Abstract
The reaction of oligosaccharide isonitriles with peptide thioacids in the presence of bulky thiophenol as activator to provide N-linked glycopeptides at room temperature is described.
A central focus of our laboratory is the development of enhanced methods for the synthesis of glycopeptides and glycoproteins.1 In this regard, we have taken note of the paucity of techniques for the efficient merger of complex glycan and peptidyl domains. Although recent years have seen breakthrough advances in one's capacity to synthesize large peptide and glycan units in isolation, the efficient merger of these fragments remains a daunting challenge. Standard glycan-peptide coupling techniques, which typically involve appendage of the carbohydrate to an aspartate residue through Lansbury2 or other standard aspartylation conditions to provide N-linked glycans, are complicated by the poor nucleophilicity of the anomeric terminus of the glycan domain, as well as by the poor solubility of the protein or peptide fragments. The efficiency of aspartylation may be compromised by a competing side reaction where, in the presence of the requisite base, the peptide undergoes ring closure to provide an aspartimide side product.3 The development of more efficient aspartylation methods thus remains an important goal.
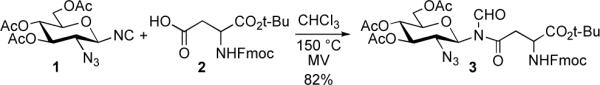
Toward this end, we recently disclosed the development of a novel isonitrile-based “two-component coupling” (2CC) reaction. As outlined in Scheme 1, we have been able to accomplish 2CC-based aspartylation between a monosaccharide isonitrile 1 and an aspartic acid 2, through microwave heating at 150 °C, to provide glycopeptide 3 in 85% yield.4
Scheme 1.
As shown in Scheme 1, the 2CC reaction originally required high temperatures, which could well be problematic for with maintenance of sensitive polypeptidic glycan structures. We also sought to develop a milder version of the 2CC reaction that could be applied to the research objective at hand.5 Chupp and coworkers had reported on the reaction of a very simple thio acid with an isonitrile at room temperature to afford N-thioformyl amide in modest yields.6 Our group7 has already successfully synthesized a series of dipeptides in good yields at room temperature, by employing a similar strategy. Unfortunately, this method suffers a significant limitation when extended to the coupling of dipeptide thioacids with dipeptide isonitriles, presumably due to the competing formation of oxazolones and thioanhydrides. With these issues in mind, we hoped to develop a modified 2CC protocol, which might allow for efficient glycan aspartylation at room temperature.4-5
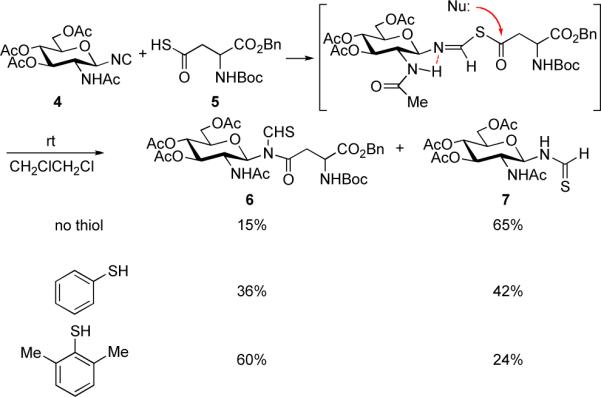
Our initial attempts to couple monosaccharide isonitrile 4 with aspartic thioacid 57 provided the desired N-thioformyl glycopeptide 6 in poor yield, due to formation of high levels of thioformamide 7 (Scheme 2). In the light of earlier precedent, we explored the introduction of thiophenol to assist in the overall addition rearrangement sequence. We were pleased to discover that the coupling yield was increased to 36% in the presence of thiophenol as activator. When 2,6-dimethyl thiophenol was utilized as the mediating agent, the coupling efficiency was further improved (60%). Nonetheless, significant amounts of 7 were still observed. We surmised that perhaps the hydrogen atom of the -NHAc group was associating with the lone pair of the adjacent nitrogen of the FCMA, thus activating it for thioformamide formation to nucleophilic attack.
Scheme 2.
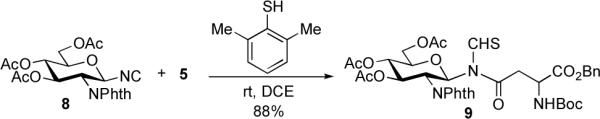
Accordingly, we prepared the -NPhth containing monosaccharide isonitrile, 8.8 Indeed, coupling of 8 with aspartic thioacid 5, in the presence of bulky 2,6-dimethyl thiophenol as activator, proceeded smoothly to provide the desired product 9 in 88% yield (Scheme 3). Notably, little or no thioformamide side product was observed.
Scheme 3.
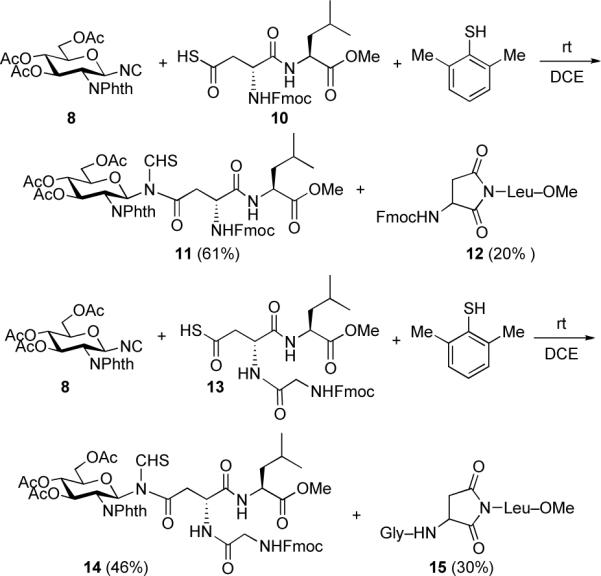
Armed with these encouraging findings, we sought to prepare more complex N-thioformyl glycopeptides (Scheme 4). The required dipeptide thioacid 10 and tripeptide thioacid 13 were synthesized from a previously known procedures.7 In the event, monosaccharide isonitrile 8 was coupled with dipeptide thioacid 10 under the above described conditions (DCE, 2,6-dimethyl thiophenol, rt). This reaction led to formation of the desired product, 11, in 61% yield. The modest yield reflected the formation of still substantial levels of undesired aspartimide side product 12.3 Coupling of 8 with tripeptide thioacid 13 provided N-linked glycopeptide 14 in 46% yield.
Scheme 4.
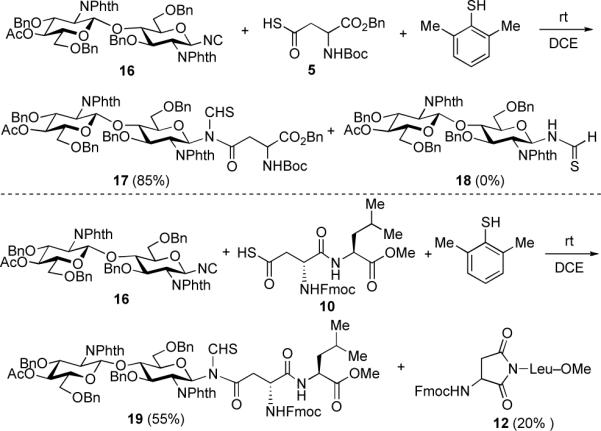
To evaluate the scope of this methodology for the assembly of more complex glycopeptides, we prepared disaccharide isonitrile 16 from a known procedure,8 taking advantage of the feasibility of reducing an anomeric azide to the corresponding amine under basic conditions without affecting the acetate group. The coupling of 16 with aspartic thioacid 5 was performed in the presence of bulky 2,6-dimethyl thiophenol as a mediator, to afford the N-thioformyl glycopeptide 17 in excellent yield without formation of thioformamide (18). Similarly, aspartylation of 16 with dipeptide 10 provided 19 in 55% yield, along with the aspartimide side product, 12 (Scheme 5).
Scheme 5.
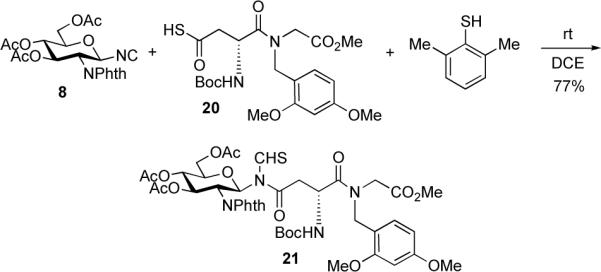
We next sought to identify an appropriate peptide protecting group which would mitigate against aspartimide formation and be compatible with other protecting groups as well as with the glycosidic bond. It seemed that a 2,4-dimethoxy benzyl group would indeed fulfill this demand. Happily, the reaction of 8 with protected dipeptide thioacid 20, in the presence of 2,6-dimethyl thiophenol, afforded the fully protected glycopeptide 21 in 77% yield (Scheme 6). Notably, no aspartimide or thioformamide side products were observed.
Scheme 6.
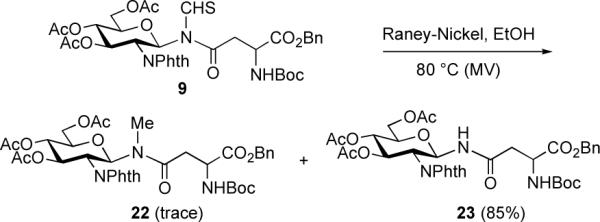
N-methylated natural peptides could well be valuable in the development of peptide-based drugs, due to their remarkable biological and pharmacological profiles, such as long in vivo half-life and bioavailability.9-10 The direct adduct of our 2CC aspartylation protocol is an N-thioformyl amide (cf. 9). We hoped to expand the general utility of this protocol. It could be valuable to convert the thioformyl function to an N-Me group. Toward this end, we followed a precedent by Chupp6 and coworkers, using Raney-nickel as catalyst. Unfortunately, our initial attempts to employ those conditions were unsuccessful. Careful monitoring of the reaction progress indicated that the starting material was very sensitive to base. When the Raney-nickel catalyst was washed to neutrality with water directly prior to the reaction, the reduction of 9 proceeded at 80 °C (microwave, EtOH) to afford N-linked glycopeptide 23 as the major product (85% yield), along with trace amounts of N-methyl glycopeptide 22 (Scheme 7).
Scheme 7.
In summary, we have developed a modified 2CC aspartylation protocol which allows for the synthesis of N-thioformyl glycopeptides in good efficiency. These thioformyl groups can be converted to N-formyl groups. The latter can be converted to NH functions as previously described. The results of further investigations in glycopeptide synthesis will be disclosed in due course.
Supplementary Material
Acknowledgments
This work was supported by the NIH (CA103823 to S.J.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herzner H, Reipen T, Schulz M, Kunz H. Chem. Rev. 2000;100:4495–4537. doi: 10.1021/cr990308c. [DOI] [PubMed] [Google Scholar]
- 2.Cohen-Anisfeld ST, Lansbury PT. J. Am. Chem. Soc. 1993;115:10531–10537. [Google Scholar]
- 3.a) Bodanszky M, Natarajan S. J. Org. Chem. 1975;40:2495–2499. doi: 10.1021/jo00905a016. [DOI] [PubMed] [Google Scholar]; b) Bodanszky M, Kwei JZ. Int. J. Pept. Protein Res. 1978;12:69–74. [PubMed] [Google Scholar]
- 4.Li X, Danishefsky SJ. J. Am. Chem. Soc. 2008;130:5446–5448. doi: 10.1021/ja800612r.; Li X, Yuan Y, Berkowitz w. F., Todaro LJ, Danishefsky SJ. J. Am. Chem. Soc. 2008;130:13222–13224. doi: 10.1021/ja8047078.; Li X, Yuan Y, Kan C, Danishefsky SJ. J. Am. Chem. Soc. 2008;130:13225–13227. doi: 10.1021/ja804709s.. We are also now pursuing the possibility that this 2CC methodology could provide an opportunity for the development of alternative aspartylation methods which might minimize formation of aspartimide side product
- 5.Wu X, Li X, Danishefsky SJ. Tetrahedron Lett. 2009;50:1523–1525. doi: 10.1016/j.tetlet.2009.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chupp JP, Leschinsky KL. J. Org. Chem. 1975;40:66–71. [Google Scholar]
- 7.Yuan Y, Zhu JL, Li X, Wu X, Danishefsky SJ. Tetrahedron Lett. 2009;50:2329–2333. doi: 10.1016/j.tetlet.2009.02.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiesa MV, Schmidt RR. Eur. J. Org. Chem. 2000:3541–3554. [Google Scholar]
- 9.Gilon C, Dechantsreiter MA, Burkhart F, Friedler A, Kessler H. Synthesis of peptides and peptidomimetics. In: Goodman M, Felix A, Moroder L, Tonolio C, editors. Houben-Weyl Methods of Organic Chemistry. E22c. GeorgThiemeVerlag; Stuttgart, Germany: 2002. pp. 215–291. [Google Scholar]
- 10.Chatterjee J, Gilon C, Hoffman A, Kessler H. Acc. Chem. Res. 2008;41:1331. doi: 10.1021/ar8000603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


