Abstract
Background: Pancreatic neuroendocrine tumors (PNETs) are uncommon neoplasms that can present with symptoms of hormone overproduction. We evaluated the incidence, prognosis, and temporal trends of PNETs.
Patients and methods: We analyzed all cases of PNETs registered in the Surveillance, Epidemiology, and End Results database from 1973 to 2000. Age-adjusted incidence and survival rates were calculated and survival trends over time were evaluated.
Results: We identified 1483 cases of PNETs. The crude annual incidence per 1 000 000 was 1.8 in females and 2.6 in males and increased with advancing age. The incidence increased over the study period. Most patients (90.8%) had nonfunctional tumors. Advanced stage, higher grade, and age were the strongest predictors of worse survival. Patients with functional tumors had better outcomes than patients with nonfunctional tumors in both univariate and multivariate analysis (P = 0.004). Survival time increased over the period from 1973 to 2000. No differences were seen in the distribution of stage or age at diagnosis among time periods.
Conclusion: PNETs are uncommon neoplasms but the incidence may be increasing. Age, grade, stage, and functional status predict survival in patients with PNETs. Survival has improved over time, but this is not explained by earlier diagnosis or stage migration.
Keywords: epidemiology, incidence, islet cell tumors, pancreatic neuroendocrine tumors
introduction
Pancreatic neuroendocrine tumors (PNETs) are uncommon neuroendocrine neoplasms with reported incidence of <1 per 100 000 persons per year in population-based studies from Europe and Asia [1–7]. Autopsy studies have indicated that these tumors are much more common, ranging from 0.8% to 10% in patients undergoing a postmortem examination, suggesting that people frequently harbor asymptomatic PNETs [8, 9]. PNETs, commonly referred to as islet cell tumors, pancreatic endocrine tumors, or islet cell carcinomas, are thought to arise from pluripotent cells within the exocrine pancreas and comprise <2% of all pancreatic tumors [5, 10]. PNETs may produce hormones, such as insulin, gastrin, glucagon, vasoactive intestinal peptide (VIP), and somatostatin, though not all hormone-producing PNETs cause symptoms related to hormone overproduction. PNETs that secrete excessive amounts of hormones without resulting in symptoms of hormone overproduction are considered nonfunctional by many clinicians. Unfortunately, there is no universally accepted definition of functional and nonfunctional PNETs. Therapy for PNETs is primarily surgical resection for localized disease and selected patients with metastatic disease. Although somatostatin analogues have proven to be very effective in ameliorating symptoms of hormone overproduction, options regarding systemic therapy for advanced disease continue to be limited.
The incidence of PNETs in the United States is not currently known but previous studies have suggested it was <1 per 100 000 per year [5, 6]. Information regarding survival and prognostic predictors of patients with PNETs is limited and largely derived from single-center surgical series that may not accurately reflect the general population of patients with PNETs. The objective of our study was to provide insights into the epidemiology and prognosis of PNETs and to evaluate trends in survival over the past three decades using a population-based registry. The Surveillance, Epidemiology, and End Results (SEER) registry currently collects information relating to cancer incidence and survival from population-based cancer registries covering ∼26% of the USA population, which is an increase from 9.5% in the early years of the SEER registry (1973–1975), as the number geographic areas included in the SEER has increased. SEER coverage currently encompasses diverse populations in the United States, including 23 percent of African-Americans, 40% of Hispanics, 42% of American Indians and Alaska Natives, 53% of Asians, and 70% of Hawaiian/Pacific Islanders [11].
patients and methods
Cases of PNETs diagnosed from January 1973 to December 2000 were obtained from the 13 population-based cancer registries of the SEER program [12]. Note that not all the registries commenced collection of cancer cases in 1973. A combination of topographical codes (International Classification of Diseases for Oncology, 3rd Edition, ICD-O-3: C250, C251, C252, C253, C254, C257, C258, and C259) and histology codes (8150, 8151, 8152, 8153, 8155, 8240, 8241, and 8246) was used to identify the cases. Tumors of all grades were included in the analysis. Histological grading was reported on the scale of 1 (well-differentiated tumors) through 4 (poorly differentiated or anaplastic tumors). Information regarding tumor grade was obtained through the individual institutions reporting to the SEER program and was not standardized among these participant sites. Tumors arising in the duodenum were excluded. For the purpose of this study, functionality of tumors was defined by histology codes as functional (8151, 8152, 8153, 8155) or nonfunctional (8150, 8240, 8241, 8246) because the SEER database offers no information on symptoms at presentation. We included carcinoid and enterochromaffin tumors of the pancreas (histology codes 8240–8242) and neuroendocrine carcinoma of the pancreas (8246) in order to capture all neuroendocrine pancreatic tumors. Insulinomas are not registered by SEER unless they show a malignant behavior with locally advanced or metastatic disease, so benign insulinomas were thus excluded. Tumors with mixed histology such as adenocarcinoid and atypical carcinoid tumors were also excluded. Tumor staging was reported according to the staging system used by SEER. Tumors were considered localized if they were confined to the pancreas, regional if there was extension into adjacent organs or metastases to regional lymph nodes, and distant if metastases to other organs were present.
The incidence rates were calculated as the number of new cases per 1 000 000 person-years, age adjusted to the 2000 US population. Data were from the original nine SEER registries. Rates were computed using the SEER*Stat 6.1.4 software [12]. Incidence rates (overall and by gender) were modeled as a linear function of year of diagnosis.
Differences between functional status and other factors [e.g., sex, surgery (yes/no), stage at diagnosis, race, age, and year of diagnosis quartiles] were investigated using a chi-square statistic.
Survival was defined as the number of months between date of diagnosis and dates of death (if known) or last follow-up (last known alive date or 31 December 2000). Kaplan–Meier curves were plotted and log-rank statistics computed to detect differences between survival curves for various factors (sex, race, stage, surgery, age, and year of diagnosis tertiles). Multivariate Cox regression models were used to estimate hazard ratios for sex, age, race, stage, and functional status [13]. All statistical analyses were carried out using SAS version 9 (SAS Institute Inc., Cary, NC).
results
patient characteristics
We identified 1483 patients aged 18 or older with PNETs during the period 1973–2000 (Table 1). There was a male predominance, with 819 men (55.2%) and 664 women (44.8%). The majority of the PNETs (N = 1346, 90.8%) were nonfunctional tumors, while 37 (2.5%) were malignant insulinomas and 100 (6.7%) were malignant functional tumors other than insulinoma. There were 63 gastrinomas (4.2%), 23 glucagonomas (1.6%), and 14 VIPomas (0.9%). No cases of somatostatinoma were registered. The mean age of the patients was 58.5 years (standard deviation: 14.9, range 19–95 years). Patients with functional tumors were younger at the time of diagnosis than patients with nonfunctional tumors (mean age 55.2 years versus 58.8 years, P = 0.006). The vast majority of patients were White (84.3%), 9.4% were Black, and 4.7% were of Asian origin.
Table 1.
Characteristics of 1483 patients with pancreatic endocrine tumors in the SEER registry 1973–2000
| Mean (range) | SD | |
| Age (all patients) | 58.5 (19–95) | 14.9 |
| Nonfunctional | 58.8 (19–95) | 14.7 |
| Functional | 55.2 (19–93) | 16.3 |
| N | % | |
| Sex | ||
| Male | 819 | 55.2 |
| Female | 664 | 44.8 |
| Race | ||
| White | 1247 | 84.1 |
| Black | 139 | 9.4 |
| Asian | 69 | 4.7 |
| Other | 28 | 1.9 |
| Stage | ||
| Localized | 164 | 11.1 |
| Regionally advanced | 307 | 20.7 |
| Metastatic | 893 | 60.2 |
| Unstaged | 119 | 8.0 |
| Grade | ||
| 1 | 88 | 5.9 |
| 2 | 77 | 5.2 |
| 3 | 106 | 7.2 |
| 4 | 40 | 2.7 |
| Not graded | 1172 | 79.0 |
| Number of primaries | ||
| 1 | 1310 | 88.3 |
| ≥2 | 173 | 11.7 |
| Functional statusa | ||
| Nonfunctional PETs | 1346 | 90.8 |
| Malignant insulinoma | 37 | 2.5 |
| Malignant gastrinoma | 63 | 4.2 |
| Malignant glucagonoma | 23 | 1.6 |
| Malignant VIPoma | 14 | 0.9 |
Based upon histology codes.
SEER, Surveillance, Epidemiology, and End Results.
The majority of patients (88.3%) had a solitary primary tumor while 11.7% had two or more tumors. Information on tumor grade was available for only 311 patients (21.0%). Among these, 88 (28.3%) had well-differentiated (grade 1) tumors while 77 (24.8%), 106 (34.1%), and 40 (12.9%) had grade 2, grade 3, and grade 4 tumors respectively.
Most patients had either metastatic (60.2%) or regionally advanced (20.7%) tumors at the time of diagnosis. Information regarding staging was missing in 119 cases (8.0%). Patients with functional tumors were as likely to present with metastatic and regionally advanced tumor as patients with nonfunctional tumors when all patients with information on tumor stage were analyzed (87.0% versus 88.1%, P = 0.72).
incidence
The crude annual overall incidence of PNETs (per 1 000 000) was 2.2 (1.8 in females and 2.6 in males), and the incidence increased with advancing age at diagnosis (Table 2). The incidence of PNETs in both sexes also increased over the study period (by 0.05 cases per 1 000 000 per year, P < 0.0001). The observed increase in incidence was greater in males (0.07/1 000 000 per year, P < 0.0001) than in females (0.03/1 000 000 per year, P = 0.0014). The crude annual overall incidence per million increased from 1.6 and 2.0 in 1973–1975 to 2.0 and 3.8 in 1996–2000 in females and males, respectively. The incidence was slightly higher for Blacks compared with Whites (2.5 versus 2.2 per 1 000 000). The incidence did not differ among the different SEER registries. The annual overall incidence of all functional tumors was 0.2 cases per million. The annual incidence of malignant insulinomas and gastrinomas was 0.1 cases per million. Other functional tumors were even rarer.
Table 2.
Incidence of PETs per 1 000 000 by age at diagnosis based upon nine SEER sites 1973–2000
| Age at time of diagnosis | All PETs |
Functional PETs |
Nonfunctional PETs |
||||||
| Overall | Female | Male | Overall | Female | Male | Overall | Female | Male | |
| 15–19 | 0.1 | 0.1 | 0.2 | 0 | 0 | 0 | 0.1 | 0.1 | 0.2 |
| 20–29 | 0.4 | 0.5 | 0.3 | 0.1 | 0.1 | 0.1 | 0.3 | 0.4 | 0.2 |
| 30–39 | 1.1 | 1.1 | 1.1 | 0.2 | 0.2 | 0.1 | 0.9 | 0.9 | 1.0 |
| 40–49 | 2.5 | 2.3 | 2.7 | 0.2 | 0.2 | 0.2 | 2.3 | 2.1 | 2.4 |
| 50–59 | 4.4 | 3.8 | 5.0 | 0.4 | 0.4 | 0.5 | 3.9 | 3.4 | 4.5 |
| 60–69 | 6.5 | 5.2 | 8.1 | 0.5 | 0.5 | 0.4 | 6.1 | 4.7 | 7.6 |
| 70–79 | 7.6 | 5.6 | 10.3 | 0.7 | 0.5 | 0.9 | 6.9 | 5.1 | 9.4 |
| 80+ | 4.6 | 3.5 | 7.0 | 0.3 | 0.3 | 0.3 | 4.3 | 3.2 | 6.7 |
PETs, pancreatic endocrine tumors; SEER, Surveillance, Epidemiology, and End Results.
survival and prognostic factors
The median overall survival (OS) for all cases was 28 months (range 0–346 months), and the survival closely paralleled the stage at diagnosis (P < 0.001) (Tables 3 and 4). Patients with functional tumors survived longer than patients with nonfunctional tumors in a univariate analysis where the median OS was 54 months for the functional tumors versus 26 months (P < 0.001) (Tables 3 and 4, Figure 1). The 5- and 10-year OS was 47.6% versus 31.3% and 33.7% versus 17.0% for functional PNETs versus nonfunctional PNETs, respectively. Table 4 shows prognostic predictors in our patients. Age at diagnosis and stage were strongly associated with survival in a univariate analysis (P < 0.001 for both). Male sex predicted shortened survival in a univariate analysis (median OS was 24 months versus 35 months for men and women respectively, P = 0.011). Higher grade also predicted worse survival (P < 0.001). Grade 1 and 2 tumors versus grades 3 and 4 were grouped for the survival analysis as there was no significant difference in survival between grade 1 versus 2 and grade 3 versus 4. The median OS was 51 months for patients whose tumors were either grade 1 or 2, 30 months for patients with tumors that were not assigned a grade, and 7.5 months in patients with grade 3 or 4 tumors (P < 0.001). Having multiple primaries did not predict survival in a univariate analysis (P = 0.30) nor did race (P = 0.52). When Blacks were compared with Whites, the former had a slightly longer median OS but the difference was not significant (32 months versus 29 months, P = 0.52). Information regarding surgical therapy was only available for the years 1998–2000 and was limited to 307 patients (20.7%). Resection of any type predicted better outcome with a median OS of 58 months in the surgery group versus 15 months in the group who did not have surgery (P < 0.001).
Table 3.
OS of patients with PETs according to stage and functional status based upon nine SEER sites 1973–2000
| Stage | Overall |
Functional |
Nonfunctional |
|||||||||
| Number | Median OS in months (95% CI) | 5-year OS (%) | 10-year OS (%) | Number | Median OS in months (95% CI) | 5-year OS (%) | 10-year OS (%) | Number | Median OS in months (95% CI) | 5-year OS (%) | 10-year OS (%) | |
| Overall | 1483 | 28 (25–32) | 32.8 | 18.6 | 137 | 54 (37–74) | 47.6 | 33.7 | 1346 | 26 (23–30) | 31.3 | 17.0 |
| Localized | 164 | 100 (68–148) | 61.9 | 45.5 | 16 | 184 (97–184) | 86.7 | 67.4 | 148 | 95 (61–124) | 59.1 | 43.1 |
| Regionally advanced | 307 | 69 (52–86) | 53.5 | 36.6 | 32 | a | 60.7 | 60.7 | 275 | 65 (52–79) | 52.7 | 33.8 |
| Metastatic | 893 | 17 (14–19) | 19.5 | 7.1 | 75 | 29 (20–45) | 32.1 | 14.8 | 818 | 16 (13–18) | 18.3 | 6.3 |
| Unstaged | 119 | 41 (25–60) | 41.0 | 25.2 | 14 | 100 (15–b) | 56.3 | 45.0 | 105 | 38 (22–54) | 39 | 22.9 |
Insufficient number of events to calculate median OS.
The mean survival time and its standard error were underestimated because the largest observation was censored and the estimation was restricted to the largest event time.
OS, overall survival; PETs, pancreatic endocrine tumors; SEER, Surveillance, Epidemiology, and End Results; CI, confidence interval.
Table 4.
Analysis of potential factors influencing OS
| Variable | Univariate analysis |
Multivariate analysis |
||
| Median OS in months (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Age group | ||||
| 18–50 | 52 (45–70) | 1.00 (reference) | ||
| 51–60 | 44 (33–55) | 1.23 (1.03–1.48) | 0.02 | |
| 61–70 | 19 (14–26) | 1.86 (1.56–2.21) | <0.0001 | |
| 71–95 | 9.5 (6–12) | <0.001 | 2.92 (2.46–3.46) | <0.0001 |
| Sex | ||||
| Male | 24 (21–27) | 1.00 (reference) | ||
| Female | 35 (30–40) | 0.011 | 0.91 (0.80–1.03) | 0.12 |
| Race | ||||
| Non-White | 26 (20–35) | 1.00 (reference) | ||
| White | 29 (24–33) | 0.79 | 0.85 (0.72–1.01) | 0.06 |
| Stage | ||||
| Localized | 100 (68–148) | 1.00 (reference) | ||
| Regionally advanced | 69 (52–86) | 1.12 (0.87–1.46) | 0.37 | |
| Metastatic | 17 (14–19) | <0.001 | 2.87 (2.29–3.61) | <0.0001 |
| Grade | ||||
| Grade 1 or 2 | 51 (37–68) | 1.00 (reference) | ||
| Unknown | 30 (26–34) | 1.17 (0.95–1.44) | 0.14 | |
| Grade 3 or 4 | 7.5 (5–10) | <0.001 | 2.28 (1.75–2.98) | <0.0001 |
| Functional status | ||||
| Nonfunctional | 26 (23–30) | 1.00 (reference) | ||
| Functional | 54 (37–74) | <0.001 | 0.71 (0.57–0.89) | 0.004 |
| Any surgery | ||||
| Yes | 58 (51–a) | Not includedb | ||
| No | 15 (9–21) | <0.001 | ||
| Year of diagnosis | ||||
| 1973–1980 | 14 (9–22) | 1.00 (reference) | ||
| 1981–1990 | 30 (23–34) | 0.75 (0.63–0.90) | 0.002 | |
| 1991–2000 | 33 (27–38) | 0.01 | 0.65 (0.55–0.77) | <0.0001 |
The mean survival time and its standard error were underestimated because the largest observation was censored and the estimation was restricted to the largest event time.
Not included due to limited number of patients with information regarding surgery.
OS, overall survival; CI, confidence interval.
Figure 1.
Overall survival (OS) of patients with PETs according to functional status based upon nine SEER sites 1973–2000.
We carried out a multivariate analysis including stage, age at diagnosis, sex, functional status, grade, race (White versus non-White), and year of diagnosis (in tertiles) as covariates (Table 4). The strongest predictors were age at diagnosis, stage, grade, and tertile year of diagnosis (P < 0.001). Patients with functional tumors had a better prognosis than patients with nonfunctional tumors after adjusting for other variables (hazard ratio 0.71, P = 0.004). Sex, multiple primaries, and race did not reach statistical significance.
temporal trends
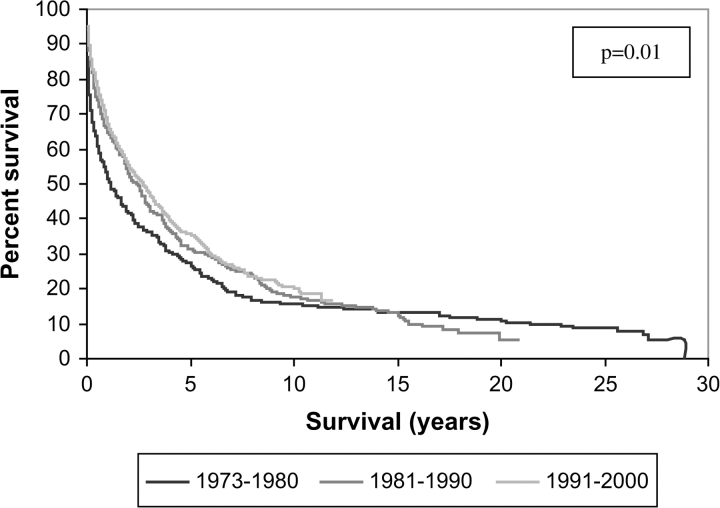
Survival has increased over time: patients diagnosed in the later tertiles of the observation period (1981–1990 and 1991–2000) had longer OS than patients diagnosed in the earliest tertile (1973–1980) (P = 0.01) (Table 5; Figure 2). The increased survival retained statistical significance after adjusting for other prognostic predictors in a multivariate analysis (Table 5). The survival increase is not explained by stage migration as patients in the later tertiles were as likely to be diagnosed with regionally advanced or metastatic disease (P = 0.21). There was no significant difference in the age at diagnosis among the diagnostic tertiles (P = 0.30), but there was a significantly higher number of functional tumors diagnosed in the period 1981–1990 compared with the earlier and later periods (P = 0.002). When analyzed separately in a univariate analysis, the survival of patients with localized and metastatic disease increased significantly over time (P = 0.013 and P = 0.002, respectively), and there was a trend toward increased survival in the patients with regionally advanced disease, although it did not meet statistical significance (P = 0.077).
Table 5.
Survival trends over time
| Year of diagnosis (diagnosis tertile) | Total number | Total events (deaths) | Median overall survival (months) | Stage at diagnosis (percent) |
Age at diagnosis | Percent with functional tumors | ||
| Localized | Regionally advanced | Metastatic | ||||||
| 1973–1980 | 244 | 225 | 14 | 12.6 | 23.7 | 63.7 | 57.1 | 5.3 |
| 1981–1990 | 387 | 341 | 30 | 9.2 | 25.5 | 65.3 | 58.7 | 13.2 |
| 1991–2000 | 852 | 589 | 33 | 13.1 | 20.9 | 66.0 | 58.8 | 8.6 |
| P value | 0.01 | 0.21 | 0.30 | 0.002 | ||||
Figure 2.
Overall survival (OS) of patients with PETs according to the year of diagnosis based upon nine SEER sites 1973–2000.
discussion
This is the largest population-based study focusing on the incidence and prognosis of PNETs in the United States. Carriaga and Henson [5] have previously reported on malignancies of the liver, gallbladder, bile ducts, and pancreas, including PNETs, using SEER data up to 1987. Their study provided limited information on the incidence of PNETs and survival and suggested that the annual incidence was <0.6/100 000, which is in agreement with a much earlier report from Connecticut, USA, that suggested the incidence was <0.1/100 000 [6]. We provide an updated evaluation of the incidence and prognosis of these uncommon tumors, and we report trends toward increasing incidence and improved survival over time. We report a crude annual incidence of 0.22 per 100 000 (2.2 per 1 000 000) with a male predominance. Investigators in Europe and Asia have reported similar annual incidences ranging from 0.12 to 0.4 cases per 100 000 [2–4, 7]. Slightly higher incidence among males has also been reported in a French study conducted in a well-defined population [7]. The incidence of PNETs increases with age and peaks in the sixth and seventh decade. We observed a trend toward increasing incidence over time, which is in keeping with the findings of other investigators in France and Michigan, USA [7, 14].
Unfortunately, there is no universal agreement on the definitions of functional and nonfunctional tumors. It is a common practice to label PNETs as functional if the patients have symptoms of hormone overproduction and nonfunctional if patients are asymptomatic in regard to hormonal symptoms even though they may have elevated hormone levels. Using histological codes from the SEER registry to determine the functionality of PNETs has significant limitations. The SEER registry does not provide data on the clinical presentation or laboratory values which are used to make the distinction between functional and nonfunctional tumors. The majority of patients had nonfunctional tumors according to the histological codes in the registry. A possible explanation for the high proportion of nonfunctional tumors is the absence of benign insulinomas from the SEER registry, but insulinomas are among the most common types of PNETs [15]. The SEER registry does not include benign insulinomas but given their very favorable prognosis [16], it seems reasonable to exclude them from this analysis. Another limitation of the SEER data is the lack of centralized pathology review. The registry relies on pathology reports provided by the participating institutions, and, therefore, there is a potential for misclassification of these tumors. This could result in underreporting of functional tumors in those patients who had symptoms of hormone overproduction but whose tumors were not identified with one of the histology codes for functional tumors. It is also possible that data on smaller tumors that were felt to be benign were not submitted to the registry. Smaller functional tumors localized to the pancreas and without local invasion and distant metastases may thus be underrepresented given the way registry data are collected. Our results regarding the effects of functionality on survival have to be viewed with these limitations in mind. Larger studies with detailed information on symptoms of hormone overproduction and measurements of hormone blood levels are needed to definitively answer the question of the effect of functional status on survival. The lack of centralized pathology review and the absence of uniform grading system also limits the conclusions that can be drawn regarding the effect of tumor grade on prognosis, but information on tumor grade was absent for the majority of the cases. For the purpose of the multivariate analysis on prognostic factors, we included patients with missing data on tumor grade as a separate group. We acknowledge the fact that the high number of cases with missing tumor grade limits the conclusions that can be drawn regarding tumor grade as a prognostic factor. We have prospectively evaluated 214 consecutive patients with PNETs seen at the Mayo Clinic and after excluding 12 cases of benign insulinoma, the proportion of nonfunctional tumors was 83.6% or slightly lower than we report using the SEER, Halfdanarson TR, Bamlet WR and Petersen GM (unpublished data). The current study shows that patients with functional tumors as determined by histological codes have a more favorable prognosis than patients with nonfunctional tumors, a finding that has been a matter of debate [17–21]. The prognostic value of the functional status of the tumors retained significance after adjusting for other important prognostic factors such as age and stage at diagnosis, suggesting that the biology of functional tumors differs from the nonfunctional tumors. This is the largest study reported that suggests that functional status of PNETs may have prognostic significance but these results have to be viewed with caution given the limitations of the SEER database. Not surprisingly, higher age at diagnosis, more advanced stage, and higher grade were the strongest predictors of worse survival.
Survival of patients with PNETs seems to have increased over the time that SEER has been collecting data, and our findings are consistent with those of other investigators [22]. The reason for increased survival over the years is not clear. Diagnosis later in the observation period predicted better survival compared with diagnosis earlier in the period after adjusting for other major variables known to affect survival such as stage, age, and functional status. One explanation for increased survival is that with the advent of improved imaging techniques, patients may be diagnosed at an earlier stage than was previously done. Computerized tomography became widely available in the 1980s and has resulted in more accurate diagnosis and staging of abdominal tumors. When we analyzed the entire cohort by comparing patients diagnosed before and after 1980 and 1985, we found no difference in the stage at diagnosis. Patients diagnosed after 1980 and 1985 were as likely to present with regionally advanced or metastatic disease, arguing against stage migration as an explanation for the increasing survival. It is possible that an improvement in the therapy of PNETs has improved the prognosis. More aggressive surgery, including resection of liver metastases and peritoneal metastases, may improve survival and has frequently been reported as a viable option in patients with resectable metastases, but there have been no controlled trials carried out to evaluate the impact of extensive debulking on survival, and such trials are not likely to be done [23–26]. Unfortunately, information regarding surgery in the SEER registry is very limited and available only for a small proportion of the patients. Furthermore, all analyses of the impact of surgery would likely suffer from significant bias as there is no information on the performance status of the individual patients. It is also possible that improvement in the medical therapy of patients with PNETs may have resulted in improved survival. Somatostatin analogues (SSAs) have been increasingly used over the last one to two decades and have been shown to be very active in ameliorating symptoms of hormone overproduction [27]. It is not known if SSAs alter the natural history of PNETs, but these drugs have been shown to possess antiproliferative activity [28]. Objective tumor responses following therapy with SSAs are uncommon, but stable disease is observed more frequently, even among nonfunctional tumors [27, 29, 30]. It is therefore possible that the introduction of SSAs may have improved the survival of patients with PNETs.
In addition to the lack of centralized pathology review and limited information regarding the surgery, there are other limitations of the SEER registry. One limitation is the lack of important clinical information such as the performance score of the patient and the burden of the tumors as well as information regarding medical therapy and interventional procedures such as hepatic artery embolization. The increase in the incidence of PNETs and the possible effects on survival merits further study. Although the rising incidence may be a real phenomenon, it is possible that improvement in pathologic diagnosis and greater awareness among pathologists has resulted in the observed increase in incidence of PNETs.
In conclusion, this large population-based study using SEER data provides an up-to-date estimate of the incidence and prognosis of PNETs and suggests that the functional status of the tumor may be an independent prognostic factor after adjusting for other major determinants of survival. The survival of patients with PNETs also seems to have increased over time but the exact cause of the observed survival increase remains to be explained.
funding
Mayo Clinic SPORE in Pancreatic Cancer (P50 CA 102701).
References
- 1.Buchanan KD, Johnston CF, O'Hare MM, et al. Neuroendocrine tumors. A European view. Am J Med. 1986;81:14–22. doi: 10.1016/0002-9343(86)90581-4. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson B, Öberg K, Skogseid B. Neuroendocrine pancreatic tumors. Clinical findings in a prospective study of 84 patients. Acta Oncol. 1989;28:373–377. doi: 10.3109/02841868909111209. [DOI] [PubMed] [Google Scholar]
- 3.Watson RG, Johnston CF, O'Hare MM, et al. The frequency of gastrointestinal endocrine tumours in a well-defined population—Northern Ireland 1970–1985. Q J Med. 1989;72:647–657. [PubMed] [Google Scholar]
- 4.Lam KY, Lo CY. Pancreatic endocrine tumour: a 22-year clinico-pathological experience with morphological, immunohistochemical observation and a review of the literature. Eur J Surg Oncol. 1997;23:36–42. doi: 10.1016/s0748-7983(97)80140-0. [DOI] [PubMed] [Google Scholar]
- 5.Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75:171–190. doi: 10.1002/1097-0142(19950101)75:1+<171::aid-cncr2820751306>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Moldow RE, Connelly RR. Epidemiology of pancreatic cancer in Connecticut. Gastroenterology. 1968;55:677–686. [PubMed] [Google Scholar]
- 7.Lepage C, Bouvier AM, Phelip JM, et al. Incidence and management of malignant digestive endocrine tumours in a well defined French population. Gut. 2004;53:549–553. doi: 10.1136/gut.2003.026401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig Dis Sci. 1991;36:933–942. doi: 10.1007/BF01297144. [DOI] [PubMed] [Google Scholar]
- 9.Grimelius L, Hultquist GT, Stenkvist B. Cytological differentiation of asymptomatic pancreatic islet cell tumours in autopsy material. Virchows Arch A Pathol Anat Histol. 1975;365:275–288. doi: 10.1007/BF00471177. [DOI] [PubMed] [Google Scholar]
- 10.Vortmeyer AO, Huang S, Lubensky I, Zhuang Z. Non-islet origin of pancreatic islet cell tumors. J Clin Endocrinol Metab. 2004;89:1934–1938. doi: 10.1210/jc.2003-031575. [DOI] [PubMed] [Google Scholar]
- 11.Overview of the SEER Program. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute; http://seer.cancer.gov/about/ (1 February 2007, date last accessed) [Google Scholar]
- 12.Surveillance, Epidemiology, and End Results (SEER) Program. ( www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 9 Regs Public-Use, Nov 2004 Sub 1973–2002, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2005, based on the November 2004 submission. [Google Scholar]
- 13.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York: John C. Wiley & Sons; 1999. [Google Scholar]
- 14.Fitzgerald TL, Hickner ZJ, Schmitz M, et al. Presented at the 2007 Gastrointestinal Cancers Symposium in Orlando, FL: 2007. Increasing incidence of nonfunctional neuroendocrine tumors of the pancreas. [Google Scholar]
- 15.Klöppel G, Heitz PU. Pancreatic endocrine tumors. Pathol Res Pract. 1988;183:155–168. doi: 10.1016/S0344-0338(88)80043-8. [DOI] [PubMed] [Google Scholar]
- 16.Service FJ, McMahon MM, O'Brien PC, Ballard DJ. Functioning insulinoma—incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc. 1991;66:711–719. doi: 10.1016/s0025-6196(12)62083-7. [DOI] [PubMed] [Google Scholar]
- 17.Cubilla AL, Hajdu SI. Islet cell carcinoma of the pancreas. Arch Pathol. 1975;99:204–207. [PubMed] [Google Scholar]
- 18.Phan GQ, Yeo CJ, Hruban RH, et al. Surgical experience with pancreatic and peripancreatic neuroendocrine tumors: review of 125 patients. J Gastrointest Surg. 1998;2:472–482. [PubMed] [Google Scholar]
- 19.White TJ, Edney JA, Thompson JS, et al. Is there a prognostic difference between functional and nonfunctional islet cell tumors? Am J Surg. 1994;168:627–629. doi: 10.1016/s0002-9610(05)80134-5. discussion 629–630. [DOI] [PubMed] [Google Scholar]
- 20.Sarmiento JM, Farnell MB, Que FG, Nagorney DM. Pancreaticoduodenectomy for islet cell tumors of the head of the pancreas: long-term survival analysis. World J Surg. 2002;26:1267–1271. doi: 10.1007/s00268-002-6714-9. [DOI] [PubMed] [Google Scholar]
- 21.Thompson GB, van Heerden JA, Grant CS, et al. Islet cell carcinomas of the pancreas: a twenty-year experience. Surgery. 1988;104:1011–1017. [PubMed] [Google Scholar]
- 22.Fesinmeyer MD, Austin MA, Li CI, et al. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1766–1773. doi: 10.1158/1055-9965.EPI-05-0120. [DOI] [PubMed] [Google Scholar]
- 23.Touzios JG, Kiely JM, Pitt SC, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241:776–783. doi: 10.1097/01.sla.0000161981.58631.ab. discussion 783–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 25.Schurr PG, Strate T, Rese K, et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg. 2007;245:273–281. doi: 10.1097/01.sla.0000232556.24258.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumors. Surg Oncol Clin N Am. 2003;12:231–242. doi: 10.1016/s1055-3207(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 27.Delaunoit T, Rubin J, Neczyporenko F, et al. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumors. Mayo Clin Proc. 2005;80:502–506. doi: 10.4065/80.4.502. [DOI] [PubMed] [Google Scholar]
- 28.Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol. 2006;17:1733–1742. doi: 10.1093/annonc/mdl105. [DOI] [PubMed] [Google Scholar]
- 29.Shah T, Caplin M. Biotherapy for metastatic endocrine tumours. Best Pract Res Clin Gastroenterol. 2005;19:617–636. doi: 10.1016/j.bpg.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Aparicio T, Ducreux M, Baudin E, et al. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer. 2001;37:1014–1019. doi: 10.1016/s0959-8049(01)00073-9. [DOI] [PubMed] [Google Scholar]