Abstract
The aims of the present study were to establish if nalfurafine, a kappa opioid agonist, inhibits compulsive scratching in mice elicited by the s.c. administration (behind the neck) of 5′-guanidinonaltrindole (GNTI), a kappa opioid antagonist; to assess if nalfurafine prevents c-fos expression provoked by GNTI or compound 48/80, two chemically diverse pruritogens; and to distinguish on the basis of neuroanatomy, those neurons in the brainstem activated by either GNTI-induced itch or formalin-induced pain (both compounds given s.c. to the right cheek). Pretreatment of mice with nalfurafine (0.001–0.03 mg/kg, s.c.) attenuated GNTI (0.3 mg/kg)-evoked scratching dose-dependently. A standard antiscratch dose of nalfurafine (0.02 mg/kg) had no marked effect on the spontaneous locomotion of mice. Tolerance did not develop to the antiscratch activity of nalfurafine. Both GNTI and compound 48/80 provoked c-fos expression on the lateral side of the superficial layer of the dorsal horn of the cervical spinal cord and pretreating mice with nalfurafine inhibited c-fos expression induced by both pruritogens. In contrast to formalin, GNTI did not induce c-fos expression in the trigeminal nucleus suggesting that pain and itch sensations are projected differently along the sensory trigeminal pathway. Our data indicate that the kappa opioid system is involved, at least in part, in the pathogenesis of itch; and that nalfurafine attenuates excessive scratching and prevents scratch-induced neuronal activity at the spinal level. On the basis of our results, nalfurafine holds promise as a potentially useful antipruritic in human conditions involving itch.
Keywords: itch, pain, kappa opioid receptor agonist, compound 48/80, kappa opioid receptor antagonist
INTRODUCTION
Itch is a sensation that provokes the desire or reflex to scratch. Although antihistamines can relieve itching in various allergies, they do not lessen itch in skin, liver or kidney diseases. Gabapentin (a γ-aminobutyric acid analog), cholestyramine (anion exchange resin), naloxone and nalmefene (opioid receptor antagonists) have been tested clinically, but an efficacious treatment against itch has yet to be identified (Patel et al., 2007; Bergasa, 2008). More promisingly, nalfurafine (a kappa opioid agonist) was reported to be safe and effective in patients with pruritus of chronic renal failure (Wikström et al., 2005).
The suppressing activity of kappa agonists against different pruritogens has been reported in rodents and monkeys (Gmerek and Cowan, 1988; Cowan and Kehner, 1997; Kehner et al., 1999; Kamei and Nagase, 2001; Ko et al., 2003). The antipruritic activity of nalfurafine (a 4,5-epoxymorphinan analgesic) (Nagase et al., 1998; Endoh et al., 1999; Endoh et al., 2000; Endoh et al., 2001) was demonstrated for the first time against substance P- and histamine-induced scratching (Togashi et al., 2002) using the standard mouse model described by Kuraishi et al. (1995). In this procedure, scratching behavior is used as a quantitative index for the study of itch. Subsequent studies established that nalfurafine attenuates scratching elicited by compound 48/80 (a mast cell activator), chloroquine (an antimalarial agent) and agmatine (a bioamine) in mice (Inan and Cowan, 2004; Wang et al., 2005; Inan and Cowan, 2006a); scratching secondary to cholestasis induced by chronic ethynylestradiol injections in rats (Inan and Cowan, 2006b); and scratching associated with intravenously administered morphine in monkeys (Wakasa et al., 2004). Taken together, nalfurafine clearly possesses a broad spectrum of antiscratch (and probably antipruritic) activities. The antiscratch activity of nalfurafine against substance P in mice (Togashi et al., 2002) and morphine in monkeys (Ko and Husbands, 2009) was attenuated by nor-binaltorphimine, a kappa opioid receptor antagonist. These reports implicate kappa receptors in mediating the antipruritic effects of nalfurafine, however, the universality of this compound’s antiscratch activity invokes mechanisms additional to a possible direct interaction with kappa opioid receptors.
Compound 48/80 is a frequently used pruritogen in animal studies. It induces a rapid release of inflammatory mediators like histamine from mast cells located in connective tissue and skin (Enerbäck, 1966; Koibuchi et al., 1985). While Sugimoto et al. (1998) reported that scratching associated with compound 48/80 may be suppressed by various H1 antagonists, Inagaki et al. (2002) found that other members of that class (ketotifen and terfenadine) antagonized the increased vascular permeability induced by compound 48/80 but not the scratching behavior. Also, compound 48/80 elicits scratching in mast cell-deficient mice suggesting that the behavior is not mediated by mast cell activation (Inagaki et al., 2002). Previously, we reported that a selective kappa opioid receptor antagonist, 5′-guanidinonaltrindole (GNTI) (Jones and Portoghese, 2000; Black et al., 2003), precipitates immediate, excessive scratching in mice (Cowan and Inan, 2009). This compulsive behavior is not markedly affected when the mice are pretreated orally with fexofenadine or JNJ 10191584, selective antagonists at H1 and H4 receptors, respectively (unpublished data).
The primary afferent neurons activated by histamine in human skin have been identified by Schmelz et al. (1997). Mechano-insensitive itch-mediating C fibers have slow conduction velocities and represent only 5% of afferent C fibers in skin. Also, only cowhage-activated mechano-sensitive fibers are described in human skin (Namer et al., 2008). Andrew and Craig (2001) reported that neurons sensitive to histamine are located on the superficial part of the dorsal horn lamina I spinothalamic tract. Since antihistaminics are not effective against all kinds of itch, an additional pathway is required to explain other clinical itch conditions.
The present studies were designed to answer the following key questions: a) does nalfurafine inhibit excessive scratching elicited by GNTI in mice? b) do compound 48/80 and GNTI activate similar neuron populations in the dorsal horn? and c) does nalfurafine influence c-fos expression provoked by compound 48/80 and GNTI? Additionally, in order to examine a possible overlap or difference between neurons activated by either itch or pain stimuli, we performed c-fos immunohistochemistry in brainstem sections obtained from mice injected with saline, formalin or GNTI using the model recently described by Shimada and LaMotte (2008).
EXPERIMENTAL PROCEDURES
Animals
Male, Swiss-Webster mice (25–30 g, n = 6–10, Ace Laboratories, Boyertown, PA) were used. Animals were housed under a 12 h light/dark cycle with food and water available ad libitum in the Animal Facility for at least 4 days. Experiments were carried out between 10:00 AM and 5:00 PM. Experimental procedures were approved by Temple University Institutional Animal Care and Use Committee, in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals.
We first conducted behavioral studies to assess the antiscratch activity of nalfurafine. All test agents, except compound 48/80, were administered in a dose volume of 0.25 ml/ 25 g. We used fixed s.c. doses of GNTI (0.3 mg/kg) and compound 48/80 (50 µg in 100 µl) to induce scratching, based on previous results (Cowan and Kehner, 1997; Cowan et al., 2002; Cowan and Inan, 2009).
Effect of nalfurafine on GNTI-induced scratching behavior
Mice were acclimated individually in rectangular observation boxes for at least 2 h, then injected s.c. in the flank with either saline or nalfurafine (0.001–0.03 mg/kg). Twenty min later, GNTI (0.3 mg/kg, s.c.) was administered midline behind the neck of each mouse. The number of hindleg scratches directed to the back of the neck was counted for 30 min. We also examined if nalfurafine would inhibit scratching when given after this behavior had started. Thus, mice were injected s.c. with either saline or 0.3 mg/kg of GNTI. Five min later, nalfurafine (0.001–0.03 mg/kg) was administered s.c. and the number of scratches was counted for 25 min.
Tolerance study
We conducted a subchronic study to investigate if tolerance develops to the antiscratch activity of nalfurafine. Since tolerance does not develop to the pruritic action of GNTI (Cowan et al., 2002), we only performed multiple injections with nalfurafine. Every day for 10 days, mice were pretreated (at −20 min) with a fixed dose of nalfurafine (0.02 mg/kg, s.c.) then challenged with GNTI and the number of scratches counted for 30 min.
Measurement of locomotor activity
We examined whether or not the antiscratch effect of nalfurafine might be a result of behavioral depression since sedation is a known side effect of kappa agonists. Mice were placed individually in a cage (27 cm × 48 cm × 20 cm) and acclimated for 1 h. They were injected s.c. with either saline or nalfurafine (0.02 or 0.04 mg/kg) and monitored for total distance traveled over 1 h using a Digiscan D Micro System (AccuScan, Columbus, OH).
Application of pain and itch stimuli to the same site
We used a recently described mouse model for administrating itch and pain stimuli (Shimada and LaMotte, 2008). After acclimation in their individual observation boxes, each mouse was injected s.c. with saline, GNTI (30 µg in 10 µl) or 5% formalin (10 µl) into the right cheek and observed under blind conditions for 10 min. We preferred the s.c. route, rather than the intradermal route, for consistency with the experiments described above. Since both pain and itch stimuli elicited their behavioral effects within the first 10 min of Shimada and LaMotte’s original study (2008), we also observed mice for 10 min. Animals were scored for wiping (indicating pain), grooming and scratching (indicating itch) of the injected cheek. Two h after the injections, 3 mice from each group were perfused intracardially (described in the next section) and brains were removed to investigate (using c-fos immunohistochemistry) whether pain and itch stimulate different neurons in brainstem sections.
Determination of c-fos immunoreactivity in cervical spinal cord and brainstem sections
We investigated whether a) GNTI and/or compound 48/80 provokes c-fos expression and pretreatment with nalfurafine inhibits this effect in the dorsal horn of the cervical spinal cord, and b) pain and scratching (itch) activate different types of neuron in the brainstem. Mice were injected s.c. with either saline or nalfurafine (0.02 mg/kg). Twenty min later, they were administered (s.c. behind the neck) saline, compound 48/80 (50 µg in 100 µl) or GNTI (0.3 mg/kg). For another aim, mice (n=3) were injected s.c. in the right cheek with saline, formalin or GNTI. Two h later, the animals were deeply anesthetized with urethane (1.2 g/kg, i.p.) and perfused intracardially with ice-cold 0.1 M phosphate buffer saline (PBS) followed by 4% paraformaldehyde/0.2% picric acid in 0.1 M PBS. The cervical spinal cords and brains were removed and postfixed in 4% paraformaldehyde solution overnight at 1°C. Tissue samples were transferred to 30% sucrose solution for at least 3–4 days before sectioning. In order to investigate if GNTI-induced c-fos expression is a consequence of scratching behavior, we first anesthetized mice with urethane and then administered GNTI. Again, two h after GNTI injection, mice were perfused. Cervical spinal cord (C5–C7) sections (35 µm) and brainstem sections (50 µm) were cut at −19°C using a cryostat. Free floating sections were kept in PBS solution at 4°C until immunohistochemistry was performed. Ten cervical spinal cord sections were randomly selected and every third brainstem section was utilized to conduct immunohistochemistry.
Tissues were processed for c-fos immunoreactivity by the avidin-biotin complex procedure as described previously (Brailoiu et al., 2005). They were first treated with 3% H2O2 to reduce endogenous peroxidase, washed two times for 10 min with PBS, and blocked with 20% normal goat serum (1:20) at room temperature for 2 h. The sections were then incubated on a shaker for 2 days at 4°C with rabbit c-fos antibody 1:4000 dilution (F7799, Sigma, St. Louis, MO). After thorough rinsing, sections were incubated in biotinylated anti-rabbit immunoglobulin G secondary antibody (1:300 dilution, Vector Laboratories, Burlingame, CA) for 2 h at room temperature. Following two 10 min rinses with PBS, sections were incubated in avidin-biotin-peroxidase complex at room temperature for 90 min (1:150 dilution, Vectastain ABC Elite kit, Vector Laboratories). Following three 10 min washes in Tris-buffered saline, sections were reacted with 0.05% diaminobenzidine (Sigma, St. Louis, MO)/ 0.001% H2O2 solution for 5–7 min and washed three times for 10 min with Tris-buffered saline. Sections were mounted on slides with 0.25% gel alcohol, air-dried, dehydrated with absolute alcohol (50%, 70%, 95%, 100%, 100% for 10 min) followed by xylene (two times for 10 min) and coverslipped with Permount. C-fos positive nuclei were observed under a light microscope and counted at 40x magnification (for aim a).
Determination of c-fos mRNA using the reverse transcriptase-PCR technique
We measured c-fos mRNA levels in mice injected s.c. with either saline or GNTI (0.3 mg/kg) using the RT-PCR technique. Two h after the injections, each mouse was decapitated and the cervical spinal cord was dissected out. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. First-strand cDNA was synthesized using the SuperScript II first-strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA). PCR was performed with a 5′ primer (AAACCGCATGGAGTGTGTTGTTCC) and a 3′ primer (TCAGACCACCTCGACAATGCATGA) for c-fos, and a 5′ primer (TCGTACCACAGGCATTGTGATGGA) and a 3′ primer (ACTCCTGCTTGCTGATCCACATCT) for β-actin under the following conditions: 94°C for 2 min, 30 cycles at 94°C for 30 sec, 55°C for 45 sec and 72°C for 45 sec and finally 72°C for 10 min for c-fos and 94°C for 2 min, 29 cycles at 94°C for 30 sec, 55°C for 45 sec and 72°C for 1 min and finally 72°C for 10 min for β-actin. The amplified products were subjected to electrophoresis in a 1% agarose gel and stained with ethidium bromide. The image was acquired with a FujiFilm Las-1000 imaging system (FujiFilm Medical Systems, Stamford, CT). The digital images were quantified with Image Gauge software (FujiFilm Medical Systems, Stamford, CT).
Compounds
GNTI (Tocris, Ellisville, MO), compound 48/80 (Sigma, St. Louis, MO) and nalfurafine (a generous gift from Adolor Company, Exton, PA) were dissolved in saline.
Data analysis
Statistical comparisons were performed with one-way ANOVA and Student’s t-test. All data are expressed as the mean ± standard error of the mean (s.e.m.). P< 0.05 was considered statistically significant.
RESULTS
Nalfurafine attenuates GNTI-induced scratching
Mice started scratching 2–3 min after the GNTI injection. This strong, stereotyped behavior continued through the 30 min observation period. Mice pretreated with saline gave 578 ± 78 scratches in 30 min. Pretreatment (at −20 min) with nalfurafine (0.001–0.03 mg/kg) decreased GNTI (0.3 mg/kg)-induced scratching in a dose-dependent manner (Fig. 1a). Furthermore, post treatment (at +5 min) with nalfurafine (0.02 and 0.03 mg/kg) decreased the number of scratches by 50% compared to the control group (Fig. 1b).
Figure 1.
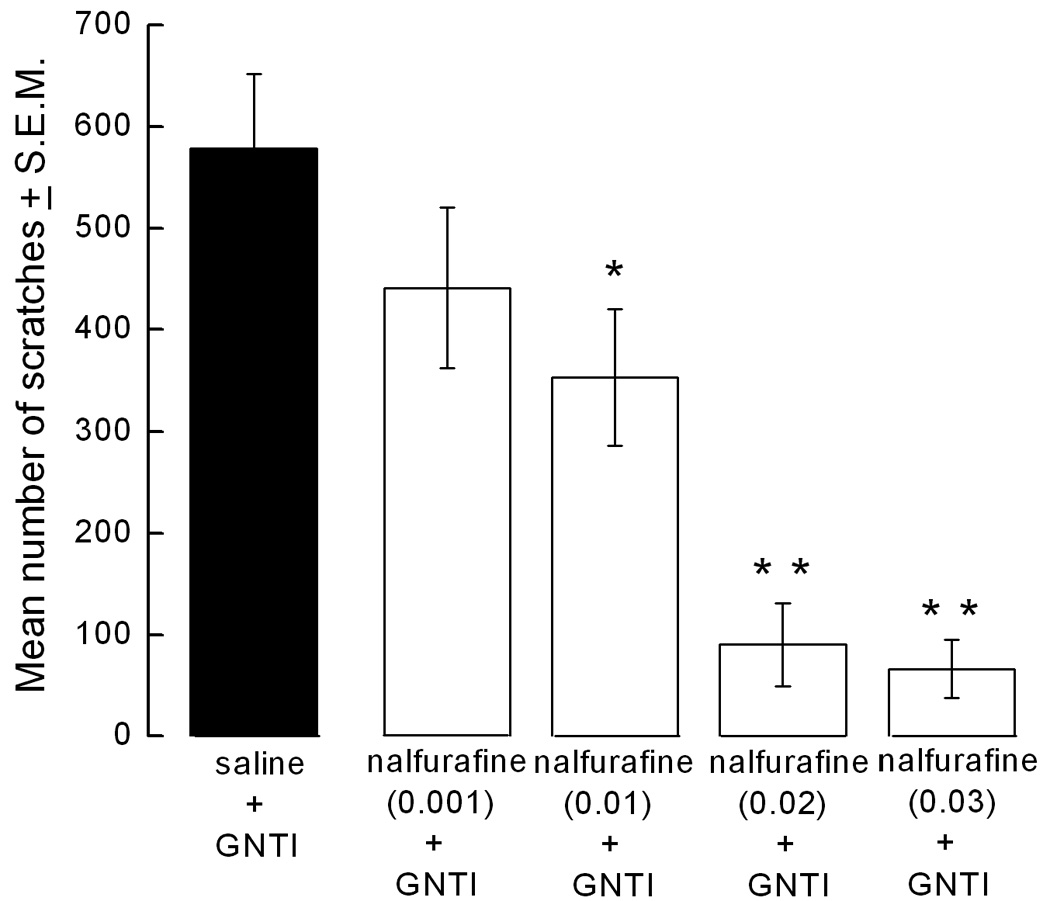
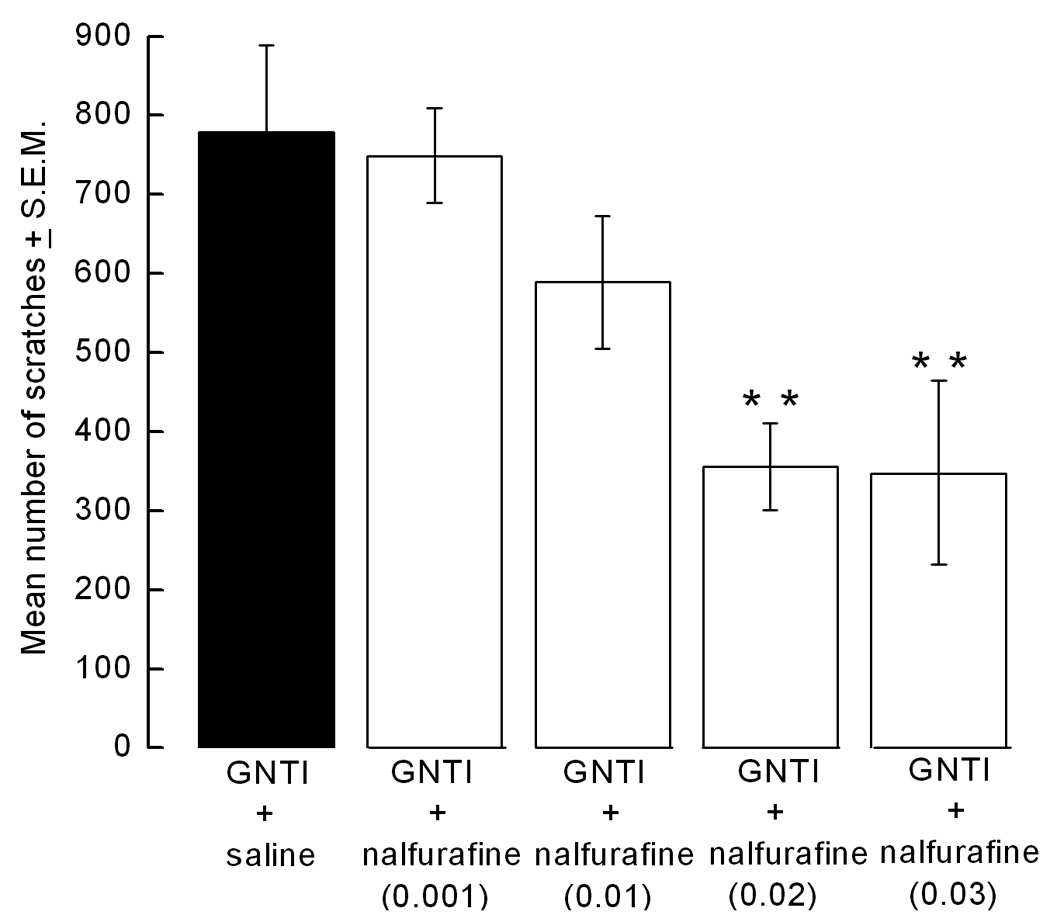
Attenuating effect of nalfurafine on GNTI-induced scratching. a) Pretreatment with nalfurafine (0.001–0.03 mg/kg) inhibits GNTI (0.3 mg/kg)-induced scratching in a dose-dependent manner (*p<0.05, **p<0.01 compared to control). b) Nalfurafine (0.02–0.03 mg/kg) reverses GNTI (0.3 mg/kg)-induced scratching (**p<0.01).
Tolerance study
Tolerance did not develop to the antiscratch activity of nalfurafine over a period of 10 days. The mean number of scratches recorded on day 1 was 32 ± 14 and on day 10 was 16 ± 7 (Fig. 2).
Figure 2.
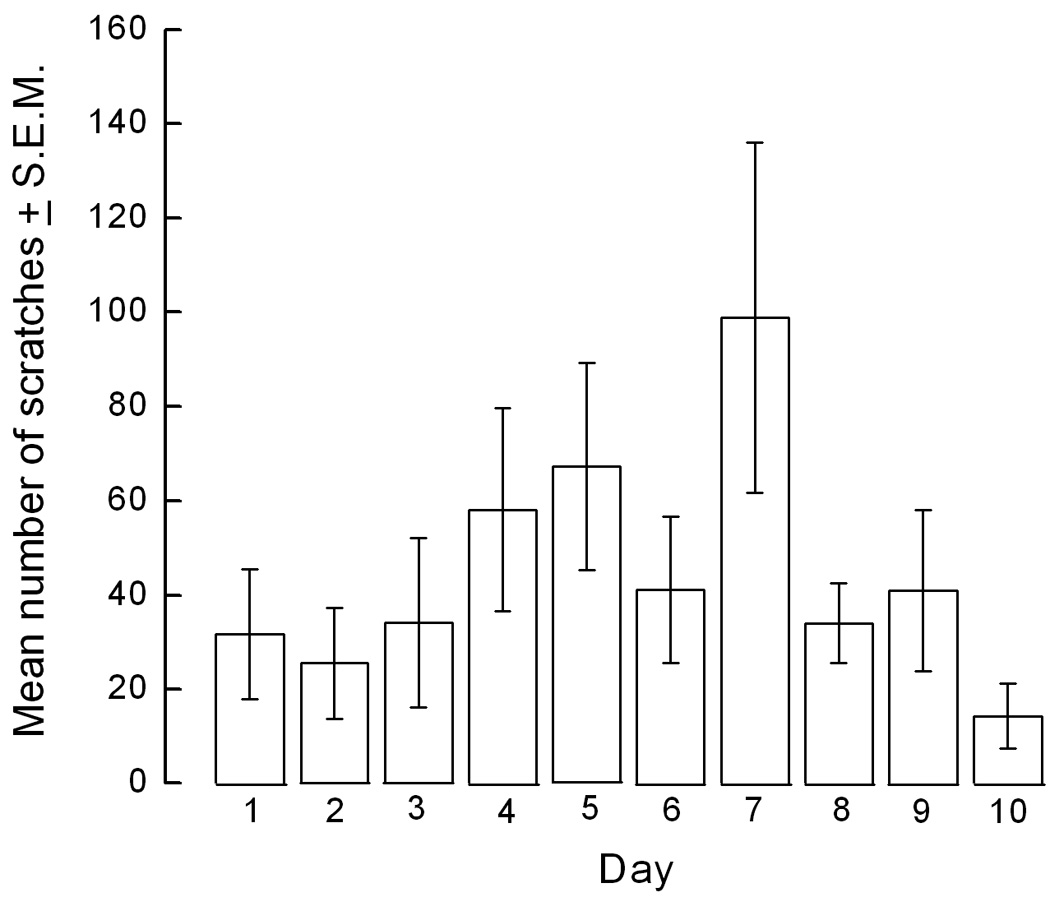
Tolerance does not develop to the antiscratch activity of nalfurafine. Mice were pretreated with either saline or nalfurafine (0.02 mg/kg) and then with GNTI (0.3 mg/kg) and the number of scratches was counted for 30 min for 10 consecutive days.
Locomotor activity
Ambulation was not suppressed in mice injected with our standard dose of nalfurafine (0.02 mg/kg) compared to saline-injected animals. A higher dose of nalfurafine (0.04 mg/kg) inhibited locomotion (p< 0.01) (Fig. 3).
Figure 3.
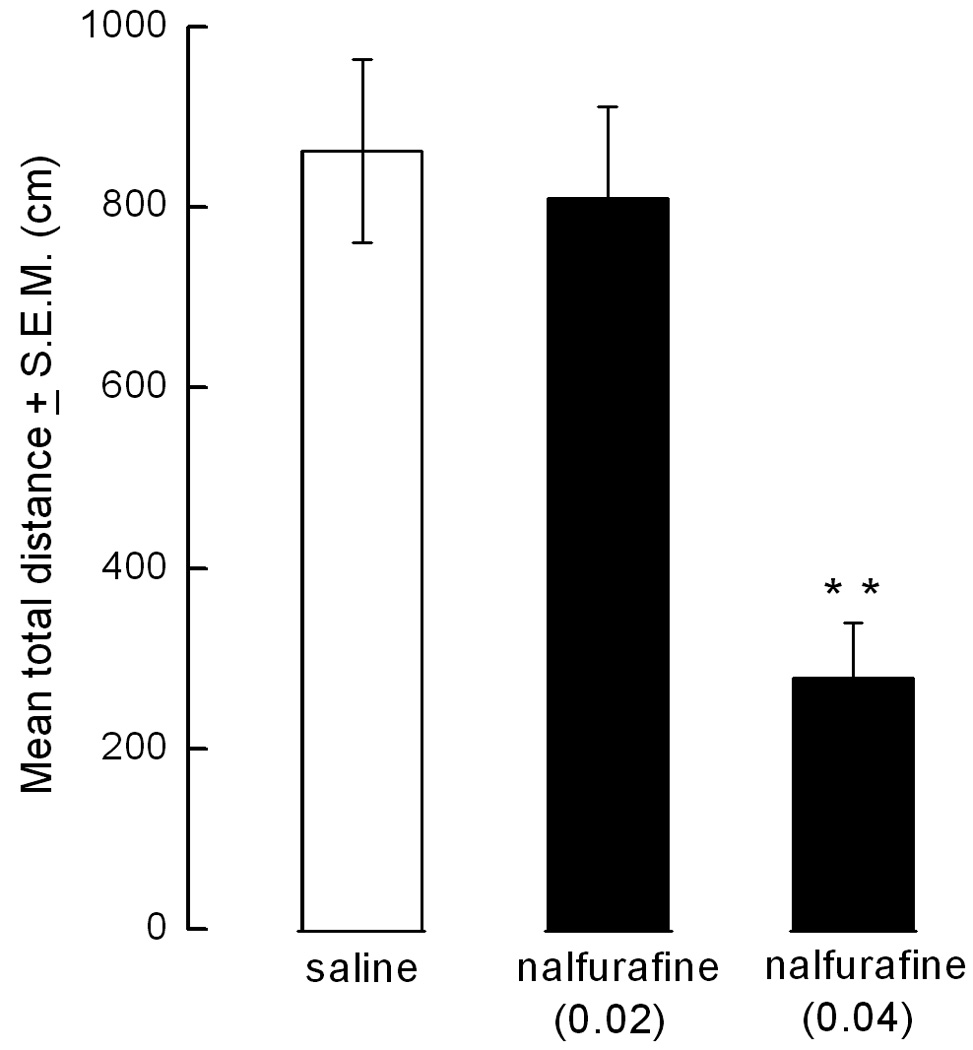
Antiscratch dose of nalfurafine has no effect on locomotion. Nalfurafine (0.02 mg/kg) does not markedly affect locomotion of mice (n=8) whereas the higher dose of nalfurafine (0.04 mg/kg) antagonizes locomotion significantly (p<0.01 compared to control and 0.02 mg/kg of nalfurafine).
GNTI and compound 48/80 induce c-fos expression
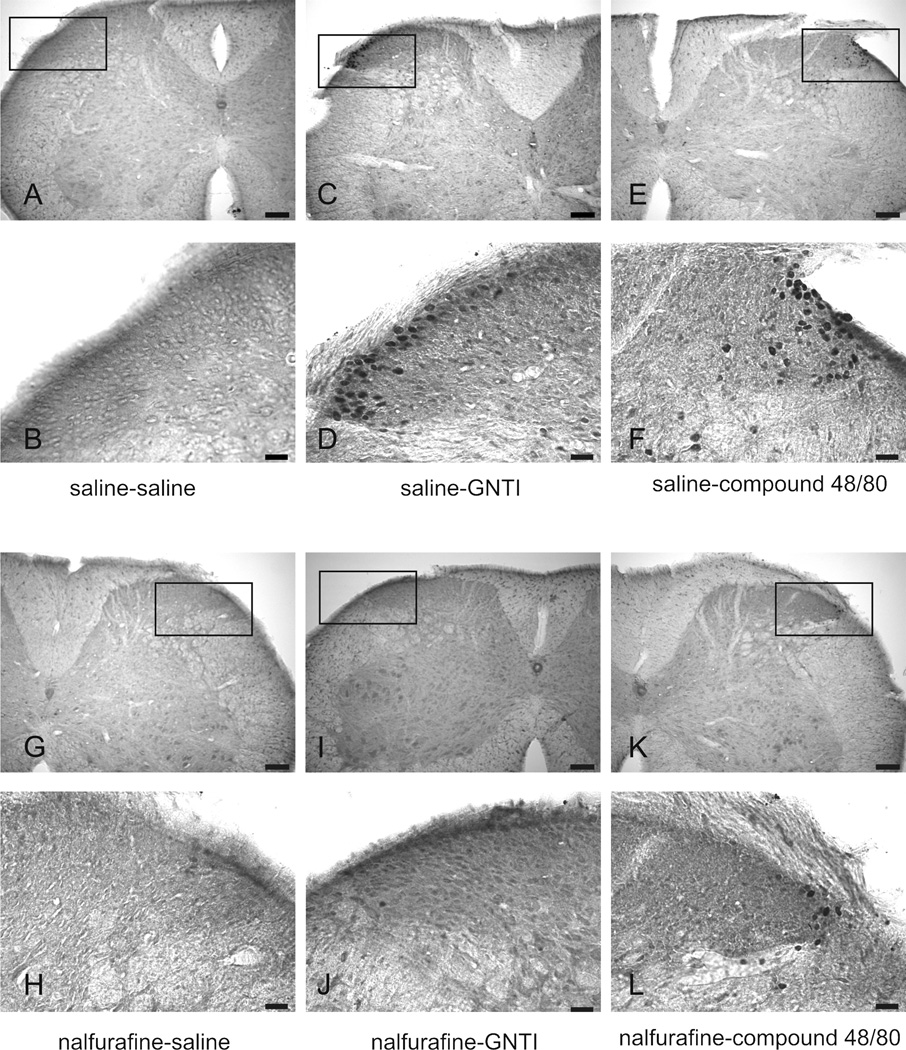
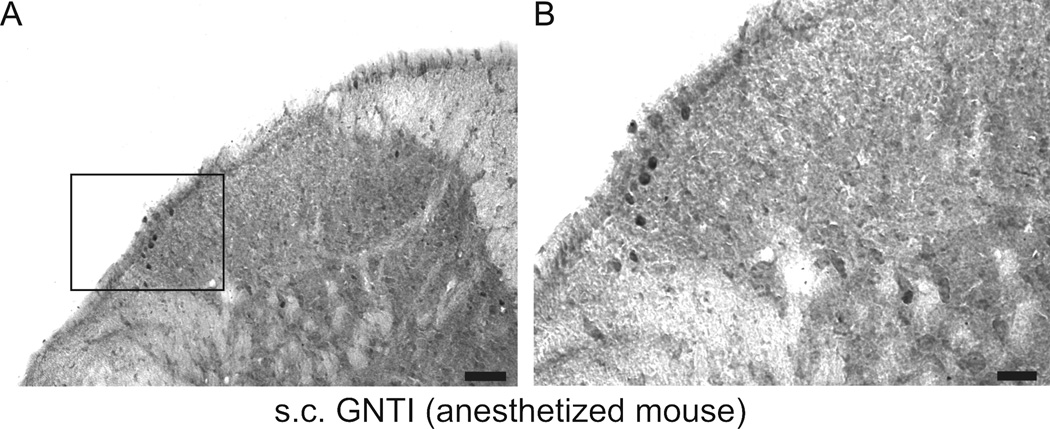
GNTI and compound 48/80, two chemically different scratch-inducing agents, significantly increased the number of c-fos positive nuclei in the lateral side of the superficial layers of the dorsal horn of cervical spinal cord sections (p<0.01, compared to saline + saline and nalfurafine + saline control groups) (Fig. 4a and 4b). GNTI caused more c-fos expression than did compound 48/80. While nalfurafine by itself had no effect on c-fos expression, pretreatment with nalfurafine (0.02 mg/kg) significantly reduced the number of c-fos cells induced by both GNTI and compound 48/80 (p< 0.01, Fig. 4a and 4b). Similarly, c-fos expression was observed on the lateral side of the dorsal horn in anesthetized mice injected with GNTI (Fig. 4c). The animals did not move or scratch during the 2 h period.
Figure 4.
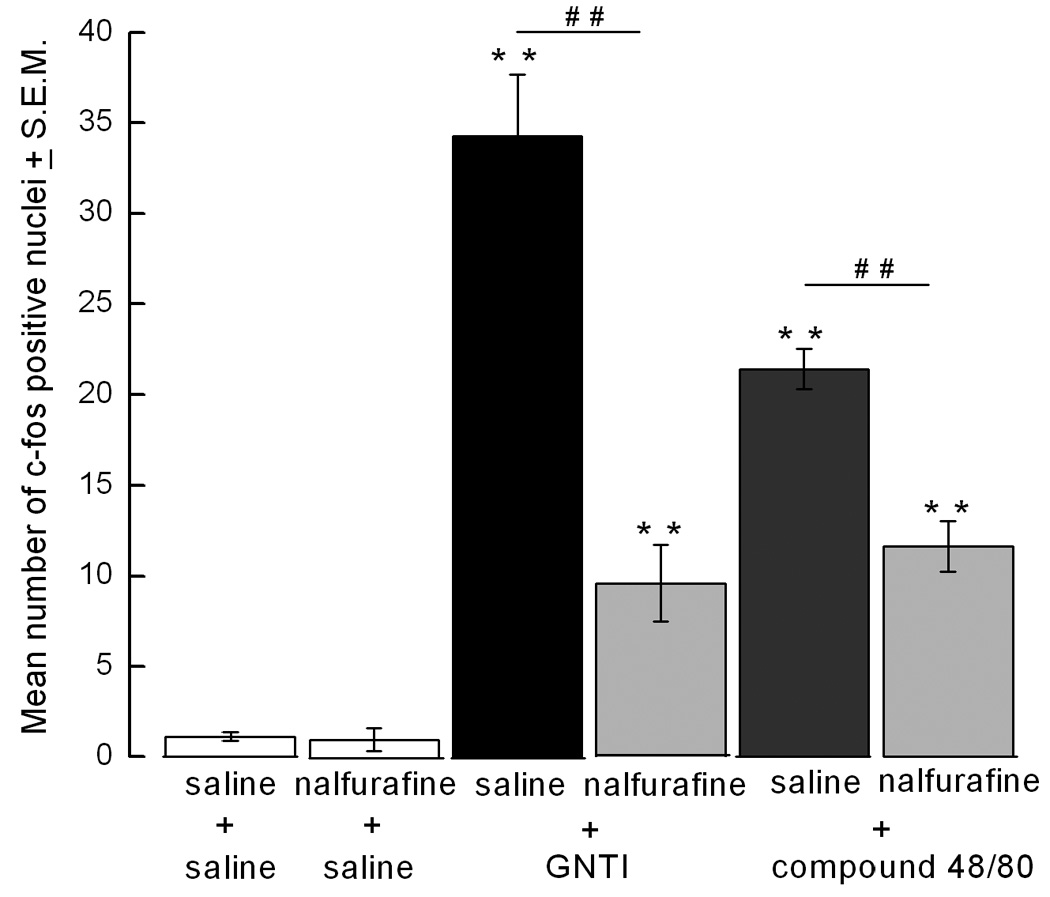
a). Representative photomicrographs of c-fos expression in the spinal cord. C-fos is not detected in the superficial layers of the dorsal horn following saline injection (A and B). GNTI (C, D) and compound 48/80 (E, F) induce c-fos expression on the lateral side of the superficial lamina of the dorsal horn of cervical spinal cord. Nalfurafine inhibits c-fos expression induced by GNTI (I, J) and compound 48/80 (K, L). Injection of nalfurafine does not evoke c-fos expressin (G, H). (Scale bars: for A, C, E, G, I and K 100 µm; for B, D, F, H, J and L 25 µm). b). The number of c-fos positive nuclei was counted and averaged from 10 randomly chosen cervical sections for each animal in each group (n=6) (**p<0.01 represents significance compared to saline-saline and nalfurafine-saline, ##p<0.01 represents significance between saline-GNTI and nalfurafine-GNTI, as well as saline-compound 48/80 and nalfurafine-compound 48/80). c). A representative picture of c-fos expression induced by GNTI in mice (n=2) anesthetized with urethane before GNTI injection. Localization of c-fos expressing neurons in urethane anesthetized mice is similar to the localization of c-fos expressing neurons in awake animals (Scale bar for A is 50 µm and for B is 25 µm).
GNTI increases c-fos mRNA levels in the cervical spinal cord
β-actin bands were observed with every mouse whereas c-fos bands (369 bp) were only observed in mice given GNTI. A representative picture of bands is shown in Fig 5a. The c-fos mRNA level (presented as % of control) was increased significantly in mice injected with GNTI compared to mice injected with saline (p< 0.001, Fig 5b).
Figure 5.
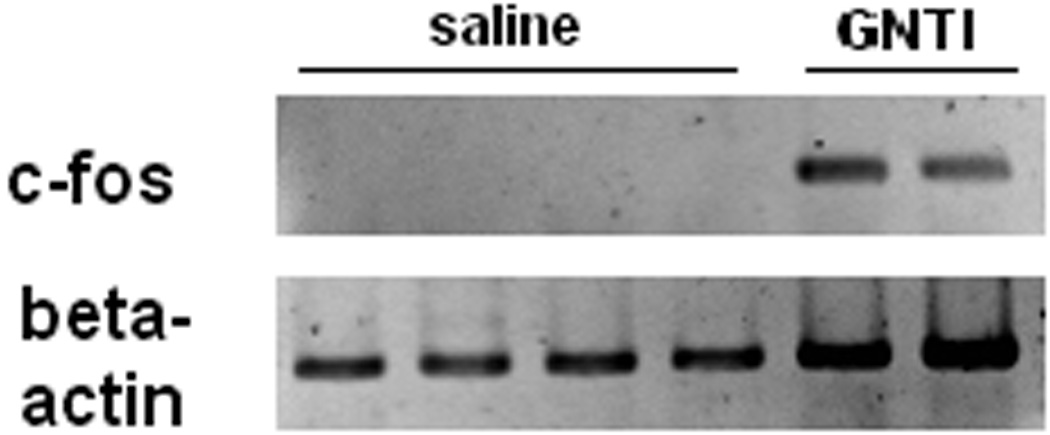
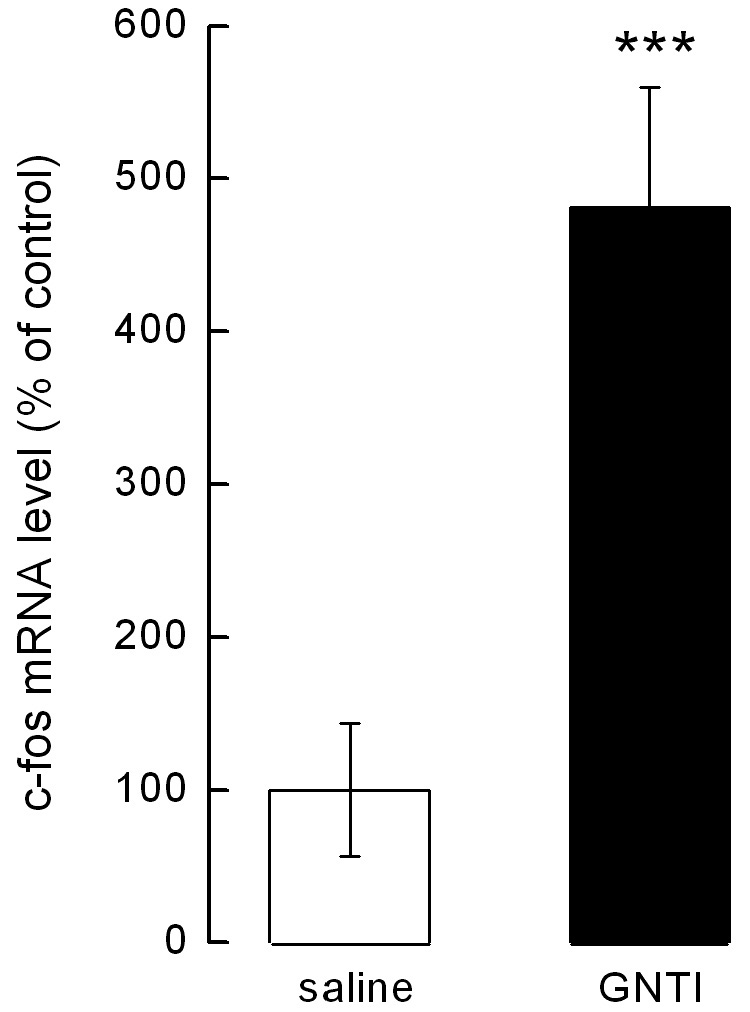
GNTI increases c-fos mRNA levels. a). A representative picture of β-actin and c-fos bands in mice injected with either saline or GNTI (0.3 mg/kg). b). GNTI significantly increases c-fos mRNA level compared to saline (p<0.001).
GNTI induces only an itch sensation
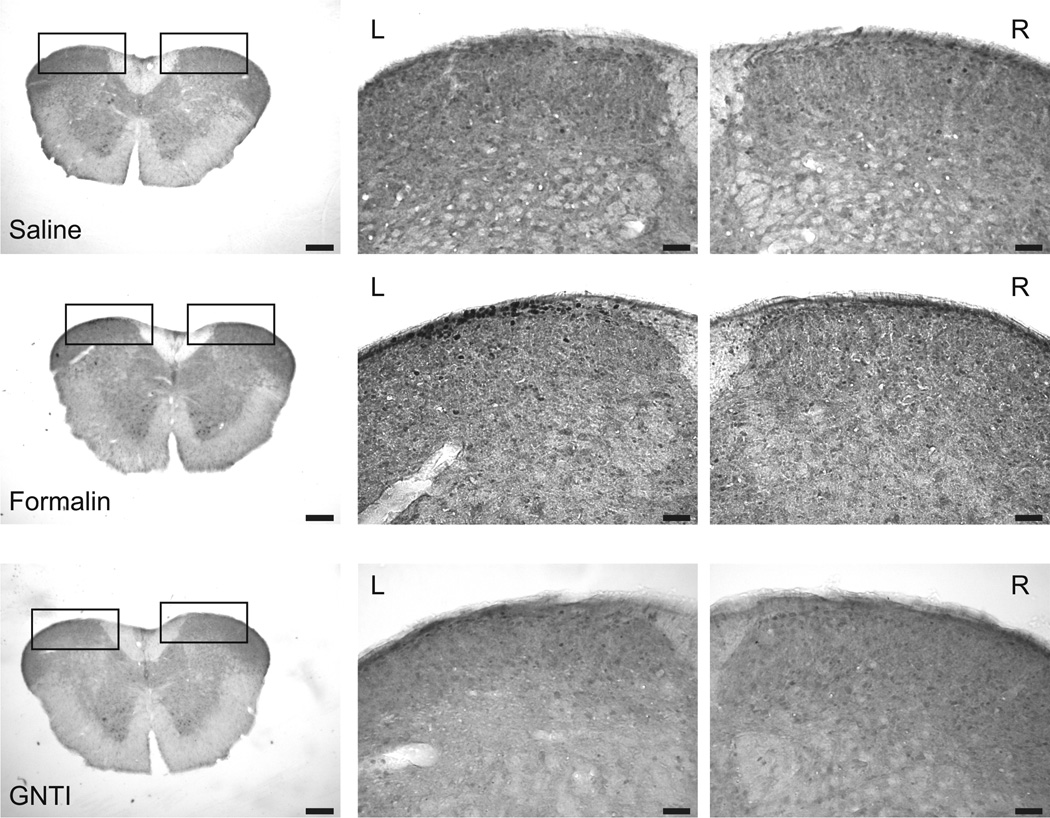
Mice given saline did not wipe or scratch the injected cheek. Animals injected with formalin wiped with the ipsilateral forelimb. Administration of GNTI induced scratching and grooming but not wiping (Fig. 6a, *p<0.05 represents GNTI vs saline and formalin; # #p<0.01 represents formalin vs saline and GNTI).
Figure 6.
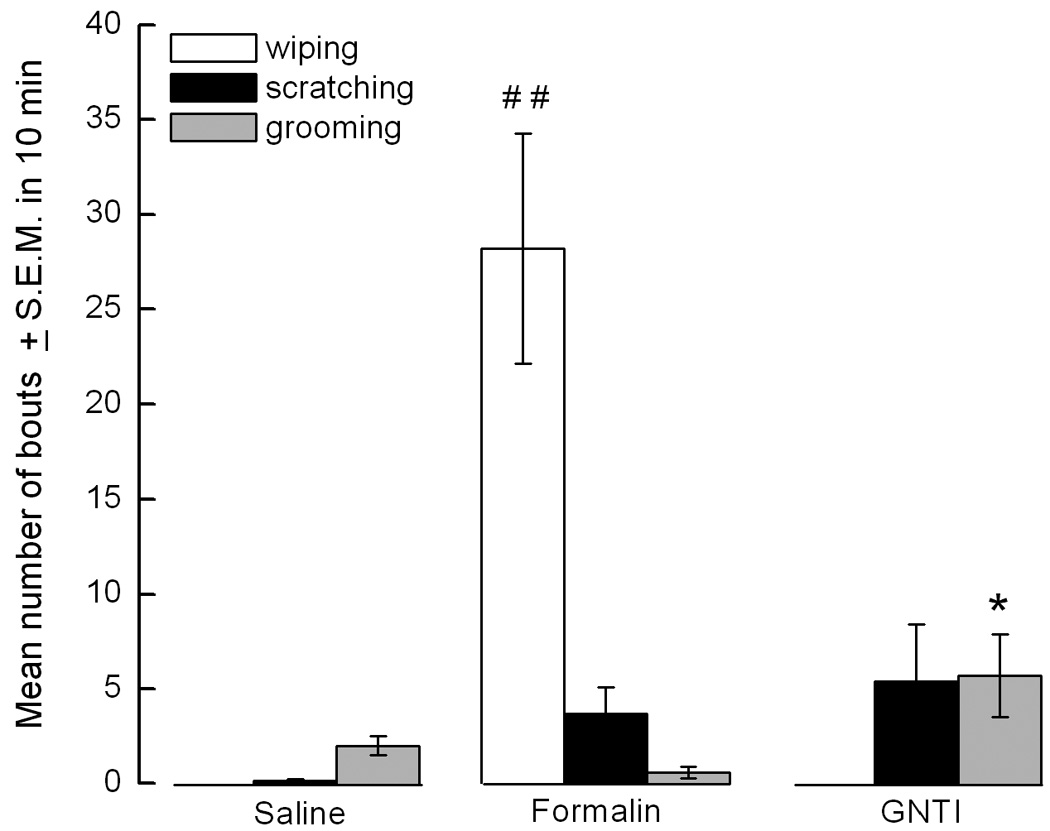
a). GNTI injection (s.c.) to the right cheek of mice induced grooming (*p<0.05 compared to saline and formalin) and scratching to the injected side. Wiping behavior was not observed. Formalin elicited wiping (# #p<0.01 compared to saline and GNTI). b). Representative pictures of c-fos expression in sections, from the junction of cervical spinal cord and medulla, obtained from mice given saline, formalin and GNTI, respectively. Formalin induced c-fos expression in the superficial layer of the dorsal horn (n=3) (Scale: 250 and 50 µm) .
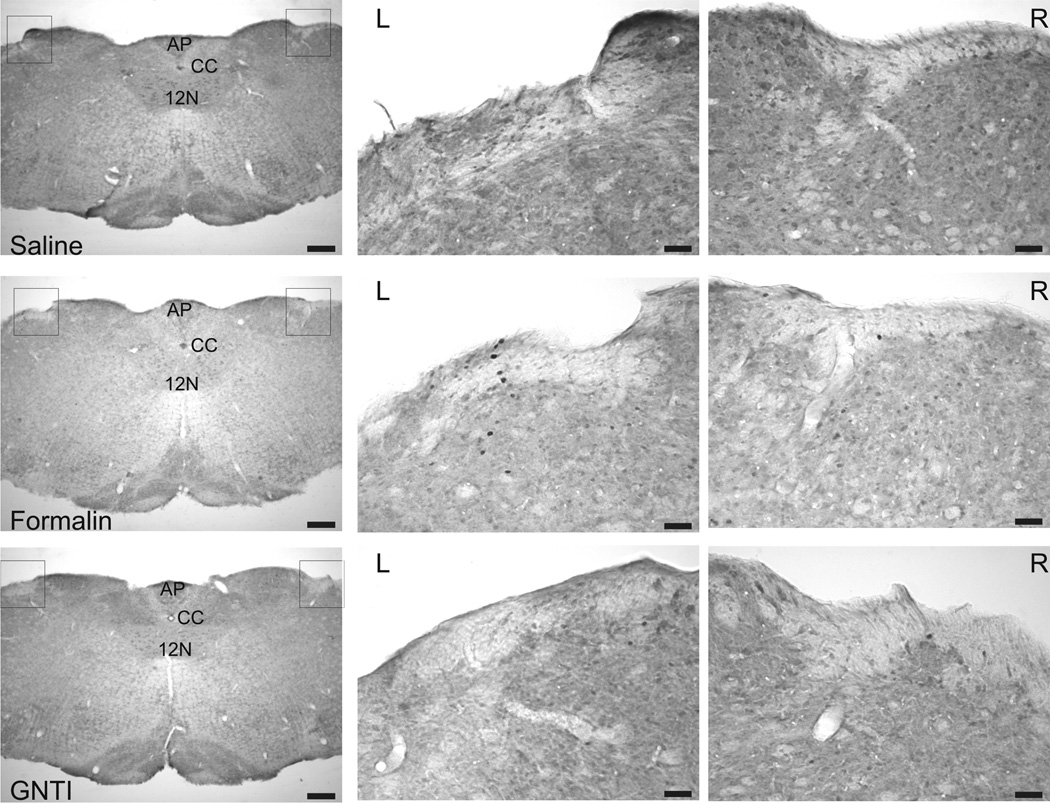
c) Representative pictures of c-fos expression in brain stem sections of mice injected with saline, formalin and GNTI. C-fos expressed neurons were observed in trigeminal nucleus in sections obtained from mice injected with formalin (n=3) (AP: area postrema; CC: central canal, 12N: 12th cranial nerve, Paxinos and Franklin, Fig. 93) (Scale: 250 and 50 µm).
C-fos expression in the brainstem following formalin and GNTI
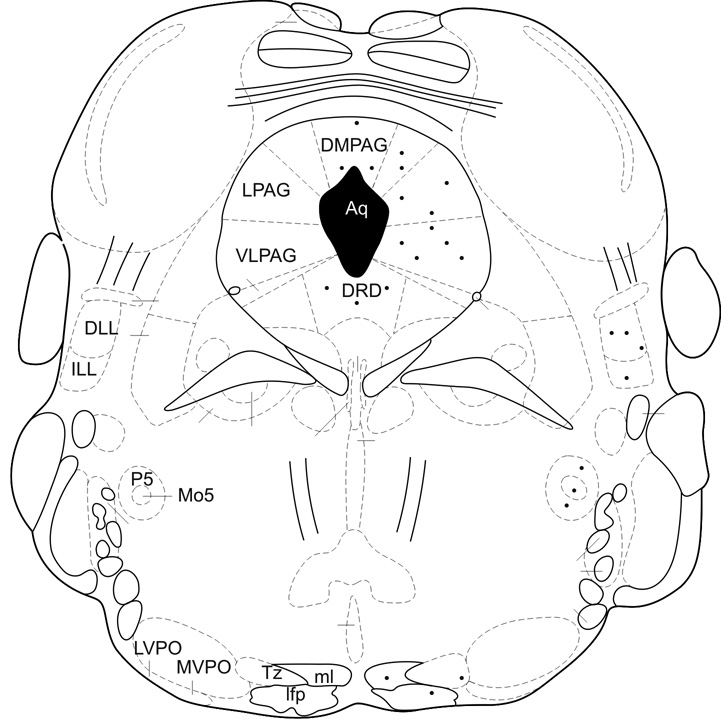
Formalin induced c-fos expression in the superficial layer of the dorsal horn of the junction sections between the cervical spinal cord and medulla. However, c-fos expression was not observed in sections obtained from mice injected with saline or GNTI (Fig. 6b). Similarly, only formalin provoked c-fos expression in the trigeminal nucleus (Fig. 6c) (Paxinos and Franklin, 2001, Fig. 93). Both formalin and GNTI elicited c-fos expression in the periaquaductal grey (PAG); dorsal and intermediate nucleus of the lateral lemniscus (DLL, ILL); peritrigeminal zone (P5) and motor trigeminal nucleus (Mo5); medial lemniscus (ml); longitudinal fasiculus of pons (lfp) and nucleus of trapezoid body (Tz). Figure 7a shows a schematic diagram representative of c-fos expression induced by formalin and GNTI (modified from Paxinos and Franklin, 2001, Fig. 71). Saline did not evoke c-fos expression (Fig. 7b). A representative picture of c-fos expression in the PAG area is shown in figure 7b. Additionally, both GNTI and formalin induced c-fos expression in the central (LPBC) and external (LPBE) areas of the lateral parabrachial nucleus. Saline did not provoke c-fos expression in these areas (Fig. 8) (Paxinos and Franklin, 2001, Fig. 73). Since we aimed to investigate whether itch and pain sensations follow the same pathway when both stimuli are applied to the same area, we used only 3 mice for each group for brain stem sections and did not obtain quantitative data.
Figure 7.
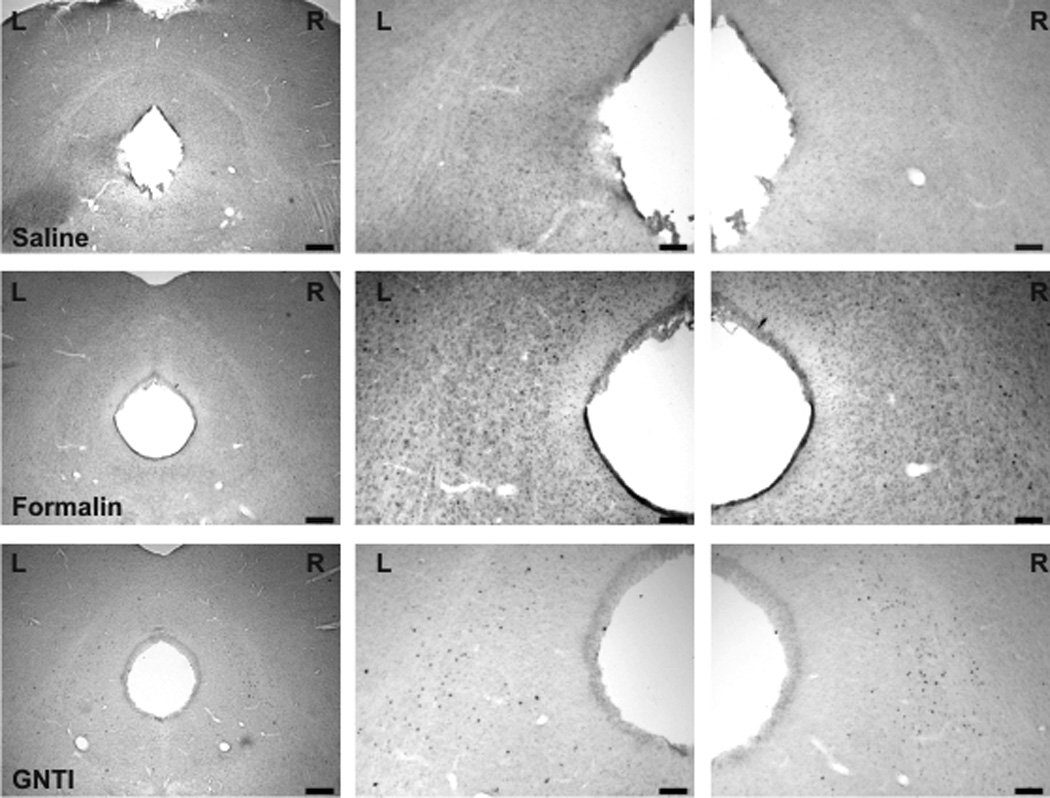
a). A schematic representation of c-fos expression evoked by GNTI-induced itch and formalin-induced nociception in the higher level of the brainstem (DMPAG: dorsomedial periaqueductal gray; DLPAG: dorsolateral periaqueductal gray; LPAG: lateral periaqueductal gray; VLPAG: ventrolateral periaqueductal gray; DRD; dorsal raphe nucleus, dorsal part; DLL: dorsal nucleus of lateral lemniscus; ILL: intermediate nucleus of lateral lemniscus; P5: peritrigeminal zone; Mo5: motor trigeminal nucleus; LVPO: lateroventral periolivary nucleus; MVPO: medioventral periolivary nucleus; Tz: nucleus of trapezoid body; ml: medial lemniscus; lfp: longitudinal fasciculus of pons (modified from Paxinos and Franklin, 2001, Fig. 71). b) A representative picture of c-fos expression in the PAG area in sections obtained from mice injected with saline, formalin and GNTI (Scale: 250 and 100 µm).
Figure 8.
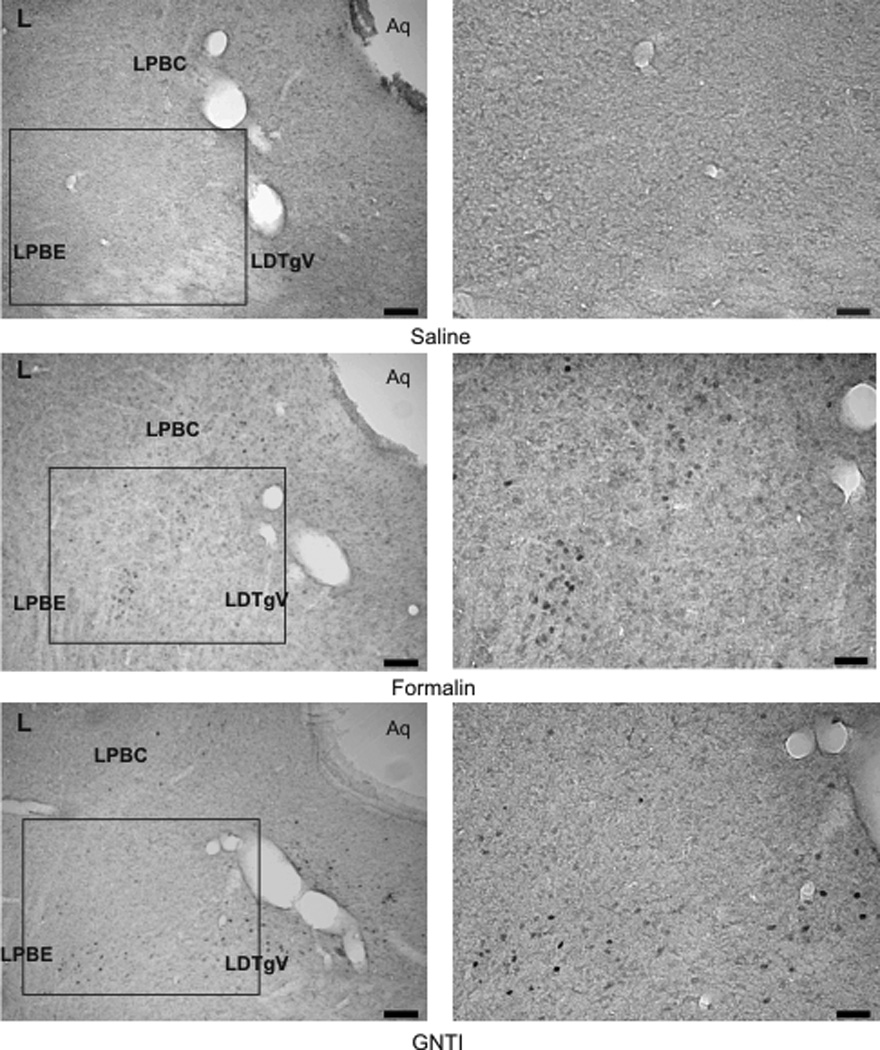
A representative photomicrograph of c-fos expression induced by GNTI and formalin in the parabrachial area of pons (LPBC: lateral parabrachial nucleus, central; LPBE: lateral parabrachial nucleus, external; LDTgV: laterodorsal tegmental nucleus, ventral) (Paxinos and Franklin, 2001, Fig. 73) (Scale: 100 and 50 µm).
DISCUSSION
We report here for the first time that nalfurafine, a kappa opioid receptor agonist with antiscratch activity against several chemically diverse pruritogens, inhibits c-fos expression elicited by both GNTI and compound 48/80 in mice. Our data show that nalfurafine attenuates scratching behavior and prevents neuronal activation by both pruritogens. Our c-fos results suggest that potential antipruritic activity of nalfurafine occurs at the spinal level.
To our knowledge, nalfurafine is the first clinically tested antipruritic to demonstrate an acute inhibitory effect at the neuronal level. Chronic daily injection of olopatadine (an antihistamine used clinically for allergic rhinitis and eczema) to mice suppressed both scratching and spinal c-fos expression induced by contact dermatitis due to repeated application of diphenylcyclopropenone (Hamada et al., 2006). Naltrexone has been studied for antiscratch activity along with inhibition of c-fos immunoreactivity elicited by serotonin in rats (Nojima et al., 2003b). Pretreatment with naltrexone inhibited scratching behavior but this opioid antagonist had no marked effect on c-fos expression induced by serotonin.
Systemically administered GNTI antagonizes kappa agonist-induced diuresis in rats over the dose range 0.10–3 mg/kg (Inan et al., 2009). The same doses of GNTI elicit intense scratching when injected s.c. behind the neck of mice (Cowan and Inan, 2009). This scratching is inhibited by over 80% when the mice are pretreated with nalfurafine (0.02 and 0.03 mg/kg). Importantly, administering these doses of nalfurafine after scratching has started still decreased the stereotyped behavior by 50%, a finding of direct relevance for clinical dermatology. Additionally, the antiscratch activity of nalfurafine is not due to behavioral depression. Our standard dose of nalfurafine (0.02 mg/kg) did not markedly affect locomotor activity. Previously, Suzuki and his colleagues (2004) reported, in agreement with our results, that behavioral depression was associated only with a higher dose of nalfurafine (0.04 mg/kg) on wheel running in mice.
The repeated administration of opioids often causes tolerance to the agonist effect being measured. Efficacy of the compound is decreased over time. In our study, the subchronic injection of nalfurafine did not diminish the antiscratch activity. This result is potentially important regarding the clinical development of nalfurafine as an antipruritic.
Our results show that GNTI and compound 48/80 induce c-fos expression in the lateral part of the superficial layer of the dorsal horn of the spinal cord suggesting that both compounds activate a group of sensory neurons located on the lateral side of lamina I and II. Nakano et al. (2008) reported that histamine, SLIGRL-NH2 (protease-activated receptor-2 agonist), as well as mosquito allergen, provoke c-fos expression in different neurons in the dorsal horn of lamina II. While histamine activated neurons towards the inner side of lamina II, SLIGRL-NH2 and mosquito allergen activated neurons located more towards the outer side of the dorsal horn of lamina II. Similar to our results, stimulation of the group of neurons located on the lateral side of the superficial layer of the dorsal horn was also associated with serotonin- (Nojima et al., 2003b) and dry skin- (Nojima et al., 2003a) induced scratching in rats. These results suggest that different pruritogens stimulate different neuron groups in the dorsal horn. GNTI injection to mice anesthetized with urethane also induced c-fos expression in the lateral side of the dorsal horn. A few c-fos positive nuclei were observed in anesthetized animals in comparison to awake animals. However, it should be noted that Buritova and Besson (2001) reported that pretreatment with urethane significantly reduced (70%) the number of c-fos positive nuclei associated with the intraplantar injection of carrageenan in rats. Also, administration of urethane before cocaine blocked c-fos expression induced by cocaine in rat striatum (Kreuter et al., 2004). In our experiment, urethane reduced c-fos expression induced by GNTI. However, GNTI still evoked c-fos expression on the lateral side of the superficial layer of the dorsal horn.
Lamina I neurons play a role in signaling of pain and thermal stimulation. Neurons that are involved in pain transmission receive input from Aδ- and polymodal C-nociceptors, which are mechano-sensitive and have fast conduction velocities different from C-nociceptors in itch stimulation (Andrew and Craig, 2001). There are many similarities between pain and itch. Brain areas activated by pain and itch have been revealed using central imaging techniques. Thus, thalamus, insular cortex, anterior cingulated cortex, prefrontal cortex, orbitofrontal cortex, supplementary motor and premotor areas, cerebellum and primary somatosensory cortex are regions activated by both algogenic and pruritogenic stimulation (Ikoma et al., 2006).
In order to examine any overlap or difference in the projection of pain and itch, we applied either an algogen or a pruritogen to the right cheek of mice using a recently described model which differentiates pain and itch behaviorally (Shimada and LaMotte, 2008). Our results revealed that mice injected with GNTI only groomed and scratched but did not wipe the injected cheek suggesting that GNTI is a pure pruritogen. These results reinforce the role of nalfurafine as an effective antipruritic agent. Also, our results show that when GNTI is administered locally, a higher dose of GNTI, compared to systemic administration, is required to evoke a mild scratching effect (30 µg vs. 8–9 µg/mouse). This might be a reason why GNTI did not induce c-fos expression in the superficial layer of the dorsal horn around the junction of the cervical spinal cord and medulla. Also, the itch stimulus might not reach the threshold to induce c-fos expression in this area. GNTI and formalin both induced c-fos expression in brain areas that are involved in ascending and descending sensory processing (parabrachial nucleus and PAG). Since formalin provoked c-fos expression in the trigeminal nucleus is similar to that following experimental tooth movement in rats (Yamashiro et al., 1997) and c-fos expression in the PAG area is similar to that following noxious stimulation of the superior sagittal sinus in the cat (Knight et al., 2005), it may be proposed that pain and itch sensations are projected differently in the brain stem.
Taken together, our results indicate that a) the kappa opioid system is involved, at least in part, in the pathogenesis of scratch, b) two chemically diverse pruritogens, GNTI and compound 48/80, induce c-fos immunoreactivity on the lateral side of the superficial layer of the dorsal horn, and nalfurafine inhibits scratching behavior as well as c-fos expression elicited by these compounds suggesting nalfurafine holds promise as a potentially useful antipruritic in human conditions involving itch.
Acknowledgements
This work was supported in part by NIH Grants NS18710 and P30DA013429. The study was presented at the 39th Annual Meeting of the International Narcotics Research Conference, held in Charleston, SC, USA, on July 13–18, 2008. There is no conflict of interest.
Abbreviations
- GNTI
5′-guanidinonaltrindole
- PBS
phosphate buffer saline
- RT-PCR
reverse transcriptase polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nature Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Bergasa NV. Update on the treatment of the pruritus of cholestasis. Clin Liver Dis. 2008;12:219–234. doi: 10.1016/j.cld.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Black SH, Chauvignac C, Grundt P, Miller CN, Wood S, Traynor JR, Lewis JW, Husbands SM. Guanidino N-substituted and N,N-disubstituted derivatives of the κ-opioid antagonist GNTI. J Med Chem. 2003;46:5505–5511. doi: 10.1021/jm0309203. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Dun SL, Ohsawa M, Yin D, Yang J, Chang JK, Brailoiu E, Dun NJ. Kiss-1 expression and metastatin-like immunoreactivity in the rat brain. J Comp Neurol. 2005;481:314–329. doi: 10.1002/cne.20350. [DOI] [PubMed] [Google Scholar]
- Buritova J, Besson JM. Urethane anaesthesia could partly mask antinociceptive effects of non-steroidal anti-inflammatory drugs: a spinal c-fos protein study. Brain Res. 2001;891:281–284. doi: 10.1016/s0006-8993(00)03254-6. [DOI] [PubMed] [Google Scholar]
- Cowan A, Kehner GB. Antagonism by opioids of compound 48/80-induced scratching in mice. Br J Pharmacol. 1997;122 Suppl:169P. [Google Scholar]
- Cowan A, Inan S, Kehner GB. GNTI, a kappa receptor antagonist, causes scratching in mice. Pharmacologist. 2002;44 Supp. 1:A51. [Google Scholar]
- Cowan A, Inan S. Kappa-opioid antagonists as pruritogenic agents. In: Dean RL, Bilsky EJ, Negus SS, editors. Opioid receptors and antagonists: from bench to clinic (volume in the contemporary neuroscience series) New York: Humana Press; 2009. pp. 533–542. Chapter 28. [Google Scholar]
- Endoh T, Matsuura H, Tajima A, Izumimoto N, Tajima C, Suzuki T, Saitoh A, Suzuki T, Narita M, Tseng L, Nagase H. Potent antinociceptive effects of TRK-820, a novel kappa-opioid receptor agonist. Life Sci. 1999;65:1685–1694. doi: 10.1016/s0024-3205(99)00417-8. [DOI] [PubMed] [Google Scholar]
- Endoh T, Tajima A, Suzuki T, Kamei J, Narita M, Tseng L, Nagase H. Characterization of the antinociceptive effects of TRK-820 in the rat. Eur J Pharmacol. 2000;387:133–140. doi: 10.1016/s0014-2999(99)00815-8. [DOI] [PubMed] [Google Scholar]
- Endoh T, Tajima A, Izumimoto N, Suzuki T, Saitoh A, Suzuki T, Narita M, Kamei J, Tseng LF, Mizoguchi H, Nagase H. TRK-820, a selective kappa-opioid agonist, produces potent antinociception in cynomolgus monkeys. Jpn J Pharmacol. 2001;85:282–290. doi: 10.1254/jjp.85.282. [DOI] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in gastrointestinal mucosa: 3. Reactivity towards compound 48/80. Acta Pathol Microbiol Scand. 1966;66:313–322. doi: 10.1111/apm.1966.66.3.313. [DOI] [PubMed] [Google Scholar]
- Gmerek DE, Cowan A. Role of opioid receptors in bombesin-induced grooming. Ann N Y Acad Sci. 1988;525:291–300. doi: 10.1111/j.1749-6632.1988.tb38614.x. [DOI] [PubMed] [Google Scholar]
- Hamada R, Seike M, Kamijima R, Ikeda M, Kodama H, Ohtsu H. Neuronal conditions of spinal cord in dermatitis are improved by olopatadine. Eur J Pharmacol. 2006;547:45–51. doi: 10.1016/j.ejphar.2006.06.058. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nature Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Igeta K, Kim JF, Nagao M, Shiraishi N, Nakamura N, Nagai H. Involvement of unique mechanisms in the induction of scratching behavior in BALB/c mice by compound 48/80. Eur J Pharmacol. 2002;448:175–183. doi: 10.1016/s0014-2999(02)01933-7. [DOI] [PubMed] [Google Scholar]
- Inan S, Cowan A. Kappa opioid agonists suppress choloroquine-induced scratching in mice. Eur J Pharmacol. 2004;502:233–237. doi: 10.1016/j.ejphar.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Inan S, Cowan A. Agmatine-induced stereotyped scratching in mice is antagonized by nalfurafine, a kappa opioid agonist. Pharmacologist. 2006a;48:38. [Google Scholar]
- Inan S, Cowan A. Nalfurafine, a kappa opioid receptor agonist, inhibits scratching behavior secondary to cholestasis induced by chronic ethynylestradiol injections in rats. Pharm Biochem Behav. 2006b;85:39–43. doi: 10.1016/j.pbb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Inan S, Lee DY, Liu-Chen LY, Cowan A. Comparison of the diuretic effects of chemically diverse kappa opioid agonists in rats: nalfurafine, U50,488H, and salvinorin A. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:263–270. doi: 10.1007/s00210-008-0358-8. [DOI] [PubMed] [Google Scholar]
- Jones RM, Portoghese PS. 5′-Guanidinonaltrindole, a highly selective and potent κ-opioid receptor antagonist. Eur J Pharmacol. 2000;396:49–52. doi: 10.1016/s0014-2999(00)00208-9. [DOI] [PubMed] [Google Scholar]
- Kamei J, Nagase H. Norbinaltorphimine, a selective kappa-opioid receptor antagonist, induces an itch-associated response in mice. Eur J Pharmacol. 2001;418:141–145. doi: 10.1016/s0014-2999(01)00941-4. [DOI] [PubMed] [Google Scholar]
- Kehner GB, Gaul F, Zang WY, Daubert JD, Cassel JA, DeHaven RN, DeHaven-Hudkins DL, Cowan A. Stereoselective antipruritic effects of enadoline and GR 94839 in mice. FASEB J. 1999;13:A801. [Google Scholar]
- Knight YE, Classey JD, Lasalandra MP, Akerman S, Kowacs F, Hoskin KL, Goadsby PJ. Pattrens of fos expression in the rostral medulla and caudal pons evoked by noxious craniovascular stimulation and periaqueductal gray stimulation in the cat. Brain Res. 2005;1045:1–11. doi: 10.1016/j.brainres.2005.01.091. [DOI] [PubMed] [Google Scholar]
- Ko MC, Lee H, Harrison C, Clark MJ, Song HF, Naughton NN, Woods JH, Traynor JR. Activation of kappa-opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J Pharmacol Exp Ther. 2003;305:173–179. doi: 10.1124/jpet.102.044909. [DOI] [PubMed] [Google Scholar]
- Ko MC, Husbands SM. Effects of atypical κ-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates. J Pharmacol Exp Ther. 2009;328:193–200. doi: 10.1124/jpet.108.143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koibuchi Y, Ichikawa A, Nakagawa M, Tomita K. Histamine release induced from mast cells by active components of compound 48/80. Eur J Pharmacol. 1985;115:163–170. doi: 10.1016/0014-2999(85)90687-9. [DOI] [PubMed] [Google Scholar]
- Kreuter JD, Mattson BJ, Wang B, You Z-B, Hope BT. Cocaine-induced fos expression in rat striatum is blocked by chloral hydrate or urethane. Neurosci. 2004;127:233–242. doi: 10.1016/j.neuroscience.2004.04.047. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–232. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- Nagase H, Hayakawa J, Kawamura K, Kawai K, Takezawa Y, Matsuura H, Tajima C, Endo T. Discovery of a structurally novel opioid kappa-agonist derived from 4,5-epoxymorphinan. Chem Pharm Bull. 1998;46:366–369. doi: 10.1248/cpb.46.366. [DOI] [PubMed] [Google Scholar]
- Nakano T, Andoh T, Lee J, Kuraishi Y. Different dorsal horn neurons responding to histamine and allergic itch stimuli. NeuroReport. 2008;19:723–726. doi: 10.1097/WNR.0b013e3282fdf6c5. [DOI] [PubMed] [Google Scholar]
- Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Carstens MI, Carstens E. C-fos expression in superficial dorsal horn of cervical spinal cord associated with spontaneous scratching in rats with dry skin. Neurosci Lett. 2003a;347:62–64. doi: 10.1016/s0304-3940(03)00609-8. [DOI] [PubMed] [Google Scholar]
- Nojima H, Simons CT, Cuellar JM, Carstens MI, Moore JA, Carstens E. Opioid modulation of scratching and spinal c-fos expression evoked by intradermal serotonin. J Neurosci. 2003b;23:10784–10790. doi: 10.1523/JNEUROSCI.23-34-10784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TS, Freedman BI, Yosipovitch G. An update on pruritus associated with CKD. Am J Kidney Dis. 2007;50:11–20. doi: 10.1053/j.ajkd.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. California: Academic Press; 2001. [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mice. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Umakoshi K, Nojiri N, Kamei C. Effects of histamine H1 receptor antagonists on compound 48/80-induced scratching behavior in mice. Eur J Pharmacol. 1998;351:1–5. doi: 10.1016/s0014-2999(98)00288-x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Izumimoto N, Takezawa Y, Fujimura M, Togashi Y, Nagase H, Tanaka T, Endoh T. Effect of repeated administration TRK-820, a kappa-opioid receptor agonist, on tolerance to its antinociceptive and sedative actions. Brain Res. 2004;995:167–175. doi: 10.1016/j.brainres.2003.09.057. [DOI] [PubMed] [Google Scholar]
- Togashi Y, Umeuchi H, Okano K, Ando N, Yoshitaka Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H. Antipruritic activity of the κ-opioid receptor agonist, TRK-820. Eur J Pharmacol. 2002;435:259–264. doi: 10.1016/s0014-2999(01)01588-6. [DOI] [PubMed] [Google Scholar]
- Wakasa Y, Fujuwara A, Umeuchi H, Endoh T, Okano K, Tanaka T, Nagase H. Inhibitory effects of TRK-820 on systemic skin scratching induced by morphine in rhesus monkeys. Life Sci. 2004;75:2947–2957. doi: 10.1016/j.lfs.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzbrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of three distinct κ ligands (salvinorin A, TRK-820 and 3FLB) on κ opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- Wikström B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, Ogasawara M, Kawashima Y, Ueno K, Mori A, Ueno Y. κ-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005;16:3742–3747. doi: 10.1681/ASN.2005020152. [DOI] [PubMed] [Google Scholar]
- Yamashiro T, Nakagawa K, Satoh K, Moriyama H, Takada K. C-fos expression in the trigeminal sensory complex and pontine parabrachial areas following experimental tooth movement. NeuroReport. 1997;8:2351–2353. doi: 10.1097/00001756-199707070-00049. [DOI] [PubMed] [Google Scholar]