Abstract
Objective To test whether an association between pain response and depression in females is present during preadolescence using a controlled pain stimulus and a clinically relevant assessment of depressive symptoms. Method In a sample of 232 girls, pain threshold and tolerance were assessed at age 10 years using the cold pressor task, and a diagnostic interview was used to assess depression symptoms at 10 and 11 years of age. Results Response to pain at age 10 was associated with depressive symptoms at ages 10 and 11; race and pubertal stage moderated the association. Pain response and depression were more strongly associated among girls who had reached advanced stages of pubertal development and among European American girls. Conclusions The results add to the existing literature on the co-occurrence of depression and pain by demonstrating modest but consistent concurrent and prospective associations between response to pain and depression among girls during preadolescence.
Keywords: depression, girls, pain, preadolescence, puberty, race.
Individual differences in pain and depression are robustly related in adults, with each condition being associated with the severity of, impairment from, and service use for the other condition (Bair, Robinson, Katon, & Kroenke, 2003). The co-occurrence of pain and depression is common, with high rates observed in both psychiatric (Munoz et al., 2005) and primary care settings (Lepine & Briley, 2004). There also are significant sex differences in the experience of pain and depression. Compared with males, females report more depression symptoms and more pain, both naturalistically (Haugland, Wold, Stevenson, Aaroe, & Woynarowska, 2001) and in response to a pain stimulus in a laboratory (Jackson, Lezzi, Chen, Ebnet, & Eglitis, 2005). Individuals who experience pain and depressive symptoms are high rate health care consumers who use more general medical services, but fewer mental health services than individuals with depression only (Bao, Strum, & Croghan, 2003). As such, the co-occurrence of pain and depression is an issue of considerable public health significance, especially for females.
The etiology of the co-occurrence of pain and depression is not known. Individual differences in depression and pain experience may be causally related, may co-occur relatively independently, or may covary over time implying a possible subtype of depressive disorder. As the biological systems involved in both pain and mood regulation are becoming more clearly articulated, it appears that the neural circuitry involved in either the processing and/or regulation of pain and depression overlaps to some extent (Brannan et al., 2005; Eisenberger & Lieberman, 2004). If the systems accounting for differences in pain response do overlap, then as individual differences in depression symptoms emerge, so too should individual differences in pain response. Establishing the developmental timing of the association between depression and pain response is important for generating testable hypotheses about the mechanisms by which these two psychological processes are related.
Thus far, however, there is only indirect evidence that pain and depressive symptoms are associated during childhood and adolescence. For example, Egger and colleagues (Egger, Costello, Erkanli, & Angold, 2004) reported that girls with depression were close to 13 times more likely to report musculoskeletal pains and four times more likely to report headaches than girls without depression. But there are other developmental clues about the timing of the co-occurrence of individual differences in response to pain and depression. Many individuals with depressive disorders experience the onset of depression during adolescence, which is when sex differences in depression emerge. Interestingly, there also are age-related changes in pain tolerance (Lu et al., 2005) and physical complaints (LeResche, Mancl, Drangsholt, Saunders, & Von Korff, 2005), with sex differences in physical complaints emerging during adolescence (Kolip, 1997; LeResche et al., 2005). Thus, pubertal development may play a role in the interface between pain and depression. To date, however, there have been no studies in which depression and pain response are assessed using independent methods and operational definitions. Measuring such differences using rigorous, reliable and valid measures would add significantly to efforts aimed at testing the hypothesis that high rates of co-occurrence of individual differences in pain response and depression emerge in childhood. Moreover, establishing whether pubertal stage moderates the association between pain response and depression would provide some basis for exploring the role of pubertal hormones in the development of individual differences in pain, depression, and their co-occurrence.
We have previously reported that African-American girls have higher levels of depression symptoms than European-American girls (Keenan et al., in press), which is consistent with findings from several other studies. This suggests that there may be specific socio-cultural stressors for African Americans, such as living in a low-income environment, that increase the possibility of transitioning from vulnerability, to being symptomatic. Such stressors may impact a variety of biological systems, some of which may affect pain sensation and depression. In fact, African-American adults usually report higher pain ratings and demonstrate greater pain sensitivity in response to a pain stimulus than other racial groups (Campbell, Hughes, Girdler, Maixner, & Sherwood, 2004; Klatzkin, Mechlin, Bunevicius, & Girdler, 2007; Weisse, Foster, & Fisher, 2005). Whether such racial differences are due to differences in socio-cultural factors, such as living in a low-income environment is not known. Controlling for poverty would partially answer this question. Nor is it known whether higher pain ratings among African Americans can be explained by higher ratings of depression; the threshold and tolerance of pain may be lower only among those individuals with high levels of depression.
In the present study, we begin to address these specific issues by testing whether pain response using a controlled pain stimulus is associated with concurrent and later depressive symptoms using a clinically meaningful assessment of DSM-IV depressive symptoms in a nonclinical sample of preadolescent girls. We hypothesize that depression and pain response at age 10 will be associated with depressive symptoms at age 10 and at age 11, with higher levels of depression being associated with a lower pain threshold and lower pain tolerance. We further hypothesize that pubertal stage of development and race will moderate the association between pain response and depression, with African-American girls and girls at more advanced stages of pubertal maturation showing a stronger association between pain response and depressive symptoms.
Method
Sampling of Participants
Participants are girls and their biological mothers recruited from the Pittsburgh Girls Study (PGS), for which a stratified, random household sampling, with over-sampling of households in low-income neighborhoods, was used to identify girls who were between the ages of 5 and 8 years. Neighborhoods in which at least 25% of the families were living at or below the poverty level were fully enumerated (i.e., all homes were contacted to determine if the household contained an eligible girl), and a random selection of 50% of the households in the remaining neighborhoods were enumerated during 1998 and 1999. The enumeration identified 3,118 separate households in which an eligible girl resided. From these households, families who moved out of state and families in which the girl would be age-ineligible by the start of the study were excluded. When two age-eligible girls were enumerated in a single household, one girl was randomly selected for participation. Of the 2,992 eligible families, 2,875 (96%) were successfully recontacted to determine their willingness to participate in the longitudinal study, and 85% of those families agreed to participate resulting in a sample of 2,451 (see Keenan, Hipwell, Duax, Stouthamer-Loeber, & Loeber, 2004 for more details).
We selected girls to participate in the Emotions sub-study (PGS-E) from the youngest participants in the PGS who either screened high on measures of depressive symptoms by their self- and parent-report at age 8, or who were included in a random selection from the remaining girls. This sampling strategy was used in order to increase the base rate of depression as the girls moved into adolescence. The measures used to screen for depressive symptoms were the Short Moods and Feelings Questionnaire (Angold, Costello, Messer, & Pickles, 1995) and the Child Symptom Inventory (Gadow & Sprafkin, 1996). Girls whose scores fell at or above the 75th percentile by their own report, their mother's report, or by both informants comprised the screen high group (n = 135). There were significantly more African-American than European-American girls in the screen high group. One hundred and thirty-six girls were randomly selected from those scoring below the 75th percentile and were matched to the screen high group on race. Eight families were not eligible at the time of recruitment for the PGS-E because the biological mother had died, the family had moved, or the family was no longer participating in the main study and could not be contacted. Of the 263 remaining eligible families, 232 (88.2%) agreed to participate and completed the laboratory assessment, 25 (9.5%) families refused to participate, and 6 (2.3%) agreed but could not be scheduled for an assessment.
In the present study, we include data from the second and third years of the study when the girls were 10 and 11 years of age, respectively. Pain response was measured at age 10. At age 10, 227 (97.8%) girls completed the assessment, 4 (1.7%) declined to participate, and 1 (0.4%) could not be scheduled, and one girl had moved out of state and was interviewed by phone. Thus, the CPT was administered to 226 girls at age 10. At age 11, 225 (97.0%) girls completed the assessment. The total number of girls who completed the CPT at age 10 and who were administered the diagnostic interviews at ages 10 and 11 was 224. These are the participants included in the present study.
The average age of the mothers was 35.7 years (SD = 7.0). Most of the mothers (71.9%) were single parents, and 16.4% had received <12 years of education. Fifty-two percent of families received some form of public assistance (e.g., food stamps, Medicaid, or monies from public aid). Families of African-American/Multi-racial girls were more likely than families of European-American girls to be receiving public assistance (63.1% vs. 26.1%; χ2 = 2.649, p < .001).
Procedures
At age 10, girls and their mothers completed an assessment of DSM-IV symptoms of depression, and girls were administered the Cold Pressor Task (CPT) to assess individual differences in pain response. At age 11, the girls and their mothers were again administered an assessment of DSM-IV symptoms of depression. Written informed consent was obtained. The University of Pittsburgh Institutional Review Board approved all study procedures. Assessments were conducted by research assistants who were European American females in their early 20s.
Measures
Assessment of Depression
Current symptoms of depression (i.e., past month) were measured using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997), a semi-structured diagnostic interview administered to both the girl and her mother. In the K-SADS-PL, symptoms of depression are rigorously assessed in terms of being present most of the time, and the interviewer is trained to probe for information necessary to determine whether a behavior meets symptom criteria. All symptoms of depression were assessed, regardless of whether disturbance in mood (i.e., sadness or irritability) or anhedonia were endorsed. We used symptom counts of depression as dependent measures in the present study because rates of major depressive disorder were low, and because we were interested in testing whether associations between pain response and depressive symptoms could be observed before the onset of major depression. We also used parent and child report separately. The level of association between the two informants was statistically significant but relatively low at age 10 (Spearman's ρ = .27), thus combining informants may not be indicated. In addition, keeping maternal reports separate from child reports provided two independent tests of the association between response to pain and depression symptoms.
The reliability of the K-SADS-PL was assessed by having a second interviewer listen to, and code, responses from the digital video of the K-SADS-PL interview to assess inter-rater agreement. Twenty-five percent of the interviews were randomly selected for this purpose. Intra-class correlation coefficients for total number of symptoms were .94 and .98 by caregiver report and .96 and .97 by child report at ages 10 and 11, respectively.
Assessment of Response to Pain
We assessed individual differences in pain response using the CPT, which has been used with child and adolescent populations (Piira, Taplin, Goodenough, & von Baeyer, 2002; Tsao, Glover, Bursch, Ifekwunigwe, & Zeltzer, 2002; von Baeyer, Piira, Chambers, Trapanotto, & Zeltzer, 2005). During the consent process the CPT was explained to both the mother and the girl. After the mother left the room the research assistant restated the description of the CPT to the girl by describing that the girl would be asked to put her dominant arm in a tank of cold water with her palm open and facing up, and with her arm submerged past her wrist but below her elbow. She was told that she should keep her arm in the water for as long as possible but to remove it if it becomes too uncomfortable for her. In a seated position, the participant first immersed her dominant arm in a tank of warm water (37°C) for 2 min, to control for differences in baseline body temperature, and then in the tank of cold water (10°C) for a maximum of 3 min. In order to maintain the temperature in the latter tank, water was circulated through ice by a pump. The pump and ice were separated from the immersion chamber by a mesh screen.
Participants verbally recorded their level of physical discomfort every 15 s using the Faces Pain Scale-Revised (FPS-R; Hicks, von Baeyer, Spafford, van Korlaar, & Goodenough, 2001) for a total of 12 pain ratings. When the Faces Pain Scale was originally developed, smiling or crying faces were not included as anchor points to ensure that pain, rather than affect, was being measured (Bieri, Reeve, Champion, Addicoat, & Ziegler, 1990). The FPS-R contains six faces displaying differing levels of pain labeled 0, 2, 4, 6, 8, and 10. The FPS-R is highly correlated with pain ratings on a visual analog scale (r = .93) and demonstrates sex and age invariance (Hicks et al., 2001). Two variables were generated from the CPT: immersion time in seconds, which we refer to as ‘pain tolerance,’ and latency to highest pain rating over the 12 15-s interval pain ratings, which we refer to as ‘pain threshold’ (girls who withdrew their arms prematurely provided a final pain rating at withdrawal). The range of scores for the pain threshold was 1 (those who reached their highest pain rating in the first 15 s interval) to 12 (those who reached their highest pain rating in the final 15-s interval). Pain tolerance and threshold were moderately correlated (Spearman's ρ = .48), providing evidence of construct validity, but also indicating a sufficient level of independence between the two measures.
Assessment of Pubertal Development
Pubertal development was measured using maternal report on the Peterson Physical Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988). The PDS includes four questions about growth spurt, body hair, breast development, and changes in skin scored on 4-point scales and one yes/no question about onset of menstruation. In the present study, we used maternal report on the PDS, which in comparison with child self-report has been shown to be more closely associated with results from a physical exam (Dorn, Dahl, Woodward, & Biro, 2006). Girls were classified into four groups: prepubertal (n = 32), beginning pubertal (n = 30), mid-pubertal (n = 144), and advanced/postpubertal (n = 18) based on categories developed by Crockett (unpublished data). This approach has been widely used in other studies (e.g., Ge, Conger, & Elder, 2001), is consistent with the normal sequence of pubertal development identified by Tanner (1962) and has shown good reliability and validity (Robertson et al., 1992) and convergent validity with Tanner staging by physical exam (Brooks-Gunn, Warren, Rosso, & Gargiulo, 1987). The main difference is that the Crockett staging includes menarche in the algorithm and the Tanner staging does not.
Assessment of Race
Sixty-seven girls (29.9%) were identified by their mothers as European American only, 146 (65.2%) were identified by their mothers as African American only, 10 girls (4.5%) were identified as African American and another race, and one girl (0.4%) was identified as Asian American only. The 10 girls identified by mothers as African American and another race significantly differed from European American girls in their level of depression symptoms by maternal and youth report, but did not differ from African-American girls who were not identified as multi-racial. Thus, these 10 girls were combined with the 146 African-American girls and the one Asian-American girl was excluded from analyses involving race.
Statistical Analyses
The analyses were carried out in three steps. First, we tested the association between pain threshold and tolerance at age 10 and youth and maternal report of depressive symptoms at ages 10 and 11 by computing Spearman's ρ coefficients for nonparametric data. Second, we tested the main effects of race and pubertal stage of development on pain response using analysis of variance. Third, the potential moderating effect of race and pubertal stage of development on the association between pain response and depression symptoms was tested by computing generalized linear models with a Poisson distribution specified for the dependent measure of symptom counts and robust estimators of the standard errors.
Results
Descriptive Statistics
Descriptive statistics for depression symptoms and pain response are presented in Table I. Most girls in the present sample reported one or more symptoms of depression, indicating that the screen used to enrich the sample for depression symptoms was successful. The most commonly endorsed symptoms by youth report at age 10 years were: sleep disturbance 26.2%; appetite disturbance 25.3%; disturbance in concentration 22.9%; fatigue 16.3%; and feelings of worthlessness or guilt 11.9%. The most commonly endorsed symptoms by caregiver report were: sleep disturbance 26.2%; appetite disturbance 36.6%; disturbance in concentration 26.4%; and guilt 15.4%. African-American girls reported significantly higher levels of depressive symptoms than European-American girls (mean = 1.5 vs. 0.8, p < .001 by youth report and mean = 1.3 vs. 0.7, p < .01 by caregiver report).
Table I.
Descriptive Statistics for Depression Symptoms and Pain Response (n = 224)
| Mean | SD | Range | |
|---|---|---|---|
| Depression symptoms age 10 (youth) | 1.28 | 1.53 | 0–7 |
| Depression symptoms age 11 (youth) | 1.29 | 1.66 | 0–9 |
| Depression symptoms age 10 (mother) | 1.14 | 1.31 | 0–7 |
| Depression symptoms age 11 (mother) | 1.06 | 1.33 | 0–8 |
| Pain threshold (interval in which highest pain rating is reported) | 3.31 | 2.38 | 1–11 |
| Pain tolerance (immersion time in seconds) | 112.83 | 72.56 | 3–180 |
Pain threshold measured via 12, 15-s interval pain ratings with a possible range of scores from 1 (those who reached their highest pain rating in the first 15-s interval) to 12 (those who reached their highest pain rating in the last 15-s interval).
Univariate Tests of the Association Between Pain Response and Symptoms of Depression
Pain response was concurrently correlated with youth report of depression symptoms at age 10, and prospectively correlated with youth report of depression symptoms at age 11, with correlations ranging from −.185 to −.252 (Table II), representing small effect sizes. Pain response also was associated with caregivers’ reports of the girls’ symptoms of depression concurrently at age 10, and prospectively at age 11, although the association between pain tolerance and age 10 symptoms of depression was only marginally significant (Spearman's ρ = −.108, p = .05) (Table II). The negative correlations indicate that lower pain tolerance and pain threshold are associated with higher levels of depression symptoms.
Table II.
Concurrent and Prospective Associations Between Pain Response and Depression Symptom Counts by Youth and Caregiver Report (n = 224)
| Depression symptom count (youth report) |
Depression symptom count (caregiver report) |
|||
|---|---|---|---|---|
| Pain response (age 10) | Age 10 | Age 11 | Age 10 | Age 11 |
| Pain tolerance | −.185** | −.205** | −.108* | −.213** |
| Pain threshold | −.252** | −.167** | −.183*** | −.184** |
Spearman's ρ; *p = .05; **p < .01.
Effects of Race and Pubertal Stage on Pain Response
African-American girls had lower pain tolerance than European-American girls [means = 106.7 s vs. 128.1 s; F(1, 221) = 4.12, p < .05) and lower pain threshold (mean interval = 3.1 vs. 3.9; F (1,221) = 5.75, p < .05]. This is not surprising given that African-American girls had higher depression symptoms than European-American girls, and depression symptoms were associated with pain response. There was no effect of pubertal stage on pain tolerance (F[3,220] = 0.22, p > .05), or threshold (F[3,220] = 0.23, p > .05).
Multivariate Tests of the Association Between Pain Response and Symptoms of Depression
Generalized linear models were computed with a Poisson distribution specified for the dependent measure of symptom counts and robust estimators of the standard errors. Race, pubertal stage, the two pain response variables, the interaction of pubertal stage and each pain response variable, and the interaction of race and each pain response variable were included as independent measures. Prior to computing interaction terms, the pain threshold and tolerance variables were centered at the mean. Four separate models were tested with youth and caregiver report of depression symptoms at ages 10 and 11 as the dependent measures. In addition, in order to explore whether effects of race were due to the greater representation of African-American families living on low incomes, receipt of public assistance was included in each model.
At age 10 youth reported depressive symptoms were associated with race (B = .439, Wald χ2 = 4.14, p < .05) and the interaction of race and pain tolerance (B = .007, Wald χ2 = 4.91, p < .05). The model fit, as measured by the likelihood ratio chi-square was good (χ2 [9] = 59.10, p < .001) (Table III).
Table III.
Concurrent Association Between Pain Response at age 10 and Youth-reported Depression Symptoms at age 10 (n = 224)
| 95% Wald (CI) |
Hypothesis test |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | B | SE | Lower | Upper | Wald χ2 | df | Sig. |
| (Intercept) | −.142 | 0.8611 | −1.830 | 1.545 | 0.027 | 1 | .869 |
| Public Assistance | .305 | 0.1670 | −0.023 | 0.632 | 3.278 | 1 | .068 |
| Race | .439 | 0.2157 | 0.016 | 0.862 | 4.145 | 1 | .042 |
| Pubertal stage | .140 | 0.1122 | −0.080 | 0.360 | 1.549 | 1 | .213 |
| Pain threshold | −.007 | 0.0073 | −0.022 | 0.007 | 0.981 | 1 | .322 |
| Pain tolerance | −.051 | 0.2759 | −0.592 | 0.489 | 0.035 | 1 | .852 |
| Race × threshold | −.131 | 0.1293 | −0.384 | 0.123 | 1.023 | 1 | .312 |
| Race × tolerance | .007 | 0.0032 | 0.001 | 0.014 | 4.906 | 1 | .027 |
| Puberty × threshold | .071 | 0.0594 | −0.046 | 0.187 | 1.411 | 1 | .235 |
| Puberty × tolerance | −.003 | 0.0014 | −0.005 | −0.000 | 3.628 | 1 | .057 |
Dependent variable: youth-reported depression symptoms at age 10.
Neither of the pain response variables explained variance in caregiver reported symptoms of depression at age 10: receipt of public assistance (B = .303, Wald χ2 = 4.17, p < .05) and race (B = .364, Wald χ2 = 4.17, p < .05) were the only significant correlates.
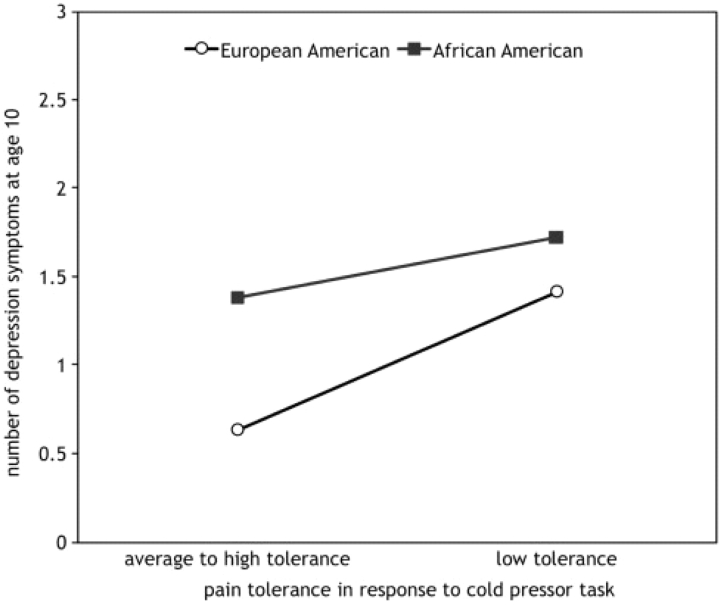
In order to demonstrate the moderation effect and size of the effect of race on the association between pain response and youth-reported depression symptoms the average number of depression symptoms for African-American and European-American girls with low pain tolerance (1 SD below the mean) and average to high tolerance (within 1 SD of the mean or higher) was calculated and plotted. As depicted in Fig. 1, there is a negligible difference in the number of depression symptoms between African-American girls with low (mean = 1.7 symptoms; 95% CI = 1.3–2.1) and average to high (mean = 1.4 symptoms; 95% CI = 1.1–1.7) pain tolerance. In contrast, European-American girls with low pain tolerance had average depression symptoms that were twice as high (mean = 1.4 symptoms; 95% CI = 0.6–2.3) as European American girls with average to high tolerance (mean = 0.6 symptoms; 95% CI = 0.2–1.0). The effect size of the difference is in the small to medium range (η = .26). The fact that the confidence intervals overlap, however, suggests that the interaction needs to be interpreted with caution.
Figure 1.
Interaction between race and pain tolerance on youth-reported symptoms of depression at age 10 (n = 224).
In testing the prospective association between pain response at age 10 and depressive symptoms at age 11 in the multivariate model, we first controlled for depression symptoms at age 10. Youth-reported symptoms at age 11 were predicted by depressive symptoms at age 10 (B = .296, Wald χ2 = 70.07, p < .001), receipt of public assistance (B = .321, Wald χ2 = 4.62, p < .05), race (B = .464, Wald χ2 = 3.93, p < .05), pubertal stage (B = .211, Wald χ2 = 4.09, p < .05), and the interaction of pubertal stage and pain threshold (B = .085, Wald χ2 = 4.10, p < .05) (Table IV). The likelihood ratio chi-square for the overall model was χ2 [10] = 162.54, p < .001.
Table IV.
Prospective Association Between Pain Response at age 10 and Youth-reported Symptoms at age 11 (n = 224)
| 95% Wald (CI) |
Hypothesis test |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | B | SE | Lower | Upper | Wald χ2 | df | Sig. |
| (Intercept) | −.655 | 0.7598 | −2.144 | 0.834 | 0.743 | 1 | .389 |
| MDD symptoms (age 10) | .296 | 0.0353 | 0.227 | 0.365 | 70.073 | 1 | .000 |
| Public Assistance | .321 | 0.1496 | 0.028 | 0.615 | 4.617 | 1 | .032 |
| Race | .464 | 0.2341 | 0.005 | 0.923 | 3.927 | 1 | .048 |
| Pubertal stage | .211 | 0.1042 | 0.006 | 0.415 | 4.087 | 1 | .043 |
| Pain threshold | −.011 | 0.0080 | −0.027 | 0.005 | 1.905 | 1 | .168 |
| Pain tolerance | −.004 | 0.1998 | −0.396 | 0.387 | 0.000 | 1 | .982 |
| Race × threshold | −.140 | 0.1031 | −0.342 | 0.062 | 1.848 | 1 | .174 |
| Race × tolerance | .005 | 0.0039 | −0.002 | 0.013 | 1.848 | 1 | .174 |
| Puberty × threshold | .085 | 0.0422 | 0.003 | 0.168 | 4.105 | 1 | .043 |
| Puberty × tolerance | .000 | 0.0012 | −0.002 | 0.002 | 0.002 | 1 | .965 |
Dependent variable: youth-reported symptoms of depression at age 11.
Caregiver reports of girls’ depression symptoms at age 11 were predicted by depressive symptoms at age 10 (B = .329, Wald χ2 = 97.50, p < .001) and the interaction of pubertal stage and pain threshold (B = −.072, Wald χ2 = 5.26, p < .05). The likelihood ratio χ2 for the overall model was χ2 [10] = 122.72, p < .001.
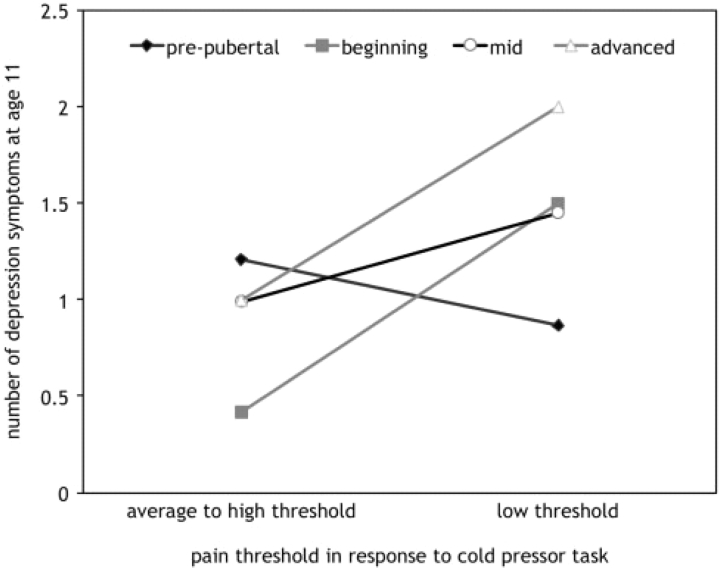
To demonstrate the moderation effect of pubertal state on the association between pain threshold at age 10 on caregivers’ reports of girls’ depression symptoms at age 11 and to provide information on the effect size, the average number of depression symptoms for girls with low pain thresholds (1 SD below the mean) compared to girls with average and high pain thresholds (within 1 SD of the mean or higher) was plotted for each of the four pubertal stages (pre-, beginning, mid-, and advanced). As depicted in Fig. 2, prepubertal girls with low pain thresholds had slightly lower depression symptoms (mean = 0.9 symptoms; 95% CI = −.04 to 1.8) than pre-pubertal girls with average to high pain thresholds (mean = 1.2 symptoms; 95% CI = 0.68–1.7). The reverse was true for girls at the other pubertal stages. For girls at beginning, mid-stages or advanced stages of puberty, low pain threshold predicted higher depression symptoms 1 year later. For example, among girls at the most advanced state of puberty (n = 18), those with low pain thresholds had on average twice as many symptoms of depression 1 year later (mean = 2.0 symptoms; 95% CI = 1.0–3.0) as those with average to high pain thresholds (mean = 1.0 symptoms; 95% CI = 0.2–1.8). The effect size of this difference was in the small to medium range (η = .25). Overlapping confidence intervals indicates that the interaction needs to be interpreted with caution.
Figure 2.
Interaction between pubertal stage and pain threshold at age 10 on maternal report of depression symptoms at age 11 (n = 224).
Discussion
The results from the present study add to the existing literature in several ways. First, in the few studies demonstrating an association between pain and depressive symptoms in children and adolescents, self-report of somatic complaints served as the measure of pain, leaving open the possibility that shared method variance accounted for the association. Using an experimental protocol, such as the CPT to assess individual differences in response to pain, avoids this problem, thus extending the results of previous studies. Moreover, laboratory-based pain reactivity has been associated with individual differences in nurse visits and school absences (Tsao et al., 2002), suggesting that using a controlled stimulus like the CPT can generate ecologically valid data.
Second, depressive symptoms were measured using a DSM-IV based diagnostic interview, for which inter-rater reliability was high. Thus, in contrast to broad-based checklists, the dependent measure in the present study is clinically relevant and specific to depression. Although the rate of depressive disorder is relatively low at this age, even sub-threshold levels of depression symptoms (e.g., two symptoms) are clinically relevant and not benign (Keenan et al., in press). Predicting changes of one or two symptoms during preadolescence from individual differences in response to pain, therefore, is important from a targeted prevention perspective. Third, establishing that pain response and depression are solidly correlated in preadolescence, when rates of depression and pain conditions are relatively low, provides further opportunity to elucidate the developmental co-occurrence of pain and depression before the two phenomena become chronic and difficult to disentangle.
Specifically, individual differences in response to pain and depressive symptoms during preadolescence were modestly associated, and these results were highly consistent across informants and over time. Previous studies of the cold pressor test in children have revealed associations between individual differences in pain-related coping (Lu, Tsao, Myers, Kim, & Zeltzer, 2007), anticipatory anxiety (Tsao et al., 2004) and efficacy about managing pain (Piira et al., 2002) and pain intensity and tolerance. Broader measures of coping, anxiety, and self-efficacy also are related to depression (Garber, 2006), and it may be that these dimensions of psychological functioning mediate the association between depressed mood and pain response. Given the results of the present study, conducting future tests that incorporate these potential mediators is warranted.
Race and pubertal stage of development moderated the association between pain response and symptoms of depression in the present study. African-American girls had both higher levels of depressive symptoms and lower pain tolerance and pain threshold, even after controlling for low income. This is consistent with race effects reported in studies using the CPT with adults (Klatzkin et al., 2007; Weisse et al., 2005). In contrast to our hypothesis, however, the association of pain and depression was stronger for European-American girls than African-American girls, at least concurrently and by youth report, thus raising the possibility that the nature of the association between pain and depression differs by race. As stated earlier, individual differences in depression and pain threshold and tolerance may be causally related, may co-occur relatively independently, or may covary over time implying a possible subtype of depressive disorder. The pattern of results observed in the present study are consistent with the hypothesis that pain and depression typically covary in European Americans, but reflect relatively independent processes in African Americans, although they may still share causal factors. Note, however, that the confidence intervals around the means for depression scores for the European-American girls with high and low pain tolerance were overlapping. In addition, race did not moderate the association between pain response and maternal report of girls’ depressive symptoms. Thus, additional hypothesis testing about the effect of race on the association between depression and pain will be needed to determine whether the moderation effects revealed in the present study are spurious findings. If replicated, then the next step will be to test hypotheses about the mechanisms by which race moderates the association between pain response and depression. For example, differences in cultural beliefs about pain and depression, and differences in experiences in seeking and getting treatment for painful conditions may explain the moderating effects of race.
It is perhaps not surprising that pubertal stage moderated the association between pain response and depressive symptoms. It is impressive, however, that such an effect was captured early in development by both informants and while controlling for age (all girls were the same age). Increases in depressive symptoms among girls appear as early as Tanner stage 3, which is roughly equivalent to mid-puberty, but is most pronounced when girls reach Tanner stage 5 (i.e., postpubertal) (Angold, Costello, & Worthman, 1998). The results of prospective tests of pain response on depression symptoms indicated that pain response and maternal report of depressive symptoms were essentially disassociated among prepubertal girls: at all other stages of pubertal development low pain threshold was associated with higher levels of depression symptoms 1 year later. This suggests that systems that trigger the activity of the adrenal and gonadal axes may also be part of the circuitry involved in the processing and regulation of pain and depression. For example, the developmental-sex differences in pain may be due to the influences of estradiol and progesterone on levels of cortisol and beta-endorphins, both of which influence pain sensation (Laatikainen, 1991; Tsigos & Chrousos, 2002). As an alternative to a more biological interpretation of the moderating effects of puberty, there may be a social–emotional or social cognition interpretation. For example, it is possible that the psychosocial experiences of puberty, and menarche in particular, result in a change in the psychological experience of pain that is independent of age. A limitation of the present study, however, is that the majority of the sample had reached the midlevel of pubertal development and fewer participants represented the more extreme ends. Thus, these associations should be interpreted with caution and require replication in a more diverse sample with respect to pubertal stage before positing more complex models of the effect of pubertal development on the association between pain response and depression.
If the results from the present study are replicable then hypotheses regarding the mechanism by which pain and depression are associated can be proposed and tested. One possibility is that atypicalities in shared neural systems account for the associations observed in the present study. Developmental changes in physical complaints (LeResche et al., 2005), depressive symptoms (Wichstrom, 1999), and cortisol reactivity (Stroud, Papandonatos, Williamson, & Dahl, 2004) in females all appear to occur in mid to late puberty. A substantial amount of research exists linking the functioning of the HPA axis and depression in adults (Plotsky, Owens, & Nemeroff, 1998) and there is some evidence for the same association in children and adolescents (Goodyer et al., 1996). Cortisol concentrations increase significantly following the administration of the CPT in adults (Dixon, Thorn, & Ward, 2004; Mechlin, Maixner, Light, Fisher, & Girdler, 2005), and such increases in cortisol have been associated with self-reports of pain intensity and unpleasantness among females but not among males (Zimmer, Basler, Vedder, & Lautenbacher, 2003). Thus, one could speculate that the sex and age effects on pain, depression, and cortisol reactivity point toward problems in the functioning of the HPA-axis as a potential candidate involved in the developmental etiology of co-occurring pain and depression.
There are a number of study limitations that may have affected the results and therefore should be addressed in future research. The first is the confounding of race and experimenter. In the present study there was complete concordance between sex of subject and experimenter, but typically a lack of concordance between race of subject and experimenter. Although concordance of sex and race does not appear to be associated with pain response to a stimulus, there may be interaction effects. For example, in one study of young adults, race differences in response to the CPT were found only in the context of a female experimenter as opposed to a male experimenter (Weisse et al., 2005). Additional research is needed to explore the nature of the impact of experimenter race on subject reporting.
The second limitation is the lack of assessment of somatic complaints and symptoms. There are a number of studies demonstrating an association between somatic complaints and depressive symptom in children (e.g., Egger et al., 2004). There is also evidence that response to the CPT is associated with more ecologically valid day-to-day experiences of pain (Tsao et al., 2002). Including a measure of somatic complaints and impairment from such complaints in addition to response to the CPT would have broadened the clinical utility of the present results. The third limitation is the lack of inclusion of anxiety symptoms. Although anxiety disorders were assessed, symptoms were only assessed if initial screening criteria were met. Thus, a continuous measure of anxiety symptoms for all girls was not available. Controlling for the variance in somatic complaints due to anxiety symptoms, however, does not wipe out the association between self-reported pain and depression (McCauley, Carlson, & Calderon, 1991). Finally, although the use of the CPT as a controlled pain stimulus is a strength of the study, it is important to note that pain threshold and tolerance in response to the CPT is a subjective experience that can be influenced by a number of individual differences factors such as catastrophic thinking about pain and anticipatory anxiety (e.g., Tsao et al., 2004). Incorporating such measures may provide a clearer picture of the psychological processes linking pain and depression.
In summary, the present results indicate that as early as age 10, individual differences in response to a pain stimulus are associated with current and future depression symptoms in girls. Continued investigation into the adolescent period, when depressive disorders increase among females, is needed to comprehensively test the developmental unfolding of the association of pain and depression and determine the impact of pubertal development on this association.
Acknowledgements
This study was funded by National Institute of Mental Health grant R01 MH66167 to Dr Keenan. The authors thank Christine Chambers and Carl Von Baeyer for consultation on use of the cold pressor task, and the families participating in the Learning About Girls' Emotions Study.
Conflicts of interest: None declared.
References
- Angold A., Costello E. J., Messer S. C., Pickles A. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International Journal of Methods in Psychiatric Research. 1995;5:237–249. [Google Scholar]
- Angold A., Costello E. J., Worthman C. M. Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Bair M. J., Robinson R. L., Katon W., Kroenke K. Depression and pain comorbidity. Archives of Internal Medicine. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Bao Y., Strum R., Croghan T. W. A national study of the effect of chronic pain on the used of health care by depressed persons. Psychiatric Services. 2003;54:693–697. doi: 10.1176/appi.ps.54.5.693. [DOI] [PubMed] [Google Scholar]
- Bieri D., Reeve R. A., Champion D. A., Addicoat L., Ziegler J. B. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: Development, initial validation, and preliminary investigation for ratio scale properties. Pain. 1990;41:139–150. doi: 10.1016/0304-3959(90)90018-9. [DOI] [PubMed] [Google Scholar]
- Brannan S. K., Mallinckrodt C. H., Brown E. B., Wohlreich M. M., Watkin J. G., Schatzberg A. F. Duloxteine 60 mg once daily in the treatment of painful physical symptoms in patients with major depressive disorder. Journal of Psychiatric Research. 2005;39:43–53. doi: 10.1016/j.jpsychires.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J., Warren M. P., Rosso J., Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child Development. 1987;58:829–841. [PubMed] [Google Scholar]
- Campbell T. S., Hughes J. W., Girdler S. S., Maixner W., Sherwood A. Relationship of ethnicity, gender, and ambulatory blood pressure to pain sensitivity: Effects of individualized pain rating scales. The Journal of Pain. 2004;5:183–191. doi: 10.1016/j.jpain.2004.02.305. [DOI] [PubMed] [Google Scholar]
- Crockett L. Pubertal development Scale: Pubertal categories. Pennsylvania State University, Department of Human Development and Family Studies, University Park: Unpublished manuscript; [Google Scholar]
- Dixon K. E., Thorn B. E., Ward L. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: A path analytic description. Pain. 2004;112:188–196. doi: 10.1016/j.pain.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Dorn L. D., Dahl R. E., Woodward H. R., Biro F. Defining the boundaries of early adolescence: A user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10:30–56. [Google Scholar]
- Egger H. L., Costello E. J., Erkanli A., Angold A. Somatic complaints and psychopathology in children and adolescents: Stomach aches, musculoskeletal pains, and headaches. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;38:852–860. doi: 10.1097/00004583-199907000-00015. [DOI] [PubMed] [Google Scholar]
- Eisenberger N. I., Lieberman M. D. Why rejection hurts: A common neural alarm system for physical and social pain. Trends in Cognitive Science. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Gadow K. D., Sprafkin J. Child Symptom Inventory. Stony Brook, NY: State University of New York at Stony Brook; 1996. [Google Scholar]
- Garber J. Depression in children and adolescents: Linking risk research and prevention. American Journal of Preventive Medicine. 2006;31:S104–S125. doi: 10.1016/j.amepre.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Ge X., Conger R. D., Elder G. H. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Goodyer I., Herbert J., Altham P., Pearson J., Secher S., Shiers H. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychological Medicine. 1996;26:245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Haugland S., Wold B., Stevenson J., Aaroe L. E., Woynarowska B. Subjective health complaints in adolescence. European Journal of Public Health. 2001;11:4–10. doi: 10.1093/eurpub/11.1.4. [DOI] [PubMed] [Google Scholar]
- Hicks C. L., von Baeyer C. L., Spafford P. A., van Korlaar I., Goodenough B. The Faces Pain Scale – Revised: Toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Jackson T., Lezzi T., Chen H., Ebnet S., Eglitis K. Gender, interpersonal transactions, and the perception of pain: An experimental analysis. Journal of Pain. 2005;6:228–236. doi: 10.1016/j.jpain.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D. A., Rao U., Flynn C., Moreci P., et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keenan K., Hipwell A. E., Duax J., Stouthamer-Loeber M., Loeber R. Phenomenology of depression in young girls. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1098–1106. doi: 10.1097/01.chi.0000131137.09111.d0. [DOI] [PubMed] [Google Scholar]
- Keenan K., Hipwell A. E., Feng X., Hinze A. E., Babinski D. E., Henneberger A., et al. Subthreshold symptoms of depression in girls are stable and predictive of depressive disorders. Journal of the American Academy of Child and Adolescent Psychiatry. doi: 10.1097/CHI.0b013e3181886eab. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzkin R. R., Mechlin B., Bunevicius R., Girdler S. S. Race and histories of mood disorders modulate experimental pain tolerance in women. Journal of Pain. 2007;8:861–868. doi: 10.1016/j.jpain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolip P. Gender differences in health status during adolescence: A remarkable shift. International. Journal of Adolescent Medicine and Health. 1997;9:9–17. doi: 10.1515/IJAMH.1997.9.1.9. [DOI] [PubMed] [Google Scholar]
- Laatikainen T. J. Corticotropin-releasing hormone and opioid peptides in reproduction and stress. Annals of Medicine. 1991;23:489–496. doi: 10.3109/07853899109150508. [DOI] [PubMed] [Google Scholar]
- Lepine J. P., Briley M. The epidemiology of pain in depression. Human Psychopharmacology. 2004;19:3–7. doi: 10.1002/hup.618. [DOI] [PubMed] [Google Scholar]
- LeResche L., Mancl L. A., Drangsholt M. T., Saunders K., Von Korff M. Relationship of pain and symptoms to pubertal development in adolescents. Pain. 2005;118:201–209. doi: 10.1016/j.pain.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Lu Q., Tsao J. C., Myers C. D., Kim S. C., Zeltzer L. K. Coping predictors of children's laboratory-induced pain tolerance, intensity, and unpleasantness. The Journal of Pain. 2007;8:708–717. doi: 10.1016/j.jpain.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Lu Q., Zeltzer L. K., Tsao J. C., Kim S. C., Turk N., Naliboff B. D. Heart rate mediation of sex differences in pain tolerance in children. Pain. 2005;118:185–193. doi: 10.1016/j.pain.2005.08.008. [DOI] [PubMed] [Google Scholar]
- McCauley E., Carlson G., Calderon R. The role of somatic complaints in the diagnosis of depression in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:631–635. doi: 10.1097/00004583-199107000-00016. [DOI] [PubMed] [Google Scholar]
- Mechlin M., Maixner W., Light K. C., Fisher J. M., Girdler S. S. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosomatic Medicine. 2005;67:948–956. doi: 10.1097/01.psy.0000188466.14546.68. [DOI] [PubMed] [Google Scholar]
- Munoz R. A., McBride M. E., Brnabic A. J. M., Lopez C. J., Hetem L. A. B., Secin R., et al. Major depressive disorder in Latin Americans: The relationship between depression severity, painful somatic symptoms, and quality of life. Journal of Affective Disorders. 2005;86:93–98. doi: 10.1016/j.jad.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Petersen A. C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Piira T., Taplin J. E., Goodenough B., von Baeyer C. L. Cognitive-behavioural predictors of children's tolerance of laboratory-induced pain: Implications for clinical assessment and future directions. Behavior Research and Therapy. 2002;40:571–584. doi: 10.1016/s0005-7967(01)00073-0. [DOI] [PubMed] [Google Scholar]
- Plotsky P. M., Owens M. J., Nemeroff C. B. Psychoneuroendocrinology of depression: Hypothalamic-pituitary-adrenal axis. Psychiatric Clinics of North America. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Robertson E., Skinner M., Love M., Elder G., Conger R., Dubas J., et al. The Pubertal Development Scale: A rural and suburban comparison. Journal of Early Adolescence. 1992;12:174–186. [Google Scholar]
- Stroud L., Papandonatos G., Williamson D., Dahl R. Sex differences in the effects of pubertal development on responses to a corticotropin-releasing hormone challenge: The Pittsburgh Psychobiologic Studies. In: Dahl R. E., Spear L. P., editors. Adolescent brain development: Vulnerabilities and opportunities. New York: Academy of Sciences; 2004. pp. 348–351. [DOI] [PubMed] [Google Scholar]
- Tanner J. M. Growth at adolescence. 2nd. New York: Lippincott; 1962. [Google Scholar]
- Tsao J. C., Glover D. A., Bursch B., Ifekwunigwe M., Zeltzer L. K. Laboratory pain reactivity and gender: Relationship to school nurse visits and school absences. Developmental and Behavioral Pediatrics. 2002;23:217–224. doi: 10.1097/00004703-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Tsao J., Myers C., Craske M., Bursch B., Kim S., Zeltzer L. K. Role of anticipatory anxiety and anxiety sensitivity in children's and adolescents’ laboratory pain responses. Journal of Pediatric Psychology. 2004;29:379–388. doi: 10.1093/jpepsy/jsh041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C., Chrousos G. P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- von Baeyer C. L., Piira T., Chambers C. T., Trapanotto M., Zeltzer L. K. Guidelines for the Cold Pressor Task as an experimental pain stimulus for use with children. Journal of Pain. 2005;6:218–227. doi: 10.1016/j.jpain.2005.01.349. [DOI] [PubMed] [Google Scholar]
- Weisse C. S., Foster K. K., Fisher E. A. The influence of experimenter gender and race on pain reporting: Does racial or gender concordance matter? Pain Medicine. 2005;6:80–87. doi: 10.1111/j.1526-4637.2005.05004.x. [DOI] [PubMed] [Google Scholar]
- Wichstrom L. The emergence of gender difference in depressed mood during adolescence: The role of intensified gender socialization. Developmental Psychology. 1999;35:232–245. [PubMed] [Google Scholar]
- Zimmer C. P., Basler H. D. P., Vedder H., Lautenbacher S. P. Sex differences in cortisol response to noxious stress. Clinical Journal of Pain. 2003;19:233–239. doi: 10.1097/00002508-200307000-00006. [DOI] [PubMed] [Google Scholar]