osteocytes (small orange arrow) and megakaryocytes (black arrow head) had positive staining. Moreover, sFRP4 as well as sFRP1 mRNA were detectable in unseparated BM cells and elevated in day 14.5 pregnant mice (sFRP1: 1st experiment 120%, 2nd experiment 560% of control levels, sFRP4: 1st experiment 170%, 2nd experiment 180% of control levels). Also, a single injection of estrogen significantly elevated sFRP1 transcripts to 150-160% of steady state levels (Fig. 3E).
Figure 3.
SFRP1 is identified as a possible mediator in hormone regulation of early events in lymphopoiesis.
A cloning strategy comprised of two independent steps was conducted (A) and the 17 genes identified by the first screen as differing in fetal MC and adult MSC are summarized (B). The second screen selected sFRP1 (shaded in B). RT-PCR using specific primers for sFRP1 was carried out to see the expression in fetal MC, adult MSC, and estrogen-treated adult MSC (C). (D) BM sections were made from a day 19.5 pregnant mouse and expression of sFRP1 protein was analyzed by immunohistochemical staining. A brown reaction product indicates sFRP1 staining. (E) Male mice given a single injection (1 mg/head) of estrogen were killed at the indicated time, and real-time PCR was performed to see the expression of sFRP1 gene in BM. The data show mean ± SD values from 4 mice in each group, and the figures are representative of 2 independent trials. Significant differences from control (0h) values are indicated by an asterisk (p<0.05).
Independent lines of investigation with murine cells had also suggested that sFRPs might participate in regulation of lymphopoiesis. Some evidence suggests ERα mediates estrogen suppression of lymphopoiesis (13, 43). Indeed, an ERα selective, but not a ERβ selective agonist suppressed B lineage growth in culture (Fig. 4A, 4B). Lymphoid progenitors were less abundant in ERα-/- mice than in normal animals and resistant to estrogen treatment (Fig. 4C). This is in contrast to ERβ-/- mice where lymphopoiesis was normally suppressed by hormone treatment (Fig. 4D). Our search was then focused on genes that might be selectively affected by signals delivered via ERα.
Figure 4.
Estrogen induces the sFRP family via ERα.
(A, B) ERα selective, but not ERβ selective agonists inhibited in vitro generation of B lymphocytes. Lin- BM cells were cultured in stromal cell-free conditions for 7 days. Each culture contained estrogen, ERα selective agonist (Propyl Pyrazole Triol) or ERβ selective agonist (ERB-041) in various concentrations. At the end of culture, numbers of B lineage (CD19+) cells (A) and myeloid (Mac1+) cells were evaluated. Averages and SD of triplicate cultures are shown. (C, D) Estrogen suppresses early pro-B cells in vivo via ERα. ERα deficient, ERβ deficient or control mice were injected sub-cutaneously with slow-release estrogen containing pellets. One week after injection, BM cells were harvested and subjected to flow cytometry to determine Lin- TdT+ cell numbers. (E) BMS2 or ERα-/- stromal cells were exposed to estrogen and gene arrays were performed. The sFRP3 mRNA results are presented as relative levels of expression.
Although a now obsolete membrane gene array method was used, some 260 genes were up regulated by at least 2 fold when BMS2 stromal cells were exposed to estrogen, and an additional 70 were suppressed to at least that degree. BMS2 is an extensively studied stromal cell line derived from normal mice (44). Estrogen increased expression of approximately 85 genes in stromal cells established from marrow of ERα-/- mice (data not shown). Comparison of the two sets of results suggested that approximately 160 genes are ERα-dependent and thus potential candidates for hormone mediated regulation of lymphopoiesis. We found that sFRP3 was among those genes and strongly induced in estrogen-treated BMS2 cells (Fig. 4E).
Further analysis revealed that transcripts for sFRP1, sFRP2 and sFRP3, were elevated when a third stromal cell line, OP42 was exposed to estrogen, while sFRP4 expression was constitutive. We then asked if estrogen directly induced sFRPs in hematopoietic cells. Another gene array involving the Lin- ckitHi Sca1+ fraction of normal bone marrow also identified sFRP1, sFRP2 and sFRP4 as estrogen inducible genes (data not shown). Thus, members of the sFRP family are normally present in bone marrow and probably up-regulated in multiple cell types in response to estrogen.
Selective suppression of early stages of lymphopoiesis by sFRP1
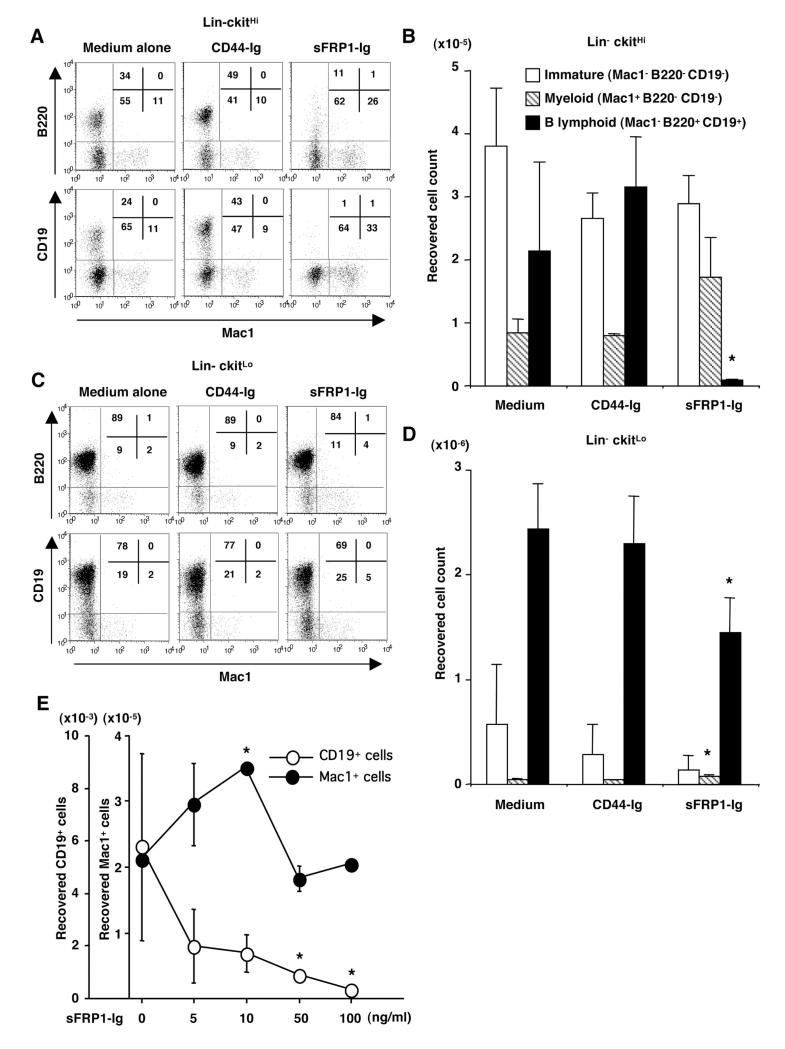
The above results suggested that sFRPs could be hormone inducible, negative regulators of lymphopoiesis. In particular, sFRP1 might account for the fact that one stromal line supports B lymphopoiesis in the absence, but not in the presence of estrogen. To test this notion, substantial amounts of stable sFRP1-Ig fusion protein, along with a control CD44-Ig were prepared. They were first added at 100 ng/ml to stromal cell-free cultures of BALB/c mouse BM to determine if there was direct biological activity on lymphoid progenitors. Starting populations used in these experiments included stem cell/ELP enriched Lin- c-kitHi, as well as pro-lymphocyte/CLP enriched Lin- c-kitLo subsets. After 10-11 days of culture with just medium or CD44-Ig, the Lin- c-kitHi fraction produced 20-50% CD45R/B220+ CD19+ cells (Fig. 5A). In striking contrast, lymphocytes represented only 1% of cells recovered from cultures containing sFRP-Ig. Undifferentiated CD11b/Mac1- B220- CD19- and CD11b/Mac1+ B220- CD19- myeloid cells were also abundant in this set of cultures. It is significant that sFRP1-Ig had no influence on percentages or absolute numbers of these non-lymphoid cells (Fig. 5A and 5B). Lymphocytes predominated in cultures initiated with the Lin- c-kitLo fraction, and their numbers were reduced to a lesser, but still significant degree by sFRP1-Ig (Fig. 5C and 5D).
Figure 5.
SFRP1 inhibits early B lymphopoiesis in culture.
Sorted Lin- c-kitHi (A, B) or Lin- c-kitLo cells (C, D) were cultured in stromal-free conditions with medium alone, CD44-Ig or sFRP1-Ig, and growth of myeloid and B lineage in each culture was evaluated. The percentages of four fractions are shown in boxes. The absolute number of undifferentiated cells (Mac1- CD45R/B220- CD19-), myeloid cells (Mac1+ CD45R/B220- CD19-), and B lineage cells (Mac1- CD45R/B220+ CD19+) recovered from culture of Lin- c-kitHi (C) or Lin- c-kitLo cells (D) was calculated. The data represent the mean ± SD values from triplicate cultures. Significant differences from control (CD44-Ig) values are indicated by an asterisk (p<0.05). Data represent one of six similar experiments. (E) Lin- c-kitHi cells (3000 cells/well) were cultured in stromal-free condition with the indicated concentration of sFRP1-Ig. Absolute numbers of CD19+ cells (○) or Mac1+ cells (●) recovered were calculated and plotted. Significant differences from control values (at sFRP1-Ig 0 ng/ml) are indicated by an asterisk (p<0.05).
We then performed a dose response analysis of sFRP1-Ig in the culture of Lin- c-kitHi cells to see if different outcomes would be obtained at different doses. The suppressive effect on CD19+ lineage was dose-dependent (Fig. 5E). Essentially similar results were obtained when recombinant human sFRP1 was used instead of the fusion protein (see below), showing that the suppression was specific to sFRP1. Proportions and absolute numbers of Mac1+ cells were not significantly reduced but rather increased by 5-10 ng/ml of the protein (Fig. 5E).
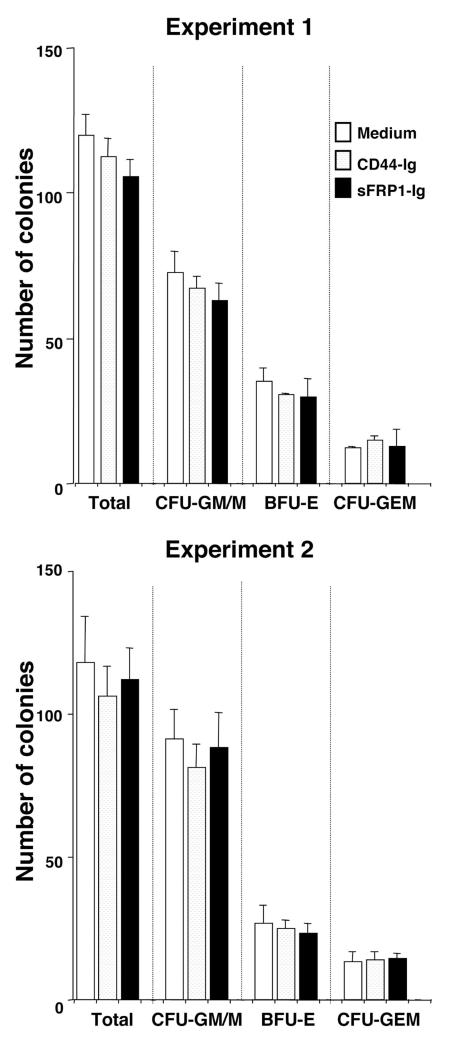
In separate experiments, sFRP1-Ig had no influence on IL-7 dependent clonal proliferation of pre-B cells (data not shown). Furthermore, there was no effect on myeloid-erythroid progenitors in methylcellulose assays (Fig. 6). Collectively, these results suggested that sFRP1 is a potential negative regulator of B lymphopoiesis, and one that is preferentially active on very primitive cells.
Figure 6.
SFRP1-Ig does not affect myelo-erythroid colony formation in methylcellulose cultures.
Five hundred Lin- c-kitHi cells were cultured in Iscove’s MDM-based methylcellulose medium supplemented with 50 ng/ml of rm SCF, 10 ng/ml of rm IL-3, 10 ng/ml of rh IL-6, and 3 units /ml of rh erythropoietin (Methocult GF 3434; StemCell Technologies, Vancouver, Canada) with medium alone, 100ng/ml of CD44-Ig, or 100ng/ml of sFRP1-Ig. After 9 days, colonies were enumerated and classified as CFU-GM/M, BFU-E or CFU-GEM(M) according to shape and color under an inverted microscope.
Stabilization of β-catenin by sFRP1 in hematopoietic progenitors
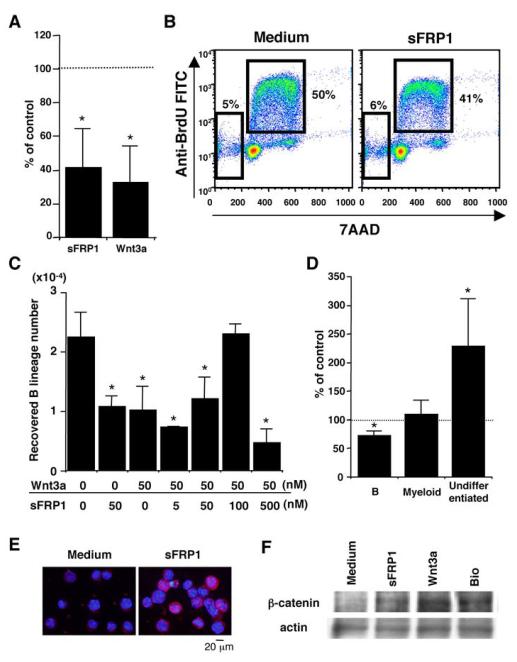
The sFRPs were originally described as inhibitors for Wnt by blocking their interactions with Frizzled receptors (15). More recently, sFRPs have been shown to function in some circumstances in Wnt independent fashion (19, 20). Although we found that sFRP1 directly influenced lymphocyte growth in stromal-free cultures, hematopoietic cells have some capacity to produce their own Wnt (45). Wnt can be divided into functionally distinct classes that can employ different signal transduction mechanisms (46). Therefore, we compared commercial sFRP1 and Wnt3a preparations for a possible influence on lymphocyte production. Since Lin- c-kitHi cells were particularly sensitive in the experiments described above, these were used to initiate stromal cell-free cultures. Interestingly, the 2 proteins showed similar results. Fifty nM of sFRP1 or Wnt3a reduced the yields of Mac1- CD19+ CD45R/B220+ B lineage cells by 42% and 33%, respectively (Fig. 7A). The suppressive effect was also evident when the proteins were used at 5-10 nM (data not shown). The suppression was unlikely due to the induction of apoptosis because neither cell viability nor proportion of cells with subdiploid DNA content was significantly increased in the presence of sFRP1 through the culture period (Fig. 7B and data not shown). Similar suppression for B lineage was observed when the culture was initiated with sFRP2, sFRP3 or sFRP4 (data not shown). Of note, sFRP1 and sFRP4 transcripts were at least as abundant in fetal liver as in adult BM (data not shown). However, generation of B lineage cells from E14.5 fetal liver Lin (TER119, Gr-1)- c-kitHi Sca1+ cells was not suppressed by either sFRP1-Ig or sFRP1 (data not shown).
Figure 7.
SFRP1 stimulates the β-catenin pathway in hematopoietic progenitors.
(A) Lin- c-kitHi cells were cultured in stromal-free condition containing 50 nM of sFRP1 or Wnt3a, and the recovered cells were classified according to their surface phenotype. The data summarize 4 independent experiments, showing mean ± SD values of % control. The normalized control (medium) value is shown at 100% with a dotted line. Significant differences (p<0.05, indicated by an asterisk) from control value were constantly observed regarding B lymphoid cells in the 4 trials. (B) Cell cycle and apoptosis analyses were performed with Lin- c-kitHi cells after 36h of stromal free culture. Percentages of cells in the subdiploid fraction and S phase are shown in each panel. (C) SFRP1 and Wnt3a were added to the same cultures at the indicated concentration to test their mutual influence. Significant differences from control (Wnt3a 0 nM, sFRP1 0 nM) values are indicated by an asterisk (p<0.05). Similar results were obtained in 2 independent experiments. (D) Lin- c-kitHi cells were cultured in stromal-free condition containing 5 nM of 6-bromoindirubin-37-oxime (BIO), a GSK-3-specific inhibitor, and the recovered cells were classified. (E) Cytospin preparations of Lin- c-kitHi cells incubated with 50 nM of sFRP1 for 24h were subjected to immunohistochemistry with an anti-β-catenin Ab. (F) LSK cells were incubated with 50 nM of sFRP1, Wnt3a, or BIO for 24h, respectively. Then 1 × 104 cells of each were subjected to Western-blotting for β-catenin. The membrane was re-blotted with an anti-actin Ab. The data are representative of 2 independent experiments.
Similar cultures were then initiated where sFRP1 and Wnt3a were added together in different ratios (Fig. 7C). As expected, each reduced B lymphopoiesis by approximately 60% when added alone. However, there was mutual interference when 100 nM of sFRP1 was added to cultures containing 50 nM of Wnt3a. These results demonstrate that while each of these ligands can suppress lymphocyte formation, they cross-antagonize each other when present at the same time.
The best studied of major signaling pathways attributed to Wnt involves stabilization of β-catenin and its subsequent accumulation in the nucleus (18). Recent reports showed that artificial β-catenin expression strongly inhibited lymphopoiesis in vitro and in vivo (24, 47, 48). Therefore, we wondered if sFRP1 would stimulate canonical Wnt signaling, because it appears to act independently of Wnt on lymphoid progenitors. As a positive control, we used 6-bromoindirubin-37-oxime (BIO), a specific pharmacological inhibitor of glycogen synthase kinase-3 (GSK-3). B lymphopoiesis was suppressed by 70% of control when BIO was added to BM cultures at 5 nM corresponding with its IC50, (Fig. 7D). In contrast, numbers of Mac1+ myeloid cells were unchanged, and cells with an immature phenotype expanded by more than 2 fold (Fig. 7D).
We then sought evidence for β-catenin stabilization in Lin- c-kit+ cells incubated in the presence of those proteins for 24h. As a positive control, we again used 50 nM of BIO. Fluorescence microscopy revealed that some of the sFRP1 or Wnt3a-treated Lin- c-kit+ cells (20% of sFRP1-treated, 29% of Wnt3a-treated in this experiment) showed cytoplasmic and nuclear accumulation of β-catenin, while cells treated with medium alone did not (Fig. 7E and data not shown). Western-blotting analyses were performed for highly enriched LSK cells. Fig. 7F showed that BIO, sFRP1 and Wnt3a all caused accumulation of β-catenin in treated cells.
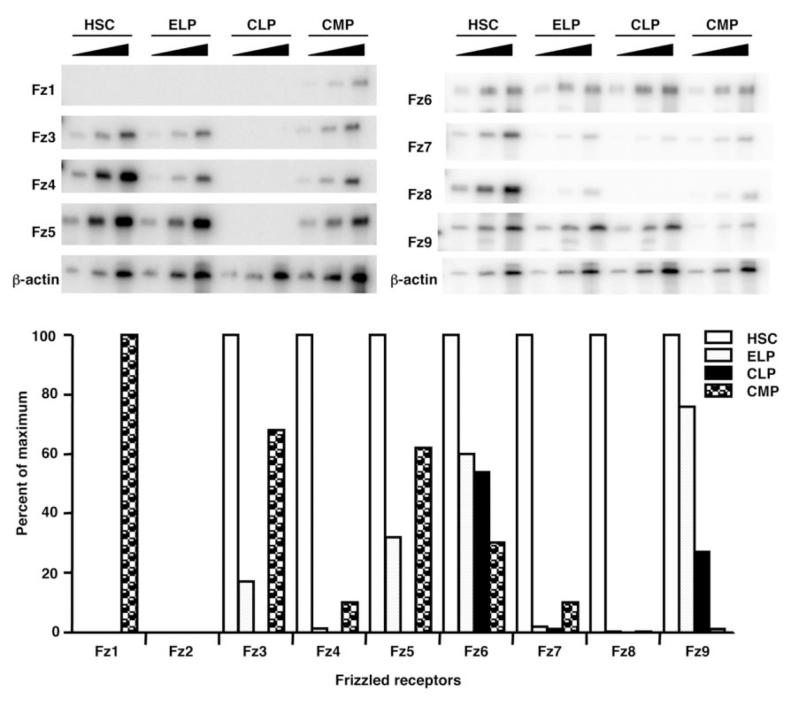
Wnt receptors tend to be down-regulated as lymphopoiesis proceeds
The results shown above suggested that sFRP1 and Wnt3a can stimulate the β-catenin pathway and preferentially influence early steps in lymphopoiesis. To better understand this vulnerability, we examined the expression pattern of Frizzled receptors on stem and progenitor cells. Interestingly, HSC highly expressed 7 out of 9 Frizzled receptors, which markedly declined as they differentiated to ELP (Fig. 8). Only two receptors, Frizzled 6 and 9, remained detectable in the lymphoid-restricted Lin- c-kitLo Sca1Lo IL-7Rα+ common lymphoid progenitors (CLP). Most Frizzled receptors also declined with commitment to the myeloid lineage, but Frizzled 1 emerged on common myeloid progenitors (CMP). Also noteworthy is the fact that stem/progenitor cells expressed Frizzled 4, a receptor utilized by Wnt5a for canonical signaling (29). These results indicate how maturing lymphoid lineage cells might become less responsive to Wnt signaling as they differentiate.
Figure 8.
Frizzled receptors diminish along with lymphoid lineage specification.
The indicated progenitors were isolated and semi-quantitative RT-PCR was carried out to amplify transcripts for the indicated Frizzled receptors in each population. The bar graphs represent the results of [α-32P]dCTP incorporation on the linear parts of PCR amplification curves normalized according to β-actin expression.
Discussion
Sex steroids likely contribute to normal steady state regulation as well as pregnancy-related suppression of lymphopoiesis, but progress has been slow in identifying relevant molecular mechanisms. Not only are there multiple cellular targets for hormone action in BM, but many responsive genes. Lymphopoietic activity declines with senescence due to as yet unknown mechanisms, although involvement of age-related hormones has been speculated (49, 50). The present study focused on the hematopoietic microenvironment as represented by stromal cells, and a number of observations point to sFRP1 as one potential mediator of estrogen action. It was hormone inducible in stromal cells that normally support lymphopoiesis, and constitutive in ones that did not. Two recombinant forms of sFRP1 suppressed early stages in B lymphopoiesis while sparing non-lymphoid progenitors. It was similar in this respect to recombinant Wnt3a. These and other studies show that the activity of sFRP1 is context dependent, utilizing the Wnt signaling pathway even when other Wnts are absent.
Much has been learned about founders of the immune system, and it is now possible to identify cells that initially transcribe lymphoid genes. These ELP are included in the rare Lin- Sca1+ c-kitHi Flk2+ CD27+ Thy1.1- VCAM-1- subset of BM. Low level expression of a human μ transgene, TdT, Rag1 and/or IL-7Rα are indications that these progenitors are “primed” for lymphopoiesis, and viable cells enriched on the basis of a Rag1 or an Ikaros reporter are highly potent in this regard (10, 51). The size of this population may be carefully regulated inasmuch as it supplies progenitors needed to replenish T, B, NK and some dendritic cells. Our comparison of pregnant and estrogen treated mice now implicates sex steroids in that process. While Rag1+ ELP appeared to be the most hormone sensitive of BM cells, we also recorded reductions in a companion Rag1- Flk2+ population.
It has been repeatedly documented that the immune system is altered during pregnancy (52, 53). Substantial down-regulation in both T and B lymphopoiesis are also reported (38, 39), and estrogen is likely to play a pivotal role in changes related to maternal immunity. However, there has been little information regarding physiological changes in the earliest stages of lymphopoiesis. Our present data clearly described that the first step of lymphopoiesis in BM are particularly suppressed during pregnancy.
A major goal of this study was to identify molecules involved in the estrogen-induced down-regulation of early stages of lymphopoiesis. Our previous studies showed that the hormone has direct effect on lymphocyte formation in culture (12). However, this does not precisely replicate changes that occur in BM of pregnant or estrogen treated animals. For example, CLP/pro-lymphocytes were particularly sensitive in stromal-free cultures while dramatic changes in more primitive ELP were recorded in the in vivo experiments described above. Consequently, the emphasis of our present study was on the environment and we exploited stromal cells to identify candidate hormone-regulated molecules.
Culture systems that support human B lymphopoiesis are inefficient (54), inspiring a search for better stromal cells. One was completely non-supportive, while commercially available human MSC were more effective than murine stromal cells that are commonly used. These findings were reported in more detail elsewhere (55), and we only used them here as a means of further narrowing our search for hormone mediators. Importantly, sFRP1 was identified as a candidate regulator of lymphopoiesis via independent approaches.
Our previous findings suggested that estrogen induces suppressive factors for lymphopoiesis in stromal cells (14). Gene screening approaches are now highly efficient and yield a surfeit of candidates. Levels of at least 330 genes changed substantially when stromal cells were exposed to estrogen in culture. While either of the two known ER could be utilized, the list of candidate genes was reduced to approximately 160 when stromal cells prepared from ERα-/- mice were stimulated. Several findings and our present results suggest that this receptor is important in regulation of lymphopoiesis (13, 43, and Fig. 4A-D). SFRPs remained among the candidates that were markedly inducible in wild type, but not in ERα-/- stromal cells. Other genes were eliminated from consideration when we found that lymphopoiesis was normally suppressed in BM of the corresponding knockout mice. These included Fas, p53, p21, Nur77 and Bax (unpublished observations).
While sFRPs produced by estrogen stimulated stromal cells can arrest the earliest stages of lymphopoiesis in bone marrow, there may be additional hormone-dependent regulatory mechanisms. For example, estrogen might directly cause sFRP production in some hematopoietic cells. Rolink and colleagues recently found two-fold reductions in bone marrow IL-7 mRNA during pregnancy (56). Residual IL-7 responding progenitor in pregnant mouse marrow expanded when exposed to high concentrations of this essential cytokine. However, pregnancy fluctuations in IL-7 would seem not to account for depletion of the most primitive lymphopoietic cells, because these ELP lack IL-7 receptors (10).
Given their wide tissue distribution, sFRPs could have multiple roles (57). Transcripts for sFRP1 were substantially induced when stromal cells were exposed to estrogen. Stromal cells are rare among maturing blood cells in BM, but hormone treatment caused 1.5 fold increases in sFRP1 expression (Fig. 3E). Targeting of the sFRP1 gene resulted in increased trabecular bone formation (58). It is interesting that osteoblastic stromal cells that line trabecular bone can make sFRP1 (Fig. 3D) because they are thought to provide one niche for HSC (59, 60).
SFRP1 was originally isolated as an extracellular inhibitor for Wnt signaling that contains a CRD homologous to the putative Wnt-binding domain of Frizzled (61). While several studies verified that sFRP1 is a Wnt antagonist, Üren et al also demonstrated a biphasic action of sFRP1, and at low concentrations it potentiates the activity of Wingless, a Wnt homolog in Drosophila (62). Our data (Fig. 7) are consistent with those reports and show that sFRP1 itself may potentially transduce Wnt-like signals in early hematopoietic progenitors when exogenous Wnt ligands are not provided.
Many of the functions ascribed to the 5 sFRPs relate to their interactions with Wnt, but there is accumulating evidence that they can signal independently of Wnt. For example, Bafico et al showed that the CRD of sFRP1 could directly bind to Frizzled (63). Although it was first speculated that the interaction created nonfunctional receptor complexes, later studies revealed that sFRP1 enhanced retinal neurogenesis via Frizzled 2, independently of canonical Wnt signaling (20). Our data indicates that a similar mechanism may be operable in hematopoietic cells. Although Frizzled 2 was not detected in hematopoietic progenitors, sFRP1 may interact with other Frizzled receptors, and directly affect early events in lymphoid differentiation. Interestingly, one early study showed that sFRP2, in contrast to sFRP1, stabilized β-catenin in MCF7 breast cancer cells and enabled the cells to resist TNF-induced apoptosis (64). Comparing expression patterns of Frizzled on MCF7 might provide a hint about which receptor is used by sFRP1 on hematopoietic progenitors, but there are obviously many possibilities.
It is controversial if and how members of the complicated Wnt family regulate hematopoiesis under normal conditions because it has been difficult to design loss of function approaches that could target the 19 Wnt ligands and multiple signaling pathways (65). That is also the case for the sFRP family, comprised of 5 members whose function might be redundant. Eliminating β-catenin is embryonic lethal, but conditional targeting of that and the related γ-catenin genes in hematopoietic cells did not compromise HSC (66, 67). On the other hand, there was evidence that this manipulation failed to block constitutive Wnt signaling (68). Furthermore, artificial β-catenin accumulation retards hematopoiesis and sustained stimulation of this pathway leads to BM failure (47, 48). Wnt signals may normally contribute to stem cell integrity, because introduction of constitutively active β-catenin allowed multipotential cells to be expanded in culture and even made committed progenitors multipotential (22, 24, 25).
Our observations implicate the sFRP family as possible mediators in hormone regulation of the earliest events in lymphopoiesis. Myelo-erythroid progenitors were unaffected by exposure to sFRP1 in culture, suggesting that it is similar to estrogen with respect to lineage specificity. However, further investigation of T, NK and plasmacytoid dendritic lineages is needed to determine if that is the case. The available information suggests that sFRP1 functions in a context dependent fashion, acting as agonists or antagonists, depending on the presence of other ligands and particular receptors. Although the complexity of the Wnt family is intimidating, there is reason to believe that further study will lead to new therapeutic strategies for lymphoid malignancies.
Acknowledgments
We thank Drs. K.L. Medina, Y. Baba and H. Tanaka for helpful discussion, and C.S.R. Meka and F. Katsube for technical expertise. We also thank Drs. H. Harris and C.R. Lyttle at Wyeth Research for providing the ER selective ligands and the ER knockout mice, and Drs N. Sakaguchi and H. Igarashi at Kumamoto University for the Rag1/GFP knock-in mice.
1. This work was partly supported by grants AI20069 and AI058162 from the National Institute of Health.
References
- 1.Busslinger M. Transcriptional control of early B cell development. Annu. Rev. Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg EV, Taghon T. Molecular genetics of T cell development. Annu. Rev. Immunol. 2005;23:601–649. doi: 10.1146/annurev.immunol.23.021704.115737. [DOI] [PubMed] [Google Scholar]
- 3.Medina KL, Pongubala JM, Reddy KL, Lancki DW, Dekoter R, Kieslinger M, Grosschedl R, Singh H. Assembling a gene regulatory network for specification of the B cell fate. Dev. Cell. 2004;7:607–617. doi: 10.1016/j.devcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Adolfsson J, Månsson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Nakano T. Hematopoietic stem cells: generation and manipulation. Trends in Immunol. 2003;24:589–594. doi: 10.1016/j.it.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Kincade PW, Medina KL, Smithson G, Scott DC. Pregnancy: a clue to normal regulation of B lymphopoiesis. Immunol. Today. 1994;15:539–544. doi: 10.1016/0167-5699(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 7.Smithson G, Beamer WG, Shultz KL, Christianson SW, Shultz LD, Kincade PW. Increased B lymphopoiesis in genetically sex steroid-deficient hypogonadal (hpg) mice. J. Exp. Med. 1994;180:717–720. doi: 10.1084/jem.180.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina KL, Kincade PW. Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proc. Natl. Acad. Sci. USA. 1994;91:5382–5386. doi: 10.1073/pnas.91.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat. Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi H, Kouro T, Yokota T, Comp PC, Kincade PW. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc. Natl. Acad. Sci. USA. 2001;98:15131–15136. doi: 10.1073/pnas.011513098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kouro T, Medina KL, Oritani K, Kincade PW. Characteristics of early murine B-lymphocyte precursors and their direct sensitivity to negative regulators. Blood. 2001;97:2708–2715. doi: 10.1182/blood.v97.9.2708. [DOI] [PubMed] [Google Scholar]
- 13.Thurmond TS, Murante FG, Staples JE, Silverstone AE, Korach KS, Gasiewicz TA. Role of estrogen receptor alpha in hematopoietic stem cell development and B lymphocyte maturation in the male mouse. Endocrinology. 2000;141:2309–2318. doi: 10.1210/endo.141.7.7560. [DOI] [PubMed] [Google Scholar]
- 14.Smithson G, Medina K, Ponting I, Kincade PW. Estrogen suppresses stromal cell-dependent lymphopoiesis in culture. J. Immunol. 1995;155:3409–3417. [PubMed] [Google Scholar]
- 15.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 16.Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc. Natl. Acad. Sci. USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin K, Wang S, Julius MA, Kitajewski J, Moos M, Jr., Luyten FP. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc. Natl. Acad. Sci. USA. 1997;94:11196–11200. doi: 10.1073/pnas.94.21.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 19.Collavin L, Kirschner MW. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development. 2003;130:805–816. doi: 10.1242/dev.00306. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez J, Esteve P, Weinl C, Ruiz JM, Fermin Y, Trousse F, Dwivedy A, Holt C, Bovolenta P. SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat. Neurosci. 2005;8:1301–1309. doi: 10.1038/nn1547. [DOI] [PubMed] [Google Scholar]
- 21.Dosen G, Tenstad E, Nygren MK, Stubberud H, Funderud S, Rian E. Wnt expression and canonical Wnt signaling in human bone marrow B lymphopoiesis. BMC Immunol. 2006;7:13. doi: 10.1186/1471-2172-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 23.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baba Y, Garrett KP, Kincade PW. Constitutively active beta-catenin confers multilineage differentiation potential on lymphoid and myeloid progenitors. Immunity. 2005;23:599–609. doi: 10.1016/j.immuni.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baba Y, Yokota T, Spits H, Garrett KP, Hayashi S, Kincade PW. Constitutively active beta-catenin promotes expansion of multipotent hematopoietic progenitors in culture. J. Immunol. 2006;177:2294–2303. doi: 10.4049/jimmunol.177.4.2294. [DOI] [PubMed] [Google Scholar]
- 26.Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4:349–360. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 27.Malhotra S, Baba Y, Garrett KP, Staal FJT, Gerstein R, Kincade PW. Contrasting responses of lymphoid progenitors to canonical and non-canonical Wnt signals. J. Immunol. 2008;181 doi: 10.4049/jimmunol.181.6.3955. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3–independent b-catenin degradation. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikels AJ, Nusse R. Purified Wnt5a Protein Activates or Inhibits β-Catenin—TCF Signaling Depending on Receptor Context. PLoS Biol. 2006;4:0570–0582. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokota T, Kouro T, Hirose J, Igarashi H, Garrett KP, Gregory SC, Sakaguchi N, Owen JJT, Kincade PW. Unique properties of fetal lymphoid progenitors identified according to RAG1 gene expression. Immunity. 2003;19:365–375. doi: 10.1016/s1074-7613(03)00231-0. [DOI] [PubMed] [Google Scholar]
- 31.Kuwata N, Igarashi H, Ohmura T, Aizawa S, Sakaguchi N. Cutting edge: absence of expression of RAG1 in peritoneal B-1 cells detected by knocking into RAG1 locus with green fluorescent protein gene. J. Immunol. 2001;163:6355–6359. [PubMed] [Google Scholar]
- 32.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shughrue PJ, Askew GR, Dellovade TL, Merchenthaler I. Estrogen-binding sites and their functional capacity in estrogen receptor double knockout mouse brain. Endocrinology. 2002;143:1643–1650. doi: 10.1210/endo.143.5.8772. [DOI] [PubMed] [Google Scholar]
- 34.Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ER alpha and ER beta, in estrogen target tissues in vivo through the use of an ER alpha-selective ligand. Endocrinology. 2002;143:4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- 35.Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, Frail DE, Henderson RA, Zhu Y, Keith JC., Jr. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144:4241–4249. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 36.Kondo M, Weissman IL, Akashi K. Identification of Clonogenic Common Lymphoid Progenitors in Mouse Bone Marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 37.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 38.Medina KL, Smithson G, Kincade PW. Suppression of B lymphopoiesis during normal pregnancy. J. Exp. Med. 1993;178:1507–1515. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rijhsinghani AG, Bhatia SK, Tygrett LT, Waldschmidt TJ. Effect of pregnancy on thymic T cell development. Am. J. Reprod. Immunol. 1996;35:523–528. doi: 10.1111/j.1600-0897.1996.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 40.LeBien TW. Fates of human B-cell precursors. Blood. 2000;96:9–23. [PubMed] [Google Scholar]
- 41.Nishihara M, Wada Y, Ogami K, Ebihara Y, Ishii T, Tsuji K, Ueno H, Asano S, Nakahata S, Maekawa T. A combination of stem cell factor and granulocyte colony-stimulating factor enhances the growth of human progenitor B cells supported by murine stromal cell line MS-5. Eur. J. Immunol. 1998;28:855–864. doi: 10.1002/(SICI)1521-4141(199803)28:03<855::AID-IMMU855>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 42.Oritani K, Medina KL, Tomiyama Y, Ishikawa J, Okajima Y, Ogawa M, Yokota T, Aoyama K, Takahashi I, Kincade PW, Matsuzawa Y. Limitin: An interferon-like cytokine that preferentially influences B-lymphocyte precursors. Nat. Med. 2000;6:659–666. doi: 10.1038/76233. [DOI] [PubMed] [Google Scholar]
- 43.Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J. Immunol. 1998;161:27–34. [PubMed] [Google Scholar]
- 44.Pietrangeli CE, Hayashi S, Kincade PW. Stromal cell lines which support lymphocyte growth: characterization, sensitivity to radiation and responsiveness to growth factors. Eur. J. Immunol. 1988;18:863–872. doi: 10.1002/eji.1830180606. [DOI] [PubMed] [Google Scholar]
- 45.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 46.Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Mol. Cell Biol. 1994;14:6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive β-catenin activation. Nat. Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 48.Kirstetter P, Anderson K, Porse BT, Jacobsen SEW, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 49.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J. Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 50.Montecino-Rodriguez E, Dorshkind K. Evolving patterns of lymphopoiesis from embryogenesis through senescence. Immunity. 2006;24:659–662. doi: 10.1016/j.immuni.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida T, Ng SY, Zuniga-Pflucker JC, Georgopoulos K. Early hematopoietic lineage restrictions directed by Ikaros. Nat. Immunol. 2006;7:382–391. doi: 10.1038/ni1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falkoff R. Maternal immunologic changes during pregnancy: a critical appraisal. Clin. Rev. Allergy. 1987;5:287–300. [PubMed] [Google Scholar]
- 53.Poole JA, Claman HN. Immunology of pregnancy. Implications for the mother. Clin. Rev. Allergy. Immunol. 2004;26:161–170. doi: 10.1385/CRIAI:26:3:161. [DOI] [PubMed] [Google Scholar]
- 54.Kouro T, Yokota T, Welner R, Kincade PW. In vitro differentiation and measurement of B cell progenitor activity in culture. Current Protocols in Immunology. 2005:22F.2.1–22F.2.12. doi: 10.1002/0471142735.im22f02s66. [DOI] [PubMed] [Google Scholar]
- 55.Ichii M, Oritani K, Yokota T, Nishida M, Takahashi I, Shirogane T, Ezoe S, Saitoh N, Tanigawa R, Kincade PW, Kanakura Y. Regulation of human B lymphopoiesis by the transforming growth factor-beta superfamily in a newly established coculture system using human mesenchymal stem cells as a supportive microenvironment. Exp. Hematol. 2008;36:587–597. doi: 10.1016/j.exphem.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Bosco N, Ceredig R, Rolink A. Transient decrease in interleukin-7 availability arrests B lymphopoiesis during pregnancy. Eur. J. Immunol. 2008;38:381–390. doi: 10.1002/eji.200737665. [DOI] [PubMed] [Google Scholar]
- 57.Leimeister C, Bach A, Gessler M. Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech. Dev. 1998;75:29–42. doi: 10.1016/s0925-4773(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 58.Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol. Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 60.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 61.Finch PW, He X, Kelley MJ, Üren A, Schaudies RP, Popescu NC, Rudikoff S, Aaronson SA, Varmus HE, Rubin JS. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc. Natl. Acad. Sci. USA. 1997;94:6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Üren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. Secreted Frizzled-related Protein-1 Binds Directly to Wingless and Is a Biphasic Modulator of Wnt Signaling. J. Biol. Chem. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- 63.Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA. Interaction of Frizzled Related Protein (FRP) with Wnt Ligands and the Frizzled Receptor Suggests Alternative Mechanisms for FRP Inhibition of Wnt Signaling. J. Biol. Chem. 1999;274:16180–16187. doi: 10.1074/jbc.274.23.16180. [DOI] [PubMed] [Google Scholar]
- 64.Melkonyan HS, Chang WC, Shapiro JP, Mahadevappa M, Fitzpatrick PA, Kiefer MC, Tomei LD, Umansky SR. SARPs: a family of secreted apoptosis-related proteins. Proc. Natl. Acad. Sci. USA. 1997;94:13636–13641. doi: 10.1073/pnas.94.25.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staal FJ, Clevers HC. WNT signalling and haematopoiesis: a WNT-WNT situation. Nat. Rev. Immunol. 2005;5:21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- 66.Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koch U, Wilson A, Cobas M, Kemler R, MacDonald HR, Radtke F. Simultaneous loss of β- and γ-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 68.Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, Huelsken J, Held W. Long-term, multilineage hematopoiesis occurs in the combined absence of β-catenin and γ-catenin. Blood. 2007;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]