Abstract
The melanocortins, a family of peptides produced from the post-translational processing of pro-opiomelanocortin (POMC), regulate ingestive behavior and energy expenditure. Loss of function mutations of genes encoding POMC, or of either of two melanocortin receptors expressed in the central nervous system (MC3R, MC4R), are associated with obesity. The analyses of MC4R knockout mice indicate that activation of this receptor is involved in the regulation of appetite, the adaptive metabolic response to excess caloric consumption, and negative energy balance associated with cachexia induced by cytokines. In contrast, MC3R knockout mice exhibit a normal, or even exaggerated, response to signals that induce a state of negative energy balance. However, loss of the MC3R also results in an increase in adiposity. This article discusses the regulation of energy balance by the melanocortins. Published and newly presented data from studies analyzing of energy balance of MC3R and MC4R knockout mice indicate that increased adiposity observed in both models involves an imbalance in fat intake and oxidation.
Keywords: Melanocortin, Energy balance, MC3R
1. Introduction
Environments where high calorie foods are available with minimal physical cost are associated with an increased incidence of obesity and a cluster of metabolic abnormalities called the Metabolic Syndrome. Insulin resistance is the defining feature of the Metabolic Syndrome [18,24,59,63]. Simply stated, energy intake and energy expenditure must be balanced over long periods to prevent excess weight gain and insulin resistance. Independently of adipose mass, the balance of fat consumption with oxidation in key insulin sensitive tissues is also thought to be important for weight maintenance and the prevention of insulin resistance [46]. Faced with an epidemic of obesity and the Metabolic Syndrome, there is an urgent need to identify therapeutic targets that will reduce appetite, increase energy expenditure and/or fat oxidation, or a combination of both.
The melanocortin system is considered a promising target for the treatment of eating disorders and obesity [35,36]. In the brain, the melanocortin system can be defined as neurons expressing pro-opiomelanocortin (POMC), agouti-related peptide (AgRP), or melanocortin receptors whose activity is affected by peptides released from POMC and AgRP neurons. Melanocortin receptors have seven transmembrane domains, increase adenylyl cyclase activity and cyclic AMP, and are activated by melanocyte stimulating hormones (α-, β-, γ-MSH) released from POMC neurons [15]. Of the five cloned melanocortin receptors, two (MC3R, MC4R) have been identified as important down stream effectors regulating energy homeostasis in response to neuropeptides secreted by POMC and AgRP neurons.
The location of POMC neurons (arcuate nucleus of the hypothalamus, nucleus tractus solitarius of the brain stem) and AgRP neurons (arcuate nucleus of the hypothalamus) suggests a role in the regulation of satiety and energy expenditure [20]. In support of this hypothesis, chronic central and peripheral administration of non-selective melanocortin receptor agonists reduces food consumption and causes weight loss [42]. The central administration of AgRP, an antagonist of the MC3R and MC4R, increases appetite, weight gain and adiposity when administered centrally or overexpressed in transgenic mice [25,51,52,55]. Targeted deletion of either the Mc3r and Mc4r genes is associated with increased adiposity [8]. Collectively, these data suggest that both receptors might be therapeutic targets for treating obesity and eating disorders. However, central administration of the non-specific melanocortin agonist melanotan-II (MTII) elicits a conditioned taste aversion (CTA) response in rats, suggesting that co-activation of MC3R and MC4R is associated with aversive sensations, such as nausea and anxiety [6,61]. In contrast, reduced food intake associated with central administration of the selective MC4R agonist Ro27-3225 is not associated with CTA [6], suggesting that MC4R-specific agonists might be best suited for the chronic treatment of obesity.
The five melanocortin receptors exhibit a broad distribution of expression in the body, and are involved in a diverse array of physiologic functions. Testing the specificity of action will therefore be an important step in the initial investigation of novel melanocortin receptor agonist and antagonist compounds. The generation of mice lacking functional MC3R or MC4R has provided important tools for investigating specificity of action for novel melanocortin receptor agonists and antagonists, and in the general investigation of melanocortin receptor function. This paper discusses results from published studies examining the phenotype of the two melanocortin receptor knockouts, and presents new phenotype data from MC3R and MC4R knockout mice backcrossed onto the C57BL/6J (B6) strain.
2. Materials and methods
2.1. Animal husbandry
Melanocortin-3 receptor knockout (Mc3r−/−) and Mc4r−/− mice were derived from breeding colonies of heterozygotes maintained in the Department of Comparative Biology at the Pennington Biomedical Research Center. Both strains have been backcrossed at least 10 generations onto the B6 background. Genotyping of Mc3r−/− and Mc4r−/− mice used a polymerase-chain reaction (PCR)-based assay as described previously [2,9]. Leptin-deficient (Lepob/Lepob) mice were purchased from Jackson Laboratories (Bar Harbor, ME). All experiments were approved by the Institutional Animal Care and Use Committee. Fat mass (FM) and fat free mass (FFM) of mice were measured by nuclear magnetic resonance spectroscopy (NMR) using a Bruker Mice Minispec Analyzer (Bruker Optics Inc., Billerica, MA). Mice were fed purified low or high fat diets purchased from Research Diets (New Brunswick, NJ), as previously described [2].
2.2. Analysis of food intake, spontaneous activity, and oxygen consumption
Food intake, movement in the X and Z-axes, and oxygen consumption and carbon dioxide respiration (VO2, VCO2) were measured simultaneously using a 16 chamber Comprehensive Laboratory Animal Monitoring System (CLAMS) from Columbus Instruments (Columbus, OH). The metabolic cages are housed within a temperature-controlled incubator (Powers Scientific, Inc., Pipersville, PA), and temperature kept at constant 28 °C to minimize inter-experimental variability due to thermal stress. Derivation of energy expenditure (EE, kJ/h) and percent energy from fat or carbohydrate oxidation (F%, C%) using indirect calorimetry were calculated as described previously [2].
3. Transgenics studies to define the role of melanocortin receptors in regulation energy balance
3.1. Ingestive behavior of melanocortin receptor knockout mice
The initial characterization of the Mc3r−/− and Mc4r−/− mice used F2–F4 offspring on a mixed genetic background (129/Sv and B6) [3,9–12,38–40,57]. Subsequent experiments have used Mc3r−/− or Mc4r−/− mice backcrossed 8–12 generations onto the B6 strain [2,23,28,37]. Feeding behavior involves neural pathways that affect motivation and reward, that respond to satiety signals, or sense metabolites such as glucose and free fatty acids (FFA), and is a complex behavioral trait reflecting the interaction of genetic and laboratory environment variables [1,17,56]. Differences in the phenotype of ingestive behavior of the melanocortin receptor knockouts in the literature might therefore occur due to variation in genetic background and the methods used to analyze ingestive behaviors. However, at this time the obese phenotype of the homozygous melanocortin receptor knockout mice appears robust, with independent groups reporting comparable phenotypes for Mc3r−/− [9,11] and Mc4r−/− mice [12,30].
3.1.1. Mc4r−/− mice on out bred (129;B6) backgrounds
When analyzed as grams ingested per animal, Mc4r−/− mice were originally reported to be hyperphagic when fed standard rodent chow [12,30]. However, both lean tissue mass and longitudinal growth are significantly increased in mice and humans with abnormal MC4R function [14,30]. The definition of ‘normal’ food consumption when comparing data from mice varying in nose-anus length by 10–12% [30], and in fat-free mass by over 20% [2], is not clear. It is reasonable to speculate that the increased energy requirements of ambulation and tissue maintenance associated with the substantial increase in lean mass is a significant factor in the increased food consumption of the Mc4r−/− mice. Certainly, a large body of clinical data obtained from human subjects suggests that resting energy expenditure, an estimate of the kJ required for maintaining a constant body weight, correlates significantly with fat free mass [45].
While the interpretation of data from the investigation of food intake using standard rodent chow is not clear, Mc4r−/− mice do not appear to control appetite normally when introduced to palatable or high fat diets [10,64]. On standard chows with low fat content (10–13% kJ/fat), food intake of Mc4r−/− mice can be within the normal range observed for wild type controls [10]. However, a marked hyperphagia is observed following the introduction of diets with increased palatability or fat content [10]. The effects of diet on ingestive behavior of Mc4r−/− mice has also been reported for lethal yellow (AY/a) mice, that aberrantly express an antagonist of the MC4R, agouti, in the brain [64].
The effects of high fat diets on the ingestive behavior of AY/a and Mc4r−/− mice is similar to that observed in some experiments examining the effect of lesions of the ventromedial hypothalamus (VMH) [41]. The sensitivity of hyperphagia of Mc4r−/− and AY/a mice to diet has recently been linked to the regulation of brain-derived neurotrophic factor (BDNF) in the VMH. BDNF mRNA expression is reduced in the VMH of AY/a and Mc4r−/− mice, and in food deprived mice, while intracerebroventricular administration of the nonspecific melanocortin receptor agonist increases BDNF mRNA expression. The anorectic response to BDNF administered intracerebroventricularly is also regulated by diet: BDNF is an anorexigen when administered intracerebroventricularly to Ay/a mice fed high fat diets, but has not effect on the ingestive behavior Ay/a mice fed low fat chow [64]. BDNF receptor (TrkB) hypomorphs, which have a reduced expression of TrkB [64], and a neural specific knockouts of the Bdnf gene [47], also exhibit the increase longitudinal growth and obese phenotype observed for Mc4r−/− and Ay/a mice. Whether BDNF neurons in the VMH express MC4R, or are innervated by MC4R-positive neurons, is not clear. Similarly, the hypothalamic and extra-hypothalamic sites that the VMH BDNF neurons project to that affect ingestive behavior are also not known.
Data from pair feeding studies, and from examination of the feeding response of Mc4r−/− mice to melanocortin agonists, has been suggested as evidence that abnormal regulation of appetite contributes to the increased weight gain. Hyperphagia on a standard chow diet has been reported for young (3–5 weeks) Mc4r−/− mice [62]; pair feeding of Mc4r−/− mice with wild type littermates from an early age (5 weeks) reduces weight gain and adiposity [38,57]. These results suggest that hyperphagia probably contributes to the increase of both lean and adipose tissue mass of Mc4r−/− mice [57]. However, pair-feeding does not completely prevent increased adiposity of Mc4r−/− mice [57], suggesting a preferential partitioning of nutrients to storage as triglyceride in adipose tissue.
If activation of the MC4R is critical for the anorectic actions of melanocortin receptors agonists, then MC4R knockout mice should not respond by reducing food intake. This result has been reported by two groups, with the inhibition of food intake associated with either peripheral or central administration of the non-specific melanocortin agonist melanotan-II not observed in Mc4r−/− mice [12,38]. The MC4R is thus critical, at least acutely, for the suppression of food intake in response to the release of MSH from POMC neurons, while the MC3R has either no or at best a very minor role [11].
MC4R neurons are also important targets for peripheral factors that regulate energy balance. Seeley and colleagues reported that central administration of a non-selective melanocortin receptor antagonist effectively inhibited the reduction of food intake associated with leptin treatment [54]. It was therefore, of interest to determine the response of Mc4r−/− mice to leptin. Adult obese Mc4r−/− mice are clearly resistant to leptin administered centrally or peripherally [38]. Leptin resistance is also observed in obese lethal yellow (AY/a) mice [7,26]. However, a caveat to this result is that young lean Mc4r−/− mice, or Mc4r−/− mice pair-fed with wild type littermates to attenuate obesity, still exhibit a reduction in food intake following peripheral leptin treatment, although with reduced effectiveness when compared to wild type mice [38]. This would suggest that, in lean mice, leptin could suppress appetite through mechanisms not involving the MC4R.
3.1.2. Mc4r−/− mice on the B6 background
The B6 strain is a widely used model of diet-induced obesity, developing a phenotype of obesity, hyperinsulinemia, and leptin resistance when fed ‘western’ style diets high in fat content [60]. Backcrossing the null melanocortin receptor alleles onto the B6 strain will facilitate comparison of the melanocortin receptor knockouts with other frequently used mouse models of obesity and insulin resistance. The spontaneous leptin gene mutant (Lepob/Lepob) and Ay/a mice are also maintained on the B6 strain.
We have reported that hyperphagia of female Mc4r−/− mice on the B6 background is sensitive to dietary fat content when compared to wild type B6 and Lepob/Lepob mice, with increased caloric intake only observed in Mc4r−/− mice [2]. Male Mc4r−/− mice on the B6 background are also not hyperphagic when fed low fat rodent chow [23]. Mc4r−/− mice on the B6 background have also been used to examine the function of this receptor in neural pathways responding to peripheral satiety factors [22,27] and cachexia [37]. The results of these experiments suggest that, while a role for the MC4R likely exists in the satiating effects of anorexic compounds, considerably redundancy exists in the neural pathways that inhibit food intake in response to factors secreted from the gut and adipose. For example, Mc4r−/− mice on the B6 background do not reduce food intake when treated with cholecystokinin (CCK) [22], suggesting that activation of MC4R in the brain stem is critical for CCK's effects on satiety. However, loss of the CCK-A receptor, which is required for inhibition of food intake by CCK, does not cause hyperphagia or obesity in mice [33]. It is therefore unclear whether the loss of CCK's satiety signal contributes significantly to the obese phenotype of Mc4r−/− mice. On the other hand, the anorectic response to PYY, a gut peptide that inhibits food intake [5], is conserved in Mc4r−/− mice [27].
3.1.3. Mc3r−/− mice on out bred (129;B6) backgrounds
Examination of feeding behavior of Mc3r−/− mice has yielded mixed results, with the published evidence suggesting at best a subtle contribution of the MC3R to the regulation of ingestive behavior. In contrast to Mc4r−/− mice, there is no published evidence for hyperphagia of Mc3r−/− mice on mixed background fed either low fat or high fat diets. Intake of low fat rodent chow by Mc3r−/− mice is either modestly reduced [11], or normal [9], when compared to wild type littermates. Food intake of Mc3r−/− mice was also not significantly different when given access to chow diet with modestly increased fat content [9] or a purified very high fat diet [11]. Moreover, the inhibition of food intake in response to MTII administered peripherally is retained in Mc3r−/− mice, suggesting that the MC3R is not critical for the inhibition of food intake in response to treatment with non-specific melanocortin receptor agonists [11].
Mc3r−/− mice on an out bred background exhibit a very modest obese phenotype, with only minor changes in body weight, when fed standard rodent chow with low fat content (12.5% kJ/fat) [11]. However, we observed evidence for sexual dimorphism for Mc3r−/− mice fed breeder chow with modestly high fat content (25% kJ/fat); with females exhibiting a marked increase in body weight not observed in males (Fig. 1). Subsequent analysis of body composition by dual X-ray absorptiometry (DEXA) indicated that fat as a percentage of total body weight can exceed 45% in 6 month old male Mc3r−/− mice fed the breeder chow diet (Mc3r−/− mice, 45.2 ±1.6%; Mc3r+/− mice, 36.1 ± 2.4%; Mc3r+/+ mice, 31.8 ± 5.2%, P < 0.05 Mc3r−/− versus Mc3r+/+ mice, n = 6−12/group). Female Mc3r−/− mice fed a high fat diet (60% kJ/fat) exhibit significantly greater weight gain due to differences in metabolism rather than ingestive behavior [11]. However, direct comparisons of weight gain and adiposity of Mc3r−/− and wild type littermates fed low fat or high fat diets have not been reported.
Fig. 1.
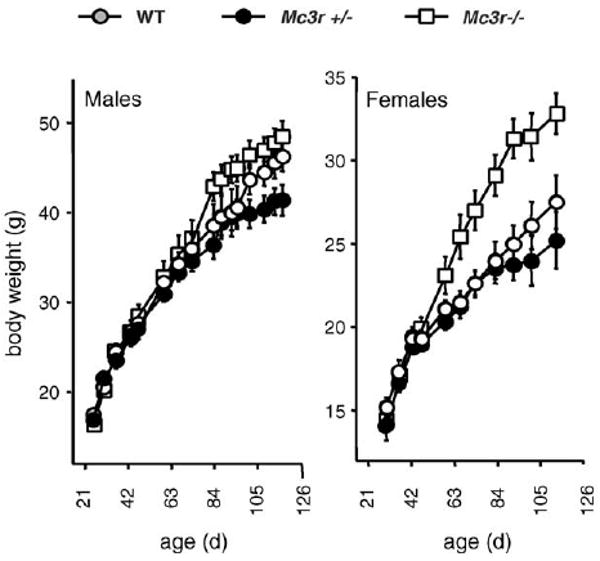
Growth curve of MC3R mutants and wild type littermates on a mixed 129; B6 background. Some of the data for this growth curve was published in the initial analysis [9]. Male and female Mc3r−/− mice (n = 9−18), heterozygotes (Mc3r+/−, n = 22−31), and wild type (Mc3r+/+, n = 9−18) mice were weaned onto mouse breeder chow (Purina 5015, 25% kJ/fat) and body weights recorder every 7–10 days.
3.1.4. Mc3r−/− mice on the B6 background
Comparisons of the response of Mc3r−/− mice with Mc4r−/− mice on the B6 background to hormones affecting satiety and anorexia are also consistent with the MC3R having a minor role in regulating ingestive behavior. Peripheral injections of CCK inhibit food intake of Mc3r−/− mice, but are not effective in Mc4r−/− mice [22]. While anorexia associated with tumors and endotoxin are markedly reduced in Mc4r−/− mice, it is conserved in Mc3r−/− mice, and might even be exaggerated, suggesting that MC3R acts to restrain the inhibitory outputs to feeding behavior from POMC neurons [37]. However, inhibition of the MC3R might be associated with an increased appetite. Increased food intake associated with intracerebroventricular administration of AgRP is conserved in Mc4r−/− mice [39]. A second independent study also reported a modest, but not statistically significant, increase in food intake of Mc4r−/− mice in response to centrally administered AgRP [23]. These results suggest that inhibition of the MC3R might increase food intake, and could be a factor in the orexigenic response to centrally administered AgRP [52].
The introduction of high fat diets to F2–F4 out bred Mc3r−/− mice results in accelerated weight gain due to increased feed efficiency (weight gain per kJ ingested) [11]. We have also observed that Mc3r−/− mice on the B6 background exhibit a disproportionate increase in adiposity compared to wild type mice when fed purified high fat diets that is not associated with hyperphagia (Fig. 2A) [9]. To investigate the mechanisms associated with the diet-induced increase in adiposity of Mc3r−/− on the B6 background, energy balance was measured using pre-obese 8-week-old mice housed in the comprehensive laboratory animal monitoring system (CLAMS). The food intake data for this experiment are shown in Fig. 2B–D. Male B6 Mc3r−/− and wild type B6 mice (2.5–3 months old, n = 8/group) that had been weaned onto a low fat diet were acclimated to the CLAMS chambers for 7 days, with temperature maintained at a constant 28 °C. There was no significant difference in body weight of the two groups. However, modest but significant differences were evident for FFM (FFM in g: Mc3r+/+, 19.2 ± 0.4; Mc3r−/−, 17.4 ± 0.4; P < 0.01) and FM (FM in g: Mc3r+/+, 4.0 ± 0.2; Mc3r−/−, 5.3 ± 0.2; P < 0.01).
Fig. 2.
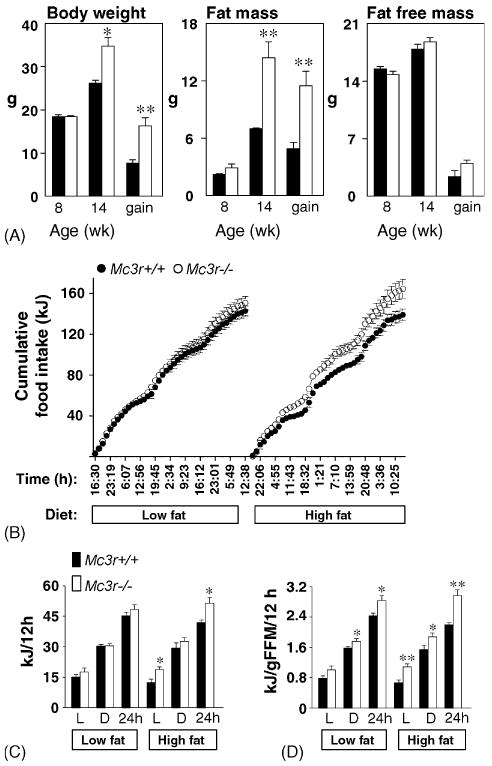
Diet-dependent hyperphagia of lean male Mc3r−/− mice that is restricted to the lights-on period. (A) Disproportionate weight gain of male Mc3r−/− mice on the B6 background when fed high fat diet is due to increase in fat mass, as determined by NMR. Mice were weaned on purified low fat diet (10% kJ/fat), and body composition measured at 8 week. Mice were then fed a purified high fat diet (45% kJ/fat) for 6 week (n = 6/group). (B) Cumulative food intake (in kJ) of purified low fat or high fat diet was measured for 3 days periods indicated hyperphagia of Mc3r−/− mice relative to wild type littermates on the high fat diet. (C and D) Mean intake during the lights-on (L) or dark (D) periods was calculated, showing a significant increase in food consumption of Mc3r−/− mice during the lights on period. Historical data suggest that Mc3r−/− mice exhibit a modest reduction in longitudinal growth and lean mass [9,11]. For the mice used in this experiment, fat free mass (FFM) was significantly lower in Mc3r−/− mice compared to wild type littermates (FFM in g: 17.4 ± 0.4 g compared to 19.2 ± 0.4 g, P < 0.01), accompanied by a modest increase in fat mass (FM) (FM in g: 5.3 ± 0.2 g compared to 4.0 ± 0.2 g, P < 0.01). When energy consumption was adjusted for fat free mass, energy consumption per gram of FFM was significantly higher irrespective of diet. Significantly different from wild type (WT), *P < 0.05, **P < 0.01.
When analyzed as kJ ingested per mouse, cumulative food intake of Mc3r−/− mice was not significantly different from WT when fed a low fat diet (Research Diets 12450B, 10% kJ/fat) (Fig. 2B and C). However, Mc3r−/− mice became mildly hyperphagic after the introduction of a purified high fat diet (Research Diets 12492, 60% kJ/fat). Interestingly, the hyperphagia of Mc3r−/− mice on the high fat diet was due primarily to an increase in food intake during the lights-on period (Fig. 2C). When adjusted for difference in fat free mass, there also was evidence of disproportionately high food consumption of Mc3r−/− mice on the low fat diet, although not to the same degree observed on the high fat diet (Fig. 2D). The results of this experiment measuring food intake using the CLAMS system indicate that the MC3R might also function to reduce food consumption, especially in response to high diets. Surprisingly, the hyperphagia of Mc3r−/− mice fed high fat diets is most pronounced during the daytime, suggesting that the MC3R might function in the circadian regulation of ingestive behavior. One of the densest sites of MC3R mRNA expression is in the VMH [48]. Chemical lesions of the VMH have been shown to increase food intake during the daytime, and to possibly function in the integration of feeding with circadian rhythms [13].
3.2. Energy expenditure and energy balance of melanocortin receptor knockout mice
Energy homeostasis involves long-term coordination of energy expenditure with energy consumption [34,49,50]. Hyperphagia owing to highly palatable calorie dense (i.e., ‘western’) diets or to a protein-deficient diet is associated with an increase in energy expenditure, or ‘diet-induced thermogenesis’ (DIT) [53,58]. DIT involves an increase in sympathetic nervous activity and stimulation of β-adrenergic receptors; mice lacking all of the three β-adrenergic receptors (‘β-less’ mice) do not exhibit DIT and rapidly gain weight when fed high fat diets [4]. This latter study elegantly demonstrates that an inappropriately low metabolic rate can be a mechanism for increasing adiposity, as food intake of the‘β-less’ mice was not significantly different from wild type controls.
3.2.1. Regulation of energy expenditure by the MC4R
The melanocortin system, acting in the central nervous system, potentially regulates energy expenditure through effects on sympathetic nervous activity [21,44]. Young lean Mc4r−/− mice also exhibit reduced physical activity, providing another mechanism for reduced energy expenditure [10,12,57]. The interpretation of data from experiments using indirect calorimetry to compare energy expenditure of obese melanocortin receptor knockout with lean mice is confounded by the issue of adjusting for metabolic size [29]. However, the results from studies investigating the effect of melanocortin ligands on energy expenditure have yielded informative data indicating that the MC4R regulates energy expenditure. Administration of non-specific melanocortin receptor agonists, such as melanotan-II, either centrally or peripherally increases oxygen consumption in lean and obese rodents [12,16,31,32,42]. In Mc4r−/− mice, the increase in oxygen consumption associated with peripheral administration of high doses of MTII is not observed, suggesting a crucial role for this receptor in the regulation of thermogenesis by the melanocortin system [12].
The MC4R also appears to be involved in the metabolic adaptation to hyperphagia and the transition between low- and high-fat diets. Mc4r−/− mice fed high fat diet exhibit, in addition to a more severe and prolonged hyperphagia, an increase in feed efficiency (weight gain per kJ ingested) [10]. Increased weight gain as a function of food intake suggests differences in the efficiency of digestion and nutrient absorption, or a reduction in energy expenditure. It was subsequently demonstrated that DIT, a 10–20% increase in oxygen consumption associated with hyperphagia observed in wild type mice, is not observed in Mc4r−/− mice [10]. Reduced DIT and low fatty acid oxidation, indicated by an increase in the respiratory exchange ratio, was also observed for Mc4r−/− mice on the B6 background [2]. The result from the latter study suggested that an integrated measurement of energy balance (i.e., energy consumed less energy expended) is more informative compared to independent measurements of energy expenditure and food intake. In two separate experiments, we have observed that the change in body weight over a 3–4 days period in the metabolic chambers is positively correlated with energy balance for males (r2 = 0.67) and females (r2 = 0.56). The approach of estimating energy balance (i.e., kJ ingested–kJ oxidized) also negates the necessity for using denominators, such as body weight or lean mass, when comparing heavy and lean strains of mice.
3.2.2. RER of Mc4r−/− mice
A high RER indicates a reduced use of fatty acid oxidation for daily energy expenditure, a condition associated with increased risk for weight gain and insulin resistance [46]. There are two independent reports of a higher RER of Mc4r−/− mice on different genetic background. Male mice on a mixed background fed low fat standard chow exhibit a higer RER [12]. We have also observed a higher RER in male Mc4r−/− mice on the B6 background (data not shown). Our group has also observed a higher RER for female Mc4r−/− mice on the B6 background fed a purified high fat diet [2]. Overall, the data support the hypothesis that the obese phenotype of Mc4r−/− mice is associated with reduced fatty acid oxidation.
3.2.3. Measurement of energy expenditure of Mc3r−/− mice
The initial investigation of energy expenditure of Mc3r−/− mice on the mixed 129;B6 background indicated that energy expenditure is not significantly different compared to wild type littermates [9,11]. Mc3r−/− mice on the mixed 129;B6 background were also distinguished from Mc4r−/− mice by exhibiting a normal DIT response to hyperphagia [9]. Both groups involved in the initial investigation of the phenotype of Mc3r−/− mice reported reduced spontaneous physical activity, measured using a photobeam system [11] or wheel cages [9]. We have also observed a reduction in physical activity of pre-obese Mc3r−/− mice on the B6 background, with reduced movements in the X and Z-axes (Fig. 4 and data not shown). The reduction in physical activity occurs during the dark, when mice are most active, with no significant difference observed during daytime. This observation raises an interesting question: how can energy expenditure can be within a normal range when physical activity is reduced was not explained. The discrepancy between physical activity and oxygen consumption data might suggest a modest elevation in basal or resting metabolic rate that is not of sufficient magnitude to be measured using indirect calorimetry.
Fig. 4.
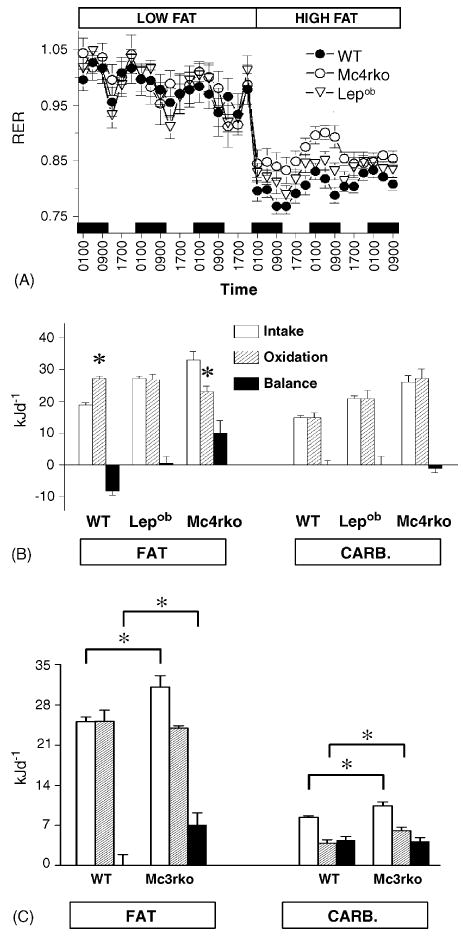
Impaired changes in substrate oxidation in melanocortin receptor knockout mice are associated with increased fat balance. Obese female Mc4r−/− mice exhibit a delayed reduction in the RER following the transition from a low- to high-fat diet (A). (B) The balance of nutrient intake and oxidation is calculated using the mean RER over 3d for mice fed the high fat diet; the balance of carbohydrate intake and oxidation is comparable between all Mc4r−/− and wild type mice, with an imbalance limited to fat metabolism. In A and B, obese female leptin deficient (Lepob/Lepob) mice were included in the study as an obese control. (C) Lean male Mc3r−/− mice exhibit a comparable imbalance of fat intake and oxidation when fed a high fat diet. In an experiment using lean male Mc3r−/− and wild type littermates at 3 months of age, fat balance was significantly higher in Mc3r−/− mice compared to wild type, whereas the balance of carbohydrate intake and oxidation was similar between strains. (A and B) reproduced with permission from [2]. Significantly different intake (B), or between groups indicated by bars (C), *P < 0.05.
Using the CLAMS, we have investigated energy expenditure of lean male B6 Mc3r−/− mice with wild type B6 littermates exposed to purified low or high fat diets. The food intake data for this group is shown in Fig. 2. As reported previously, energy expenditure of male Mc3r−/− mice appeared normal when compared to wild type B6 mice (Fig. 3A). In this experiment, energy expenditure of Mc3r−/− mice and wild type mice was not significantly affected by diet. However, the decline in the RER correlating with increased fatty oxidation was observed following introduction of the high fat diet, irrespective of genotype (Fig. 3B). It should be noted that the transition from the purified low fat to high fat diets did not result in a marked increase in total caloric consumption (Fig. 2), observed previously in studies using standard and breeder chow diets [10]. Indeed, the changes in total energy consumption on the low and high fat diets leading to hyperphagia of Mc3r−/− mice were modest, varying by 8–9% (energy consumption on the low and high fat diet for Mc3r−/− mice, 47.6 ± 2.6 kJ day−1 versus 51.8 ± 3.3 kJ day−1, P = 0.36 by paired Student's t-test; for wild type mice, 45.3 ± 1.7 kJ day−1 versus 41.9 ± 1.3 kJ day−1, P = 0.19 by paired Student's t-test). Without marked increases in total caloric consumption, a robust increase in energy expenditure would not be expected in mice maintaining a positive energy balance.
Fig. 3.
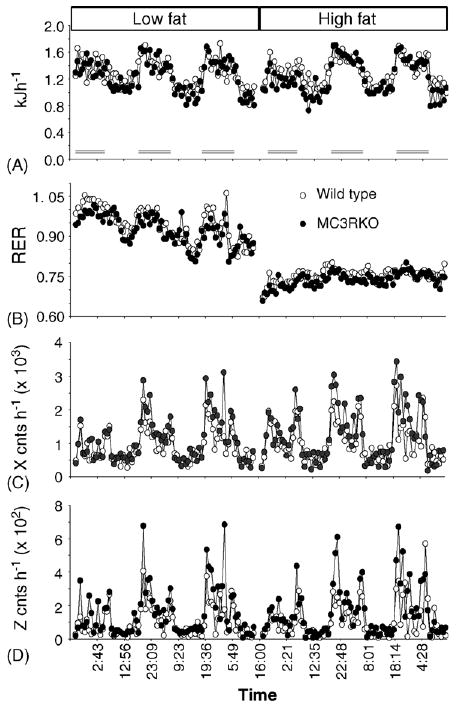
Energy expenditure of lean male Mc3r−/− mice fed a low-fat or high-fat diet. Lean male Mc3r−/− mice exhibit a normal diurnal variation in energy expenditure which is not affected by dietary fat content (A), but exhibit a modest increase in the RER consistent with reduced fatty acid oxidation (B). The dark periods are indicated by the bars shown in (A). Spontaneous physical activity, indicated by movements in the X-axis (C) and Z-axis (D) was reduced for Mc3r−/− mice in the period.
3.2.4. RER of Mc3r−/− mice
In the two initial investigations of the phenotype of Mc3r−/− mice, a modest increase in the RER was observed by one of the groups [9]. In the pre-obese male B6 Mc3r−/− mice used in the experiment reported in this manuscript, a modest increase in the RER was also observed, irrespective of diet (Fig. 3B, Table 1). We have observed a similar increase in the RER in pre-obese female B6 Mc3r−/− mice (data not shown). While the difference in RER appears modest (0.962 compared to 0.925), the expected range for RER data is 0.7–1.0. A difference of 0.04 would therefore indicate at 12–14% difference in substrate utilization [19]. We have used energy expenditure and RER data to estimate the balance of fat and carbohydrate [2,19]. The results from our analysis of female B6 Mc4r−/− mice are shown in Fig. 4A and B. The results of that experiment suggest that an imbalance of fat consumption and oxidation contributes to increased adiposity. In that experiment, the RER of Mc4r−/− mice had returned to within normal range by the third day. However, it should be noted that the mice used for this experiment were already obese, possibly activating MC4R-independent mechanisms for preventing extreme obesity [2].
Table 1. Increased RER of lean male Mc3r−/− mice compared to wild type littermates.
| Diet | Period | Wild type | MC3R−/− | P-value |
|---|---|---|---|---|
| LF | Light | 0.899 ± 0.007 | 0.902 ± 0.018 | 0.883 |
| Dark | 0.925 ± 0.006 | 0.962 ± 0.012 | 0.022 | |
| 24 h | 0.915 ± 0.004 | 0.939 ± 0.015 | 0.173 | |
| HF | Light | 0.734 ± 0.007 | 0.759 ± 0.004 | 0.001 |
| Dark | 0.741 ± 0.004 | 0.754 ± 0.040 | 0.041 | |
| 24 h | 0.745 ± 0.005 | 0.762 ± 0.004 | 0.016 |
RER data recorded over a 3-day period on each diet was broken down according to light period, or per 24 h. On the low fat diet (LF), RER of Mc3r−/− mice is significantly increased during the dark period. On the high fat diet, RER was significantly increased during the light and dark periods. This data was presented in hourly form in Fig. 3. P-values were determined by Student's t-test.
In the current experiment, it was evident that Mc3r−/− mice also have a significantly higher positive fat balance compared to wild type controls (Fig. 4C). The balance of total energy consumption and oxidation (i.e., 24 h energy intake less 24 h energy expenditure) for Mc3r−/− mice and wild type littermates was not significantly different on the low fat diet (17.2 ± 2.0 kJ day−1 versus 15.7 ± 2.2 kJ day−1, P = 0.6). However, on the high fat diet energy balance was significantly higher for Mc3r−/− mice (18.6 ± 3.1 kJ day−1 versus 10.3 ± 2.2 kJ day−1, P = 0.05). The difference in the energy balance of Mc3r−/− and wild type mice was primarily due to fat balance, which was higher in Mc3r−/− mice compared to wild type mice. There was no difference in carbohydrate balance, which exhibited a comparable positive value in both Mc3r−/− mice and wild type littermates (Fig. 2C). The magnitude of the imbalance in fat intake and oxidation, estimated to be in the order of 7 kJ day−1, was comparable to that previously observed in female Mc4r−/− mice (Fig. 1B) [2]. Assuming 1 g of fat contains 9 kcal, or 37.7 kJ, the imbalance of fat intake and oxidation for both melanocortin receptor knockout models, in the range of 7–10 kJ day−1, is roughly equivalent to 180 mg of triglyceride.
4. Summary and conclusions
The melanocortin system is involved in the regulation of energy intake, energy expenditure, and the balance of substrate oxidation. Loss of MC3R or MC4R function is associated with obesity. The obese phenotype of MC4R mutant mice is sensitive to diets with increased dietary fat content, an observation made by three laboratories. One of the important down stream effectors discussed by this review for the regulation of food intake, and possibly metabolism, by the MC4R when challenged with palatable high fat diets is BDNF in the ventromedial nucleus of the hypothalamus. The obese phenotype of MC3R knockout mice is also probably sensitive to high diets, perhaps more so than MC4R knockout mice. At this time, no mechanism other than reduced activity has been identified for the obese phenotype of MC3R knockout mice. Moreover, there has been no attempt to identify down stream targets of MC3R action in the central nervous system.
For both Mc3r−/− and Mc4r−/− mice on the B6 background, our data indicate an imbalance in fat consumption and oxidation compared to wild type littermates as a mechanism explaining increased adiposity on high fat diets. The mechanism by which MC3R and MC4R regulate balance fat consumption and oxidation is not clear. Both Mc3r−/− and Mc4r−/− mice are reported to have reduced physical activity, which could contribute to reduced oxidation of fatty acids by skeletal muscle. Whether MC3R expressed in the hypothalamus, like the MC4R, is involved in the regulation of sympathetic nervous activity has not been investigated.
An interesting and novel finding reported here is that Mc3r−/− mice on the B6 background can exhibit a modest hyperphagia when fed a purified high fat diet. The hyperphagia of male Mc3r−/− mice on the B6 background was not observed for Mc3r−/− mice on a mixed 129;B6 background. This could indicate an effect of genetic strain on a complex behavioral phenotype. Alternatively, the methods used to measure food intake and the use of purified diets, as opposed to standard rodent chows, might also explain the difference in phenotype.
The increased consumption of high fat diet by Mc3r−/− mice mice on the B6 background appears to be limited to the lights-on period. Whether the hyperphagia associated with the introduction of the high fat diet persists, or is a transient response to the transition from a low to high fat diet, requires further experiments. In addition, whether this data indicates a potential role for the MC3R in the circadian regulation of food consumption remains to be determined. Interestingly, one of the brain regions with the highest density of MC3R mRNA expression is the VMH [48]. The VMH has been suggested to be an important site for coordinating physical activity with feeding time [13]. Whether VMH neurons expressing the MC3R are involved in this behavioral response to restricting food intake is not known, but could be investigated using conditional Mc3r gene knockouts or site-specific rescue with viral vectors.
The measurement of energy balance is important for studies analyzing phenotypes of knockout or transgenic models of obesity. However, there is still considerable uncertainty over how to adjust for differences in lean and fat mass when comparing energy expenditure of obese and lean mice [29,43]. Common denominators that have been used include body weight, body weight to the three quarter power, or fat free mass. In all cases, assumptions are made about body composition and the contribution of different organs and tissues to energy expenditure of the whole organism, often leading to errors in the interpretation of metabolic data [29,43]. We believe that the experiments described in this review demonstrate a useful approach for the analysis of metabolism in rodent models of obesity. It appears to this author that calculating the balance of energy intake and energy expenditure is a preferable approach, thereby eliminating the use of denominators normally used to adjust energy expenditure.
Acknowledgments
The author thanks M. Josephine Babin, Dr. Jennifer McClaine, Emily Meyer and Diana Albarado for technical assistance. Dr.'s Leslie Kozak, James Delany, and Eric Ravussin are thanked for comments and advice on calorimetry data and mouse studies. The melanocortin receptor knockout mice were kindly provided by Dr. Roger Cone (The Vollum Institute and the Center for the Study of Weight Regulation, Portland, Oregon) and Dr. Dennis Huszar (Millenium Pharmaceuticals, Cambridge, Massachusetts). This research is supported by the grants from the Pennington Biomedical Research Foundation, the Louisiana Board of Regents, the American Diabetes Association, and DK068330.
References
- 1.Accili D, Kido Y, Nakae J, Lauro D, Park BC. Genetics of type 2 diabetes: insight from targeted mouse mutants. Curr Mol Med. 2001;1(1):9–23. doi: 10.2174/1566524013364040. [DOI] [PubMed] [Google Scholar]
- 2.Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, et al. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology. 2004;145(1):243–52. doi: 10.1210/en.2003-0452. [DOI] [PubMed] [Google Scholar]
- 3.Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144(5):1753–60. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- 4.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297(5582):843–5. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 5.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 6.Benoit SC, Schwartz MW, Lachey JL, Hagan MM, Rushing PA, Blake KA, et al. A novel selective melanocortin-4 receptor agonist reduces food intake in rats and mice without producing aversive consequences. J Neurosci. 2000;20(9):3442–8. doi: 10.1523/JNEUROSCI.20-09-03442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1(4):619–25. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 8.Butler AA, Cone RD. Knockout studies defining different roles for melanocortin receptors in energy homeostasis. Ann N Y Acad Sci. 2003;994:240–5. doi: 10.1111/j.1749-6632.2003.tb03186.x. [DOI] [PubMed] [Google Scholar]
- 9.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141(9):3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 10.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4(6):605–11. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- 11.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 12.Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9(2):145–54. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 13.Choi S, Wong LS, Yamat C, Dallman MF. Hypothalamic ventromedial nuclei amplify circadian rhythms: do they contain a food-entrained endogenous oscillator? J Neurosci. 1998;18(10):3843–52. doi: 10.1523/JNEUROSCI.18-10-03843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coll AP, Farooqi IS, Challis BG, Yeo GS, O' Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab. 2004;89(6):2557–62. doi: 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- 15.Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, et al. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res. 1996;51:287–317. [PubMed] [Google Scholar]
- 16.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24(1):155–63. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 17.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284(5420):1670–2. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 18.Diamond J. The double puzzle of diabetes. Nature. 2003;423(6940):599–602. doi: 10.1038/423599a. [DOI] [PubMed] [Google Scholar]
- 19.Elia M, Livesey G. Theory and validity of indirect calorimetry during net lipid synthesis. Am J Clin Nutr. 1988;47(4):591–607. doi: 10.1093/ajcn/47.4.591. [DOI] [PubMed] [Google Scholar]
- 20.Ellacott KL, Cone RD. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res. 2004;59:395–408. doi: 10.1210/rp.59.1.395. [DOI] [PubMed] [Google Scholar]
- 21.Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S78–82. doi: 10.1038/sj.ijo.0801918. [DOI] [PubMed] [Google Scholar]
- 22.Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004 doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- 23.Fekete C, Marks DL, Sarkar S, Emerson CH, Rand WM, Cone RD, et al. Effect of Agouti-related protein in regulation of the hypothalamic-pituitary-thyroid axis in the melanocortin 4 receptor knockout mouse. Endocrinology. 2004;145(11):4816–21. doi: 10.1210/en.2004-0476. [DOI] [PubMed] [Google Scholar]
- 24.Friedman JM. A war on obesity, not the obese. Science. 2003;299(5608):856–8. doi: 10.1126/science.1079856. [DOI] [PubMed] [Google Scholar]
- 25.Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL. Overexpression of Agrt leads to obesity in transgenic mice. Nat Genet. 1997;17(3):273–4. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- 26.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94(16):8878–83. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halatchev IG, Ellacott KL, Fan W, Cone RD. PYY3-36 inhibits food intake through a melanocortin-4 receptor-independent mechanism. Endocrinology. 2004 doi: 10.1210/en.2003-1754. [DOI] [PubMed] [Google Scholar]
- 28.Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, et al. Central serotonin and melanocortin pathways regulating energy homeostasis. Ann N Y Acad Sci. 2003;994:169–74. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- 29.Himms-Hagen J. On raising energy expenditure in ob/ob mice. Science. 1997;276(5315):1132–3. doi: 10.1126/science.276.5315.1132. [DOI] [PubMed] [Google Scholar]
- 30.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 31.Hwa JJ, Ghibaudi L, Gao J, Parker EM. Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2001;281(2):R444–51. doi: 10.1152/ajpregu.2001.281.2.R444. [DOI] [PubMed] [Google Scholar]
- 32.Jonsson L, Skarphedinsson JO, Skuladottir GV, Atlason PT, Eiriksdottir VH, Franzson L, et al. Melanocortin receptor agonist transiently increases oxygen consumption in rats. Neuroreport. 2001;12(17):3703–8. doi: 10.1097/00001756-200112040-00020. [DOI] [PubMed] [Google Scholar]
- 33.Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, et al. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J Clin Invest. 1999;103(3):383–91. doi: 10.1172/JCI4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–4. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 35.MacNeil DJ, Howard AD, Guan X, Fong TM, Nargund RP, Bednarek MA, et al. The role of melanocortins in body weight regulation: opportunities for the treatment of obesity. Eur J Pharmacol. 2002;440(2–3):141–57. doi: 10.1016/s0014-2999(02)01425-5. [DOI] [PubMed] [Google Scholar]
- 36.Marks DL, Butler AA, Cone RD. Melanocortin pathway: animal models of obesity and disease. Ann Endocrinol (Paris) 2002;63(2 Pt 1):121–4. [PubMed] [Google Scholar]
- 37.Marks DL, Butler AA, Turner R, Brookhart G, Cone RD. Differential role of melanocortin receptor subtypes in cachexia. Endocrinology. 2003;144(4):1513–23. doi: 10.1210/en.2002-221099. [DOI] [PubMed] [Google Scholar]
- 38.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21(1):119–22. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 39.Marsh DJ, Miura GI, Yagaloff KA, Schwartz MW, Barsh GS, Palmiter RD. Effects of neuropeptide Y deficiency on hypothalamic agouti-related protein expression and responsiveness to melanocortin analogues. Brain Res. 1999;848(1–2):66–77. doi: 10.1016/s0006-8993(99)01962-9. [DOI] [PubMed] [Google Scholar]
- 40.Ni XP, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest. 2003;111(8):1251–8. doi: 10.1172/JCI16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oku J, Bray GA, Fisler JS, Schemmel R. Ventromedial hypothalamic knife-cut lesions in rats resistant to dietary obesity. Am J Physiol. 1984;246(6 Pt 2):R943–8. doi: 10.1152/ajpregu.1984.246.6.R943. [DOI] [PubMed] [Google Scholar]
- 42.Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes. 2002;51(5):1337–45. doi: 10.2337/diabetes.51.5.1337. [DOI] [PubMed] [Google Scholar]
- 43.Poehlman ET. Reduced metabolic rate after caloric restriction-can we agree on how to normalize the data? J Clin Endocrinol Metab. 2003;88(1):14–5. doi: 10.1210/jc.2002-021672. [DOI] [PubMed] [Google Scholar]
- 44.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23(14):5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2002;967:363–78. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 47.Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15(10):1748–57. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 48.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, et al. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA. 1993;90(19):8856–60. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr. 2000;71(6):1421–32. doi: 10.1093/ajcn/71.6.1421. [DOI] [PubMed] [Google Scholar]
- 50.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R183–92. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 51.Rosenfeld RD, Zeni L, Welcher AA, Narhi LO, Hale C, Marasco J, et al. Biochemical, biophysical, and pharmacological characterization of bacterially expressed human agouti-related protein. Biochemistry. 1998;37(46):16041–52. doi: 10.1021/bi981027m. [DOI] [PubMed] [Google Scholar]
- 52.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139(10):4428–31. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 53.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281(5726):31–5. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 54.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, et al. Melanocortin receptors in leptin effects. Nature. 1997;390(6658):349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 55.Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11(5):593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 56.Smith BK, Andrews PK, West DB. Macronutrient diet selection in thirteen mouse strains. Am J Physiol Regul Integr Comp Physiol. 2000;278(4):R797–805. doi: 10.1152/ajpregu.2000.278.4.R797. [DOI] [PubMed] [Google Scholar]
- 57.Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA. 2000;97(22):12339–44. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stock MJ. Gluttony and thermogenesis revisited. Int J Obes Relat Metab Disord. 1999;23(11):1105–17. doi: 10.1038/sj.ijo.0801108. [DOI] [PubMed] [Google Scholar]
- 59.Strauss RS, Pollack HA. Epidemic increase in childhood overweight. Jama. 2001;286(22):2845–8. doi: 10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- 60.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37(9):1163–7. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 61.Thiele TE, van Dijk G, Yagaloff KA, Fisher SL, Schwartz M, Burn P, et al. Central infusion of melanocortin agonist MTII in rats: assessment of c-Fos expression and taste aversion. Am J Physiol. 1998;274(1 Pt 2):R248–54. doi: 10.1152/ajpregu.1998.274.1.R248. [DOI] [PubMed] [Google Scholar]
- 62.Weide K, Christ N, Moar KM, Arens J, Hinney A, Mercer JG, et al. Hyperphagia, not hypometabolism, causes early onset obesity in melanocortin-4 receptor knockout mice. Physiol Genomics. 2003;13(1):47–56. doi: 10.1152/physiolgenomics.00129.2002. [DOI] [PubMed] [Google Scholar]
- 63.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 64.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6(7):736–42. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]


